Abstract
Background: At present, only one systematic review has investigated the effect of levothyroxine (LT4) in the treatment of euthyroid pregnant women with thyroid autoimmunity, but some problems [such as merging different types of research for meta-analysis, lacking neonatal outcomes, and so on] exist in this study, satisfactory results can not be provided. So, this systematic review was performed to investigate the effect of LT4 in euthyroid pregnant women with thyroid autoimmunity, in the hope of providing more comprehensive evidence for clinical use.
Methods: Medline (Ovid), Embase (Ovid), and Cochrane Central Register of Controlled Trials were electronically searched from database inception to March 2022. We included cohort studies and RCTs that evaluated the impact of LT4 therapy on pregnancy and neonatal outcomes in euthyroid pregnant women with thyroid autoimmunity. Meta-analyses of different types of studies were performed separately, and meta-analyses were further performed by only including researches with low and moderate risk of bias. We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to evaluate the quality of evidence, and used TSA to test the sufficiency of the evidence.
Results: Finally, 2,901 euthyroid pregnant women with thyroid autoimmunity in six RCTs and five cohort studies were included. In all outcomes, no statistically significant differences were found between LT4 group and control group, including miscarriage [RR = 0.85, 95%CI (0.69,1.05), p = 0.14, I 2 = 1%], preterm birth [RR = 0.80, 95%CI (0.59,1.08), p = 0.14, I2 = 0%], preeclampsia [RR = 0.68, 95%CI (0.12, 3.91), p = 0.66, I 2 = 0%], placenta abruption [Peto’ OR = 0.14, 95%CI (0.00, 6.94), p = 0.32, I 2 = 0%], birth weight [MD = -36.00, 95%CI (-170.41, 98.41), p = 0.60, I 2 = 0%], gestational age at delivery [MD = -0.10, 95%CI (-0.61, 0.41), p = 0.70, I 2 = 0%] and neonatal admission [RR = 1.33, 95%CI (0.21, 8.58), p = 0.76, I 2 = 0%]. The results for all outcomes were insufficient and inconclusive as demonstrated by TSA. The GRADE assessments showed that the quality of evidence of 4 outcomes (miscarriage, preterm birth, birth weight and gestational age at delivery) were moderate, and 3 outcomes (preeclampsia, placenta abruption and neonatal admission) were low or very low.
Conclusion: For pregnancy and neonatal outcomes in euthyroid pregnant women with thyroid autoimmunity, we did not find benefit of LT4 treatment in this study.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022346745, identifier CRD42022346745.
Keywords: levothyroxine, pregnancy outcomes, neonatal outcomes, euthyroid pregnant women, thyroid autoimmunity
1 Introduction
Thyroid autoimmunity is characterized by elevated thyroid peroxidase antibody (TPOAb) and/or thyroglobulin antibody (TgAb) and found in 2%–17% of pregnant women (Benhadi et al., 2007; Pearce et al., 2008; Abbassi-Ghanavati et al., 2010; Ashoor et al., 2010; Moreno-Reyes et al., 2013). According to the 2017 American Thyroid Association (ATA) guideline, TPOAb-positive pregnant women with 2.5–4.0 mIU/L thyroid-stimulating hormone (TSH) in early pregnancy are considered as euthyroid pregnant women with thyroid autoimmunity (Alexander et al., 2017). Currently, levothyroxine (LT4) is widely used in these women. However, in this issue, the recommendations provided in different guidelines differ markedly. For example, LT4 is recommended for pregnant women with TSH 2.5–4.0 mIU/L and TPOAb in early pregnancy in the 2019 Chinese Medical Association (CMA) guideline (Association CM, 2019), but LT4 is weakly recommended for them in the 2017 American Thyroid Association (ATA) guideline; What’s more, LT4 therapy is not recommended for pregnant women with TSH 2.5–4.0 mIU/L and TPOAb in early pregnancy in the 2020 American College of Obstetricians and Gynecologists (ACOG) guideline (Autho r Anonymous, 2020).
At present, only one meta-analysis explored the impact of LT4 treatment in pregnant euthyroid women with thyroid autoimmunity (Sun et al., 2020). However, a series of problems exist in this study. Firstly, data of different types of studies (cohort studies and RCTs) were synthesized in one meta-analysis (Cochrane Training, 2022). Because of methodological heterogeneity, it may result in incorrect results (Cochrane Training, 2022). Secondly, only pregnancy outcomes were assessed in this study, and neonatal outcomes were not assessed. Thirdly, in this study, neither the GRADE method nor TSA were performed to evaluate the quality of the current evidence and to test whether the current studies had enough statistical power to reach a firm conclusion. Both of them are important for high-quality systematic review and meta-analysis.
So, we performed a systematic review, meta-analysis and TSA to evaluate the effect of LT4 treatment on pregnancy and neonatal outcomes in euthyroid pregnant women with thyroid autoimmunity, in the hope of providing more comprehensive and credible evidence for clinical use.
2 Materials and methods
This study was reported in line with the PRISMA (Moher et al., 2009).
2.1 Inclusion criteria
2.1.1 Participants
Euthyroid pregnant women with positive TPOAb and/or TgAb. The diagnosis was based on the diagnostic criteria in the 2017 ATA guideline.
2.1.2 Interventions and controls
LT4 compared with no treatment or placebo. Dosages and treatment duration were not limited.
2.1.3 Outcomes
Primary outcomes: 1) pregnancy outcomes: miscarriage, preterm birth, preeclampsia.
Secondary outcomes: 1) pregnancy outcomes: placenta abruption; 2) neonatal outcomes: gestational age at delivery, birth weight, neonatal admission.
2.1.4 Types of studies
RCTs and cohort studies were enrolled in this systematic review.
2.2 Exclusion criteria
Full-text not available; outcome data not available; duplicate publications.
2.3 Data sources and retrieval strategy
We searched databases including Medline (Ovid), EMbase (Ovid) and the Cochrane Central Register of Controlled Trials from inception to March 2022. The references of the included studies were also checked for additional studies. The retrieval strategy of Medline (Ovid) is shown in Supplementary Table S1.
2.4 Study selection and data extraction
The titles and abstracts of all studies were screened by 2 researchers independently to determine which studies were potentially relevant. Then, two reviewers independently assessed the full texts. If there were any disagreements, a third researcher resolved it.
2 researchers independently extracted the data using a form. The extracted information included basic information of the study, basic information of the pregnant women, interventions and comparations, sample sizes and outcome data.
2.5 Assessment of risk of bias
2 researchers assessed the risk of bias, and we resolved the disagreements were by discussion with a third researcher.
For RCTs, we used the RoB2 tool recommended by the Cochrane Handbook to evaluated the risk of bias (Sterne et al., 2019) and this tool provided a bias summary for every study rated as “low risk of bias”, “some concerns (moderate risk of bias)” or “high risk of bias”.
For cohort studies, we used the Newcastle-Ottawa Quality Assessment Scale (NOS) to evaluated the risk of bias (Stang, 2010). 3 aspects were included in this tool and a score of 7–9 was rated as “low risk of bias”, 4–6 was rated as “moderate risk of bias”, and 0–3 was rated as “high risk of bias”.
2.6 Quality of evidence
For each outcome, the GRADE was used in evaluating the quality of the evidence (Atkins et al., 2004). 5 aspects (risk of bias, reporting biases, imprecisions, inconsistencies and indirectness) were considered and the quality of evidence was classified into 4 levels: high, moderate, low and very low (Balshem et al., 2011).
2.7 Statistical analysis
Meta-analyses were performed by RevMan 5.4. For dichotomous data, we calculated the relative risk (RR) or odds ratio (OR) with 95% confidence interval (CI) to evaluate the effect measure, and for continuous data, we calculated the mean difference (MD) with 95% CI to evaluate the effect measure. Besides, we calculated the Peto’s OR with 95% CI for dichotomous outcomes with zero-events. We assessed heterogeneity by I-squared (I 2 ) test. If heterogeneity was acceptable (I 2 ≤ 50%), we used a fixed effect model, otherwise, we used a random effect model.
2.7.1 Meta-analysis
We separately performed meta-analyses of different types of studies. We also performed meta-analyses by only including researches with low and moderate risk of bias to evaluate the influence of poor-quality studies. For each outcome, we drew conclusions by following the rules below (Guyatt et al., 2011):
If the evidence quality of RCTs was higher than the evidence quality of cohort studies, conclusion was drew based on the meta-analysis result of RCTs. Otherwise, conclusion was drew based on the meta-analysis result of cohort studies. If there were no obvious difference in the meta-analysis result between all studies and studies with low and moderate risk of bias, conclusion was drew based on the result of all studies. If obvious difference exist between them, conclusion was drew based on the result of studies with low and moderate risk of bias.
2.7.2 TSA analysis
We conducted TSA using the TSA viewer version 0.9.5.10 Beta to test whether the included studies had enough statistical power to reach a firm conclusion (Copenhagen Trial Unit, 2022).
3 Results
3.1 Results of search and study selection
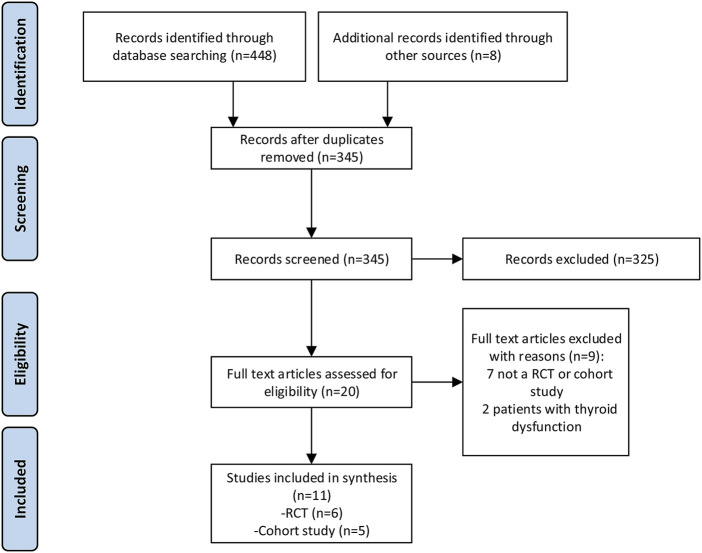
In the initial search, we identified a total of 448 studies. Finally, 11 studies (6 RCTs and 5 cohort studies) met our inclusion criteria and were enrolled in this study (Figure 1).
FIGURE 1.
Study flow diagram.
3.2 Study characteristics
11 studies (Negro et al., 2005; Negro et al., 2006; Revelli et al., 2009; Lepoutre et al., 2012; Mosaddegh et al., 2012; Negro et al., 2016; Nazarpour et al., 2017; Wang et al., 2017; Dhillon-Smith et al., 2019; Dal Lago et al., 2021; Tsunemi et al., 2021) comprising 2,901 euthyroid pregnant women with thyroid autoimmunity were included. These studies took place in a variety of countries: 3 RCTs and 2 cohort studies in Italy, 1 RCT and 1 cohort study in Iran, 1 RCT in China, 1 RCT in United Kingdom, 1 cohort study in Japan and 1 cohort study in Belgium. Details of the included studies are summarized in Table 1.
TABLE 1.
Characteristics of the included studies.
| Study | Types of study | Country | Sample size | Age (year) | TSH (normal range; mIU/L) | Baseline TSH (median/mean, LT4/control) | Defintion of TPOAb positivity (IU/ml) | ||
|---|---|---|---|---|---|---|---|---|---|
| LT4 | Control | LT4 | Control | ||||||
| Negro 2005 | RCT | Italy | 36 | 36 | 29.2 ± 4.0 | 30.1 ± 5.0 | 0.27–4.2 | 1.9/1.7 mIU/L | >100 |
| Negro 2006 | RCT | Italy | 57 | 58 | 30.0 ± 5.0 | 30.0 ± 6.0 | 0.27–4.2 | 1.6/1.7 mIU/L | >100 |
| Negro 2016 | RCT | Italy | 198 | 195 | 28.9 ± 5.2 | 29.9 ± 5.1 | 0.5–2.5 | 1.4/1.4 mIU/L | >16 |
| Nazarpour 2017 | RCT | Iran | 18 | 24 | 26.6 ± 5.8 | 27.0 ± 4.7 | 0.1–4 | 3.7/3.2 mIU/L | >50 |
| Wang 2017 | RCT | China | 300 | 300 | 31.3 ± 3.9 | 31.7 ± 3.8 | 0.5–4.78 | 2.9/2.1 mIU/L | ≥60 |
| Dhillon-Smith 2019 | RCT | United Kingdom | 476 | 476 | 32.5 ± 4.9 | 32.7 ± 4.9 | 0.44–3.63 | 2.1/2.0 mIU/L | >99% concordance in the U.K. NEQAS IIA |
| Revelli 2009 | Cohort study | Italy | 55 | 38 | 35.1 ± 4.1 | 37.0 ± 3.5 | NR | 2.1/2.0 mIU/L | ≥35 |
| Lepoutre 2012 | Cohort study | Belgium | 49 | 47 | 31.5 ± 5.5 | 32.5 ± 5.3 | 0.2–3.5 | NR mIU/L | ≥9 |
| Mohammad 2012 | Cohort study | Iran | 39 | 6 | NR | NR | NR | NR mIU/L | >40 |
| Alessandro 2021 | Cohort study | Italy | 227 | 230 | 36.3 ± 4.4 | 34.7 ± 4.4 | 0.4–2.5 | 1.7/1.4 mIU/L | unknown |
| Tsunemi A 2021 | Cohort study | Japan | 25 | 11 | 36.1 ± 5.3 | 36.8 ± 5.0 | 2.5-URL | 3.4/3.3 mIU/L | ≥16 |
RCT, randomized controlled trial; LT4, levothyroxine; TSH, thyroid-stimulating hormone; NR, not reported; URL, upper reference limit.
3.3 Assessment of risk of bias
The risk of bias assessments are shown in Supplementary Tables S2, S3. For RCTs, the following features were determined to be at high risk of bias: randomization process in 1 RCT (Nazarpour et al., 2017), deviations from intended interventions in 2 studies (Negro et al., 2006; Negro et al., 2016), measurement of outcomes in 2 studies (Negro et al., 2006; Negro et al., 2016). Overall, 3 RCTs were at a high risk of bias (Negro et al., 2006; Negro et al., 2016; Nazarpour et al., 2017), 2 RCTs had some concerns (moderate risk of bias) (Negro et al., 2005; Wang et al., 2017), and 1 RCT were at a low risk of bias (Dhillon-Smith et al., 2019). For cohort studies, 4 studies were at a high risk of bias (Revelli et al., 2009; Lepoutre et al., 2012; Mosaddegh et al., 2012; Dal Lago et al., 2021), and 1 study was at moderate risk of bias (Tsunemi et al., 2021).
3.4 Meta-analysis results
3.4.1 Primary outcome
3.4.1.1 Miscarriage
6 RCTs and 5 cohort studies reported miscarriage.
For RCTs, the results found no significant difference between LT4 group and control group in miscarriage [RR = 0.85, 95%CI (0.69,1.05), p = 0.14, I 2 = 1%] (Table 2). When only including researches with low and moderate risk of bias, there was still no obvious difference between two groups in miscarriage [RR = 0.91, 95%CI (0.71,1.17), p = 0.47, I 2 = 12%]. (Table 2).
TABLE 2.
Results of meta-analysis for all outcomes.
| Outcome | Included studies | Number of studies | Number of patients | MD/RR/OR | 95% CI | P | I 2 (%) | Model |
|---|---|---|---|---|---|---|---|---|
| /Peto’s OR | ||||||||
| Primary outcomes | ||||||||
| Miscarriage | all RCTs | 5 | 1,313 | 0.85 | 0.69-1.05 | 0.14 | 1 | Fix |
| RCTs with low to moderate risk of bias | 2 | 585 | 0.91 | 0.71-1.17 | 0.47 | 12 | Fix | |
| all cohort study | 5 | 397 | 0.12 a | 0.07-0.19 | <0.001 | 62 | Fix | |
| Cohort study with low to moderate risk of bias | 1 | 19 | 1.5 | 0.12-18.36 | 0.75 | 0 | Fix | |
| Preterm birth | all RCTs | 5 | 1,282 | 0.80 | 0.59-1.08 | 0.14 | 0 | Fix |
| RCTs with low to moderate risk of bias | 1 | 540 | 0.85 | 0.53-1.36 | 0.49 | 0 | Fix | |
| all Cohort study | 2 | 134 | 0.95 a | 0.24-3.79 | 0.94 | 0 | Fix | |
| Preeclampsia | all RCTs | 1 | 115 | 0.68 | 0.12-3.91 | 0.66 | 0 | Fix |
| Secondary outcomes | ||||||||
| Placenta abruption | all RCTs | 1 | 115 | 0.14 a | 0.00-6.94 | 0.32 | 0 | Fix |
| all cohort study | 1 | 96 | 7.09 a | 0.14-357.80 | 0.33 | 0 | Fix | |
| Birth weight | all RCTs | 1 | 375 | −36.00 | -170.41-98.41 | 0.60 | 0 | Fix |
| Gestational age at delivery | all RCTs | 1 | 374 | −0.10 | -0.61-0.41 | 0.70 | 0 | Fix |
| Neonatal admission | all RCTs | 1 | 42 | 1.33 | 0.21-8.58 | 0.76 | 0 | Fix |
Peto’s OR; RCTs, randomized controlled trials; MD, mean difference; OR, odds ratio; RR, relative risk; CI, confidence interval; I 2 , statistical heterogeneity. According to the pre-defined rules, the meta-analysis results of all RCTs were used to draw conclusions for each outcome.
For cohort studies, the results of all studies showed that LT4 group compared with the control group had a lower risk of miscarriage [Peto’ OR = 0.12, 95%CI (0.07, 0.19), p < 0.01, I 2 = 62%] (Table 1). When we excluded studies with high risk of bias, no obvious difference between two groups in miscarriage were found [RR = 1.50, 95%CI (0.12,18.36), p = 0.75, I 2 = 0%]. (Table 2).
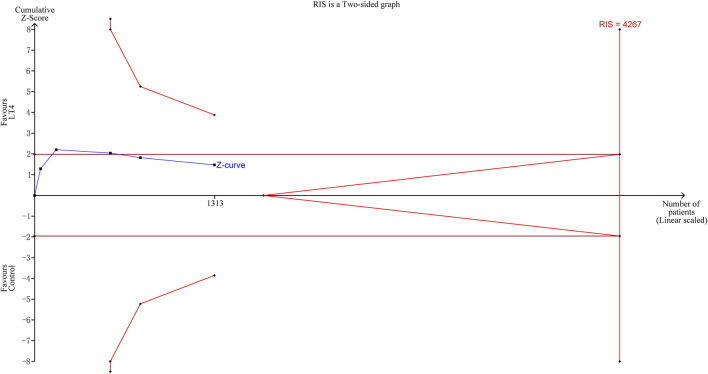
TSA indicated that the cumulative information size (n = 1,313) was 31% of required information size (RIS) (n = 4,267). The cumulative Z curve did not cross the trial sequential monitoring boundary or the futility boundary, indicating that current evidence was insufficient and inconclusive. (Figure 2).
FIGURE 2.
Trial sequential analysis of miscarriage. The risk of type I error was set at 5% with a power of 80%. The relative risk reduction (RRR) was set at 20%. The variance was calculated from the data obtained from the included trials.
The GRADE assessment showed that the quality of evidence for miscarriage was moderate (Supplementary Table S4).
3.4.1.2 Preterm birth
5 RCTs and 2 cohort studies reported preterm birth.
For RCTs, the results found no significant difference between LT4 group and control group in preterm birth [RR = 0.80, 95%CI (0.59,1.08), p = 0.14, I 2 = 0%]. When only including researches with low and moderate risk of bias, there was still no obvious difference between two groups in preterm birth [RR = 0.85, 95%CI (0.53,1.36), p = 0.49, I 2 = 0%]. (Table 2).
For cohort studies, the results found no significant difference between LT4 group and control group in preterm birth [Peto’ OR = 0.95, 95%CI (0.24, 3.79), p = 0.94, I 2 = 0%]. (Table 2).
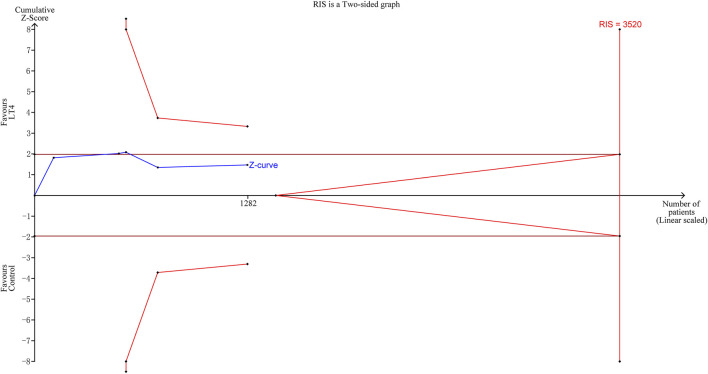
TSA indicated that the cumulative information size (n = 1,282) was 36% of RIS (n = 3,520). The cumulative Z curve did not cross the trial sequential monitoring boundary or the futility boundary, indicating that current evidence was insufficient. (Figure 3).
FIGURE 3.
Trial sequential analysis of preterm birth. The risk of type I error was set at 5% with a power of 80%. The relative risk reduction (RRR) was set at 20%. The variance was calculated from the data obtained from the included trials.
The GRADE assessment showed that the quality of evidence for preterm birth was moderate. (Supplementary Table S4).
3.4.1.3 Preeclampsia
A total of 1 RCT reported preeclampsia.
the results found no significant difference between LT4 group and control group in preeclampsia [RR = 0.68, 95%CI (0.12, 3.91), p = 0.66, I 2 = 0%]. (Table 2).
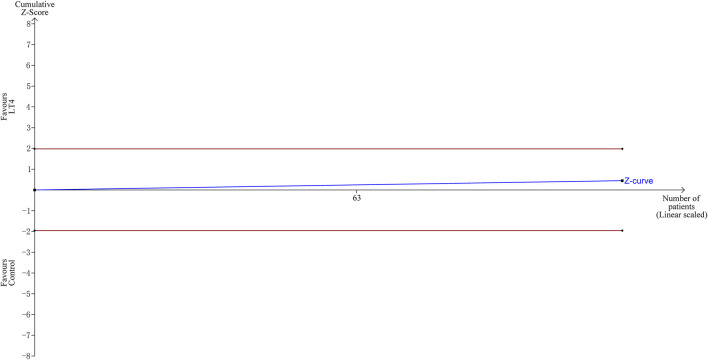
TSA indicated that the cumulative information size (n = 115) was 1% of RIS (n = 13493). The cumulative Z-curve did not cross the trial sequential monitoring boundary or the futility boundary, indicating that current evidence was insufficient and inconclusive. (Figure 4).
FIGURE 4.
Trial sequential analysis of preeclampsia. The risk of type I error was set at 5% with a power of 80%. The relative risk reduction (RRR) was set at 20%. The variance was calculated from the data obtained from the included trials.
The GRADE assessment showed that the quality of evidence for preeclampsia was very low. (Supplementary Table S4).
3.4.2 Secondary outcomes
The meta-analyses of RCTs found no significant difference between LT4 group and control group in placenta abruption [Peto’ OR = 0.14, 95%CI (0.00, 6.94), p = 0.32, I 2 = 0%], birth weight [MD = -36.00, 95%CI (-170.41, 98.41), p = 0.60, I 2 = 0%], gestational age at delivery [MD = -0.10, 95%CI (-0.61, 0.41), p = 0.70, I 2 = 0%] or neonatal admission [RR = 1.33, 95%CI (0.21, 8.58), p = 0.76, I 2 = 0%]. The meta-analyses of cohort studies also found no significant difference between two groups in placenta abruption [Peto’ OR = 7.09, 95%CI (0.14, 357.80), p = 0.33, I 2 = 0%]. (Table 2).
TSA indicated that the current evidences for placenta abruption, birth weight, gestational age at delivery and neonatal admission were insufficient and inconclusive. (Supplementary Figures S1–S4).
The GRADE assessment showed that for birth weight and gestational age at delivery, the quality of evidence was moderate; for placenta abruption and neonatal admission, the quality of evidence was very low. (Supplementary Table S4).
4 Discussion
4.1 Principle findings
To our knowledge, this study firstly explored the impact of LT4 in euthyroid pregnant women with thyroid autoimmunity using the TSA method and the GRADE approach, and is the most comprehensive systematic review assessing this effect. Our study did not find obvious differences between two groups (LT4 vs control group) in all pregnancy and neonatal outcomes (miscarriage, preterm birth, preeclampsia, placenta abruption, birth weight, gestational age at delivery and neonatal admission). TSA indicated that the result for all primary and secondary outcomes were insufficient and inconclusive. The GRADE assessments indicated that the quality of evidences for 4 outcomes (miscarriage, preterm birth, birth weight and gestational age at delivery) were moderate, and the quality of evidences for the other 3 outcomes (preeclampsia, placenta abruption and neonatal admission) were low or very low quality.
4.2 Compared with previous studies
Many systematic reviews have assessed the role of LT4 treatment in pregnant women with thyroid autoimmunity (Rao et al., 2019; Sun et al., 2020; Wang et al., 2020; Lau et al., 2021). However, these studies synthesized the data of both pregnant women with subclinical hypothyroidism and euthyroid pregnant women. Rao et al., in 2019 (Rao et al., 2019) enrolled 5 RCT and 2 cohort study of women with thyroid autoimmunity and found that LT4 significantly in reduced the risk of pregnancy loss. However, patients with subclinical hypothyroidism were also included and an important RCT published in 2019 (Dhillon-Smith et al., 2019) were not enrolled. This RCT assessed the impact of LT4 in euthyroid women who had thyroid autoimmunity and a history of miscarriage or infertility, and this study reached an unexpected conclusion that LT4 did not reduce the adverse outcomes (Dhillon-Smith et al., 2019). Wang et al. (Wang et al., 2020)in 2020 assessed the impact of LT4 in pregnant patients with thyroid autoantibodies and 6 RCT were included with a conclusion that LT4 showed no obvious benefit for the target patients. Lorraine Lau et al. (Lau et al., 2021) in 2021 also assessed the role of LT4 in pregnant women with thyroid autoimmunity and did not found difference between LT4 and control groups. The two researches also enrolled patients with subclinical hypothyroidism. Only one study (Sun et al., 2020) restricted the population to euthyroid pregnant women with thyroid autoimmunity. However, a series of problems exist in this study, including synthesizing different types of studies (RCTs and cohort studies) for meta-analysis, lacking neonatal outcomes, lacking TSA analysis, lacking evaluation of the quality of evidence. Thus, the above-mentioned systematic reviews cannot provide guidance on the need for LT4 therapy in euthyroid pregnant women with thyroid autoimmunity. Our study resolved these problems and provided more comprehensive and accurate results than the above-mentioned study.
4.3 Explanation of unexpected findings
Although significant differences between LT4 and control groups were not found in our study, trends toward decreased risks of some outcomes (such as miscarriage and preterm birth) exist. Moreover, results of TSA indicated that statistical power of the current RCTs for these outcomes were not enough to reach firm conclusions. So, in our study, these negative results might be induced by the relatively small sample size and be altered by future high-quality studies.
4.4 Implications for clinical recommendations
Currently, LT4 is frequently applied in euthyroid pregnant women with thyroid autoimmunity, particularly in China. Besides, the two most widely used guidelines (the 2017 ATA guideline (Alexander et al., 2017) and the 2019 CMA guideline (Association CM, 2019)) both recommend LT4 treatment for euthyroid pregnant women with thyroid autoimmunity, although the strength of recommendations differs among these two guidelines. However, our study did not find evidence of benefit for LT4 therapy in euthyroid pregnant women with thyroid autoimmunity.
Several studies have demonstrated that the presence of TPOAb in euthyroid pregnant women is associated with an increased risk of miscarriage, that is 2–3 times higher than in pregnant women without TPOAb (Chen and Hu, 2011; Thangaratinam et al., 2011). Most clinical studies mainly focused on the adverse impact of low thyroid hormone level on pregnant women, but some studies also demonstrated the adverse impact of high thyroid hormone level. Animal studies have found that high level of thyroid hormone adversely affect brain development (Marta et al., 1998), and a large prospective cohort study in 2016 investigated the association of maternal thyroid function with child intelligence quotient (IQ) and brain morphology (Korevaar et al., 2016). This cohort study found that both low and high maternal free thyroxine concentrations during pregnancy were associated with lower child IQ and lower grey matter and cortex volume. When excluding women with overt hypothyroidism and overt hyperthyroidism, all associations did not change, indicating that LT4 treatment during pregnancy may increase the potential risk of adverse offspring outcomes, regardless of the thyroid function.
Based on our results and the potential adverse impact of LT4 on offspring outcomes, the widespread use of LT4 in euthyroid pregnant women with thyroid autoimmunity may not be appropriate, and some revision may be required for the recommendations of these two guidelines. Moreover, the 2020 ACOG guideline does not recommend LT4 therapy for euthyroid pregnant women with thyroid autoimmunity (Autho r Anonymous, 2020), which is supported by our results and need to be taken into consideration in future clinical practice. In addition, according to our TSA results, large sample size RCTs of high quality are still warranted in the future to reach a firm conclusion. So, pros and cons of LT4 must be fully weight before making the decision.
4.5 Strengths and limitations
Our study has many strengths. Firstly, we restricted study population to euthyroid pregnant women with thyroid autoimmunity only and assessed both pregnancy and neonatal outcomes, which could precisely and comprehensively answer the clinical question of whether LT4 therapy is required in euthyroid pregnant women with thyroid autoimmunity. Secondly, our study used the well recommended statistical method to deal with zero-events in meta-analysis (Sweeting et al., 2004; Xu et al., 2021), and performed TSA to test whether the current evidence was sufficient, which further ensured the reliability of our study.
Our study also has some limitations. Firstly, the number of included researches and the total sample size were relatively limited, which induced inadequate statistical power to draw firm conclusions for some outcomes. Secondly, we failed to evaluate the impact of LT4 therapy on childhood outcomes due to lack of information from the original studies. Thirdly, the risk of publication bias cannot be ruled out due to the limited number of enrolled researches.
5 Conclusion
Our study found no evidence of benefit of LT4 therapy on pregnancy and neonatal outcomes in euthyroid pregnant women with thyroid autoimmunity. This finding do not support LT4 therapy for euthyroid pregnant women with thyroid autoimmunity. Further and large sample size RCTs of high quality are still needed to clarify this issue.
Funding Statement
This study was supported by Science and Technology Plan Project of Sichuan Province (2020YFS0035, 2019YFS0410).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
X-FJ, QW, LiZ, and LlZ conceptualized the research question. JC, MZ, and HL made the retrieval strategies, JC and MZ performed the study selection and data extraction, JC and X-FJ assessed the risk of bias and quality of evidence, MZ conducted the meta-analyses and TSA analysis, X-FJ, DL, and KZ made all figures and tables, JC and X-FJ wrote the manuscript, QW, LlZ, LiZ, LnZ, DL, HL, and KZ reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1054935/full#supplementary-material
References
- Abbassi-Ghanavati M., Casey B. M., Spong C. Y., McIntire D. D., Halvorson L. M., Cunningham F. G. (2010). Pregnancy outcomes in women with thyroid peroxidase antibodies. Obstet. Gynecol. 116, 381–386. 10.1097/AOG.0b013e3181e904e5 [DOI] [PubMed] [Google Scholar]
- Alexander E. K., Pearce E. N., Brent G. A., Brown R. S., Chen H., Dosiou C., et al. (2017). 2017 guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27, 315–389. 10.1089/thy.2016.0457 [DOI] [PubMed] [Google Scholar]
- Ashoor G., Maiz N., Rotas M., Jawdat F., Nicolaides K. H. (2010). Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 20, 989–993. 10.1089/thy.2010.0058 [DOI] [PubMed] [Google Scholar]
- Association CM (2019). Guideline on diagnosis and management of thyroid diseases during pregnancy and postpartum. Chin. J. Endocrinol. Metab. 35, 636–665.2nd edition. 10.3760/cma.j.issn.1000-6699.2019.08.003 [DOI] [Google Scholar]
- Atkins D., Best D., Briss P. A., Eccles M., Falck-Ytter Y., Flottorp S., et al. (2004). Grading quality of evidence and strength of recommendations. Bmj 328, 1490. 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Author Anonymous (2020). Thyroid disease in pregnancy: ACOG practice bulletin, number 223. Obstet. Gynecol., 135: e261–e274. 10.1097/AOG.0000000000003893 [DOI] [PubMed] [Google Scholar]
- Balshem H., Helfand M., Schünemann H. J., Oxman A. D., Kunz R., Brozek J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- Benhadi N., Wiersinga W. M., Reitsma J. B., Vrijkotte T. G. M., van der Wal M. F., Bonsel G. J. (2007). Ethnic differences in TSH but not in free T4 concentrations or TPO antibodies during pregnancy. Clin. Endocrinol. (Oxf) 66, 765–770. 10.1111/j.1365-2265.2007.02803.x [DOI] [PubMed] [Google Scholar]
- Chen L., Hu R. (2011). Thyroid autoimmunity and miscarriage: A meta-analysis. Clin. Endocrinol. (Oxf) 74, 513–519. 10.1111/j.1365-2265.2010.03974.x [DOI] [PubMed] [Google Scholar]
- Cochrane Training (2022). Cochrane Handbook for systematic reviews of interventions. Version 6.3. Available at: https://training.cochrane.org/handbook/current.
- Copenhagen Trial Unit (2022). User manual for trial sequential analysis (TSA). Available at: https://ctu.dk/tsa/ (Accessed July, 2022).
- Dal Lago A., Galanti F., Miriello D., Marcoccia A., Massimiani M., Campagnolo L., et al. (2021). Positive impact of levothyroxine treatment on pregnancy outcome in euthyroid women with thyroid autoimmunity affected by recurrent miscarriage. J. Clin. Med. 10, 2105. 10.3390/jcm10102105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon-Smith R. K., Middleton L. J., Sunner K. K., Cheed V., Baker K., Farrell-Carver S., et al. (2019). Levothyroxine in women with thyroid peroxidase antibodies before conception. N. Engl. J. Med. 380, 1316–1325. 10.1056/NEJMoa1812537 [DOI] [PubMed] [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G., Kunz R., Brozek J., Alonso-Coello P., et al. (2011). GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J. Clin. Epidemiol. 64, 407–415. 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- Korevaar T. I., Muetzel R., Medici M., Chaker L., Jaddoe V. W. V., de Rijke Y. B., et al. (2016). Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 35–43. 10.1016/S2213-8587(15)00327-7 [DOI] [PubMed] [Google Scholar]
- Lau L., Benham J. L., Lemieux P., Yamamoto J., Donovan L. E. (2021). Impact of levothyroxine in women with positive thyroid antibodies on pregnancy outcomes: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 11, e043751. 10.1136/bmjopen-2020-043751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepoutre T., Debiève F., Gruson D., Daumerie C. (2012). Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol. Obstet. Invest. 74, 265–273. 10.1159/000343759 [DOI] [PubMed] [Google Scholar]
- Marta C. B., Adamo A. M., Soto E. F., Pasquini J. M. (1998). Sustained neonatal hyperthyroidism in the rat affects myelination in the central nervous system. J. Neurosci. Res. 53, 251–259. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Bmj 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Reyes R., Glinoer D., Van Oyen H., Vandevijvere S. (2013). High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: A population-based study. J. Clin. Endocrinol. Metab. 98, 3694–3701. 10.1210/jc.2013-2149 [DOI] [PubMed] [Google Scholar]
- Mosaddegh M. H., Ghasemi N., Jahaninejad T., Mohsenifar F., Aflatoonian A. (2012). Treatment of recurrent pregnancy loss by Levothyroxine in women with high Anti-TPO antibody. Iran. J. Reprod. Med. 10, 373–376. [PMC free article] [PubMed] [Google Scholar]
- Nazarpour S., Ramezani Tehrani F., Simbar M., Tohidi M., Alavi Majd H., Azizi F. (2017). Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur. J. Endocrinol. 176, 253–265. 10.1530/EJE-16-0548 [DOI] [PubMed] [Google Scholar]
- Negro R., Formoso G., Mangieri T., Pezzarossa A., Dazzi D., Hassan H. (2006). Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J. Clin. Endocrinol. Metab. 91, 2587–2591. 10.1210/jc.2005-1603 [DOI] [PubMed] [Google Scholar]
- Negro R., Mangieri T., Coppola L., Presicce G., Casavola E. C., Gismondi R., et al. (2005). Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: A prospective study. Hum. Reprod. 20, 1529–1533. 10.1093/humrep/deh843 [DOI] [PubMed] [Google Scholar]
- Negro R., Schwartz A., Stagnaro-Green A. (2016). Impact of levothyroxine in miscarriage and preterm delivery rates in first trimester thyroid antibody-positive women with TSH less than 2.5 mIU/L. J. Clin. Endocrinol. Metab. 101, 3685–3690. 10.1210/jc.2016-1803 [DOI] [PubMed] [Google Scholar]
- Pearce E. N., Oken E., Gillman M. W., Lee S. L., Magnani B., Platek D., et al. (2008). Association of first-trimester thyroid function test values with thyroperoxidase antibody status, smoking, and multivitamin use. Endocr. Pract. 14, 33–39. 10.4158/EP.14.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Zeng Z., Zhou F., Wang H., Liu J., Wang R., et al. (2019). Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: A systematic review and meta-analysis. Hum. Reprod. Update 25, 344–361. 10.1093/humupd/dmz003 [DOI] [PubMed] [Google Scholar]
- Revelli A., Casano S., Piane L. D., Grassi G., Gennarelli G., Guidetti D., et al. (2009). A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: Effects of levothyroxine, acetyl-salicylic acid and prednisolone adjuvant treatments. Reprod. Biol. Endocrinol. 7, 137. 10.1186/1477-7827-7-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Sun X., Hou N., Wang H., Ma L., Sun J., Liu Y. (2020). A meta-analysis of pregnancy outcomes with levothyroxine treatment in euthyroid women with thyroid autoimmunity. J. Clin. Endocrinol. Metab., 105. dgz217. 10.1210/clinem/dgz217 [DOI] [PubMed] [Google Scholar]
- Sweeting M. J., Sutton A. J., Lambert P. C. (2004). What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 23, 1351–1375. 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- Thangaratinam S., Tan A., Knox E., et al. (2011). Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 342, d2616. 10.1136/bmj.d2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi A., Uchida T., Kuroda K., Ikemoto Y., Ochiai A., Goto H., et al. (2021). Effect of thyroxine treatment on pregnancy outcomes in infertile Japanese women with TSH levels between 2.5 μIU/mL and the upper reference limit: A retrospective study. Endocr. J. 68, 171–177. 10.1507/endocrj.EJ20-0380 [DOI] [PubMed] [Google Scholar]
- Wang H., Gao H., Chi H., Zeng L., Xiao W., Wang Y., et al. (2017). Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: A randomized clinical trial. Jama 318, 2190–2198. 10.1001/jama.2017.18249 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang Y., Tan H., Bai Y., Zhou L., Fang F., et al. (2020). Effect of levothyroxine on pregnancy outcomes in women with thyroid autoimmunity: A systematic review with meta-analysis of randomized controlled trials. Fertil. Steril. 114, 1306–1314. 10.1016/j.fertnstert.2020.06.034 [DOI] [PubMed] [Google Scholar]
- Xu C., Furuya-Kanamori L., Zorzela L., Lin L., Vohra S. (2021). A proposed framework to guide evidence synthesis practice for meta-analysis with zero-events studies. J. Clin. Epidemiol. 135, 70–78. 10.1016/j.jclinepi.2021.02.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.