Abstract
Background/Aims
Endosonography is associated with a long learning curve. We aimed to assess variables that may influence the diagnostic outcomes in endoscopic ultrasound-guided fine-needle aspiration/biopsy (EUS-FNA/B) of solid pancreatic tumors regarding the level of endoscopists' experience.
Methods
Consecutive patients undergoing EUS-guided puncture of solid pancreatic tumors (eight endosonographers, including six trainees) were prospectively enrolled. An experienced endosonographer was defined as having performed at least 250 EUS examinations, including 75 FNA/Bs. The final diagnosis was determined by cytopathology, histopathology, or clinical follow-up.
Results
In total, 283 EUS-FNA/Bs of solid pancreatic tumors (75.6% malignant) in 239 patients (median age 69 years, 57.6% males) were enrolled. Trainees performed 149/283 (52.7%) of the interventions. Accuracy and sensitivity for detecting malignancy were significantly higher in the expert group than in the trainee group (85.8% vs 73.2%, p=0.01 and 82.5% vs 68.4%, p=0.02). Solid lesions evaluated by an expert using FNB needles showed the best odds for a correct diagnosis (odds ratio, 3.07; 95% confidence interval, 1.15 to 8.23; p=0.02). More experienced endoscopists achieved better accuracy in sampling via the transduodenal approach (86.7% vs 68.5%, p<0.001), in the sampling of malignant lesions (82.5 vs 68.4, p=0.02), and in the sampling of lesions located in the pancreatic head (86.1 vs 69.1, p=0.02). In cases involving these factors, we observed a moderate improvement in the diagnostic accuracy after 40 attempts.
Conclusions
Transduodenal approach, pancreatic head lesions, and malignancy were recognized as the most important clinical factors affecting the learning curve in EUS-FNA/B of solid pancreatic lesions.
Keywords: Competence, Endosonography, Fine-needle aspiration/biopsy, Learning, Pancreatic tumor
INTRODUCTION
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) emerged as the preferred technique for tissue acquisition of pancreatic lesions to rule out malignancy.1 Performed by an experienced endosonographer, it has been reported to be safe and more accurate than alternative methods such as computed tomography- or ultrasound-guided FNA.2
Obtaining adequate pancreatic specimens can be challenging and time-consuming, requiring considerable technical and cognitive skills of the operator. Interventional EUS has a long learning curve, and the amount of training and the number of procedures needed to reach technical proficiency is still a subject of debate.3
The outcome of EUS-FNA varies in diagnostic accuracy (76% to 98%) and sensitivity for malignancy (64% to 95%) depending on the different clinical variables and methodology used in previous studies.4
Factors that determine the diagnostic performance of EUS have been discussed in detail, and many high-quality randomized comparative studies on sampling techniques, rapid on-site evaluation (ROSE) of specimens, needle type and size have been published to date. Only a few studies addressed the operators’ experience, even though it significantly impacts diagnostic outcomes. According to the study of Harewood et al.,5 the diagnostic accuracy of EUS-FNA in endosonographers without prior experience was only 33% and improved after a short period of mentored training up to 91%.
ROSE may be useful for self-assessment of EUS-sampling by trainees during the learning process, which may help guide the number of FNA passes and evaluate which parts of the lesions should be targeted. It is an effective way to identify and correct technical errors during the procedure.6
Unfortunately, due to limited personal and financial resources in Europe, an experienced cytopathologist is available only in a few referral centers. For this purpose, needles with a specially designed tip were developed to obtain core samples with preserved histological architecture. They showed high diagnostic accuracy (88% to 91%)7 even if an on-site cytopathologist was not present.
Theoretically, the new core biopsy needles should improve the diagnostic performance of less experienced endoscopists. However, very few studies have addressed the factors that may influence the outcome of EUS-guided tissue acquisition during the training. It is unknown how the choice of needle type interferes with diagnostic accuracy during the learning curve of EUS-FNA of pancreatic lesions.
In the previous series reporting the learning curve, the sensitivity for malignancy ranged between 44% and 95%, increased with operators’ experience, and reached the required level of 80% after performing at least 20 to 30 FNAs.5,8
Severe adverse events were rare (1% to 3%) irrespective of endoscopists’ experience, while one study reported fewer minor events even late in the learning curve after performing 200 procedures.8 That indicates the learning curve may continue long after fellowship and accomplished minimum number of procedures as defined by the American Society for Gastrointestinal Endoscopy and European Society for Gastrointestinal Endoscopy (ESGE).9
We aimed to identify the clinical factors that may impact the diagnostic performance of EUS-guided fine-needle aspiration/biopsy (FNA/B) in solid pancreatic lesions during the training in centers with no cytopathologist on-site.
MATERIALS AND METHODS
1. Patients and study design
All consecutive patients between February 2017 to May 2021 (Klinikum Klagenfurt) and December 2018 to May 2021 (University Hospital St. Pölten), referred for EUS-guided tissue acquisition of solid pancreatic lesions, were prospectively enrolled.
A standardized data collection on patient demographics, endosonographic characteristics, procedure- and sedation-related adverse events was implemented in both centers. Information on the following factors associated with the performance of EUS-FNA was collected: tumor type (malignant and benign), sampling route (trans-gastral and transduodenal), location of the lesion (pancreatic head, uncinate, body, and tail), size of the lesion (<20 mm, 20–40 mm, >40 mm), needle type (FNA and FNB), needle size (19 gauge and 22 gauge), number of needle passes, use of suction, sampling technique, the physical status of the patient (American Society of Anesthesiologists classification), use of anticoagulation (ongoing and discontinued), level of operators experience, histopathological and cytopathological findings.
In all cases, laboratory tests (complete blood count, liver enzymes, prothrombin time, and international normalized ratio) has been available before the procedure. All patients were monitored overnight, and a complete blood count was controlled four hours after the intervention. Follow-up, including physical examination and laboratory analysis, was performed in the outpatient clinic.
This study was approved by the Carinthia ethics committee (approval number: A40/18+A 22/19), and the study protocol conforms to the ethical guidelines of the Declaration of Helsinki (as revised Brasil 2013) as reflected in a priori approval by the institution’s human research committee. Written informed consent was obtained from all patients.
2. Study end points
The primary end point was to identify the clinical factors that impact the diagnostic performance of EUS-guided puncture of solid pancreatic masses during the training in centers with no cytopathologist on-site. Secondary outcomes included assessing the learning curve, comparing FNA and FNB techniques regarding the level of experience, and evaluating adverse events.
3. Endoscopic ultrasound
EUS-guided FNA was conducted using a linear array device (EG-3870UTK, Pentax Medical Co., Montvale, NJ, USA [Klagenfurt], and GF-UCT180 Olympus Medical Co., Tokyo, Japan [St. Pölten]) using the fanning technique with a sampling of multiple areas within the lesion (10 to 15 motions) as described previously.4 For the suction technique, a 10 mL syringe was used. The aspirate was placed on a glass slide, and ethanol-fixed smears were prepared. Visible core specimens were macroscopically evaluated for the presence of whitish (likely to be tumor tissue) or hemorrhagic parts and collected into formalin for subsequent histological analysis. The tissue samples obtained by a second investigator, who took over the procedure in the same examination if the initial endosonographer had difficulties getting an adequate sample, were marked and analyzed separately. The needle type and size were selected according to the endoscopists’ preferences and center availability (Table 1). Deep sedation was performed with propofol-based regimes combined with midazolam, administered either by a physician or a specially trained endoscopy nurse. Sedation-related complications were defined as persistently low oxygen saturation (<90%), arterial hypotension with sustained systolic pressure below 90 mm Hg, or cardiac arrhythmias requiring therapy. Procedure-related complications were defined according to the ESGE guidelines.6 In addition, we also documented minor bleedings, as described in the previous study.10
Table 1.
Performance of EUS-Guided FNA/B for Trainees and Experts Regarding the Needle Type and Sampling Technique
| Variable | No. | 22 Gauge§, % | Stylet | Suction, % | Passes, mean±SD | Adequacy, % | Accuracy, % | Sensitivity, % |
|---|---|---|---|---|---|---|---|---|
| Expert | ||||||||
| FNA | ||||||||
| EZ Shot3 (Olympus)* | 36 | 0 | No | 72.2 | 2.8±0.9 | 97.2 | 80.6 | 75.0 |
| FNB | ||||||||
| ProCore (Cook) | 8 | 100 | No | 12.5 | 2.5±0.6 | 87.5 | 75.0 | 66.7 |
| Acquire (Boston) | 77 | 100 | No | 15.8 | 3.0±1.1 | 96.1 | 89.5 | 88.9 |
| Shark Core (Medtronic) | 13 | 84.6 | No | 42.9 | 3.4±1.0 | 100 | 85.7 | 77.8 |
| Trainee | ||||||||
| FNA | ||||||||
| EZ Shot3 (Olympus)* | 104 | 3.2 | No | 82.7 | 2.8±0.7 | 96.1 | 72.2 | 66.7 |
| FNB | ||||||||
| ProCore (Cook) | 2 | 100 | No | 0 | 2.0±1.0 | 100 | 100 | 100 |
| Acquire (Boston) | 7 | 85.7 | No | 42.9 | 3.0±0.9 | 100 | 71.4 | 57.1 |
| Shark Core (Medtronic) | 36 | 80.6 | No | 50.0 | 2.9±0.5 | 97.2 | 69.4 | 64.3 |
EUS, endoscopic ultrasound; FNA, fine-needle aspiration; FNB, fine-needle biopsy.
*Flexible nitinol needle; §By all participants only 19-gauge and 22-gauge needles were used.
4. Experience of endosonographers
An experienced endosonographer was defined as someone who had performed at least 250 EUS examinations, including 75 FNA/Bs.9 Eight endoscopists participated in the study: two with considerable endosonographic experience (>10 years and >150 EUS/year), and six with no previous experience in EUS-guided tissue sampling but with advanced expertise in conventional endoscopy and different levels of expertise in diagnostic EUS only. Two of them reached the suggested threshold for competency over the study period. During the training, the first 15 to 20 FNA/Bs were performed under direct supervision. After that, a second experienced endoscopist assisted, depending on case difficulty and trainees’ demand.
5. Diagnostic interpretation
On-site evaluation by cytopathologists to ensure specimen adequacy was not available in the participating centers. The final diagnosis was determined according to the following criteria: (1) histopathological or cytological analysis of specimens obtained by EUS-FNA/B, (2) surgical pathology, (3) autopsy reports, (4) clinical and radiological findings in follow-up in combination with tumor markers in a period at least 6 months. In cases where trainees could not complete the procedure and required the mentor to take over, the examinations were counted as false negatives for trainee endoscopists and were additionally analyzed in the expert group. Cases where hands-on assistance by an experienced endosonographer was provided (e.g., by scope manipulation to optimize visualization) or verbal instruction was given were analyzed in the trainee group.
6. Statistical analysis
Numerical variables with normal distribution are expressed as means with standard deviation, and in the case of non-normal distribution as medians with range intervals. According to distribution normality, a parametric t-test or nonparametric Mann-Whitney U test was used to compare the variables. Differences in proportions expressed as percentages were tested with the chi-square and the Fisher exact test. In the second step, the independent discriminative values of variables reaching a univariate statistical significance were assessed by stepwise logistic regression. Odds ratio (OR) and 95% confidence interval (CI) were reported. A p-value <0.05 was considered as significant for each statistical test. The learning curve was assessed by the cumulative sum (Cusum) analysis, as described in previous literature.3 The parameters used for Cusum analysis were set as follows: type I and II error rates at 0.10 as generally accepted, and the acceptable (p0) and unacceptable (p1) failure rates at 0.10 and 0.20.11 Then, intermediate values were calculated: P=ln (p1/p0), Q=ln [(1–p1)/(1–p0)], and s=Q/(P+Q). Therefore, for each success (s), a decrement of 0.15, and each failure (1–s), an increment of 0.85 was drawn on the Cusum plot.
RESULTS
1. Baseline characteristics
A total of 239 patients (median age 69 years, range 19 to 92 years) with 283 EUS-FNA/B interventions (183 Klagenfurt and 100 St. Pölten) on solid pancreatic lesions were enrolled. The baseline patient characteristics are shown in Table 2. In 149 out of 283 interventions were performed by trainees (52.7%) with different expertise in diagnostic EUS only (range, 14 to 33 examinations before first FNA).
Table 2.
Baseline Characteristics
| Variable | Total (n=283) | Expert (n=134) | Trainee (n=149) | p-value |
|---|---|---|---|---|
| Age, yr | 66.1±14.9 | 66.2±14.8 | 68.2±13.3 | 0.23 |
| Male sex | 163 (57.6) | 78 (58.2) | 85 (57.0) | 0.93 |
| Tumor size, cm | 3.0±0.9 | 3.0±1.0 | 3.1±1.3 | 0.76 |
| Location | ||||
| Head | 153 (54.1) | 72 (53.7) | 81 (54.4) | 0.91 |
| Uncinate | 48 (17.0) | 21 (15.7) | 27 (18.1) | 0.71 |
| Body | 49 (17.3) | 23 (17.2) | 26 (17.4) | 0.91 |
| Tail | 33 (11.7) | 18 (13.4) | 15 (10.1) | 0.50 |
| Puncture | ||||
| Trans-gastric | 83 (29.3) | 42 (31.3) | 41 (27.5) | 0.57 |
| Transduodenal | 200 (70.7) | 92 (68.7) | 108 (72.5) | 0.57 |
| FNB | 143 (50.5) | 98 (73.1) | 45 (30.2) | <0.001 |
| 19 Gauge | 136 (48.1) | 38 (28.4) | 98 (65.8) | <0.001 |
| Passes | 2.9±0.8 | 3.0±0.8 | 2.8±0.8 | 0.77 |
| ASA ≥3 | 82 (29.0) | 38 (28.4) | 44 (29.5) | 0.94 |
| Final diagnosis | ||||
| Adenocarcinoma | 181 (64.0) | 82 (61.2) | 99 (66.4) | 0.43 |
| NET | 13 (4.6) | 4 (3.0) | 9 (6.0) | 0.36 |
| Metastasis | 14 (4.9) | 9 (6.7) | 5 (3.4) | 0.32 |
| Lymphoma | 3 (1.1) | 2 (1.5) | 1 (0.7) | 0.94 |
| Chronic pancreatitis | 24 (8.5) | 19 (14.2) | 5 (3.4) | 0.002 |
| Acute inflammation | 17 (6.0) | 6 (4.5) | 11 (7.4) | 0.44 |
| Autoimmune | 16 (5.7) | 6 (4.5) | 10 (6.7) | 0.59 |
| Other tumors | 15 (5.3) | 6 (4.5) | 9 (6.0) | 0.77 |
Data are presented as mean±SD or number (%).
FNB, fine-needle biopsy; ASA, American Society of Anesthesiologists classification; NET, neuroendocrine tumor.
2. Diagnostic performance
Overall, sensitivity, specificity, and accuracy for detecting malignancy were 74.8%, 92.8%, and 79.2%, respectively. Accuracy and sensitivity were significantly higher in the expert group than in the trainee group (85.8% vs 73.2%, p=0.01 and 82.5% vs 68.4%, p=0.02). The experience was the only significant variable in logistic regression analysis to predict correct diagnosis (OR, 2.08; 95% CI, 1.05 to 4.13; p=0.03). The diagnostic accuracy for EUS-FNB performed by experts was significantly higher than trainees (87.8% vs 73.3%, p=0.05). Solid lesions evaluated by an expert using FNB needles showed the best odds for a correct diagnosis (OR, 3.07; 95% CI, 1.15 to 8.23; p=0.02). The experience level seems less important when performing EUS-FNA (OR, 1.50; 95% CI, 0.58 to 3.89; p=0.39) since there was no significant difference in diagnostic outcomes (Table 3). Furthermore, in the trainee group, the accuracy of FNB was similar to FNA (73.3% vs 73.1%, p=0.86). The FNB needles were found to have a significantly higher negative predictive value for malignancy when used by an experienced operator (72.7% vs 40%, p=0.03). The proportion of samples adequate for cytologic and histologic evaluation was 96.5% (10 cases with insufficient material).
Table 3.
Diagnostic Outcomes of EUS-FNA/B Regarding the Level of Experience
| Experience | Expert | Trainee | p-value |
|---|---|---|---|
| FNA* | |||
| Sensitivity | 75.0 (21/28) | 67.9 (55/81) | 0.64 |
| Specificity | 100 (8/8) | 91.3 (21/23) | 0.97 |
| PPV | 100 (21/21) | 100 (55/55) | |
| NPV | 53.3 (8/15) | 42.9 (21/49) | 0.68 |
| Accuracy | 80.6 (29/36) | 73.1 (76/104) | 0.50 |
| FNB† | |||
| Sensitivity | 85.5 (59/69) | 69.4 (25/36) | 0.06 |
| Specificity | 92.3 (27/29) | 88.9 (8/9) | 0.71 |
| PPV | 100 (59/59) | 100 (25/25) | |
| NPV | 72.7 (27/39) | 40.0 (8/20) | 0.03 |
| Accuracy | 87.8 (86/98) | 73.3 (33/45) | 0.05 |
| Total‡ | |||
| Sensitivity | 82.5 (80/97) | 68.4 (80/117) | 0.02 |
| Specificity | 94.6 (35/37) | 90.6 (29/32) | 0.86 |
| PPV | 100 (80/80) | 100 (80/80) | |
| NPV | 62.5 (35/54) | 42.0 (29/69) | 0.03 |
| Accuracy | 85.8 (115/134) | 73.2 (109/149) | 0.01 |
Data are presented as % (number/number).
EUS, endoscopic ultrasound; FNA/B, fine-needle aspiration/biopsy; PPV, positive predictive value; NPV, negative predictive value.
*Expert (n=36) and trainee (n=104); †Expert (n=98) and trainee (n=45); ‡Expert (n=134) and trainee (n=149).
3. Effects of clinical factors on the diagnostic accuracy and sensitivity
More experienced endoscopists achieved better accuracy and sensitivity for malignancy in sampling via the transduodenal approach (86.7% vs 68.5%, p<0.001 and 85.3% vs 63.5%, p<0.001), regardless of the sampling technique. Lesions located in the pancreatic head were more likely to be diagnosed correctly when an expert performed the procedure (Table 4).
Table 4.
Accuracy and Sensitivity of EUS-FNA/B for Trainees and Experts According to Different Clinical Variables
| Variable | Accuracy, % | Sensitivity, % | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FNA+FNB | FNA | FNB | FNA+FNB | FNA | FNB | ||||||||||||||||||
| E | T | p-value | E | T | p-value | E | T | p-value | E | T | p-value | E | T | p-value | E | T | p-value | ||||||
| Puncture | |||||||||||||||||||||||
| Trans-gastral | 84.1 | 85.4 | 0.89 | 72.7† | 80.8 | 0.91 | 90.0 | 93.3 | 0.85 | 75.9 | 81.3 | 0.84 | 66.7† | 73.7 | 0.95 | 83.3 | 92.3 | 0.84 | |||||
| Transduodenal | 86.7 | 68.5 | 0.004* | 84.0 | 70.5 | 0.28 | 87.7 | 63.3 | 0.01* | 85.3 | 63.5 | 0.004* | 78.9 | 66.1 | 0.44 | 87.8 | 56.5 | 0.007* | |||||
| Location | |||||||||||||||||||||||
| Head | 86.1 | 69.1 | 0.02* | 81.2 | 71.9 | 0.67 | 88.7 | 62.5 | 0.01* | 84.6 | 64.1 | 0.02* | 72.7 | 67.4 | 0.98 | 89.7 | 55.6 | 0.009* | |||||
| Uncinate | 85.7 | 66.7 | 0.24 | 88.9† | 66.7 | 0.42 | 83.3 | 66.7† | 0.84 | 83.3 | 61.9 | 0.26 | 87.5† | 62.5 | 0.43 | 80.0† | 60.0† | 0.83 | |||||
| Body | 82.6 | 76.9 | 0.88 | 60.0† | 70.6 | 0.92 | 88.9 | 88.9† | 0.51 | 71.4 | 72.7 | 0.76 | 60.0† | 64.3 | 0.71 | 77.8† | 87.5† | 0.90 | |||||
| Tail | 88.9 | 100 | 0.54 | 83.3† | 100† | 0.83 | 91.7 | 100† | 0.71 | 84.6 | 100 | 0.58 | 75.0† | 100† | 0.91 | 88.9† | 100† | 0.75 | |||||
| Tumor type | |||||||||||||||||||||||
| Malignant | 82.5 | 68.4 | 0.02* | 75.0 | 69.1 | 0.73 | 85.5 | 69.4 | 0.08 | 82.5 | 68.4 | 0.02* | 75.0 | 67.9 | 0.64 | 85.5 | 69.4 | 0.08 | |||||
| Benign | 94.6 | 87.5 | 0.53 | 100† | 87.0 | 0.71 | 93.1 | 88.9† | 0.76 | - | - | - | - | - | - | ||||||||
| Tumor size | |||||||||||||||||||||||
| <20 mm | 88.2 | 66.7 | 0.24 | 83.3† | 62.5 | 0.68 | 100† | 80.0† | 0.85 | 78.6 | 62.5 | 0.57 | 80.0† | 61.5 | 0.85 | 100† | 66.7† | 0.78 | |||||
| 20–40 mm | 80.0 | 72.0 | 0.36 | 71.4 | 72.0 | 0.83 | 82.1 | 74.1 | 0.63 | 76.6 | 65.8 | 0.28 | 64.7 | 64.3 | 0.80 | 80.0 | 69.6 | 0.55 | |||||
| >40 mm | 94.4 | 80.9 | 0.43 | 100† | 92.3 | 0.75 | 92.3 | 62.5† | 0.26 | 93.3 | 81.3 | 0.64 | 100† | 90.0 | 0.92 | 90.9 | 66.7† | 0.55 | |||||
| ASA | |||||||||||||||||||||||
| <3 | 87.5 | 74.3 | 0.02* | 75.9 | 74.3 | 0.93 | 93.9 | 74.3 | 0.01* | 84.1 | 68.8 | 0.04* | 69.6 | 68.5 | 0.86 | 93.3 | 69.2 | 0.01* | |||||
| ≥3 | 81.6 | 70.5 | 0.36 | 100† | 70.6 | 0.20 | 77.4 | 70.0† | 0.95 | 78.6 | 67.6 | 0.48 | 100† | 66.7 | 0.33 | 73.9 | 70.0† | 0.84 | |||||
| Needle type | |||||||||||||||||||||||
| FNA | 80.6 | 73.1 | 0.50 | 75.0 | 67.9 | 0.64 | |||||||||||||||||
| FNB | 87.8 | 73.3 | 0.05* | 85.5 | 69.4 | 0.08 | |||||||||||||||||
| Needle size | |||||||||||||||||||||||
| 19 Gauge | 81.6 | 72.4 | 0.37 | 80.6 | 69.3 | 0.30 | 100† | 87.5† | 0.42 | 75.9 | 67.9 | 0.57 | 75.0 | 65.7 | 0.51 | 100† | 87.5† | 0.18 | |||||
| 22 Gauge | 87.5 | 74.5 | 0.07 | 100† | 80.0 | 0.41 | 87.5 | 70.3 | 0.26 | 85.3 | 69.2 | 0.08 | 100† | 81.8 | 0.35 | 85.3 | 64.3 | 0.02* | |||||
| Anticoagulants | |||||||||||||||||||||||
| Yes | 80.0 | 72.7 | 0.59 | 90.9 | 75.0 | 0.47 | 75.0 | 66.7 | 0.84 | 76.9 | 70.5 | 0.76 | 88.9 | 73.3 | 0.60 | 70.6 | 64.3† | 0.98 | |||||
| No | 87.9 | 73.4 | 0.01* | 76.0 | 71.9 | 0.90 | 93.1 | 76.7 | 0.04* | 84.5 | 67.1 | 0.02* | 68.4 | 64.7 | 0.99 | 92.0 | 72.7 | 0.07 | |||||
EUS, endoscopic ultrasound; FNA/B, fine-needle aspiration/biopsy; E, expert; T, trainee; ASA, American Society of Anesthesiologists classification.
*p<0.05; †Number of cases <10.
The tumor size seems to play a role in the diagnostic accuracy independent of the operator’s experience. Experts reached better diagnostic performance for small lesions (<20 mm), more evident when performing FNA than FNB. However, the final analysis did not reach statistical significance due to the low number of cases in this group.
4. Learning curve evaluation
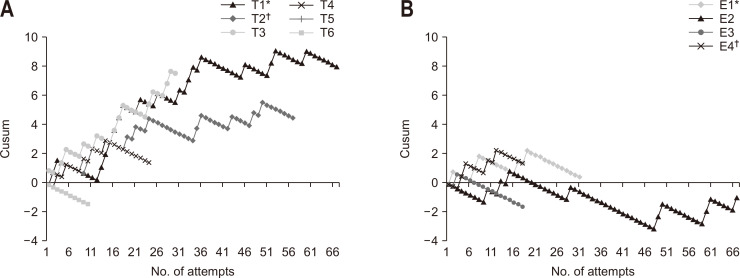
Overall, the trainees did not achieve acceptable performance over the study period since the curves deviated upward due to predominantly failed attempts (Fig. 1). When comparing FNA and FNB curves in the trainee group, there was no difference in the average Cusum score at attempt 10 (0.8 vs 0.6) and 20 (3.3 vs 3.3). Overall, the diagnostic performance of trainees was poor within the first 30 attempts. After that, a moderate improvement (flattened curve) was observed. The data for trainees 1 and 2 suggest that the learning curve continues beyond the threshold to reach competency. The endosonographers (experts 2 and 3) with over 10 years of experience (>150 EUS/year) showed a downward deviation and were the only sustainable toward acceptable performance. The impact of the clinical variables on the accuracy of EUS-FNA/B during the first 50 attempts overall is presented in Table 5.
Fig. 1.
Cumulative sum (Cusum) analysis for successful endoscopic ultrasound-guided tissue acquisition for trainees (A) and experienced investigators (B).
T, trainee; E, expert. Over the study period trainee 1 (T1*) and trainee 2 (T2†) reached the suggested number of procedures to achieve competency. In panel B, they are presented as expert 1 (E1*) and expert 4 (E4†).
Table 5.
The Impact of the Clinical Variables on the Accuracy of EUS-FNA/B during the First 50 Attempts Overall
| Variable | Trainees (No. of attempts) | Experts | |||||
|---|---|---|---|---|---|---|---|
| 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | All | ||
| Lesion | |||||||
| Head | 65.4 (17/26) | 61.1 (11/18) | 71.4 (10/14) | 64.3 (9/14) | 81.8 (9/11) | 86.1 (56/65) | |
| Tail | 100 (3/3) | 100 (7/7) | 100 (3/3) | - | 100 (2/2) | 88.9 (16/18) | |
| Malignant | 63.0 (17/27) | 61.3 (19/31) | 72.4 (21/29) | 64.7 (11/17) | 68.8 (11/16) | 82.5 (80/97) | |
| Benign | 76.9 (10/13) | 88.9 (8/9) | 100 (5/5) | 100 (3/3) | 100 (4/4) | 94.6 (35/37) | |
| <20 mm | 50.0 (1/2) | 50.0 (2/4) | 50.0 (4/8) | 75.0 (3/4) | 66.7 (2/3) | 88.2 (15/17) | |
| >40 mm | 40.0 (2/5) | 100 (3/3) | 100 (3/3) | 83.3 (5/6) | 100 (3/3) | 94.4 (17/18) | |
| Puncture | |||||||
| Transduodenal | 65.6 (21/32) | 57.7 (15/26) | 72.7 (16/22) | 64.7 (11/17) | 81.3 (13/16) | 86.7 (78/90) | |
| Trans-gastral | 75.0 (6/8) | 85.7 (12/14) | 83.3 (10/12) | 100 (3/3) | 75.0 (3/4) | 85.0 (37/44) | |
| FNB | 50.0 (4/8) | 76.5 (13/17) | 66.7 (8/12) | 100 (2/2) | 75.0 (3/4) | 87.8 (86/98) | |
| FNA | 71.9 (23/32) | 60.9 (14/23) | 81.8 (18/22) | 66.7 (12/18) | 75.0 (12/16) | 80.6 (29/36) | |
| 19 Gauge | 74.1 (20/27) | 65.5 (19/29) | 81.8 (18/22) | 66.7 (12/18) | 76.5 (13/17) | 81.6 (31/38) | |
| 22 Gauge | 76.9 (7/13) | 72.7 (8/11) | 66.7 (8/12) | 100 (2/2) | 66.7 (2/3) | 87.5 (84/96) | |
| Patient | |||||||
| Anticoagulants | 53.3 (8/15) | 76.5 (13/17) | 70.0 (7/10) | 90.0 (9/10) | 85.7 (6/7) | 80.0 (28/35) | |
| ASA ≥3 | 57.1 (8/14) | 66.7 (6/9) | 71.4 (10/14) | 100 (7/7) | 100 (5/5) | 81.6 (31/38) | |
Data are presented as % (number/number).
EUS, endoscopic ultrasound; FNA/B, fine-needle aspiration/biopsy; ASA, American Society of Anesthesiologists classification.
5. Adverse events
Four out of six procedure-related adverse events occurred early in the learning process within the first 20 attempts. Two major bleedings occurred (0.7%), both were immediately stopped by endoscopic intervention. Minor bleeding events were documented in 32 of 283 cases (11.3%). Overall, there was no need for a blood transfusion, and none of the patients presented as hemodynamically unstable. One patient developed pancreatitis, one complained of severe abdominal pain, and another one presented with a mucosal injury at the gastroesophageal junction and was treated conservatively. Four of seven sedation-related complications (2.5%) involved trainees (five with prolonged hypoxia and two with hypotonia). No intensive care admission was needed.
DISCUSSION
The present study investigated the clinical factors that may impact the learning curve of EUS-FNA/B of solid pancreatic lesions and has been one of the few of this type.
We showed that endosonographer experience is likely to be a strong determining factor for the diagnostic outcome of EUS-guided sampling during the training period. A significant difference in accuracy between experts and trainees has been observed, especially when performing EUS-FNB. In this group, the odds of establishing the correct diagnosis were three times greater for experts than for trainees. The experience level seems particularly important when EUS-guided tissue sampling is performed using the novel FNB needles previously associated with high diagnostic yield. Compared to the experts, failed attempts in challenging examinations may contribute to the overall poor performance using FNB needles in the trainee group, particularly in the early stages of training. In contrast, the inferior performance of FNA needles may result from needle design itself and histopathological limitations, especially in centers without ROSE and qualified cytopathologists available rather than endoscopists’ experience.
A recent retrospective study concluded that the FNB needles might shorten the learning curve of EUS-guided tissue acquisition in centers without ROSE.12 In contrast to our study, no information on FNA experience was provided. We also analyzed the first attempts overall, where the highest failure rate is expected, and found no difference in Cusum scores between FNA and FNB at attempts 10 and 20. A moderate improvement with flattened curves was observed after a trainee performed 30 procedures, but our data shows that the learning curve may continue beyond the suggested threshold.
There is a disagreement among standards of different societies regarding the training program and procedural thresholds. The American Society for Gastrointestinal Endoscopy recommends 50 FNA procedures, of which 25 should be performed on the pancreas.13 In the recently published curriculum, the ESGE proposes that a minimum of 250 EUS and 75 FNA/B procedures are required before a trainee is likely to demonstrate acceptable performance.9
In this context, higher thresholds seem more appropriate since there is some evidence that the learning curve may continue long beyond the proposed case volume. Wani et al.14 evaluated the minimum standards for training in advance endoscopy in a prospective multicentric study. They reported an average of 110 EUS-FNAs were required to achieve competence.
Substantial variability in learning curves has been described, and caseload alone does not ensure competence in EUS-FNA.11,14 The latter depends on training quality, endoscopists’ talent, skills, and previous experience in advanced endoscopy using side-viewing devices. Therefore, an individualized approach to assess the competency in EUS would be more appropriate. Initially, it would be reasonable to re-assess each trainee’s diagnostic performance after 30 FNAs performed in short periods (e.g., every 10th procedure), at least until consistently acceptable diagnostic results and a low adverse events rate can be reached.
In our experience, the trainees who had the most experience in ultrasound imaging were more confident in EUS during the learning process. It seems that adequate familiarity with transabdominal ultrasound before attending the EUS training should be the standard of care. However, no supporting data addressing this question can be found.
It is reassuring that, although particular attention to safety is needed, our data show that performing supervised FNA by trainees may be safe and effective even early during the learning curve. In contrast to some other studies,5 the caseload of diagnostic EUS before the first puncture was significantly lower for trainees participating in the present study.
Currently, ESGE does not recommend ROSE over off-site evaluation since there was no clear evidence of its positive impact in a recent randomized study.15 Other studies suggested that on-site pathologists may provide better patient care by reducing the number of needle passes needed to establish the diagnosis.16 This seems to have a low impact on the procedure time and complication rate.
Studies evaluating novel FNB needles with a specially designed tip as used in our centers are lacking. Those needles seem to be more effective than FNA needles, particularly when ROSE is not available.16 Little is known how the choice of needle type interferes with diagnostic accuracy during the learning curve of EUS-guided tissue sampling.
In our experience, trainees more frequently decided to use larger FNA rather than FNB needles, which could contribute to a lower overall diagnostic performance in the trainee group. However, when comparing the same sampling technique using FNB only, we observed still better accuracies in the expert group. More attention to needle choice during EUS training, particularly in technically more challenging situations, is needed.
Many high-quality studies have confirmed the low impact of the needle size on the diagnostic outcomes in EUS-guided sampling of solid pancreatic lesions. Most of them compared 22- and 25-gauge FNA needles,17 while data on the more flexible nitinol 19-gauge needle used in our study are scarce. Using these needles, we did not observe any technical failure, irrespective of the experience level.
Theoretically, the lesions located in the pancreatic body and tail are better to reach than those in the pancreatic head or uncinate process. With an echoendoscope straightened, the FNA may be easier to perform even in less experienced hands. Our results show the best performance for lesions in the pancreatic tail, which was similarly high for both trainees and experienced endoscopists.
A study from Japan, including 788 lesions, demonstrated a strong correlation between diagnostic outcomes and mass size, with inferior accuracy for lesions <10 mm.18 We observed sensitivity of only 62.5% for lesions <20 mm in the trainee group, considerably less than in the expert group (78.6%). Still, statistical significance was not reached because of the low number of cases included in the trainee group.
There is a lack of high-quality data on the impact of endoscopists’ experience on adverse events in EUS. A national study from the United States on a large cohort of patients who received EUS has identified trainee participation as a risk factor for unexpected cardiopulmonary events.19 Another study revealed that trainee involvement during EUS could be associated with an increased risk of adverse events, particularly in the first 3 months of the training. The overall adverse event incidence was reported to be 3.4%,20 slightly higher than in the previous literature (1% to 3%)8,21 and compared to results presented here (2.1%). In this context, the amount of training may be more appropriate than the duration alone to evaluate adverse events. We observed that four out of six events occurred early in the learning curve in trainees who had performed less than 30 FNAs. Only two major bleedings occurred and were stopped immediately by an endoscopic intervention. The first event was more a consequence of the operators’ inexperience: more careful examination of the biopsy-pathway could probably have identified the large submucosal vessel or more stable needle handling could have prevented its puncture.
In conclusion, transduodenal approach, pancreatic head lesions, and malignancy were recognized as the most important clinical factors affecting the learning curve of EUS-FNA/B of solid pancreatic lesions and were frequently associated with failed attempts in the trainee cohort.
In cases involving these factors, we observed a moderate improvement in the diagnostic accuracy of EUS-FNA/B after 40 attempts. However, in our experience, the learning curve continues beyond the suggested threshold of 75 procedures to reach adequate competency.
FNB needles increase the accuracy and sensitivity for malignancy in centers without ROSE when performed by an experienced endosonographer. For trainees, similar results for both techniques, FNA and FNB, were observed.
Early in the training process, additional attention to avoid complications is warranted. Further studies and agreement on standards between different societies would be highly desirable and should be encouraged.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: M.R., S.B., M.P.R. Data acquisition, analysis, and interpretation: M.R., S.B., M.K., G.E., C.U., J.P. Drafting of the manuscript: M.R., S.B. Critical revision of the manuscript for important intellectual content: J.W.E., A.M., M.P.R. Statistical analysis: M.R., S.B. Administrative, technical, or material support; study supervision: M.R., M.P.R. Approval of final manuscript: all authors.
REFERENCES
- 1.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 2.Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–975. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Wani S, Coté GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013;77:558–565. doi: 10.1016/j.gie.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014;46:1056–1062. doi: 10.1055/s-0034-1377558. [DOI] [PubMed] [Google Scholar]
- 5.Harewood GC, Wiersema LM, Halling AC, Keeney GL, Salamao DR, Wiersema MJ. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002;55:669–673. doi: 10.1067/mge.2002.123419. [DOI] [PubMed] [Google Scholar]
- 6.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 7.Fabbri C, Fuccio L, Fornelli A, et al. The presence of rapid on-site evaluation did not increase the adequacy and diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition of solid pancreatic lesions with core needle. Surg Endosc. 2017;31:225–230. doi: 10.1007/s00464-016-4960-4. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: a learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–708. doi: 10.1016/S0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 9.Johnson G, Webster G, Boškoski I, et al. Curriculum for ERCP and endoscopic ultrasound training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2021;53:1071–1087. doi: 10.1055/a-1537-8999. [DOI] [PubMed] [Google Scholar]
- 10.Razpotnik M, Bota S, Kutilek M, et al. The bleeding risk after endoscopic ultrasound-guided puncture of pancreatic masses. Scand J Gastroenterol. 2021;56:205–210. doi: 10.1080/00365521.2020.1863458. [DOI] [PubMed] [Google Scholar]
- 11.Wani S, Hall M, Keswani RN, et al. Variation in aptitude of trainees in endoscopic ultrasonography, based on cumulative sum analysis. Clin Gastroenterol Hepatol. 2015;13:1318–1325. doi: 10.1016/j.cgh.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin MY, Wu CL, Kida M, Chang WL, Sheu BS. Confirming whether fine needle biopsy device shortens the learning curve of endoscopic ultrasound-guided tissue acquisition without rapid onsite evaluation. Clin Endosc. 2021;54:420–427. doi: 10.5946/ce.2020.184.e128c636087b4daf9317afc705ac14b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–814. doi: 10.1016/S0016-5107(01)70082-X. [DOI] [PubMed] [Google Scholar]
- 14.Wani S, Han S, Simon V, et al. Setting minimum standards for training in EUS and ERCP: results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc. 2019;89:1160–1168. doi: 10.1016/j.gie.2019.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–1439. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–E375. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilmann P, Săftoiu A, Hollerbach S, et al. Multicenter randomized controlled trial comparing the performance of 22 gauge versus 25 gauge EUS-FNA needles in solid masses. Scand J Gastroenterol. 2013;48:877–883. doi: 10.3109/00365521.2013.799222. [DOI] [PubMed] [Google Scholar]
- 18.Sugiura R, Kuwatani M, Hirata K, et al. Effect of pancreatic mass size on clinical outcomes of endoscopic ultrasound-guided fine-needle aspiration. Dig Dis Sci. 2019;64:2006–2013. doi: 10.1007/s10620-018-5435-3. [DOI] [PubMed] [Google Scholar]
- 19.Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Khan U, Abunassar M, Chatterjee A, James PD. Advanced endoscopy trainee involvement early in EUS training may be associated with an increased risk of adverse events. J Can Assoc Gastroenterol. 2020;3:83–90. doi: 10.1093/jcag/gwy066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razpotnik M, Bota S, Essler G, Weber-Eibel J, Peck-Radosavljevic M. Impact of endoscopist experience, patient age and comorbidities on dose of sedation and sedation-related complications by endoscopic ultrasound. Eur J Gastroenterol Hepatol. 2022;34:177–183. doi: 10.1097/MEG.0000000000002084. [DOI] [PubMed] [Google Scholar]