Background:
The clinical behavior of cT4 prostate cancer is poorly understood. Previous studies have historically grouped patients with T4 disease with those with other locally advanced presentations such as extraprostatic extension (cT3a) and seminal vesicle involvement (cT3b) (1). Data remain limited regarding the natural history of clinical T4 prostate cancer and its patterns of locoregional spread. Recent evidence showing improved survival rates after local and systemic therapies suggests that more patients with T4 prostate cancer may benefit from local therapy (2–4). Specifically, use of external-beam radiation therapy (EBRT) was found in two large randomized trials to provide survival advantages over hormone therapy alone for patients with high-risk disease (5, 6). The Radiation Therapy Oncology Group (RTOG) contouring atlas recommends that elective pelvic nodal irradiation for prostate cancer include treatment of the presacral LNs in the subaortic region and the distal common iliac nodes up to the L5/S1 interspace, with the contours for the external iliac nodes extending to the top of the femoral head and those for the obturator nodes to the top of the symphysis pubis (7). Whether these nodal targets should be modified depending on local disease invasion is unknown. We therefore attempted to identify common sites of nodal disease presentation and failure for patients presenting with cT4 prostate cancer.
Methods:
With the approval of our Institutional Review Board, we retrospectively identified all men who presented with treatment-naive cT4 prostate cancer, had available pelvic imaging for review, and received treatment at our institution from 1996 through 2018. Prostate cancer diagnosis was confirmed by review of pathology slides by a genitourinary pathologist. Initial disease staging for all patients consisted of a baseline bone scan and pelvic imaging (computed tomography [CT] or magnetic resonance imaging [MRI]). T4 disease was confirmed based on findings from physical examination, cystoscopy, examination under anesthesia (e.g., cystoscopy or rectal ultrasonography), imaging or combination thereof. LN involvement and location at diagnosis were reviewed by a genitourinary radiologist (TKB). All patients’ follow-up scans were also reviewed and based on LN size, imaging characteristics, progression/regression characteristics on systemic therapy, LN failure sites were recorded. For patients who underwent pelvic LN dissection, any pathologically involved LNs and their anatomic locations were recorded. A total of 103 patients met these criteria with a median follow up of 8 years (range 0.5–14 years). Baseline patient characteristics are summarized in Table 1. LN involvement either upfront or at recurrence were considered in this analysis. Pelvic LN echelons were defined as per the RTOG contouring consensus (7). Descriptive statistics were used to present patient characteristics. Proportion of LN region involvement was compared via Chi-square test. All tests were two sided with p-value <0.05 considered significant. Statistical analyses were done using JMP Pro ver. 15 and SAS ver. 9.4 (both Cary, NC).
Table 1.
Patient Baseline characteristics
| Characteristics | Value or No. (%) |
|---|---|
| Age, years | |
| Mean | 63 |
| Median | 62.4 |
| Range | 40–88 |
| Initial PSA Level, ng/mL | |
| Mean | 58 |
| Median | 17.5 |
| Range | 0.5–900 |
| Tumor Histology | |
| Adeno (NOS) | 81 (79) |
| Adeno (ductal) | 9 (9) |
| Small Cell | 6 (6) |
| Other (NE, sarc, sq) | 7 (7) |
| Grade Group | |
| 2 | 1 |
| 3 | 4 |
| 4 | 15 |
| 5 | 77 |
| N/A (small cell histology) | 6 |
| Organ(s) Involved | |
| Bladder | 54 (52) |
| Bladder & rectum | 24 (23.3) |
| Bladder & PSW | 2 (2) |
| Rectum | 14 (14) |
| Rectum & PSW | 1 (1) |
| PSW | 5 (5) |
| Bladder, rectum & PSW | 3 (3) |
| Disease Stage | |
| T4N0M0 | 24 (23) |
| T4N1M0 | 22 (21) |
| T4NanyM1 | 57 (55) |
| Initial N Category | |
| 0 | 26 (25) |
| 1 | 77 (75) |
| Initial M Category | |
| 0 | 46 (45) |
| 1a | 13 (13) |
| 1b | 38 (37) |
| 1c | 6 (6) |
Abbreviations: NOS, not otherwise specified; duct, ductal; other, neuro-endocrine, sarcomatoid, squamous; PSW, pelvic sidewall.
Results:
Patients with any rectal involvement at diagnosis (n=42) had a significantly increased rate of perirectal and mesorectal LN involvement compared with those who did not (n=61) (45% vs 26% p <0.05) (FIGURE 1). No other LN stations were significantly associated with local disease organ invasion (all P>0.05). The perirectal and mesorectal nodal echelons are not part of the standard pelvis EBRT field and are therefore underdosed, especially when intensity-modulated radiation therapy is used (FIGURE 2). Furthermore, standard LN dissection conducted as part of prostatectomy and pelvic LN dissection include the external iliac, obturator, hypogastrics +/− presacral lymph nodes. This pattern of perirectal and mesorectal involvement with rectal invasion was present in patients presenting with non-metastatic or metastatic disease (Supplement Figure1). In contrast, the presence of bladder or pelvic side wall involvement was not associated with a distinct pattern of LN disease (all P>0.05), although only a few patients in this study (11 [11%]) had pelvic side-wall involvement.
Figure 1.
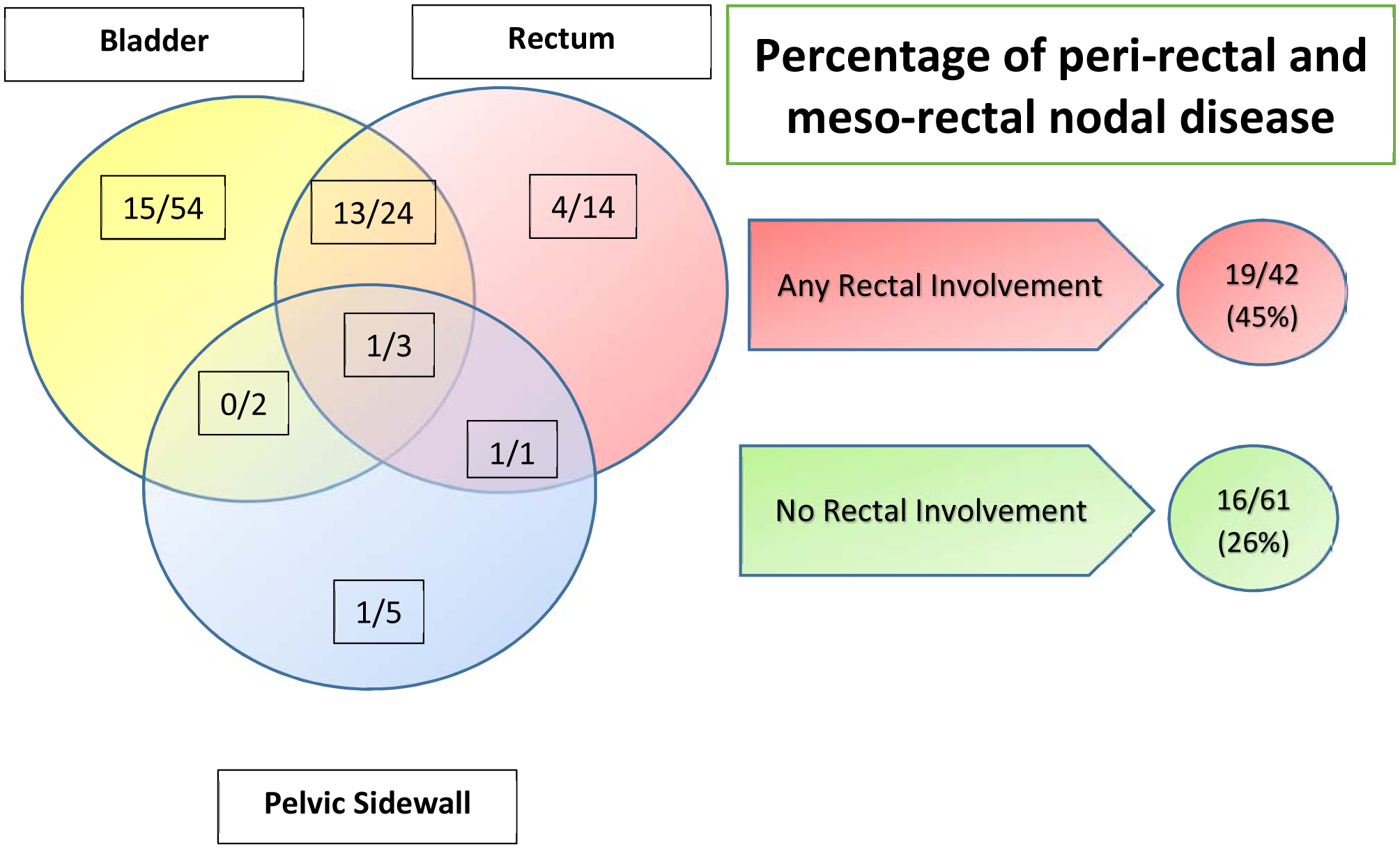
Local tissue invasion at baseline and percentages of perirectal and mesorectal lymph node involvement in 103 men with T4 prostate cancer. A total of 45% of patients with any rectal involvement at diagnosis had perirectal or mesorectal nodal disease compared with 26% of those with no rectal involvement at diagnosis. P=0.045
Figure 2.
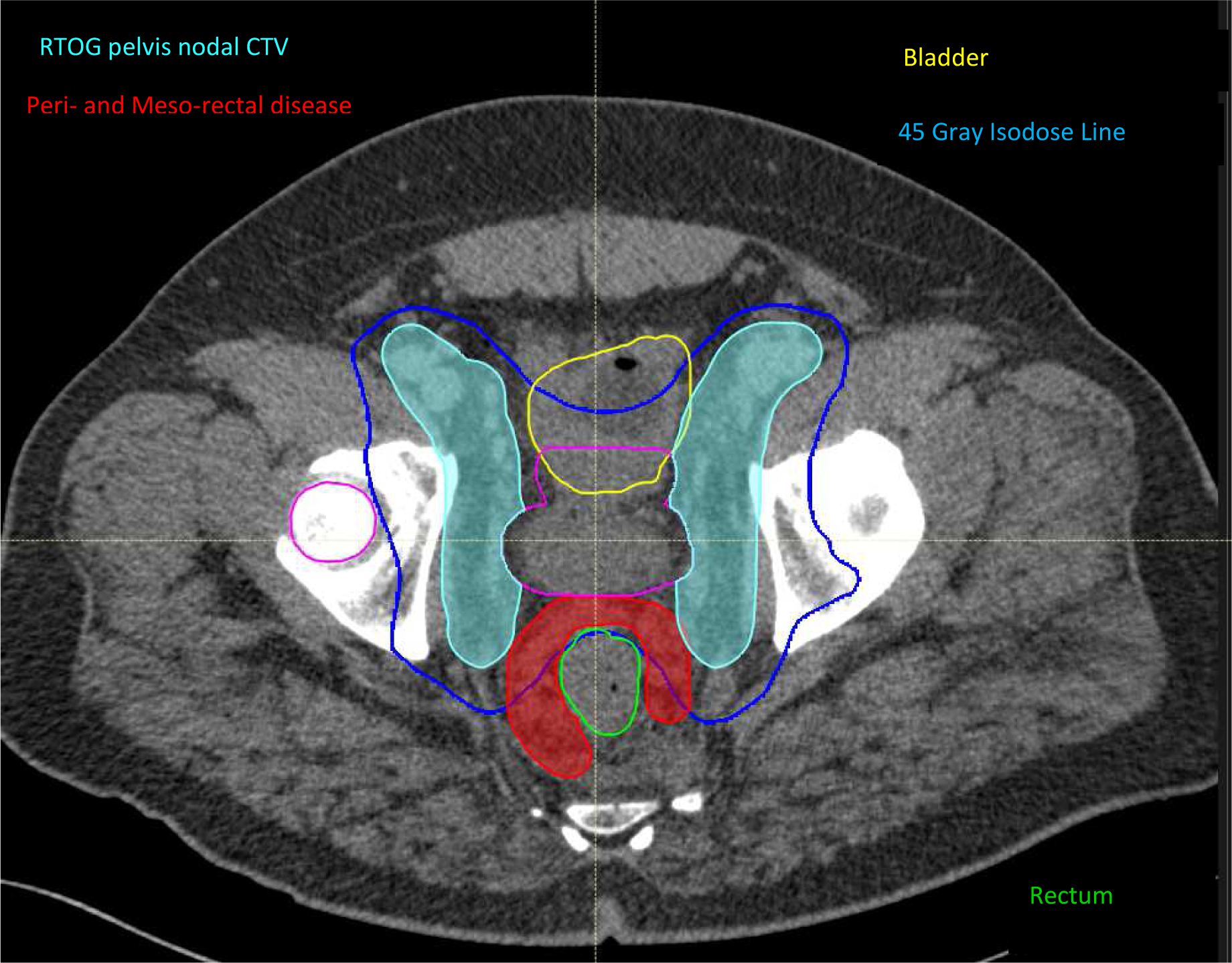
Axial view of an intensity-modulated radiation therapy plan encompassing the prostate (light blue colorwash) and pelvic lymph nodes (red colorwash) for a patient with rectal involvement. Involved mesorectal and perirectal lymph nodes (contoured in red) fall outside the usual pelvic field and are therefore underdosed.
Discussion:
The role of neoadjuvant and local therapy for T4 prostate cancer is being actively investigated (2, 8, 9). Our findings suggest that men presenting with clinical T4 prostate cancer with rectal involvement who are to receive definitive local therapy may benefit either from adjustment of pelvic EBRT fields to encompass the mesorectal and perirectal lymph nodes or from surgical removal of the involved nodes. Although adjusting radiation fields in response to invasion of surrounding organs is common for cancer of other pelvic organs, this issue has not been addressed in prostate cancer (10).
Our study had both limitations and strengths. Aside from the inherent limitations of a single-institution retrospective study, histologic confirmation of nodal disease was not routinely obtained. Furthermore, our sample size does limit the number and complexity of multivariate analyses that can be done, and thus we have chosen not to pursue such analyses. However, all patients initial and follow-up imaging were subject to thorough evaluation by experts in prostate imaging and radiation therapy. Second, most of our baseline and follow-up imaging involved either CT or MRI. Advanced prostate cancer imaging modalities such as choline or prostatespecific membrane antigen positron emission tomography/CT scans were not routinely used. Their increased sensitivity and specificity may be helpful for detecting more peri-rectal or mesorectal disease in such patients (11). The strength of this study is its inclusion of a large, relatively homogenous group of treatment-naive patients with an upfront diagnosis of T4 prostate cancer; previous studies have included patients with a variety of advanced T categories, including T3a and T3b. Moreover, with a total of 103 patients and a median follow-up period of 8 years, the current analysis represents one of the largest series with the longest follow-up. Finally, the current analysis was the first to our knowledge to address patterns of nodal disease in patients with clinical T4 prostate cancer.
Conclusion:
In summary, we found that patients who presented with T4 disease with rectal involvement had higher rates of perirectal and mesorectal LN involvement. Therefore, perirectal and mesorectal LN coverage should be considered for patients receiving definitive radiation therapy or pelvic LN dissection.
Supplementary Material
Disclosure Statement:
Dr. Frank Reports personal fees from Varian, grants and personal fees from C4 imaging, grants from Eli Lilly, grants from Elekta, grants and personal fees from Hitachi, other from Breakthrough Chronic Care, personal fees from Boston Scientific, personal fees from National Comprehensive Cancer Network (NCCN), outside the submitted work
Dr. Tang reports personal fees from Reflexion, personal fees from AstraZeneca, personal fees from Wolter Kluwer, outside the submitted work; In addition, Dr. Tang has a patent U.S. Patent #9,175,079 licensed to THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIVERSITY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Collaborating Centre for Cancer (UK). Prostate Cancer: Diagnosis and Treatment. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2014 Jan. (NICE Clinical Guidelines N [Available from: https://www.ncbi.nlm.nih.gov/books/NBK248406/.
- 2.Hajili T, Ohlmann CH, Linxweiler J, Niklas C, Janssen M, Siemer S, et al. Radical prostatectomy in T4 prostate cancer after inductive androgen deprivation: results of a single-institution series with long-term follow-up. BJU international. 2019;123(1):58–64. [DOI] [PubMed] [Google Scholar]
- 3.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. The New England journal of medicine. 2017;377(4):338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet (London, England). 2019;393(10185):2051–8. [DOI] [PubMed] [Google Scholar]
- 5.Brundage M, Sydes MR, Parulekar WR, Warde P, Cowan R, Bezjak A, et al. Impact of Radiotherapy When Added to Androgen-Deprivation Therapy for Locally Advanced Prostate Cancer: Long-Term Quality-of-Life Outcomes From the NCIC CTG PR3/MRC PR07 Randomized Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(19):2151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet (London, England). 2009;373(9660):301–8. [DOI] [PubMed] [Google Scholar]
- 7.McClinton C, Niroumand M, Sood S, Shah V, Hill J, Dusing RW, et al. Patterns of lymph node positivity on (11)C-acetate PET imaging in correlation to the RTOG pelvic radiation field for prostate cancer. Practical radiation oncology. 2017;7(5):325–31. [DOI] [PubMed] [Google Scholar]
- 8.Berglund RK, Tangen CM, Powell IJ, Lowe BA, Haas GP, Carroll PR, et al. Ten-year follow-up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on Southwest Oncology Group Study 9109. Urology. 2012;79(3):633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone PA, Ward KC, Goodman M, Assikis V, Petros JA. Radical prostatectomy for clinical T4 prostate cancer. Cancer. 2006;106(12):2603–9. [DOI] [PubMed] [Google Scholar]
- 10.Gaffney DK, King B, Viswanathan AN, Barkati M, Beriwal S, Eifel P, et al. Consensus Recommendations for Radiation Therapy Contouring and Treatment of Vulvar Carcinoma. International journal of radiation oncology, biology, physics. 2016;95(4):1191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, et al. (18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. The Lancet Oncology. 2019;20(9):1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


