Abstract
Introduction
In people living with human immunodeficiency virus (PLHIV), traditional cardiovascular risk factors, exposure to HIV per se and antiretroviral therapy (ART) are assumed to contribute to cardiometabolic diseases. Nevertheless, controversy exists on the relationship of HIV and ART with diabetes. To clarify the relationship between HIV and type 2 diabetes, this review determined, in PLHIV in Africa, diabetes and prediabetes prevalence, and the extent to which their relationship was modified by socio‐demographic characteristics, body mass index (BMI), diagnostic definitions used for diabetes and prediabetes, and HIV‐related characteristics, including CD4 count, and use and duration of ART.
Methods
For this systematic review and meta‐analysis (PROSPERO registration CRD42021231547), a comprehensive search of major databases (PubMed‐MEDLINE, Scopus, Web of Science, Google Scholar and WHO Global Health Library) was conducted. Original research articles published between 2000 and 2021 in English and French were included, irrespective of study design, data collection techniques and diagnostic definitions used. Observational studies comprising at least 30 PLHIV and reporting on diabetes and/or prediabetes prevalence in Africa were included. Study‐specific estimates were pooled using random effects models to generate the overall prevalence for each diagnostic definition. Data analyses used R statistical software and “meta” package.
Results
Of the 2614 records initially screened, 366 full‐text articles were assessed for eligibility and 61 were selected. In the systematic review, all studies were cross‐sectional by design and clinic‐based, except for five population‐based studies. Across studies included in the meta‐analysis, the proportion of men was 16–84%. Mean/median age was 30–62 years. Among 86,412 and 7976 participants, diabetes and prediabetes prevalence rates were 5.1% (95% CI: 4.3–5.9) and 15.1% (9.7–21.5). Self‐reported diabetes (3.5%) was lower than when combined with biochemical assessments (6.2%; 7.2%).
Discussion
While not statistically significant, diabetes and prediabetes were higher with greater BMI, in older participants, urban residents and more recent publications. Diabetes and prediabetes were not significantly different by HIV‐related factors, including CD4 count and ART.
Conclusions
Although HIV‐related factors did not modify prevalence, the diabetes burden in African PLHIV was considerable with suboptimal detection, and likely influenced by traditional risk factors. Furthermore, high prediabetes prevalence foreshadows substantial increases in future diabetes in African PLHIV.
Keywords: Africa, ART, CD4 count, diabetes, prediabetes, HIV, risk factors, prevalence
1. INTRODUCTION
Despite a focus on infectious diseases in Africa, there is growing acknowledgement of the increasing burden of non‐communicable diseases (NCDs) as well as the double challenge of Africans experiencing both NCDs and infectious diseases. This is particularly true in people living with human immunodeficiency virus (PLHIV) following the successful rollout of highly active antiretroviral therapy (HAART), which has been accompanied by increased longevity [1, 2, 3]. The maturing of the HIV epidemic on the continent with ageing populations has subsequently led to exposure to NCDs, and a parallel increase in cardiovascular and cardiometabolic diseases.
The aetiology of cardiometabolic diseases in PLHIV is multifactorial. Together with traditional cardiovascular risk factors, such as ageing, obesity, unhealthy lifestyles and so on, exposure to HIV per se and HAART are assumed to contribute to cardiometabolic diseases [1, 2]. The use of HAART long‐term has been linked to dysregulation of glucose metabolism and dyslipidaemia, chronic systemic inflammation, endothelial dysfunction and an increase in cardiovascular disease (CVD) risk [1, 3–5].
Nevertheless, controversy exists, and debate is ongoing on the relationship of HIV and HAART with type 2 diabetes mellitus (hereafter referred to as diabetes); both increased risk and no difference have been described in European populations in high‐income countries [6]. In Africa, the global region with the greatest HIV burden (over 25 million individuals) [7], a meta‐analysis of a few heterogeneous studies published between 2008 and 2016, and with moderate‐to‐high risk of bias, revealed no significant association between prevalent diabetes and HIV or antiretroviral therapy (ART) [4]. In contrast, systematic reviews of studies prior to 2017 conducted in PLHIV globally have reported significant relationships between ART use and diabetes or prediabetes [5, 8]. Nevertheless, these reviews have highlighted the need for further research to explore the interactions between prediabetes and/or diabetes with ART in PLHIV [5].
Diabetes in PLHIV in Africa is poorly understood with insufficient information on the epidemiology and influences of this complex condition. This is of concern because of the increasing diabetes burden in general populations in Africa attributable to traditional risk factors [9], and likely a similar pattern in PLHIV. Moreover, unlike HIV, diabetes is inadequately detected and poorly controlled in Africa leading to a rising burden linked to premature death [9, 10]. Diabetes has the potential to threaten the advances in longevity achieved with the advent of ART in PLHIV in Africa [5]. Exploring and understanding the link between HIV and diabetes is important to maintain the advances made in the battle against HIV. Such information can inform strategies and interventions to effectively address comorbid diabetes in PLHIV [1, 2, 6].
This systematic review and meta‐analysis aimed to determine the pooled prevalence of diabetes and its precursor state, prediabetes, among adult PLHIV in Africa. Additionally, the meta‐analysis examined the magnitude of diabetes and prediabetes prevalence by socio‐demographic characteristics (age, gender and urban/rural residence), body mass index (BMI), diagnostic definitions used for diabetes and prediabetes, and HIV‐related characteristics (CD4 count, and use and duration of ART), among other predictive characteristics.
2. METHODS
This systematic review, focusing on the prevalence of prediabetes and diabetes in PLHIV in Africa (including North Africa), was registered in the PROSPERO registry for systematic reviews (registration number CRD42021231547) [11]. The systematic review and meta‐analyses were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA guidelines) [12].
2.1. Search strategy
A comprehensive electronic search was conducted across major databases, including PubMed‐MEDLINE, Scopus, Web of Science, Google Scholar and WHO Global Health Library. This was supplemented with manual scanning of reference lists of relevant articles and reviews. The search terms comprised combinations of MeSH terms, CINAHL headings and free words relating to prevalence, diabetes, prediabetes and HIV/AIDS. The search terms for PubMed‐MEDLINE are presented in Table S1 and were adapted accordingly for the other databases. The search was filtered for original research articles conducted in Africa and published from 01 January 2000 to 31 December 2021 in English and French languages.
2.2. Selection of eligible studies and diagnostic criteria
Observational studies (cross‐sectional, case–control and cohort studies) comprising at least 30 people, that reported on the prevalence of diabetes and/or prediabetes among adult PLHIV in Africa, were included. Studies reporting outcomes in pregnant women, children or type 1 diabetes only were excluded.
Criteria for diabetes included self‐report and/or biochemical testing using the following methods: oral glucose tolerance test (OGTT), fasting blood glucose (FBG) only, glycated haemoglobin (HbA1c) or random blood glucose (RBG). Prediabetes was determined on biochemical assessments only of the latter tests. Although the cut‐off points for diagnosing diabetes and prediabetes were not predefined, the biochemical cut‐points to diagnose diabetes across most studies were as follows: FBG ≥7 mmol/L and/or 2‐hour blood glucose ≥11.1 mmol/L; HbA1c ≥6.5%; and RBG ≥11.1 mmol/L. Prediabetes was generally diagnosed as follows: impaired fasting glycaemia (IFG): FBG: 6.1–6.9 mmol/L; impaired glucose tolerance (IGT): 2‐hour blood glucose: 7.8–11.0 mmol/L; HbA1c: 5.7–6.4% and RBG 7.8–11.0 mmol/L.
For the overall estimate of diabetes prevalence, each study was included once only irrespective of the number of criteria used for diagnosis. A tiered approach was used to include a single prevalence estimate as follows: (1) OGTT; (2) FBG; and (3) RBG. For example, if a study reported diabetes prevalence using both OGTT and FBG, the OGTT‐based diabetes prevalence was selected. Studies with self‐report diabetes estimates or data extracted from clinic folders were included. However, studies that utilized HbA1c only for the diagnosis of diabetes were excluded from the overall diabetes prevalence estimate because HbA1c has not yet been recommended for diabetes diagnostic purposes in African populations.
Similarly, for the overall estimate of prediabetes prevalence, the tiered approach was as follows: (1) OGTT; (2) IGT; (3) IFG; and (4) RBG. Two pooled prevalence estimates for prediabetes, with and without studies that used HbA1c only, were calculated. The studies that utilized HbA1c only were excluded from the sub‐group analyses.
2.3. Screening and data extraction
The studies were independently reviewed (KAN and NP) by title and abstract for eligibility, followed by an assessment of the relevant full texts. Disagreements were resolved by discussion and consensus or in consultation with a third investigator (APK). Relevant data for this review, extracted using a data extraction form designed for this review, included the following: (1) Manuscript details (author names and year of publication); (2) Study characteristics (country, study design, year of survey, study population, setting, sample size and sampling method); (3) Definitions (criteria used to define prediabetes or diabetes); and (4) Participant socio‐demographic and lifestyle characteristics (age, gender, smoking and alcohol use), HIV‐related factors (HIV stage, severity [CD4 count and viral load], duration of HIV diagnosis, ART regimen and duration of ART use) and comorbidities (obesity, hypertension, dyslipidaemia and co‐infections, such as tuberculosis and hepatitis).
2.4. Assessment of the methodological quality of included studies
The methodological quality of the included studies was evaluated using a checklist adapted from Hoy et al. [13] and used in previous systematic reviews [14]. The representativeness of the sample, the sampling technique, the response rate, the data collection method, the measurement tools, the case definitions and the statistical reporting were evaluated. Each of the nine questions were scored as low [1] or high (0) risk of bias. The total scores determined the risk of bias as follows: low: 7–9, moderate: 4–6 and high: 0–3.
The interrater disagreement was resolved by consensus or in consultation with a third investigator (APK). The precision (C) or margin of error was estimated for each included study, considering the sample size (SS) and the observed prevalence (p) of diabetes/prediabetes from the formula SS = Z2_p_(1–p)/C2, where Z is the z‐value fixed at 1.96 across studies (corresponding to the 95% confidence interval). The desirable margin of error was 5% (0.05) or lower.
2.5. Data synthesis and analyses
Data analyses were conducted using the R statistical software and the “meta” package. For each included study, the unadjusted prevalence of diabetes and prediabetes were estimated overall and across the major sub‐groups of interest. The study‐specific estimates were pooled using random effects models to generate the overall prevalence of diabetes and prediabetes for each diagnostic definition. The variance of the raw prevalence was stabilized using the Freeman–Tukey double arc‐sine transformation before pooling the data to minimize the effect of extreme prevalence on the overall estimates. Data are presented as prevalence (%) and 95% confidence intervals (CI). A p‐value <0.05 described statistically significant differences in findings within each diagnostic criterion overall, and by sub‐group analyses.
Heterogeneity among studies was assessed using I 2, Cochran's Q and H statistics. I 2 values of <50% represented low heterogeneity and >75% described high heterogeneity. Potential sources of heterogeneity were explored by comparing the prevalence of diabetes and prediabetes between sub‐groups of interest. These comparisons used the Q‐test based on the Analysis of the Variance. Differences in major characteristics, such as study design, study populations, and diagnostic criteria and cut points for diabetes and prediabetes, were used to define sub‐groups of interest, for example discrete categories (gender, setting, year of publication, diagnostic criteria and ART use) or by using median values of the summary estimates for continuous characteristics (age, BMI, sample size and ART duration).
The presence of publication bias was assessed using the funnel plots. This was supplemented by formal statistical assessments using the Egger test of bias [15]. A p‐value <0.05 illustrated a significant asymmetry of the funnel plot and evidence of publication bias. The Duval and Tweedie trim‐and‐fill was used to adjust estimates for the effects of publication bias.
Ethical approval was not required as this was secondary analyses of published data.
3. RESULTS
3.1. The review processes and data extraction
After duplicate removals from the 4083 records identified, titles and abstracts of 2614 records were screened, and 366 full‐text articles were assessed for eligibility (Figure 1). Of these, 61 fulfilled the eligibility criteria and were included in this review. One article [16] reported surveys at three time points which were counted separately, making a total of 63 studies included in the meta‐analysis.
Figure 1.
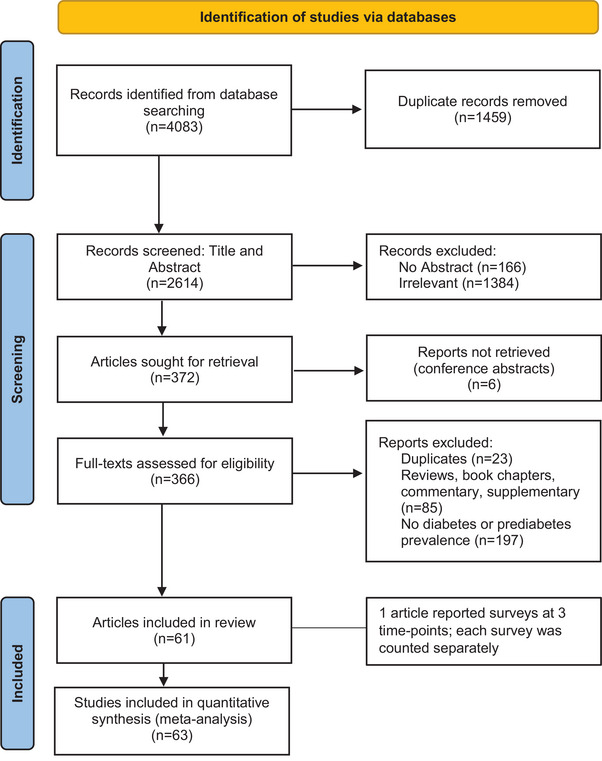
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) diagram.
All relevant HIV‐related factors (HIV staging and viral load) and co‐morbidities (hypertension, dyslipidaemia and co‐infections) were not extracted as planned because of the lack of such data or an inadequate number of studies reporting the requisite data for meaningful analyses.
3.2. Methodological quality of the included studies
The risk of bias assessment for the included studies is summarized in Figure 2. Eight studies had a low risk of bias and 55 studies had a moderate risk of bias. Among the latter, 33 studies involved less than 500 participants, while 16 studies reported using some form of random selection approach to select participants.
Figure 2.
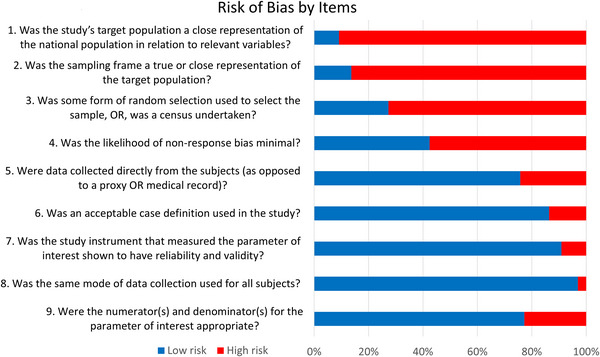
Risk of bias assessments for the included studies.
3.3. General characteristics of the included studies
Studies were published between 2008 and 2021 (Table 1). One study was published before 2010 [17], nine were published from 2011 to 2015 [18, 19, 20, 21, 22, 23, 24, 25, 26] and 5–10 studies were published yearly thereafter. The highest number of included studies were from South Africa (n = 18) [16, 18, 34–24, 27, 39–33], followed by Ethiopia (n = 8) [26, 40–46] and Tanzania (n = 7) [19, 22, 25, 47–50], with four studies each from Cameroon [19, 51–53], Ghana [21, 54–56], Kenya [17, 57–59], Malawi [60, 61, 62, 63] and Uganda [64, 65, 66, 67].
Table 1.
Summary of the characteristics and methodological quality of the included studies
| Authors | Published year | Country | Area | Study site | Study type | Study period | Sampling | N | Male % | Mean/median age | Selection criteria | Quality grade (risk) | Margin of error |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manuthu et al. [17] | 2008 | Kenya | Urban | Clinic‐based | Cross‐sectional | 2006 | Non‐random | 295 | ART naïve: 44; on ART: 40 | ART naïve: 36.5; on ART: 39.4 | Adult PLHIV on ART ≥4 weeks and not changing ART regimen in the past year, and without history of diabetes or taking lipid‐lowering agents. | Moderate | 0.045 |
| Dave et al. [18] | 2011 | South Africa | Urban | Clinic‐based | Cross‐sectional | NR | Non‐random | 849 | 23 | − | PLHIV on first‐line ART regimen (d4T, 3TC and efavirenz or nevirapine) >6 months and ART naïve, and without history of diabetes or impair glucose tolerance. | Moderate | 0.017 |
| Nagu et al. [19] | 2012 | Tanzania | Urban | Clinic‐based | Cross‐sectional | 2004–2009 | Non‐random | 41,891 | 29 | 36 (10) | PLHIV >15 years, ART naïve (83% >18 years) | Moderate | 0.002 |
| Ngatchou et al. [20] | 2013 | Cameroon | Urban | Clinic‐based | Cross‐sectional | 2009–2010 | Unspecified | 108 | 26 | 39 (11) | PLHIV ≥18 years, ART naive | Moderate | 0.082 |
| Ngala et al. [21] | 2013 | Ghana | Urban | Clinic‐based | Cross‐sectional | 2009–2010 | Unspecified | 164 | 42 | 38.2 (0.65) | PLHIV ≥18 years, on ART ≥6 months, without previous diabetes, hypertension or family history of diabetes, hypertension. | Moderate | 0.038 |
| Kagaruki et al. [22] | 2014 | Tanzania | Urban; rural | Clinic‐based | Cross‐sectional | 2011–2012 | Non‐random | 671 | 30 | 38.7 (10.1 | PLHIV ≥18 years | Low | 0.015 |
| Sawadogo et al. [23] | 2014 | Burkina Faso | Urban | Clinic‐based | Cross‐sectional | 2011 | Random | 400 | 29 | 41.4 (8.8) | PLHIV ≥18 years, on ART ≥6 months | Moderate | 0.011 |
| Rabkin et al. [24] | 2015 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 175 | 26 | 45.4 (8.8) | PLHIV ≥30 years on ART ≥1 year | Moderate | 0.029 |
| Maganga et al. [25] | 2015 | Tanzania | Urban | Clinic‐based | Cross‐sectional | 2012–2013 | Non‐random | 301 | ART naïve: 41; on ART: 23 | ART naïve: 37 (32–44); on ART: 40 (38–47) | PLHIV ≥18 years ART naive, or on ART ≥2 years | Moderate | 0.013 |
| Mohammed et al. [26] | 2015 | Ethiopia | Urban; rural | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 393 | 33 | 37.9 (11.2) | PLHIV ≥18 years | Moderate | 0.024 |
| Divala et al. [60] | 2016 | Malawi | Urban; rural | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 952 | 28 | 43 (10.2) | PLHIV ≥18 years | Moderate | 0.013 |
| Mugisha et al. [64] | 2016 | Uganda | Urban; rural | Population‐based | Cross‐sectional | 2012–2013 | Non‐random | 244 | 40 | 57 | PLHIV ≥50 years | Moderate | 0.03 |
| Isa et al. [76] | 2016 | Nigeria | Urban | Clinic‐based | Cross‐sectional | 2011–2013 | Non‐random | 2632 | 35 | 37.4 (9.7) | PLHIV ≥18 years on ART | Moderate | 0.006 |
| Rhee et al. [51] | 2016 | Cameroon | Urban | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 500 | 27 | 42.5 (36.5–49.5) | PLHIV ≥16 years on ART (>80% age ≥18 years), and without diabetes history | Moderate | 0.017 |
| Levitt et al. [27] | 2016 | South Africa | Urban; rural | Population‐based | Cross‐sectional | 2008–2010 | Non‐random | 940 | 24 | 33.6 (8.77) | PLHIV ≥18 years, without diabetes history | Moderate | 0.014 |
| Magodoro et al. [1] | 2016 | Zimbabwe | Urban | Clinic‐based | Cross‐sectional | 2015 | Non‐random | 1400 | 31 | 42 (36–50) | PLHIV ≥18 years on ART | Moderate | 0.009 |
| Abebe et al. [46] | 2016 | Ethiopia | Urban; rural | Clinic‐based | Cross‐sectional | 2013–2014 | Random | 462 | 31 | ART naïve: 34.6 (9.9); on ART: 37.5 (9.2) | PLHIV ≥15 years (93% ≥18 years) | Moderate | 0.025 |
| Sinxadi et al. [28] | 2016 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2007–2008 | Non‐random | 107 | 27 | 38 (31–45) | PLHIV ≥18 years on EFV for ≥6 months and adherence to ART, without previous diabetes | Moderate | 0.026 |
| Traoré et al. [77] | 2016 | Morocco | Urban | Clinic‐based | Retrospective | − | Non‐random | 1800 | − | − | PLHIV treated at the Infectious Diseases out‐patient department of the University Hospital Center of Casablanca (Ibn Rochd), Morocco | Moderate | 0.008 |
| Ekrikpo et al. [78] | 2017 | Nigeria | Urban | Clinic‐based | Cross‐sectional | 2002–2016 | Non‐random | 1818 | 40 | 34.3 (9.9) | PLHIV ≥18 years, ART naive | Moderate | 0.011 |
| Noumegni et al. [52] | 2017 | Cameroon | Urban | Clinic‐based | Cross‐sectional | 2015–2016 | Non‐random | 452 | 20 | 44.4 (9.8) | PLHIV ≥18 years, ART naïve or on ART; without CVD history | Moderate | 0.014 |
| PrayGod et al. [50] | 2017 | Tanzania | Urban | Clinic‐based | Cross‐sectional | 2015 | Non‐random | 273 | 35 | 38.9 (9.7) | PLHIV ≥18 years on ART for 2–3 years | Moderate | 0.014 |
| Gaziano et al. [29] | 2017 | South Africa | Rural | Population‐based | Cross‐sectional | 2014–2015 | Random | 4576 | 46 | 61.7 (13.1) | PLHIV ≥40 years | Moderate | 0.015 |
| Van Heerden et al. [30] | 2017 | South Africa | Rural | Population‐based | Cross‐sectional | 2011–2012 | Non‐random | 189 | 19 | − | PLHIV ≥18 years | Moderate | 0.02 |
| Shankalala et al. [79] | 2017 | Zambia | Urban | Clinic‐based | Cross‐sectional | 2015 | Non‐random | 270 | 31 | 46 (38–51) | PLHIV ≥18 years on ART ≥24 months | Moderate | 0.039 |
| Kazooba et al. [65] | 2017 | Uganda | Rural | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 1024 | 35 | 44.8 (8) | PLHIV ≥18 years | Moderate | 0.011 |
| Labhardt et al. [31] | 2017 | South Africa | Rural | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 1166 | 34 | 44.4 (35.3–54.4) | PLHIV ≥16 years, on NNRTI‐based first‐line ≥6 months | Low | 0.008 |
| Pfaff et al. [61] | 2018 | Malawi | Urban | Clinic‐based | Cross‐sectional | 2015–2017 | Non‐random | 2979 | − | − | PLHIV ≥18 years | Moderate | 0.003 |
| Ngu et al. [53] | 2018 | Cameroon | Urban | Clinic‐based | Cross‐sectional | − | Non‐random | 311 | 16 | 43.4 (10.6) | PLHIV ≥21 years | Moderate | 0.035 |
| Mathabire Rücker et al. [62] | 2018 | Malawi | Urban | Clinic‐based | Cross‐sectional | 2015–2016 | Non‐random | 379 | 26 | 47 (42–52) | PLHIV ≥ 30 years on ART ≥10 years | Moderate | 0.025 |
| Kansiime et al. [66] | 2018 | Uganda | Urban | Clinic‐based | Cross‐sectional | 2017 | Non‐random | 387 | 34 | 42 (20–75) | PLHIV ≥18 years on ART ≥2 months | Moderate | 0.021 |
| Ataro et al. [45] | 2018 | Ethiopia | Urban | Clinic‐based | Cross‐sectional | 2017 | Non‐random | 425 | 30 | 39.7 (8.9) | PLHIV ≥years on ART ≥6 months | Moderate | 0.024 |
| Rabkin et al. [68] | 2018 | Swaziland | Urban | Clinic‐based | Cross‐sectional | 2015–2016 | Non‐random | 1826 | 38 | 47 (40–82) | PLHIV ≥40 years | Moderate | 0.009 |
| Katoto et al. [80] | 2018 | DRC | Urban | Clinic‐based | Cross‐sectional | 2016 | Non‐random | 495 | 38 | 43 (36–51) | PLHIV ≥18 years | Moderate | 0.027 |
| Osoti et al. [57] | 2018 | Kenya | Urban; rural | Clinic‐based | Cross‐sectional | 2014 | Non‐random | 300 | 36 | 40 (33–46) | PLHIV ≥18 years | Moderate | 0.031 |
| Fiseha and Belete [40] | 2019 | Ethiopia | Urban; peri‐urban | Clinic‐based | Cross‐sectional | 2018 | Unspecified | 408 | 33 | 37 (10) | PLHIV ≥ 18 years on ART ≥12 months | Low | 0.027 |
| Muchira et al. [67] | 2019 | Uganda | Urban; rural | Clinic‐based | Cross‐sectional | NR | Non‐random | 118 | 51.7 | 51.3 (7.1) | PLHIV ≥40 years on ART ≥3 years | Moderate | 0.071 |
| Faurholt‐Jepsen et al. [41] | 2019 | Ethiopia | Urban | Clinic‐based | Cross‐sectional | 2010–2012 | Non‐random | 332 | 33 | 32.9 (8.8) | PLHIV ≥ 18 years, ART naïve | Moderate | 0.036 |
| Juma et al. [58] | 2019 | Kenya | Rural | Clinic‐based | Cross‐sectional | 2013–2015 | Non‐random | 1502 | 31 | 30 (31–48) | PLHIV ≥18 years, ART naïve or on ART | Moderate | 0.003 |
| Hyle et al. [32] | 2019 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2015–2016 | Non‐random | 458 | 22 | 38 (33–44) | PLHIV ≥21 years, on ART | Moderate | 0.021 |
| Nguyen et al. [33] | 2019 | South Africa | Urban; rural | Clinic‐based | Cross‐sectional | 2014–2015 | Random | 748 | 21 | 38 (35–42) | PLHIV ≥18 years, ART naïve or on ART | Low | 0.013 |
| Nkinda et al. [48] | 2019 | Tanzania | Urban | Clinic‐based | Cross‐sectional | 2018 | Non‐random | 240 | 25 | 47 (10) | PLHIV ≥18 years, on first‐line ART regimen | Moderate | 0.039 |
| Appiah et al. [54] | 2019 | Ghana | Urban | Clinic‐based | Cross‐sectional | 2013–2014 | Non‐random | 345 | 28 | 41 (11) | PLHIV ≥18 years | Moderate | 0.021 |
| Zungu et al. [34] | 2019 | South Africa | Urban; rural | Clinic‐based | Cross‐sectional | 2015–2016 | Random | 2648 | 23 | − | PLHIV educators ≥18 years | Moderate | 0.009 |
| Sogbanmu et al. [35] | 2019 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2016–2017 | Random | 335 | 31 | − | PLHIV adults, ART naive | Moderate | 0.026 |
| Jeremiah et al. [47] | 2020 | Tanzania | Urban | Clinic‐based | Cross‐sectional | 2016–2017 | Non‐random | ART naïve: 956; on ART: 336 | 39 | 41 (11) | PLHIV ≥ 18 years, ART naïve or on ART | Moderate | 0.009 |
| Kato et al. [49] | 2020 | Tanzania | Urban | Clinic‐based | Cross‐sectional | 2017 | Non‐random | 612 | 30 | 47 (42–52) | PLHIV ≥18 years: ART naïve or on ART ≥5 years | Moderate | 0.021 |
| Gebrie et al. [42] | 2020 | Ethiopia | Urban; rural | Clinic‐based | Cross‐sectional | 2019 | Random | 407 | 40 | 38.6 (10.29) | PLHIV ≥18 years on ART ≥6 months | Low | 0.027 |
| Duguma et al. [43] | 2020 | Ethiopia | Urban; rural | Clinic‐based | Cross‐sectional | 2019 | Random | 271 | 37 | 38.5 (8.98) | PLHIV ≥18 years on ART ≥3 months | Moderate | 0.038 |
| Woldesemayat [44] | 2020 | Ethiopia | Urban | Clinic‐based | Cross‐sectional | 2016 | Random | 382 | 39 | 35 (10) | PLHIV ≥18 years on ART | Low | 0.014 |
| Singano et al. [63] | 2021 | Malawi | Urban | Clinic‐based | Cross‐sectional | 2018 | Non‐random | 1316 | 30 | 44 (38–53) | PLHIV ≥18 years on ART ≥6 months | Moderate | 0.008 |
| Umar and Naidoo [36] | 2021 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2005–2009 | Random | 1203 | 34 | 29–48 (60%) | PLHIV ≥18 years on ART ≥6 months | Moderate | 0.016 |
| Chiwandire et al. [16] | 2021 | South Africa | Urban; rural | Clinic‐based | Cross‐sectional | 2005 | Random | 978 | 32 | − | PLHIV ≥25 years | Moderate | 0.012 |
| Chiwandire et al. [16] | 2021 | South Africa | Urban; rural | Clinic‐based | Cross‐sectional | 2008 | Random | 1023 | 31 | − | PLHIV ≥25 years | Moderate | 0.011 |
| Chiwandire et al. [16] | 2021 | South Africa | Urban; rural | Clinic‐based | Cross‐sectional | 2017 | Random | 2483 | 29 | − | PLHIV ≥25 years | Moderate | 0.008 |
| Rajagopaul and Naidoo [37] | 2021 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2017 | Non‐random | 301 | 37.5 | 41.6 (11) | PLHIV ≥18 years, on ART | Moderate | 0.016 |
| Kubiak et al. [38] | 2021 | South Africa | Urban | Clinic‐based | Cross‐sectional | 2017–2019 | Non‐random | 1207 | 44.4 | 31.3 (9.5) | PLHIV ≥18 years, ART naive | Moderate | 0.008 |
| Njoroge et al. [59] | 2021 | Kenya | Urban; rural | Clinic‐based | Cross‐sectional | 2018 | Random | 600 | 36.2 | 46.8 (41.6–53.1) | PLHIV >35 years old, on ART for at least 5 years | Low | 0.017 |
| Chezac et al. [81] | 2021 | Zimbawe | Urban | Clinic‐based | Cross‐sectional | 2010 | Non‐random | 203 | 37 | − | PLHIV on ART enrolled at Chitungwiza Central Hospital's Opportunistic Infections Clinic in 2010 | Moderate | 0.034 |
| Sanuade et al. [55] | 2021 | Ghana | Urban | Clinic‐based | Cross‐sectional | – | Random | 525 | 84 | 33.6 (5.0) | PLHIV ≥18 years | Moderate | 0.031 |
| Sarfo et al. [56] | 2021 | Ghana | Urban; rural | Clinic‐based | Cross‐sectional | – | Non‐random | 502 | 24.8 | 44 | PLHIV ≥30 years | Moderate | 0.022 |
| Hird et al. [39] | 2021 | South Africa | Urban | Population‐based | Cross‐sectional | 2014 | Random | 487 | − | − | PLHIV ≥18 years | Low | 0.01 |
| Hema et al. [82] | 2021 | Burkina Faso | Urban | Clinic‐based | Cross‐sectional | 2018 | Non‐random | 4259 | 26.1 | 45 (38–52) | PLHIV >18 years old on ART | Moderate | 0.008 |
Abbreviation: PLHIV, people living with HIV.
All studies were cross‐sectional and clinic‐based, except five studies that were population‐based (four from South Africa and one from Uganda). Forty studies were conducted in urban settings only, five in rural settings only [29–31, 58, 65] and 18 in both urban and rural settings. Most studies (n = 44) recruited unselected samples, while 16 studies used random sampling techniques; three studies did not specify the sampling technique.
Most studies (n = 44) had less than 1000 participants and 11 studies had between 1000 and 2000 participants; the sample sizes ranged from 107 to 41,891 participants. The proportion of males in the studies ranged from 16% to 84%. The mean/median age ranged from 30 to 62 years. Most studies were conducted in ≥18‐year‐old adults but a few focused on older adults (≥30 years: n = 3 [24, 56, 62]; ≥40 years: n = 3 [29, 67, 68]; ≥ 50 years: n = 1 [64]).
3.4. Biochemical tests utilized in included studies
Among the 63 included studies, 35 defined diabetes using biochemical criteria and/or a self‐reported diagnosis, 20 defined diabetes using biochemical criteria only and 11 studies described self‐reported diabetes only (Table S2). The most common biochemical tests used were OGTT and FBG (Table S2). Seven studies used HbA1c alone to diagnose diabetes and were excluded from the overall diabetes prevalence estimate [24, 31, 38, 51, 59, 62, 68] because HbA1c has been shown to underperform in African populations [69]. Estimates from 56 studies were pooled to determine the overall diabetes prevalence. However, five studies reported diabetes prevalence in sub‐groups only and were counted as separate studies. These included four that determined diabetes prevalence separately in ART naïve and ART users [18, 25, 49, 57], and a single study that described diabetes prevalence in ART naïve, first‐line ART users and second‐line ART users [18]. Thus, a total of 62 studies were pooled to derive the overall diabetes prevalence estimate (Table 2).
Table 2.
Summary statistics for the meta‐analyses of the prevalence studies on diabetes in people with HIV in Africa using random effects model and double‐arcsine transformations
| Group | Sub‐group | Criteria | N studies | N participants | N cases | Prevalence (95 CI) | H (95 CI) | I 2 (95 CI) | p‐heterogeneity | p‐dif criteria | p‐diff sub‐groups | p‐Egger test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Any criteria | 62 | 86,412 | 3559 | 5.05 [4.27–5.89] | 4.59 [4.25–4.94] | 95.2 [94.5–95.9] | <0.001 | 0.937 | 0.002 | ||
| Biochemical criteria only | 0.165 | |||||||||||
| OGTT | 13 | 5213 | 217 | 3.31 [1.93–5.02] | 2.95 [2.37–3.67] | 88.5 [82.1–92.6] | <0.001 | 0.390 | ||||
| FBG | 9 | 2405 | 229 | 7.89 [4.56–12.00] | 3.29 [2.56–4.23] | 90.7 [84.7–94.4] | <0.001 | 0.401 | ||||
| RBG | 2 | 459 | 37 | 6.30 [1.40–19.46] | 4.33 [2.47–7.58] | 94.7 [83.6–98.3] | <0.001 | – | ||||
| HbA1c | 7 | 4155 | 287 | 5.06 [1.70–9.99] | 5.82 [4.74–7.14] | 97.0 [95.5–98.0] | <0.001 | 0.632 | ||||
| Mixed criteria | 4 | 6750 | 293 | 3.08 [0.00–12.30] | 14.70 [12.57–17.18] | 99.5 [99.4–99.7] | <0.001 | 0.541 | ||||
| Self‐report only | 0.274 | |||||||||||
| Self‐report | 13 | 14,797 | 470 | 3.48 [2.17–5.07] | 4.63 [3.91–5.48] | 95.3 [93.5–96.7] | <0.001 | 0.264 | ||||
| Patient folder | 7 | 5453 | 332 | 5.00 [2.85–7.71] | 3.94 [3.03–5.11] | 93.5 [89.1–96.2] | <0.001 | 0.417 | ||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 5 | 1932 | 138 | 6.18 [2.49–11.31] | 3.96 [2.89–5.43] | 93.6 [88.0–96.6] | <0.001 | 0.846 | ||||
| FBG | 14 | 10,001 | 702 | 7.16 [5.31–9.26] | 3.49 [2.88–4.22] | 91.8 [87.9–94.4] | <0.001 | 0.683 | ||||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | − | ||||
| HbA1c | 4 | 2991 | 153 | 4.86 [2.40–8.10] | 3.18 [2.11–4.78] | 90.1 [77.6–95.6] | <0.001 | 0.998 | ||||
| Mixed criteria | 13 | 55,145 | 1740 | 4.15 [3.05–5.41] | 4.52 [3.81–5.36] | 95.1 [93.1–96.5] | <0.001 | 0.215 | ||||
| Age | ||||||||||||
| Median age, 41 years old | ≥39 years | Any criteria | 25 | 18,378 | 1053 | 5.96 [4.51–7.60] | 4.20 [3.70–4.77] | 94.3 [92.7–95.6] | <0.001 | 0.002 | 0.129 | 0.448 |
| Biochemical criteria only | 0.375 | |||||||||||
| OGTT | 2 | 1440 | 125 | 11.99 [3.71–24.02] | 3.74 [2.05–6.83] | 92.8 [76.1–97.9] | <0.001 | 0.016 | 0.523 | |||
| FBG | 6 | 1580 | 131 | 7.67 [3.70–12.87] | 3.30 [2.41–4.52] | 90.8 [82.8–95.1] | <0.001 | 0.891 | 0.986 | |||
| RBG | 1 | 270 | 33 | 12.22 [8.56–16.42] | − | − | − | − | ||||
| HbA1c | 5 | 2461 | 254 | 6.91 [2.75–12.71] | 4.40 [3.27–5.92] | 94.8 [90.7–97.1] | <0.001 | 0.019 | 0.176 | |||
| Mixed criteria | 3 | 3771 | 280 | 4.48 [0.00–20.26] | 15.84 [13.22–18.98] | 99.6 [99.4–99.7] | <0.001 | − | 0.915 | |||
| Self‐report | 0.091 | |||||||||||
| Self‐report | 7 | 6561 | 209 | 3.78 [2.39–5.47] | 2.99 [2.21–4.06] | 88.8 [79.5–93.9] | <0.001 | 0.686 | 0.180 | |||
| Patient folder | 2 | 1535 | 37 | 2.40 [1.64–3.28] | 1.04 [–] | 8.3 [–] | 0.296 | 0.774 | − | |||
| Biochemical criteria and/or self‐report | 0.346 | |||||||||||
| OGTT | 2 | 546 | 54 | 6.66 [0.00–30.20] | 7.54 [5.01–11.36] | 98.2 [96.0–99.2] | <0.001 | 0.919 | ||||
| FBG | 7 | 7057 | 487 | 6.89 [3.84–10.72] | 4.74 [3.76–5.99] | 95.6 [92.9–97.2] | <0.001 | 0.802 | 0.997 | |||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 3 | 2707 | 129 | 3.96 [1.52–7.46] | 3.43 [2.14–5.50] | 91.5 [78.2–96.7] | <0.001 | 0.050 | 0.736 | |||
| Mixed criteria | 8 | 4359 | 165 | 3.89 [2.88–5.04] | 1.71 [1.17–2.49] | 65.7 [27.1–83.9] | 0.004 | 0.328 | 0.296 | |||
| <39 years | Any criteria | 24 | 55,455 | 1868 | 4.50 [3.45–5.68] | 3.99 [3.49–4.56] | 93.7 [91.8–95.2] | <0.001 | 0.131 | 0.002 | ||
| Biochemical criteria only | 0.095 | |||||||||||
| OGTT | 7 | 2142 | 54 | 2.33 [1.60–3.18] | 1.13 [1.00–1.68] | 21.2 [0.0–64.5] | 0.267 | 0.883 | ||||
| FBG | 3 | 825 | 98 | 8.28 [2.44–16.99] | 3.42 [2.13–5.48] | 91.4 [78.0–96.7] | <0.001 | 0.070 | ||||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 1 | 1207 | 26 | 2.15 [1.40–3.06] | − | − | − | |||||
| Mixed criteria | 0 | − | − | − | − | − | − | |||||
| Self‐report | 0.148 | |||||||||||
| Self‐report | 3 | 2147 | 75 | 3.48 [2.74–4.30] | 1.00 [1.00–3.10] | 0.0 [0.0–89.6] | 0.699 | 0.327 | ||||
| Patient folder | 1 | 382 | 8 | 2.09 [0.86–3.81] | − | − | − | |||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 3 | 1386 | 84 | 5.90 [4.18–7.89] | 1.41 [1.00–2.62] | 49.7 [0.0–85.4] | 0.136 | 0.815 | ||||
| FBG | 6 | 2482 | 178 | 7.42 [5.41–9.72] | 2.05 [1.37–3.07] | 76.2 [46.6–89.4] | 0.001 | 0.034 | ||||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | |||||
| HbA1c | 1 | 284 | 24 | 8.45 [5.47–11.99] | − | − | − | |||||
| Mixed criteria | 5 | 47,131 | 1490 | 5.14 [3.32–7.32] | 4.88 [3.70–6.45] | 95.8 [92.7–97.6] | <0.001 | 0.165 | ||||
| Gender | ||||||||||||
| Men | Any criteria | 22 | 8142 | 423 | 4.85 [3.59–6.29] | 2.67 [2.24–3.18] | 86.0 [80.0–90.1] | <0.001 | 0.223 | 0.524 | 0.994 | |
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 2 | 540 | 8 | 1.43 [0.53–2.68] | 1.00 | 00.0 | 0.923 | 0.278 | ||||
| FBG | 2 | 170 | 15 | 8.54 [4.64–13.38] | 1.00 | 00.0 | 0.325 | 0.249 | ||||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 2 | 581 | 13 | 1.85 [0.79–3.25] | 1.00 | 00.0 | 0.737 | 0.625 | ||||
| Mixed criteria | 0 | − | − | − | − | − | − | |||||
| Self‐report | 0.798 | |||||||||||
| Self‐report | 6 | 2428 | 92 | 3.36 [1.89–5.22] | 2.20 [1.49–3.25] | 79.3 [54.8–90.5] | <0.001 | 0.990 | 0.647 | |||
| Patient folder | 3 | 567 | 19 | 3.71 [0.95–7.95] | 2.03 [1.12–3.69] | 75.8 [20.3–92.7] | 0.016 | 0.589 | 0.308 | |||
| Biochemical criteria and/or self‐report | 0.115 | |||||||||||
| OGTT | 1 | 157 | 10 | 6.37 [3.01–10.80] | − | − | − | 0.889 | ||||
| FBG | 5 | 1873 | 164 | 8.05 [4.95–11.81] | 2.30 [1.51–3.51] | 81.1 [56.1–91.9] | <0.001 | 0.119 | 0.763 | |||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 0 | − | − | − | − | − | − | |||||
| Mixed criteria | 6 | 3104 | 141 | 4.28 [2.59–6.35] | 2.45 [1.69–3.55] | 83.4 [65.2–92.1] | <0.001 | 0.958 | 0.963 | |||
| Women | Any criteria | 22 | 17,846 | 827 | 4.47 [3.66–5.36] | 2.60 [2.17–3.11] | 85.2 [78.8–89.7] | <0.001 | 0.324 | 0.946 | ||
| Biochemical criteria only | 0.009 | |||||||||||
| OGTT | 2 | 1298 | 32 | 2.48 [1.40–3.85] | 1.41 [–] | 49.8 [–] | 0.158 | – | ||||
| FBG | 2 | 419 | 26 | 6.14 [3.99–8.68] | 1.00 [–] | 0.0 [–] | 0.493 | – | ||||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 2 | 801 | 20 | 2.85 [0.84–5.87] | 1.61 [1.00–3.35] | 61.5 [0.0–91.1] | 0.107 | – | ||||
| Mixed criteria | 0 | − | − | − | − | − | − | |||||
| Self‐report | 0.636 | |||||||||||
| Self‐report | 6 | 6370 | 262 | 3.46 [2.16–5.05] | 3.07 [2.21–4.26] | 89.4 [79.5–94.5] | <0.001 | 0.179 | ||||
| Patient folder | 3 | 1178 | 32 | 2.80 [1.25–4.88] | 1.71 [1.00–3.18] | 65.6 [0.0–90.1] | 0.054 | 0.663 | ||||
| Biochemical criteria and/or self‐report | 0.297 | |||||||||||
| OGTT | 1 | 591 | 37 | 6.26 [4.44–8.37] | ||||||||
| FBG | 5 | 4673 | 280 | 5.82 [4.41–7.40] | 1.70 [1.05–2.75] | 65.3 [9.1–86.7] | 0.021 | 0.957 | ||||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 0 | − | − | − | − | − | − | |||||
| Mixed criteria | 6 | 5206 | 203 | 4.28 [2.66–6.24] | 3.13 [2.26–4.33] | 89.8 [80.5–94.7] | <0.001 | 0.096 | ||||
| BMI | ||||||||||||
| Median BMI, 23 kg/m2 | BMI≥23 kg/m2 | Any criteria | 13 | 5913 | 408 | 7.06 [4.66–9.89] | 3.72 [3.07–4.50] | 92.8 [89.4–95.1] | <0.001 | 0.002 | 0.106 | 0.433 |
| Biochemical criteria only | 0.095 | |||||||||||
| OGTT | 5 | 1718 | 49 | 2.71 [1.97–3.57] | 1.00 [1.00–2.19] | 0.0 [0.0–79.2] | 0.532 | 0.175 | 0.441 | |||
| FBG | 2 | 272 | 39 | 14.93 [1.49–37.95] | 4.36 [2.49–7.62] | 94.7 [83.9–98.3] | <0.001 | 0.314 | – | |||
| HbA1c | 1 | 1207 | 26 | 2.15 [1.40–3.06] | − | − | − | 0.011 | – | |||
| Self‐report | 0.685 | |||||||||||
| Self‐report | 2 | 1206 | 41 | 3.38 [2.42–4.49] | 1.00 | 00.0 | 0.418 | 0.284 | ||||
| Patient folder | 1 | 502 | 15 | 2.99 [1.66–4.68] | − | − | − | – | ||||
| Biochemical criteria and/or self‐report | 0.418 | |||||||||||
| OGTT | 2 | 1054 | 99 | 10.97 [2.78–23.55] | 5.07 [3.02–8.49] | 96.1 [89.1–98.6] | <0.001 | 0.303 | – | |||
| FBG | 3 | 1265 | 112 | 8.02 [1.84–17.86] | 5.34 [3.70–7.71] | 96.5 [92.7–98.3] | <0.001 | 0.773 | 0.938 | |||
| HbA1c | 1 | 502 | 35 | 6.97 [4.90–9.38] | − | − | − | 0.439 | ||||
| Mixed criteria | 2 | 2276 | 128 | 5.60 [4.69–6.59] | 1.00 [–] | 00.0 [–] | 0.909 | 0.430 | ||||
| BMI<23 kg/m2 | Any criteria | 14 | 13,511 | 614 | 4.51 [2.81–6.57] | 4.91 [4.20–5.73] | 95.8 [94.3–97.0] | <0.001 | 0.606 | 0.832 | ||
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 1 | 273 | 4 | 1.47 [0.31–3.31] | − | − | − | |||||
| FBG | 2 | 543 | 36 | 6.54 [4.57–8.81] | − | − | − | |||||
| HbA1c | 1 | 118 | 8 | 6.78 [2.84–12.13] | 1.00 | 00.0 | 0.498 | |||||
| Mixed criteria | 1 | 1316 | 2 | 0.15 [0.00–0.46] | − | − | − | |||||
| Self‐report | − | |||||||||||
| Self‐report | 3 | 1678 | 61 | 6.43 [1.46–14.36] | 3.98 [2.57–6.14] | 93.7 [84.9–97.4] | <0.001 | |||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 2 | 638 | 37 | 5.61 [2.60–9.64] | 1.95 [1.00–4.11] | 73.7 [0.0–94.1] | 0.051 | |||||
| FBG | 5 | 5516 | 397 | 7.34 [3.96–11.62] | 3.76 [2.72–5.21] | 92.9 [86.5–96.3] | <0.001 | 0.978 | ||||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | |||||
| HbA1c | 1 | 284 | 24 | 8.45 [5.47–11.99] | − | − | − | |||||
| Mixed criteria | 5 | 5577 | 190 | 4.53 [2.41–7.26] | 4.02 [2.94–5.50] | 93.8 [88.5–96.7] | <0.001 | 0.083 | ||||
| Area | ||||||||||||
| Combined | Any criteria | 21 | 13,590 | 765 | 5.99 [4.68–7.45] | 3.23 [2.75–3.80] | 90.4 [86.7–93.1] | <0.001 | <0.001 | 0.308 | 0.163 | |
| Biochemical criteria only | 0.126 | |||||||||||
| OGTT | 4 | 1612 | 47 | 2.81 [2.03–3.70] | 1.00 [1.00–2.56] | 0.0 [0.0–84.7] | 0.395 | 0.621 | 0.162 | |||
| FBG | 3 | 418 | 13 | 3.00 [1.02–5.82] | 1.39 [1.00–2.58] | 48.4 [0.0–85.0] | 0.143 | 0.002 | 0.103 | |||
| RBG | 0 | − | − | − | − | − | − | |||||
| HbA1c | 1 | 118 | 8 | 6.78 [2.84–12.13] | − | − | − | 0.360 | ||||
| Mixed criteria | 0 | − | − | − | − | − | − | 0.818 | ||||
| Self‐report | 0.950 | |||||||||||
| Self‐report | 7 | 8212 | 369 | 4.36 [3.13–5.78] | 2.69 [1.95–3.72] | 86.2 [73.7–92.8] | <0.001 | <0.001 | 0.934 | |||
| Patient folder | 2 | 832 | 36 | 4.46 [1.72–8.35] | 2.29 [1.11–4.74] | 81.0 [19.0–95.5] | 0.021 | 0.757 | ||||
| Biochemical criteria and/or self‐report | 0.008 | |||||||||||
| OGTT | 3 | 1360 | 111 | 8.29 [2.93–15.98] | 4.21 [2.76–6.41] | 94.4 [86.9–97.6] | <0.001 | 0.342 | 0.757 | |||
| FBG | 7 | 2824 | 248 | 9.48 [6.43–13.04] | 2.97 [2.18–4.03] | 88.6 [79.0–93.8] | <0.001 | 0.194 | 0.098 | |||
| HbA1c | 2 | 2328 | 125 | 5.72 [3.88–7.87] | 1.76 [1.00–3.71] | 67.9 [0.0–92.8] | 0.077 | 0.679 | ||||
| Mixed criteria | 2 | 1552 | 69 | 4.43 [3.46–5.52] | 1.00 | 00.0 | 0.394 | <0.001 | ||||
| Urban | Any criteria | 37 | 68,894 | 2652 | 4.80 [3.81–5.88] | 4.88 [4.44–5.36] | 95.8 [94.9–96.5] | <0.001 | 0.217 | 0.033 | ||
| Biochemical criteria only | 0.001 | |||||||||||
| OGTT | 9 | 3601 | 170 | 3.38 [1.48–5.97] | 3.47 [2.72–4.42] | 91.7 [86.5–94.9] | <0.001 | 0.324 | ||||
| FBG | 6 | 1987 | 216 | 10.70 [6.56–15.68] | 3.21 [2.33–4.42] | 90.3 [81.6–94.9] | <0.001 | 0.861 | ||||
| RBG | 1 | 270 | 33 | 12.22 [8.56–16.42] | − | − | − | |||||
| HbA1c | 5 | 3537 | 260 | 5.04 [1.01–11.78] | 7.03 [5.63–8.78] | 98.0 [96.8–98.7] | <0.001 | 0.644 | ||||
| Mixed criteria | 3 | 5584 | 272 | 3.58 [0.00–18.00] | 17.99 [15.24–21.22] | 99.7 [99.6–99.8] | <0.001 | 0.624 | ||||
| Self‐report | 0.178 | |||||||||||
| Self‐report | 5 | 5419 | 86 | 2.64 [0.90–5.21] | 4.33 [3.21–5.85] | 94.7 [90.3–97.1] | <0.001 | 0.019 | ||||
| Patient folder | 5 | 4621 | 296 | 5.21 [2.51–8.78] | 4.56 [3.41–6.09] | 95.2 [91.4–97.3] | <0.001 | 0.542 | ||||
| Biochemical criteria and/or self‐report | 0.697 | |||||||||||
| OGTT | 2 | 572 | 27 | 3.47 [0.00–12.74] | 4.23 [2.40–7.45] | 94.4 [82.6–98.2] | <0.001 | |||||
| FBG | 6 | 6074 | 409 | 5.53 [3.00–8.74] | 3.75 [2.80–5.02] | 92.9 [87.2–96.0] | <0.001 | 0.508 | ||||
| HbA1c | 2 | 663 | 28 | 3.91 [0.00–14.17] | 4.81 [2.83–8.18] | 95.7 [87.5–98.5] | <0.001 | |||||
| Mixed criteria | 10 | 52,459 | 1585 | 3.76 [2.62–5.10] | 4.53 [3.72–5.51] | 95.1 [92.8–96.7] | <0.001 | 0.526 | ||||
| Rural | Any criteria | 5 | 3928 | 142 | 3.83 [0.94–8.40] | 5.59 [4.33–7.23] | 96.8 [94.7–98.1] | <0.001 | 0.382 | 0.621 | ||
| Biochemical criteria only | 0.065 | |||||||||||
| RBG | 1 | 189 | 4 | 2.12 [0.45–4.76] | − | − | − | |||||
| HbA1c | 1 | 500 | 19 | 3.80 [2.28–5.67] | − | − | − | |||||
| Mixed criteria | 1 | 1166 | 21 | 1.80 [1.11–2.65] | − | − | − | |||||
| Self‐report | − | − | − | |||||||||
| Self‐report | 23 | 1166 | 15 | 1.29 [0.71–2.02] | − | − | − | |||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| FBG | 2 | 1103 | 45 | 5.98 [1.02–14.28] | 2.56 [1.27–5.18] | 84.8 [37.9–96.3] | 0.010 | |||||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | |||||
| Mixed criteria | 1 | 1134 | 86 | 7.58 [6.11–9.20] | − | − | − | |||||
| Study setting | ||||||||||||
| Clinic‐based | Any criteria | 54 | 77,474 | 3133 | 5.21 [4.32–6.19] | 4.78 [4.41–5.17] | 95.6 [94.9–96.3] | <0.001 | 0.970 | 0.193 | 0.003 | |
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 12 | 4726 | 207 | 3.45 [1.94–5.33] | 3.00 [2.39–3.76] | 88.9 [82.5–92.9] | <0.001 | 0.165 | 0.387 | |||
| FBG | 9 | 2405 | 229 | 7.89 [4.56–12.00] | 3.29 [2.56–4.23] | 90.7 [84.7–94.4] | <0.001 | − | 0.400 | |||
| RBG | 1 | 270 | 33 | 12.22 [8.56–16.42] | <0.001 | |||||||
| HbA1c | 6 | 3668 | 280 | 5.88 [1.97–11.61] | 5.84 [4.67–7.30] | 97.1 [95.4–98.1] – | <0.001 | 0.028 | 0.748 | |||
| Mixed criteria | 4 | 6750 | 293 | 3.08 [0.00–12.30] | 14.70 [12.57–17.18] | 99.5 [99.4–99.7] | <0.001 | − | 0.541 | |||
| Self‐report | 0.164 | |||||||||||
| Self‐report | 8 | 7669 | 144 | 2.93 [1.47–4.85] | 4.06 [3.20–5.14] | 93.9 [90.2–96.2] | <0.001 | 0.234 | 0.001 | |||
| Patient folder | 7 | 5453 | 332 | 5.00 [2.85–7.71] | 3.94 [3.03–5.11] | 93.5 [89.1–96.2] | <0.001 | − | 0.417 | |||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 5 | 1932 | 138 | 6.18 [2.49–11.31] | 3.96 [2.89–5.43] | 93.6 [88.0–96.6] | <0.001 | − | 0.846 | |||
| FBG | 14 | 10,001 | 702 | 7.16 [5.31–9.26] | 3.49 [2.88–4.22] | 91.8 [87.9–94.4] | <0.001 | − | 0.683 | |||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | − | ||||
| HbA1c | 4 | 2991 | 153 | 4.86 [2.40–8.10] | 3.18 [2.11–4.78] | 90.1 [77.6–95.6] | <0.001 | − | 0.998 | |||
| Mixed criteria | 12 | 54,011 | 1654 | 3.88 [2.84–5.06] | 4.21 [3.50–5.07] | 94.4 [91.8–96.1] | <0.001 | <0.001 | 0.347 | |||
| Community‐based | Any criteria | 8 | 8938 | 426 | 4.18 [2.98–5.57] | 2.83 [2.12–3.79] | 87.6 [77.7–93.1] | <0.001 | 0.441 | |||
| Biochemical criteria only | 0.693 | |||||||||||
| OGTT | 1 | 487 | 10 | 2.05 [0.95–3.53] | − | − | − | |||||
| RBG | 1 | 189 | 4 | 2.12 [0.45–4.76] | − | − | − | |||||
| HbA1c | 1 | 487 | 7 | 1.44 [0.54–2.72] | − | − | − | |||||
| Self‐report | ||||||||||||
| Self‐report | 5 | 7128 | 326 | 4.32 [3.10–5.73] | 2.54 [1.70–3.79] | 84.5 [65.2–93.1] | <0.001 | 0.761 | ||||
| Biochemical criteria and/or self‐report | ||||||||||||
| Mixed criteria | 1 | 1134 | 86 | 7.58 [6.11–9.20] | – | – | – | |||||
| Publication year | ||||||||||||
| Median publication year 2018 | 2018 or later | Any criteria | 34 | 28,020 | 1547 | 5.78 [4.42–7.29] | 5.00 [4.54–5.51] | 96.0 [95.2–96.7] | <0.001 | 0.559 | 0.063 | 0.138 |
| Biochemical criteria only | 0.722 | |||||||||||
| OGTT | 3 | 2449 | 127 | 3.90 [1.21–7.99] | 4.19 [2.75–6.40] | 94.3 [86.8–97.6] | <0.001 | 0.740 | 0.120 | |||
| FBG | 6 | 1863 | 177 | 6.72 [3.25–11.27] | 3.33 [2.43–4.55] | 91.0 [83.1–95.2] | <0.001 | 0.601 | 0.067 | |||
| HbA1c | 5 | 3480 | 261 | 5.56 [1.21–12.65] | 7.01 [5.61–8.75] | 98.0 [96.8–98.7] | <0.001 | 0.548 | 0.765 | |||
| Mixed criteria | 3 | 5584 | 272 | 3.58 [0.00–18.00] | 17.99 [15.24–21.22] | 99.7 [99.6–99.8] | <0.001 | 0.818 | 0.624 | |||
| Self‐report | 0.359 | |||||||||||
| Self‐report | 10 | 12,925 | 427 | 3.64 [2.08–560] | 5.13 [4.27–6.15] | 96.2 [94.5–97.4] | <0.001 | 0.686 | 0.333 | |||
| Patient folder | 5 | 2620 | 166 | 5.16 [2.60–849] | 3.21 [2.25–4.58] | 90.3 [80.3–95.2] | <0.001 | 0.858 | 0.349 | |||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 5 | 1932 | 138 | 6.18 [2.49–11.31] | 3.96 [2.89–5.43] | 93.6 [88.0–96.6] | <0.001 | − | 0.846 | |||
| FBG | 8 | 6608 | 562 | 10.21 [7.99–12.67] | 2.45 [1.79–3.36] | 83.3 [68.7–91.1] | <0.001 | <0.001 | 0.029 | |||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | – | – | – | |||||
| HbA1c | 4 | 2991 | 153 | 4.86 [2.40–8.10] | 3.18 [2.11–4.78] | 90.1 [77.6–95.6] | <0.001 | 0.998 | ||||
| Mixed criteria | 8 | 6718 | 195 | 4.15 [2.05–6.92] | 4.60 [3.69–5.73] | 94.4 [91.6–96.3] | <0.001 | 0.737 | 0.007 | |||
| Before 2018 | Any criteria | 28 | 58,392 | 2012 | 4.18 [3.33–5.12] | 3.53 [3.10–4.03] | 92.0 [89.6–93.9] | <0.001 | 0.871 | 0.055 | ||
| Biochemical criteria only | 0.012 | |||||||||||
| OGTT | 10 | 2764 | 90 | 3.12 [1.61–5.06] | 2.49 [1.89–3.29] | 83.9 [72.0–90.8] | <0.001 | 0.361 | ||||
| FBG | 3 | 542 | 52 | 10.86 [2.49–23.83] | 3.94 [2.55–6.10] | 93.6 [84.6–97.3] | <0.001 | 0.269 | ||||
| RBG | 2 | 459 | 37 | 6.30 [0.14–19.46] | 4.33 [2.47–7.58] | 94.7 [83.6–98.3] | <0.001 | |||||
| HbA1c | 2 | 675 | 26 | 3.81 [2.46–5.42] | 1.00 | 00.0 | 0.833 | |||||
| Mixed criteria | 1 | 1166 | 21 | 1.80 [0.11–2.65] | – | – | – | |||||
| Self‐report | 0.597 | |||||||||||
| Self‐report | 3 | 1872 | 43 | 2.96 [0.92–6.04] | 2.91 [1.75–4.86] | 88.2 [67.2–95.8] | <0.001 | 0.068 | ||||
| Patient folder | 2 | 2833 | 166 | 4.64 [0.63–12.01] | 7.13 [4.67–10.89] | 98.0 [95.4–99.2] | <0.001 | |||||
| Biochemical criteria and/or self‐report | 0.566 | |||||||||||
| FBG | 6 | 3393 | 140 | 3.90 [2.28–5.91] | 2.70 [1.90–3.84] | 86.3 [72.4–93.2] | <0.001 | 0.921 | ||||
| Mixed criteria | 5 | 48,427 | 1545 | 4.28 [2.88–5.95] | 4.64 [3.48–6.18] | 94.8 [91.4–96.8] | <0.001 | 0.053 | ||||
| CD4 count level | ||||||||||||
| Median CD4 count = 358 cells/μl | CD4 count≥358 cells/μl | Any criteria | 14 | 9805 | 588 | 5.55 [3.28–8.36] | 4.93 [4.22–5.75] | 95.9 [94.4–97.0] | <0.001 | 0.002 | 0.298 | 0.955 |
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 2 | 780 | 25 | 3.55 [1.34–6.66] | 1.46 [1.00–2.93] | 53.0 [0.0–88.3] | 0.144 | 0.303 | ||||
| FBG | 4 | 843 | 52 | 6.05 [4.50–7.80] | 1.00 [1.00–2.56] | 0.0 [0.0–84.7] | 0.520 | 0.718 | 0.262 | |||
| HbA1c | 2 | 293 | 15 | 5.05 [2.65–8.11] | 1.05 | 9.9 | 0.292 | 0.111 | ||||
| Mixed criteria | 1 | 1166 | 21 | 1.80 [1.11–2.65] | − | − | − | <0.001 | ||||
| Self‐report | 0.309 | |||||||||||
| Self‐report | 4 | 2490 | 73 | 4.14 [1.55–7.83] | 3.61 [2.47–5.29] | 92.3 [83.6–96.4] | <0.001 | 0.164 | 0.087 | |||
| Patient folder | 2 | 884 | 23 | 2.58 [1.62–3.75] | 1.00 | 00.0 | 0.426 | 0.464 | – | |||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 2 | 988 | 49 | 3.09 [0.01–10.41] | 4.13 [2.33–7.33] | 94.1 [81.5–98.1] | <0.001 | 0.218 | ||||
| FBG | 5 | 5642 | 446 | 9.47 [5.31–14.64] | 4.28 [3.17–5.79] | 94.5 [90.0–97.0] | <0.001 | 0.506 | 0.560 | |||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | – | ||||
| HbA1c | 1 | 502 | 35 | 6.97 [4.90–9.38] | − | − | − | 0.439 | ||||
| Mixed criteria | 2 | 1058 | 56 | 5.29 [4.01–6.73] | 1.00 | 00.0 | 0.704 | |||||
| CD4 count<358 cells/μl | Any criteria | 13 | 9974 | 374 | 4.06 [2.72–5.65] | 3.42 [2.80–4.19] | 91.5 [87.2–94.3] | <0.001 | 0.027 | 0.289 | ||
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 4 | 1211 | 28 | 2.22 [1.43–3.17] | 1.00 [1.00–2.56] | 0.0 [0.0–84.7] | 0.641 | 0.690 | ||||
| FBG | 2 | 272 | 30 | 10.13 [0.00–44.88] | 6.69 [4.31–10.39] | 97.8 [94.6–99.1] | <0.001 | |||||
| HbA1c | 2 | 1707 | 45 | 2.82 [1.42–4.67] | 1.88 [1.00–3.96] | 71.6 [0.0–93.6] | 0.060 | |||||
| Mixed criteria | 1 | 1316 | 2 | 0.15 [0.00–0.46] | − | − | − | |||||
| Self‐report | − | − | − | 0.916 | ||||||||
| Self‐report | 1 | 1316 | 29 | 2.20 [1.47–3.07] | − | − | − | |||||
| Patient folder | 1 | 1033 | 22 | 2.13 [1.33–3.11] | − | − | − | |||||
| Biochemical criteria and/or self‐report | − | − | − | 0.229 | ||||||||
| OGTT | 1 | 332 | 25 | 7.53 [4.91–10.64] | − | − | − | |||||
| FBG | 2 | 740 | 58 | 7.78 [5.78–10.05] | 1.09 | 16.5 | 0.273 | |||||
| HbA1c | 1 | 284 | 24 | 8.45 [5.47–11.99] | − | − | − | |||||
| Mixed criteria | 5 | 7050 | 277 | 4.78 [2.65–7.47] | 4.58 [3.43–6.12] | 95.2 [91.5–97.3] | <0.001 | 0.167 | ||||
| ART use | ||||||||||||
| Combined | Any criteria | 18 | 18,294 | 926 | 5.38 [3.82–7.18] | 4.92 [4.29–5.64] | 95.9 [94.6–96.9] | <0.001 | 0.928 | 0.827 | 0.142 | |
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 2 | 967 | 23 | 2.18 [1.02–3.75] | 1.36 | 46.3 | 0.172 | 0.404 | ||||
| FBG | 2 | 1020 | 134 | 12.97 [8.38–18.37] | 2.42 [1.18–4.95] | 82.9 [28.8–95.9] | 0.015 | 0.006 | ||||
| RBG | 1 | 189 | 4 | 2.12 [0.45–4.76] | <0.001 | |||||||
| HbA1c | 1 | 500 | 19 | 3.80 [2.28–5.67] | − | − | − | 0.644 | ||||
| Mixed criteria | 1 | 2979 | 13 | 0.44 [0.23–0.71] | − | − | − | <0.001 | ||||
| Self‐report | 0.001 | |||||||||||
| Self‐report | 9 | 11,739 | 396 | 3.43 [1.84–5.48] | 5.21 [4.31–6.31] | 96.3 [94.6–97.5] | <0.001 | 0.918 | 0.484 | |||
| Patient folder | 2 | 2130 | 165 | 7.70 [6.60–8.88] | 1.00 | 00.0 | 0.324 | 0.005 | ||||
| Biochemical criteria and/or self‐report | ||||||||||||
| OGTT | 1 | 748 | 47 | 6.28 [4.65–8.14] | − | − | − | 0.956 | ||||
| FBG | 3 | 1719 | 96 | 6.60 [2.87–11.67] | 3.43 [2.14–5.50] | 91.5 [78.2–96.7] | <0.001 | 0.847 | 0.196 | |||
| Mixed criteria | 3 | 5065 | 150 | 3.61 [0.50–9.31] | 7.95 [5.97–10.59] | 98.4 [97.2–99.1] | <0.001 | 0.304 | 0.283 | |||
| No ART | Any criteria | 17 | 48,048 | 1633 | 5.16 [3.56–7.01] | 4.05 [3.46–4.74] | 93.9 [91.6–95.6] | <0.001 | 0.927 | 0.040 | ||
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 5 | 2220 | 119 | 3.22 [0.96–6.67] | 3.60 [2.58–5.02] | 92.3 [85.0–96.0] | <0.001 | 0.015 | ||||
| FBG | 2 | 244 | 33 | 12.67 [0.00–41.12] | 5.21 [3.14–8.66] | 96.3 [89.8–98.7] | <0.001 | |||||
| HbA1c | 3 | 2476 | 200 | 5.53 [0.04–18.54] | 9.93 [7.75–12.73] | 99.0 [98.3–99.4] | <0.001 | 0.901 | ||||
| Mixed criteria | 1 | 954 | 223 | 23.38 [20.74–26.12] | − | − | − | |||||
| Self‐report | − | − | − | |||||||||
| Self‐report | 0 | − | − | − | − | − | − | |||||
| Patient folder | 1 | 244 | 8 | 3.28 [1.65–6.42] | − | − | − | |||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 2 | 638 | 37 | 5.61 [2.60–9.64] | 1.95 [1.00–4.11] | 73.7 [0.0–94.1] | 0.051 | |||||
| FBG | 5 | 1112 | 98 | 8.07 [4.09–13.17] | 2.65 [1.79–3.93] | 85.8 [68.8–93.5] | <0.001 | 0.964 | ||||
| RBG | 1 | 285 | 1 | 0.35 [0.00–1.50] | − | − | − | 0.960 | ||||
| HbA1c | 2 | 528 | 40 | 7.55 [5.42–9.98] | 1.00 | 00.0 | 0.421 | 0.100 | ||||
| Mixed criteria | 4 | 44,213 | 1411 | 6.03 [3.12–9.77] | 5.25 [3.87–7.13] | 96.4 [93.3–98.0] | <0.001 | 0.138 | ||||
| ART use | Any criteria | 35 | 20,070 | 1000 | 4.72 [3.54–6.05] | 3.99 [3.57–4.45] | 93.7 [92.2–94.9] | <0.001 | 0.761 | 0.652 | ||
| Biochemical criteria only | 0.001 | |||||||||||
| OGTT | 8 | 2026 | 75 | 3.79 [1.78–6.45] | 2.63 [1.94–3.56] | 85.5 [73.4–92.1] | <0.001 | 0.221 | ||||
| FBG | 5 | 1141 | 62 | 4.83 [2.83–7.30] | 1.68 [1.04–2.72] | 64.5 [6.7–86.5] | 0.023 | 0.415 | ||||
| RBG | 1 | 270 | 33 | 12.22 [8.56–16.42] | − | − | − | |||||
| HbA1c | 5 | 1179 | 68 | 5.03 [2.79–7.84] | 1.91 [1.20–3.02] | 72.5 [30.9–89.0] | 0.005 | 0.385 | ||||
| Mixed criteria | 3 | 2817 | 57 | 2.67 [0.06–8.50] | 6.67 [4.84–9.19] | 97.8 [95.7–98.8] | <0.001 | 0.265 | ||||
| Self‐report | ||||||||||||
| Self‐report | 4 | 3058 | 74 | 3.52 [1.43–6.45] | 3.40 [2.29–5.03] | 91.3 [80.9–96.1] | <0.001 | 0.089 | ||||
| Patient folder | 5 | 3079 | 159 | 4.17 [1.56–7.90] | 4.13 [3.04–5.62] | 94.1 [89.2–96.8] | <0.001 | 0.694 | ||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 2 | 546 | 54 | 6.66 [0.00–30.20] | 7.54 [5.01–11.36] | 98.2 [96.0–99.2] | <0.001 | |||||
| FBG | 10 | 7170 | 508 | 6.90 [4.63–9.58] | 3.41 [2.71–4.31] | 91.4 [86.3–94.6] | <0.001 | 0.979 | ||||
| RBG | 1 | 1217 | 6 | 0.49 [0.16–0.98] | − | − | − | |||||
| HbA1c | 3 | 2463 | 113 | 3.96 [1.32–7.87] | 3.30 [2.04–5.34] | 90.8 [76.0–96.5] | <0.001 | 0.831 | ||||
| Mixed criteria | 7 | 5867 | 179 | 3.48 [2.42–4.72] | 2.11 [1.46–3.05] | 77.6 [53.4–89.2] | <0.001 | 0.102 | ||||
| ART duration | ||||||||||||
| Median duration of ART use = 4.5 years | On ART for ≥4.5 years | Any criteria | 12 | 9824 | 514 | 4.56 [2.77–6.74] | 4.21 [3.49–5.07] | 94.4 [91.8–96.1] | <0.001 | 0.031 | 0.550 | 0.570 |
| Biochemical criteria only | <0.001 | |||||||||||
| OGTT | 1 | 150 | 27 | 18.00 [12.23–24.59] | <0.001 | |||||||
| FBG | 4 | 977 | 51 | 4.41 [2.16–7.35] | 1.87 [1.11–3.15] | 71.3 [18.1–89.9] | 0.015 | − | 0.355 | |||
| RBG | 1 | 270 | 33 | 12.22 [8.56–16.42] | − | − | − | − | ||||
| HbA1c | 2 | 497 | 33 | 6.57 [4.52–8.96] | 1.00 | 00.0 | 0.872 | − | ||||
| Mixed criteria | 1 | 1316 | 1 | 0.15 [0.00–0.46] | − | − | − | 0.001 | ||||
| Self‐report | 0.096 | |||||||||||
| Self‐report | 3 | 1856 | 63 | 5.48 [1.63–11.29] | 3.69 [2.35–5.81] | 92.7 [81.9–97.0]‐ | <0.001 | 0.101 | 0.102 | |||
| Patient folder | 2 | 1415 | 30 | 2.10 [1.40–2.93] | 1.00 | 00.0 | 0.962 | 0.280 | ||||
| Biochemical criteria and/or self‐report | 0.005 | |||||||||||
| OGTT | 1 | 240 | 2 | 0.83 [0.01–2.50] | − | − | − | <0.001 | ||||
| FBG | 4 | 5844 | 379 | 6.35 [2.96–10.87] | 4.64 [3.34–6.45] | 95.4 [91.0–97.6] | <0.001 | 0.960 | 0.839 | |||
| HbA1c | 1 | 379 | 4 | 1.06 [0.22–2.39] | − | − | − | <0.001 | ||||
| Mixed criteria | 2 | 1916 | 61 | 3.50 [1.36–6.56] | 2.92 [1.49–5.72] | 88.3 [55.2–96.9] | 0.003 | 0.255 | ||||
| On ART for <4.5 years | Any criteria | 12 | 7191 | 300 | 3.68 [1.89–5.99] | 4.37 [3.65–5.24] | 94.8 [92.5–96.4] | <0.001 | 0.234 | 0.725 | ||
| Biochemical criteria only | 0.336 | |||||||||||
| OGTT | 4 | 926 | 22 | 2.28 [1.14–3.75] | 1.21 [1.00–2.02] | 31.7 [0.0–75.5] | 0.222 | 0.450 | ||||
| Mixed criteria | 1 | 1166 | 21 | 1.80 [1.11–2.65] | − | − | − | |||||
| Self‐report | 0.325 | |||||||||||
| Self‐report | 2 | 1624 | 28 | 1.90 [0.66–3.74] | 2.06 [1.00–4.32] | 76.5 [0.0–94.6] | 0.039 | |||||
| Patient folder | 1 | 502 | 15 | 2.99 [1.66–4.68] | − | − | − | |||||
| Biochemical criteria and/or self‐report | <0.001 | |||||||||||
| OGTT | 1 | 332 | 25 | 7.53 [4.91–10.64] | − | − | − | |||||
| FBG | 3 | 1234 | 95 | 6.16 [0.85–15.63] | 5.50 [3.84–7.89] | 96.7 [93.2–98.4] | <0.001 | 0.633 | ||||
| RBG | 1 | 1502 | 7 | 0.47 [0.17–0.89] | − | − | − | |||||
| HbA1c | 2 | 786 | 59 | 7.48 [5.73–9.44] | 1.00 | 00.0 | 0.439 | |||||
| Mixed criteria | 5 | 3861 | 217 | 5.61 [3.38–8.35] | 2.31 [1.70–3.14] | 81.3 [65.6–89.9] | <0.001 | 0.786 |
Abbreviations: FBG, fasting blood glucose; OGTT, oral glucose tolerant test; RBG, random blood glucose.
– not computable.
Two overall pooled prediabetes prevalence estimates were calculated across studies that utilized HbA1c (n = 30) and those that did not (n = 24). The six studies that diagnosed prediabetes using HbA1c alone were excluded from the sub‐group analyses [31, 38, 51, 59, 62, 67] (Tables S2 and S3). Among the studies that described prediabetes, IGT was the most frequently reported form, followed by IFG.
3.5. Prevalence of diabetes
The diabetes prevalence rates by biochemical tests and/or self‐reported diabetes are illustrated in the forest plots in Figure 3. Overall, 3559 of the 86,412 participants included in the overall pooled estimate had diabetes, corresponding to a prevalence of 5.1% (95% CI: 4.3–5.9). Self‐reported diabetes prevalence per se, at 3.5% (2.2–5.1), was much lower than when combined with biochemical assessments (OGTT and/or self‐report: 6.2% [2.5–11.3]; FBG and/or self‐report: 7.2% [5.3–9.3]). For these data, the I 2 was between 92% and 95%, and the p‐heterogeneity was <0.001.
Figure 3.
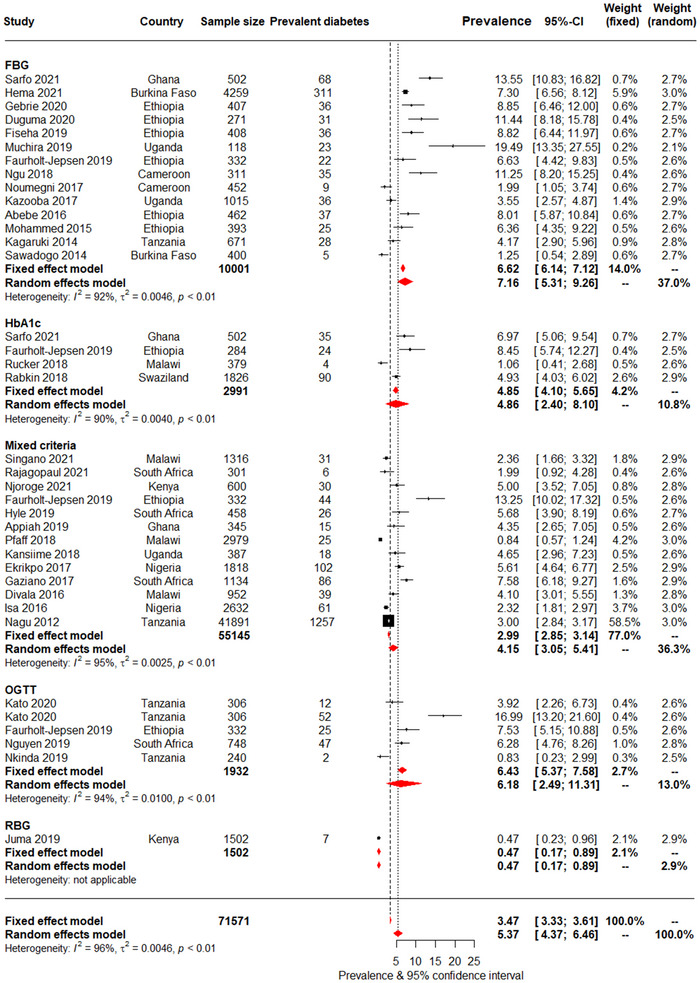
Pooled prevalence of diabetes across studies using biochemical tests and/or self‐reports to diagnose diabetes. Each diagnostic criterion included biochemical test and/or self‐reported diabetes. For each study, the black box represents the study estimate (prevalence of diabetes) and the horizontal bar denotes the 95% confidence intervals (95% CI). The size of the boxes is proportional to the inverse variance. The diamonds at the lower tail of the figure are for the pooled effect estimates from both random and fixed effects models. The proportional contribution of each study (weight) to the pooled estimates is also shown separately for fixed and random effects models, together with the prevalence estimates and measures of heterogeneity. The vertical line is centred on the pooled estimates.
Although not significantly different, diabetes prevalence was generally higher in participants who were older (cut‐point 39 years: 6.0% [4.5–7.6] vs. 4.5% [3.5–5.7]), had higher BMI (cut‐point 23 kg/m2: 7.1% [4.7–9.9] vs. 4.5% [2.8–6.6]), lived in urban versus rural areas (4.8% [3.8–5.9] vs. 3.8% [0.9–8.4]) and in studies published after versus before 2018 (5.8% [4.4–7.3] vs. 4.2% [3.3–5.1]). Diabetes prevalence was also not significantly different by HIV‐related factors of CD4 count, ART use or duration of ART use. There was also substantial heterogeneity for the diabetes prevalence by sub‐group analyses; the I 2 was between 92% and 97%, and p‐heterogeneity <0.001.
For the overall diabetes prevalence, there was some evidence of publication bias overall (p = 0.002 for the Egger test). There was also evidence of bias among studies conducted in clinic‐based settings (p = 0.003), urban areas (p = 0.033) and in participants younger than 40 years of age (p = 0.002). In trim and fill analyses however, imputed studies always had implausible effect estimates, with diabetes prevalence always lower than 1%, and null in about half of imputed studies (Figures S1–S4). This is unlikely and suggests that publication bias was a spurious finding (Figure S5).
3.6. Prevalence of prediabetes
The prediabetes prevalence is presented in Table S4 and Figure 4. Prevalence was similarly high across studies that did not use HbA1c and those that did: 15.1% (95% CI: 9.7–21.5) versus 15.2% (10.8–20.1). There was no significant difference in prediabetes prevalence by sub‐groups (Table S4). However, prediabetes was higher in older (≥39 years) compared with younger participants (22.5% [11.6–35.7] vs. 9.7% [5.5–14.8]), in women compared with men (10.0% [3.9–18.5] vs. 6.2% [0.3–17.6]), in those with higher BMI (≥25 kg/m2) compared with BMI <25 kg/m2 (18.1% [5.2–36.2] vs. 10.3% [3.9–18.5]) and in publications after 2017 than before (17.1% [8.7–27.5] vs. 13.2% [7.7–19.8]). Prediabetes prevalence was similar by CD4 counts, ART use and duration of ART use, although point‐estimates were higher in ART naïve and participants with shorter duration of ART use.
Figure 4.
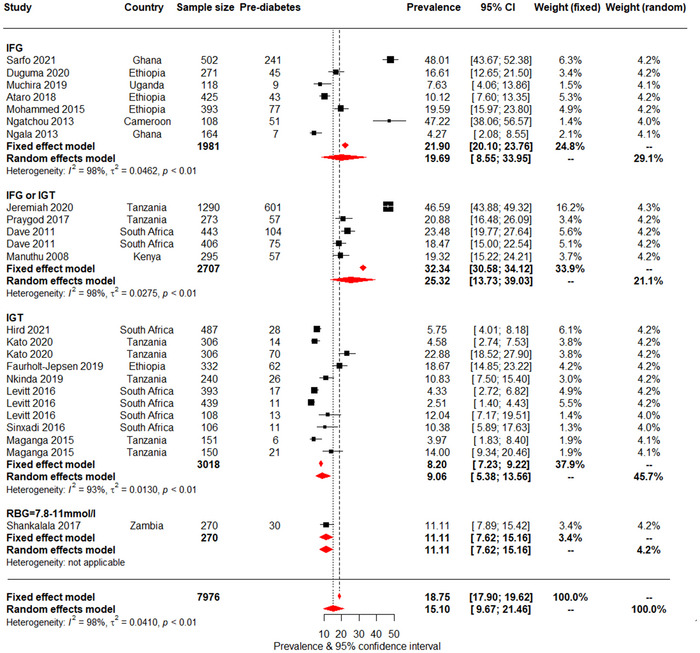
Pooled prediabetes prevalence in people living with HIV, presented by biochemical tests. For each study, the black box represents the study estimate (prevalence of diabetes) and the horizontal bar denotes the 95% confidence intervals (95% CI). The size of the boxes is proportional to the inverse variance. The diamonds at the lower tail of the figure are for the pooled effect estimates from both random and fixed effects models. The proportional contribution of each study (weight) to the pooled estimates is also shown separately for fixed and random effects models, together with the prevalence estimates and measures of heterogeneity. The vertical line is centred on the pooled estimates.
Considerable heterogeneity was also apparent across studies for prediabetes prevalence with I 2 between 95% and 99%, and p‐heterogeneity <0.001. There was some evidence of publication bias when studies that used HbA1c alone were not accounted for (p = 0.015 for the Egger test), but not when these studies were included in the analysis (p = 0.197). Evidence of bias was also apparent across clinic‐based studies (p = 0.008) and those in urban settings (p = 0.017). In trim and fill analyses, imputed studies systematically had very high effect estimates, with prediabetes prevalence of 50% or higher. This is implausible and suggests that findings of publication bias were spurious findings (Figures S6–S8).
4. DISCUSSION
This systematic review and meta‐analysis conducted in adult PLHIV in Africa illustrates an established burden of diabetes, at 5.1%, and a high prediabetes prevalence of 15.2%. This diabetes prevalence accords with the 5.3% age‐adjusted diabetes prevalence in the Africa region reported in the 2021 International Diabetes Federation (IDF) Atlas [70]. The age‐adjusted IGT and IFG prevalence rates in the Africa region were 12.6% and 8.0%, respectively. The comparative prevalence rates of diabetes and prediabetes in PLHIV compared with general populations likely suggest similar influences in their development of dysglycaemia.
The prevalence of self‐reported diabetes only (3.5%), which reflects diabetes awareness or detection rather than true prevalence, was lower than combined self‐report with biochemically assessed diabetes (OGTT: 6.2%; FBG: 7.2%). Further, although not statistically significant, general trends in sub‐group analyses conformed with traditional diabetes risk factors and were in the expected direction; prevalence rates were higher with older age, greater BMI and in urban residents. Notably, a rising prevalence of diabetes and prediabetes over time was suggested by higher rates in recent versus earlier publications. There were no clear trends for diabetes and prediabetes prevalence by HIV‐related factors.
The lower prevalence of known or self‐reported diabetes compared with diabetes prevalence identified on combined biochemical analyses with self‐report suggests that a substantial proportion of PLHIV with co‐morbid diabetes were undiagnosed for the latter condition. This is likely similar to general populations in Africa where a substantial proportion of diabetes is undiagnosed [9, 10]. However, unlike general populations, these PLHIV are in regular contact with health services and would be expected to have all co‐morbidities, including diabetes identified. Unfortunately, in practice, ART is generally provided by international donors in Africa with little funding or care provided for NCDs [71]. Consequently, there are disparities in management with the free treatment provided for HIV but a minimal focus on diabetes and other CVDs, such as hypertension and dyslipidaemia.
This is a missed opportunity to holistically manage the rise in NCD comorbidities in PLHIV in Africa. Policymakers should be alerted to the tangible shift in approach that is urgently required for the care of this vulnerable population. There needs to be a swing from a focus on HIV itself to a more comprehensive approach that encompasses the care of neglected NCD co‐morbidities. This is important if the momentum gained in increasing life expectancy in PLHIV in Africa is to be maintained. Screening for diabetes should be included in routine assessments of PLHIV in Africa, which is currently not standard practice [8].
The urgent need for this shift in approach for the care of diabetes and other NCD co‐morbidities in African PLHIV is underscored by the high burden of prediabetes demonstrated in this review; almost one in six people were affected. Generally, it is predicted that 5–10% of individuals with prediabetes will progress to diabetes annually [72]. This likely foretells of a substantial increase in diabetes prevalence in this vulnerable population in future. The effectiveness of HAART with increased longevity and subsequent ageing, and the uptake of unhealthy lifestyle behaviours will likely translate to the high prediabetes burden progressing to diabetes. A recent systematic review demonstrated that, similar to general populations, traditional risk factors, such as older age, diabetes family history, overweight/obesity and so on, were among the main contributors to the development of dysglycaemia in PLHIV globally, including in Africa [5]. The higher prevalence of diabetes and prediabetes with older age and higher BMI in the current review likely corroborates the influence of traditional risk factors in the development of dysglycaemia in African PLHIV. Therefore, the large burden of prediabetes in African PLHIV with the potential for conversion to diabetes in the future possibly mirrors the diabetes trends predicted for general populations in Africa.
Reinforcing the future expansion of diabetes in PLHIV in Africa, although not significant, was the higher prevalence of diabetes and prediabetes illustrated in recent years. This increasing pattern is likely a reflection of the diabetes trend predicted in general populations in Africa. The 4.7% diabetes prevalence estimated in Africa in 2019 is expected to rise to 5.2% by 2045 with a more than doubling of the absolute numbers [10]. The current literature and this review likely underline a shift in the disease burden from communicable diseases to NCDs in Africa with diabetes a significant disease entity in the region [9, 73], even in PLHIV.
Similar to the findings of a systematic review conducted a few years ago but using different eligibility criteria for included studies [4], the current review found no statistically significant difference in diabetes prevalence by ART status. Two additional systematic reviews, one conducted in Sub‐Saharan Africa [6] and the other in a few longitudinal studies in PLHIV globally [8], reported no association between ART use and FBG. Nevertheless, the uncertainty of the evidence is highlighted by the overall findings in the review by Nduka and colleagues, which included mainly cross‐sectional studies. They reported an association between ART use and diabetes, diagnosed on mean FBG levels [8]. Moreover, a systematic review by Nansseu and colleagues of longitudinal studies conducted in PLHIV globally reported an association between a cumulative exposure to some ART drugs and incident diabetes and prediabetes, but this finding was not consistent across studies [5]. Despite their differences in the eligibility criteria for included studies, these systematic reviews underscore the absence of clear irrefutable evidence linking the development of diabetes with ART [74].
Over the last few years, there has been a change to the use of newer ART drugs with fewer metabolic effects [5]. Studies conducted in populations using newer drugs would have been unlikely to be included in the reviews of studies published prior to 2017. Further research detailing the newer ART drugs used in recent studies and their specific contributions to the development of diabetes, if any, is required. This includes dolutegravir, an integrase inhibitor, which has been found to be more effective and better tolerated than older ART medications, leading to its recommended use as a preferred first‐ and second‐line ART by the World Health Organization [75]. Recent evidence from Africa describes greater odds of hyperglycaemia in PLHIV treated with dolutegravir compared with other ART regimens even after adjusting for potential confounders of age, BMI and co‐morbid hypertension [75]. If a wider body of research confirms these findings, systematic screening for diabetes and prediabetes prior to the use of dolutegravir may need to be incorporated into HIV treatment guidelines [75].
4.1. Strengths and limitations
The strengths of this review include the following: (1) using a review protocol with a comprehensive and systematic search strategy examining five separate databases and the reference lists of eligible studies; (2) evaluating a large number of participants from different studies; and (3) using the Freeman–Tukey double arc‐sine transformation which stabilized the variance of primary studies before combining the data; this limited the effect on the pooled estimates of studies with small or large prevalence rates.
The limitations of this review include the following: (1) the restriction to English and French languages may have excluded eligible studies in other languages and introduced a language bias; (2) the inability to examine, because of insufficient data, the associations by ART drug category, which may have been clinically relevant; (3) the inability to describe, because of insufficient data, the associations by adiposity category, which may have underscored the importance of the relation of traditional risk factors with diabetes; (4) the inclusion of only cross‐sectional studies precluded any causal inferences; (5) few (six) eligible studies had a low risk of bias; (6) the substantial heterogeneity among included studies; and (7) the inability to explore the association with a family history of diabetes, which is a key risk factor for diabetes; this was because of insufficient data.
5. CONCLUSIONS
As the diabetes epidemic worsens in Africa, adult PLHIV are affected as severely, and by similar socio‐demographic and anthropometric factors, as Africans without HIV. Furthermore, the high prevalence of prediabetes portends a likely increase in future diabetes. Policymakers in African countries must be alerted to the need to integrate cost‐effective and efficient screening, prevention and treatment of diabetes with HIV care; this will maintain the momentum and secure the advances made in optimizing HIV management. Otherwise, the future will witness a substantial proportion of PLHIV in Africa succumbing to premature diabetes and CVD‐related morbidity and mortality. Evidence‐based research is needed to provide guidance on the best strategies and approaches for the integration of diabetes and CVD prevention and care with HIV management. This review, comprising cross‐sectional studies, highlights the lack of associations between diabetes and HIV‐related factors of CD4 count, ART use and duration of ART use. Longitudinal studies are, therefore, needed to clearly elucidate the influences, both traditional and HIV related, on the development of diabetes in African PLHIV.
COMPETING INTERESTS
None to declare for all co‐authors.
AUTHORS’ CONTRIBUTIONS
Study conception (NP, KAN and APK), protocol drafting (KAN, NP and APK), protocol operationalization (NP, KAN and APK), data analysis and interpretation (KAN, NP and APK), drafting the manuscript (NP, KAN and APK), critical revision of the manuscript (JH, AES, JCC and JBN) and approval of the final version (all co‐authors).
FUNDING
NP, KAN, JH and APK are supported by the South African Medical Research Council. AES is supported by the intramural programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Minority Health and Health Disparities of the National Institutes of Health (NIH, Bethesda, Maryland, USA).
DATA ACCESS, RESPONSIBILITY AND ANALYSIS
KAN and APK had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis; both are guarantors.
Supporting information
Figure S1: Forest plot showing the overall pooled prevalence of diabetes in people living with HIV, from the trim and fill analyses.
Figure S2: Forest plot showing the pooled prevalence of diabetes in people living with HIV in clinic‐based studies, from the trim and fill analyses.
Figure S3: Forest plot showing the pooled prevalence of diabetes in people living with HIV in urban settings, from the trim and fill analyses.
Figure S4: Forest plot showing the pooled prevalence of diabetes in people younger than 40 years old living with HIV, from the trim and fill analyses .
Figure S5: Funnel plots for studies that reported prevalence of diabetes in people living with HIV (A) overall and (B) in clinic‐based settings from the trim and fill analyses. Black dots identify the actual studies while clear dots identify imputed studies.
Figure S6: Forest plot showing the pooled prevalence of pre‐diabetes in people living with HIV, from the trim and fill analyses.
Figure S7: Forest plot showing the pooled prevalence of pre‐diabetes in people living with HIV in studies in clinical settings, from the trim and fill analyses.
Figure S8: Forest plot showing the pooled prevalence of pre‐diabetes in people living with HIV in studies in urban areas, from the trim and fill analyses.
ACKNOWLEDGEMENTS
None.
DATA AVAILABILITY STATEMENT
The study is based on aggregation of publicly available data from primary studies. As such, there are no data to be shared.
REFERENCES
- 1. Magodoro IM, Esterhuizen TM, Chivese T. A cross‐sectional, facility based study of comorbid non‐communicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes. 2016;9:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dimala CA, Atashili J, Mbuagbaw JC, Wilfred A, Monekosso GL. A comparison of the diabetes risk score in HIV/AIDS patients on highly active antiretroviral therapy (HAART) and HAART‐naïve patients at the Limbe Regional Hospital, Cameroon. PLoS One. 2016;11(5):e0155560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abebe M, Kinde S, Belay G, Gebreegziabxier A, Challa F, Gebeyehu T, et al. Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a cross‐sectional comparative study. BMC Res Notes. 2014;7:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prioreschi A, Munthali RJ, Soepnel L, Goldstein JA, Micklesfield LK, Aronoff DM, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta‐analysis. BMJ Open. 2017;7(3):e013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV‐infected adults on antiretroviral therapy: a systematic review and meta‐analysis. Epidemiology. 2018;29(3):431–41. [DOI] [PubMed] [Google Scholar]
- 6. Dillon DG, Gurdasani D, Riha J, Ekoru K, Asiki G, Mayanja BN, et al. Association of HIV and ART with cardiometabolic traits in sub‐Saharan Africa: a systematic review and meta‐analysis. Int J Epidemiol. 2013;42(6):1754–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joint United Nations Programme on HIV/AIDS (UNAIDS) . Fact sheet – World AIDS Day 2021. Geneva: UNAIDS; 2021. [Google Scholar]
- 8. Nduka CU, Stranges S, Kimani PK, Sarki AM, Uthman OA. Is there sufficient evidence for a causal association between antiretroviral therapy and diabetes in HIV‐infected patients? A meta‐analysis. Diabetes Metab Res Rev. 2017;33(6):1‐31. [DOI] [PubMed] [Google Scholar]
- 9. Peer N, Baatiema L, Kengne A‐P. Ischaemic heart disease, stroke, and their cardiometabolic risk factors in Africa: current challenges and outlook for the future. Expert Rev Cardiovasc Ther. 2021;19(2):129–40. [DOI] [PubMed] [Google Scholar]
- 10. International Diabetes Federation . IDF Diabetes Atlas Ninth edition 2019. Brussels, Belgium; 2019. [Google Scholar]
- 11. Nguyen K, Peer N, Hill J, Kengne AP. Prevalence of diabetes and prediabetes among African adults living with HIV: a systematic review and meta‐analysis. PROSPERO 2021 CRD42021231547. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021231547 [DOI] [PMC free article] [PubMed]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen KA, Peer N, Mills EJ, Kengne AP. A meta‐analysis of the metabolic syndrome prevalence in the global HIV‐infected population. PLoS One. 2016;11(3):e0150970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiwandire N, Zungu N, Mabaso M, Chasela C. Trends, prevalence and factors associated with hypertension and diabetes among South African adults living with HIV, 2005–2017. BMC Public Health. 2021;21(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Manuthu EM, Joshi MD, Lule GN, Karari E. Prevalence of dyslipidemia and dysglycaemia in HIV infected patients. East Afr Med J. 2008;85(1):10–7. [DOI] [PubMed] [Google Scholar]
- 18. Dave JA, Lambert EV, Badri M, West S, Maartens G, Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor‐based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV‐infected patients. J Acquir Immune Defic Syndr. 2011;57(4):284–9. [DOI] [PubMed] [Google Scholar]
- 19. Nagu TJ, Kanyangarara M, Hawkins C, Hertmark E, Chalamila G, Spiegelman D, et al. Elevated alanine aminotransferase in antiretroviral‐naïve HIV‐infected African patients: magnitude and risk factors. HIV Med. 2012;13(9):541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ngatchou W, Lemogoum D, Ndobo P, Yagnigni E, Tiogou E, Nga E, et al. Increased burden and severity of metabolic syndrome and arterial stiffness in treatment‐naïve HIV+ patients from Cameroon. Vasc Health Risk Manag. 2013;. 9:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ngala RA, Fianko K. Dyslipidaemia and dysglycaemia in HIV‐infected patients on highly active anti‐retroviral therapy in Kumasi Metropolis. Afr Health Sci. 2013;13(4):1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kagaruki GB, Mayige MT, Ngadaya ES, Kimaro GD, Kalinga AK, Kilale AM, et al. Magnitude and risk factors of non‐communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health. 2014;14:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sawadogo A, Sanou S, Hema A, Kamboule BE, Kabore NF, Sore I, et al. Metabolic syndrome and cardiovascular risk patients under antiretrovirals in a day hospital at Bobo‐Dioulasso (Burkina Faso). Bull Soc Pathol Exot. 2014;107(3):151–8. [DOI] [PubMed] [Google Scholar]
- 24. Rabkin M, Mutiti A, Chung C, Zhang Y, Wei Y, El‐Sadr WM. Missed opportunities to address cardiovascular disease risk factors amongst adults attending an urban HIV clinic in South Africa. PLoS One. 2015;. 10(10):e0140298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maganga E, Smart LR, Kalluvya S, Kataraihya JB, Saleh AM, Obeid L, et al. Glucose metabolism disorders, HIV and antiretroviral therapy among Tanzanian adults. PLoS One. 2015;. 10(8):e0134410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mohammed AE, Shenkute TY, Gebisa WC. Diabetes mellitus and risk factors in human immunodeficiency virus‐infected individuals at Jimma University Specialized Hospital, Southwest Ethiopia. Diabetes Metab Syndr Obes. 2015;. 8:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levitt NS, Peer N, Steyn K, Lombard C, Maartens G, Lambert EV, et al. Increased risk of dysglycaemia in South Africans with HIV; especially those on protease inhibitors. Diabetes Res Clin Pract. 2016;119:41–7. [DOI] [PubMed] [Google Scholar]
- 28. Sinxadi PZ, McIlleron HM, Dave JA, Smith PJ, Levitt NS, Haas DW, et al. Plasma efavirenz concentrations are associated with lipid and glucose concentrations. Medicine. 2016;95(2):e2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaziano TA, Abrahams‐Gessel S, Gomez‐Olive FX, Wade A, Crowther NJ, Alam S, et al. Cardiometabolic risk in a population of older adults with multiple co‐morbidities in rural South Africa: the HAALSI (Health and Aging in Africa: Longitudinal Studies of INDEPTH Communities) study. BMC Public Health. 2017;17(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Heerden A, Barnabas RV, Norris SA, Micklesfield LK, van Rooyen H, Celum C. High prevalence of HIV and non‐communicable disease (NCD) risk factors in rural KwaZulu‐Natal, South Africa. J Int AIDS Soc. 2017;20(2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Labhardt ND, Müller UF, Ringera I, Ehmer J, Motlatsi MM, Pfeiffer K, et al. Metabolic syndrome in patients on first‐line antiretroviral therapy containing zidovudine or tenofovir in rural Lesotho, Southern Africa. Trop Med Int Health. 2017;22(6):725–33. [DOI] [PubMed] [Google Scholar]
- 32. Hyle EP, Bekker L‐G, Martey EB, Huang M, Xu A, Parker RA, et al. Cardiovascular risk factors among ART‐experienced people with HIV in South Africa. J Int AIDS Soc. 2019;22(4):e25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen KA, Peer N, de Villiers A, Mukasa B, Matsha TE, Mills EJ, et al. Glycated haemoglobin threshold for dysglycaemia screening, and application to metabolic syndrome diagnosis in HIV‐infected Africans. PLoS One. 2019;. 14(1):e0211483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zungu NP, Mabaso ML, Kumalo F, Sigida S, Mlangeni L, Wabiri N, et al. Prevalence of non‐communicable diseases (NCDs) and associated factors among HIV positive educators: findings from the 2015/6 survey of Health of Educators in Public Schools in South Africa. PLoS One. 2019;. 14(2):e0209756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sogbanmu O, Obi L, Ter G, Okoh A, Iweriebor B, Nwodo U, et al. Diagnosing diabetes mellitus with glycated haemoglobin in newly diagnosed HIV‐positive patients in Buffalo City Municipality, South Africa: a cross‐sectional study. Open Public Health J. 2019;12:263–8. [Google Scholar]
- 36. Umar DM, Naidoo P. Prevalence and predictors of diabetes mellitus among persons living with HIV: a retrospective cohort study conducted in 4 public healthcare facilities in KwaZulu‐Natal. BMC Public Health. 2021;21(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rajagopaul A, Naidoo M. Prevalence of diabetes mellitus and hypertension amongst the HIV‐positive population at a district hospital in eThekwini, South Africa. Afr J Prim Health Care Fam Med. 2021;13(1):e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kubiak RW, Kratz M, Motala AA, Galagan S, Govere S, Brown ER, et al. Clinic‐based diabetes screening at the time of HIV testing and associations with poor clinical outcomes in South Africa: a cohort study. BMC Infect Dis. 2021;21(1):789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hird TR, Partap U, Moodley P, Pirie FJ, Esterhuizen TM, O'Leary B, et al. HIV infection and anaemia do not affect HbA(1c) for the detection of diabetes in black South Africans: evidence from the Durban Diabetes Study. Diabet Med. 2021;38(11):e14605. [DOI] [PubMed] [Google Scholar]
- 40. Fiseha T, Belete AG. Diabetes mellitus and its associated factors among human immunodeficiency virus‐infected patients on anti‐retroviral therapy in Northeast Ethiopia. BMC Res Notes. 2019;12(1):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Faurholt‐Jepsen D, Olsen MF, Andersen AB, Kæstel P, Abdissa A, Amare H, et al. Hyperglycemia and insulin function in antiretroviral treatment‐naive HIV patients in Ethiopia: a potential new entity of diabetes in HIV? AIDS. 2019;33(10):1595–602. [DOI] [PubMed] [Google Scholar]
- 42. Gebrie A, Tesfaye B, Gebru T, Adane F, Abie W, Sisay M. Diabetes mellitus and its associated risk factors in patients with human immunodeficiency virus on anti‐retroviral therapy at referral hospitals of Northwest Ethiopia. Diabetol Metab Syndr. 2020;. 12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duguma F, Gebisa W, Mamo A, Tamiru D, Woyesa S. Diabetes mellitus and associated factors among adult HIV patients on highly active anti‐retroviral treatment. HIV AIDS. 2020;12:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woldesemayat EM. Chronic diseases multimorbidity among adult people living with HIV at Hawassa University Comprehensive Specialized Hospital, Southern Ethiopia. Int J Chronic Dis. 2020;. 2020:2190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ataro Z, Ashenafi W, Fayera J, Abdosh T. Magnitude and associated factors of diabetes mellitus and hypertension among adult HIV‐positive individuals receiving highly active antiretroviral therapy at Jugal Hospital, Harar, Ethiopia. HIV AIDS. 2018;. 10:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abebe SM, Getachew A, Fasika S, Bayisa M, Girma Demisse A, Mesfin N. Diabetes mellitus among HIV‐infected individuals in follow‐up care at University of Gondar Hospital, Northwest Ethiopia. BMJ Open. 2016;6(8):e011175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeremiah K, Filteau S, Faurholt‐Jepsen D, Kitilya B, Kavishe BB, Krogh‐Madsen R, et al. Diabetes prevalence by HbA1c and oral glucose tolerance test among HIV‐infected and uninfected Tanzanian adults. PLoS One. 2020;. 15(4):e0230723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nkinda L, Patel K, Njuguna B, Ngangali JP, Memiah P, Bwire GM, et al. C‐reactive protein and interleukin‐6 levels among human immunodeficiency virus‐infected patients with dysglycemia in Tanzania. BMC Endocr Disord. 2019;19(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kato I, Tumaini B, Pallangyo K. Prevalence of non‐communicable diseases among individuals with HIV infection by antiretroviral therapy status in Dar es Salaam, Tanzania. PLoS One. 2020;15(7):e0235542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. PrayGod G, Changalucha J, Kapiga S, Peck R, Todd J, Filteau S. Dysglycemia associations with adipose tissue among HIV‐infected patients after 2 years of antiretroviral therapy in Mwanza: a follow‐up cross‐sectional study. BMC Infect Dis. 2017;17(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rhee JY, Bahtila TD, Palmer D, Tih PM, Aberg JA, LeRoith D, et al. Prediabetes and diabetes among HIV‐infected adults in Cameroon. Diabetes Metab Res Rev. 2016;32(6):544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Noumegni SRN, Nansseu JR, Ama VJM, Bigna JJ, Assah FK, Guewo‐Fokeng M, et al. Insulin resistance and associated factors among HIV‐infected patients in sub‐Saharan Africa: a cross sectional study from Cameroon. Lipids Health Dis. 2017;16(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ngu RC, Choukem S‐P, Dimala CA, Ngu JN, Monekosso GL. Prevalence and determinants of selected cardio‐metabolic risk factors among people living with HIV/AIDS and receiving care in the South West Regional Hospitals of Cameroon: a cross‐sectional study. BMC Res Notes. 2018;11(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Appiah LT, Sarfo FS, Huffman MD, Nguah SB, Stiles JK. Cardiovascular risk factors among Ghanaian patients with HIV: a cross‐sectional study. Clin Cardiol. 2019;42(12):1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanuade OA, Baatiema L, Christian AK, Puplampu P. Cardiovascular risk factors among patients with human immunodeficiency viral infection at a tertiary hospital in Ghana: a cross‐sectional study. Pan Afr Med J. 2021;. 38:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sarfo FS, Norman B, Nichols M, Appiah L, Osei Assibey S, Tagge R, et al. Prevalence and incidence of pre‐diabetes and diabetes mellitus among people living with HIV in Ghana: evidence from the EVERLAST Study. HIV Med. 2021;22(4):231–43. [DOI] [PubMed] [Google Scholar]
- 57. Osoti A, Temu TM, Kirui N, Ngetich EK, Kamano JH, Page S, et al. Metabolic syndrome among antiretroviral therapy‐naive versus experienced HIV‐infected patients without preexisting cardiometabolic disorders in western Kenya. AIDS Patient Care STDs. 2018;32(6):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Juma K, Nyabera R, Mbugua S, Odinya G, Jowi J, Ngunga M, et al. Cardiovascular risk factors among people living with HIV in rural Kenya: a clinic‐based study. Cardiovasc J Afr. 2019;. 30(1):52–6. [DOI] [PubMed] [Google Scholar]
- 59. Njoroge A, Augusto O, Page ST, Kigondu C, Oluka M, Puttkammer N, et al. Increased risk of prediabetes among virally suppressed adults with HIV in Central Kenya detected using glycated haemoglobin and fasting blood glucose. Endocrinol Diabetes Metab. 2021;4(4):e00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health. 2016;16(1):1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pfaff C, Singano V, Akello H, Amberbir A, Berman J, Kwekwesa A, et al. Early experiences integrating hypertension and diabetes screening and treatment in a human immunodeficiency virus clinic in Malawi. Int Health. 2018;10(6):495–501. [DOI] [PubMed] [Google Scholar]
- 62.Mathabire Rücker SC, Tayea A, Bitilinyu‐Bangoh J, Bermúdez‐Aza EH, Salumu L, Quiles IA, et al. High rates of hypertension, diabetes, elevated low‐density lipoprotein cholesterol, and cardiovascular disease risk factors in HIV‐infected patients in Malawi. AIDS. 2018;32(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singano V, van Oosterhout JJ, Gondwe A, Nkhoma P, Cataldo F, Singogo E, et al. Leveraging routine viral load testing to integrate diabetes screening among patients on antiretroviral therapy in Malawi. Int Health. 2021;13(2):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mugisha JO, Schatz EJ, Randell M, Kuteesa M, Kowal P, Negin J, et al. Chronic disease, risk factors and disability in adults aged 50 and above living with and without HIV: findings from the Wellbeing of Older People Study in Uganda. Glob Health Action. 2016;9:31098. doi: 10.3402/gha.v9.31098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kazooba P, Kasamba I, Mayanja BN, Lutaakome J, Namakoola I, Salome T, et al. Cardiometabolic risk among HIV‐POSITIVE Ugandan adults: prevalence, predictors and effect of long‐term antiretroviral therapy. Pan Afr Med J. 2017;. 27:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kansiime S, Mwesigire D, Mugerwa H. Prevalence of non‐communicable diseases among HIV positive patients on antiretroviral therapy at joint clinical research centre, Lubowa, Uganda. PLoS One. 2019;. 14(8):e0221022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Muchira J, Stuart‐Shor E, Manne‐Goehler J, Lo J, Tsai AC, Kakukire B, et al. Validity of hemoglobin A1c for diagnosing diabetes among people with and without HIV in Uganda. Int J STD AIDS. 2019;30(5):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rabkin M, Palma A, McNairy ML, Gachuhi AB, Simelane S, Nuwagaba‐Biribonwoha H, et al. Integrating cardiovascular disease risk factor screening into HIV services in Swaziland: lessons from an implementation science study. AIDS. 2018;32(Suppl 1):S43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Briker SM, Aduwo JY, Mugeni R, Horlyck‐Romanovsky MF, DuBose CW, Mabundo LS, et al. A1C underperforms as a diagnostic test in Africans even in the absence of nutritional deficiencies, anemia and hemoglobinopathies: insight from the Africans in America Study. Front Endocrinol. 2019;. 10:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. International Diabetes Federation . IDF Diabetes Atlas‐10th Edition. International Diabetes Federation (IDF); 2021. [PubMed] [Google Scholar]
- 71. Bloomfield GS, Velazquez EJ. HIV and cardiovascular disease in sub‐Saharan Africa: the Sutton Law as applied to global health. J Am Coll Cardiol. 2013;. 61:2395. [DOI] [PubMed] [Google Scholar]
- 72. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high‐risk state for diabetes development. Lancet. 2012;379(9833):2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. NCD Risk Factor Collaboration (NCD‐RisC) – Africa Working Group . Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population‐based studies. Int J Epidemiol. 2017;46(5):1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Daultrey H, Youseff E, Wright J, Davies K, Chakera AJ, Levett T. The investigation of diabetes in people living with HIV: a systematic review. Diabet Med. 2021;38(4):e14454. [DOI] [PubMed] [Google Scholar]
- 75. Namara D, Schwartz JI, Tusubira AK, McFarland W, Birungi C, Semitala FC, et al. The risk of hyperglycemia associated with use of dolutegravir among adults living with HIV in Kampala, Uganda: a case–control study. Int J STD AIDS. 2022;33(14):1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Isa SE, Oche AO, Kang'ombe AR, Okopi JA, Idoko JA, Cuevas LE, et al. Human immunodeficiency virus and risk of type 2 diabetes in a large adult cohort in Jos, Nigeria. Clin Infect Dis. 2016;63(6):830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Traoré Y, Bensghir R, Ihbibane F, OuladLashen A, Sodqi M, Marih L, et al. Diabetes and human immunodeficiency virus infection: epidemiological, therapeutic aspects and patient experience. Presse Med. 2016;45(6 Pt 1):e139–43. [DOI] [PubMed] [Google Scholar]
- 78. Ekrikpo UE, Akpan EE, Ekott JU, Bello AK, Okpechi IG, Kengne AP. Prevalence and correlates of traditional risk factors for cardiovascular disease in a Nigerian ART‐naive HIV population: a cross‐sectional study. BMJ Open. 2018;8(7):e019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shankalala P, Jacobs C, Bosomprah S, Vinikoor M, Katayamoyo P, Michelo C. Risk factors for impaired fasting glucose or diabetes among HIV infected patients on ART in the Copperbelt Province of Zambia. J Diabetes Metab Disord. 2017;. 16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Katoto PDMC, Thienemann F, Bulabula ANH, Esterhuizen TM, Murhula AB, Lunjwire PPM, et al. Prevalence and risk factors of metabolic syndrome in HIV‐infected adults at three urban clinics in a post‐conflict setting, eastern Democratic Republic of the Congo. Trop Med Int Health. 2018;23(7):795–805. [DOI] [PubMed] [Google Scholar]
- 81. Cheza A, Tlou B, Zhou DT. Incidence of non‐communicable diseases (NCDs) in HIV patients on ART in a developing country: case of Zimbabwe's Chitungwiza Central Hospital—a retrospective cohort study (2010–2019). PLoS One. 2021;16(5):e0252180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hema A, Poda GEA, Tougouma J‐B, Meda ZC, Kabore F, Zoungrana J, et al. Sur‐risque de diabète sucré et d'hypertension artérielle chez les personnes infectées par le VIH suivis à l'hôpital de jour du CHU Souro Sanou, Bobo‐Dioulasso, Burkina Faso, 2018. Rev Epidemiol Sante Publique. 2021;69(2):72–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Forest plot showing the overall pooled prevalence of diabetes in people living with HIV, from the trim and fill analyses.
Figure S2: Forest plot showing the pooled prevalence of diabetes in people living with HIV in clinic‐based studies, from the trim and fill analyses.
Figure S3: Forest plot showing the pooled prevalence of diabetes in people living with HIV in urban settings, from the trim and fill analyses.
Figure S4: Forest plot showing the pooled prevalence of diabetes in people younger than 40 years old living with HIV, from the trim and fill analyses .
Figure S5: Funnel plots for studies that reported prevalence of diabetes in people living with HIV (A) overall and (B) in clinic‐based settings from the trim and fill analyses. Black dots identify the actual studies while clear dots identify imputed studies.
Figure S6: Forest plot showing the pooled prevalence of pre‐diabetes in people living with HIV, from the trim and fill analyses.
Figure S7: Forest plot showing the pooled prevalence of pre‐diabetes in people living with HIV in studies in clinical settings, from the trim and fill analyses.
Figure S8: Forest plot showing the pooled prevalence of pre‐diabetes in people living with HIV in studies in urban areas, from the trim and fill analyses.
Data Availability Statement
The study is based on aggregation of publicly available data from primary studies. As such, there are no data to be shared.


