Abstract
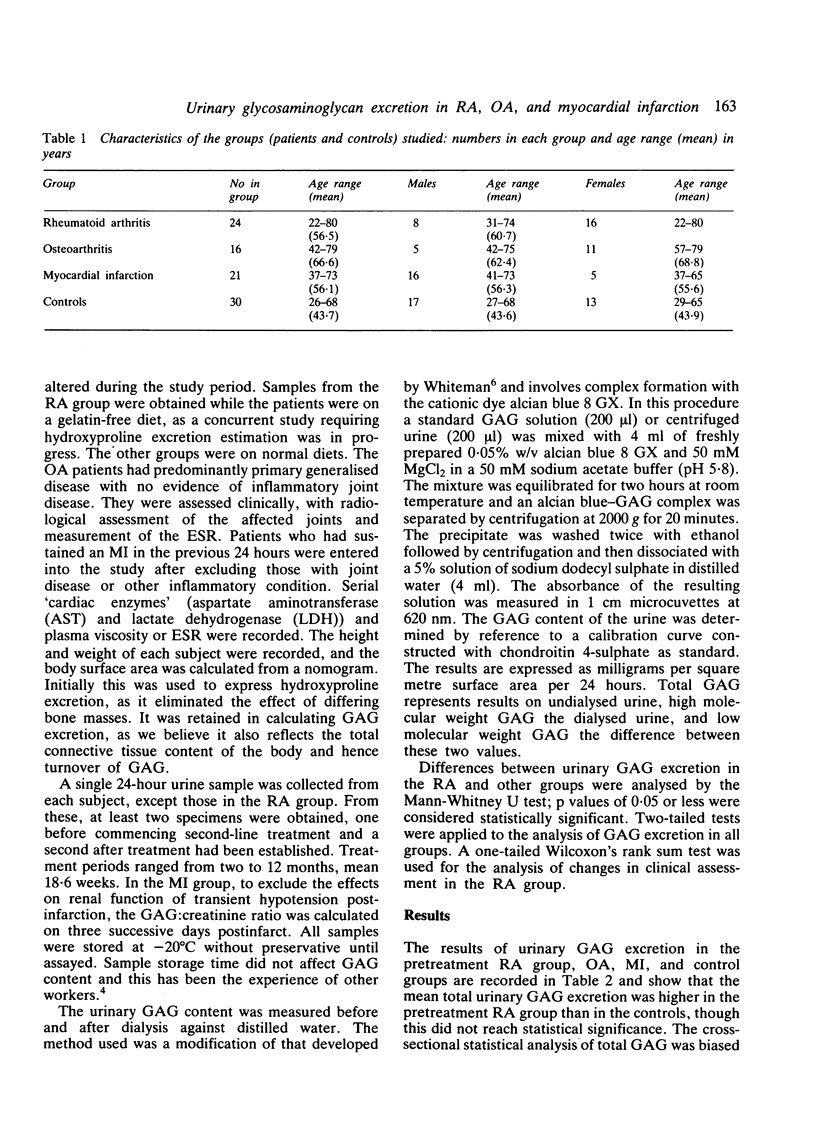
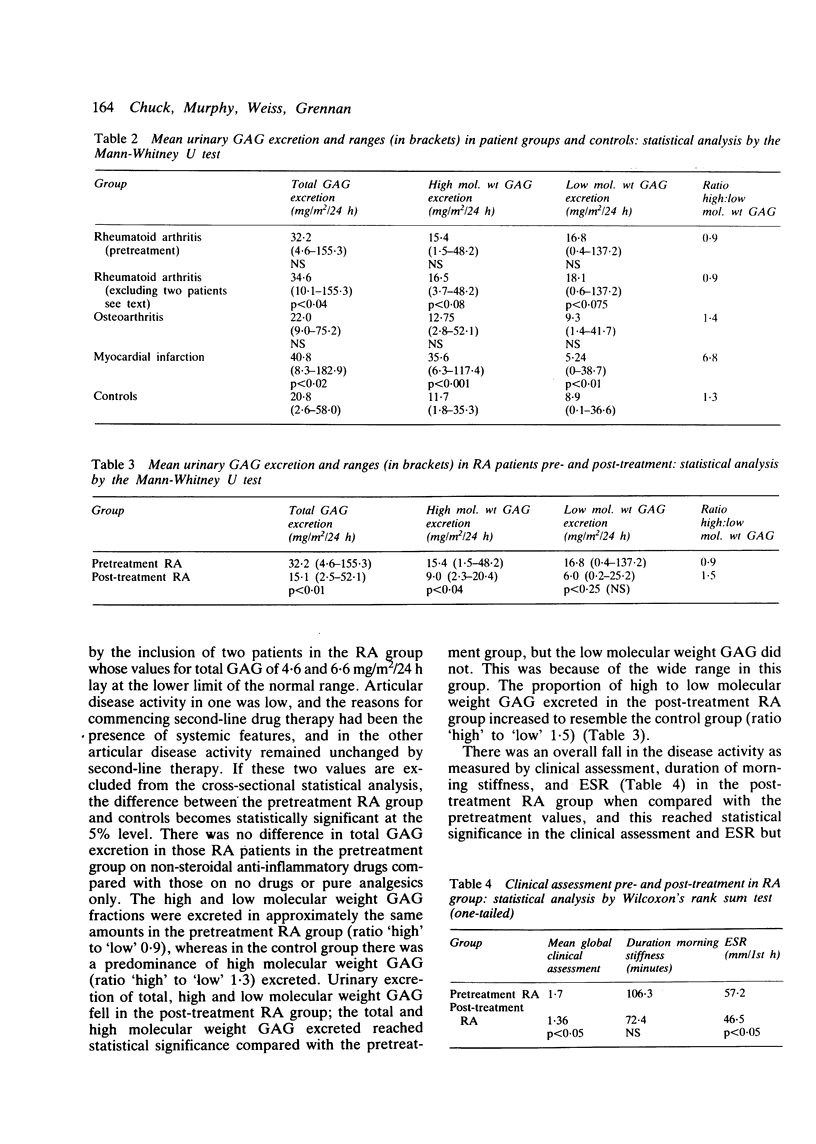
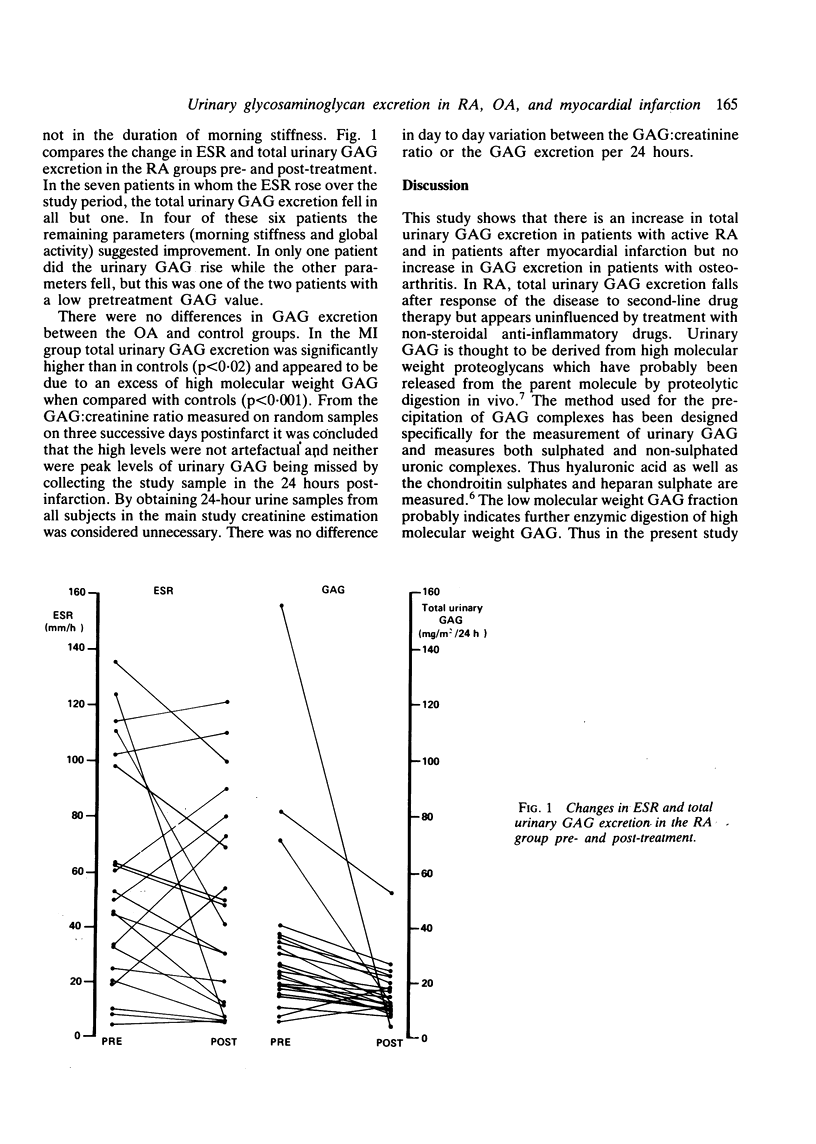
Urinary glycosaminoglycan (GAG) excretion was measured in 24 patients with active rheumatoid arthritis (RA) before and after treatment with conventional second-line agents. Urinary GAG excretion was also measured in normal controls, patients with osteoarthritis (OA), and patients with acute myocardial infarction (MI). Total GAG excretion was increased in the RA group and fell after second-line therapy (p less than 0.01). More low than high molecular weight GAG was excreted in the active RA group, and this pattern was reversed after treatment. Excretion of total, high and low molecular weight GAG in the OA group did not differ significantly from controls. Total GAG excretion was increased in the MI group when compared with controls (p less than 0.02) and consisted mainly of high molecular weight GAG. The serial measurement of urinary GAG provides a further index of disease activity and may help to monitor response to treatment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DI FERRANTE N. Urinary excretion of acid mucopolysaccharides by patients with rheumatoid arthritis. J Clin Invest. 1957 Oct;36(10):1516–1520. doi: 10.1172/JCI103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd J. T., Wexler B. C. Myocardial connective tissue metabolism in response to injury. II. Investigation of the mucopolysaccharides involved in isoproterenol-induced necrosis and repair in rat hearts. Circ Res. 1970 Jan;26(1):101–109. doi: 10.1161/01.res.26.1.101. [DOI] [PubMed] [Google Scholar]
- Judd J. T., Wexler B. C. Sulfur 35 uptake in acid mucopolysaccharides of the rat heart following injury. Am J Physiol. 1973 Feb;224(2):312–317. doi: 10.1152/ajplegacy.1973.224.2.312. [DOI] [PubMed] [Google Scholar]
- Mbuyi J. M., Dequeker J., Teblick M., Merlevede M. Relevance of urinary excretion of alcian blue-glycosaminoglycans complexes and hydroxyproline to disease activity in rheumatoid arthritis. J Rheumatol. 1982 Jul-Aug;9(4):579–583. [PubMed] [Google Scholar]
- Murata K., Takeda M. Compositional changes of urinary acidic glycosaminoglycans in progressive systemic sclerosis. Clin Chim Acta. 1980 Nov 20;108(1):49–59. doi: 10.1016/0009-8981(80)90291-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky A. I., Howell D. S., Woessner J. F., Jr Neutral proteases and cathepsin D in human articular cartilage. J Clin Invest. 1974 Apr;53(4):1044–1053. doi: 10.1172/JCI107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetlar M. R., Davitt W. F., Rosett R. L., Crass M. F., 3rd, Lautsch E. V., Kischer C. Glycosaminoglycan changes in healing myocardial infarction. Proc Soc Exp Biol Med. 1978 Jun;158(2):210–214. doi: 10.3181/00379727-158-40173. [DOI] [PubMed] [Google Scholar]
- Smith E. B. Acid glycosaminoglycan, collagen and elastin content of normal artery, fatty streaks and plaques. Adv Exp Med Biol. 1974;43(0):125–139. doi: 10.1007/978-1-4684-3243-5_6. [DOI] [PubMed] [Google Scholar]
- Whiteman P. The quantitative determination of glycosaminoglycans in urine with Alcian Blue 8GX. Biochem J. 1973 Feb;131(2):351–357. doi: 10.1042/bj1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]


