This randomized clinical trial investigates the 5-year survival outcomes of laparoscopy-assisted distal gastrectomy compared with open distal gastrectomy for the management of advanced gastric cancer.
Key Points
Question
Is laparoscopy-assisted distal gastrectomy (LADG) for locally advanced gastric cancer noninferior to open distal gastrectomy (ODG) in terms of 5-year outcomes?
Findings
In this randomized clinical trial of 507 patients with locally advanced gastric cancer, the 5-year relapse-free survival as a primary end point was 73.9% and 75.7% for the ODG and LADG groups, respectively.
Meaning
Study results provide robust evidence suggesting that laparoscopic gastrectomy for locally advanced gastric cancer could become an appropriate treatment approach when performed by skilled surgeons.
Abstract
Importance
Evidence of implementation of laparoscopic gastrectomy for locally advanced gastric cancer is currently insufficient, as the primary end point in previous prospective studies was evaluated at a median follow-up time of 3 years. More robust evidence is necessary to verify noninferiority of laparoscopic gastrectomy.
Objective
To compare 5-year survival outcomes between laparoscopy-assisted distal gastrectomy (LADG) and open distal gastrectomy (ODG) with D2 lymph node dissection for locally advanced gastric cancer.
Design, Setting, and Participants
This was a multicenter, open-label, noninferiority, prospective randomized clinical trial. Between November 26, 2009, and July 29, 2016, eligible patients with histologically proven gastric carcinoma from 37 institutes in Japan were enrolled. Two interim analyses and final analysis were performed in October 2014, May 2018, and November 2021, respectively.
Interventions
Patients were randomly assigned (1:1) to either the ODG or LADG group. The procedures were performed exclusively by qualified surgeons.
Main Outcomes and Measures
The primary end point was 5-year relapse-free survival, and the noninferiority margin for the hazard ratio (HR) was set at 1.31. The secondary end points were 5-year overall survival and safety.
Results
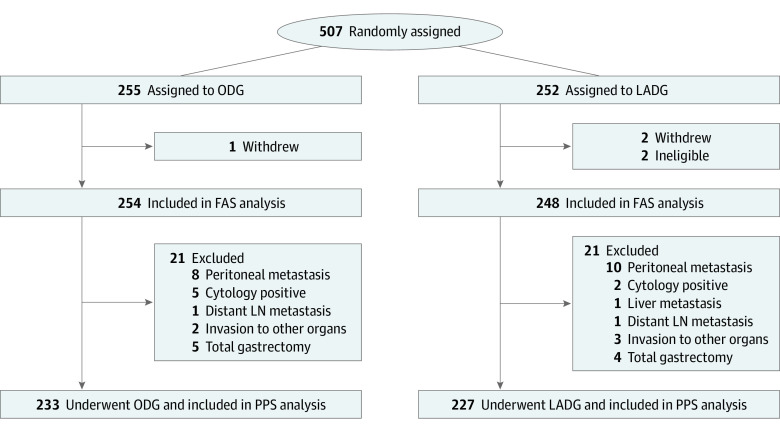
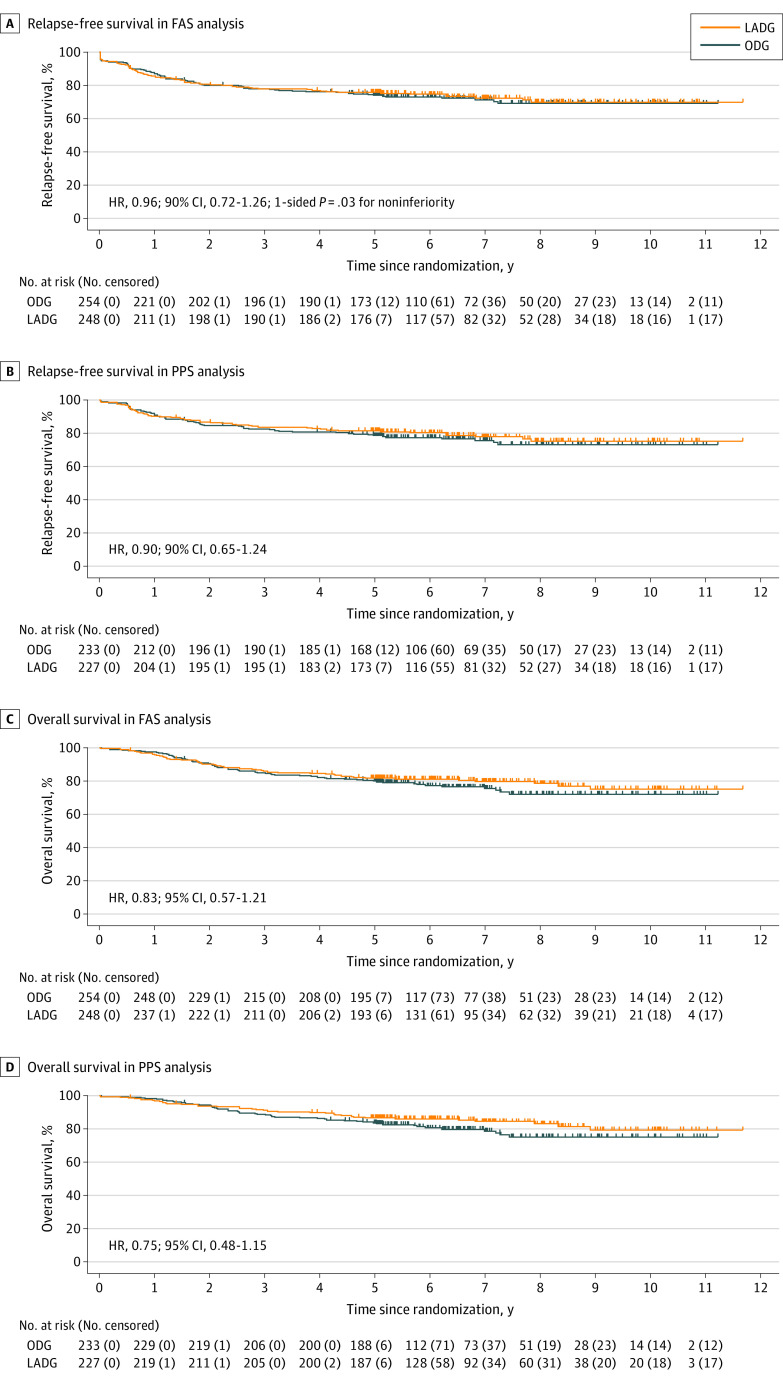
A total of 502 patients were included in the full-analysis set: 254 (50.6%) in the ODG group and 248 (49.4%) in the LADG group. Patients in the ODG group had a median (IQR) age of 67 (33-80) years and included 168 males (66.1%). Patients in the LADG group had a median (IQR) age of 64 (34-80) years and included 169 males (68.1%). No significant differences were observed in severe postoperative complications between the 2 groups in the safety analysis (ODG, 4.7% [11 of 233] vs LADG, 3.5% [8 of 227]; P = .64). The median (IQR) follow-up for all patients after randomization was 67.9 (60.3-92.0) months. The 5-year relapse-free survival was 73.9% (95% CI, 68.7%-79.5%) and 75.7% (95% CI, 70.5%-81.2%) for the ODG and LADG groups, respectively, and the HR was 0.96 (90% CI, 0.72-1.26; noninferiority 1-sided P = .03). Further, no significant difference was observed in overall survival time between the 2 groups, and the HR was 0.83 (95% CI, 0.57-1.21; P = .34). The pattern of recurrence was similar between the 2 groups.
Conclusions and Relevance
Results of this study show that on the basis of 5-year follow-up data, LADG with D2 lymph node dissection for locally advanced gastric cancer, when performed by qualified surgeons, was proved noninferior to ODG. This laparoscopic approach could become a standard treatment for locally advanced gastric cancer.
Trial Registration
UMIN Clinical Trial Registry: UMIN000003420
Introduction
Gastric cancer (GC) has high mortality rates worldwide; thus, finding a cure is critical. Minimally invasive techniques have significantly improved surgical outcomes. Since its introduction in Japan in 1991,1 the number of patients with GC treated with laparoscopy-assisted distal gastrectomy (LADG) and lymph node dissection (LND) has steadily increased.2 Retrospective studies and large-scale randomized clinical trials (RCTs) have shown the advantages of minimally invasive surgery for GC. Recently, large-scale RCTs were conducted in Japan and other East Asian countries to evaluate the safety and effectiveness of LADG for stage I GC.3,4,5 Based on their results, LADG is now recognized as a standard treatment for stage I GC, alongside open surgery, according to the latest Japanese GC treatment guidelines.6
For advanced GC (AGC), laparoscopic gastrectomy is considered technically difficult owing to the large tumor size and LN metastasis. To clarify the safety and effectiveness of LADG with D2 LND for AGC, outcomes of several prospective studies have been reported recently (ie, multicenter randomized clinical trials of comparing laparoscopic distal gastrectomy to open distal gastrectomy in terms short- and long-term outcomes [KLASS-02 and CLASS-01]).7,8,9,10 However, the primary end point of these studies was fundamentally based on statistical hypotheses regarding 3-year outcomes, and the median follow-up time was approximately 3 years. Alternatively, 5-year follow-up data as secondary analysis of these studies were subsequently published.11,12 Although these secondary analyses seem informative for clinical practice, a primary end point on a longer follow-up period is clearly more robust for verifying noninferiority of LADG for locally AGC. To better understand the use of LADG with D2 LND for locally AGC, we conducted a multicenter randomized phase 2/3 study (JLSSG0901).13 Here, we present the outcomes of phase 3 for which the primary end point was 5-year survival outcomes.
Methods
Study Design and Participants
This study was an open-label, multi-institutional, prospective, randomized phase 2/3 trial conducted by the Japanese Laparoscopic Surgery Study Group (JLSSG) to investigate safety and efficacy of LADG. The protocol was approved by the JLSSG protocol review committee (JLSSG0901) and the institutional review boards of each of the 37 participating hospitals (Supplement 1 and eAppendix in Supplement 2). All patients provided written informed consent before randomization. Phase 2 demonstrated the technical feasibility of laparoscopic D2 LND for AGC for the treatment of anastomotic leakage and pancreatic fistula formation. Phase 3 investigated the safety and noninferiority of LADG compared with open distal gastrectomy (ODG). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Between November 26, 2009, and July 29, 2016, patients from 37 institutes in Japan were included in this study based on the following criteria: (1) age of 20 to 80 years with histologically proven gastric adenocarcinoma, (2) body mass index (BMI) less than 30 (BMI is calculated as weight in kilograms divided by height in meters squared), (3) Eastern Cooperative Oncology Group performance status 0 or 1 (considered potentially curable by distal gastrectomy), and (4) clinical diagnosis of muscularis propria (MP), subserosa (SS), and serosal exposure (SE), N0-2 without bulky node metastasis, or M0 lesions without involvement of other organs.13 The criteria are detailed in eTable 1 in Supplement 2. Factor N was preoperatively determined by the anatomical position according to the 13th Japanese Classification of Gastric Carcinoma (JCGC).14
Randomization and Masking
Patient enrollment and randomization and data management were conducted by the Data Center, Clinical Trial Support Division, General Clinical Research Center of Oita University Hospital, Yufu, Japan. Group assignment was conducted using the minimization method according to the clinical depth of invasion (MP vs SS/SE), clinical N category (N0 vs N1 vs N2), and the institution. The procedure was not concealed from the investigators or patients.
Procedures
To train instructors, the Endoscopic Surgical Skill Qualification System (ESSQS) was established by the Japan Society for Endoscopic Surgery.15 Surgeons and institutes were strictly evaluated; all surgeons had ESSQS certification and had performed a specified number of both laparoscopic gastrectomy and open gastrectomy procedures. Furthermore, a central review by the committee was performed, using intraoperative photographs to assess surgical quality.
In both groups, distal gastrectomy with D2 LND was performed according to Japanese GC treatment guidelines.16 The procedures have been described in detail in our previous report.13 Staging laparoscopy in the ODG group was recommended for patients at high risk of peritoneal dissemination. Adjuvant chemotherapy (oral 5-fluorouracil agents) was administered 1 year postoperatively when pathological stages II and III (except M/SM and SS with N0) were confirmed after surgery according to Japanese GC treatment guidelines.16 Patients were followed up for 5 years after the last patient enrollment, with computed tomography scans and blood tests performed every 6 months.
The case report forms were recorded according to both the 13th and 14th editions of the JCGC and were used to document operative and pathological information. Moreover, the data were described as per the 7th edition of the Union for International Cancer Control TNM classification.17 The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 was used to classify complication grades.
Outcomes
The primary end point was 5-year relapse-free survival (RFS), defined as the time from randomization to relapse, death from any cause, or last date of contact with the patient. The surgery day was considered an event when any unresectable factors were found intraoperatively, which is synonymous with curability C according to the 13th edition of the JCGC. The secondary end points included 5-year overall survival (OS; time from randomization to death from any cause), morbidity and mortality rates, proportion of LADG completion, number of harvested LNs, early postoperative course, and recurrence sites.
Statistical Analysis
The expected 5-year RFS rate for the ODG group was assumed to be 65% based on a previous study,18 and it was determined to be clinically unacceptable if the rate decreased by 8% or greater in the LADG group. The corresponding noninferiority margin for the hazard ratio (HR) was 1.31. The required number of participants was calculated with a 1-sided α of .05, statistical power of 75%, 4-year enrollment period, and 5-year follow-up period (follow-up until 9 years after study initiation). Thus, the planned sample size was set at 500 (250 per group) according to the Lachin and Foulkes method.19 The initial 180 patients were assigned to phase 213 and also included in the subsequent phase 3. During patient enrollment, it became apparent that the number of participants was lower than necessary; thus, we extended the enrollment period to 7.5 years with an unchanged 500 total sample size.
Two interim analyses were planned. Multiplicity was adjusted using the Lan-DeMets method with the O’Brien-Fleming alpha-spending function.20 The first and second interim analyses were performed in October 2014 and May 2018, respectively. The data and safety monitoring committee independently reviewed the results of the interim analyses and approved the continuation of the planned follow-up. In the final analysis, the statistical significance level was 4.99% considering the multiplicity adjustment.
RFS and OS curves were estimated using the Kaplan-Meier method. HRs and associated CIs were estimated using the Cox regression model after adjusting for randomization factors, except for the institution. Regarding the sensitivity analysis, we analyzed RFS using a stratified Cox regression model with randomization factors, except for institutions as strata. Subgroup RFS analyses were conducted using the Cox regression model. Fisher exact test and Wilcoxon rank sum test were used to analyze the categorical and continuous variables, respectively. For the primary analysis, the P value was 1-sided; for all other analyses, P values were 2-sided.
Statistical analyses were performed using SAS, version 9.4 (SAS Institute) and R, version 4.1.1 (R Foundation for Statistical Computing). Efficacy end points were analyzed based on the full-analysis set (FAS) and per-protocol set (PPS). The FAS excluded the patients who withdrew consent or were ineligible from all randomized patients, and the PPS was defined as patients who completed the protocol treatment. Additionally, the safety end points were analyzed in the PPS.
Results
Patients
Overall, 507 patients were randomly assigned to either the ODG (n = 255) or LADG group (n = 252) between November 26, 2009, and July 29, 2016 (Figure 1). One and 2 patients in the ODG and LADG groups, respectively, withdrew consent after randomization. Two patients in the LADG group were found ineligible after randomization. Of the 502 patients included in the FAS, 254 patients (50.6%) in the ODG group and 248 patients (49.4%) in the LADG group were included in the efficacy analysis. Patients in the ODG group had a median (IQR) age of 67 (33-80) years and included 168 males (66.1%) and 86 females (33.9%). Patients in the LADG group had a median (IQR) age of 64 (34-80) years and included 169 males (68.1%) and 79 females (31.9%). Subsequently, 42 patients were excluded owing to tumor extension or noncurative factors such as distant metastasis. The remaining 460 patients who underwent curative distal gastrectomy with D2 LND according to the protocol were analyzed as the PPS. All patients in the FAS received the assigned treatment without switching to an alternative. No substantial differences were observed in baseline characteristics (Table 1).
Figure 1. Consolidated Standards for Reporting Trials (CONSORT) Diagram.
The full-analysis set (FAS) excluding ineligible patients and those who withdrew was included in the efficacy analysis. LADG indicates laparoscopy-assisted distal gastrectomy; LN, lymph node; ODG, open distal gastrectomy; PPS, per-protocol set.
Table 1. Baseline Patient Characteristics and Pathological Outcomes.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Full-analysis set | Per-protocol set | |||
| ODG (n = 254) | LADG (n = 248) | ODG (n = 233) | LADG (n = 227) | |
| Characteristics of the patients | ||||
| Age, median (range), y | 67 (33-80) | 64 (34-80) | 66 (33-80) | 64 (34-80) |
| Sex | ||||
| Male | 168 (66.1) | 169 (68.1) | 160 (68.7) | 156 (68.7) |
| Female | 86 (33.9) | 79 (31.9) | 73 (31.3) | 71 (31.3) |
| ECOG performance status | ||||
| 0/1 | 250/4 | 247/1 | 230/3 | 226/1 |
| BMI,a median | 22.5 | 22.3 | 22.7 | 22.3 |
| Tumor location | ||||
| Lower | 133 (52.4) | 105 (42.3) | 120 (51.5) | 96 (42.3) |
| Middle | 120 (47.2) | 142 (57.3) | 112 (48.1) | 130 (57.3) |
| Upper | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) |
| Tumor size, median (IQR), cm | 4 (3-5) | 4 (3-5) | 4 (3-5) | 4 (3-5) |
| Clinical depth of invasion | ||||
| MP | 113 (44.5) | 122 (49.2) | 109 (46.8) | 118 (52) |
| SS | 104 (40.9) | 85 (34.3) | 92 (39.5) | 83 (36.6) |
| SE | 37 (14.6) | 41 (16.5) | 32 (13.7) | 26 (11.5) |
| Clinical N categoryb | ||||
| N0 | 138 (54.3) | 130 (52.4) | 127 (54.5) | 125 (55.1) |
| N1 | 94 (37.0) | 84 (33.9) | 90 (38.6) | 74 (32.6) |
| N2 | 22 (8.7) | 34 (13.7) | 16 (6.9) | 28 (12.3) |
| Clinical stageb | ||||
| IB | 124 (48.8) | 118 (47.6) | 115 (49.4) | 114 (50.2) |
| II | 83 (32.7) | 85 (34.3) | 77 (31) | 80 (35.2) |
| III | 47 (18.5) | 45 (18.1) | 41 (17.6) | 33 (14.5) |
| Pathological results | ||||
| Pathological T categoryc | ||||
| T1 | 59 (23.2) | 70 (28.2) | 59 (25.3) | 69 (30.4) |
| T2 | 65 (25.6) | 57 (23) | 65 (27.9) | 57 (25.1) |
| T3 | 65 (25.6) | 65 (26.2) | 63 (27) | 60 (26.4) |
| T4 | 63 (24.8) | 54 (21.8) | 46 (19.7) | 41 (18.1) |
| TX | 2 (0.8) | 2 (0.8) | 0 | 0 |
| Pathological N categoryc | ||||
| N0 | 110 (43.3) | 102 (41.1) | 109 (46.8) | 100 (44) |
| N1 | 58 (22.8) | 56 (22.6) | 53 (22.8) | 56 (24.7) |
| N2 | 40 (15.7) | 37 (14.9) | 39 (16.7) | 35 (15.4) |
| N3 | 43 (16.9) | 50 (20.2) | 32 (13.7) | 36 (15.9) |
| NX | 3 (1.2) | 3 (1.2) | 0 | 0 |
| Pathological stagec | ||||
| IA | 45 (17.7) | 53 (21.4) | 45 (19.3) | 52 (22.9) |
| IB | 50 (19.7) | 39 (15.7) | 50 (21.5) | 39 (17.2) |
| II | 69 (27.2) | 65 (26.2) | 67 (28.8) | 64 (28.2) |
| III | 78 (30.7) | 78 (31.5) | 71 (30.4) | 72 (31.7) |
| IV | 12 (4.7) | 13 (5.2) | 0 | 0 |
| Curabilityb | ||||
| A/B/C | 180/62/12 | 183/52/13 | 180/52/1 | 180/45/2 |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LADG, laparoscopy-assisted distal gastrectomy; N, node; ODG, open distal gastrectomy; T, tumor.
Calculated as weight in kilograms divided by height in meters squared.
According to the 13th edition of Japanese Classification of Gastric Carcinoma. N category indicates the following: N0, no evidence of lymph node metastasis; N1, metastasis to group 1 lymph node based on the classification of regional lymph nodes; N2, metastasis to group 2 lymph node based on the classification of regional lymph nodes. Clinical stage indicates the following: stage IB, T2N0M0; stage II, T2N1M0 or T3N0M0; stage III, T2N2M0 or T3N1M0 or T3N2M0. Curability A indicates curative resection with D2 or greater lymph node dissection for MP or SS disease with N0 or N1 without distant metastasis. Curability B indicates no residual but not fulfilling criteria for Curability A. Curability C indicates definite residual disease.
According to the 7th edition of the Union for International Cancer Control TNM classification. Pathological T category (T1, tumor invasion of mucosa or submucosa; T2, tumor invasion of muscularis propria; T3, tumor invasion of subserosa; T4, serosal exposure or tumor invades adjacent structures. Pathological N category (N0, no regional lymph node metastasis; N1, metastasis in 1 to 2 regional lymph nodes; N2, metastasis in 3 to 6 regional lymph nodes; N3, metastasis in 7 or more regional lymph nodes; NX, regional lymph nodes cannot be assessed). Pathological stages include stage IA, T1N0M0; stage IB, T1N1M0 or T2N0M0; stage II, T1N2M0 or T1N3M0 or T2N1M0 or T2N2M0 or T3N0M0; stage III, T2N3M0 or T3N2-3M0 or T4N1-N3M0; stage IV, any T any NM1.
Safety Outcomes
The surgical results, early postoperative course, mortality, and pathological results are summarized in eTable 2 in Supplement 2. The distribution of surgical procedures, reconstruction, and degree of LND were similar in both groups; the number of harvested LNs was 43 in each group. Compared with the ODG group, the LADG group was associated with a longer operating time (median [IQR], 291 [236-345] minutes vs 205 [175-240] minutes; P < .001) and lower estimated blood loss (median [IQR], 30 [10-90] mL vs 141 [80-270] mL; P < .001). No patients required conversion to open surgery due to intraoperative complications. We noticed no significant differences in reoperation (within 30 days after the initial operation), readmission (within 30 days after initial discharge), or 30-day and in-hospital mortality rates between the 2 groups. No significant difference in frequency of postoperative chemotherapy was found between the 2 groups.
Comparisons of the intraoperative and postoperative complications, according to the CTCAE, version 4.0 in the PPS category are presented in eTable 3 in Supplement 2. No significant differences were observed in the incidence of intraoperative complications. Any postoperative complications in the ODG and LADG groups were observed in 25 of 233 patients (10.7%) and 26 of 227 patients (11.5%), respectively, whereas those of grade 3 and higher were observed in 11 of 233 patients (4.7%) and 8 of 227 patients (3.5%; P = .64), respectively. Among postoperative complications, no significant difference was observed in the incidence of anastomotic leakage and pancreatic fistula formation between the 2 groups. Regarding late complications, the incidence of cholecystitis in all complication grades was significantly higher in the ODG group than in the LADG group (11 of 233 [4.7%] vs 2 of 227 [0.9%]; P = .02); however, no significant difference was observed in the incidence of complications greater than or equal to grade 3 (5 of 233 [2.1%] vs 2 of 227 [0.9%]; P = .45) (eTable 4 in Supplement 2).
Efficacy Outcomes
The median (IQR) follow-up for all patients after randomization was 67.9 (60.3-92.0) months. The RFS curves are shown in Figures 2A and B. The 5-year RFS was 73.9% (95% CI, 68.7%-79.5%) in the ODG group and 75.7% (95% CI, 70.5%-81.2%) in the LADG group. The HR for RFS in the LADG vs ODG groups was 0.96 (90% CI, 0.72-1.26; noninferiority 1-sided P = .03). The HR by sensitivity analysis using a stratified Cox regression was 0.93 (90% CI, 0.70-1.23). In the FAS analysis, 139 of 502 patients (27.7%; 72 of 254 [28.3%] and 67 of 248 [27.0%] in the ODG and LADG groups, respectively) had recurrence, death, or curability C. When curability C was excluded from the events for RFS analysis, the 5-year RFS was 77.6% (95% CI, 72.5%-83.0%) and 79.9% (95% CI, 74.9%-85.2%) in the ODG and LADG groups, respectively, whereas the HR for RFS in the LADG vs ODG groups was 0.92 (90% CI, 0.68-1.26). In the PPS analysis, the 5-year RFS was 78.4% (95% CI, 73.3%-83.9%) in the ODG group and 81.4% (95% CI, 76.4%-86.6%) in the LADG group. The HR for RFS in the LADG vs ODG group was 0.90 (90% CI, 0.65-1.24). The OS curves are shown in Figures 2C and D. In the FAS analysis, the 5-year OS was 79.8% (95% CI, 75.0%-84.9%) and 81.7% (95% CI, 77.0%-86.7%) in the ODG and LADG groups, respectively, whereas the HR for 5-year OS in the LADG vs ODG groups was 0.83 (95% CI, 0.57-1.21; P = .34). In the PPS analysis, the 5-year OS was 83.6% (95% CI, 79.0%-88.5%) in the ODG group and 86.6% (95% CI, 82.3%-91.2%) in the LADG group. The HR for 5-year OS in the LADG vs ODG groups was 0.75 (95% CI, 0.48-1.15; P = .19).
Figure 2. Survival Analyses.
A, Relapse-free survival in the full-analysis set (FAS) analysis. B, Relapse-free survival in the per-protocol set (PPS) analysis. C, Overall survival in the FAS analysis. D, Overall survival in the PPS analysis. LADG indicates laparoscopy-assisted distal gastrectomy; ODG, open distal gastrectomy.
After curative resection, 46 of 254 patients (18.1%) and 44 of 248 patients (17.7%) in the ODG and LADG groups, respectively, showed recurrence. The recurrence pattern was similar between the 2 groups (Table 2). Furthermore, 13 of 254 patients (5.1%) and 11 of 248 patients (4.4%) in the ODG and LADG groups, respectively, died of causes other than recurrence.
Table 2. Distribution of the Recurrence Sites.
| Recurrence sites | Full-analysis set | P value | Per-protocol set | P value | ||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | |||||
| ODG (n = 254) | LADG (n = 248) | ODG (n = 233) | LADG (n = 227) | |||
| Lymph node | 16 (6.3) | 9 (3.6) | .22 | 16 (6.9) | 9 (4.0) | .22 |
| Peritoneum | 11 (4.3) | 19 (7.7) | .13 | 10 (4.3) | 14 (6.2) | .53 |
| Liver | 12 (4.7) | 7 (2.8) | .35 | 11 (4.7) | 6 (2.6) | .32 |
| Lung | 1 (0.4) | 1 (0.4) | >.99 | 1 (0.4) | 1 (0.4) | >.99 |
| Bone | 4 (1.6) | 1 (0.4) | .37 | 4 (1.7) | 1 (0.4) | .37 |
| Remnant stomach | 0 (0) | 2 (0.8) | .24 | 0 (0) | 2 (0.9) | .499 |
| Othersa | 2 (0.8) | 5 (2.0) | .28 | 1 (0.4) | 4 (1.8) | .37 |
Abbreviations: LADG, laparoscopy-assisted distal gastrectomy; ODG, open distal gastrectomy.
Others include ovary, bone marrow, and pleura.
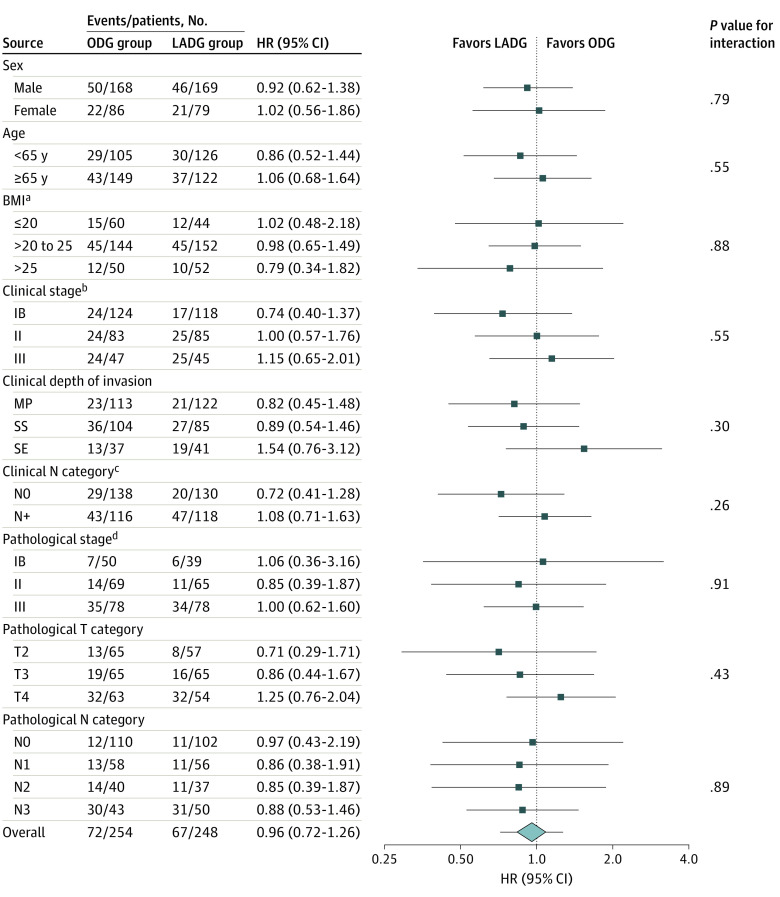
Subgroup analyses of 5-year RFS in the FAS were performed for sex (male vs female), age (<65 vs ≥65 years), BMI (≤20 vs >20 to 25 vs >25), clinical stage (IB vs II vs III), clinical depth of invasion (MP vs SS vs SE), clinical N category (N0 vs N+), pathological stage (IB vs II vs III), pathological T category (T2 vs T3 vs T4), and pathological N category (N0 vs N1 vs N2 vs N3), according to the 7th Union for International Cancer Control TNM classification (Figure 3). Although no significant interactions were observed between the treatment effect and any of the baseline factors, patients with a BMI greater than 25 and pathological positive metastatic nodes in the LADG group tended to have better survival rates than patients in the ODG group. Contrarily, patients in the LADG group with T4 disease tended to have worse survival rates than patients in the ODG group. Among patients with stage III disease, survival rates were identical in both groups.
Figure 3. Subgroup Analysis of Relapse-Free Survival .
BMI indicates body mass index; HR, hazard ratio; LADG, laparoscopy-assisted distal gastrectomy; MP, muscularis propria; N, node; ODG, open distal gastrectomy; SE, serosal exposure; SS, subserosa; T, tumor.
aCalculated as weight in kilograms divided by height in meters squared.
bClinical stage indicates the following: stage IB, T1N1M0 or T2N0M0; stage II, T1N2M0 or T1N3M0 or T2N1M0 or T2N2M0 or T3N0M0; stage III, T2N3M0 or T3N2-3M0 or T4N1-N3M0.
cClinical N category indicates the following: N0, no regional lymph node metastasis; N+, metastasis in lymph nodes. Pathological N category indicates the following: N0, no regional lymph node metastasis; N1, metastasis in 1 to 2 regional lymph nodes; N2, metastasis in 3 to 6 regional lymph nodes; N3, metastasis in 7 or more regional lymph nodes.
dClinical and pathological T category indicates the following: T2, tumor invasion of MP; T3, tumor invasion of SS; T4, SE or tumor invades adjacent structures.
Discussion
Although 2 pivotal studies have been conducted in Asian countries, the efficacy and safety of laparoscopic gastrectomy for locally AGC remain controversial. Herein, we focused on the 5-year survival of patients because advanced cancers have the potential for late recurrence (even 3 years after the initial surgery). As a result, the noninferiority of LADG to ODG was confirmed in the primary end point of 5-year RFS as well as favorable 5-year OS as a secondary end point in the LADG group. The recurrence rates and pattern were found to be similar between the 2 groups. Moreover, this RCT revealed a favorable postoperative recovery from LADG, suggesting that LADG with D2 LND, performed by qualified surgeons, could become a standard treatment for locally AGC.
Laparoscopic gastrectomy for locally AGC is technically demanding; therefore, a meticulous trial design and quality control were essential. This study has several advantages. First, the design was that of a randomized phase 2/3 study; we confirmed the technical safety of LADG with D2 LND for locally AGC in phase 2 before proceeding to phase 3.13 Hence, we completed enrollment for the phase 3 part without any major difficulties. Second, we aimed to ensure high-quality surgical interventions in this trial. To achieve this, the participating surgeons were required to have ESSQS certification,5 which was established in 2004 to maintain laparoscopic technical skills, a standardized laparoscopic surgery process. Recent studies have shown that ESSQS-certified surgeons were more likely to deliver favorable LADG outcomes.21,22 Furthermore, we performed a central review of D2 LND quality by assessing close-up photographs of the operative field after dissection, which benefited surgical quality assessment.23
Among postoperative complications, pancreatic fistula formation is considered a concern during LADG; its high frequency after LADG for early GC and AGC was demonstrated in a large-scale prospective study conducted using a national clinical database.24 However, this incidence in our study was extremely lower than that of previous RCTs. The amount of bleeding during LADG (30 mL) was minimal in our study. Considering recent reports on the negative impact of intraoperative blood loss on long-term outcomes after curative gastrectomy for AGC,25 safer techniques could contribute to more favorable patient outcomes.
Moreover, we investigated the association of unexpected recurrence or death after laparoscopic surgery for AGC. A 5-year follow-up period was believed to be required to understand this association in clinical settings because approximately one-third of recurrences are observed after the first 3 years.26,27 Therefore, we defined the 5-year RFS as a primary end point as opposed to the 3-year RFS in the CLASS-01 and KLASS-02 studies.9,10 The verification of noninferiority is based on the statistical hypothesis regarding the primary end point, leading to more verifiable and robust results than outcomes of the secondary end points. Therefore, it seems difficult to accurately judge the noninferiority in 5-year follow-up outcomes of studies for which primary end points are defined as 3-year RFS.11,12
Laparoscopic surgery for AGC is associated with concerns such as peritoneal dissemination or unexpected recurrence after gastric wall damage caused by forceps, ie, crushing of LNs and adipose tissue or pneumoperitoneum. Furthermore, the effect of laparoscopic surgery on long-term results is unclear, especially in patients with stage III GC, LN metastasis, or serosal invasion. In fact, the recurrence rate tended to be higher in patients with stage III GC in the CLASS-01 trial. Here, no significant interaction was noticed between treatment efficacy and number of patients with stage III GC. Patients with metastatic node-positive cancer in the LADG group showed better survival rates than those in the ODG group. This may be attributed to high-magnification views enabling precise maneuvering during laparoscopic LND. Conversely, it may be a concern that patients with T4 disease in the LADG group had worse survival rates than those in the ODG group in FAS; however, the HR decreased for RFS in the PPS category. A previous large-scale retrospective study indicated no interaction between laparoscopic gastrectomy and T4 disease.28 As statistical power in this subgroup analysis was insufficient to detect any interaction due to the small sample number and events, further large-scale studies with intraoperative randomization limited to patients with T4 disease are required.
Strengths and Limitations
One of the strengths of our study is that robust evidence was obtained by an accurate long follow-up with high follow-up rates of nearly 99%. Evaluating the 5-year results of these RCTs could be beneficial for decision-making in practice toward GC treatments.
This study also has some limitations. First, despite the eligibility criteria set for AGC, 74% of lesions were pathologically deeper than those of T2, and 63% of patients had a disease advanced beyond stage II. A recent prospective large cohort study showed that 15% of clinical T2 cases had pathological T1 disease, suggesting that contamination from earlier diseases could not be completely avoided.29 Per policy, treatment was decided according to the preoperative diagnosis; however, crucially, the results proved noninferiority of LADG to ODG. Second, patients with BMI of 30 or greater were excluded from this study. Although obesity is a risk factor for postoperative complications that can lead to unfavorable long-term outcomes, a recent meta-analysis has shown that laparoscopic surgery can reduce complication rates in these patients.30 In the subgroup analysis, we noticed that LADG was efficient in patients with BMI greater than 25; therefore, laparoscopic surgery could favorably affect survival outcomes in this population. Ultimately, verifying the use of laparoscopic surgery in obese patients with AGC is warranted. Third, patients with large tumors, such as large types 3 and 4 requiring total gastrectomy, were not included in this study. To expand the indications for laparoscopic total gastrectomy in AGC, associated factors need to be considered, such as the dissection range and anastomosis methods. In Western countries, the frequency of AGC requiring total gastrectomy is high,31,32 and the data for extrapolating our results to Western populations may be insufficient. Therefore, further studies are required to expand the indications for total gastrectomy in patients with AGC. Finally, our findings are based on the surgery for selected patients performed by qualified surgeons. Indeed, only approximately 10% of all patients who had distal gastrectomy in the participating institutions during the study period were enrolled in this study. Therefore, implementation to real-life clinical practice should be carefully considered according to each institution’s experiences.
Conclusions
In conclusion, findings of this RCT reveal that LADG with D2 LND for locally AGC was safely performed by trained and qualified surgeons without major surgical complications, and noninferiority of this procedure compared with ODG concerning the 5-year RFS was established. We conclude that a laparoscopic approach could become the standard treatment for locally AGC.
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Surgical Results, Early Postoperative Course, Mortality
eTable 3. Comparison of Intraoperative and Postoperative Complications According to CTCAE Version 4.0 Between the 2 Groups Per Protocol Set
eTable 4. Late Complications in the Per Protocol Set
eAppendix. Institutions That Participated in the Study
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4(2):146-148. [PubMed] [Google Scholar]
- 2.Shiroshita H, Inomata M, Akira S, et al. Current status of endoscopic surgery in Japan: the 15th National Survey of Endoscopic Surgery by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg. 2022;15(2):415-426. doi: 10.1111/ases.13012 [DOI] [PubMed] [Google Scholar]
- 3.Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20(4):699-708. doi: 10.1007/s10120-016-0646-9 [DOI] [PubMed] [Google Scholar]
- 4.Kim HH, Han SU, Kim MC, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506-513. doi: 10.1001/jamaoncol.2018.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142-151. doi: 10.1016/S2468-1253(19)30332-2 [DOI] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350-1357. doi: 10.1200/JCO.2015.63.7215 [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Hyung WJ, Yang HK, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT). Ann Surg. 2019;270(6):983-991. doi: 10.1097/SLA.0000000000003217 [DOI] [PubMed] [Google Scholar]
- 9.Hyung WJ, Yang HK, Park YK, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Long-Term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol. 2020;38(28):3304-3313. doi: 10.1200/JCO.20.01210 [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Huang C, Sun Y, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321(20):1983-1992. doi: 10.1001/jama.2019.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Liu H, Hu Y, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg. 2022;157(1):9-17. doi: 10.1001/jamasurg.2021.5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song SY, Hur H, Hyung WJ, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: 5-year outcomes of the KLASS-02 randomized clinical trial. JAMA Surg. 2022;157(10):879-886. doi: 10.1001/jamasurg.2022.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaki N, Etoh T, Ohyama T, et al. A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg. 2015;39(11):2734-2741. doi: 10.1007/s00268-015-3160-z [DOI] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association . Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer. 1998;1(1):10-24. doi: 10.1007/s101209800016 [DOI] [PubMed] [Google Scholar]
- 15.Tanigawa N, Lee SW, Kimura T, et al. The Endoscopic Surgical Skill Qualification System for gastric surgery in Japan. Asian J Endosc Surg. 2011;4(3):112-115. doi: 10.1111/j.1758-5910.2011.00082.x [DOI] [PubMed] [Google Scholar]
- 16.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14(2):113-123. doi: 10.1007/s10120-011-0042-4 [DOI] [PubMed] [Google Scholar]
- 17.Sobin LH, Gospondarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell; 2010. [Google Scholar]
- 18.Sasako M, Sano T, Yamamoto S, et al. ; Japan Clinical Oncology Group . D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453-462. doi: 10.1056/NEJMoa0707035 [DOI] [PubMed] [Google Scholar]
- 19.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42(3):507-519. doi: 10.2307/2531201 [DOI] [PubMed] [Google Scholar]
- 20.Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659-663. doi: 10.2307/2336502 [DOI] [Google Scholar]
- 21.Akagi T, Endo H, Inomata M, et al. Clinical impact of Endoscopic Surgical Skill Qualification System (ESSQS) by Japan Society for Endoscopic Surgery (JSES) for laparoscopic distal gastrectomy and low anterior resection based on the National Clinical Database (NCD) registry. Ann Gastroenterol Surg. 2020;4(6):721-734. doi: 10.1002/ags3.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi S, Kagawa T, Kuroda S, et al. Accreditation as a qualified surgeon improves surgical outcomes in laparoscopic distal gastrectomy. Surg Today. 2021;51(12):1978-1984. doi: 10.1007/s00595-021-02309-2 [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, Inomata M, Akagi T, et al. Quality control by photo documentation for evaluation of laparoscopic and open colectomy with D3 resection for stage II/III colorectal cancer: Japan Clinical Oncology Group Study JCOG 0404. Jpn J Clin Oncol. 2014;44(9):799-806. doi: 10.1093/jjco/hyu083 [DOI] [PubMed] [Google Scholar]
- 24.Hiki N, Honda M, Etoh T, et al. Higher incidence of pancreatic fistula in laparoscopic gastrectomy: real-world evidence from a nationwide prospective cohort study. Gastric Cancer. 2018;21(1):162-170. doi: 10.1007/s10120-017-0764-z [DOI] [PubMed] [Google Scholar]
- 25.Misawa K, Kurokawa Y, Mizusawa J, et al. ; Stomach Cancer Study Group of the Japan Clinical Oncology Group . Negative impact of intraoperative blood loss on long-term outcome after curative gastrectomy for advanced gastric cancer: exploratory analysis of the JCOG1001 phase III trial. Gastric Cancer. 2022;25(2):459-467. doi: 10.1007/s10120-021-01266-6 [DOI] [PubMed] [Google Scholar]
- 26.Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric Cancer. 2006;9(2):74-81. doi: 10.1007/s10120-006-0360-0 [DOI] [PubMed] [Google Scholar]
- 27.Zhu Z, Li L, Xu J, et al. Laparoscopic vs open approach in gastrectomy for advanced gastric cancer: a systematic review. World J Surg Oncol. 2020;18(1):126. doi: 10.1186/s12957-020-01888-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita T, Uyama I, Terashima M, et al. ; LOC-A Study Group . Long-term outcomes of laparoscopic vs open surgery for clinical stage II/III gastric cancer: a multicenter cohort study in Japan (LOC-A study). Ann Surg. 2019;269(5):887-894. doi: 10.1097/SLA.0000000000002768 [DOI] [PubMed] [Google Scholar]
- 29.Fukagawa T, Katai H, Mizusawa J, et al. ; Stomach Cancer Study Group of the Japan Clinical Oncology Group . A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer. 2018;21(1):68-73. doi: 10.1007/s10120-017-0701-1 [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Zhao B, Huang Y, Lu H, Luo R, Huang B. Feasibility of laparoscopy gastrectomy for gastric cancer in the patients with high body mass index: a systematic review and meta-analysis. Asian J Surg. 2020;43(1):69-77. doi: 10.1016/j.asjsur.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 31.van der Veen A, Brenkman HJF, Seesing MFJ, et al. ; LOGICA Study Group . Laparoscopic versus open gastrectomy for gastric cancer (LOGICA): a multicenter randomized clinical trial. J Clin Oncol. 2021;39(9):978-989. doi: 10.1200/JCO.20.01540 [DOI] [PubMed] [Google Scholar]
- 32.van der Wielen N, Straatman J, Daams F, et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer. 2021;24(1):258-271. doi: 10.1007/s10120-020-01109-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Surgical Results, Early Postoperative Course, Mortality
eTable 3. Comparison of Intraoperative and Postoperative Complications According to CTCAE Version 4.0 Between the 2 Groups Per Protocol Set
eTable 4. Late Complications in the Per Protocol Set
eAppendix. Institutions That Participated in the Study
Nonauthor Collaborators
Data Sharing Statement