Abstract
Aeromonas hydrophila is a freshwater, facultatively anaerobic, chemo-organoheterotrophic bacterium that distressed fishes with gastroenteritis, septicemia and causes a disease known as Motile Aeromonas Septicemia (MAS), which affects the aquatic environment. Haemolysin, aerolysin, cytosine, gelatinase, enterotoxin and antimicrobial peptides have been identified as virulence factors in A. hydrophila. Medicinal herbs/plants and their uses are the instant, easily available, cost-effective, efficient and eco-friendly approach for socio-economic, sustainable development of modern aquaculture practice. Phytotherapy either through a dip or by incorporation into the diets is an alternative approach to synthetic pharmaceuticals to diminish the pathogenicity of aquatic environmental pathogens. Due to the presence of remarkable phytoconstituents like flavonoids, alkaloids, pigments, terpenoids, steroids and essential oils, the medicinal plant exhibits anti-microbial, appetite-stimulating, anti-stress, growth-promoting and immunostimulatory activities. Aqua-industry preferred phytotherapy-based techniques/compounds to develop resistance against a variety of aquatic pathogens in culturable fishes because they are inexpensive and environment-friendly. As a result, this review elaborates on the diverse applications of phytotherapy as a promising tool for disease management in aquaculture and a major step toward organic aquaculture.
Keywords: Aeromonas hydrophila, Motile Aeromonas Septicemia (MAS), Immunostimulants, Phytotherapy, Herbalism, Pathogenicity, Hemorrhagic septicemia
Highlights
-
•
Aeromonas spp. causes significant economic losses to aquaculture.
-
•
Medicinal plants are biocompatible and cost-efficient.
-
•
Potent bioactive compounds of medicinal plants exhibit antimicrobial activities.
-
•
Phytotherapy represents a promising tool for the sustainability of the aquaculture.
1. Introduction
Aeromonas hydrophila is a freshwater, facultatively anaerobic, chemo-organoheterotrophic bacterium that causes disease in fishes, amphibians, reptiles, birds and mammals with gastroenteritis, septicemia and necrotizing fasciitis being the most prevalent kinds of disease [[1], [2], [3], [4]]. Aeromonas species can be found in a variety of aquatic and environmental habitats including sediment, estuaries, seaweed, sea grass, used water, drinking water and food [5,6]. Genus Aeromonas comprises Gram-negative, motile bacilli or coccobacilli rods, non-spore-forming with rounded ends that size 1–3.5 μm across and belongs to the Aeromonadaceae family of Gammaproteobacteria. They are facultatively anaerobic, catalase, oxidase and indol-positive, able to convert nitrate to nitrite and are generally resistant to the vibrio static agent O/129. In a microbiological survey, A. hydrophila is prevalent in the Chesapeake Bay and its tributaries with concentrations ranging from ca. 4.6 × 102/g in sediment and <0.3/l to 5 × 103/ml in the water column [7]. Kaper et al. [8] found that A. hydrophila in shellfish growing waters had cell counts ranging from 3 to 2400 cells/100 ml in water and from 3 to 4600 cells/100 g in oysters.
Carps are the major group of freshwater fish that are important as food sources and study models all around the world. Aeromonas sp. and Pseudomonas sp. are the most prevalent bacteria isolated from carp culture systems [9]. A. hydrophila is a widely investigated bacteria due to its occurrence in the estuaries [10], food [11], water [12], antibiotic resistance and potential to cause disease in animals and humans [13]. Recent research found motile species of Aeromonas especially A. hydrophila are the main causing agents for a variety of infections [14]. Aeromoniasis was shown to be the most prevalent bacterial disease occurring whole year in Indian major carps Catla catla, Labeo rohita, Cirrhinus mrigala and exotic carps such as Hypophthalmichthys molitrix, Ctenopharyngodon idella and Cyprinus carpio. H. molitrix was the most sensitive to Aeromonas of the six fish species tested [9]. A. hydrophila has a natural habitat in water and can thrive at temperatures ranging from 0 to 45 °C with an optimum temperature of 22–32 °C. In fish, A. hydrophila infection is a zoonotic disease, i.e., it may be transmitted from animals to humans and vice versa [15]. Stress conditions such as crowding, low dissolved oxygen, higher organic content, physical injuries, temperature fluctuation and factory pollution may cause A. hydrophila infection [16,17].
A. hydrophila is classified as a primary or secondary pathogen [18,19]. When a pathogen causes disease in stressed fish on its own, it is referred to as a primary pathogen. Generally, A. hydrophila is found as a secondary invader [20]. Because secondary pathogens have a limited invasive capacity, they depend on the existence of primary infection to infect. A. hydrophila is usually considered a secondary pathogen that infects a fish that has already been infected with another infection [21]. A. hydrophila can also act as an opportunistic invader, infecting fish under stressed conditions or along with other pathogens [22]. It is considered an efficient biomarker of a stressed or polluted aquatic environment [23]. The term “opportunistic pathogen” means, if a chance is given, A. hydrophila always has the potential of causing disease [20].
In India, “Mrgayurveda” a subdiscipline of Ayurveda, focuses on animal life and the use of herbal medicines to treat animal diseases [24]. Phytotherapy is a medical practice that focuses more on traditional approaches rather than modern medication. It highly involves the knowledge and usage of medical herbalism. Although the aquaculture industry has only just begun using phytotherapy, it is gradually being recognized as a treatment option in place of synthetic pharmaceuticals [25]. This biodegradable and environmental-friendly application is known as phytotherapy or more often commonly called herbalism. Globally, the use of medicinal herbs in aquaculture has drawn considerable interest and has become a subject of active scientific research [24,26]. It has been observed that medicinal plants contain a wide range of appetite-stimulating, growth-promoting, antibacterial, immunostimulant, anti-inflammatory, antistress, anticancer qualities and their usage in traditional medicine has been recognized across the world for thousands of years. The most common medicinal plants incorporated in fish diets as powder and extracts are Azadirachta indica, Withania somnifera, Allium sativum, Zingiber officinale, Ocimum sanctum, Tinospora cordifolia, Aloe barbadensis etc. [27]. They appear to be administered to fish without causing any negative side effects, unlike chemotherapeutics. Additionally, they are cost-effective, readily accessible, biocompatible and contribute a significant role in sustainable and rural community development [28] (Table 1).
Table 1.
List of medicinal plants and their potent bioactive compounds for the possible therapeutic use in various diseases of aquaculture.
| Scientific name | Common name | Part used | Bioactive compounds | Properties | References |
|---|---|---|---|---|---|
| Scutellaria baicalensis | Chinese skullcap | Aerial part | Baicalin, baicalein, 7-O-glucuronide and oroxylin A | Antimicrobial, antioxidant, anticancer, and anti-inflammatory | [29] |
| Castanea sativa | Sweet chestnut | Phenolic extract of shell | Trigalloyl-HHDP-glucose, gallic acid and quercetin | Antibacterial and antioxidant | [30] |
| Pandanus tectorius | Screw pine | Leave powder extract | p-hydroxybenzaldehyde, syringaldehyde, E-ferulaldehyde, E-sinapinaldehyde, vanillin and 5-hydroxymethylfurfual | Antibacterial and antioxidant | [31] |
| Aloe vera | Aloe-vera | Leaves | 7-hydroxyaloin A and 7-hydroxyaloin B, (8-O-methyl-7-hydroxyaloin A and 8-O-methyl-7-hydroxyaloin B | Antibacterial, antifungal, and antiviral properties | [32] |
| Elaeagnus angustifolia | Russian olive | Leaves extract | Cyanidin-3-O-glucoside, gallic acid and anthocyanin | Antimicrobial, antioxidant and antimutagenic | [33] |
| Coffea arabica | Arabian coffee | Coffee silver skin | Chlorogenic acids, caffeine, trigonelline, melanoidins and diterpenes | Antibacterial | [34] |
| Citrus limon | Lemon | Lemon peels | Caffeoyl N-Tryptophan, hydroxycinnamoyl-Oglucoside acid, vicenin 2, eriocitrin, kaempferol-3-O- rutinoside, and quercetin-3-rutinoside | Antibacterial and antifungal | [35] |
| Nigella sativa | Black cumin | Seed | Thymoquinone, thymohydroquinone, dithymoquinone, p-cymene, carvacrol, 4-terpineol, t-anethole, sesquiterpene, α-pinene, and thymol | Antibacterial | [36] |
| Arum maculatum | Cuckoo pint | Leaves | 1,1-diphenyl 2-picrylhydrazyl free radical (DPPH), β-Carotene and tocopherols | Antimicrobial activity, antioxidant properties, antibacterial, antimutagenic, anticarcinogenic and cardioprotective activities | [37] |
| Aloe barbadensis | Aloe vera | Leaf | Aloe-emodin, aloin, aloesin, emodin and acemannan | Antifungal, antibacterial, antiviral and anthelmintic | [38] |
| Thymus vulgaris | Common thyme | Oil | Borneol, carvacrol, cymol, linalool, thymol, tannin, apigenin, luteolin, saponins and triterpenic acid | Antibacterial, antifungal and antioxidant | [39] |
| Achillea cucullata | Gandrain | Oil | Camphor, 1,8-cineole and isoborneol | Antioxidant, antibacterial antimicrobial and enzyme-inhibition activity | [40] |
| Anisomeles malabarica | Malabar catmint | Leaves | β-sitosterol, ovatodiolide, anisomelicacid, malabaric acid, anisomelol and triterpene betulinic acid | Antioxidant, antibacterial and antibacterial activities | [41] |
| Cynara cardunculus | Cardoon | Oil | 5-O-caffeoylquinic, 3,5-O-dicaffeoylquinic acid, luteolin-7-O-glucoside, luteolin-7-O-malonylhexoside, palmitic, linoleic, stearic, caproic and oleic acid | Antioxidant, anti- inflammatory, antifungal and antibacterial | [42] |
| Melocanna baccifera | Muli bamboo | Leaf | β-sitosterol, E-phytol, β-amyrin, syringic acid, blumenol B and tianshic acid | Antifungal, antibacterial, antiprotozoal, antibacterial antitussive and immunomodulatory | [43] |
| Thymus linearis | Himalayan thyme | Oil | Thymol, carvacrol, thymyl acetate and β-caryophyllene | Antimicrobial, antibacterial antioxidant activity and antiseptic | [44] |
| Excoecaria agallocha | Mangrove | Leaf | Squalene, tochopherol, terpenoids | Antimicrobial, antibacterial and immunomodulatory | [45] |
| Mentha piperita | Peppermint | Oil | Menthone, iso-menthone, menthol, germacrene D, α-pinene, Limonene, 1,8-cineole and menthone | Antimicrobial, antibacterial and Immunostimulant | [46] |
| Ocimum sanctum | Tulsi | Leaves | Ursolic acid, oleanolic acid and salrigenin | Antioxidative, antimicrobial, antistress, antibacterial antidiabetic and antiviral | [47] |
| Citrus medica | Fingered citron | Fruit | Limonene, geranial and neral | Antifungal and antibacterial | [48] |
| Zingiber officinale | Zinger | Root | Zingiberene, β-bisabolene, α-farnesene, β-sesquiphellandrene, andα-curcumene, 6-gingerol and 6-shogaol | Antioxidants, antibacterial, anti-inflammatory and Antimicrobial | [49] |
| Cinnamomum cassia | Chinese cinnamon | Tree bark | Cinnamaldehyde, cinnamon oil, eugenol, salicylaldehyde and trans-cinnamic acid | Antioxidant, anti- inflammatory and antibacterial | [50] |
| Eriobotrya japonica | Japanese medlar | Leaves | Corosolic acid, 3-epicorosolic acid, euscaphic acid, oleanolic acid, maslinic acid (9), methyl arjunolate and betulinic acid | Antioxidant, anti- inflammatory and antibacterial | [51] |
| Tinospora cardifolia | Guduchi | Leaves | Berberine, choline, tinosporin, tinocordiside, furanolactone and β-sitosterol | Antibacterial | [52] |
| Withania somnifera | Ashwagandha | Root | Withaniol, withasomnine, somnirol, somnitol, withanic acid, phytosterol and ipuranol | Antibacterial | [53] |
| Toona sinensis | Chinese cedar | Leaves | Ursolic acid, bBetulic acid, cedrellin, phytol and scopoletin | Antibacterial, antiviral, antioxidant, anti-cancer and anti-inflammatory | [54] |
| Punica granatum | Pomegranate | Leaves | Ellagic and gallic tannins | Antiviral and antibacterial | [55] |
| Thymus daenensis | Thyme | Oil | Thymol, p-cymene, 1,8-cineole, γ-terpinene and carvacrol | Antiseptic, antimicrobial, antispasmodic, antibacterial antioxidant and antitussive agent | [56] |
| Indigofera suffruticosa | Indian indigo | Leaves | Syringic acid, p-coumaric acid, vanillin, syringaldehyde, salicylic acid, quercetin, isoliquiritigenin, and formononetin | Antibacterial | [57] |
| Camellia sinensis | Tea plant | Leaves and buds | Catechins, epicatechins, theaflavins, flavonol glycosides, l-theanine, caffeine and theobromine | Antiparasitic and antibacterial | [58] |
| Allium sativum | Garlic | Tuber | Allicin, alliin, diallyl sulfide, diallyl disulfide, diallyl trisulfide, ajoene, and S-allyl-cysteine | Hypolipidemic, antibacterial, antimicrobial, antihypertensive and hepatoprotective | [59] |
| Carica papaya | Pawpaw | Seeds | Tannins, papain, nicotine, cyanogenicglucosides and quercetin | Antioxidative, antibacterial and antimicrobial | [60] |
The enhancement and acceleration of the aquaculture sector growth require the development and production of effective, safe and pollution-free herbal compounds. Herbal medicines are inexpensive and have excellent results. Additionally, they are eco-friendly and green [61]. Pharmacology and toxicology of numerous herbal medicines and compound preparations lack functions that treat aquatic animals, prevent disease, promote growth and improve the quality of aquatic products. These abilities are found in effective ingredients, content, structure, extraction and relationships among effective ingredients. Various nations are now actively pursuing new methods of green farming and investing more money in scientific research. As current society develops to environmental protection and healthy direction, aquaculture is also no exception [62]. With the help of a combination of extract chemicals or erstwhile immunostimulants, they can be used as a whole plant or specific part. Being environmentally cheaper, medicinal plants show minimum side effects and are hence used as an option for antibiotics in the fisheries industry. The relevance of plants as natural and undamaging composite has probable in aquaculture as a substitute for antibiotics [63]. The aquaculture sector relies on phytotherapy since they have proven benefits such as improving the delivery system, bioavailability, and sustained discharge of bioactive compounds [64].
2. Characters of Aeromonas hydrophila
2.1. Morphological characters
Features such as capsule formation and motile with flagella formation were observed [65]. Isolates of A. hydrophila produce lateral flagella for surface movement/swarming and polar flagella for suspension movement. Polar flagella production in A. piscicola AH-3 has been examined with mutations in flaAB, flaH, fliA, fliM, maf-1 and flrC eliminate polar flagella production and resulting in decreased adhesion and biofilm formation [66]. In addition to having single lateral flagellin, A. piscicola AH-3 contains glycosylated polar and lateral flagella. On the other hand, A. hydrophila AH-1, has two lateral flagellins but just one glycosylated polar flagellum [67]. In A. piscicola AH-3, mutations in the pseudaminic acid biosynthesis genes pseB and pseI prevented the production of both polar and lateral flagellin, whereas, in A. hydrophila AH-1, only the development of polar flagella was impacted. Thus, in glycosylation-negative A. hydrophila AH-1 mutants, lateral flagella production was unaffected [68].
2.2. Physiological characters
Maximum temperature for growth in nutrient broth (30, 37 and 41 °C); growth factor requirements using a mineral-ammonium medium containing glucose or succinate as the sole source of carbon and energy; growth in peptone water in the presence or absence of sodium chloride; catalase production; growth in KCN medium; methyl red and Voges-Proskauer reactions [69].
2.3. Carbohydrate metabolism
Production of acid and gas from glucose and glycerol: acid production from L-arabinose, L-rhamnose, L-xylose, D-mannose, D-cellobiose, D-lactose, D-maltose, D-sucrose, D-trehalose, D-mannitol, D-dulcitol, D-sorbitol, salicin, sorbose, raffinose, erythritol, mucate, adonitol, meso-inositol, melibiose; esculin hydrolysis; production of butanediol-dehydrogenase and β-galactosidase [70].
2.4. Nitrogenous compound metabolism
Production of urease, lysine decarboxylase, phenylalanine deaminase, tryptophan deaminase, ornithine decarboxylase, arginine dihydrolase, H2S production on Kligler's medium and from cysteine on cysteine-iron agar, tetrathionate reductase; indole formation in peptone water [71].
2.5. Extracellular enzymes
Biochemical and physiological characteristics of the A. hydrophila isolates have been shown in Table 2 [72]. They were found to possess the same characteristics as those tested [73, 74]. Production of elastase, lipase, gelatinase, pectinase, RNAase and DNAase [75].
Table 2.
The comparative study of characteristics of A. hydrophila isolates [72].
| Characters | Characterization [73] | Characterization [74] |
|---|---|---|
| Gram stain | – | – |
| Shape | Rod | Rod |
| Motility | + | + |
| Oxidase | + | + |
| Catalase | + | + |
| OF test | F | F |
| Acid and gas production from glucose | + | + |
| Acid production from | ||
| Lactose | + | + |
| Sucrose | + | + |
| Maltose | + | + |
| Mannitol | + | + |
| Inositol | – | – |
| Sorbitol | – | – |
| Rhamnose | – | – |
| Methyl-red test | – | – |
| Voges-Proskauer | + | + |
| Indole | + | + |
| H2S production | + | + |
| Arginine decomposition | + | + |
| Lysine decarboxylation | – | – |
| Ornithine decarboxylation | – | – |
| Citrate utilization | + | + |
| Growth in 4 °C | – | – |
| Growth in 5 °C | – | – |
| Growth in 37 °C | + | + |
| Growth in 40 °C | + | + |
| Growth in 0% NaCl | + | + |
| Growth in 1% NaCl | + | + |
| Growth in 2% NaCl | + | + |
| Growth in 3% NaCl | + | + |
| Growth in 4% NaCl | – | – |
2.6. Structural proteins, phospholipids and polysaccharides
O-antigens, capsules and S-layer proteins serve as protection mechanisms against host defences. Capsules have anti-phagocytic activity, improve resistance to the complement system and promote adhesion in Aeromonas sp. [76,77]. O-antigens are a type of lipopolysaccharide with a variety of structural properties that act as colonization factors. A. piscicola at 20 °C, AH-3 produces O-antigens but not at 37 °C, resulting in O-antigens-deficient strains that are unable to colonize hosts and have low T3SS component expression [78]. A. hydrophila has eight distinct O-antigen gene clusters, and all epidemic strains isolated from channel catfish (Ictalurus punctatus) share a homologous O-antigen gene cluster [79]. The S-layer protein gene (ahsA) encodes an exterior paracrystalline layer in A. hydrophila TF7 (genomic data lacking). During insertional mutagenesis of spsD S-protein secretion, this layer is removed [80].
3. A. hydrophila growth in culture media
Rimler Shotts agar was used as a selective medium to isolate A. hydrophila (HiMedia). Plates were incubated at 37 °C for 28 h. Using an automated microbial analyzer all cultures were identified to the species level (Biolog, US). For further characterization, selected A. hydrophila colonies were subcultured in Tryptic Soya Broth (Difco). In the RS-medium, A. hydrophila formed yellow colonies. Gram staining of these colonies gives a Gram-negative reaction, microscopically examination gives rod-shaped, motile colonies, biochemical tests give oxidase-positive, antibiotic and fermentative resistance tests give novobiocin resistance, indicating that the colonies are made up of aeromonads. By using an automated microbial analyzer, all isolates were identified as A. hydrophila [81].
Rimler-Shotts (RS) medium was created, which is a modification of various enterobacteria-specific media. It was made up of L-ornithine hydrochloride 0.8 g; L-lysine-hydrochloride 6.5 g; sodium thiosulfate 5 g; agar 13.5 g; maltose 3.5 g; sodium deoxycholate 1.0 g; L-cysteine-hydrochloride 0.3 g; novobiocin 0.005 g; sodium chloride 5.0 g; bromothymol blue 0.03 g; ferric ammonium citrate 6.8 g; yeast extract 3.0 g and sufficient water in quantity to make 1 L. Stirring was used to dissolve the components, the pH was adjusted to 7.0 and the liquid was brought to a boil for 1 min, cooled to 45 °C and poured into plates. Plates were refrigerated until they were required. When organisms were inoculated on RS media, four distinct kinds of colonies were obtained. The first was yellow indicating that maltose fermentation had occurred. The second was yellow with a black center and showed a similar reaction to the first but with H2S added. The third sort of colony displayed hues of greenish-yellow to green, indicating lysine or ornithine decarboxylation, or both. The fourth type had a black center and was green, implying the same reaction as the third but with H2S production [[82], [83], [84]]. The most basic (maltose fermentation) or acidic reaction was produced by choosing and combining the components in this medium (decarboxylation of lysine or ornithine, or both). Sodium thiosulfate and L-cysteine hydrochloride, or both, were largely required for the production of hydrogen sulphide, with ferric ammonium citrate being used to aid in the visualization of this reaction. Gram-positive organisms and Vibrio spp. were eliminated with the addition inhibitors of sodium deoxycholate and novobiocin. The use of novobiocin to suppress the development of Vibrio spp. reduces the misunderstanding that can occur when distinguishing these organisms from anaerogenic strains of A. hydrophila [85].
Smooth, spherical, small, convex and yellowish colonies of A. hydrophila (CAHH14 strain) were seen on the RS-plate. It passes biochemical tests for motility, catalase, oxidase and the O/F test, and it is resistant to the novobiocin and 0/129 disc [86]. All Aeromonas strains were cultivated for 24–36 h in Tryptic soy broth (TSB) (Difco) at 28–30 °C. The accuracy tests were conducted using TSA as a control medium, as well as for colony separation from recovery media. In the selectivity experiments, the reference agar used was plate count agar (Difco). For the recovery of A. hydrophila from water samples, the following selective media were used. DNTA consisted of 30 mg of ampicillin (Sigma, USA) and 0.1% toluidine blue (Sigma, USA) added to DNase agar (Difco). MacConkey agar (Difco) with 1% trehalose was used as MCT (Difco). A. hydrophila AB3-15 was cultivated as a lawn culture in Roux bottles for 24 h on Tryptic soya agar (TSA) and collected in sterile phosphate-buffered saline (PBS) pH 7.0. A live vaccination was created using recently obtained cells, and a dead vaccine was created by heating the harvested cells in a water bath at 60 °C for 1 h (Table 3) [87,88]. Rimler Shotts agar, a selective medium, was used to isolate A. hydrophila (HiMedia). The plates were incubated for 48 h at room temperature (RT 28 °C). Using differential biochemical assays, all cultures were identified at the species level. For additional molecular characterization, selected A. hydrophila colonies were sub-cultured in peptone water [89].
Table 3.
The list of selective media of pure culture used in the growth of A. hydrophila [87].
| Culture medium | Acronym |
|---|---|
| Pril-xylose-ampicillin modified agar | PXAm |
| Inositol-brilliant green-bile salts agar | IBB |
| Bile salts-brilliant green agars | BBG |
| Rimler-Shotts agar | RS |
| Rimler-Shotts agar (without lysine) | RSm |
| mA agar | mA |
| DNase-toluidine blue-ampicillin agar | DNTA |
| Xylose-sodium deoxycholate-citrate agar | XDC |
| Starch-bile salts agar | SB |
| MacConkey-trehalose agar | McT |
| Dextrin-fuchsin-sulfite agar | DFS |
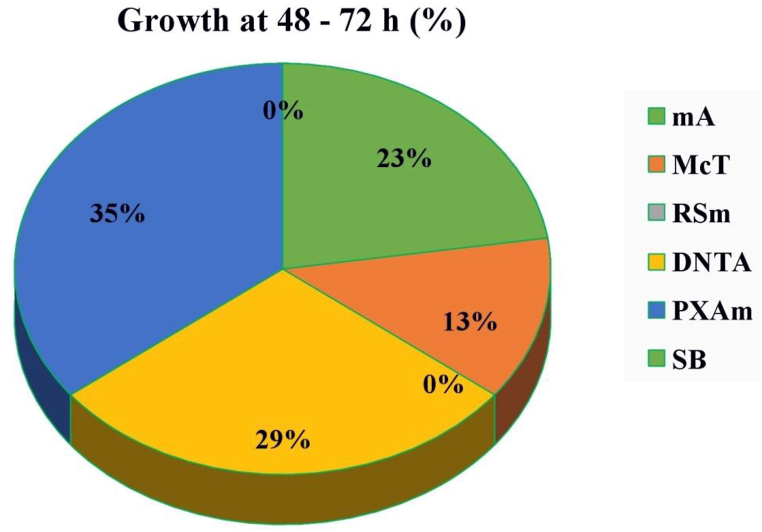
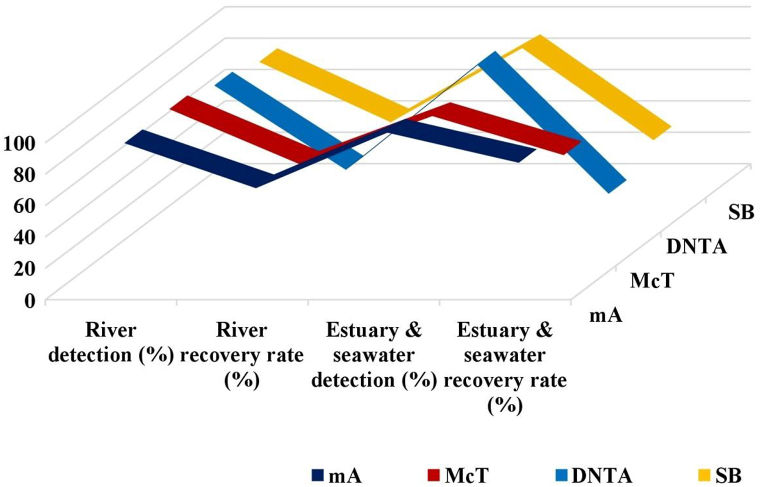
The various strains of A. hydrophila grew in all 6 media tested. On mA, McT, PXAm and DNTA agars, one strain developed after 48 h. However, due to their ability to significantly reduce the growth of both Gram-positive and Gram-negative flora, these four media were the most selective (other than Aeromonas and Plesiomonas spp.) (Fig. 1) [90]. mA agar has a high specificity; the percentage of colonies identified as A. hydrophila was greater than 75% and only 3% of the colonies on this medium had false-negative results (Fig. 2) [90]. The percentage of non-typical colonies identified as A. hydrophila in the other medium ranged from 20 to 33.3% for DNTA and McT agars, respectively, while the percentage of typical colonies that were positively verified ranged from 22.2 to 60% for SB and McT agars, respectively [91].
Fig. 1.
Qualitative growth of A. hydrophila on the selective recovery media [90].
Fig. 2.
Comparison of the efficiency of different media for recovery of A. hydrophila from water samples [90].
The total bacterial count was done on Tryptone soya agar (TSA, Oxoid) plates and Aeromonas-like bacteria were isolated on AIM plates [92]. Small, convex, round, smooth, translucent and yellow colonies were formed by A. hydrophila isolates (Ah1 and Ah12) on both Aeromonas Isolation Agar (AIA) medium and Rimler Shotts (RS) medium. A. hydrophila was a small rod with polar flagella that moved swarmingly and was Gram-negative. A. hydrophila isolates consume sucrose, lactose, fructose, dextrose, glucose, D-maltose, D-galactose, D-ribose, glycerol, sorbitol, trehalose, starch, rhamnose, L-arginine, salicin, D-mannose, amygdalin and arabinose but do not ferment raffinose; A. hydrophila strains do not grow in 4.8% NaCl but do in nutritious broth and other basal media with 0.2% NaCl [65].
4. Gross clinical and pathological symptoms
A. hydrophila has been identified as the causative agent of many symptoms related to gastroenteritis, systemic infections and bacterial endocarditis in humans and other species. A. hydrophila have reportedly been linked to necrotic septicemic and ulcerative disorders in amphibians, reptiles and fishes [93]. It is recognized as an opportunistic pathogen of homeothermic and poikilothermic hosts [89]. A. hydrophila causes disease in fish known as Motile Aeromonas Septicemia (MAS), Hemorrhagic septicemia, ulcer disease or red-sore disease [94]. Bacterial infections cause heavy mortality in both wild and cultured freshwater fish. A. hydrophila, A. sobria and A. caviae are the most prevalent Aeromonas sp. [95]. According to Taylor [96] A. hydrophila and A. sobria are the pathogens that cause Motile Aeromonas septicemia (MAS) in fish and other aquatic species. In West Bengal, Karunasagar et al. [97] investigated outbreaks of infectious dropsy brought on by A. hydrophila in three of the most common species of Indian major carps. Bacterial fish disease, particularly bacterial hemorrhagic septicemia and Motile Aeromonas Septicemia in freshwater fish resulted in substantial losses [9,98,99]. Aeromonas infection was responsible for 45.45% of exotic carp diseases, followed by 6.25% of Indian main carp diseases. Aeromonas spp. was previously described in exotic carp, H. molitrix, C. idella and C. carpio [92,100]. Motile Aeromonas spp. has been isolated from water, healthy or diseased fish, food products, human feces and other clinical/environmental samples [101]. Fish are stressed when the quality of the water deteriorates, which increases their susceptibility to infections from opportunistic pathogens such as Aeromonas species [102,103]. The increasing prevalence of Aeromonas in diseased populations of Indian major carps and exotic carps shows that it is evolving into a significant pathogen as the carp culture system is intensified [9].
The majority of freshwater fish affected by A. hydrophila are catfish, various species of bass and a variety of tropical ornamental fish. A. hydrophila causes infections in fish resulting red mouth, bloated abdomen, blood on the exterior surface and around the anal scale sloughing, surface lesions and septicemia [92,93]. Clinical indications such as loss of balance, abnormal movement, reddish lesions on the fin bases and anal area and a greyish-white lesion that extended up to the caudal fin were observed in each group of intramuscularly injected fish in a moribund state. The liver was found to be enlarged, unsmooth and irregular after the dissection of the freshly dead fish [104]. It is believed to be the cause of fatal hemorrhagic septicemia and epizootic ulcerative syndrome (EUS), which are characterized by internal symptoms like ascetic fluid accumulation, organ damage, anaemia, especially to the kidney and liver as well as external symptoms like blisters, dropsy, abscesses, gill and anal haemorrhages, exophthalmia, scale protrusion, tail rot and fin rot [105,106]. A. hydrophila was isolated from dropsy-infected common crap (C. carpio) in Meghalaya which caused enormous mortality [86]. Saprolegnia declina infection in salmonids, spring viraemia of carp and myxobacterial and other protozoan infections in the larval branchial cavity are only a few of the diseases that A. hydrophila makes worse [107]. Fish mortality caused by A. hydrophila causes significant economic losses in the Southeast Asian fish farming business [19,108]. The skin abnormalities resembled furunculosis, assuming the shape of very big conspicuous bulges filled with clear exudate that, when ruptured, revealed haemorrhagically altered muscle. The skin lesions started as depigmented patches surrounded by a hyperaemic zone with ulcer formation. Inside the abdominal cavity, some fish had exophthalmos, inflammation around the pectoral fins, hyperaemia of the swim-bladder wall and petechial haemorrhages on the liver. Low erythrocyte counts, low haematocrit and haemoglobin levels were used to identify severe anaemia. Lower levels of total protein, cholesterol, triacylglycerol and total calcium, as well as an increase in urea, were found in clinical chemistry examinations of the diseased fish. Among the enzymes and isoenzymes examined, α-hydroxybutyryl dehydrogenase, lactate dehydrogenase, alanine aminotransferase and γ-glutamyl transferase all reported catalytic concentrations exceeding multiples of the normal range [95]. The symptoms vary due to a variety of factors such as the presence or absence of septicemia, organisms virulence and fish resistance to infection and stress factors linked with the fish. The diagnosis of this disease based solely on symptoms is extremely unreliable and may have devastating financial effects on the fish producer due to the variety of symptoms [94].
5. Pathogenicity of A. hydrophila
The A. hydrophila is advocated as an indicator of the presence of harmful chemicals in surface waters, such as phenol, but this was not supported by further investigations. Another potential application of aeromonads as a water quality indicator is the link between aerogenic and anaerogenic strains [109]. The anaerogenic strains of A. hydrophila predominated in sewage-polluted river water, whereas aerogenic strains predominated in unpolluted waters [8,110,111]. A. hydrophila outbreaks are generally thought to be associated with changes in host susceptibility caused by environmental changes such as elevated temperature which is connected to the generation of virulence elements including cytotoxins and hemolysins as well as elevated nitrite levels in farmed fish and hypoxic circumstances [112]. The ability of A. hydrophila to form biofilms, use particular metabolic pathways and regulate the expression of virulence factors via quorum sensing are all examples of virulence factors. The disease is also brought on by the production and/or secretion of virulence factors like hemolysins, cytotoxins, adhesins, proteases and lipases [113]. According to research endotoxin, haemolysin, enterotoxin and cytotoxin are now known to be produced by aeromonads [8,[114], [115], [116], [117], [118], [119], [120]].
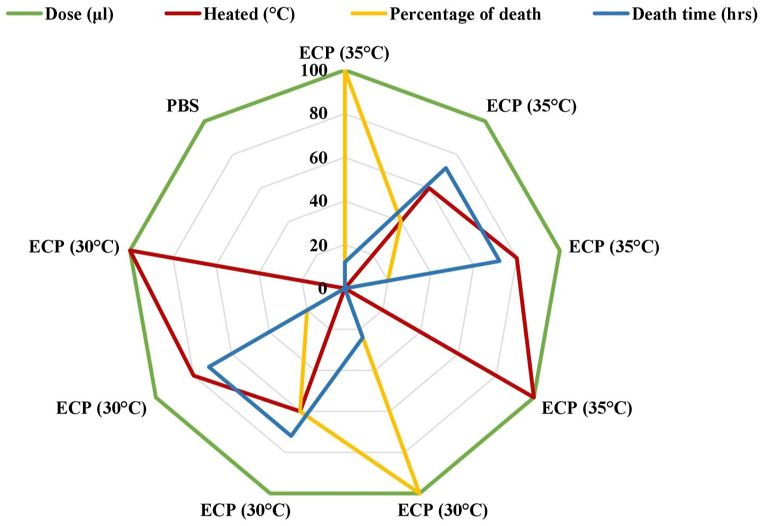
According to Daskalov [15] A. hydrophila pathogenicity and virulence are dependent on its ability to produce components related to gastroenteritis. Endotoxins, exotoxins, siderophores, cytotoxins, adhesins, invasins, S-layers and flagella are examples of these properties. Elastase, collagenase, metalloprotease, enolase, lipase and serine protease are all degradative enzymes found in A. hydrophila spp. that can contribute to virulence [121]. Slime formation, haemolysin, proteolytic activity, antimicrobial peptides, enterotoxin, lipolytic activity, aerolysin, cytosine and gelatinase have all been identified as virulence factors in A. hydrophila. These elements are used by A. hydrophila as a mechanism of defence, survival and pathogenicity establishment [12]. The uncontrollable accumulation of bacterial microcolonies on surfaces that are encased in a polysaccharide matrix is known as a biofilm. Bacterial resistance to conventional antibiotics and chronic infections come from biofilm formation [122]. Bacterial resistance to antimicrobial agents and host defences is provided by biofilms [123]. The primary virulence factors that influence pathogenicity are extracellular toxins (hemolysin, enterotoxin and protease), structural traits (pilli, S-layer and lipopolysaccharide), adhesion and invasion [4,124]. In refrigerated conditions, Aeromonas species can develop and produce toxins, demonstrating that refrigeration is ineffective in controlling the infection [122]. Studies on the proteolytic activity of ECP of A. hydrophila found that the culture grown at 30 °C had the highest proteolytic activity. However, the ECP from the culture grown at 35 °C exhibited only minimal proteolytic activity (Fig. 3) [86]. The proteolytic effect and found that at 28 °C, specific strains of A. hydrophila have the least proteolytic effect [125]. This result was solely due to ECP obtained from cultures grown at various temperatures during incubation [86]. Aeromonas strain causes fluid accumulation in adult rabbit's ligated ileal loops, similar to Vibrio cholerae toxigenic strains [8].
Fig. 3.
Lethal toxicity of A. hydrophila (CAHH14 strain) to rohu [86].
Many environmental conditions like temperature, pH values, salt levels and others influence the production of virulence traits. A. hydrophila, which has been associated with human gastroenteritis, is probably capable of growing in foods at refrigeration temperatures currently thought to be sufficient for preventing the growth of food-borne pathogens [15]. Human disease is most likely caused by adhesion to and colonization of mucosa followed by fluid buildup or epithelial alteration [126]. Studies have shown that pathogens produced toxins more quickly at 28 °C and that the inclusion of 1–5% NaCl or a pH of more or less than 7.2 decreased the formation of hemolysin and cytotoxin [121]. In research, it was found that out of 69 strains of A. hydrophila about 47 strains produce hemolysin titer at 10 °C while only 6 strains produce hemolysin titer at 37 °C. Regardless of the hemolytic titer, 40% (4 strains) of A. hydrophila were enterotoxin after growing at 37 °C, while 30% (3 strains) were enterotoxin after growing at 10 °C [127]. When the bacteria were cultured at 35 °C for 30 h, the largest amount of haemolysin was produced. The bacteria's highest level of proteolytic activity was seen after 36 h of growth at 30 °C [86].
Extracellular Products (ECP) were collected using a modified procedure [128]. Centrifugation at 2800 rpm for 45 min was used to extract the bacterial cells from the culture broth samples. Using the recovered supernatant fluid as a source of crude ECP, the hemolytic and proteolytic activities were assessed [129,130]. The approach was used to determine the hemolytic activity and proteolytic activity of ECP [86]. The rohu was shown to be fatal to the hemolytic and proteolytic toxin generated by A. hydrophila (LD50 1.7 × 104 CFU/ml). Heating ECP reduced its lethality while boiling it at 100 °C for 10 min rendering it completely inactive. This shows that the temperature affected the protease and hemolytic activities of A. hydrophila ECP [125]. Fish mortality is significantly influenced by the heat-labile potential pathogenic component of ECPs (protease and hemolysin) when fish are injected with untreated ECP of A. hydrophila (CAHH14 strain) [131].
6. Antibiotic resistance pattern
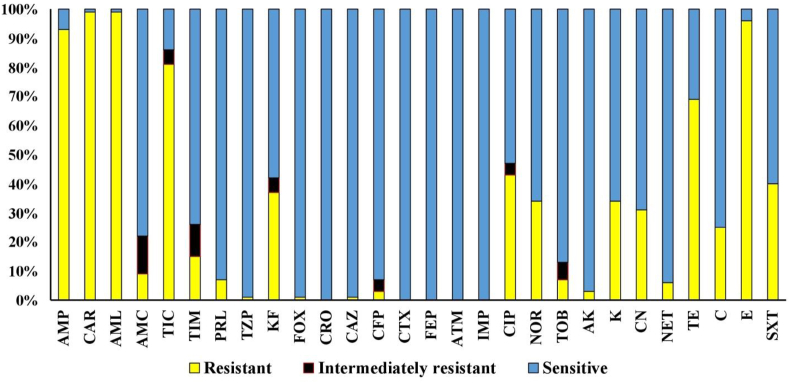
The A. hydrophila isolates were sensitive to sulfamethoxazole, streptomycin, chloramphenicol, neomycin and trimethoprim/sulfamethoxazole [132]. Ampicillin, bacitracin, penicillin, tetracycline and streptomycin were all resistant, whereas erythromycin, gentamycin, kanamycin, nalidixic acid, neomycin and sulfisoxazole were all susceptible. Oxytetracycline, chlortetracycline, tetracycline, neomycin, trimethoprim/sulfamethoxazole and chloramphenicol were all effective against the majority of the isolates [15]. Multiple antibiotic resistances were found in 319 strains of A. hydrophila isolated from fish and prawns [133]. Methicillin and rifampicin resistance was the most common, followed by bacitracin and novobiocin resistance, although chloramphenicol sensitivity was the most common [15]. The A. hydrophila strains was sensitive to azithromycin, ofloxacin, oxytetracycline, doxycycline, streptomycin, chlortetracycline, nitrofurazone and norfloxacin but resistant to ampicillin, amoxicillin, bacitracin, cloxacillin, cefuroxime, co-trimoxazole, cephalexin, erythromycin and flumequine [65]. It was found except Aeromonas diversa, 96% of the Aeromonas spp. tested sensitive for ciprofloxacin (Fig. 4) [7,134]. They found that 63% of the 90 Aeromonas strains isolated from freshwater fish were susceptible to ciprofloxacin. In order to control the bacterial population in India's fields and hatcheries, a variety of antimicrobial drugs (oxytetracycline, ciprofloxacin, nitrofurantoin, furazolidone or chloramphenicol) were used [135].
Fig. 4.
Susceptibility profile (%) to antibiotics of A. hydrophila (n = 67) isolates [7].
The A. hydrophila isolates were resistant to quinolones, aminoglycosides, fluoroquinolones, cephalosporins of the third and fourth generations and other frequently used antibiotics but susceptible to cephalosporins (ceftazidime, cefuroxime, cefpodoxime, ceftriaxone, cefalotin, cefoxitin, cefotaxime and cephalexin), norfloxacin, nitrofurantoin, quinolones, chloramphenicol, tetracycline, kanamycin, aminoglycosides, amoxicillin, sulphamethoxazole, imipenem, streptomycin, oxytetracycline, doxycycline, gentamicin, ticarcillin, ofloxacin, pefloxacin, ciprofloxacin, neomycin, oxacillin, gatifloxacin, amikacin and levofloxacin identified in several global environmental samples, clinical samples and diseased fish samples [65]. Oxysentin, acimox and oxy-D Vet had the lowest, medium and greatest inhibitory zones respectively. The sensitivity of the bacterium A. hydrophila to oxysentin, acimox and oxy-D Vet was determined to be low, medium and extremely sensitive respectively. A prescribed combination of acimox and oxy-D Vet may be used to cure anal erosion. Caudal fin ray loss, ulcerative lesions and hemorrhagic lesions all fully recovered [104]. In Taiwanese isolates of Aeromonas discovered growing resistance to trimethoprim, sulphamethoxazole, tetracyclines, certainly extended-spectrum cephalosporins (ceftriaxone, cefotaxime and cefotaxime) and tobramycin [136]. Cotrimoxazole often works well against Aeromonas species despite neither sulphamethoxazole nor trimethoprim being very powerful against these bacteria, because the two medications work well together [137]. The Aeromonas species (A. hydrophila, A. caviae and A. sobria) found in this investigation were all sensitive to ofloxacin, pefloxacin and ciprofloxacin based on the antibiotic profile. All three species (A. caviae, A. sobria and A. hydrophila) were tetracycline, nitrofurantoin and augmentin resistant, whereas ceftriaxone, gentamycin, cotrimoxazole and amoxicillin were randomly sensitive. Resistance to oxytetracycline is common in environmental Aeromonas isolates [138]. The majority of the isolates in the study were resistant to first-generation quinolones (oxolinic acid and pipemidic acid) but clinically responsive to fluoroquinolones ranging from pefloxacin (54% to ciprofloxacin 98%) [136]. This study supports Aeromonas sp. sensitivity to ciprofloxacin, pefloxacin and ofloxacin. All of the Aeromonas species identified in this investigation were completely sensitive to these antibiotics. The frequency and character of antibiotic resistance differed depending on the source of the strains [139]. Aeromonas spp. resistance to frequently used antibiotics is a growing issue in ornamental fish. Previously, it was discovered that the species Aeromonas was becoming more resistant to β-lactam antibiotics [140,141].
Ampicillin, carbenicillin, amoxicillin, cephalothin and cefoxitin were the least effective β-lactam antibiotics for A. hydrophila, whereas amikacin was the most effective aminoglycoside antibiotic (84%). Furthermore, A. hydrophila exhibited resistance to quinolones (ciprofloxacin and norfloxacin) of around 40% [7]. Compared to other species, A. hydrophila isolates had greater rates of ciprofloxacin and norfloxacin resistance (43% and 34%, respectively). Quinolones are artificial antibiotics that are frequently used as the initial line of treatment for human infections with Aeromonas [137,142]. Fourteen antibacterial agents from 9 different antibiotic groups including cephalosporins, aminoglycosides, tetracyclines, chloramphenicol, nitrofurantoin, fluoroquinolones, sulphonamides, penicillin and polymixin were used to test the 8 isolates of A. hydrophila found in 15 samples of fish taken from retail stores in Mhow city that were tested in-vitro. Cefuroxime, ciprofloxacin, ceftriaxone, cefotaxime, gentamycin, chloramphenicol, nalidixic acid, kanamycin, nitrofurantoin and ofloxacin had the highest sensitivity (100%) followed by co-trimoxazole (62.2%) and oxytetracycline (50%). Antibiotics ampicillin and colistin were both resistant to all of the isolates, that means none of the isolates tested positive for penicillin or the polymyxin group of antibiotics. All A. hydrophila isolates were positive for multiple drug resistance [143].
7. Experimental induction of A. hydrophila infection
The intramuscular injection technique resulted in 100% death at a dosage of 2.8 × 106 CFU/fish and 60% mortality at a dose of 2.8 × 105 CFU/fish of the experimental fish. H. molitrix was found to be sensitive to A. hydrophila as evidenced by 100% mortality at 2.8 × 106 CFU/fish and 60% mortality at 2.8 × 105 CFU/fish. The post-infection mortality days ranged from 2 to 5 days and 4 to 9 days respectively. Through experimental infections in carps (rohu, catla and mrigal) 100% of L. rohita died at a dose of 6.7 × 106 CFU/fish and 80% died at a dose of 6.7 × 105 CFU/fish, with post-infection mortality days ranging from 1 to 4 days and 3 to 11 days respectively [144]. Cirrhinus cirrhosus mortality was 100% at a dosage of 6.7 × 106 CFU/fish and 60% at a dose of 6.7 × 105 CFU/fish, with post-infection mortality days ranging from 2 to 5 days and 4 to 12 days, respectively [104].
The pathogenicity of A. hydrophila, a bacterial isolate found in naturally diseased singhi fish (Heteropneustes fossilis) was tested for pathogenicity against catfishes (H. fossilis and C. batrachus), carps (L. rohita, C. catla and C. cirrhosus) and perch (A. testudineus) with average body weights of 20.4 g for H. fossilis, 25.6 g for C. batrachus, 35.2 g for L. rohita, 25.7 g for C. catla, 30.5 g for C. cirrhosus and 20.3 g for A. testudineus. Intramuscular injections of 6.7 × 106 and 6.7 × 105 CFU/fish were performed. Pathogenicity of injected A. hydrophila was confirmed at 30 °C water temperature by the death of 60 to 100% of all examined fishes within 2 to 11 days. All of the examined fishes had A. hydrophila infections in their livers, kidneys and intestines. According to studies, the bacterial load in catfish livers ranged from 5.5 × 108 CFU/g in H. fossilis to 5.6 × 107 CFU/g in the intestines of C. batrachus. The liver of C. batrachus and the kidney of H. fossilis were found to have the lowest bacterial load 2.4 × 103 CFU/g and 2.2 × 102 CFU/g respectively. The liver of C. catla had 4.9 × 109 CFU/g, the intestine of L. rohita had 7.7 × 108 CFU/g, and the intestine of C. cirrhosus had 5.8 × 108 CFU/g of bacteria respectively. In the kidneys of C. catla, L. rohita and C. cirrhosus, the lowest bacterial loads were 2.7 × 104 CFU/g, 3.3 × 104 CFU/g and 5.6 × 103 CFU/g respectively [144].
Twelve fish from each group were exposed to the pathogenic A. hydrophila strain 018 after a 60-day feeding period (obtained from Aquatic Animal Health Management Division, CIFE, Mumbai). The A. hydrophila was cultured in a BOD incubator for 24 h on nutrient broth at 30 °C before being collected by centrifuging the culture broth at 10,000 rpm for 10 min at 4 °C. The final concentration was maintained at 1.8 × 108 CFU/ml by serial dilution after the cells had been washed three times in sterile PBS (pH 7.4). Each experimental group fish received an intraperitoneal injection of 0.2 ml of bacterial solution. For ten days, all groups were monitored for mortality. A. hydrophila was found to be the cause of mortality when tissues from dead fish were obtained for bacteriological culture. Fish exposed to A. hydrophila after the challenge had damaged hepatocytes, oedema and leucocytic infiltration in parenchymatous tissues, haemosiderosis and acute bleeding in the kidney [88]. Ictalurus punctatus, a channel catfish, was exposed to the pathogen A. hydrophila by abrading its skin and submerging it in a suspension of the pathogen, which caused a lesion to form. Lesions developed that were comparable to those brought on by intraperitoneal (IP) injection [145]. Injecting common infections such as A. hydrophila, Aquaspirillum sp., Pseudomonas sp., Streptococcus sp. and Streptococcus sp. into healthy C. batrachus and Ophiocephalus striatus resulted in mild, mild-to-moderate and severe dermo-muscular necrotic lesions [146]. Anguilla anguilla eels were exposed to extracellular products isolated from A. hydrophila and A. jandaei, which had LD50 values of 107 and 108 CFU/fish respectively and caused degenerative changes and ulceration [147].
8. Isolation and calculation of A. hydrophila
The A. hydrophila was isolated from Thai pangus, it has the bacterial load of 4.8 × 106 to 7.2 × 107 CFU/g in the gut, 2.6 × 106 CFU/g in the liver and 2.4 × 103 to 3.70 × 106 CFU/g in the kidney [113]. A. hydrophila was isolated from H. fossilis [72]; they found that the liver had the greatest bacterial load 2.4 × 107 CFU/g, while the kidney had the lowest 2.1 × 102 CFU/g. The total bacterial load detected in the sampled fish gut, liver and kidney were 1.0 × 105 to 1.5 × 105 CFU/g, 2.7 × 102 to 4.5 × 104 CFU/g and 1.0 × 103 to 2.2 × 103 CFU/g, respectively [92]. Moribund fish liver, kidney and intestine were homogenized and sterile PS was used to make two consecutive decimal dilutions of 10−1 and 10−2 from the stock solution for each organ. To assess the pathogens pathogenesis in the organs of the experimentally infected fish, the colonies that developed were counted using a computerized colony counter [148]. H. fossilis kidney had the lowest bacterial load 2.1 × 102 CFU/g, while the H. fossilis liver had the highest 2.42 × 107 CFU/g bacterial load. When singhi fish were experimentally infected with the selected A. hydrophila isolate (CK602), 100% of the fish died within 1 to 9 days at a dosage of 1.92 × l07 CFU/fish [72]. A haemolysin-negative mutant of A. hydrophila was used to immunize fingerlings of C. catla, L. rohita and C. mrigala. The highest antibody titers were found in C. catla followed by C. mrigala and L. rohita. Fish that had received an immunization showed good resistance to homologous challenges. When faced with heterologous challenges C. mrigala and L. rohita displayed a moderate level of resistance [97].
9. Prevention and control of A. hydrophila
Disinfectants and antimicrobial medicines have shown minimal effectiveness in the prevention or control of aquatic animal diseases. One of the most revolutionary technologies that have emerged in response to these challenges is the use of “immunostimulants” which fill the gap left by vaccines and probiotics [149]. The best way to avoid A. hydrophila infection is to never have it. This may sound absurd, but fish are considerably less susceptible to this disease if stress factors such as handling, stocking levels, diet, transportation and water quality are minimized. To limit the possibility of this disease arising, excellent cleanliness and filtration processes are essential. Treatment should begin as soon as the diagnosis of A. hydrophila infection in fish is established [94]. Using antibiotics to prevent disease and promote growth may lead to the emergence of drug-resistant microorganisms and the buildup of antibiotic residues in fish and the environment [150,151]. Furthermore, chemotherapy has the potential to destroy or disrupt the natural bacteria in the digestive tract, which is helpful to fish [152].
The positive benefits of some beneficial bacteria in aquaculture have been widely established [[153], [154], [155]], these helpful bacteria are referred to as probiotic bacteria. Probiotic bacteria are being used to manage possible infections, which is an alternate technique that is gaining popularity in the aquaculture industry [156]. Probiotics are microorganisms that enhance the host health. They are used in aquaculture to control disease and as supplementary nutrients [157]. To develop a vaccine for trout as well as a detection kit for food-borne diseases caused by A. hydrophila in Korea, the isolation and characterization of A. hydrophila in Korea is required as the initial step. Diverse techniques including PCR, biochemical/physiological assays, randomly amplified polymorphic DNA (RAPD), plasmid profiling and gel electrophoresis of total membrane and extracellular proteins were used to characterize and compare different strains of A. hydrophila to the type strain. Hemolysin, haemagglutinin, cytotoxin, protease and surface array proteins were among the virulence factors [158]. A study indicates that when the fingerlings were intra-peritoneally challenged with A. hydrophila there was a rise in TLC in the control (infected) group, but the levamisole-supplemented groups had a decrease in leucocyte count. This is mostly due to the fish immune systems reaction to the bacterial invasion. The gradual restoration of leucocyte counts to normal in the immunostimulant-supplemented groups may be indicative of the repair of systemic injury [159].
Antimicrobial peptides or proteins (AMPs) are the first line of defence molecules occurring naturally in all multicellular species. AMPs have a significant role in innate host defence in nature. Because they include all of the major AMP types including cathelicidins, hepcidins, defensins, histone-derived peptides and piscidines, fish is regarded as a notable source of antimicrobial peptides. AMPs are thought to be a very promising all-natural antibiotic replacement. The fish peptides have broad-spectrum antibacterial activity, eliminating infections that affect both fish and humans. Additionally, their genes are highly reactive against microorganisms and innate immunostimulatory chemicals and they have immunomodulatory properties. Later studies have shown that several of the unique characteristics of fish peptides such as their capacity to function even in conditions of extremely high salt concentrations, make them promising candidates for development as therapeutic antimicrobials. Numerous biological effects of AMPs have been seen, including the neutralization of endotoxin, immunomodulatory action and stimulation of angiogenesis [160].
When fish were challenged intraperitoneally with A. hydrophila the total serum protein concentration was lowest in the control (infected) group and highest in the D3 group at the end of the experimental trial [161]. Gudding et al. [162] found that total serum protein content was considerably increased in levan-fed common carp fingerlings against A. hydrophila infection, whereas lower values were reported in the control (infected) group. Two antibiotics Remet-30®, a potentiated sulfonamide and Terramycin®, oxytetracycline are the only ones now approved for use in therapy. According to Swann and White [94] terramycin®, oxytetracycline and Remet-30®, a potentiated sulfonamide is the only antibiotics on the market right now (Table 4).
Table 4.
The recommended doses of useful drugs currently used in aquaculture.
| Product | Sponsor | Dosage |
|---|---|---|
| Terramycin® | Pfizer, Inc. | 2.5–3.75 g/100 lb of fish per day for 10 days in a feed |
| Remet-30® | Hoffman-La Roche, Inc. | 50 mg/kg of fish per day for 5 days |
Another approach to using antibiotics is a dip or bath. However, the efficacy or effectiveness of this strategy is debatable. This method has drawbacks such as destroying indoor tank systems biofilters and perhaps preventing the uptake of antibiotics into the fish. Inadequate dose levels, overdose, bacterial drug resistance and the chelation of calcium to hard water in the case of Terramycin® used in a dip or bath are all potential problems with antibiotic therapy. Remember that many fish may be stressed even if they show no symptoms of this disease, and the increased handling required for therapy could be fatal for these species [94]. Fish vaccines have the potential to significantly reduce certain disease-related losses, therefore reducing antibiotic use. As a result, overall unit costs are reduced, and manufacturing is more predictable. Fish vaccines are preferable to antibiotics because they are made of natural biological materials that do not leave a residue on the product or the environment and do not result in the development of a disease-causing organism that is resistant to them; however, there are some drawbacks, such as the decreased value of the cultivated species and the slowed growth rate of some species [20,162].
Multiple injections of β-glucan produced from barley might improve immune response and disease resistance in L. rohita fingerlings against infections caused by opportunistic pathogens A. hydrophila and E. tarda. β-glucans are glucose polymers present in the plant, fungus and bacterium cell walls that have been shown to have immunostimulatory activities in fish [163]. Because they are comparable to fungal or bacterial Gram-negative polysaccharides, fish recognize these polysaccharides as foreign agents. Following exposure, the immune system of fish develops an inflammatory response similar to that of a disease, providing excellent protection against opportunistic infections [164]. Numerous investigations have found that β-glucan increases fish resistance to a variety of bacterial infections by increasing complement and lysozyme levels, as well as improving the phagocytic, respiratory burst and bactericidal activities of fish phagocytes. By administering various doses of β-glucan four times every two weeks, as was shown in the fourth week following the challenge both by I.P. injection and bath immersion, the mortality (%) due to infections with A. hydrophila and E. tarda was significantly reduced (P < 0.05). The group of fish that received 10 mg of β-glucan/kg of body weight four times had the lowest mortality (%) rate [163].
9.1. Probiotics
The possibility of inhibiting two A. hydrophila strains by bacteriocin-producing lactic acid bacteria isolated from retail slices of beef [165]. According to Vescovo et al. [166] A. hydrophila survival in ready-to-use mixed salad greens may be hampered by combinations of carbon dioxide, Lactobacillus casei and low storage temperature. According to Santos et al. [167] and Daskalov et al. [15] Lactococcus lactis sub-sp. Lactis strain 388 displayed inhibitory effects against three strains of A. hydrophila.
9.2. Polyphosphates/NaCl
According to Palumbo et al. [168] A. hydrophila in BHI broth was shown to be inactivated by a mixture of 2% of any polyphosphate (sodium pyrophosphate, sodium tripolyphosphate and hexaphos or sodaphos) and 3.5% NaCl and this inactivation was temperature-dependent. The polyphosphate NaCl mixture inhibited bacterial growth in ground pork during refrigerated storage. According to Velazquez et al. [169] the growth of A. hydrophila was completely inhibited by concentrations between 0.5 and 3.0% of four phosphates (tetrasodium pyrophosphate, sodium acid pyrophosphate, trisodium phosphate and sodium tripolyphosphate) in modified completely defined synthetic medium (mCDS) and cooked ground meat medium. A stronger inhibitory impact (bactericidal and bacteriolytic effects) was produced by sodium acid pyrophosphate (0.5%) [15].
9.3. Heating
D-values (1.5, 0.10 and 0.03 min) were obtained at 51, 57 and 60 °C indicating that such heat methods can provide a significant safety factor in the inactivation of A. hydrophila in liquid egg [15,170].
9.4. High hydrostatic pressure
The A. hydrophila response to high hydrostatic pressure, which ranged from 51 to 304 MegaPascals (MPa), was examined for 15 min. The results showed A. hydrophila has the potential to repair or proliferate after being exposed to pressure in pork [171].
9.5. Smoking
According to Boyle et al. [172] several A. hydrophila strains were sensitive to the concentration of smoke from various types of wood smoke. Fish is traditionally preserved by the cold-smoking method.
10. Control of A. hydrophila by herbal treatment in aquaculture
Antibiotics should not be used routinely during fish culture to reduce disease risk because they may harm the indigenous microflora of juveniles or adults and may increase the possibility of developing antibiotic-resistant bacteria [173]. As a result, eco-friendly disease-prevention methods are needed to support long-term fish culture. Immunostimulants, which improve fish resistance by increasing non-specific defence mechanisms are an intriguing option for disease management. Immunostimulants are a class of biological or synthetic compounds that, when used as an adjuvant with a vaccination, stimulate non-specific defence mechanisms as well as specific immune responses [174]. Although food modification is an excellent method for improving non-specific immunity in fish, research has been conducted to examine the impact of dietary variables on the immune system [88]. The use of herbs to prevent A. hydrophila ulcerative dermatitis, either through dip therapy or by adding herbs into feeds, is another innovative alternative approach to the control of aquaculture disease [175,176]. According to Hao et al. [177] plant extracts (eugenol and pimento extracts) were shown to be the most efficient in suppressing A. hydrophila growth [15,20].
In vivo testing must be carried out to examine the immunomodulatory and disease resistance impacts of plant materials to assess their full potential on fish health and disease resistance. One of the most critical elements in determining the effectiveness and safety of phytotherapy is dosage, which is directly related to the material used. While too low of a dosage may not have the intended impact on fish, too high of a dosage may be toxic and have detrimental consequences on fish development, survival and immunological function [178,179]. One of the factors considered essential to the effectiveness of the experiment is the time duration of treatment. Choosing the ideal treatment time is crucial for obtaining numerous benefits. To maximize the impact of plant-enriched diets on fish immunity and disease resistance, several researchers have focused on finding the ideal treatment time. On the other hand, environmentally friendly ingredients should be recommended (powdered plants or extracts with low-toxicity solvents). According to the plant and the type of material used, the dose of the plant provides an essential characteristic that must be evaluated carefully [[180], [181], [182]] (Table 5).
Table 5.
Disease resistance of various medicinal plants against various fish pathogens used in aquaculture.
| Medicinal plants | Common name | Against fish | Pathogen | Disease resistance | References |
|---|---|---|---|---|---|
| Bougainvillea glabra | Paper flower | Cyprinus carpio | Aeromonas hydrophila | yes | [183] |
| Allium hirtifolium | Mooseer | Oncorhynchus mykiss | Streptococcus iniae | yes | [184] |
| Andrographis paniculata | King of bitters | Pangasianodon hypopthalmus | Aeromonas hydrophila | yes | [185] |
| Eichhornia crassipes | Water hyacinth | Oncorhynchus mykiss | Streptococcus iniae | yes | [186] |
| Ginkgo biloba | Maidenhair trees | Cyprinus carpio | Aeromonas hydrophila | yes | [187] |
| Sargassum angustifolium | Narrow leaf weed | Oncorhynchus mykiss | Yersinia rukeri | Yes | [188] |
| Aloe barbadensis | Aloe vera | Oncorhynchus mykiss | Saprolegnia parasitica | Yes | [38] |
| Melocanna baccifera | Muli bamboo | Labeo rohita | Saprolegnia parasitica | Yes | [43] |
| Thymus vulgaris | Common thyme | Cyprinus carpio | Saprolegnia spp. | Yes | [189] |
| Phyllanthus niruri | stonebreaker | Oreochromis mossambicus | Vibrio harveyi | Yes | [190] |
| Urtica dioica | Stinging nettle | Labeo victorianus | Aeromonas hydrophila | Yes | [182] |
| Ocimum sanctum | Tulsi | Labeo rohita | Aeromonas hydrophilia | Yes | [47] |
| Euphorbia hirta | Asthma-plant | Cyprinus carpio | Aeromonas hydrophila | Yes | [191] |
| Thymus vulgaris | Common thyme | Oreochromis mossambicus | Streptococcus iniae | Yes | [192] |
| Sophora flavescens | Kushen | Oreochromis niloticus (GIFT) | Streptococcus agalactiae | Yes | [193] |
| Cynodon dactylon | Bermuda grass | Catla catla | Aeromonas hydrophilia | Yes | [194] |
| Lactuca indica | Indian lettuce | Epinephelus bruneus | Streptococcus iniae | Yes | [195] |
| Withania somnifera | Ashwagandha | Labeo rohita | Aeromonas hydrophila | Yes | [53] |
| Zingiber officinale | Ginger | Oncorhynchus mykiss | Aeromonas hydrophila | Yes | [196] |
| Aegle marmelos | Golden apple | Oreochromis mossambicus | Vibrio harveyi | Yes | [197] |
| Solanum trilobatum | Purple fruited pea eggplant | Oreochromis mossambicus | Aeromonas hydrophilia | Yes | [198] |
| Piper longum | Indian long pepper | Epinephalus tauvina | Vibrio harveyi | Yes | [199] |
| Echinacea purpurea | Purple coneflower | Oreochromis niloticus | Pseudomonas fluorescens | Yes | [200] |
| Tinospora cordifolia | Guduchi | Oreochromis mossambicus | Aeromonas hydrophila | No | [201] |
| Allium sativum | Garlic | Oreochromis niloticus | Aeromonas hydrophila | Yes | [202] |
Neem, Azadirachta indica is an Indian plant that has been researched extensively across the world. In India, neem is known as “Sarva roga nivarak” or “healer of all ailments” and is considered an important part of the Ayurvedic tradition. According to research, the water-soluble portion of the alcoholic extract of A. indica leaves showed hypoglycemic, hypolipidemic, hepatoprotective, antifertility, hypotensive and anti-serotonin activities. Neem A. indica tree oil possesses antibacterial properties that are effective against a variety of Gram-positive and Gram-negative bacteria, including strains of Mycobacterium TB and streptomycin-resistant bacteria [203]. Some of the bioactive substances that give neem its antibacterial effects include azadirachtin, nimbidinin, nimbinin, nimbidic acid, nimbidin, nimbin, nimbolide, margolone, isomargolonone, margolonone, tetra-notriterpenoids and limnoids [204,205]. The most well-known biopesticide is A. indica, which has been categorized by WHO/UNEP as a naturally occurring pesticide with “high” environmental effects. Myxobolasis, trichodinosis, gyrodactylosis, argulosis, scuticocliates and other parasite diseases in farmed tropical freshwater fish have all been treated with natural remedies including plant extracts [206]. Azadirachtin is beneficial against Argulus spp. [207]. and its influence on physiological and serum biochemical markers in Carassius auratus has also been studied [208]. Fish treated with azadirachtin exhibited significantly higher TEC, TLC, total Ig, total protein, NBT activity, serum lysozyme activity and myeloperoxidase levels than the control group (P < 0.05) in all treatment groups. Similar results were obtained for SGOT, SGPT and blood glucose levels, however, PCV and Hb did not differ substantially (P < 0.05) between the treatment and control groups. Azadirachtin exhibited considerably (P < 0.05) improved relative percentage survival (42.60%) against A. hydrophila infection at a dosage of 4 g/kg compared to the control. Azadirachtin EC 25% (4 g/kg) was shown to have increased serum lysozyme, NBT activity, leucocyte counts, protein profiles and resistance to A. hydrophila infection in this study, suggesting that it might be used as an immunostimulant in aquaculture [149].
Many synthetic and herbal immunostimulants have been found to improve fish immunological health by increasing phagocytic, lysozyme and complement activities as well as immunoglobulin levels in response to several causative extremities [209]. Traditional medicine has used a variety of plants to treat and control several diseases [210]. According to reports, natural plant products with active principal components like flavonoids, alkaloids, pigments, terpenoids, steroids, essential oils and phenolics have appetite-stimulating, anti-stress, growth-promoting, tonic immunostimulatory and anti-microbial properties in finfish and shrimp larviculture [149,211]. The growing interest in the use of herbal immunostimulants to improve fish defence systems and protect them from diseases is relatively new. There are many different types of herbal plants, but the Ocimum sanctum (Tulsi) is regarded as the “Queen of Herbs” and its medicinal benefits are well-documented in Hindu mythology. The bioactive principle in O. sanctum leaf extracts such as ursolic acid, oleanolic acid and saligenin have immunomodulatory properties [212]. Eugenol, methyl eugenol and caryophyllene are among the several components found in tulsi O. sanctum leaves in addition to water-soluble phenolic compounds [47,213]. After being exposed to A. hydrophila, the control group displayed significantly damaged hepatocytes, oedema and leucocytic infiltration in parenchymatous tissues, as well as severe bleeding and haemosiderosis in the kidney. In contrast, the T5 group supplemented with 1.25% levan only experienced mild renal tubule deterioration. The T5 group shows the highest relative survival percentage of juveniles after being challenged with A. hydrophila followed by the T4 group [88].
The plant species that have shown the greatest promise for usage in the aquaculture industry are garlic (Allium sativum), ginger (Zingiber officinale), pomegranate (Punica granatum), Bermuda grass (Cynodon dactylon) and ashwagandha (Withania somnifera). Allicin and ajoene, two components of pure garlic have been found to have an impact on aquaculture and to be effective against harmful microorganisms (A. hydrophila, fish protozoa Spironucleus vortens and Ichthyophthirius multifiliis) by stimulating the immune system [214]. Pomegranates contain a variety of phytochemicals, such as the bioactive polyphenol ellagitannins, which have antioxidant and anti-inflammatory properties. The chemical makeup of C. dactylon includes tannins (catechins), phenolic substances (gallic acid), flavonoids (quercetin) and anthocyanins (cyanidin). Bermuda grass (C. dactylon) has antiviral, antiparasitic, immunostimulant, antibacterial and growth-regulating properties in fish and shellfish [180,215,216]. Various characteristics of W. somnifera include antiviral, antibacterial, growth-promoting effects and immunostimulant [53]. Along with certain sesquiterpenoids and zingiberene as the primary component, ginger is made up of a mixture of zingerone, shogaols and gingerols (Table 6) [250].
Table 6.
List of medicinal plant species wherein plant part used, their doses and experimental conditions developed through the exposure of various treatments and optimization strategies.
| Medicinal plant species | Common name | Part used | Doses | Experimental conditions | Fish used | References |
|---|---|---|---|---|---|---|
| Asparagus racemosus | Shatavari | Root extract | 100 mg/kg | FRP tanks | Labeo rohita | [217] |
| Achyranthes aspera | Prickly chaff flower | Leaf and seed powder | 0.5% | Aquarium | Clarias batrachus | [218] |
| Azadirachta indica | Neem | Leaf extracts | 5, 7 and 10% | Tanks | Oncorhynchus mykiss | [219] |
| Allium sativum | Garlic | Root powder | 0.5, 1.5, 3 and 10% | Fibre pond | Lates calcarifer | [220] |
| Nymphaea alba | Water lily | Whole plant | 10, 20 and 30% | Plastic aquaria | Clarias gariepinus | [221] |
| Phyllanthus emblica | Amla | Fruit extract | 20 mg/kg | Tanks | Oreochromis niloticus | [222] |
| Thymus vulgaris | Thyme | Flower oil | 2% | Fiberglass tank | Oncorhynchus mykiss | [223] |
| Zingiber officinale | Ginger | Rhizomes of ginger | 5 g, 10 g, 15 g, and 20 g/kg | Semi-intensive culture system | Labeo rohita | [224] |
| Artemisia absinthium | Common wormwood | Aqueous extract | 0.5, 1 and 1.5% | Concrete tank | Cyprinus carpio | [225] |
| Coffea arabica | Coffee | Coffee silver skin | 10, 20, 40 and 80 g/kg | Biofloc system | Oreochromis niloticus | [34] |
| Psidium guajava | Guava | Leaves extracts | 100, 150, 200 and 250 leaves mg/kg | Plastic tanks | Cyprinus carpio | [226] |
| Nigella sativa | Black seed | Seeds powder | 1 and 2.5% | Glass aquaria | Labeo rohita | [36] |
| Achyranthes aspera | Prickly chaff flower | Leaf and seed powder | 0.25, 0.5% leaves and 0.5% seeds | Hapa (pond) | Labeo rohita | [227] |
| Isatis tinctoria | Woad | Leaves extract | 1, 1.5, 2 & 2.5% | Tanks | Pseudotropheus acei | [228] |
| Radix bupleuri | Chaihu | Root extract | 200–800 mg/kg | Cage | Epinephelus lanceolatus and Epinephelus fuscoguttatus | [229] |
| Ziziphus jujube | Fruit extract | Fruit extract | 0.25, 0.5 and 1% | Tank | Cyprinus carpio | [230] |
| Citrus sinensis | Common jujube | Peels | 5, 10 and 29 g/kg | Biofloc system | Oreochromis niloticus | [231] |
| Curcuma longa | Turmeric | Root powder | 2.0 g/kg | Fiberglass tank | Cyprinus carpio | [232] |
| Mespilus germanica | Spanish cherry | Leaves extract | 0.25, 0.5 and 1% | Fiberglass tank | Cyprinus carpio | [233] |
| Urtica dioica | Stinging nettle | Plant methanolic extracts | 0.1 and 0.5 g/kg | Aquarium | Oncorhynchus mykiss | [234] |
| Musa acuminata | Banana | Peel flour | 0, 1, 3, 5 and 7% | Plastic tank | Labeo rohita | [235] |
| Chlorophytum borivilianum | Safed musli | Root (Polysaccharide) | 0.4% | Plastic quarantine tank | Labeo rohita | [236] |
| Ocimum sanctum | Holy basil or Tulsi | Leaf extracts | 0.05, 0.1, 0.2, 0.5 and 1% | Rectangular plastic tub | Labeo rohita | [47] |
| Mentha piperita | Peppermint | Leaves extract | 1, 2, 3, 4 and 5 g/kg | Round blue tanks | Lates calcarifer | [237] |
| Euphorbia hirta | Hairy spurge | Leaf extract | 5, 10, 20, 25 and 50 g/kg | Tanks | Cyprinus carpio | [191] |
| Nigella sativa | Black cumin | Seed oil | 1, 2, 3% | Aquarium | Oncorhynchus mykiss | [238] |
| Sophora flavescens | Chinese medicinal herb | Root powder | 0.025, 0.05, 0.1, 0.2 and 0.4% | Concrete tanks | Oreochromis niloticus | [193] |
| Viscum album | Mistletoe | Leaf extract | 50 mg/kg | FRP tank | Oreochromis niloticus | [239] |
| Lindera aggregate | Chinese spicebush | Root | 60 mg/l | Glass aquarium | Carassius auratus | [50] |
| Pseudolarix kaempferi | Golden larch | Leaf extract | 12.5 and 25 mg/ml | Glass petri dishes | Carassius auratus eggs | [240] |
| Eriobotrya japonica | Japanese plum | Leaf extract | 0.1, 1 and 2% | Recirculating cement tanks | Epinephelus bruneus | [241] |
| Prunella vulgaris | Carpenter's herb | Ethanol extract | 0.01, 0.1 and 1% | Recirculating cement tanks | Paralichthys olivaceus | [242] |
| Aegle marmelos | Bel | Leaf extract | 5 g/kg | Tanks | Cyprinus carpio | [243] |
| Datura metel | Dhatura | Leaf extract | 100 mg/ml | FRP tank | Ornamental fish | [244] |
| Withania somnifera | Ashwagandha | Root powder | 2 g/kg | FRP tank | Labeo rohita | [53] |
| Camellia sinensis | Green tea | Leaf powder | 0.5 g/kg | Fiberglass tank | Oreochromis niloticus | [245] |
| Rheum officinale | Indian rhubarb | Anthraquinone extract (Bail) | 0.05, 0.1, 0.2, & 0.4% | Concrete tanks | Macrobrachium rosenbergii | [246] |
| Lonicera japonica | Japanese honeysuckle | Plant extract | 0.1% and 0.1% with 0.05% boron | Recirculation system | Oreochromis niloticus | [247] |
| Eclipta alba | Bhringraj | Leaves aqueous extract | 0.01, 0.1 and 1% | FRP tanks | Oreochromis mossambicus | [248] |
| Cinnamomum cassia | Cinnamon | Bark extract | 75.8–189.6 μg/ml | FRP tank | Cyprinus carpio | [249] |
11. Other plants and perspectives
Some algae and some mushrooms have also been researched for their competency in aquaculture because they are considered a rich source of bioactive molecules; the vast majority of algae showed high antibacterial properties and some showed immunostimulant, antiparasitic, antiviral and antifungal properties [251]. Red alga Asparagopsis taxiformis famous to secrete a variety of halogenated metabolites, showed antifungal, antibacterial and antiparasitic properties against several fish pathogens [252,253]. A. taxiformis improved the immune system of Penaeus monodon and were successful in the therapeutics of vibriosis in P. monodon, they found fascinating properties of different marine organisms such as sponges, which can repress quorum sensing of marine pathogenic bacteria such as Vibrio harveyi, it represents that they can be used as the better option of medication for the organic aquaculture (Table 7). [[254], [255], [256]].
Table 7.
List of unused quality medicinal plants in aquaculture.
| Scientific name | Common name |
|---|---|
| Swertia chiraita | Chiraita |
| Gymnema sylvestre | Gudmar |
| Commiphora wightii | Guggul |
| Lawsennia iermis | Henna/Mehdi |
| Plumbago zeylanica | Swet chitrak |
| Plumbago indica | Rakta chitrak |
| Terminalia chebula | Harida |
| Andrographis paniculata Fam | Kalmegh/Bhui neem |
| Saraca asoca | Ashok |
| Solanum nigrum | Makoi |
| Santalum album | Sandal wood |
| Casia augustifolia | Senna |
| Terminalia bellerica | Bahada |
12. Conclusions
Infectious diseases like Motile Aeromonas Septicemia (MAS) are a primary obstacle to the development and sustainability of the aquaculture industry because they cause economic harm, limit productivity and require the use of control measures that are often very expensive. However, overuse of antibiotics and other synthetic pharmaceuticals leads to the development of antibiotic-resistant strains and the accumulation of drug residues in fish tissues and water which could be hazardous to the environment and unsafe to consumers. While effective vaccine development for the number of fish pathogens is usually an expensive and time-consuming process. In addition to vaccination and conventional medications, due to the presence of potent bioactive compounds medicinal plant-derived products appear to be a promising tool for enhancing growth, survival, health status, innate and immune responses, as well as disease resistance in aquaculture. They appear to be administered to fish without causing any negative side effects, unlike chemotherapeutics. Additionally, they are inexpensive, easily available and biocompatible.
Further investigation is strongly recommended to conduct additional research to determine the ideal administration doses and timings as well as to isolate, characterize and quantify the bioactive compounds found in plants and phytoextracts to determine the most potent compounds/metabolites that could be used in new natural formulations for use in fish. Additionally, studies on their mechanism of action, the stability of plant components in aquatic environments, the digestibility in fish, as well as in vitro and in vivo toxicity testing are necessary for their safe utilization. This review article shows the efficacy of phytotherapy in aquaculture which will benefit fish farmers, researchers and pharmaceutical firms.
Author contribution statement
Anurag Semwal: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Avdhesh Kumar: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Neelesh Kumar: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of interest's statement
The authors declare no conflict of interest.
Contributor Information
Anurag Semwal, Email: anuragsemwal479@gmail.com.
Avdhesh Kumar, Email: avdheshkumar.fisheries@gbpuat-tech.ac.in.
Neelesh Kumar, Email: neeleshrajpoot74@gmail.com.
References
- 1.Cipriano R.C., Bullock G.L., Pyle S.W. Division of Fishery Research, U.S. Fish & Wildlife Publication; Washington DC: 1984. Aeromonas Hydrophila and Motile Aeromonad Septicemias of Fish.https://digitalcommons.unl.edu/usfwspubs/134 [Google Scholar]
- 2.Figueras M.J., Aldea M.J., Fernández N., Aspíroz C., Alperi A., Guarro J. Aeromonas hemolytic uremic syndrome. A case and a review of the literature. Diagn. Microbiol. Infect. Dis. 2007;58(2):231–234. doi: 10.1016/j.diagmicrobio.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Torres M.J.M., Peterson J.M., Wolf S.E. Detection of infection and sepsis in burns. Surg. Infect. 2021;22(1):20–27. doi: 10.1089/sur.2020.348. [DOI] [PubMed] [Google Scholar]
- 4.Janda J.M., Abbott S.L. The genus Aeromonas: taxonomy, pathogenicity and infection. Clin. Microbiol. Rev. 2010;23(1):35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matyar F., Kaya A., Dinçer S. Distribution and antibacterial drug resistance of Aeromonas spp. from fresh and brackish waters in Southern Turkey. Ann. Microbiol. 2007;57(3):443–447. doi: 10.1007/BF03175087. [DOI] [Google Scholar]
- 6.Martinez-Murcia A.J., Saavedra M.J., Mota V.R., Maier T., Stackebrandt E., Cousin S. Aeromonas aquariorum sp. nov., isolated from aquaria of ornamental fish. Int. J. Syst. Evol. Microbiol. 2008;58(5):1169–1175. doi: 10.1099/ijs.0.65352-0. [DOI] [PubMed] [Google Scholar]
- 7.Dias C., Mota V., Martinez-Murcia A., Saavedra M.J. Antimicrobial resistance patterns of Aeromonas spp. isolated from ornamental fish. J. Aquacult. Res. Dev. 2012;3(3) doi: 10.4172/2155-9546.1000131. [DOI] [Google Scholar]
- 8.Kaper J.B., Lockman H., Colwell R.R., Joseph S.W. Aeromonas hydrophila: ecology and toxigenicity of isolates from an estuary. J. Appl. Bacteriol. 1981;50(2):359–377. doi: 10.1111/j.1365-2672.1981.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal K.B., Mukherjee D., Guchhait A., Dash G. Phenotypic and molecular identification of bacterial species in Indian major carps and exotic carps from south 24 Parganas, West Bengal, India. Int. J. Curr. Microbiol. Appl. Sci. 2018;7(1):534–547. doi: 10.20546/ijcmas.2018.701.06. [DOI] [Google Scholar]
- 10.Odeyemi O.A., Ahmad A., Usup G. In-vitro antimicrobial activity of Aeromonas spp isolated from estuary using different screening protocols. Int. J. Pharma Sci. Res. 2012;3(2):428. [Google Scholar]
- 11.Radu S., Ahmad N., Ling F.H., Reezal A. Prevalence and resistance to antibiotics for Aeromonas species from retail fish in Malaysia. Int. J. Food Microbiol. 2003;81(3):261–266. doi: 10.1016/S0168-1605(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 12.Asmat A., Gires U. Proceedings of the Regional Symposium on Environment and Natural Resources. Vol. 1. 2002. The occurrence of aerolysin-positive Aeromonas hydrophila strains in sea water and associated with marine copepods; pp. 495–502. Malaysia. [Google Scholar]
- 13.Evangelista-Barreto N.S., Carvalho F.C.T.D., Vieira R.H.S., Dos Reis C.M.F., Macrae A., Rodrigues D.D.P. Characterization of Aeromonas species isolated from an estuarine environment. Braz. J. Microbiol. 2010;41:452–460. doi: 10.1590/S1517-83822010000200027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gracey M., Burke V., Robinson J. Aeromonas-associated gastroenteritis. Lancet. 1982;320(8311):1304–1306. doi: 10.1016/S0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- 15.Daskalov H. The importance of Aeromonas hydrophila in food safety. Food Control. 2006;17(6):474–483. doi: 10.1016/j.foodcont.2005.02.009. [DOI] [Google Scholar]
- 16.Pippy J.H., Hare G.M. Relationship of river pollution to bacterial infection in salmon (Salmo salar) and suckers (Catostomus commersoni) Trans. Am. Fish. Soc. 1969;98(4):685–690. doi: 10.1577/1548-8659(1969)98[685:RORPTB]2.0.CO;2. [DOI] [Google Scholar]
- 17.Shotts E.B., Gaines J.L., Martin L., Prestwood A.K. Aeromonas-induced deaths among fish and reptiles in a eutrophic inland lake. J. Am. Vet. Med. Assoc. 1972;161(6):603–607. [PubMed] [Google Scholar]
- 18.Shome R., Shome B.R. A typical chronic form of Aeromonas hydrophila infection in Indian major carp, Catla catla, from Andaman. Curr. Sci. 1999;76(9):1188–1190. [Google Scholar]
- 19.Thampuran N., Surendran P.K., Mukundan M.K., Gopakumar K. Bacteriological studies on fish affected by epizootic ulcerative syndrome (EUS) in Kerala, India. Asian Fish Sci. 1995;8(2):103–111. doi: 10.33997/j.afs.1995.8.2.001. [DOI] [Google Scholar]
- 20.Harikrishnan R., Balasundaram C. Modern trends in Aeromonas hydrophila disease management with fish. Rev. Fish. Sci. 2005;13(4):281–320. doi: 10.1080/10641260500320845. [DOI] [Google Scholar]
- 21.Snieszko S.F. The effects of environmental stress on outbreaks of infectious diseases of fishes. J. Fish. Biol. 1974;6(2):197–208. doi: 10.1111/j.1095-8649.1974.tb04537.x. [DOI] [Google Scholar]
- 22.Grizzle J.M., Kiryu Y. Histopathology of gill, liver, pancreas and serum enzyme levels of channel catfish infected with Aeromonas hydrophila complex. J. Aquat. Anim. Health. 1993;5(1):36–50. doi: 10.1577/1548-8667(1993)005<0036:HOGLAP>2.3.CO;2. [DOI] [Google Scholar]
- 23.Leung K.Y., Yeap I.V., Lam T.J., Sin Y.M. Serum resistance as a good indicator for virulence in Aeromonas hydrophila strains isolated from diseased fish in South‐East Asia. J. Fish. Dis. 1995;18(6):511–518. doi: 10.1111/j.1365-2761.1995.tb00355.x. [DOI] [Google Scholar]
- 24.Chakraborty S.B., Hancz C. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev. Aquacult. 2011;3(3):103–119. doi: 10.1111/j.1753-5131.2011.01048.x. [DOI] [Google Scholar]
- 25.Citarasu T. Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult. Int. 2010;18(3):403–414. doi: 10.1007/s10499-009-9253-7. [DOI] [Google Scholar]
- 26.Galina J., Yin G., Ardo L., Jeney Z. The use of immunostimulating herbs in fish. An overview of research. Fish Physiol. Biochem. 2009;35:669–676. doi: 10.1007/s10695-009-9304-z. [DOI] [PubMed] [Google Scholar]
- 27.Bulfon C., Volpatti D., Galeotti M. Current research on the use of plant derived products in farmed fish. Aquacult. Res. 2015;46(3):513–551. doi: 10.1111/are.12238. [DOI] [Google Scholar]
- 28.Torres-León C., Ramírez F.R., Aguirre-Joya J.A., Ramírez-Moreno A., Chávez-González M.L., Aguillón-Gutierrez D.R., Camacho-Guerra L., Ramírez-Guzmán N., Vélez S.H., Aguilar C.N. Medicinal plants used by rural communities in the arid zone of Viesca and Parras Coahuila in Northeast Mexico. Saudi Pharmaceut. J. 2023;31(1):21–28. doi: 10.1016/j.jsps.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y.T., Cheng E.H.C., Wang H.Y., Zhang L.H.L., Lin S.Y., Dong T.T.X., Duan R., Qin Q.W., Wang W.X., Tsim K.W.K. The extract from aerial part of Scutellaria baicalensis regulates gut microbiota in rabbit fish: replacement of antibiotic fighting against pathogenic bacteria. Aquaculture. 2023;565 doi: 10.1016/j.aquaculture.2022.739140. [DOI] [Google Scholar]
- 30.Imperatore R., Orso G., Facchiano S., Scarano P., Hoseinifar S.H., Ashouri G., Guarino C., Paolucci M. Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture. 2023;563 doi: 10.1016/j.aquaculture.2022.738878. [DOI] [Google Scholar]
- 31.Cheng C., Park S.C., Giri S.S. Effect of Pandanus tectorius extract as food additive on oxidative stress, immune status, and disease resistance in Cyprinus carpio. Fish Shellfish Immunol. 2022;120:287–294. doi: 10.1016/j.fsi.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Amri A., Bouraoui Z., Balbuena-Pecino S., Capilla E., Gharred T., Haouas Z., Guerbej H., Hosni K., Navarro I., Jebali J. Dietary supplementation with Aloe vera induces hepatic steatosis and oxidative stress together with a disruption of cellular signaling pathways and lipid metabolism related genes' expression in gilthead sea bream (Sparus aurata) Aquaculture. 2022;559 doi: 10.1016/j.aquaculture.2022.738433. [DOI] [Google Scholar]
- 33.Hoseini S.M., Mirghaed A.T., Iri Y., Hoseinifar S.H., Van Doan H., Reverter M. Effects of dietary Russian olive, Elaeagnus angustifolia, leaf extract on growth, hematological, immunological, and antioxidant parameters in common carp, Cyprinus carpio. Aquaculture. 2021;536 doi: 10.1016/j.aquaculture. [DOI] [Google Scholar]
- 34.Van Doan H., Lumsangkul C., Hoseinifar S.H., Harikrishnan R., Balasundaram C., Jaturasitha S. Effects of coffee silverskin on growth performance, immune response, and disease resistance of Nile tilapia culture under biofloc system. Aquaculture. 2021;543 doi: 10.1016/j.aquaculture.2021.736995. [DOI] [Google Scholar]
- 35.Harikrishnan R., Thamizharasan S., Devi G., Van Doan H., Kumar T.T.A., Hoseinifar S.H., Balasundaram C. Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates immuno-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol. 2020;106:675–684. doi: 10.1016/j.fsi.2020.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Latif M., Faheem M., Hoseinifar S.H., Van Doan H. Dietary black seed effects on growth performance, proximate composition, antioxidant and histo-biochemical parameters of a culturable fish, rohu (Labeo rohita) Animals. 2020;11:48. doi: 10.3390/ani11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farahmandfar R., Kenari R.E., Asnaashari M., Shahrampour D., Bakhshandeh T. Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci. Nutr. 2019;7(2):465–475. doi: 10.1002/fsn3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehrabi Z., Firouzbakhsh F., Rahimi-Mianji G., Paknejad H. Immunostimulatory effect of Aloe vera (Aloe barbadensis) on non-specific immune response, immune gene expression, and experimental challenge with Saprolegnia parasitica in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2019;503:330–338. doi: 10.1016/j.aquaculture.2019.01.025. [DOI] [Google Scholar]
- 39.Gedikoğlu A., Sökmen M., Çivit A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019;7(5):1704–1714. doi: 10.1002/fsn3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eruygur N., Koçyiğit U.M., Taslimi P., Ataş M., Tekin M., Gülçin İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. South Afr. J. Bot. 2019;120:141–145. doi: 10.1016/j.sajb.2018.04.001. [DOI] [Google Scholar]
- 41.Krishna S., Chandrasekaran S., Dhanasekar D., Perumal A. GCMS analysis, antioxidant and antibacterial activities of ethanol extract of Anisomeles malabarica (L.) R.Br. ex. Sims leaves. Asian J. Pharm. Pharmacol. 2019;5:180–187. doi: 10.31024/ajpp.2019.5.1.26. [DOI] [Google Scholar]
- 42.Scavo A., Rial C., Varela R.M., Molinillo J.M.G., Mauromicale G., Macias F.A. Influence of genotype and harvest time on the Cynara cardunculus L. sesquiterpene lactone profile. J. Agric. Food Chem. 2019;67(23):6487–6496. doi: 10.1021/acs.jafc.9b02313. [DOI] [PubMed] [Google Scholar]
- 43.Khan M.I.R., Saha R.K., Saha H. Muli bamboo (Melocanna baccifera) leaves ethanolic extract a non-toxic phyto-prophylactic against low pH stress and saprolegniasis in Labeo rohita fingerlings. Fish Shellfish Immunol. 2018;74:609–619. doi: 10.1016/j.fsi.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 44.Shirazi M. In vivo biological investigation of methanolic extract of Thymus linearis whole plant. Am. J. Ethnomed. 2018;5(1–2):1–5. doi: 10.21767/2348-9502.10002. [DOI] [Google Scholar]
- 45.Laith A.A., Mazlan A.G., Effendy A.W., Ambak M.A., Nurhafizah W.W.I., Alia A.S., Jabar A., Najiah M. Effect of Excoecaria agallocha on non-specific immune responses and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Res. Vet. Sci. 2017;112:192–200. doi: 10.1016/j.rvsc.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Adel M., Amiri A.A., Zorriehzahra J., Nematolahi A., Esteban M.Á. Effects of dietary peppermint (Mentha piperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish (Rutilus frisiikutum) Fish Shellfish Immunol. 2015;45(2):841–847. doi: 10.1016/j.fsi.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Das R., Raman R.P., Saha H., Singh R. Effect of Ocimum sanctum Linn. (Tulsi) extract on the immunity and survival of Labeo rohita (Hamilton) infected with Aeromonas hydrophila. Aquacult. Res. 2015;46(5):1111–1121. doi: 10.1111/are.12264. [DOI] [Google Scholar]
- 48.Hu Y., Ji J., Ling F., Chen Y., Lu L., Zhang Q., Wang G. Screening medicinal plants for use against Dactylogyrus intermedius (Monogenea) infection in goldfish. J. Aquat. Anim. Health. 2014;26(3):127–136. doi: 10.1080/08997659.2014.902872. [DOI] [PubMed] [Google Scholar]
- 49.Hassanin M.E., Hakim Y., Badawi M.E. Dietary effect of ginger (Zingiber officinale Roscoe) on growth performance, immune response of Nile tilapia (Oreochromis niloticus) and disease resistance against Aeromonas hydrophila. Abbassa. Int. J. Aqua. 2014;7:35–52. [Google Scholar]
- 50.Ji J., Lu C., Kang Y., Wang G.X., Chen P. Screening of 42 medicinal plants for in vivo anthelmintic activity against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus) Parasitol. Res. 2012;111:97–104. doi: 10.1007/s00436-011-2805-6. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.K., Yeo J., Kim B., Ha M., Kim V.N. Short structured RNAs with low GC content are selectively lost during extraction from a small number of cells. Mol. Cell. 2012;46(6):893–895. doi: 10.1016/j.molcel.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 52.Alexander C.P., Kirubakaran C.J.W., Michael R.D. Water soluble fraction of Tinospora cordifolia leaves enhanced the non-specific immune mechanisms and disease resistance in Oreochromis mossambicus. Fish Shellfish Immunol. 2010;29(5):765–772. doi: 10.1016/j.fsi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Sharma A., Deo A.D., Riteshkumar S.T., Chanu T.I., Das A. Effect of Withania somnifera (L. Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita (Hamilton) fingerlings. Fish Shellfish Immunol. 2010;29(3):508–512. doi: 10.1016/j.fsi.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Wu C.C., Liu C.H., Chang Y.P., Hsieh S.L. Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mossambicus. Fish Shellfish Immunol. 2010;29(2):258–263. doi: 10.1016/j.fsi.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 55.Harikrishnan R., Heo J., Balasundaram C., Kim M.C., Kim J.S., Han Y.J., Heo M.S. Effect of Punica granatum solvent extracts on immune system and disease resistance in Paralichthys olivaceus against lymphocystis disease virus (LDV) Fish Shellfish Immunol. 2010;29(4):668–673. doi: 10.1016/j.fsi.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Pirbalouti A.G., Taheri M., Raisee M., Bahrami H.R., Abdizadeh R. In vitro antifungal activity of plant extracts on Saprolegnia parasitica from cutaneous lesions of rainbow trout (Oncorhynchus mykiss) eggs. J. Food Agric. Environ. 2009;7:94–96. [Google Scholar]
- 57.Leite S.P., Vieira J.R.C., de Medeiros P.L., Leite R.M.P., de Menezes Lima V.L., Xavier H.S., de Oliveira Lima E. Antimicrobial activity of Indigofera suffruticosa. Evid. Based Complementary Altern. Med. 2006;3(2):261–265. doi: 10.1093/ecam/nel010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki K., Misaka N., Sakai D.K. Efficacy of green tea extract on removal of the ectoparasitic flagellate Ichthyobodo necator from chum salmon, Oncorhynchus keta, and masu salmon. O. masou. Aquaculture. 2006;259(1–4):17–27. doi: 10.1016/j.aquaculture.2006.05.004. [DOI] [Google Scholar]
- 59.Sahu S., Das B.K., Mishra B.K., Pradhan J., Sarangi N. Effect of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. J. Appl. Ichthyol. 2007;23(1):80–86. doi: 10.1111/j.1439-0426.2006.00785.x. [DOI] [Google Scholar]
- 60.Ekanem A.P., Obiekezie A., Kloas W., Knopf K. Effects of crude extracts of Mucuna pruriens (Fabaceae) and Carica papaya (Caricaceae) against the protozoan fish parasite Ichthyophthirius multifiliis. Parasitol. Res. 2004;92:361–366. doi: 10.1007/s00436-003-1038-8. [DOI] [PubMed] [Google Scholar]
- 61.Rashidian G., Lazado C., Mahboub H.H., Mohammadi-Aloucheh R., Prokić M., Nada H.S., Faggio C. Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) skin mucus. Int. J. Mol. Sci. 2021;22(6):3270. doi: 10.3390/ijms22063270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaolan G., Linlin L., Ke C. International Conference on Economic Management and Social Science. Atlantis Press; EMSS: 2014. Application of Chinese herbal medicine additives in aquaculture; pp. 180–183. [DOI] [Google Scholar]
- 63.Van Hai N. The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture. 2015;446:88–96. doi: 10.1016/j.aquaculture.2015.03.014. [DOI] [Google Scholar]
- 64.Jeyavani J., Sibiya A., Sivakamavalli J., Divya M., Preetham E., Vaseeharan B., Faggio C. Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: a review. Aquacult. Int. 2022;30(2):1071–1086. doi: 10.1007/s10499-022-00848-013. [DOI] [Google Scholar]
- 65.Samal S.K., Das B.K., Pal B. Isolation, biochemical characterization, antibiotic susceptibility study of Aeromonas hydrophila isolated from freshwater fish. Int. J. Curr. Microbiol. Appl. Sci. 2014;3(12):259–267. [Google Scholar]
- 66.Canals R., Altarriba M., Vilches S., Horsburgh G., Shaw J.G., Tomás J.M., Merino S. Analysis of the lateral flagellar gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 2006;188(3):852–862. doi: 10.1128/JB.188.3.852-862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peabody C.R., Chung Y.J., Yen M.R., Vidal-Ingigliardi D., Pugsley A.P., Saier M.H., Jr. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology. 2003;149(11):3051–3072. doi: 10.1099/mic.0.26364-0. [DOI] [PubMed] [Google Scholar]
- 68.Fulton K.M., Mendoza-Barbera E., Twine S.M., Tomás J.M., Merino S. Polar glycosylated and lateral non-glycosylated flagella from Aeromonas hydrophila strain AH-1 (serotype O11) Int. J. Mol. Sci. 2015;16(12):28255–28269. doi: 10.3390/ijms161226097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richard C., Giammanco G., Popoff M. Vibrio parahaemolyticus. Isolement et diagnostic bactériologique. Ann. Biol. Clin. 1974;32(1):33–40. [PubMed] [Google Scholar]
- 70.Schubert R.H. Infrasubspecific taxonomy of Aeromonas hydrophila (Chester 1901) Stanier 1943. Zentralbl. Bakteriol. Orig. B. 1969;211(3):406–409. [PubMed] [Google Scholar]
- 71.Popoff M., Veron M. A taxonomic study of the Aeromonas hydrophila-Aeromonas punctata group. Microbiology. 1976;94(1):11–22. doi: 10.1099/00221287-94-1-11. [DOI] [PubMed] [Google Scholar]
- 72.Mostafa K., Islam M.T., Sabur M.A., Rashid M.M. Experimental pathogenesis of Aeromonas hydrophila bacteria in shing Heteropneustes fossilis (Bloch) Bangladesh J. Fish. Res. 2008;12(1):27–33. [Google Scholar]
- 73.Plumb J.A. Symposium on Fish Vaccination. OlE; Paris: 1984. Immunization of warm water fish against five important pathogens; p. 222.https://agris.fao.org/agris-search/search.do?recordID=XE8534635 [Google Scholar]
- 74.Sabur M.A. Department of Aquaculture, Bangladesh Agricultural University; Bangladesh: 2006. Studies on the Ecology of the Pathogenic Bacteria Aeromonas Hydrophila in Indigenous and Exotic Carps under Polyculture Condition. [Google Scholar]
- 75.Davis B.R., Ewing W.H. Lipolytic, pectolytic, and alginolytic activities of Enterobacteriaceae. J. Bacteriol. 1964;88(1):16–19. doi: 10.1128/jb.88.1.16-19.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martínez M.J., Simon-Pujol D., Congregado F., Merino S., Rubires X., Tomás J.M. The presence of capsular polysaccharide in mesophilic Aeromonas hydrophila serotypes O: 11 and O: 34. FEMS Microbiol. Lett. 1995;128(1):69–73. doi: 10.1111/j.1574-6968.1995.tb07502.x. [DOI] [PubMed] [Google Scholar]
- 77.Merino S., Aguilar A., Rubires X., Abitiu N., Regué M., Tomás J.M. The role of the capsular polysaccharide of Aeromonas hydrophila sero group O: 34 in the adherence to and invasion of fish cell lines. Res. Microbiol. 1997;148(7):625–631. doi: 10.1111/j.1574-6968.1997.tb12572.x. [DOI] [PubMed] [Google Scholar]
- 78.Vilches S., Jimenez N., Tomás J.M., Merino S. Aeromonas hydrophila AH-3 type III secretion system expression and regulatory network. Appl. Environ. Microbiol. 2009;75(19):6382–6392. doi: 10.1128/AEM.00222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hossain M. 2012. Molecular Interactions between Phage and the Catfish Pathogen Edwardsiella Ictalurid and Comparative Genomics of Epidemic Strains of Aeromonas Hydrophila (Doctoral Dissertation)https://etd.auburn.edu/handle/10415/3429 [Google Scholar]
- 80.Thomas S.R., Trust T.J. Tyrosine phosphorylation of the tetragonal paracrystalline array of Aeromonas hydrophila: molecular cloning and high-level expression of the S-layer protein gene. J. Mol. Biol. 1995;245(5):568–581. doi: 10.1006/jmbi.1994.0047. [DOI] [PubMed] [Google Scholar]
- 81.Sarkar A., Saha M., Patra A., Roy P. Characterization of Aeromonas hydrophila through RAPD-PCR and SDS-PAGE analysis. Open J. Med. Microbiol. 2012;2(2):37–40. doi: 10.4236/ojmm.2012.22005. [DOI] [Google Scholar]
- 82.Moller V. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol. Microbiol. Scand. 1955;36(2):158–172. doi: 10.1111/j.1699-0463.1955.tb04583.x. [DOI] [PubMed] [Google Scholar]
- 83.Taylor W.I., Schelhart D. Isolation of shigellae. Appl. Microbiol. 1971;21(1):32–37. doi: 10.1128/am.21.1.32-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor W.I., Harris B. Isolation of shigellae. II. Comparison of plating media and enrichment broths. Am. J. Clin. Pathol. 1965;44(4):476–479. doi: 10.1093/ajcp/44.4_ts.476. [DOI] [PubMed] [Google Scholar]
- 85.Shotts E.B., Jr., Rimler R. Medium for the isolation of Aeromonas hydrophila. Appl. Microbiol. 1973;26(4):550–553. doi: 10.1128/am.26.4.550-553.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahu I., Das B.K., Marhual N., Samanta M., Mishra B.K., Eknath A.E. Toxicity of crude extracellular products of Aeromonas hydrophila on rohu, Labeo rohita (Ham.) Indian J. Microbiol. 2011;51(4):515–520. doi: 10.1007/s12088-011-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seidler R.J., Allen D.A., Lockman H., Colwell R.R., Joseph S.W., Daily O.P. Isolation, enumeration, and characterization of Aeromonas from polluted waters encountered in diving operations. Appl. Environ. Microbiol. 1980;39(5):1010–1018. doi: 10.1128/aem.39.5.1010-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta S.K., Pal A.K., Sahu N.P., Dalvi R., Kumar V., Mukherjee S.C. Microbial levan in the diet of Labeo rohita Hamilton juveniles: effect on non‐specific immunity and histopathological changes after challenge with Aeromonas hydrophila. J. Fish. Dis. 2008;31(9):649–657. doi: 10.1111/j.1365-2761.2008.00939.x. [DOI] [PubMed] [Google Scholar]
- 89.Divya P.R., Thomas P.C., Chandrika V., Paulton M.P. Genetic characterization of Aeromonas hydrophila using protein profiling and RAPD PCR. Asian Fish Sci. 2009;22:763–771. [Google Scholar]
- 90.Arcos M.L., de Vicente A., Morinigo M.A., Romero P.E.D.R.O., Borrego J.J. Evaluation of several selective media for recovery of Aeromonas hydrophila from polluted waters. Appl. Environ. Microbiol. 1988;54(11):2786–2792. doi: 10.1128/aem.54.11.2786-2792.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burke V.J.M.D.K., Robinson J., Gracey M., Peterson D., Partridge K. Isolation of Aeromonas hydrophila from a metropolitan water supply: seasonal correlation with clinical isolates. Appl. Environ. Microbiol. 1984;48(2):361–366. doi: 10.1128/aem.48.2.361-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rashid M.M., Hossain M.S., Ali M.F. Isolation and identification of Aeromonas hydrophila from silver carp and its culture environment from Mymensingh region. J. Bangladesh Agric. Univ. 2013;11(2):373–376. doi: 10.3329/jbau.v11i2.19943. [DOI] [Google Scholar]
- 93.Austin B., Austin D.A. Praxis publishing Ltd (Springer); Chichester, UK: 2007. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish. [Google Scholar]
- 94.Swann L., White M.R. 1991. Diagnosis and Treatment of "Aeromonas Hydrophila" Infection of Fish. Aquaculture Extension, Illinois-Indiana Sea Grant Program.https://nsgl.gso.uri.edu/ilin/iling91003.pdf [Google Scholar]
- 95.Rehulka J. Aeromonas causes severe skin lesions in rainbow trout (Oncorhynchus mykiss): clinical pathology, haematology, and biochemistry. Acta Vet. 2002;71(3):351–360. doi: 10.2754/avb200271030351. [DOI] [Google Scholar]
- 96.Taylor P.W. Multiple antimicrobial resistances in a chronic bacterial infection of koi carp. N. Am. J. Aquacult. 2003;65(2):120–125. doi: 10.1577/15488454(2003)65<120:MARIAC>2.0.CO;2. [DOI] [Google Scholar]
- 97.Karunasagar I., Rosalind G., Karunasagar I. Immunological response of the Indian major carps to Aeromonas hydrophila vaccine. J. Fish. Dis. 1991;14(3):413–417. doi: 10.1111/j.1365-2761.1991.tb00841.x. [DOI] [Google Scholar]
- 98.Roberts R.J., Wootten R., MacRae I., Millar S., Struthers W. Institute of Aquaculture, Starling University; Scotland, UK: 1989. Ulcerative Disease Survey, Bangladesh; Final to Government of Bangladesh and the Overseas Development Administration. [Google Scholar]
- 99.Lio-Po G.D., Albright L.J., Tendencia E. Proceedings of the First Symposium on Diseases in Asian Aquaculture. 1992. Aeromonas hydrophila in the epizootic ulcerative syndrome (EUS) of snakehead, Ophiocephalus striatus, and catfish, Clarias batrachus: quantitative estimation in natural infection and experimental induction of dermo-muscular necrotic lesion; pp. 461–474. Bali, Indonesia. [Google Scholar]
- 100.Yi S.W., You M.J., Lee H.B., Shin G.W. A case of Aeromonas veronii infection in Israeli carp (Cyprinus carpio): phylogenetic analysis and antimicrobial resistance. Korean J. Vet. Serv. 2012;35(3):239–243. doi: 10.7853/kjvs.2012.35.3.239. [DOI] [Google Scholar]
- 101.Beaz-Hidalgo R., Martínez-Murcia A., Figueras M.J. Reclassification of Aeromonas hydrophila subsp. Dhakensis Huys et al. 2002 and Aeromonas aquariorum Martinez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst. Appl. Microbiol. 2013;36(3):171–176. doi: 10.1016/j.syapm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 102.Karvonen A., Rintamaki P., Jokela J., Valtonen E.T. Increasing water temperature and disease risks in aquatic systems, climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 2010;40(13):1483–1488. doi: 10.1016/j.ijpara.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 103.Tam B., Gough W.A., Tsuji L. The impact of warming on the appearance of furunculosis in fish of the James Bay region, Quebec, Canada. Reg. Environ. Change. 2011;11:123–132. doi: 10.1007/s10113-010-0122-8. [DOI] [Google Scholar]
- 104.Ali M.F., Rashid M.M., Rahman M.M., Haque M.N. Pathogenicity of Aeromonas hydrophila in silver carp Hypophthalmichthys molitrix and its control trial. Sch. J. Agric. Vet. Sci. 2014;7(6):21–24. [Google Scholar]
- 105.Rahman M.H., Kawai K., Kusuda R. Virulence of starved Aeromonas hydrophila to cyprinid fish. Fish Pathol. 1997;32(3):163–168. doi: 10.3147/jsfp.32.163. [DOI] [Google Scholar]
- 106.Rahman M., Huys G., Rahman M., Albert M.J., Kuhn I., Mollby R. Persistence, transmission and virulence characteristics of Aeromonas strains in a duckweed aquaculture-based hospital sewage water recycling plant in Bangladesh. Appl. Environ. Microbiol. 2007;73(5):1444–1451. doi: 10.1128/AEM.01901-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inglis V., Roberts R.J., Bromage N.R. John Willey & Sons, Inc.; New York, United States: 1993. Bacterial Diseases of Fish. [Google Scholar]
- 108.Llobrera A.T., Gacutan R.Q. Aeromonas hydrophila associated with ulcerative disease epizootic in Laguna de Bay, Philippines. Aquaculture. 1987;67(3–4):273–278. doi: 10.1016/0044-8486(87)90211-0. [DOI] [Google Scholar]
- 109.Schubert R.H.W. A method for the determination of toxicity of pollutants in water and effluent to bacteria. Zent.bl. Bakteriol. Parasitenkd. Infekt.krankh. Hyg. 2. Abt. 1973;156(6):545–550. [PubMed] [Google Scholar]
- 110.Schubert R.H. The relation of aerogenic to anaerogenic aeromonads of the" Hydrophila-Punctata-group" in river water depending on the load of waste (author's transl) Zentralbl. Bakteriol. B. 1975;160(3):237–245. [PubMed] [Google Scholar]
- 111.Schubert R. The detection of aeromonads of the" hydrophila-punctata-group" within the hygienic control of drinking water (author's transl) Zentralbl. Bakteriol. Orig. B. 1976;161(5–6):482–497. [PubMed] [Google Scholar]
- 112.Qiao N., Shao Z. Isolation and characterization of a novel biosurfactant produced by hydrocarbon‐degrading bacterium Alcanivorax dieselolei B5. J. Appl. Microbiol. 2010;108(4):1207–1216. doi: 10.1111/j.1365-2672.2009.04513.x. [DOI] [PubMed] [Google Scholar]
- 113.Allan B.J., Stevenson R.M. Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can. J. Microbiol. 1981;27(10):1114–1122. doi: 10.1139/m81-174. [DOI] [PubMed] [Google Scholar]
- 114.Barney M.C., Rigney M.M., Rouf M.A. Vol. 93. American Society of Microbiology; 1972. Isolation and Characterization of Endotoxin from Aeromonas Hydrophila. [Google Scholar]
- 115.Bernheimer A.W., Avigad L.S. Partial characterization of aerolysin, a lytic exotoxin from Aeromonas hydrophila. Infect. Immun. 1974;9(6):1016–1021. doi: 10.1128/iai.9.6.1016-1021.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanyal S.C., Singh S.J., Sen P.C. Enteropathogenicity of Aeromonas hydrophila and Plesiomonas shigelloides. J. Med. Microbiol. 1975;8(1):195–198. doi: 10.1099/00222615-8-1-195. [DOI] [PubMed] [Google Scholar]
- 117.Wadstrom T., Ljungh Å., Wretlind B. Enterotoxin, haemolysin and cytotoxic protein in Aeromonas hydrophila from human infections. Acta Pathol. Microbiol. Scand. B. 1976;84B(2):112–114. doi: 10.1111/j.1699-0463.1976.tb01911.x. [DOI] [PubMed] [Google Scholar]
- 118.Ljungh A., Popoff M., Wadstrom T. Aeromonas hydrophila in acute diarrheal disease: detection of enterotoxin and bio typing of strains. J. Clin. Microbiol. 1977;6(2):96–100. doi: 10.1128/jcm.6.2.96-100.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ljungh Å., Wretlind B., Wadström T. Evidence for enterotoxin and two cytolytic toxins in human isolates of Aeromonas hydrophila. Toxins. 1978:947–960. doi: 10.1016/B978-0-08-022640-8.50090-1. [DOI] [Google Scholar]
- 120.Donta S.T., Haddow A.D. Cytotoxic activity of Aeromonas hydrophila. Infect. Immun. 1978;21(3):989–993. doi: 10.1128/iai.21.3.989-993.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barnett T.C., Kirov S.M., Strom M.S., Sanderson K. Aeromonas spp. possess at least two distinct type IV pilus families. Microb. Pathog. 1997;23(4):241–247. doi: 10.1006/mpat.1997.0152. [DOI] [PubMed] [Google Scholar]
- 122.Donlan R.M. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 2001;33(8):1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 123.Sudheesh P.S., Al-Ghabshi A., Al-Mazrooei N., Al-Habsi S. Comparative pathogenomics of bacteria causing infectious diseases in fish. Int. J. Evol. Biol. 2012 doi: 10.1155/2012/457264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cahill M.M. Virulence factors in motile Aeromonas species. J. Appl. Microbiol. 1990;69(1):1–16. doi: 10.1111/j.1365-2672.1990.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 125.González‐Serrano C.J., Santos J.A., García‐López M.L., Otero A. Virulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J. Appl. Microbiol. 2002;93(3):414–419. doi: 10.1046/j.1365-2672.2002.01705.x. [DOI] [PubMed] [Google Scholar]
- 126.Palumbo S.A., Williams A.C., Buchanan R.L., Phillips J.G. Model for the aerobic growth of Aeromonas hydrophila K144. J. Food Protect. 1991;54(6):429–435. doi: 10.4315/0362-028X-54.6.429. [DOI] [PubMed] [Google Scholar]
- 127.Mano S.B., Ordonez J.A., de Fernando G.G. Growth/survival of natural flora and Aeromonas hydrophila on refrigerated uncooked pork and Turkey packaged in modified atmospheres. Food Microbiol. 2000;17(6):657–669. doi: 10.1006/fmic.2000.0358. [DOI] [Google Scholar]
- 128.Sirirat T., Intuseth J., Chanphong J., Thompson K., Chinabut S., Adams A. Characterisation of Aeromonas hydrophila extracellular products with reference to toxicity, virulence, protein profiles and antigenicity. Asian Fish Sci. 1999;12(4):371–379. [Google Scholar]
- 129.Kwapinski J.B. Methods of Serological Research. Wiley; New York: 1965. The diffusion precipitation test; pp. 162–183. [Google Scholar]
- 130.Sakai D.K. Transmission of protease genes into a protease deficient mutant of Aeromonas salmonicida in river sediments. J. Fish. Dis. 1987;10(3):171–179. doi: 10.1111/j.1365-2761.1987.tb01059.x. [DOI] [Google Scholar]
- 131.Yu H.B., Kaur R., Lim S., Wang X.H., Leung K.Y. Characterization of extracellular proteins produced by Aeromonas hydrophila AH1. Proteomics. 2007;7(3):436–449. doi: 10.1002/pmic.200600396. [DOI] [PubMed] [Google Scholar]
- 132.Krovacek K., Peterz M., Faris A., Månsson I. Enter toxigenicity and drug sensitivity of Aeromonas hydrophila isolated from well water in Sweden: a case study. Int. J. Food Microbiol. 1989;8(2):149–154. doi: 10.1016/0168-1605(89)90069-X. [DOI] [PubMed] [Google Scholar]
- 133.Vivekanandhan G., Savithamani K., Hatha A.A.M., Lakshmanaperumalsamy P. Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. Int. J. Food Microbiol. 2002;76(1–2):165–168. doi: 10.1016/S0168-1605(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 134.Hatha M., Vivekanandhan A.A., Joice G.J. Antibiotic resistance pattern of motile aeromonads from farm raised fresh water fish. Int. J. Food Microbiol. 2005;98(2):131–134. doi: 10.1016/j.ijfoodmicro.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 135.Abraham T.J., Manley R., Palaniappan R., Dhevendaran K. Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J. Aquacult. Trop. 1997;12:1–8. [Google Scholar]
- 136.Ko W.C., Yu K.W., Liu C.Y., Huang C.T., Leu H.S., Chuang Y.C. Increasing antibiotic resistance in clinical isolates of Aeromonas strains in Taiwan. Antimicrob. Agents Chemother. 1996;40(5):1260–1262. doi: 10.1128/AAC.40.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jones B.L., Wilcox M.H. Aeromonas infections and their treatment. J. Antimicrob. Chemother. 1995;35(4):453–461. doi: 10.1093/jac/35.4.453. [DOI] [PubMed] [Google Scholar]
- 138.Araujo R.M., Arribas R.M., Pares R. Distribution of Aeromonas species in waters with different levels of pollution. J. Appl. Bacteriol. 1991;71(2):82–186. doi: 10.1111/j.1365-2672.1991.tb02976.x. [DOI] [PubMed] [Google Scholar]
- 139.Ashiru A.W., Uaboi-Egbeni P.O., Oguntowo J.E., Idika C.N. Isolation and antibiotic profile of Aeromonas species from tilapia fish (Tilapia nilotica) and catfish (Clarias batrachus) Pakistan J. Nutr. 2011;10(10):982–986. doi: 10.3923/pjn.2011.982.986. [DOI] [Google Scholar]
- 140.Schmidt A.S., Bruun M.S., Dalsgaard I., Pedersen K., Larsen J.L. Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl. Environ. Microbiol. 2000;66(11):4908–4915. doi: 10.1128/AEM.66.11.4908-4915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rowe‐Magnus D.A., Guerout A.M., Mazel D. Bacterial resistance evolution by recruitment of super integron gene cassettes. Mol. Microbiol. 2002;43(6):1657–1669. doi: 10.1046/j.1365-2958.2002.02861.x. [DOI] [PubMed] [Google Scholar]
- 142.Alcaide E., Blasco M.D., Esteve C. Mechanisms of quinolone resistance in Aeromonas species isolated from humans, water and eels. Res. Microbiol. 2010;161(1):40–45. doi: 10.1016/j.resmic.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 143.Kaskhedikar M., Chhabra D. Multiple drug resistance in Aeromonas hydrophila isolates of fish. Vet. World. 2010;3(2):76–77. [Google Scholar]
- 144.Sarkar M.J.A., Rashid M.M. Pathogenicity of the bacterial isolate Aeromonas hydrophila to catfishes, carps and perch. J. Bangladesh Agric. Univ. 2012;10(1):157–161. doi: 10.22004/ag.econ.209312. [DOI] [Google Scholar]
- 145.Bach R., Chen P.K., Chapman G.B. Changes in the spleen of the channel catfish Ictalurus punctatus Rafinesque induecd by infection with Aeromonas hydrophila. J. Fish. Dis. 1978;1(3):205–217. doi: 10.1111/j.1365-2761.1978.tb00023.x. [DOI] [Google Scholar]
- 146.Lio‐Po G.D., Albright L.J., Leano E.M. Experiments on virulence dose and portals of entry for Aeromonas hydrophila in walking catfish. J. Aquat. Anim. Health. 1996;8(4):340–343. doi: 10.1577/1548-8667(1996)008<0340:EOVDAP>2.3.CO;2. [DOI] [Google Scholar]
- 147.Esteve C., Biosca E.G., Amaro C. Virulence of Aeromonas hydrophila and some other bacteria isolated from European eels Anguilla anguilla reared in fresh water. Dis. Aquat. Org. 1993;16(1):15–20. [Google Scholar]
- 148.Rahman M.M., Chowdhury M.B.R. Isolation of bacterial pathogen causing an ulcer disease in farmed carp fishes of Mymensingh. Bangladesh J. Fish. Res. 1996;19:103–110. [Google Scholar]
- 149.Kumar S., Raman R.P., Pandey P.K., Mohanty S., Kumar A., Kumar K. Effect of orally administered azadirachtin on non-specific immune parameters of goldfish Carassius auratus (Linn. 1758) and resistance against Aeromonas hydrophila. Fish Shellfish Immunol. 2013;34(2):564–573. doi: 10.1016/j.fsi.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 150.Esiobu N., Armenta L., Ike J. Antibiotic resistance in soil and water environments. Int. J. Environ. Health Res. 2002;12(2):133–144. doi: 10.1080/09603120220129292. [DOI] [PubMed] [Google Scholar]
- 151.FAO/WHO/OIF . Report of a Joint FAO/OIE/WHO Expert Consultation on Antimicrobial Use in Aquaculture and Antimicrobial Resistance. WHO; Geneva, Switzerland: 2006. Antimicrobial use in aquaculture and antimicrobial resistance.ftp://ftp.fao.org/ag/agn/food/aquaculture_rep_13_16june2006 [Google Scholar]
- 152.Sugita H., Miyajima C., Deguchi Y. The vitamin B12-producing ability of the intestinal microflora of freshwater fish. Aquaculture. 1991;92:267–276. doi: 10.1016/0044-8486(91)90028-6. [DOI] [Google Scholar]
- 153.Farzanfar A. The use of probiotics in shrimp aquaculture. FEMS Microbiol. Immunol. 2006;48(2):149–158. doi: 10.1111/j.1574-695X.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 154.Vaseeharan B.A.R.P., Ramasamy P. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Lett. Appl. Microbiol. 2003;36(2):83–87. doi: 10.1046/j.1472-765X.2003.01255.x. [DOI] [PubMed] [Google Scholar]
- 155.Decamp O., Moriarty D.J., Lavens P. Probiotics for shrimp larviculture: review of field data from Asia and Latin America. Aquacult. Res. 2008;39(4):334–338. doi: 10.1111/j.1365-2109.2007.01664.x. [DOI] [Google Scholar]
- 156.Gomez-Gil B., Roque A., Turnbull J.F. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture. 2000;191(1–3):259–270. doi: 10.1016/S0044-8486(00)00431-2. [DOI] [Google Scholar]
- 157.Parthasarathy R., Ravi D. Probiotic bacteria as growth promoter and biocontrol agent against Aeromonas hydrophila in Catla catla (Hamilton, 1822) Indian J. Fish. 2011;58(3):87–93. [Google Scholar]
- 158.Lee S., Kim S., Yoojung O., Lee Y. Characterization of Aeromonas hydrophila isolated from rainbow trouts in Korea. J. Microbiol. 2000;38(1):1–7. [Google Scholar]
- 159.Maqsood S., Samoon M.H., Singh P. Immunomodulatory and growth promoting effect of dietary levamisole in Cyprinus carpio fingerlings against the challenge of Aeromonas hydrophila. Turk. J. Fish. Aquat. Sci. 2009;9(1):111–120. [Google Scholar]
- 160.Semwal A., Khati A., Kumar A., Yadav M.K., Arya D. A review on antimicrobial peptides (AMPs) Pharma Innov. 2022;11(4):1139–1146. [Google Scholar]
- 161.Rairakhwada D., Pal A.K., Bhathena Z.P., Sahu N.P., Jha A., Mukherjee S.C. Dietary microbial levan enhances cellular non-specific immunity and survival of common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 2007;22(5):477–486. doi: 10.1016/j.fsi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 162.Gudding R., Lillehaug A., Evensen Recent developments in fish vaccinology. Vet. Immunol. Immunopathol. 1999;72(1–2):203–212. doi: 10.1016/S0165-2427(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 163.Saravanan K., Sivaramakrishnan T., Praveenraj J., Kiruba-Sankar R., Haridas H., Kumar S., Varghese B. Effects of single and multi-strain probiotics on the growth, hemato-immunological, enzymatic activity, gut morphology and disease resistance in rohu, Labeo rohita. Aquaculture. 2021;540 doi: 10.1016/j.aquaculture.2021.736749. [DOI] [Google Scholar]
- 164.Dalmo R.A., Bogwald J., Ingebrigtsen K., Seljelid R. The immunomodulatory effect of laminaran [β (1,3)‐D‐glucan] on Atlantic salmon, Salmo salar L., anterior kidney leucocytes after intraperitoneal, per oral and per anal administration. J. Fish. Dis. 1996;19(6):449–457. doi: 10.1046/j.1365-2761.1996.d01-97.x. [DOI] [Google Scholar]
- 165.Lewus C.B., Kaiser A., Montville T.J. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl. Environ. Microbiol. 1991;57(6):1683–1688. doi: 10.1128/aem.57.6.1683-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vescovo M., Scolari G., Orsi C., Sinigaglia M., Torriani S. Combined effects of Lactobacillus casei inoculum, modified atmosphere packaging and storage temperature in controlling Aeromonas hydrophila in ready‐to use vegetables. Int. J. Food Sci. 1997;32(5):411–419. doi: 10.1046/j.1365-2621.1997.00121.x. [DOI] [Google Scholar]
- 167.Santos J.A., López Díaz T., García Fernández M.C., García López M.L., Otero A. Effect of a lactic starter culture on the growth and protease activity of Aeromonas hydrophila. J. Appl. Bacteriol. 1996;80(1):13–18. doi: 10.1111/j.1365-2672.1996.tb03183.x. [DOI] [Google Scholar]
- 168.Palumbo S.A., Call J.E., Cooke P.H., Williams A.C. Effect of polyphosphates and NaCl on Aeromonas hydrophila K144. J. Food Saf. 1995;15(1):77–87. doi: 10.1111/j.1745-4565.1995.tb00122.x. [DOI] [Google Scholar]
- 169.Velazquez L.D.C., Escudero M.E., de Guzmán A.M.S. Antibacterial effects of different food-related phosphates using Aeromonas hydrophila. J. Food Protect. 2001;64(2):195–200. doi: 10.4315/0362-028X-64.2.195. [DOI] [PubMed] [Google Scholar]
- 170.Sheldon B.W., Schuman J.D. Thermal and biological treatments to control psychrotrophic pathogens. Poultry Sci. 1996;75(9):1126–1132. doi: 10.3382/ps.0751126. [DOI] [PubMed] [Google Scholar]
- 171.Ellenberg L., Hoover D.G. Injury and survival of Aeromonas hydrophila 7965 and Yersinia enterocolitica 9610 from high hydrostatic pressure. J. Food Saf. 1999;19(4):263–276. doi: 10.1111/j.1745-4565.1999.tb00251.x. [DOI] [Google Scholar]
- 172.Boyle D.L., Sofos J.N., Maga J.A. Inhibition of spoilage and pathogenic microorganisms by liquid smoke from various woods. Lebensm. Wiss. Technol. 1988;21(1):54–58. [Google Scholar]
- 173.Alderman D.J., Hastings T.S. Antibiotic use in aquaculture: development of antibiotic resistance–potential for consumer health risks. Int. J. Food Sci. 1998;33(2):139–155. doi: 10.1046/j.1365-2621.1998.3320139.x. [DOI] [Google Scholar]
- 174.Nayak S.K., Swain P., Mukherjee S.C. Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.) Fish Shellfish Immunol. 2007;23(4):892–896. doi: 10.1016/j.fsi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 175.Harikrishnan R., Rani M.N., Balasundaram C. Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture. 2003;221(1–4):41–50. doi: 10.1016/S0044-8486(03)00023-1. [DOI] [Google Scholar]
- 176.Rath R.K. third ed. Scientific Publishers; Jodhpur, Rajasthan, India: 2018. Freshwater Aquaculture. [Google Scholar]
- 177.Hao Y.Y., Brackett R.E., Doyle M.P. Inhibition of Listeria monocytogenes and Aeromonas hydrophila by plant extracts in refrigerated cooked beef. J. Food Protect. 1998;61(3):307–312. doi: 10.4315/0362-028X-61.3.307. [DOI] [PubMed] [Google Scholar]
- 178.Talpur A.D., Ikhwanuddin M.H.D. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch) Aquaculture. 2012;364:6–12. doi: 10.1016/j.aquaculture.2012.07.035. [DOI] [Google Scholar]
- 179.Mo W.Y., Lun C.H.I., Choi W.M., Man Y.B., Wong M.H. Enhancing growth and non-specific immunity of grass carp and Nile tilapia by incorporating Chinese herbs (Astragalus membranaceus and Lycium barbarum) into food waste based pellets. Environ. Pollut. 2016;219:475–482. doi: 10.1016/j.envpol.2016.05.055. [DOI] [PubMed] [Google Scholar]
- 180.Kaleeswaran B., Ilavenil S., Ravikumar S. Dietary supplementation with Cynodon dactylon (L.) enhances innate immunity and disease resistance of Indian major carp, Catla catla (Ham.) Fish Shellfish Immunol. 2011;31(6):953–962. doi: 10.1016/j.fsi.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 181.Binaii M., Ghiasi M., Farabi S.M.V., Pourgholam R., Fazli H., Safari R., Alavi S.E., Taghavi M.J., Bankehsaz Z. Biochemical and hemato-immunological parameters in juvenile beluga (Huso huso) following the diet supplemented with nettle (Urtica dioica) Fish Shellfish Immunol. 2014;36(1):46–51. doi: 10.1016/j.fsi.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 182.Ngugi C.C., Oyoo-Okoth E., Mugo-Bundi J., Orina P.S., Chemoiwa E.J., Aloo P.A. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria Labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2015;44(2):533–541. doi: 10.1016/j.fsi.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 183.Giri S.S., Kim S.G., Woo K.J., Jung W.J., Lee S.B., Lee Y.M., Jo S.J., Hwang M.H., Park J., Kim J.H., Sukumaran V. Effects of Bougainvillea glabra leaf on growth, skin mucosal immune responses, and disease resistance in common carp Cyprinus carpio. Fish Shellfish Immunol. 2023;132 doi: 10.1016/j.fsi.2022.108514. [DOI] [PubMed] [Google Scholar]
- 184.Rashidian G., Mahboub H.H., Fahim A., Hefny A.A., Prokić M.D., Rainis S., Boldaji J.T., Faggio C. Mooseer (Allium hirtifolium) boosts growth, general health status, and resistance of rainbow trout (Oncorhynchus mykiss) against Streptococcus iniae infection. Fish Shellfish Immunol. 2022;120:360–368. doi: 10.1016/j.fsi.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 185.Maiti S., Saha S., Jana P., Chowdhury A., Khatua S., Ghosh T.K. Effect of dietary Andrographis paniculata leaf extract on growth, immunity, and disease resistance against Aeromonas hydrophila in Pangasianodon hypopthalmus. J. Appl. Aquacult. 2021;1–25 doi: 10.1080/10454438.2021.1959861. [DOI] [Google Scholar]
- 186.Rufchaei R., Mirvaghefi A., Hoseinifar S.H., Valipour A., Nedaei S. Effects of dietary administration of water hyacinth (Eichhornia crassipes) leaves extracts on innate immune parameters, antioxidant defence and disease resistance in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2020;515 doi: 10.1016/j.aquaculture.2019.734533. [DOI] [Google Scholar]
- 187.Bao L., Chen Y., Li H., Zhang J., Wu P., Ye K., Ai H., Chu W. Dietary Ginkgo biloba leaf extract alters immune-related gene expression and disease resistance to Aeromonas hydrophila in common carp Cyprinus carpio. Fish Shellfish Immunol. 2019;94:810–818. doi: 10.1016/j.fsi.2019.09.056. [DOI] [PubMed] [Google Scholar]
- 188.Zeraatpisheh F., Firouzbakhsh F., Khalili K.J. Effects of the macroalga Sargassum angustifolium hot water extract on hematological parameters and immune responses in rainbow trout (Oncorhynchus mykiss) infected with Yersinia rukeri. J. Appl. Phycol. 2018;30:2029–2037. doi: 10.1007/s10811-018-1395-4. [DOI] [Google Scholar]
- 189.Alsafah A.H., Al-Faragi J.K. Influence of thyme (Thymus vulgaris) as feed additives on growth performance and antifungal activity on Saprolegnia spp. in Cyprinus carpio L. J. Entomol. Zool. Stud. 2017;5(6):1598–1602. [Google Scholar]
- 190.Muthulakshmi M., Subramani P.A., Michael R.D. Immunostimulatory effect of the aqueous leaf extract of Phyllanthus niruri on the specific and nonspecific immune responses of Oreochromis mossambicus Peters. Iran. J. Vet. Res. 2016;17(3):200. doi: 10.1111/are.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pratheepa V., Sukumaran N. Effect of Euphorbia hirta plant leaf extract on immunostimulant response of Aeromonas hydrophila infected Cyprinus carpio. PeerJ. 2014;2:e671. doi: 10.7717/peerj.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gultepe N., Bilen S., Yılmaz S., Güroy D., Aydın S. Effects of herbs and spice on health status of tilapia (Oreochromis mossambicus) challenged with Streptococcus iniae. Acta Vet. 2014;83(2):125–131. doi: 10.2754/avb201483020125. [DOI] [Google Scholar]
- 193.Wu Y.R., Gong Q.F., Fang H., Liang W.W., Chen M., He R.J. Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2013;34(1):220–227. doi: 10.1016/j.fsi.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 194.Kaleeswaran B., Ilavenil S., Ravikumar S. Changes in biochemical, histological and specific immune parameters in Catla catla (Ham.) by Cynodondactylon (L.) J. King Saud Univ. Sci. 2012;24(2):139–152. doi: 10.1016/j.jksus.2010.10.001. [DOI] [Google Scholar]
- 195.Harikrishnan R., Kim J.S., Kim M.C., Balasundaram C., Heo M.S. Lactuca indica extract as feed additive enhances immunological parameters and disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture. 2011;318(1–2):43–47. doi: 10.1016/j.aquaculture.2011.04.049. [DOI] [Google Scholar]
- 196.Nya E.J., Austin B. Use of dietary ginger, Zingiber officinale Roscoe, as an immunostimulant to control Aeromonas hydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum) J. Fish. Dis. 2009;32(11):971–977. doi: 10.1111/j.1365-2761.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 197.Immanuel G., Uma R.P., Iyapparaj P., Citarasu T., Punitha Peter S.M., Michael Babu M., Palavesam A. Dietary medicinal plant extracts improve growth, immune activity and survival of tilapia Oreochromis mossambicus. J. Fish. Biol. 2009;(7):1462–1475. doi: 10.1111/j.1095-8649.2009.02212.x. [DOI] [PubMed] [Google Scholar]
- 198.Divyagnaneswari M., Christybapita D., Michael R.D. Immunomodulatory activity of Solanum trilobatum leaf extracts in Oreochromis mossambicus. Dise. Asian Aquacul. VI, Fish Health Sec. 2008;6:221–234. [Google Scholar]
- 199.Punitha S.M.J., Babu M.M., Sivaram V., Shankar V.S., Dhas S.A., Mahesh T.C., Immanuel G., Citarasu T. Immunostimulating influence of herbal biomedicines on nonspecific immunity in grouper Epinephelus tauvina juvenile against vibrio harveyi infection. Aquacult. Int. 2008;16:511–523. doi: 10.1007/s10499-007-9162-6. [DOI] [Google Scholar]
- 200.Mesalhy A.S., Fathi M.M., George J. Echinacea as immunostimulatory agent in Nile tilapia (Oreochromis niloticus) via earthen pond experiment. Int. Symp. Tilapia in Aquaculture. 2008:1033–1042. [Google Scholar]
- 201.Sudhakaran D.S., Srirekha P., Devasree L.D., Premsingh S., Michael R.D. Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J. Exp. Biol. 2006;44(9):726–732. [PubMed] [Google Scholar]
- 202.Shalaby A.M., Khattab Y.A., Abdel Rahman A.M. Effects of garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus) J. Venom. Anim. Toxins Incl. Trop. Dis. 2006;12:172–201. doi: 10.1590/S1678-91992006000200003. [DOI] [Google Scholar]
- 203.Ara I., Siddiqui B.S., Faizi S., Siddiqui S. Structurally novel diterpenoid constituents from the stem bark of Azadirachta indica (Meliaceae) J. Chem. Soc. Perkin Trans. 1989;1(2):343–345. doi: 10.1039/P19890000343. [DOI] [Google Scholar]
- 204.Mitra C.R., Garg H.S., Pandey G.N. Identification of nimbidic acid and nimbidinin from Azadirachta indica. Phytochemistry. 1971;10(4):857–864. doi: 10.1016/S0031-9422(00)97156-5. [DOI] [Google Scholar]
- 205.Siddiqui S., Faizi S., Siddiqui B.S. Constituents of Azadirachta indica: isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin, and a new protolimonoid, naheedin. J. Nat. Prod. 1992;55(3):303–310. doi: 10.1021/np50081a005. [DOI] [PubMed] [Google Scholar]
- 206.Harikrishnan R., Heo J., Balasundaram C., Kim M.C., Kim J.S., Han Y.J., Heo M.S. Effect of traditional Korean medicinal (TKM) triherbal extract on the innate immune system and disease resistance in Paralichthys olivaceus against Uronema marinum. Vet. Parasitol. 2010;170(1–2):1–7. doi: 10.1016/j.vetpar.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 207.Kumar S., Raman R.P., Kumar K., Pandey P.K., Kumar N., Mohanty S., Kumar A. In vitro and in vivo antiparasitic activity of azadirachtin against Argulus spp. in Carassius auratus (Linn. 1758) Parasitol. Res. 2012;110(5):1795–1800. doi: 10.1007/s00436-011-2701-0. [DOI] [PubMed] [Google Scholar]
- 208.Kumar S., Raman R.P., Kumar K., Pandey P.K., Kumar N., Mallesh B., Mohanty S., Kumar A. Effect of azadirachtin on haematological and biochemical parameters of Argulus-infested goldfish Carassius auratus (Linn. 1758) Fish Physiol. Biochem. 2012;39(4):733–747. doi: 10.1007/s10695-012-9736-8. [DOI] [PubMed] [Google Scholar]
- 209.Siwicki A.K. Immunostimulating influence of levamisole on nonspecific immunity in carp (Cyprinus carpio) Dev. Comp. Immunol. 1989;13(1):87–91. doi: 10.1016/0145-305X(89)90021-9. [DOI] [PubMed] [Google Scholar]
- 210.Duke J.A. first ed. CRC press; Boca Raton, Florida: 2018. CRC Handbook of Nuts. [DOI] [Google Scholar]
- 211.Citarasu T., Babu M.M., Punitha S.M.J., Venket Ramalingam K., Marian M.P. International Conference on Advanced Technologies in Fisheries and Marine Sciences. MS University; India: 2001. Control of pathogenic bacteria using herbal biomedicinal products in the larviculture system of Penaeus monodon; p. 104. [Google Scholar]
- 212.Mukherjee R., Dash P.K., Ram G.C. Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Res. Vet. Sci. 2005;79(1):37–43. doi: 10.1016/j.rvsc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 213.Chopra R.N., Nayar S.L., Chopra I.C., Asolkar L.V., Kakkar K.K., Chakre O.J., Varma B.S. CSIR; New Delhi, India: 1956. Glossary of Indian Medicinal Plants.https://www.worldcat.org/title/glossary-of-indian-medicinal-plants-with-supplement/oclc/499428255 [Google Scholar]
- 214.Millet C.O., Lloyd D., Williams C., Williams D., Evans G., Saunders R.A., Cable J. Effect of garlic and allium-derived products on the growth and metabolism of Spironucleus vortens. Exp. Parasitol. 2011;127(2):490–499. doi: 10.1016/j.exppara.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 215.Balasubramanian G., Sarathi M., Venkatesan C., Thomas J., Hameed A.S. Oral administration of antiviral plant extract of Cynodon dactylon on a large-scale production against white spot syndrome virus (WSSV) in Penaeus monodon. Aquaculture. 2008;279(1–4):2–5. doi: 10.1016/j.aquaculture.2008.03.052. [DOI] [Google Scholar]
- 216.Balasubramanian G., Sarathi M., Venkatesan C., Thomas J., Hameed A.S. Studies on the immunomodulatory effect of extract of Cyanodon dactylon in shrimp, Penaeus monodon, and its efficacy to protect the shrimp from white spot syndrome virus (WSSV) Fish Shellfish Immunol. 2008;25(6):820–828. doi: 10.1016/j.fsi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 217.Monsang S.J., Acharya A., Gon Choudhury T., Kamilya D. Dietary Asparagus racemosus ethanolic root extract modulates immune‐biochemical response, immune gene expression and provides protection against Aeromonas hydrophila in Labeo rohita fingerlings. Aquacult. Res. 2022;53(13):4795–4804. doi: 10.1111/are.15971. [DOI] [Google Scholar]
- 218.Kumar N., Sharma J., Mittal P., Chakrabarti R. Effect of leaves and seeds of Achyranthes aspera as feed supplements on the immunological and stress parameters and related gene expressions of Asian catfish (Clarias batrachus) Vet. Res. Commun. 2022;47:99–109. doi: 10.1007/s11259-022-09932-5. [DOI] [PubMed] [Google Scholar]
- 219.Abidin Z.U., Hassan H.U., Masood Z., Rafique N., Paray B.A., Gabol K., Shah M.I.A., Gulnaz A., Ullah A., Zulfiqar T., Siddique M.A.M. Effect of dietary supplementation of neem, Azadirachta indica leaf extracts on enhancing the growth performance, chemical composition and survival of rainbow trout, Oncorhynchus mykiss. Saudi J. Biol. Sci. 2022;29(4):3075–3081. doi: 10.1016/j.sjbs.2022.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wijayanto D., Nugroho R.A., Kurohman F., Nursanto D.B. The effect of garlic (Allium sativum) supplementation in feed on the growth, survival and profits of Asian seabass (Lates calcarifer) cultivation reared in freshwater media. Aquacult. Aquarium Conserv. Legis. 2022;15(4):1882–1890. [Google Scholar]
- 221.Aghoghovwia O.A., Bestman O. Growth and survival of Clarias gariepinus fingerlings fed water lily leaf meal as partial replacement for soybean meal. J. Curr. Res. Food Sci. 2022;3(1):17–20. [Google Scholar]
- 222.Van Doan H., Lumsangkul C., Sringarm K., Hoseinifar S.H., Dawood M.A., El-Haroun E., Harikrishnan R., Jaturasitha S., Paolucci M. Impacts of amla (Phyllanthus emblica) fruit extract on growth, skin mucosal and serum immunities, and disease resistance of Nile tilapia (Oreochromis niloticus) raised under biofloc system. Aquac. Rep. 2022;22 doi: 10.1016/j.aqrep.2021.100953. [DOI] [Google Scholar]
- 223.Yousefi M., Ghafarifarsani H., Hoseini S.M., Hoseinifar S.H., Abtahi B., Vatnikov Y.A., Kulikov E.V., Van Doan H. Effects of dietary thyme essential oil and prebiotic administration on rainbow trout (Oncorhynchus mykiss) welfare and performance. Fish Shellfish Immunol. 2022;120:737–744. doi: 10.1016/j.fsi.2021.12.023. [DOI] [PubMed] [Google Scholar]
- 224.Rawat P., Kaur V.I., Tyagi A., Norouzitallab P., Baruah K. Determining the efficacy of ginger Zingiber officinale as a potential nutraceutical agent for boosting growth performance and health status of Labeo rohita reared in a semi-intensive culture system. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.960897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yousefi M., Zahedi S., Reverter M., Adineh H., Hoseini S.M., Van Doan H., El-Haroun E.R., Hoseinifar S.H. Enhanced growth performance, oxidative capacity and immune responses of common carp, Cyprinus carpio fed with Artemisia absinthium extract-supplemented diet. Aquaculture. 2021;545 doi: 10.1016/j.aquaculture.2021.737167. [DOI] [Google Scholar]
- 226.Giri S.S., Kim H.J., Kim S.G., Kim S.W., Kwon J., Lee S.B., Sukumaran V., Park S.C. Effectiveness of the guava leaf extracts against lipopolysaccharide-induced oxidative stress and immune responses in Cyprinus carpio. Fish Shellfish Immunol. 2020;105:164–176. doi: 10.1016/j.fsi.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 227.Kumar N., Sharma J., Singh S.P., Singh A., Krishna V.H., Chakrabarti R. Validation of growth enhancing, immunostimulatory and disease resistance properties of Achyranthes aspera in Labeo rohita fry in pond conditions. Heliyon. 2019;5(2) doi: 10.1016/j.heliyon.2019.e01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bıyıklı M., Koca S.B., Yiğit N.Ö., Metin S., Kara N., Gürbüzer G. The effects of Isatis tinctoria extract on diseases resistance against Aeromonas hydrophila and pigmentation, growth of Pseudotropheus acei. Turkish J. Agri. Food Sci. Tech. 2019;7(2):137–141. doi: 10.24925/turjaf.v7isp2.137-141.3180. [DOI] [Google Scholar]
- 229.Zou C., Su N., Wu J., Xu M., Sun Z., Liu Q., Chen L., Zhou Y., Wang A., Ye C. Dietary Radix bupleuri extracts improves hepatic lipid accumulation and immune response of hybrid grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀) Fish Shellfish Immunol. 2019;88:496–507. doi: 10.1016/j.fsi.2019.02.052. [DOI] [PubMed] [Google Scholar]
- 230.Hoseinifar S.H., Khodadadian Zou H., Paknejad H., Ahmadifar E., Van Doan H. Non‐specific immune responses and intestinal immunity of common carp (Cyprinus carpio) fed Jujube (Ziziphus jujube) fruit extract. Aquacult. Res. 2018;49:2995–3003. doi: 10.1111/are.13759. [DOI] [Google Scholar]
- 231.Van Doan H., Hoseinifar S.H., Elumalai P., Tongsiri S., Chitmanat C., Jaturasitha S., Doolgindachbaporn S. Effects of orange peels derived pectin on innate immune response, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus) cultured under indoor biofloc system. Fish Shellfish Immunol. 2018;80:56–62. doi: 10.1016/j.fsi.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 232.Abdel‐Tawwab M., Abbass F.E. Turmeric powder, Curcuma longa L., in common carp, Cyprinus carpio L., diets: growth performance, innate immunity, and challenge against pathogenic Aeromonas hydrophila infection. J. World Aquacult. Soc. 2017;48(2):303–312. doi: 10.1111/jwas.12349. [DOI] [Google Scholar]
- 233.Hoseinifar S.H., Zou H.K., Miandare H.K., Van Doan H., Romano N., Dadar M. Enrichment of common carp (Cyprinus carpio) diet with medlar (Mespilus germanica) leaf extract: effects on skin mucosal immunity and growth performance. Fish Shellfish Immunol. 2017;67:346–352. doi: 10.1016/j.fsi.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 234.Bilen S., Ünal S., Güvensoy H. Effects of oyster mushroom (Pleurotus ostreatus) and nettle (Urtica dioica) methanolic extracts on immune responses and resistance to Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss) Aquaculture. 2016;454:90–94. doi: 10.1016/j.aquaculture.2015.12.010. [DOI] [Google Scholar]
- 235.Giri S.S., Jun J.W., Sukumaran V., Park S.C. Dietary administration of banana (Musa acuminata) peel flour affects the growth, antioxidant status, cytokine responses, and disease susceptibility of rohu, Labeo rohita. J. Immunol. Res. 2016 doi: 10.1155/2016/4086591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Giri S.S., Sen S.S., Chi C., Kim H.J., Yun S., Park S.C., Sukumaran V. Chlorophytum borivilianum polysaccharide fraction provokes the immune function and disease resistance of Labeo rohita against Aeromonas hydrophila. J. Immunol. Res. 2015 doi: 10.1155/2015/256510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Talpur A.D. Mentha piperita (Peppermint) as feed additive enhanced growth performance, survival, immune response and disease resistance of Asian seabass, Lates calcarifer (Bloch) against Vibrio harveyi infection. Aquaculture. 2014;420:71–78. doi: 10.1016/j.aquaculture.2013.10.039. [DOI] [Google Scholar]
- 238.Awad E., Austin D., Lyndon A.R. Effect of black cumin seed oil (Nigella sativa) and nettle extract (quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquaculture. 2013;388:193–197. doi: 10.1016/j.aquaculture.2013.01.008. [DOI] [Google Scholar]
- 239.Park K.H., Choi S.H. The effect of mistletoe, Viscum album coloratum, extract on innate immune response of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2012;32(6):1016–1021. doi: 10.1016/j.fsi.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 240.Cao H., Xia W., Zhang S., He S., Wei R., Lu L., Yang X. Saprolegnia pathogen from Pengze Crucian carp (Carassius auratus var. Pengze) eggs and its control with traditional Chinese herb. Isr. J. Aquac. Bamidgeh. 2012;64:1–7. [Google Scholar]
- 241.Kim J.S., Harikrishnan R., Kim M.C., Jang I.S., Kim D.H., Hong S.H., Balasundaram C., Heo M.S. Enhancement of Eriobotrya japonica extracts on non-specific immune response and disease resistance in kelp grouper Epinephelus bruneus against Vibrio carchariae. Fish Shellfish Immunol. 2011;31:1193–1200. doi: 10.1016/j.fsi.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 242.Harikrishnan R., Kim J.S., Kim M.C., Balasundaram C., Heo M.S. Prunella vulgaris enhances the non-specific immune response and disease resistance of Paralichthys olivaceus against Uronemamarinum. Aquaculture. 2011;318:61–66. doi: 10.1016/j.aquaculture.2011.05.020. [DOI] [Google Scholar]
- 243.Pratheepa V., Ramesh S., Sukumaran N. Immunomodulatory effect of Aegle marmelos leaf extract on freshwater fish Cyprinus carpio infected by bacterial pathogen Aeromonas hydrophila. Pharm. Biol. 2010;48(11):1224–1239. doi: 10.3109/13880201003713598. [DOI] [PubMed] [Google Scholar]
- 244.Ravikumar S., Selvan G.P., Gracelin A.A. Antimicrobial activity of medicinal plants along Kanyakumari coast, Tamil Nadu, India. Afr. J. Basic Appl. Sci. 2010;2(5–6):153–157. [Google Scholar]
- 245.Abdel Tawwab M., Ahmad M.H., Seden M.E., Sakr S.F. Use of green tea, Camellia sinensis L., in practical diet for growth and protection of Nile tilapia, Oreochromis niloticus (L.), against Aeromonas hydrophila infection. J. World Aquacult. Soc. 2010;41:203–213. doi: 10.1111/j.1749-7345.2010.00360.x. [DOI] [Google Scholar]
- 246.Liu B., Ge X., He Y., Xie J., Xu P., He Y., Zhou Q., Pan L., Chen R. Effects of anthraquinones extracted from Rheum officinale bail on the growth, non-specific immune response of Macrobrachium rosenbergii. Aquaculture. 2010;310(1–2):13–19. doi: 10.1016/j.aquaculture.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 247.Ardó L., Yin G., Xu P., Váradi L., Szigeti G., Jeney Z., Jeney G. Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture. 2008;275(1–4):26–33. doi: 10.1016/j.aquaculture.2007.12.022. [DOI] [Google Scholar]
- 248.Christybapita D., Divyagnaneswari M., Michael R.D. Oral administration of Eclipta alba leaf aqueous extract enhances the non-specific immune responses and disease resistance of Oreochromis mossambicus. Fish Shellfish Immunol. 2007;23(4):840–852. doi: 10.1016/j.fsi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 249.Mok J.S., Song K.C., Choi N.J., Yang H.S. Antibacterial effect of cinnamon (Cinnamomum cassia) bark extract against fish pathogenic bacteria. Korean J. Fish. Aquat. Sci. 2001;34(5):545–549. [Google Scholar]
- 250.Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 2008;46(2):409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 251.Choudhury S., Sree A., Mukherjee S.C., Pattnaik P., Bapuji M. In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fish Sci. 2005;18(3–4):285. doi: 10.33997/j.afs.2005.18.3.009. [DOI] [Google Scholar]
- 252.Genovese G., Faggio C., Gugliandolo C., Torre A., Spano A., Morabito M., Maugeri T.L. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the straits of Messina against pathogens relevant in aquaculture. Mar. Environ. Res. 2012;73:1–6. doi: 10.1016/j.marenvres.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 253.Genovese G., Leitner S., Minicante S.A., Lass‐Flörl C. The Mediterranean red alga Asparagopsis taxiformis has antifungal activity against Aspergillus species. Mycoses. 2013;56(5):516–519. doi: 10.1111/myc.12065. [DOI] [PubMed] [Google Scholar]
- 254.Manilal A., Selvin J., George S. In vivo therapeutic potentiality of red seaweed, Asparagopsis (Bonnemaisoniales, Rhodophyta) in the treatment of vibriosis in Penaeus monodon Fabricius. Saudi J. Biol. Sci. 2012;19(2):165–175. doi: 10.1016/j.sjbs.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Manilal A., Selvin J., Sugathan S. Immuno-modulatory efficacy of Indian red algae, Asparagopsis taxiformis, in Penaeus monodon. J. Appl. Aquacult. 2013;25(1):81–93. doi: 10.1080/10454438.2013.763514. [DOI] [Google Scholar]
- 256.Mai T., Tintillier F., Lucasson A., Moriou C., Bonno E., Petek S., Magre K., Al Mourabit A., Saulnier D., Debitus C. Quorum sensing inhibitors from Leucetta chagosensis Dendy, 1863. Lett. Appl. Microbiol. 2015;61(4):311–317. doi: 10.1111/lam.12461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.