Abstract
Furanic polymers, currently mainly represented by polyethylene 2,5-furandicarboxylate (PEF), also known as polyethylene furanoate, have a fantastic potential to replace fossil-based polymers: for example, polyethylene terephthalate (PET). While 2,5-furandicarboxylic acid (FDCA), a precursor of PEF, and its derived polymers have been studied extensively, 2,5-bis(hydroxymethyl)furan (BHMF) has received relatively little attention so far. Similarly to FDCA, BHMF is a biobased platform chemical derived from renewable sources such as sugars. This review highlights different polymerization techniques for BHMF-based polyesters and addresses BHMF’s relative instability during the synthesis of BHMF-derived polymers, including polycarbonates and polyurethanes. Furthermore, the degradability of furanic polyesters is discussed and BHMF’s toxicity is briefly elaborated.
Introduction
Conventional polyethylene terephthalate (PET) is indispensable in today’s society, since it is ubiquitously used in bottles, packaging, fibers, and more applications due to its favorable properties: high strength to weight ratio, light transmittance, and low permeability.1−3 Although PET is generally seen as nonbiodegradable, a great deal of research has been performed on enzymatic degradation or catalytic depolymerization of PET.1,4,5 PET is industrially produced from fossil-based ethylene and para-xylene, which are converted to ethylene glycol and terephthalic acid or dimethyl terephthalate, which act as the monomers.2 Although the production of 100% Bio-PET has been achieved, a multitude of disadvantages are present, including high feedstock costs, limited resources, and low yields.2
Biobased polyethylene furanoate (PEF) may be a greener alternative to PET due to its similarities in chemical structure and enhanced properties, such as a higher Tg, lower Tm, higher yield stress, and improved gas barrier properties, i.e. lower water, CO2, and O2 permeability.6−10 However, PEF has typically a lower elongation at break and a reduced crystallinity and is still far from cost competitive with PET.8,11 The Dutch company Avantium is one of the main drivers commercializing PEF on a large scale by a novel catalytic process for the production of furanics.7,9 PEF can be synthesized from the monomers ethylene glycol (EG) and 2,5-furandicarboxylic acid (FDCA). EG can be obtained from bioethanol, and FDCA is mostly produced from sugars through a multistep process.7−9
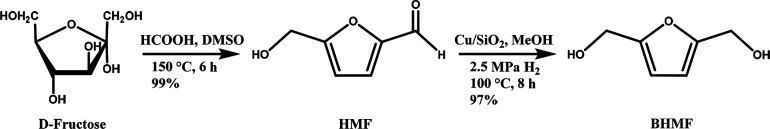
5-Hydroxymethylfurfural (HMF) is typically derived from sugars and is considered a renewable platform chemical due to its versatile reactivity and role as a precursor in many possible applications, e.g., the production of FDCA.11−13 Another less highlighted monomer derived from HMF is 2,5-bis(hydroxymethyl)furan (BHMF). BHMF can be easily obtained from HMF in very high yields, up to 99%, via an enzymatic or chemical reduction reaction.11,14−16 Synthetic routes toward BHMF have been discussed extensively in the literature, including the recent and comprehensive review by Aricò.15−18 Although BHMF is mainly studied as a monomer, it can also act as a building block for plasticizers, biodiesel additives, flame retardants, and surfactants.19−24 Both FDCA and BHMF have the advantage of a relatively higher thermal and chemical stability compared to HMF, which is susceptible to degradation to levulinic acid, humins, and other degradation products.13,15,25,26 An example of a potential synthetic production pathway for BHMF from d-fructose is illustrated in Figure 1, derived from the work of Cao et al. and Thananatthanachon and Rauchfuss.14,26 The latter converted d-fructose to HMF in DMSO with formic acid as a catalyst, yielding 99% HMF. On the other hand, Cao et al. used a low-cost reusable Cu/SiO2 catalyst for the HMF reduction to BHMF in a 97% yield. More than 30 synthetic production paths have been elaborated in the review by Aricò, for example from HMF or directly from fructose.15 A simple, cheap, and effective route directly from a stable compound is preferred over the use of unstable HMF as the starting material. Furthermore, the isolation, scale-up, and purification of the BHMF reaction mixtures need special attention.15
Figure 1.
Two step synthesis route for the production of BHMF from D-fructose, data obtained from refs. (14 and 26)
The extensively studied BHMF synthesis routes are promising for large-scale production; thus, the valorization of BHMF to biobased polymers is worth highlighting. Although BHMF is difunctional, it usually requires a second difunctional comonomer to produce a polymer. In the case of a polyester, a diacid or a dimethyl ester is necessary in order to allow polycondensation. Succinic acid and adipic acid are suitable comonomers and are (potentially) obtained from biobased resources, like carbohydrates, using micro-organisms like fungi and bacteria.27,28 This highlights that it is feasible to produce fully biobased BHMF-based polyesters.
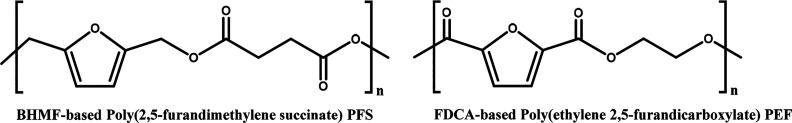
BHMF is structurally very similar to FDCA, since both monomers contain an aromatic furan ring and have short side groups at positions 2 and 5. A difference between BHMF- and FDCA-based polyesters is the position of the ester group, as illustrated in Figure 2. FDCA and BHMF possess significant differences in their melting points, which are 342 and 74–77 °C, respectively.29,30 Typically, FDCA appears as a white crystalline powder, while BHMF is more yellowish. However, this difference in color needs to be carefully taken into account, as this yellow color could also be caused by minor impurities, since BHMF has also been reported to appear as a white solid.31 While FDCA itself is acidic, when BHMF is exposed to an acidic environment (e.g., the Brønsted acid H2SO4) it degrades to humins.32 Another physiochemical difference between these two furan compounds is their thermal stability. FDCA can withstand high temperatures, for example during bulk polymerization at a temperature higher than 200 °C.33 In contrast, BHMF has limited thermal stability and its degradation is already induced at 120–130 °C.34,35 These differences might be explained by the difference in reactivity and electronegativity of the carboxylic acid groups and the hydroxy groups close to the furan ring. The carboxylic acid groups of FDCA are already in the highest oxidation state, while the hydroxyl groups of BHMF are still prone to oxidation. HMF contains, similar to BHMF, a methoxy group attached to the furan ring. It is known that this side of HMF is able to react with water to open the ring structure and form 2,5-dioxo-6-hydroxyhexanal via a multistep process, which turns subsequently into humins.36,37 A similar reaction might take place during thermal degradation of BHMF in the presence of moisture. Detailed degradation pathways of both FDCA and BHMF are an interesting topic to explore. Other comparisons between them are their toxicity properties, since it is expected that the different functional groups will influence toxicity as well, which is briefly discussed later in this review.
Figure 2.
Structural representation of poly(2,5-furandimethylene succinate) (PFS) and poly(ethylene 2,5-furandicarboxylate) (PEF).
Nevertheless, based on the chemical similarities it is expected that BHMF-based polyesters will have properties similar to those of their FDCA-based counterparts. Consequently, polymers derived from renewable BHMF are very interesting to study as sustainable alternatives for fossil-based polymers.
The current status of literature covering BHMF-based polymers is limited compared to that for other furanic polyesters. This indicates that the promising precursor BHMF is still in the shadow of other biobased building blocks and polymers. Four recent reviews by Lalanne et al., Zhang et al., Kashparova et al., and Chernyshev et al. focused mainly on the synthesis of furanics and FDCA polymers and only shortly elaborated on a few examples of BHMF-based polymers.11,38−40 To provide a comprehensive overview of the field, this review highlights and summarizes the major peer-reviewed articles on BHMF-based polymers, thereby discussing the synthesis routes and their potential. The topic is broadened by including the (bio)degradability of furanic polyesters.
BHMF-Based Polyesters
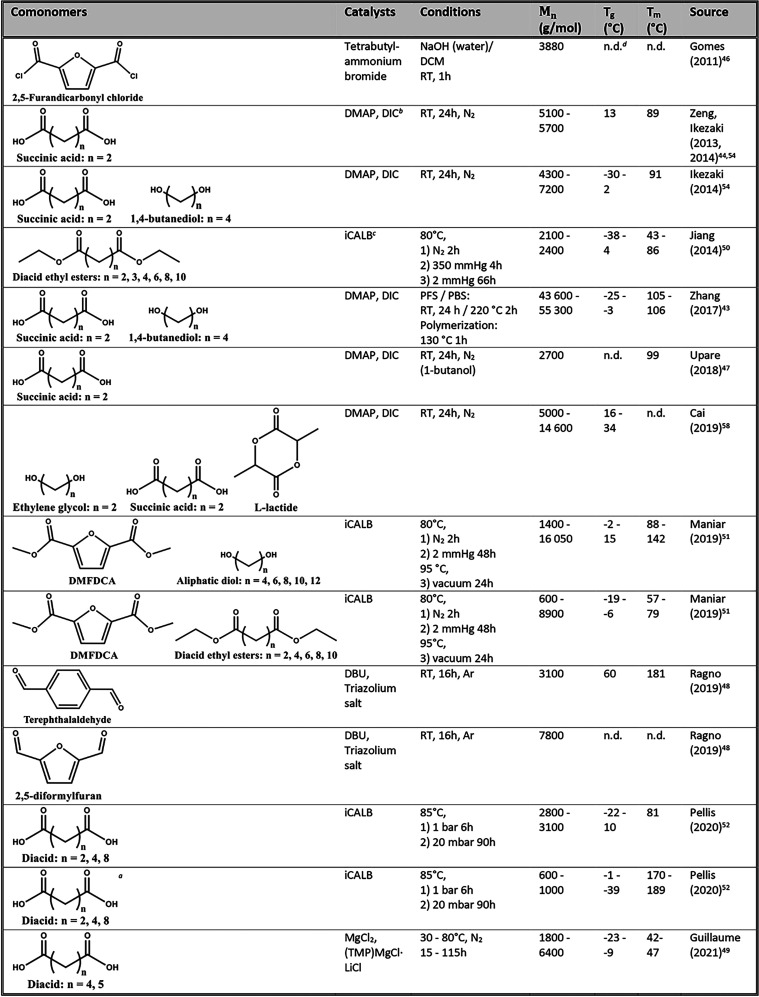
While many articles have been published on the synthesis and recyclability of FDCA-derived polyesters, not much research has been conducted on polymers based on BHMF.11,41,42 The reason for this could be BHMF’s relative thermal instability during high-temperature (bulk) polymerization.43 Therefore, most aliphatic furanic copolyesters based on BHMF reported in the literature are formed either by enzymatic polymerization or by organo-catalyzed solution polymerization at low temperature.44 In the following paragraphs, the current literature on BHMF polyesters is highlighted by polymerization method, i.e. solution polymerization, enzymatic polymerization, and Diels–Alder cross-linking. An overview of the products, including comonomers, polymerization conditions, molecular weights, and thermal properties, is displayed in Table 1.
Table 1. Synthesis of Furanic Polyesters Based on BHMF: Comonomers, Catalysts, Reaction Conditions, and Thermal Transition Points.
Note that this polyester was based on 3,4-BHMF and not on 2,5-BHMF, like all others.
N,N-Dimethyl-4-aminopyridine and N,N′-diisopropylcarbodiimide are abbreviated DMAP and DIC, respectively.
All enzymatic polymerizations were catalyzed by immobilized Candida antarctica Lipase B (iCALB).
The Tg and Tm values could not be determined for all polyesters; hence, only the reported values are reproduced.
Solution Polymerization
To the best of our knowledge, the first reported BHMF-based polyester was reported by Moore and Kelly in 1978.45 Several polyesters, based on furan or tetrahydrofuran monomers, were synthesized, including one with FDCA and BHMF units. To avoid thermal degradation during synthesis, solution polymerization in chloroform was preferred over melt polycondensation. A high-furan-content polyester was produced from 2,5-furandicarbonyl chloride and BHMF by using trimethylamine as an acid acceptor. The polyester degraded to a black insoluble material at around 200 °C. More properties were unfortunately not reported.
Gomes et al. provided additional information by synthesizing several polyesters based on FDCA and various diols, including BHMF, using three different polymerization techniques: solution polymerization, interfacial polycondensation, and polytransesterification.46 Poly(2,5-furandimethylene 2,5-furandicarboxylate) was prepared via interfacial polymerization of 2,5-furandicarbonyl chloride and BHMF under mild conditions in a biphasic system. However, only a low-molecular-weight polymer was obtained which had medium thermal stability: Td,onset = 205 °C and Td,max = 345 °C.
A different approach was taken by Upare and co-workers, who focused on the synthesis of BHMF from fructose and subsequently polymerized it with succinic acid.47 Fructose was converted to HMF in a high yield (95%) using Amberlyst-15, which was then reduced to BHMF with a CuSiO2 catalyst under an H2 atmosphere. However, due to its high solubility and low thermal stability, BHMF could not be separated from its solvent (1-butanol) by either crystallization or distillation. To address this issue, BHMF was polymerized with succinic acid to produce a solid, thermally stable polymer that could be isolated and analyzed.
Usually, a polyester is synthesized via polycondensation using a diol and a diacid or the corresponding dimethyl or diethyl ester of the diacid. However, Ragno et al. used a Lewis base organocatalyst (N-heterocyclic carbene (NHC)) in order to polymerize a diol and a dialdehyde.48 Both fossil-based and biobased monomers were used, including BHMF. Terephthalaldehyde and 2,5-diformylfuran were independently reacted with BHMF under oxidative conditions, using a triazolium salt and DBU as catalysts and THF as a solvent. The obtained polyesters, poly(2,5-furandimethylene terephthalate) and poly(2,5-furandimethylene 2,5-furandicarboxylate), showed medium and poor thermal stabilities (Td,onset = 247 and <200 °C, respectively).
Recently, Guillaume et al. described the synthesis of BHMF from FDCA followed by polymerization with either adipic acid or pimelic acid.49 FDCA was first converted to the dimethyl ester with a Lewis acid (MgCl2) as a catalyst and dimethyl dicarbonate (Moc2O) as a coupling agent. Following that, BHMF was produced via hydrogenation with a Ru-based catalyst (Gusev). The polymerization was successfully performed under mild conditions using an Mg-based catalyst and di-tert-butyl dicarbonate (Boc2O) as a coupling agent. In addition, they showed that a one-pot synthesis of BHMF-based polyester from FDCA as a starting material is possible under similar conditions.
Enzymatic Polycondensation
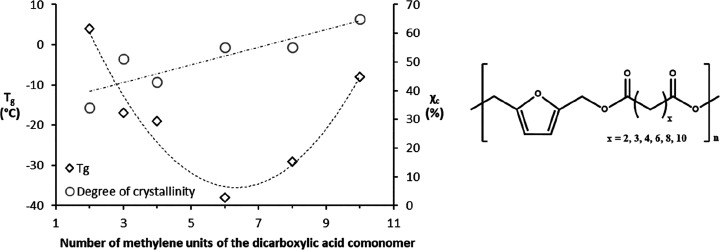
The first report covering enzymatic polymerization of BHMF with various diacid ethyl esters, containing different numbers of methylene units (Figure 3), was published by Jiang et al. in 2014.50 The immobilized enzyme Candida antarctica Lipase B (iCALB), was used as a biocatalyst in a three-stage process. Semicrystalline polymers were obtained with a low molecular weight (Mn < 2400), which was explained by ether formation via dehydration between the BHMF hydroxyl groups. It was found that increasing the number of methylene units in the chain resulted in increased crystallinity, thermal stability, and generally a higher melting temperature (Tm). This is due to the improved packing capability of the aliphatic units in the polymer chains. The glass transition temperature (Tg) of the obtained polyesters reached a minimum for poly(2,5-furandimethylene suberate), related to the equilibrium of chain flexibility and crystallinity (Figure 3).
Figure 3.
Glass transition temperature (Tg), second heating curve, and degree of crystallinity (χc) versus the number of methylene units of dicarboxylic acid comonomers in BHMF-based polyesters. Data are reprinted with permission from ref (50). Copyright 2014 American Chemical Society.
Another study by Maniar et al. covered the enzymatic synthesis of aliphatic “aromatic” copolyesters, originating from three different monomers.51 These monomers included dimethyl 2,5-furandicarboxylate (DMFDCA), BHMF, and an aliphatic diol or diacid ethyl ester, with varying numbers of methylene units. The copolyesters prepared from diols demonstrated the highest degree of polymerization and a higher thermal stability than diacid-ethyl-based polyesters. An increased aliphatic diol chain length led to a reduction of both Tg and Tm. The same held for the Tg value of the diacid-ethyl-based polyesters, but crystallization was not observed in all cases.
Similarly, Pellis et al. reported the synthesis of aliphatic-aromatic polyesters based on furan, benzene, and pyridine derivatives using the same enzyme.52 The use of 3,4-bis(hydroxymethyl)furan (3,4-BHMF) was novel, while 2,5-BHMF was also used with succinic, adipic, or sebacic acid as comonomer. The 3,4-BHMF-based polyester was obtained in lower yield and significantly lower molecular weight compared to 2,5-BHMF. However, a TGA analysis showed a significantly higher thermal stability for the 3,4-BHMF polyester compared to 2,5-BHMF. For both polymer series, it was observed that a higher number of methylene units in the chain resulted in a lower Tg value, due to enhanced chain mobility and flexibility of the aliphatic units.
Diels–Alder Cross-Linking
A Diels–Alders reaction involves the cycloaddition of a conjugated diene and an alkene to form dynamic covalent bonds. Furanic moieties in BHMF-based polymers can act as the conjugated dienes and allow the introduction of thermoreversible cross-links via Diels–Alder chemistry (Figure 4).53
Figure 4.
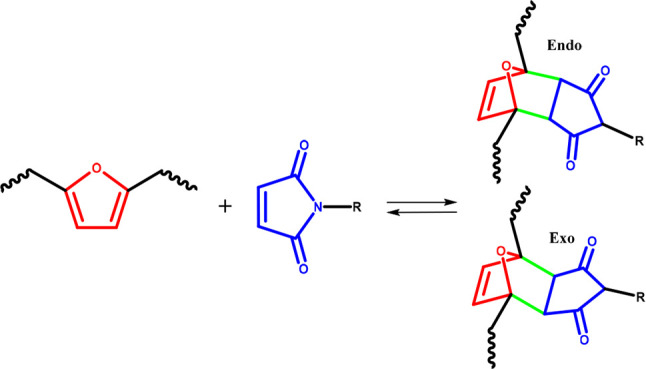
Schematic representation of the reaction between a furan unit (red) and a maleimide (blue) to form dynamic covalent bonds (green). Figure adapted and reproduced with permission from ref (53). Copyright 2022 Macromolecular Rapid Communications.
This has been demonstrated by Yoshie’s research group, which published numerous articles on self-healing polyesters based on dynamic Diels–Alder cycloaddition between the furan groups of BHMF and bismaleimides.44,54−56 This included the synthesis of low-molecular-weight non-cross-linked polyesters from the polycondensation of BHMF, succinic acid, and 1,4-butanediol to widen the properties. It was discovered that increasing the aliphatic content with the addition of 1,4-butanediol significantly reduced the Tg value.54 Subsequently, the resulting polymers were thermoreversibly cross-linked with 1,8-bis(maleimido)triethylene glycol in different ratios. This resulted in high-strength polymers with a tensile strength of up to 50 MPa. Based on toughness, the reversible network’s self-healing efficiency was reported to be as much as 74%. The polymerization conditions and thermal properties of the non-cross-linked BHMF-based polyesters are presented in Table 1.
Zhang et al. reported a similar study in which they prepared a multiblock polyester based on BHMF, succinic acid, and 1,4-butanediol.43 Two different blocks were synthesized in solution with N,N′-diisopropylcarbodiimide (DIC) and 4-dimethylaminopyridine (DMAP) as catalysts: a BHMF-succinic acid block (PFS) and a 1,4-butanediol-succinic acid block (PBS). It was found that high-molecular-weight multiblock polymers could be prepared, the different segments of which were miscible in the amorphous region. The Tg value increased with higher BHMF content in the polymer, while the thermal stability (Td5%) decreased and Tm remained relatively constant. The tensile strength decreased with higher PFS content (13 to 30 MPa), while the elongation at break increased (420 to 720%). In a follow-up study, the BHMF-based block polyesters were used for the preparation of a thermoreversible network, created by Diels–Alder cross-linking using a bismaleimide.57
Finally, Cai et al. synthesized a shape-memory polymer with self-healing properties based on BHMF, lactic acid, succinic acid, and ethylene glycol.58 Lactide and ethylene glycol were used to prepare a hydroxyl-terminated polymer, which was subsequently polymerized with BHMF and succinic acid. The addition of BHMF and succinic acid lead to a reduced Tg value of the PLA-based polymer. Using the Diels–Alder reaction, the obtained polymer was then cross-linked with varying amounts of a bismaleimide. It was shown that increasing the cross-link density resulted in a higher Tg value. The thermal properties of the non-cross-linked polymer are shown in Table 1.
Melt Polycondensation of BHMF
The use and potential of BHMF are “largely underestimated”, as Guillaume et al. asserted.49 Although the polymerization of BHMF has been proven to be possible, only low molecular weights (Mn) have been obtained, up to a maximum of 16000 for high-BHMF-content polymers. Furthermore, enzymatic pathways, catalysis under mild reaction conditions, or multiple polymerization steps are time-consuming and cost-inefficient. Melt polycondensation would give a promising outcome but is thought to be challenging due to the (thermal) instability of BHMF.43,50
However, Nasirudeen et al. reported a successful polymerization of FDCA with several diols, including BHMF.59 Polymerization was performed at 160 °C for 6 h under a N2 atmosphere, and then the temperature was raised to 200 °C and a vacuum was applied to remove excess diol, although it remains unclear if this method was also applied for the FDCA-BHMF polymer. Thermal and mechanical characterization of poly(2,5-furandimethylene 2,5-furandicarboxylate) was not reported in the article; only the water absorption and water contact angle were measured. Other polymeric details, such as the molecular weight and thermal stability of BHMF, were not provided.
In addition, Gopalakrishnan et al. reported the synthesis of FDCA-based polyesters, and mentioned the polymerization of BHMF and adipic acid via direct polycondensation.60 Synthesis details for this polyester were not given; however, it yielded an amorphous material with an onset decomposition temperature of 300 °C. Prior to publication of the article, similar information was revealed in a patent in which Gopalakrishnan is one of the named inventors.61
Synthesis of Other BHMF-Based Polymers
The potential of BHMF as a polymeric building block has also been demonstrated for other types of polymers, like polyurethanes and polycarbonates. Despite the fact that the thermal stability of BHMF has been addressed in several articles, various potential solutions have been reported.
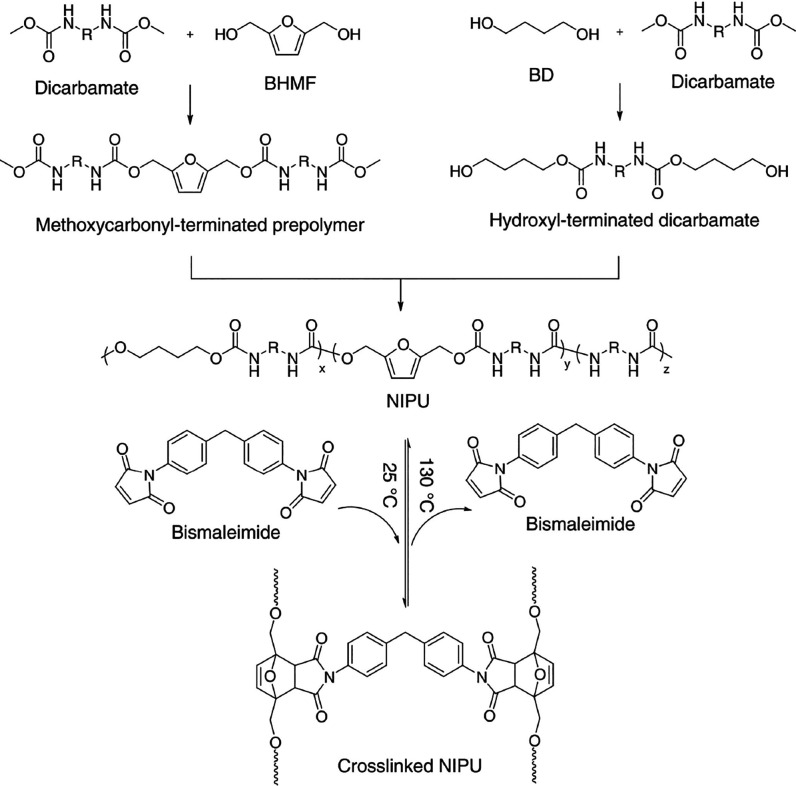
Zhang et al. reported that BHMF undergoes side reactions during the synthesis of polyurethanes at temperatures higher than 140 °C.62 The authors overcame this problem by prepolymerizing BHMF with an excess amount of dicarbamates at lower temperatures via “transurethanization”. Using a bismaleimide, this resulted in reversible Diels–Alders cross-linked polyurethanes (Figure 5).
Figure 5.
Synthesis route of nonisocyanate polyurethane (NIPU) based on BHMF, 1,4-butandiol, and dicarbamates, thermoreversibly cross-linked with a bismaleimide. Reproduced with permission from ref (62). Copyright 2019 Journal of Polymer Science.
In order to address BHMF’s thermal stability, Oh and co-workers used mechanochemical synthesis to create eco-friendly polyurethanes.63 The polymers from methylene diphenyl diisocyanate and BHMF were synthesized at room temperature for 60 min in the presence of a catalyst. This resulted in polyurethanes with a high molecular weight (Mn 59000) and moderate thermal stability (Td5% = 201 °C).
Another solution was presented by Kieber et al., who initially reported that polyurethane synthesis of BHMF and diisocyanate-functionalized isohexides led to brown discoloration at a temperature of 120 °C.34 This was attributed to BHMF decomposition, which was then prevented by polymerizing at 30 °C for 24 h with a catalyst (dibutyltin dilaurate) under a nitrogen atmosphere.
Choi et al. attempted to synthesize a BHMF-based polycarbonate via a conventional synthetic pathway.35 However, BHMF could not withstand the high temperatures (130–240 °C) and degraded into insoluble “hydrothermal carbon”. To circumvent this, copolymerization with highly reactive carbonyldiimidazole under mild conditions (40 °C) was performed via a two-step process. The temperature control was crucial and had a significant effect on the molecular weight. Interestingly, the addition of N-substituted maleimide units to the furanic units resulted in an enhanced Tg value due to increased torsional strains and reduced chain mobility.
BHMF was applied in a study by Lillie et al. as well. The research team synthesized several polymers via acyclic diene metathesis (ADMET) polymerization, including a BHMF/10-undecenoic acid based polymer.64 Fisher esterification was not possible for polyester synthesis from BMHF and 10-undecenoyl chloride due to significant decomposition of BHMF. The polyester was successfully obtained via solution polymerization in dichloromethane using 4-dimethylaminopyridine and trimethylamine.
A special type of a BHMF-based polymer was developed by Vijjamarri et al., who synthesized various biobased poly(silyl ethers).65 This unique class of polymers contains Si–O–C units in the main chain, making them susceptible for biodegradation. Various poly(silyl ethers) were produced from BHMF and different hydrosilanes by using a manganese catalyst and toluene as solvent. High-boiling-point solvents were tried as well, but without success due to the low solubility of BHMF and the formation of dark insoluble solids, which were most likely BHMF degradation products. The BHMF-derived polymer obtained had a Tg value of 15.6 °C and a decomposition temperature (Td50%) of 453 °C. A hydrolysis experiment at low pH demonstrated complete degradation in a few hours, which was attributed to the cleavage of the Si–O–C bonds.
Another way of producing BHMF-based polymers is by introducing epoxy groups by a reaction with reactive epichlorohydrin under low-temperature conditions to form 2,5-bis[(2-oxiranylmethoxy)methyl]furan (BOF) (Figure 6). Cho et al. synthesized diepoxy compounds based on furanics, including BHMF, which were studied as adhesives after photocuring.66 BHMF was reacted with epichlorohydrin in a biphasic system and a phase transfer catalyst. Curing was performed using a cationic photoinitiator (IRGACURE 250) by irradiating with UV light. After 5 min of curing, adhesive shear stresses of up to 4.7 MPa were measured, although an adhesive based on furfuryl alcohol was stronger.
Figure 6.
Reaction between BHMF and epichlorohydrin to form the diepoxide 2,5-bis[(2-oxiranylmethoxy)methyl]furan (BOF). Figure adapted from ref (66). Copyright 2012 Taylor & Francis.
In a follow-up study by the same group (Jeong et al.), furfuryl alcohol and BHMF were converted to epoxy compounds using epichlorohydrin and subsequently reacted with methacrylic acid.67 Unfortunately, the observed adhesives strengths were significantly lower compared to their previous findings, i.e. less than 0.7 MPa.
A similar approach was taken by Hu et al., who prepared thermosetting polymers based on aromatic diepoxies and standard diamine curing agents.68 One of the monomers was 2,5-bis[(2-oxiranylmethoxy)methyl]furan (BOF), which was produced from BHMF and epichlorohydrin (Figure 6). Subsequently, the epoxy monomers were mixed in various ratios (0–1.0) with diglycidyl ether of bisphenol A (DGEBA) and cured by using a diamine curing agent, PACM or EPIKURE W. They observed improved thermomechanical behavior of the BHMF-derived thermosets. This was assigned to hydrogen bonding between the furanic oxygen and epoxy-amine hydroxyl groups or due to hindered chain rotation of the furan moieties.
Recently, Wang et al. used a cobaltic nitrogen-doped carbon catalyst for the production of BHMF from HMF in high conversion and yield, 99.3% and 91.5%, respectively.69 Subsequently, BHMF was used for the production of a porous hyper-cross-linked polymer (HCP) (Figure 7), meant for CO2/H2 storage. This was achieved via a Friedel–Crafts alkylation reaction using ferric chloride as the catalyst and 1,2-dichloroethane as the solvent. The BHMF-derived HCP showed a superior CO2 absorbability and similar H2 absorption compared to benzene-based HCPs.
Figure 7.
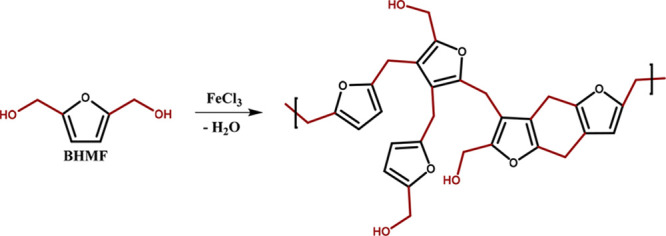
Schematic representation of BHMF conversion to a hyper-cross-linked polymer (HCP). Reproduced with permission from ref (69). Copyright 2022 Wiley-VCH GmbH.
Degradability of Furanic Polyesters
Biobased and (bio)degradable are not correlated; for example PE, and nylon can be (partly) produced from biobased resources but are not biodegradable, while commercially available poly(butylene succinate) (PBS) is often fossil-based and biodegradable.70 Biodegradability is a wide concept, since a number of materials finally will degrade in nature after a very long time. Therefore, polymers are usually referred to as compostable polymers if they meet the requirements of the standardized test ISO 17088 (2021). A compostable polymer/plastic is therein defined as “a plastic that undergoes degradation by biological processes during composting to yield CO2, water, inorganic compounds and biomass at a rate consistent with other known compostable materials and leave no visible, distinguishable or toxic/hazardous residue”.71
(Bio)degradability of polymers is often attempted to be estimated in the literature via enzymatic hydrolysis or a simple soil burial degradation test. While this is scientifically wrong and by no means an estimate of biodegradability, various literature sources are highlighted on the (bio)degradability of a furanic aliphatic polyester in the following paragraphs to provide a current overview of this field.
(Bio)degradation of PEF
To the best of the authors’ knowledge, the biodegradability of BHMF-based polyesters has not been studied yet. However, there are several reports on the degradability of other furanic polyesters, mainly PEF. For example, the Dutch company Avantium proved that PEF could be composted under industrial composition conditions (58 °C, Organic Waste Systems (OWS)).72
Pellis et al. investigated the enzymatic hydrolysis of PEF powders for various molecular weights.73 Surprisingly, a higher-molecular-weight PEF hydrolyzed more quickly by a cutinase 1 enzyme from Thermobifida ellulosilytica. This was observed by measuring the FDCA concentrations; thus, PEF can theoretically be completely depolymerized by this enzyme. It is known that other factors, like the degree of crystallinity, can have a major effect on the depolymerization rate as well, but all PEF samples had low (<1%) and similar crystallinity.
The enzymatic hydrolysis of PEF powders was also studied by Weinberger et al., who focused on the effect of crystallinity, molecular weight, and particle size.74 The authors reported PEF hydrolysis via cutinase 1 to be faster for smaller particle sizes, amorphous structures, and lower molecular masses. In addition, the enzymatic hydrolysis of PEF films was studied and it was observed that a film was completely hydrolyzed to FDCA and water-soluble oligomers after 72 h at 65 °C. Higher temperatures were found to improve enzymatic degradation of PEF, due to higher chain mobility close to the Tg value. Higher enzyme kinetics at higher temperatures are also expected to play a role.
In a follow-up study by the same authors, PEF and PET films were prepared with different crystallinities and their enzymatic hydrolysis was subsequently analyzed.75 Although weight losses were still limited after 72 h, i.e. less than 10%, it was discovered that PEF film degraded 1.7 times more quickly than PET films. Furthermore, increased crystallinity obtained through annealing near the Tg value reduced the hydrolysis rate; however, this effect was less significant for PEF.
Austin et al. developed a promising PET-digesting enzyme (PETase).76 It was found that PETase and a double mutant were able to degrade a bottle-grade PET with 15% crystallinity. The same enzymes were also capable of degrading PEF, which was visualized by a relative reduction in crystallinity (15.7%) and a change in surface morphology. PETase was only active for PEF and PET and not for the aliphatic polyesters PLA and PBS. An engineered enzyme for polyester degradation would be interesting for industrial recycling processes but is irrelevant for biodegradation in the environment.
(Bio)degradation of Furanic Copolyesters
Findings related to the enzymatic hydrolysis of other furanic copolyesters were also previously reported. Okada et al. produced furanic polyesters based on isosorbide, isomannide, difuranic esters, aliphatic segments, and combinations thereof.77 They reported that spontaneous hydrolysis and enzymatic degradation could be achieved, but degradation was limited. It was observed that a higher aliphatic content favored enzymatic degradation. This was due to the sterically hindered furanic groups, which hindered the accessibility of the ester group by enzymes. However, a higher aliphatic content increases the hydrophobicity as well, which inhibits enzymatic degradation. In soil burial degradation experiments, SEM revealed that spores were situated on the polymer surface, while filamentous fungi eroded the surface, implying that these polymers are biodegradable.
The degradability of PEF was improved by the random incorporation of lactic acid units by Matos et al.78 First, bis(hydroxyethyl)-2,5-furandicarboxylate was prepared from FDCA and ethylene glycol and subsequently copolymerized in bulk with PLA oligomers. The degradation was simulated via hydrolysis in a phosphate buffer solution at 37 °C. The copolyester consisting of 73% PEF units lost 22% of its original weight after 12 weeks. PEF/PLA copolyesters (PEF 2 wt %, PLA 7 wt %) showed an enhanced degradation compared to their homopolyesters. This was assigned to a reduced Tg value, lower crystallinity, and increased hydrophilicity, which was indicated by increased water absorption.
A similar study was performed by Wu et al., who also synthesized copolyesters from lactic acid oligomers and esters of FDCA and ethylene glycol.79 They characterized the thermal properties, molecular weight, and degradation behavior of these copolyesters in a phosphate buffer solution and garden soil. It was found that the incorporation of lactic acid units in the aliphatic furanic copolyester improved the degradation significantly, both in the phosphate buffer and in garden soil. Mass losses of up to 67% after 55 days at 35 °C in garden soil were observed for the copolyester containing 80% lactic acid units, while the degradation was only 15% w/w for the polyester consisting of 20% lactic acid. These results indicated that the introduction of furanic moieties reduced biodegradability. However, the cleavage of the ester bonds was found to be random and the molecular weight (Mn: 68000–130000) and Tg values (68–79 °C) were different for each copolyester and increased with reducing lactic acid content.
Terzopoulou et al. reported the synthesis of poly(ethylene furanoate-co-ethylene succinate), a polymer similar to PEF but containing more aliphatic units.80 The authors claimed that FDCA-based polyesters (PEF) are not biodegradable but copolymerization with biodegradable aliphatic poly(ethylene succinate) could improve this. They found that the copolyesters containing up to 50% PEF showed still some enzymatic degradation in 1 month, i.e. <6%. An increasing ethylene succinate content significantly improved the enzymatic degradation. This was explained by reducing the concentration of rigid furan structures, which hindered the access of enzymes. In addition, higher PEF contents increased the Tm and Tg values, which reduced chain mobility and hydrolysis. Nevertheless, high aliphatic contents are usually undesirable, since they lead generally to weaker mechanical properties with respect to their aromatic counterparts.
The hydrolytic stability and enzymatic hydrolysis of polyesters based on FDCA, sulfonated isophthalic acid, and various diols were studied by Haernvall and co-workers.81 The polyesters studied showed good hydrolytic stability under neutral conditions at 50 °C, since only low amounts of FDCA could be detected. The hydrolysis rate was higher when the enzyme cutinase 1 from Thermobifida cellulosilytica was added under similar conditions. An increased length of the aliphatic diol reduced the enzymatic hydrolysis. Replacement of the diol with an ether diol also improved the enzymatic degradation, caused by the increased hydrophilicity. The incorporation of ionic phthalic acid did not negatively affect the enzymatic degradation.
Finally, Papadopoulos et al. synthesized copolyesters from 2,5-dimethylfuran dicarboxylate (DMFD), adipic acid (AA), and ethylene glycol (EG) in different ratios.82 One of their experiments used the enzymatic hydrolysis of copolyester films via Rhizopus oryzae lipase and Pseudomonas cepacia lipase. It was observed that copolyesters consisting of 5% to 10% PEF and respectively 95% and 90% poly(ethylene adipate) (PEAd) showed an enhanced enzymatic degradation compared to neat aliphatic PEAd. This was explained by the reduced crystallinity and lower melting points of these copolyesters, compared to PEAd. A higher PEF content leads to higher Tg and Tm values, if crystalline regions are present, resulting in lower biodegradability. A 50/50 PEF/PEAd copolymer showed practically no hydrolysis and mass loss after 30 days.
Currently, no single standardized ISO/ASTM test exists for biodegradability of polymers, as also recently outlined by the SAPEA report “Biodegradability of plastics in the open environment”.83 Instead, multiple standardized tests are available to evaluate a polymer’s degradability under various circumstances, ranging from different aqueous systems to slurry digestion systems, under solid conditions for aerobic or anaerobic biodegradation. Examples are ISO 14851:2019 (aerobic degradability of plastic materials in an aqueous medium), ISO 13975:2019 (anaerobic degradation of plastic materials in controlled slurry digestion systems), and ISO 17556:2019 (aerobic degradability of plastic materials in soil).84−86 In addition, polymeric properties associated with biodegradability include but are not limited to molecular weight, crystallinity, Tg and Tm, polymer structure, size, and hydrophobicity. In addition, the enzyme type and environmental conditions like temperature, moisture content, pH, presence of oxygen, and nutrients are important factors as well.70 Furthermore, aliphatic aromatic polyesters are not produced in nature; thus, it is not surprising that enzymes like cutinase, which has evolved for the degradation of natural cutin polyester, work rather slow for these polyesters.73 The influence of the main polymeric properties on (bio)degradability are given in Table 2. Enzymes catalyze a certain specific reaction, and therefore enzymatic degradation results usually only in depolymerization. None of the discussed articles reported above showed the real biodegradation of furanic polyesters, as the research was based on composting tests or enzymatic depolymerizations.
Table 2. Influence of the Molecular Weight, Crystallinity, Tm and Tg, Polymer Structure, Particle Size, and Hydrophobicity on the Enzymatic Degradability of Polyesters, Derived from Tokiwa et al.70 and References Discussed in this Section.
In cases where furan-based polyesters are broken down to their monomers, these should subsequently be converted to CO2 and H2O to complete the biodegradation. It is known that the fungal biodegradation pathway of BHMF is a multistep process. BHMF is first converted to HMF, then to HMFCA, subsequently via FDCA and FCA, before it enters the tricarboxylic acid cycle (TCA), also known as the Krebs cycle, where CO2 is released.87
Based on a review of the literature on degradability and enzymatic hydrolysis of furan-based polyesters, it is logical to postulate that BHMF-based polyesters will be able to slowly degrade to their monomers, possibly through biological processes, similarly to PEF. The chemical structure and physical properties, on the other hand, will have a significant impact on the degradation rate.
Toxicity of BHMF
Polymers should meet a multitude of requirements, like thermal and physical properties, but a very important one is safety. Safety is related here to the exposure of monomers to humans, animals, or the environment, which could happen during production. In addition, exposure may occur if monomers are released during chemical recycling, such as by hydrolysis or pyrolysis, or biodegradation.10
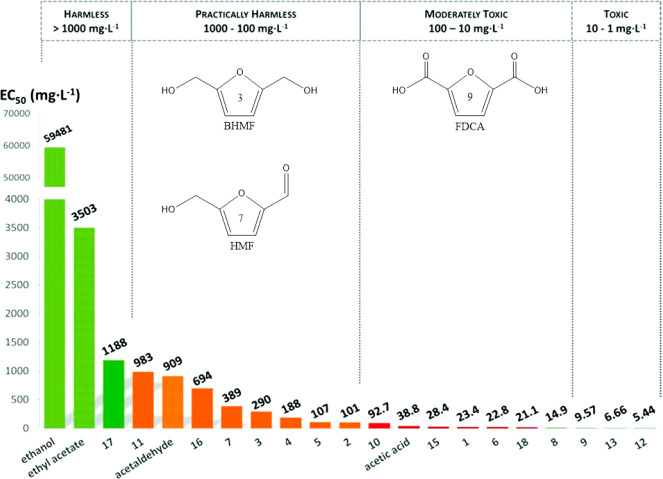
Ventura et al. did an extensive study on the toxicity of furanic platform chemicals, including BHMF, FDCA, and HMF, an intermediate in the production of BHMF and FDCA.88 The toxicity of these compounds was investigated using marine bacteria,Vibrio fischeri, based on their bioluminescence response, known as the Microtox test. In a very clear overview (Figure 8), BHMF and HMF were classified as “practically harmless”, while FDCA was considered to be “toxic”. The differences in toxicity between furanics were explained by the differences in hydrophobicity and the correlated penetration into cells. Furthermore, alcohol groups were generally regarded as less toxic: for example, the toxicity of furan-2,5-dicarbaldehyde was reduced by converting it to HMF or BHMF.
Figure 8.
Relative toxicities of BHMF (3), HMF (7), FDCA (9), other biobased compounds, and reference compounds represented as the median effective concentration (EC50), in mg L–1. The figure is modified and reproduced with permission from ref (88). Copyright 2016 The Royal Society of Chemistry.
Pan et al. reported that HMF derivatives, including FDCA and BHMF, are less toxic to microorganisms.89
Although there is still a lack of information on the toxicity of furanic monomers such as BHMF and FDCA, it is important to have information on the potential toxic effects. Most furanic polyesters are still in the development stage, and their toxicity affects not only their production but also their recycling or biodegradation.
Conclusion
This article highlights the synthesis of BHMF-based polymers and the degradation of furanic polyesters. The synthesis of BHMF-based polymers has been demonstrated to be possible via enzymatic polymerization and solution polymerization, but only low molecular weights have been achieved to date. The thermal stability issues of BHMF in polymer production was reported and addressed in the synthesis of polyurethanes, polycarbonates, and epoxy monomers. Recent studies have shown that BHMF-based polymers showed good thermal stability. A wide range of thermal properties of these BHMF-based polymers can be tuned by altering the number of methylene units in the dicarboxylic acid comonomer. Furthermore, the furanic moieties in the polymeric chains can be used for thermoreversible cross-linking by Diels–Alder chemistry using a bismaleimide. Considering the diverse properties possessed by BHMF-based polymers, understanding their property–application relationship becomes increasingly important.
Several studies reported on the degradability and compostability of furanic polyesters, but no real biodegradability tests have yet been performed. However, promising results are shown for the enzymatic degradation of these polymers. Furthermore, the degradation rate of furanic polyesters is dependent on multiple properties, including molecular weight, crystallinity, thermal properties, polymeric structure, particle size, and hydrophobicity. It is hypothesized that BHMF-based polyesters degrade slowly, but this will be highly dependent on the conditions and polymeric properties.
In summary, biobased BHMF-derived polymers show interesting properties and have the potential to replace fossil-based polymers. Understanding their production route and properties will be critically important to reach the full potential of BHMF as a renewable building block and will in turn undoubtedly lead us to technological advancements in the discipline of green polymer materials. The next step is the production of BHMF-based polymers in higher amounts to evaluate their macroscopic properties, such as mechanical and gas barrier properties, in order to consider in which field these polymers might be applicable. In addition, the development of an industrial or straightforward synthesis route is key to bring BHMF-based polymers to the next stage.
Future Perspectives
To improve the sustainability of BHMF-based polymer synthesis, several recommendations with technological readiness follow from this review. The greatest potential is attributed to the use of a melt polycondensation method that allows elimination of the use of a solvent. Melt polycondensation is a straightforward synthesis route and is also preferred in industrial processes. In addition, the molecular weight of the yielded polymers is an important parameter, and higher values are desired to obtain more insights into the macromolecular properties. Polyesters derived from BHMF and aliphatic comonomers have been reported to have limited thermal properties, i.e., low Tg and Tm values. However, several studies on BHMF copolymerization with FDCA or its derivatives and l-lactide have shown promising results to address this issue. Therefore, the use of different comonomers with BHMF is recommended, in order to gain a better understanding of the structure–property relationship of BHMF-based polyesters and reveal various possible attributes that can be produced.
A critical dimension in realizing many of the opportunities mentioned here is that the synthesis of BHMF-based polyesters is challenging due to the nature of BHMF’s instability in many systems. Also important to consider is the necessity of the use of relatively cheap and commercially available catalysts for scaling up the BHMF-based polymer production. The (bio)degradability of BHMF-based polymers is left untouched as an important unanswered question. The position and orientation of the ester bond distinguish FDCA- and BHMF-based polyesters structurally (Figure 2). This disparity is expected to have a significant impact on the accessibility of enzymes on the polymer chain and thus on the (bio)degradability. Investigating the enzymatic degradation or biodegradability of BHMF-based polyesters will leverage assessments to incentivize a more effective approach in terms of sustainability.
Although the development of BHMF-based polymers is still in its early stages, successful synthesis has been achieved for a diverse range of polymers with distinct properties. However, reported findings should be critically evaluated and more knowledge has to be obtained to gain a better understanding of this topic. Systematically implementing the recommendations discussed here will increase the current state of the art in BHMF-based polymer synthesis with an emphasis not only on the production of versatile biobased materials but also on more sustainable manufacturing routes.
Acknowledgments
This work was supported by Greenwise Campus, Regio Deals, Province of Drenthe, and Municipality of Emmen.
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
References
- Siracusa V.; Blanco I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers (Basel) 2020, 12 (8), 1641. 10.3390/polym12081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J.; Zheng M.; Sun R.; Wang A.; Wang X.; Zhang T. Synthesis of Ethylene Glycol and Terephthalic Acid from Biomass for Producing PET. Green Chem. 2016, 18 (2), 342–359. 10.1039/C5GC01771H. [DOI] [Google Scholar]
- Neaţu F.; Culică G.; Florea M.; Parvulescu V. I.; Cavani F. Synthesis of Terephthalic Acid by P-Cymene Oxidation Using Oxygen: Toward a More Sustainable Production of Bio-Polyethylene Terephthalate. ChemSusChem 2016, 9 (21), 3102–3112. 10.1002/cssc.201600718. [DOI] [PubMed] [Google Scholar]
- Salvador M.; Abdulmutalib U.; Gonzalez J.; Kim J.; Smith A. A.; Faulon J. L.; Wei R.; Zimmermann W.; Jimenez J. I. Microbial Genes for a Circular and Sustainable Bio-PET Economy. Genes (Basel) 2019, 10 (5), 373. 10.3390/genes10050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan N.; Montazer Z.; Sharma P. K.; Levin D. B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 1. 10.3389/fmicb.2020.580709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenboom J. G.; Hohl D. K.; Fleckenstein P.; Storti G.; Morbidelli M. Bottle-Grade Polyethylene Furanoate from Ring-Opening Polymerisation of Cyclic Oligomers. Nat. Commun. 2018, 9 (1), 1–7. 10.1038/s41467-018-05147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong E.; Dam M. A.; Sipos L.; Gruter G. J. M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. ACS Symp. Ser. 2012, 1105, 1–13. 10.1021/bk-2012-1105.ch001. [DOI] [Google Scholar]
- Jiang L.; Gonzalez-Diaz A.; Ling-Chin J.; Malik A.; Roskilly A. P.; Smallbone A. J. PEF Plastic Synthesized from Industrial Carbon Dioxide and Biowaste. Nat. Sustain. 2020, 3 (9), 761–767. 10.1038/s41893-020-0549-y. [DOI] [Google Scholar]
- Eerhart A. J. J. E.; Faaij A. P. C.; Patel M. K. Replacing Fossil Based PET with Biobased PEF; Process Analysis, Energy and GHG Balance. Energy Environ. Sci. 2012, 5 (4), 6407–6422. 10.1039/c2ee02480b. [DOI] [Google Scholar]
- Loos K.; Zhang R.; Pereira I.; Agostinho B.; Hu H.; Maniar D.; Sbirrazzuoli N.; Silvestre A. J. D.; Guigo N.; Sousa A. F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8 (July), 1–18. 10.3389/fchem.2020.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne L.; Nyanhongo G. S.; Guebitz G. M.; Pellis A. Biotechnological Production and High Potential of Furan-Based Renewable Monomers and Polymers. Biotechnol. Adv. 2021, 48, 107707. 10.1016/j.biotechadv.2021.107707. [DOI] [PubMed] [Google Scholar]
- Agarwal B.; Kailasam K.; Sangwan R. S.; Elumalai S. Traversing the History of Solid Catalysts for Heterogeneous Synthesis of 5-Hydroxymethylfurfural from Carbohydrate Sugars: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. 10.1016/j.rser.2017.08.088. [DOI] [Google Scholar]
- van der Klis F.; van Haveren J.; van Es D. S.; Bitter J. H. Synthesis of Furandicarboxylic Acid Esters From Nonfood Feedstocks Without Concomitant Levulinic Acid Formation. ChemSusChem 2017, 10 (7), 1460–1468. 10.1002/cssc.201700051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q.; Liang W.; Guan J.; Wang L.; Qu Q.; Zhang X.; Wang X.; Mu X. Catalytic Synthesis of 2,5-Bis-Methoxymethylfuran: A Promising Cetane Number Improver for Diesel. Appl. Catal. A Gen. 2014, 481, 49–53. 10.1016/j.apcata.2014.05.003. [DOI] [Google Scholar]
- Aricò F. Synthetic Approaches to Stable Bio-Based Diol. Pure Appl. Chem. 2021, 93 (5), 551–560. 10.1515/pac-2021-0117. [DOI] [Google Scholar]
- Xia Z. H.; Zong M. H.; Li N. Catalytic Synthesis of 2,5-Bis(Hydroxymethyl)Furan from 5-Hydroxymethylfurfual by Recombinant Saccharomyces Cerevisiae. Enzyme Microb. Technol. 2020, 134, 109491. 10.1016/j.enzmictec.2019.109491. [DOI] [PubMed] [Google Scholar]
- He A.; Hu L.; Zhang Y.; Jiang Y.; Wang X.; Xu J.; Wu Z. High-Efficiency Catalytic Transfer Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural to 2,5-Bis(Hydroxymethyl)Furan over a Zirconium-Carbon Coordination Catalyst. ACS Sustain. Chem. Eng. 2021, 9 (46), 15557–15570. 10.1021/acssuschemeng.1c05618. [DOI] [Google Scholar]
- Ojagh H.; Achour A.; Ho P. H.; Bernin D.; Creaser D.; Pajalic O.; Holmberg J.; Olsson L. Effect of DMSO on the Catalytical Production of 2,5-Bis(Hydoxymethyl)Furan from 5-Hydroxymethylfurfural over Ni/SiO 2 Catalysts. React. Chem. Eng. 2021, 7 (1), 192–200. 10.1039/D1RE00255D. [DOI] [Google Scholar]
- Howell B. A.; Lazar S. T. Biobased Plasticizers from Carbohydrate-Derived 2,5-Bis(Hydroxymethyl)Furan. Ind. Eng. Chem. Res. 2019, 58 (3), 1222–1228. 10.1021/acs.iecr.8b05442. [DOI] [Google Scholar]
- Lǎcǎtuş M. A.; Bencze L. C.; Toşa M. I.; Paizs C.; Irimie F. D. Eco-Friendly Enzymatic Production of 2,5-Bis(Hydroxymethyl)Furan Fatty Acid Diesters, Potential Biodiesel Additives. ACS Sustain. Chem. Eng. 2018, 6 (9), 11353–11359. 10.1021/acssuschemeng.8b01206. [DOI] [Google Scholar]
- Lǎcǎtuş M. A.; Dudu A. I.; Bencze L. C.; Katona G.; Irimie F. D.; Paizs C.; Toşa M. I. Solvent-Free Biocatalytic Synthesis of 2,5-Bis-(Hydroxymethyl)Furan Fatty Acid Diesters from Renewable Resources. ACS Sustain. Chem. Eng. 2020, 8 (3), 1611–1617. 10.1021/acssuschemeng.9b06442. [DOI] [Google Scholar]
- Stensrud K.; Wicklund L.. Synthesis of Non-Toxic Surfactants from 5-Hydroxymethyl-2-Furfural, Furan-2,5-Dimethanol and Bis-2,5-Dihydroxymethyl-Tetrahydrofurans. US 2017/0226075 A1, 2017.
- West R. M.; Wu R.; Silks L. A.. Furan-Based Composition. WO 2015/084813 A1, 2015.
- Howell B. A.; Han X. Effective Biobased Phosphorus Flame Retardants from Starch-Derived Bis −2,5-(Hydroxymethyl)Furan. Molecules 2020, 25 (3), 592. 10.3390/molecules25030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R. F. A.; Mitrev Y. N.; Simeonov S. P.; Afonso C. A. M. Going Beyond the Limits of the Biorenewable Platform: Sodium Dithionite-Promoted Stabilization of 5-Hydroxymethylfurfural. ChemSusChem 2018, 11 (10), 1612–1616. 10.1002/cssc.201800297. [DOI] [PubMed] [Google Scholar]
- Thananatthanachon T.; Rauchfuss T. B. Efficient Route to Hydroxymethylfurans from Sugars via Transfer Hydrogenation. ChemSusChem 2010, 3 (10), 1139–1141. 10.1002/cssc.201000209. [DOI] [PubMed] [Google Scholar]
- Jansen M. L. A.; van Gulik W. M. Towards Large Scale Fermentative Production of Succinic Acid. Curr. Opin. Biotechnol. 2014, 30, 190–197. 10.1016/j.copbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Kruyer N. S.; Peralta-Yahya P. Metabolic Engineering Strategies to Bio-Adipic Acid Production. Curr. Opin. Biotechnol. 2017, 45, 136–143. 10.1016/j.copbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Triebl C.; Nikolakis V.; Ierapetritou M. Simulation and Economic Analysis of 5-Hydroxymethylfurfural Conversion to 2,5-Furandicarboxylic Acid. Comput. Chem. Eng. 2013, 52, 26–34. 10.1016/j.compchemeng.2012.12.005. [DOI] [Google Scholar]
- Wang T.; Wei J.; Feng Y.; Liu H.; Tang X.; Zeng X.; Sun Y.; Lei T.; Lin L. Efficient Synthesis of Bio-Based Monomer 2,5-Bishydroxymethylfuran by the Solvent-Free Hydrogenation of 5-Hydroxymethylfurfural-Based Deep Eutectic Mixture. J. Chem. Technol. Biotechnol. 2020, 95 (6), 1748–1755. 10.1002/jctb.6373. [DOI] [Google Scholar]
- Wang T.; Wei J.; Liu H.; Feng Y.; Tang X.; Zeng X.; Sun Y.; Lei T.; Lin L. Synthesis of Renewable Monomer 2, 5-Bishydroxymethylfuran from Highly Concentrated 5-Hydroxymethylfurfural in Deep Eutectic Solvents. J. Ind. Eng. Chem. 2020, 81, 93–98. 10.1016/j.jiec.2019.08.057. [DOI] [Google Scholar]
- Musolino M.; Ginés-Molina M. J.; Moreno-Tost R.; Aricò F. Purolite-Catalyzed Etherification of 2,5-Bis(Hydroxymethyl)Furan: A Systematic Study. ACS Sustain. Chem. Eng. 2019, 7 (12), 10221–10226. 10.1021/acssuschemeng.9b01413. [DOI] [Google Scholar]
- Jiang M.; Liu Q.; Zhang Q.; Ye C.; Zhou G. A Series of Furan-Aromatic Polyesters Synthesized via Direct Esterification Method Based on Renewable Resources. J. Polym. Sci. Part A Polym. Chem. 2012, 50 (5), 1026–1036. 10.1002/pola.25859. [DOI] [Google Scholar]
- Kieber R. J.; Silver S. A.; Kennemur J. G. Stereochemical Effects on the Mechanical and Viscoelastic Properties of Renewable Polyurethanes Derived from Isohexides and Hydroxymethylfurfural. Polym. Chem. 2017, 8 (33), 4822–4829. 10.1039/C7PY00949F. [DOI] [Google Scholar]
- Choi E. H.; Lee J.; Son S. U.; Song C. Biomass-Derived Furanic Polycarbonates: Mild Synthesis and Control of the Glass Transition Temperature. J. Polym. Sci. Part A Polym. Chem. 2019, 57 (17), 1796–1800. 10.1002/pola.29448. [DOI] [Google Scholar]
- Patil S. K. R.; Lund C. R. F. Formation and Growth of Humins via Aldol Addition and Condensation during Acid-Catalyzed Conversion of 5-Hydroxymethylfurfural. Energy Fuels 2011, 25 (10), 4745–4755. 10.1021/ef2010157. [DOI] [Google Scholar]
- Istasse T.; Richel A. Mechanistic Aspects of Saccharide Dehydration to Furan Derivatives for Reaction Media Design. RSC Adv. 2020, 10 (40), 23720–23742. 10.1039/D0RA03892J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Wang T.; Tang X.; Peng L.; Wei J.; Lin L. Methods in the Synthesis and Conversion of 2,5-Bis-(Hydroxylmethyl)Furan from Bio-Derived 5-Hydroxymethylfurfural and Its Great Potential in Polymerization. BioResources 2018, 13 (3), 7137–7154. 10.15376/biores.13.3.Zhang. [DOI] [Google Scholar]
- Kashparova V. P.; Chernysheva D. V.; Klushin V. A.; Andreeva V. E.; Kravchenko O. A.; Smirnova N. V. Furan Monomers and Polymers from Renewable Plant Biomass. Russ. Chem. Rev. 2021, 90 (6), 750–784. 10.1070/RCR5018. [DOI] [Google Scholar]
- Chernyshev V. M.; Kravchenko O. A.; Ananikov V. P. Conversion of Plant Biomass to Furan Derivatives and Sustainable Access to the New Generation of Polymers, Functional Materials and Fuels. Russ. Chem. Rev. 2017, 86 (5), 357–387. 10.1070/RCR4700. [DOI] [Google Scholar]
- Terzopoulou Z.; Karakatsianopoulou E.; Kasmi N.; Majdoub M.; Papageorgiou G. Z.; Bikiaris D. N. Effect of Catalyst Type on Recyclability and Decomposition Mechanism of Poly(Ethylene Furanoate) Biobased Polyester. J. Anal. Appl. Pyrolysis 2017, 126, 357–370. 10.1016/j.jaap.2017.05.010. [DOI] [Google Scholar]
- Jiang Y.; Woortman A. J. J.; Alberda Van Ekenstein G. O. R.; Loos K. A Biocatalytic Approach towards Sustainable Furanic-Aliphatic Polyesters. Polym. Chem. 2015, 6 (29), 5198–5211. 10.1039/C5PY00629E. [DOI] [Google Scholar]
- Zhang Y.; Li T.; Xie Z.; Han J.; Xu J.; Guo B. Synthesis and Properties of Biobased Multiblock Polyesters Containing Poly(2,5-Furandimethylene Succinate) and Poly(Butylene Succinate) Blocks. Ind. Eng. Chem. Res. 2017, 56 (14), 3937–3946. 10.1021/acs.iecr.7b00516. [DOI] [Google Scholar]
- Zeng C.; Seino H.; Ren J.; Hatanaka K.; Yoshie N. Bio-Based Furan Polymers with Self-Healing Ability. Polymer (Guildf). 2013, 54 (20), 5351–5357. 10.1016/j.polymer.2013.07.059. [DOI] [Google Scholar]
- Moore J. A.; Kelly J. E. Polyesters Derived from Furan and Tetrahydrofuran Nuclei. Macromolecules 1978, 11 (3), 568–573. 10.1021/ma60063a028. [DOI] [Google Scholar]
- Gomes M.; Gandini A.; Silvestre A. J. D.; Reis B. Synthesis and Characterization of Poly(2,5-Furan Dicarboxylate)s Based on a Variety of Diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49 (17), 3759–3768. 10.1002/pola.24812. [DOI] [Google Scholar]
- Upare P. P.; Hwang Y. K.; Hwang D. W. An Integrated Process for the Production of 2,5-Dihydroxymethylfuran and Its Polymer from Fructose. Green Chem. 2018, 20 (4), 879–885. 10.1039/C7GC03597G. [DOI] [Google Scholar]
- Ragno D.; Di Carmine G.; Brandolese A.; Bortolini O.; Giovannini P. P.; Fantin G.; Bertoldo M.; Massi A. Oxidative NHC-Catalysis as Organocatalytic Platform for the Synthesis of Polyester Oligomers by Step-Growth Polymerization. Chem. - A Eur. J. 2019, 25 (64), 14701–14710. 10.1002/chem.201903557. [DOI] [PubMed] [Google Scholar]
- Guillaume L.; Marshall A.; Niessen N.; Ni P.; Gauvin R. M.; Thomas C. M. Multicatalysis from Renewable Resources: A Direct Route to Furan-Based Polyesters. Green Chem. 2021, 23 (18), 6931–6935. 10.1039/D1GC01889B. [DOI] [Google Scholar]
- Jiang Y.; Woortman A. J. J.; Alberda Van Ekenstein G. O. R.; Petrović D. M.; Loos K. Enzymatic Synthesis of Biobased Polyesters Using 2,5-Bis(Hydroxymethyl) Furan as the Building Block. Biomacromolecules 2014, 15 (7), 2482–2493. 10.1021/bm500340w. [DOI] [PubMed] [Google Scholar]
- Maniar D.; Jiang Y.; Woortman A. J. J.; van Dijken J.; Loos K. Furan-Based Copolyesters from Renewable Resources: Enzymatic Synthesis and Properties. ChemSusChem 2019, 12 (5), 990–999. 10.1002/cssc.201802867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis A.; Weinberger S.; Gigli M.; Guebitz G. M.; Farmer T. J. Enzymatic Synthesis of Biobased Polyesters Utilizing Aromatic Diols as the Rigid Component. Eur. Polym. J. 2020, 130, 109680. 10.1016/j.eurpolymj.2020.109680. [DOI] [Google Scholar]
- van den Tempel P.; Picchioni F.; Bose R. K. Designing End-of-Life Recyclable Polymers via Diels–Alder Chemistry: A Review on the Kinetics of Reversible Reactions. Macromol. Rapid Commun. 2022, 43, 2200023. 10.1002/marc.202200023. [DOI] [PubMed] [Google Scholar]
- Ikezaki T.; Matsuoka R.; Hatanaka K.; Yoshie N. Biobased Poly(2,5-Furandimethylene Succinate-Co-Butylene Succinate) Crosslinked by Reversible Diels-Alder Reaction. J. Polym. Sci. Part A Polym. Chem. 2014, 52 (2), 216–222. 10.1002/pola.26990. [DOI] [Google Scholar]
- Zeng C.; Seino H.; Ren J.; Hatanaka K.; Yoshie N. Self-Healing Bio-Based Furan Polymers Cross-Linked with Various Bis-Maleimides. Polymer (Guildf). 2013, 54 (20), 5351–5357. 10.1016/j.polymer.2013.07.059. [DOI] [Google Scholar]
- Zeng C.; Seino H.; Ren J.; Yoshie N. Polymers with Multishape Memory Controlled by Local Glass Transition Temperature. ACS Appl. Mater. Interfaces 2014, 6 (4), 2753–2758. 10.1021/am405287p. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Dai Z.; Han J.; Li T.; Xu J.; Guo B. Interplay between Crystallization and the Diels-Alder Reaction in Biobased Multiblock Copolyesters Possessing Dynamic Covalent Bonds. Polym. Chem. 2017, 8 (29), 4280–4289. 10.1039/C7PY00677B. [DOI] [Google Scholar]
- Cai S.; Qiang Z.; Zeng C.; Ren J. Multifunctional Poly(Lactic Acid) Copolymers with Room Temperature Self-Healing and Rewritable Shape Memory Properties via Diels-Alder Reaction. Mater. Res. Express 2019, 6 (4), 045701. 10.1088/2053-1591/aafba3. [DOI] [Google Scholar]
- Nasirudeen M. B.; Hailes H. C.; Evans J. R. G. Production of Hydrophobic Polymers from Bio-Based Resources. Fuw Trends Sci. Technol. J. 2018, 3 (2A), 336–341. [Google Scholar]
- Gopalakrishnan P.; Narayan-Sarathy S.; Ghosh T.; Mahajan K.; Belgacem M. N. Synthesis and Characterization of Bio-Based Furanic Polyesters. J. Polym. Res. 2014, 21, 1. 10.1007/s10965-013-0340-0. [DOI] [Google Scholar]
- Ghosh T.; Mahajan K.; Narayan-Sarathy S.; Balgacem M. N.; Gopalakrishnan P.. 2,5-Furan Dicarboxylic Acid-Based Polyesters Perpared from Biomass. WO 2013/103574 A1, 2013.
- Zhang L.; Michel F. C.; Co A. C. Nonisocyanate Route to 2,5-Bis(Hydroxymethyl)Furan-Based Polyurethanes Crosslinked by Reversible Diels–Alder Reactions. J. Polym. Sci. Part A Polym. Chem. 2019, 57 (14), 1495–1499. 10.1002/pola.29418. [DOI] [Google Scholar]
- Oh C.; Choi E. H.; Choi E. J.; Premkumar T.; Song C. Facile Solid-State Mechanochemical Synthesis of Eco-Friendly Thermoplastic Polyurethanes and Copolymers Using a Biomass-Derived Furan Diol. ACS Sustain. Chem. Eng. 2020, 8 (11), 4400–4406. 10.1021/acssuschemeng.9b06944. [DOI] [Google Scholar]
- Lillie L. M.; Tolman W. B.; Reineke T. M. Structure/Property Relationships in Copolymers Comprising Renewable Isosorbide, Glucarodilactone, and 2,5-Bis(Hydroxymethyl)Furan Subunits. Polym. Chem. 2017, 8 (24), 3746–3754. 10.1039/C7PY00575J. [DOI] [Google Scholar]
- Vijjamarri S.; Streed S.; Serum E. M.; Sibi M. P.; Du G. Polymers from Bioderived Resources: Synthesis of Poly(Silylether)s from Furan Derivatives Catalyzed by a Salen-Mn(V) Complex. ACS Sustain. Chem. Eng. 2018, 6 (2), 2491–2497. 10.1021/acssuschemeng.7b03932. [DOI] [Google Scholar]
- Cho J. K.; Lee J. S.; Jeong J.; Kim B.; Kim B.; Kim S.; Shin S.; Kim H. J.; Lee S. H. Synthesis of Carbohydrate Biomass-Based Furanic Compounds Bearing Epoxide End Group(s) and Evaluation of Their Feasibility as Adhesives. J. Adhes. Sci. Technol. 2013, 27 (18–19), 2127–2138. 10.1080/01694243.2012.697700. [DOI] [Google Scholar]
- Jeong J.; Kim B.; Shin S.; Kim B.; Lee J. S.; Lee S. H.; Cho J. K. Synthesis and Photo-Polymerization of Bio-Based Furanic Compounds Functionalized by 2-Hydroxypropyl Methacrylate Group(S. J. Appl. Polym. Sci. 2013, 127 (4), 2483–2489. 10.1002/app.37959. [DOI] [Google Scholar]
- Hu F.; La Scala J. J.; Sadler J. M.; Palmese G. R. Synthesis and Characterization of Thermosetting Furan-Based Epoxy Systems. Macromolecules 2014, 47 (10), 3332–3342. 10.1021/ma500687t. [DOI] [Google Scholar]
- Wang T.; Xie W.; Pang Y.; Qiu W.; Feng Y.; Li X.; Wei J.; Tang X.; Lin L. Solvent-Free Hydrogenation of 5-Hydroxymethylfurfural and Furfural to Furanyl Alcohols and Their Self-Condensation Polymers. ChemSusChem 2022, 15, e202200186. 10.1002/cssc.202200186. [DOI] [PubMed] [Google Scholar]
- Tokiwa Y.; Calabia B. P.; Ugwu C. U.; Aiba S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10 (9), 3722–3742. 10.3390/ijms10093722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO Standards. ISO 17088:2021.
- Gruter G.-J.Technology & Markets Day Path to the Future; 2019. https://www.avantium.com/wp-content/uploads/2019/06/20190606-Technology-Day_CTO_Gert-Jan_Gruter_breakout_final_.pdf.
- Pellis A.; Haernvall K.; Pichler C. M.; Ghazaryan G.; Breinbauer R.; Guebitz G. M. Enzymatic Hydrolysis of Poly (Ethylene Furanoate). 2016, 235, 47–53. 10.1016/j.jbiotec.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Weinberger S.; Canadell J.; Quartinello F.; Yeniad B.; Arias A.; Pellis A.; Guebitz G. M. Enzymatic Degradation of Poly(Ethylene 2,5-Furanoate) Powders and Amorphous Films. Catalysts 2017, 7 (11), 318. 10.3390/catal7110318. [DOI] [Google Scholar]
- Weinberger S.; Haernvall K.; Scaini D.; Ghazaryan G.; Zumstein M. T.; Sander M.; Pellis A.; Guebitz G. M. Enzymatic Surface Hydrolysis of Poly(Ethylene Furanoate) Thin Films of Various Crystallinities. Green Chem. 2017, 19 (22), 5381–5384. 10.1039/C7GC02905E. [DOI] [Google Scholar]
- Austin H. P.; Allen M. D.; Donohoe B. S.; Rorrer N. A.; Kearns F. L.; Silveira R. L.; Pollard B. C.; Dominick G.; Duman R.; Omari K. El; Mykhaylyk V.; Wagner A.; Michener W. E.; Amore A.; Skaf M. S.; Crowley M. F.; Thorne A. W.; Johnson C. W.; Lee Woodcock H.; McGeehan J. E.; Beckham G. T. Characterization and Engineering of a Plastic-Degrading Aromatic Polyesterase. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (19), E4350–E4357. 10.1073/pnas.1718804115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M.; Yokoe M.; Aoi K. Biodegradable Polymers Based on Renewable Resources. VI. Synthesis and Biodegradability of Poly(Ester Carbonate)s Containing 1,4:3,6-Dianhydro-D-Glucitol and Sebacic Acid Units. J. of Polymer Sci. Part A Polym. Chem. 1997, 35, 2729–2737. 10.1002/app.10995. [DOI] [Google Scholar]
- Matos M.; Sousa A. F.; Fonseca A. C.; Freire C. S. R.; Coelho J. F. J.; Silvestre A. J. D. A New Generation of Furanic Copolyesters with Enhanced Degradability: Poly(Ethylene 2,5-Furandicarboxylate)-Co-Poly(Lactic Acid) Copolyesters. Macromol. Chem. Phys. 2014, 215 (22), 2175–2184. 10.1002/macp.201400175. [DOI] [Google Scholar]
- Wu H.; Wen B.; Zhou H.; Zhou J.; Yu Z.; Cui L.; Huang T.; Cao F. Synthesis and Degradability of Copolyesters of 2, 5-Furandicarboxylic Acid, Lactic Acid, and Ethylene Glycol. Polym. Degrad. Stab. 2015, 121, 100–104. 10.1016/j.polymdegradstab.2015.08.009. [DOI] [Google Scholar]
- Terzopoulou Z.; Tsanaktsis V.; Bikiaris D. N.; Exarhopoulos S.; Papageorgiou D. G.; Papageorgiou G. Z. Biobased Poly(Ethylene Furanoate-: Co -Ethylene Succinate) Copolyesters: Solid State Structure, Melting Point Depression and Biodegradability. RSC Adv. 2016, 6 (87), 84003–84015. 10.1039/C6RA15994J. [DOI] [Google Scholar]
- Haernvall K.; Zitzenbacher S.; Yamamoto M.; Schick M. B.; Ribitsch D.; Guebitz G. M. Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters. Polymers (Basel) 2017, 9 (9), 403. 10.3390/polym9090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos L.; Magaziotis A.; Nerantzaki M.; Terzopoulou Z.; Papageorgiou G. Z.; Bikiaris D. N. Synthesis and Characterization of Novel Poly(Ethylene Furanoate-Co-Adipate) Random Copolyesters with Enhanced Biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. 10.1016/j.polymdegradstab.2018.08.002. [DOI] [Google Scholar]
- Science Advice for Policy by European Academies. Biodegradability of Plastics in the Open Environment; 2020.
- ISO Standards. ISO 14851:2019.
- ISO Standards. ISO 13975:2019.
- ISO Standards. ISO 17556:2019.
- Martins C.; Hartmann D. O.; Varela A.; Coelho J. A. S.; Lamosa P.; Afonso C. A. M.; Silva Pereira C. Securing a Furan-Based Biorefinery: Disclosing the Genetic Basis of the Degradation of Hydroxymethylfurfural and Its Derivatives in the Model Fungus Aspergillus Nidulans. Microb. Biotechnol. 2020, 13 (6), 1983–1996. 10.1111/1751-7915.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S. P. M.; De Morais P.; Coelho J. A. S.; Sintra T.; Coutinho J. A. P.; Afonso C. A. M. Evaluating the Toxicity of Biomass Derived Platform Chemicals. Green Chem. 2016, 18 (17), 4733–4742. 10.1039/C6GC01211F. [DOI] [Google Scholar]
- Pan X.; Wu S.; Yao D.; Liu L.; Zhang L.; Yao Z.; Pan Y.; Chang S.; Li B. Efficient Biotransformation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furancarboxylic Acid by a New Whole-Cell Biocatalyst: Pseudomonas Aeruginosa PC-1. React. Chem. Eng. 2020, 5 (8), 1397–1404. 10.1039/D0RE00018C. [DOI] [Google Scholar]