Abstract
In previous years, different pollutants, for example, organic dyes, antibiotics, heavy metals, pharmaceuticals, and agricultural pollutants, have been of note to the water enterprise due to their insufficient reduction during standard water and wastewater processing methods. MOFs have been found to have potential toward wastewater management. This Review focused on the synthesis process (such as traditional, electrochemical, microwave, sonochemical, mechanochemical, and continuous-flow spray-drying method) of MOF materials. Moreover, the properties of the MOF materials have been discussed in detail. Further, MOF materials’ applications for wastewater treatment (such as the removal of antibiotics, organic dyes, heavy metal ions, and agricultural waste) have been discussed. Additionally, we have compared the performances of some typical MOFs-based materials with those of other commonly used materials. Finally, the study’s current challenges, future prospects, and outlook have been highlighted.
1. Introduction
Groundwater sources and external water decay by organic contaminants such as organic dyes, antibiotics, heavy metal ions, and pesticides have become a severe environmental issue. Manufactured dyes have a complex structure and are coloring agents that are nonbiodegradable with heightened resilience.1,2 Additionally, organic dyes are broadly utilized for dyeing a variety of products such as leather, textiles, medicine, and plastics.3 Fabric industries eliminate large quantities of polluted wastewater that possess organic dyes.4 Coloring reagents are one of the recognized types of water pollutants that should be extracted from wastewater prior to release within aquatic techniques.5−8 Actually, a concentration of 1.0 mg/L provides sufficient coloration and can be unsuitable for consumption by human beings and also contaminate water bodies. There are vast concerns related to the contamination of water by dyes as a kind of organic contaminant due to their persistence and coloration results.9 Antibiotics are widely utilized by humans as well as animal husbandry for the treatment of bacterial infections. More than one-half of antibiotic doses are excreted through urine from the body of human beings and animals because they cannot be adsorbed 100% and are mixed with water via flow streams. Many antibiotics are combined with water sources from hospital effluents, posing detrimental effects to the ecosystem.10 Antibiotics can cause several health problems, such as skin allergies, carcinogenic effects, and hereditary genetic defects, and can lower immune power.11−13
In addition, several industries such as civil construction, electroplating, metallurgy, and ceramics frequently produce heavy metal ions released to water resources via industrial wastewater.14 Heavy metal ions are toxic and have detrimental impacts on human health, as they can cause renal and neurological bioaccumulation and are poisonous to the digestive system.15−17 For farmland, pesticides are extensively used for better crop production, and eradication of these agricultural pests contaminates water sources and can damage the digestive, reproductive, endocrine, and nervous systems.18
The growing number of inhabitants and rise in water drinking have resulted in different processing techniques to remove contaminated components of anthropogenic sources within industrial wastewater before removing them to biological processes.19−21 Therefore, advancements in techniques and analyses used to develop photocatalytic composites have been of interest to numerous investigators. Industrial wastewater, such as fabric wastewater, is of primary consideration as this enterprise delivers enormous quantities of wastewater with an intense spectrum of chemical interpretations.22 In the coloring, printing, and finishing techniques, 10–50% failure of dyes from reactive pigments to the atmosphere is expected due to procedure inadequacy. Unfortunately, all of these contaminants cannot be removed entirely or degraded by traditional wastewater processing manufacturers within the atmosphere due to their continuous character in H2O and higher potency to rays, temperature, chemicals, and microbes-based invasion.23−25
MOF is a unique three-dimensional (3D) organic–inorganic composite with extremely porous nanostructures containing metal ions/groups and organic linkers.26,27 MOF has been carefully studied for several decades and has evolved as one of the magnetic materials for scientists and inventors because of promising components with tunable pore networks, adequate adsorption places, etc. The studies have also shown an in-depth investigation of MOF materials in previous decades, demonstrating their exceptional performance within catalysis,28,29 adsorption,30,31 and water harvesting.32 However, conventional catalysts and adsorbents such as activated carbons, clay minerals, zeolites, etc., have poor adsorption and pollutant removal capacity as compared to those of MOF-based NMs.33−36
Investigators have begun to manage the resilience of MOFs within diverse conditions, to comprehend the potential decay pathways, and to endeavor to create more durable framework networks.37 The strength of MOFs may be influenced by numerous aspects, such as the working atmosphere, metal ions, organic ligands, metal–ligand organization geometry, hydrophobicity of the aperture texture, etc.38,39 Also, synthesizing MOFs-based composites could help to improve the strength and performance toward practical applications. A review in this regard is provided by Ahmadijokani et al., where they explained the electrospinning synthesis route to synthesize MOF composites and hence their applications toward wastewater management.40 Investigations on the durability of MOFs have permitted us to explain the impact of some aspects and judiciously prepare sturdy framework networks. The somewhat labile coordination binds, which sustain the framework systems, are considered liable for the little resilience of MOFs.41 Therefore, a steady MOF system should have robust coordination binds to endure the invasion of guest molecules or have steric hindrances to control the intrusion of guests into the metal nodes.
Use of MOFs as support matrixes has been studied to combine different functional materials, for example, NPs,42−44 bioentities,45 and polymers,46 which offer rise to composites with improved or unique effects as related to their parental frameworks. Between these MOF composite substances, it may further decrease the size of the support MOFs and/or the active guest materials to nanoscale control to afford MOF composites.47 Combining different functional NPs, such as Au,48 quantum dots,49 and up conversion NPs,50 within MOF networks allows the nanocomposites to maintain the properties arising from the universal crystalline and porous configurations of MOFs and even to support the exceptional biomimetic catalytic, optical-electrical, and magnetic characteristic of the NPs. Similarly, the synergistic impact of integrating these materials may stimulate unique chemical and physical effects. The emergence of nanocomposites in this area should attract concentration to the potential possibilities and issues of these nanomaterials to reduce their progress in innovation and functionalities and donate to their growth potential utility for biomedical work. Up to now, NPs comprised into MOFs for catalytic utilizations have been broadly investigated and outlined.51,52
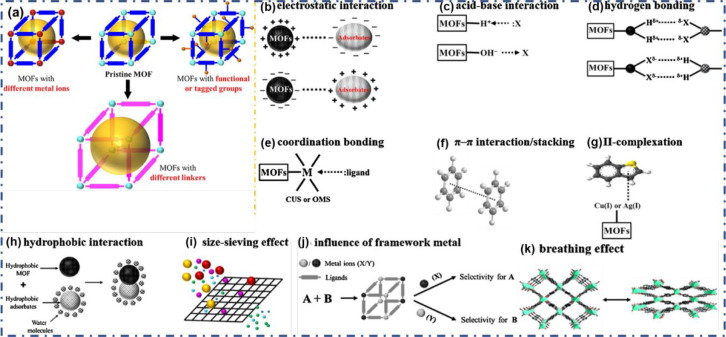
MOFs have drawn considerable concentration because of their increased porosity, designable pores, and easy preparation. Some fascinating MOFs are available, including isostructural, isoreticular, isomorphous, or similar types. As illustrated within Figure 1a, MOFs with identical crystal systems may be comprised of various metal ions (or groups) and linkers; thus, it may be convenient to examine the assistance of separately metallic components or linkers to adsorption, catalysis, and the band gap. Furthermore, MOFs can have functionality or FGs that profoundly impact the relations with adsorbates or supports (for catalysis). Figure 1b–k displays a rephrased plan for the noted tools associated with water and fuel sanctification through adsorption.53
Figure 1.
(a) Schematic representations of comparable MOFs. (b–k) Graphic expression of potential mechanisms for adsorption on the MOFs. Reprinted with permission from ref (53). Copyright 2021 Elsevier.
This Review focused on the synthesis process (such as the traditional, electrochemical, microwave, sonochemical, mechanochemical, and continuous-flow spray-drying methods) of MOF materials. Moreover, the properties of the MOF nanomaterials have been discussed in detail. Further, MOF nanomaterials’ applications for wastewater treatment (such as the removal of antibiotics, organic dyes, heavy metal ions, and agricultural waste) have been discussed. Additionally, we have compared the performance of some typical MOFs-based nanomaterials with those of other commonly used materials. Finally, the study’s current challenges, future prospects, and outlook have been highlighted.
2. Synthesis of the Metal–Organic Framework
A growing field of analysis favorably complementary to the finding of unique MOF substances is related to the effect of fabricating and processing methods that permit the substances’ design to be used explicitly within all measurement hierarchies. These constructions are based on the molecular-scale methods that alter the chemical arrangements of substances and examine how to manage MOFs’ characteristics at high order ranges through the fine-tuning of networks.54 These treatment strategies are of high functional significance in allowing MOFs to be provided in arrangements that are amenable to combination into exact design compositions and are also anticipated to enhance the handling aspects of the substances while they are made in the industrial ranges.55,56
For any MOF approach to be industrially viable, several vital factors have to be assessed: (i) a universal approach is necessary to adjust the highest digit of MOF networks with an identical piece of tools; (ii) the opportunity to bypass complicated processing prerequisites such as high temperature and pressure will decrease capital and working expenses and ease protection circumstances; (iii) a control from set to continued processing could be helpful to deliver a higher outcome per unit time and a constant steady-state process that guides toward seriously decreased rests, work expenses, reactor volumes, and constant and uniform exhibit; and (iv) an elevated space-time-yield (STY) parameter that estimates the quantity of MOF constructed per m3 reaction combination per day. Nevertheless, these techniques are such that they would be generalized to any MOF design, delivered to properly optimize the reaction or fabrication essentials. Similarly, it can be anticipated that the application of MOF superstructures on a large scale will inevitably be plagued by economic and ecological concerns of their preparation systems.
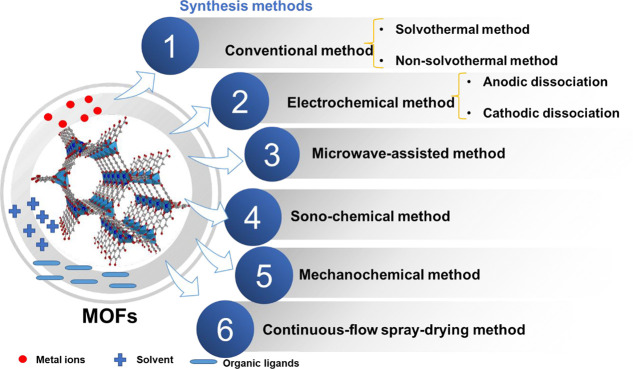
Since the discovery of MOFs, several methods such as electrochemical, microwave, sonochemical, and solvothermal have been developed to synthesize MOFs. Unreacted molecules, organic, and inorganic molecules play a pivotal role in the formation of MOFs. Two main steps that are important after the synthesis of MOFs are purification and activation,57 as impurities can reduce the adsorption ability and performance of the material. Sometimes heating is required for the activation of MOF materials, which causes the collapse of the MOF network with guest molecules. So, to simplify the activation process, we can utilize different methods, such as replacing the nonvolatile with a volatile solvent, to lessen the required deactivation temperature. In this section, we briefly describe different methods to synthesize MOFs (Table 1). Figure 2 shows the synthesis process of MOFs.
Table 1. Summary of the Synthesis Methods with Encountered Challenges.
| synthesis method | pros | cons | challenge |
|---|---|---|---|
| conventional | easy and simple | time and temperature regulation difficult | controlling morphology and degradation |
| no need of mechanical equipment | not suitable for large-scale applications | ||
| electrochemical | ambient conditions | energy use | electrode selection for practical applications |
| controlling film | uniform film thickness | ||
| microwave-assisted | accelerated nucleation and growth rate | need pairing of other methods for better results | need special setup and design of equipment |
| easy pairing with other methods | |||
| sonochemical | enhance crystallization and growth | detrimental effects of ultrasonic waves | large-scale design and application |
| quick and consistent nucleation | |||
| mechanochemical | green approach | morphology control | controlling nucleation and crystallization without solvent |
| ambient condition | |||
| solvent-free approach | |||
| continuous full spray drying | one step | design flexibility | structure and morphology control |
| industrial |
Figure 2.
Synthesis process of MOFs.
2.1. Conventional Method
Solvothermal and nonsolvothermal methods are included in conventional methods. In the solvothermal method, MOFs are developed in sealed nuclear magnetic resonance (NMR) tubes or in vials through conventional electric heating in small intervals.58 The term “solvo” indicates the solvents, such as ethanol, methanol, formamides, acetones, and water. This method is used by Huang et al.59 to fabricate Cu3(benzene tricarboxylic acid)2 (Cu3(BTC)2) and Cu(benzene dicarboxylic acid) (Cu(BDC)) MOFs for the treatment of phenol wastewater. Underneath the identical circumstances, the Cu(BDC) exhibited a higher catalytic performance as compared to that of the Cu3(BTC)2, which was primarily ascribed to the unique design of the Cu(BDC), directing accessible entry within the holes for the organics. The prepared Cu(BDC) showed a solid capability to adjust to the imitation phenol wastewater of distinct concentrations. Thus, Cu-MOFs would promise heterogeneous catalysts for the catalytic wet peroxide oxidation of organic effluents. Two types of mixtures were prepared, first with 1.94 g of Cu2+ nitrate trihydrate mixed in 30 mL of deionized water, and second with 0.84 g of 1,3,5-benzenetricarboxylic mixed in 15 mL of ethanol and dimethylformamide (DMF) each. Both of the solutions are mixed together with stirring until the suspension becomes homogeneous. The obtained solution was then transferred to an autoclave that was sealed and heated to 110 °C. Deep blue crystals were obtained after the reaction was cooled at room temperature. The authors observed a phenol conversion efficiency of 99%. Amino-functionalized Ti(IV)-based MOFs were fabricated by Wang et al.60 for the removal of Cr(VI) from wastewater via the solvothermal technique. The material possessed a higher specific surface area of 1343.9 m2 g–1. The microwave-assisted solvothermal method was utilized by Nguyen et al.61 for the development of bimetallic metal (Ni, Mg, and Sn)/MOFs, which showed 96% removal of rhodamine B, crystal violet from the wastewater.
Despite numerous benefits, time and temperature must be regulated tightly in the solvothermal approach. For instance, the difference in temperature can influence the particle morphology, and the reaction time extension may direct MOF degradation.62,63
Nonsolvothermal techniques are simpler than solvothermal for the synthesis of MOFs. Mechanical, nanoprecipitation, and emulsion are the methods that are involved in a nonsolvothermal approach for the fast growth of MOFs.64 In this strategy, complex equipment is not required; below the boiling point temperature of the solvent, and at atmospheric pressure, MOFs can be fabricated in an open vessel. Modification of pH and temperature is concerned with obtaining the maximum yield of the MOF material. For instance, to synthesize MOF-74 (Zn), Zhang et al.65 carried out an adapted method without the utilization of microwave/ultrasonic treatment, and no extra pressure was provided to the system. In brief, 239 mg of 2,5-dihydroxy terephthalic acid and 686 mg of Zn (OAc)2·2H2O were dissolved in dimethylformamide (20 mL). The obtained solution was then added to the salt solution with constant stirring for 18 h at room temperature to produce MOF-74 (Zn). The MOF-74 (Zn) was dried in air and evacuated at 270 °C. Finally, MOF-74 (Zn) was added to 1.14 mL of ethylenediamine and toluene solution, which was dried in air to yield ammoniated MOF-74 (Zn). Ammoniated MOF-74(Zn) byproducts are a luminescent sensor for selective tetrabromobisphenol A (TBBPA) detection. The fluorescence enhancement delivered an excellent linear association with the concentrations of TBBPA in the capacity of 50–400 μg/L, and its detection limit could reach 0.75 μg/L.
However, traditional methods of MOFs synthesis yield a fine powder, so they are not commercially applicable to much extent.
2.2. Electrochemical Method
An electrochemical preparation process is a standard approach that permits monitoring the film deposit operation via modifications within the involved voltage or current to prevent film consistency. This is a technique with a quick reaction rate and gentle circumstances. It may be brought out under ambient conditions, and the valuable equipment is somewhat easy.66−68 Currently, electrochemical preparation approaches may be separated within the anodic electrodeposition,69 cathodic electrodeposition,70 and electrophoretic deposition techniques.71
MOFs can be synthesized and deposited directly as well as indirectly. Required MOFs are generated directly on the surface of the electrode via electrochemical reactions in the former, while in the latter, an electrochemical reaction is a step of the procedure to synthesize MOFs. Electrochemical methods can be of two types such as anodic dissolution and cathodic electrosynthesis.
2.2.1. Anodic Dissolution
In this method, a potential or current is applied to the electrode, which is dipped inside the solution consisting of an organic ligand and electrolyte. After anodic voltage is applied, oxidation of metal takes place to form metal ions and liberated into the solution of organic linkers. A thin layer of MOF is formed on the surface of the electrode due to the reaction of metal ions and organic ligands.72
2.2.2. Cathodic Dissolution
In this system, when a specific voltage is applied to a metal precursor, organic ligand, and pro-base-containing solution in an appropriate electrolyte, MOFs are formed on the cathodic electrode.67
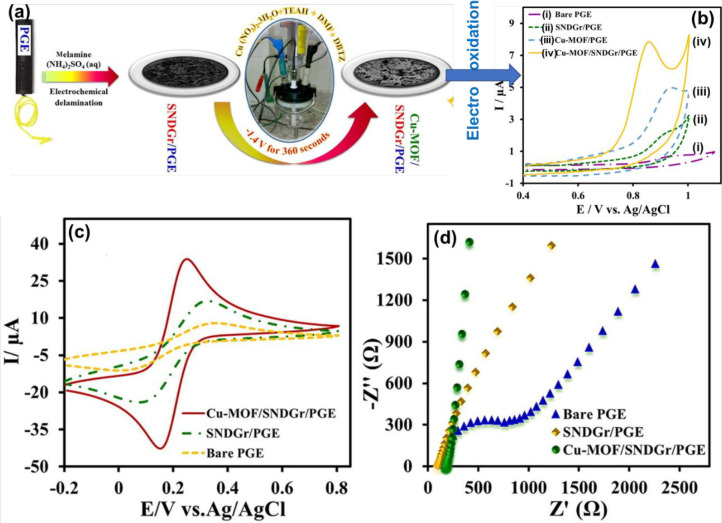
Some of the examples of MOFs synthesis via electrochemical methods are described next. Habibi et al.73 fabricated Cu-based MOF on S, N-doped graphene nanocomposite (SNDGr) via an electrochemical technique as showcased in Figure 3a for the detection of sertraline hydrochloride. Briefly, for the Cu2+ source, Cu(NO3)·3H2O (0.577 g) and triethylamine hydrochloride (0.12 g) as a supporting electrolyte were utilized and dissolved in 5 mL of dimethylformamide (DMF). This solution was considered the first solution. After that, disodium 5,5′-bitetrazole-1-ide(Na2C2N8) was dissolved in 5 mL of DMF. This solution was then added to the first solution with continuous stirring for an early 2 h, and the resulting mixture was sonicated for 5 min. Afterward, SNDGr/pencil graphite electrode (PGE) was dipped into the obtained solution, and a cathodic current of −1.4 V was applied, resulting in the formation of Cu-MOF/SNDGr/PGE. Considerable enhancement within the oxidative peak current at a lower voltage toward electrooxidation of sertraline hydrochloride (STLHC) upon the Cu-MOF/SNDGr/PGE would signal that, for quantitative electrochemical finding STLHC, a prudent sensor could be made. Eventually, on the reverse scan, as shown within Figure 3b, no reduction peak occurred, indicating the irreversible nature of the electrochemical method of the STLHC upon the all-incorporated electrodes. For electrochemical analyses, identification of the electrochemical active surface area (ECSA) is essential. The electrocatalytic description of various electrodes was studied through the cyclic voltammetry (CV) approach utilizing the typical redox electrode design, [Fe(CN)6]3–/[Fe(CN)6]4–, into 0.1 M KCl medium. A pair of finally described peaks of the CVs curves of the probes is showcased in Figure 3c. The acquired CVs indicate that the peak voltage split (ΔΕp) of Cu-MOF/SNDGr/PGE, SNDGr/PGE, and pristine PGE is 0.106, 0.171, and 0.251 V, respectively. The reductive and oxidative peak currents of Cu-MOF/SNDGr/PGE are 2- and 4-fold more elevated in comparison to the SNDGr/PGE and bare PGE concurrently. The interfacial characteristics of the probe and solution were examined by the electrochemical impedance spectroscopy (EIS) process, which was conducted within 0.1 M KCl media via utilizing the standard [Fe(CN)6]3–/[Fe(CN)6]4– redox method as the electrode. At an open circuit voltage within 1 Hz to 100 kHz, the Nyquist plots for the pristine and doped PGEs are displayed in Figure 3d. The Nyquist plot of EIS at higher frequencies comprises very short semicircles associated with the electron transfer procedure within the interface of the electrode/solution, and at lower frequencies, a direct line associated with the scattering method is shown.
Figure 3.
(a) Schematic illustration of the Cu-MOF/SNDGr/PGE and its application. (b) CVs at a sweep speed of 50 mV s–1 of different synthesized material (0.1 M, pH = 7.0) solutions comprising 11.8 μM STLHC. (c) CVs of the pristine PGE (i), SNDGr/PGE (ii), Cu-MOF/PGE (iii), and Cu-MOF/SNDGr/PGE (iv) in 0.1 M KCl comprising 5 mM [Fe(CN)6]3–/4–. (d) Nyquist spectra of pristine PGE, SNDGr/PGE, and Cu-MOF/SNDGr/PGE with the identical media. Reprinted with permission from ref (73). Copyright 2021 Elsevier.
Zhou et al.74 electrochemically synthesized a series of face-centered cubic MOFs, which efficiently decreased the energy input by approximately 90% as compared to the single distillation process, a conventional method. Overall, the electrochemical process may be involved beneath favorable processing circumstances and constantly nucleate at all temperatures, and it also permits surface changes.
2.3. Microwave-Assisted Method
Microwave treatment had a shorter reaction time for the synthesis of MOFs, which was reported in 2005 for the first time. The authors observed that a reduced time of synthesis did not affect the yield much as 44% material was obtained in 4 h in the case of the microwave-assisted method, while in the case of a traditional heated oven 45% yield was obtained after 4 days. A reduction in synthesis times was progressively obtained since 2005.75 Further, narrow sized crystals or reduced particle size MOFs can be obtained via microwave-assisted techniques in comparison to conventional ovens, and even the size of the crystal can be controlled during synthesis.76 Lev Bromberg et al.77 elucidated that MIL-101 synthesized via the microwave-assisted method exhibited a larger surface area (4004 m2/g) as compared to autoclave-synthesized MIL-101, which had a shorter surface area of 3460 m2/g. Therefore, a larger surface area, controllable particle size, and a shorter time for reaction are the significance of microwave-assisted methods for the fabrication of MOF-based materials.
Microwave-assisted syntheses of MOF have been widely used. In a Teflon vessel, a mixture of substrate and the appropriate solvent is taken, and then the vessel is sealed and kept inside a microwave unit at a certain temperature for a set time.78 Ren et al.79 designed Zn-MOFs for the effective degradation of contaminants such as tetracycline hydrochloride and Congo red from wastewater by the microwave-assisted ball-milling technique. Another team of researchers fabricated NH2-MIL-125(Ti) MOF using the microwave-assisted method for the removal of diclofenac. Here, MIL refers to Materials of Institute Lavoisier, a kind of MOF where the Ti8O8(OH)4 metal cluster is linked with six benzene-1,4-dicarboxylate units.80 MOFs were synthesized at 200 °C under microwave radiation for 15 min and showed a surface area of 1030 m2/g and a pore volume of 0.45 cm3/g, which indicates the developed porosity. In the batch test, diclofenac was completely removed within 3 h.
Electrochemical preparation and microwave radiation were suggested to deposit a patterned luminescent MOF (LMOF) upon conductive glass.81 Mainly, a film of lanthanum hydroxide is incorporated upon a fluorine-doped tin dioxide (FTO) exterior via electrochemical deposition. Afterward, beneath microwave irradiation, the hydroxide film was altered to LMOF. The microwave-aided preparation approach may significantly accelerate the MOF particles’ nucleation and evolution rate due to its rapid heating, thereby obtaining MOF substances with smaller sizes and more fair distribution that may be involved in catalysis. This method is suitable for most lanthanide ions and has effective energy transferability. Furthermore, they have potential application in the field of anticounterfeiting barcodes.
2.4. Sonochemical Method
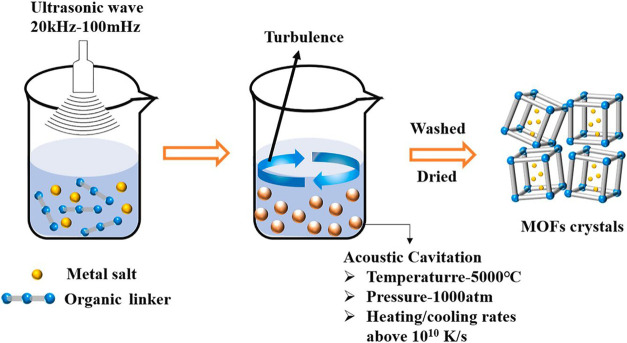
Ultrasonic rays provide energy to the reaction between the metal ion origin and the organic ligand. The sonochemical approach may enhance the crystallization and development speed during the growth procedure.82,83 ZIF-8 layers may be prepared through a sonochemical approach. Initially, Zn(NO3)2·6H2O and 2-MeImi were liquefied within DMF and mixed continuously until a clear solution was received. After that, TEA (triethylamine) was integrated within the solution, and the solution was fast assigned to the reactor and put upon the ultrasound rod. The ZIF-8 specimen was rinsed with DMF and absorbed into methanol. After filtration, the specimen was parched. The sonochemical process facilitates quick and consistent nucleation, and the outcome of ZIF-8 acquired through this process is very high.84 The synthesis of MOFs using the sonochemical technique is shown in Figure 4.85
Figure 4.
Graphical representation of the synthesis of MOFs by the sonochemical method. Reprinted with permission from ref (85). Copyright 2021 Elsevier.
The reactions between organic ligand and metal ion sources are initiated by ultrasonic radiation in the case of the sonochemical synthesis of MOFs. Under mild conditions, it can improve the surface morphology and growth rate and crystallization.86 MOF-177 was synthesized by Jung et al.83 by utilizing 1-methyl-2-pyrrolidinone as a solvent via the sonochemical technique with a Pyrex reactor attached to a sonicator. The fabrication was conducted in He with no usage of external cooling. Crystals were obtained within 40 min and stored for 3 days in dichloromethane, and then vacuum-dried for 1 day. The surface area of the obtained MOF was in the range of 4200–4900 m2/g, and the product yield was 95.6%. However, the desire for green synthetic methods is increasing daily to minimize the detrimental impacts on the ecological system. Thus, we also discuss some green synthesis for the preparation of MOFs.
2.5. Mechanochemical Method
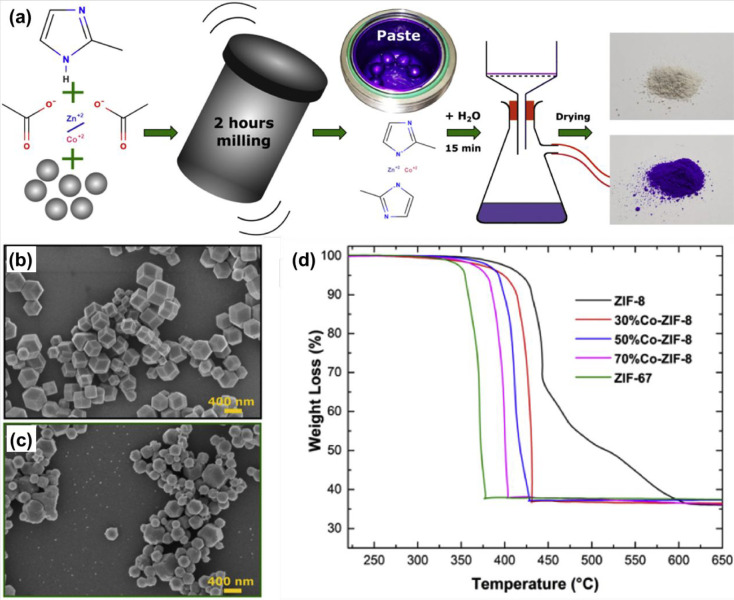
Mechanochemical synthesis gained more attention as a greener method than did solution-based routes as the energy is directly transferred between solid-phase reactants, and a minimal amount of solvent (or solvent-free conditions) is required to assist the mechanochemical reactions known as liquid-assisted grinding. In addition, these reactions can generally take place at room temperature and are less time-consuming.87 Typically, ball-milling/grinding of the mixture of solid precursors in a ball-miller is involved in the mechanochemical synthesis, which provides an opportunity to utilize insoluble metal sources.88 Zeolitic imidazole frameworks ZIF-8, Co-containing ZIF-8, and ZIF-67 were synthesized by Taheri et al.89 where the authors found that ZIF-8 is more appropriate for water-related applications with a surface area of 1881 m2/g as compared to ZIF-76, which had a surface area of 1525 m2/g. Ball-milling was utilized for 2 h to fabricate MOF-based materials. Figure 5a illustrates the synthesis process of Co-containing ZIF-8, Figure 5b,c represents the SEM images of ZIF-8 and ZIF-67, and Figure 5d shows the thermogravimetric analysis from 300 to 650 °C with a weight loss of nearly 63%, which confirms the decomposition of ZIF to metal oxides. The selection of suitable MOFs for water treatment was directed in this study. Various zirconium-based MOFs are synthesized using the mechanochemical technique. For instance, the UiO-66 analogue was fabricated via a mechanochemical approach without acidic additives using a liquid-assisted grinding process with water.90 This provides new paths for converting biomolecules into MOFs that can be used as composites in numerous applications.
Figure 5.
(a) Representation of the synthetic process of Co-containing ZIF-8; (b,c) SEM pictures of ZIF-8 and ZIF-67; and (d) thermogravimetric analysis of the synthesized materials. Reprinted with permission from ref (89). Copyright 2020 Elsevier.
2.6. Continuous-Flow Spray-Drying Method
For the production of MOFs on a large scale for the industrial exploitation and continuous synthesis of MOFs in the form of NPs, composites, and spherical structures, the continuous-flow spray-drying technique attracted much attention due to its cost-effectiveness and environmentally friendly process.91 This technique is a combination of both spray-drying and continuous-flow techniques. Several members of the MOFs family involving UiO-66 and Fe-BTC/MIL-100 were synthesized by Garzón-Tovar et al.91 Briefly, they introduced a continuous-flow reactor at the nozzle of the spray dryer. Initially, in a continuous flow reactor, the precursor solution was injected, which contains metal salt and organic linker, and it was heated to a temperature that promotes nucleation. The outlet of the flow reactor was directly connected to the entrance of the spray dryer; the solution was automatically injected into the spray dryer. Here, the growth of the MOFs was confined to the atomized droplets and collected as micro spherical beads. In addition, the utilized solvent could be recovered, making the process cost-effective and waste-efficient. UiO-66-NH2 and Zr-fumarate beads were synthesized by Avci-Camur et al.92 Briefly, a mixture of 2-aminoterephthalic acid with H2O and CH3COOH in an equimolar ratio with ZrOCl2·8H2O was inserted into the coil-flow reactor and placed in a silicone bath. The resulting yellow-colored slurry was spray-dried, and the beads were collected and dried at 75 °C. The surface area of UiO-66-NH2 was 840 m2/g. This synthesis method is integrated as a green approach in many industrial sectors for the continuous one-step preparation of MOF beads.
3. Properties of the Metal–Organic Frameworks
The adsorptive and catalytic properties of the MOF-based materials have great potential in drug delivery,93,94 luminescence,95,96 sensing and degradation,97,98 and in the removal of toxic pollutants from wastewater. They possess tunable pore sizes, outstanding thermo-chemical stability, and large amounts of surface area. We briefly explain the adsorptive and catalytic properties of MOF-based materials in separation, sensing, and environmental remediation. Figure 6 shows the proposed properties of the MOFs.
Figure 6.
Properties of the MOFs materials.
3.1. Adsorptive Properties
Adsorptive elimination of poisonous contaminants from wastewater is considered to be one of the most challenging and outstanding research areas toward the protection of the environment and management of water due to cost-effectiveness, user and eco-friendly synthesis, facile operation, and recyclability. Unsaturated metal sites and charge on the MOF-based composites play a significant role in dye adsorption and removal properties as these active adsorption sites of MOF can interact supermolecularly with the molecules of dyes.99 Generally, the mitigation or elimination of toxic molecules from aqueous solutions via adsorption requires a known amount of MOF-based material in polluted aqueous solution for the particular time period, so that pollutant molecules get adsorbed on the surface of MOF, which ultimately can be removed by mechanical methods. Therefore, thermodynamic water stability is an important factor in adsorption.
3.1.1. Present State of MOF Materials as Adsorbent
Decades of essential investigation on reticular chemistry have afforded universal MOF systems and allowed a precise acquaintance of their effects. This has paved the path for a paradigm change from basic science to the advanced investigation. While previous years have seen a considerable improvement in the chemistry and application of MOFs, there remains ample space for study, especially in the context of commercialization. Concerning the emerging area of MOFs, more steps must be dedicated to the fundamental investigation into their properties to fully employ their potential in applied analysis. In contrast, using the extensive body of actual study on MOFs, placing and optimizing substances for typical utilizations, and pushing reticular materials from the lab to industrial applications is essential.100
As discussed in the previous sections, the adsorbents’ porosity, pore geometry, and distinct adsorption locations are crucial in efficient adsorptive reduction. The pores of solids can be classified on the basis of their sizes.101 Conventionally, the topography of adsorbents has been overwhelmed via substances, for example, zeolites, activated carbon, silica, waxes, and alumina. These substances propose a generous concession among price, ability, and selectivity. The majority of aluminosilicate zeolite, untreated alumina, and activated carbon (AC) substances are hydrophilic and absorb from organic media. In contrast, AC is hydrophobic and thus competently works to absorb organic contaminants from water. Therefore, AC is presently the considerable standard adsorbent substance for wastewater processing.
A recent study on adsorption substances primarily concentrates on producing and optimizing specific adsorbents to utilize them for application. Other agricultural, municipal waste, and industrial rivulets have been supposed to produce low-price adsorbents. In contrast, adsorbents with extraordinary capability or selectivity have also been developed. MOFs are classified as engineering materials with porous organic networks that make them unique with extraordinary performance as nonconventional adsorbents.
3.1.2. Effects of Morphology on the Adsorptive Property
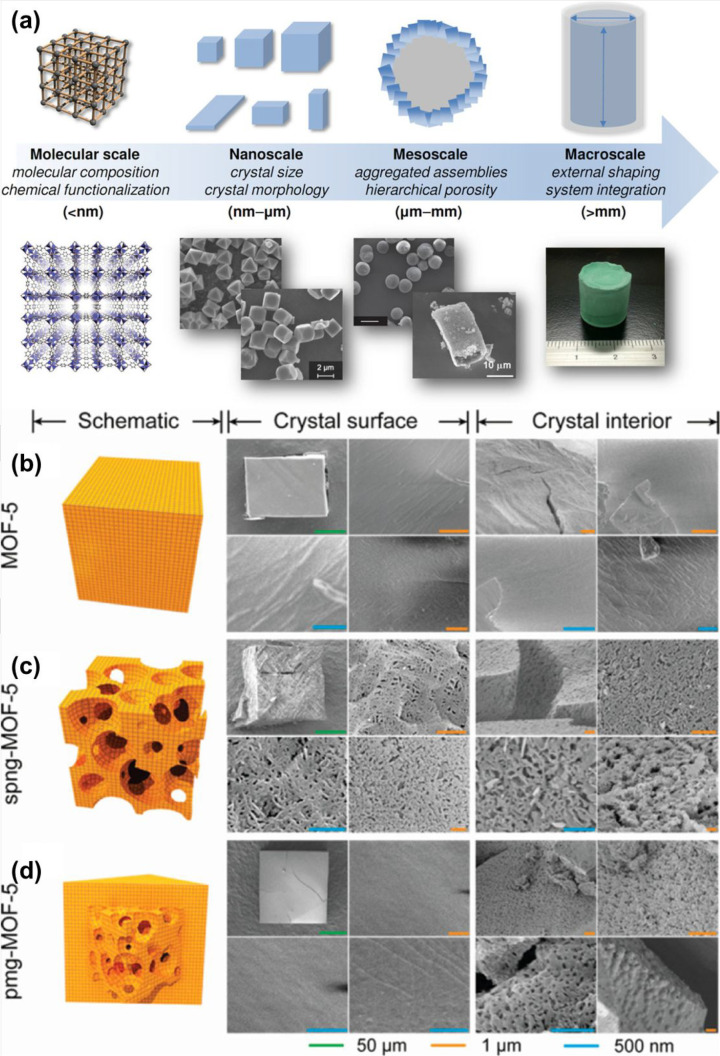
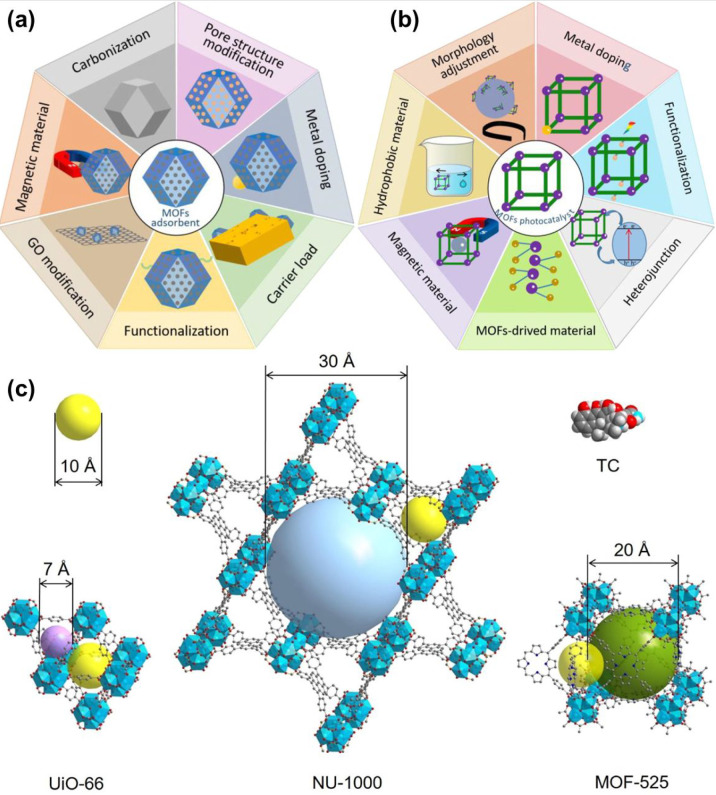
It is clearly understood that the structural morphology and orientation could significantly impact the performance of MOFs as adsorptive materials. In regard to this, Oliver et al.102 showed the effect of high order structurization on the adsorptive characteristics of MOFs. Significantly, from an essential viewpoint, the high order structurization of MOFs delivers the option for unique effects to occur that are self-sustaining of the molecular arrangement and system configuration of the MOF. The measurement scales appropriate to MOFs’ optimization are shown in Figure 7a. In the molecular range, the modular manufactured process authorizes the metal and organic ingredients to be rationally chosen, such that their positions are comprised into the resultant framework. It permits parameters, for example, the chemical functionalities and pore dimensions, to be finely adjusted according to the chosen substance characteristics. On the nanoscale, the characteristics of particular crystals are managed, for example, crystal dimensions and morphology that may show benefits, such as the adsorption kinetics. Individual crystals may be employed as assembling unions to make big groups at the mesoscale, directing refined architectures, for example, hollow spheres, thin layers, and monolithic designs. Ultimately, structuralization on the macroscale delivers ways to shape the MOF designs into the preferred structure. These scales provide many fascinating possibilities to increase the adsorptive effects of MOF techniques for the mesoscale and nanoscale structuralization of MOF.
Figure 7.
Measurement ranges applicable to the optimization of MOFs (a). Schematic graphs and scanning electron microscopy (SEM) pictures of the crystal interior as well as the exterior for (b) cubic, (c) spng, and (d) pmg-like crystals of MOF-5. Reprinted with permission from ref (102). Copyright 2018 John Wiley and Sons.
However, in the cubic crystals brought within traditional syntheses (Figure 7b), higher amounts of 4-(dodecyloxy) benzoic acid (DBA) were delivered into a sponge-type (spng) crystal with pores that reproduced via the whole crystal (Figure 7c), while a “pomegranate” (pmg) surface, in which a thick texture retained a hierarchically porous network, was kept at low concentrations of the additive (Figure 7d). An uptake shape was shown by the adsorption isotherm of N2 toward the two hierarchically porous structures compatible with the high density of micropores originating from the MOF-5 network class. The N2 adsorption isotherms toward the two hierarchically porous structures showed an uptake shape compatible with a high density of micropores originating from the MOF-5 network class. However, a noticeable impact was not shown by mesopores as the supported macro- and mesopores even at high heat would have to be very large for their influence to be exerted on the adsorption profile of N2.
Solids with a high porosity, large surface area, and high metal ion densities are desirable for catalysis. Numerous MOFs have a surface area of about 5000 m2/g or more,103 and the presence of metallic nodes brings out the catalytic performance for several reactions. One of the reasons MOFs are more advantageous than other solid catalysts is the probability of structure and pore dimension prediction by knowing the structure of the linker and directionality of coordination among the metal clusters. For example, by replacing one linker with another having increased dimensions but the same geometry of carboxylate units, reticular MOFs can be obtained.104 MIL-101(Cr) is frequently used as an acid catalyst in aqueous solutions. It comprises two zeotypic mesopores of 29 and 34 Å diameter and a surface area of 4100 m2/g and is highly stable in catalytic reactions.105
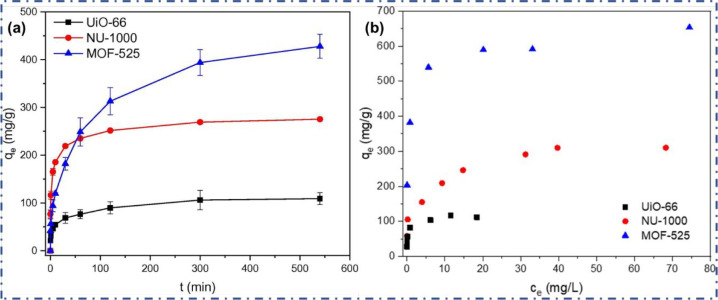
The clusters of the framework act as nanosized oxides, and the surrounding linkers work as antennae.106 MOFs with transition metals such as Co, Cr, and Fe act as oxidation catalysts.107 MOF provides different active sites such as metal nodes with exchangeable coordination positions that are unconnected to the linkers, modified ligands with attached active sites, or inclusion within the void volume of the active species utilized as oxidation catalysts. Depending on the type of oxidation, these catalysts attain a different level of efficiency.108 Liu et al.109 fabricated Zr-based MOFs (UiO-66, NU-1000, and MOF-525) and reported adsorption isotherms corresponding to each morphology. Adsorption kinetics and isotherms are affected significantly by the different morphologies of the material. For instance, an adsorption equilibrium was reached in 120 min in the case of MOF-525, while in the cases of UiO-66 and NU-1000, it takes only 40 min to reach the adsorption equilibrium as described in Figure 8a. Sips model of adsorption isotherm was most suitable for the adsorption of TC on MOFs as showcased in Figure 8b. The adsorption capacity of TC was maximum for MOF-525 among the three prepared Zr-based MOFs, which is even higher than for other adsorbents, that is, 9.5, 3, and 5.4 times higher than those of granular AC, MWCNTs, and GO, respectively.
Figure 8.
(a) Adsorption of TC on UiO-66, NU-1000, and MOF-525 with time and (b) adsorption isotherms of TC on as-synthesized MOFs. Reprinted with permission from ref (110). Copyright 2021 Elsevier.
3.1.3. Effect of Pore Size on the Adsorptive Properties
The pore size and geometry of the MOF-based materials are crucial for the adsorptive removal of dyes. Because of their higher surface area, porous MOFs can absorb more significant quantities of dyes than can nonporous MOFs. Flexible or adjustable design results in several MOF frameworks with multiple pore sizes, dimensions, and geometries. An exhaustive review by Cai et al.27 provides insights into this aspect, where they considered composites and MOF derivatives to explain the effects of mesopores on the microporous structures. For instance, MOFs such as IRMOF-01 and PCN-222 have square grid channels, while PCN-224 and MOF-74 have hexagonal channels.111,112 If the pore size of the MOF-based composites is larger than the size of the dye molecules, then a considerable amount of dye can be adsorbed on the surface of the MOF. However, if the size of the molecules of dyes is more significant as compared to the MOF frameworks, then the adsorption of dye molecules is ruled out within the pores of MOF.113 Also, Cui et al.114 reported the regime upon the pore chemistry and size within metal coordination systems with hexafluoro silicate and organic linkers toward preferential binding, as well as the tidy group of acetylene molecules via combined host–guest and guest–guest relations. The significance of these binding relations affords high adsorption ability and selectivity for acetylene at room temperature. Experimental breakthrough curves exhibit their efficiency in separating acetylene/ethylene combinations.
3.1.4. Effect of Functional Groups on the Adsorptive Properties
The adherence of adsorbates is promoted by particular functional groups present in MOF-based materials, such as amino groups interacting with acidic dyes and sulfonic acid interacting with basic dyes and pore geometries of porous MOFs.99
Dyes can facilely be degraded via the oxidation process. The Fenton advanced oxidation process is considered a potential method for removing dyes.115 Li et al.116 fabricated magnetic porous Fe3O4/carbon octahedra via two-step calcination of Fe-based MOF to eliminate methylene blue. Within 1 h, this material shows 100% removal efficiency in the presence of H2O2 by a Fenton-like heterogeneous reaction.
3.2. Catalytic Properties
Solids with high porosity, large surface area, and high metal ion densities are desirable for catalysis. Numerous MOFs have a surface area of about 5000 m2/g or more,103 and the presence of metallic nodes brings out the catalytic performance for several reactions. One of the reasons MOFs are more advantageous than other solid catalysts is the probability of structure and pore dimension prediction by knowing the structure of the linker and directionality of coordination among the metal clusters. For example, by replacing one linker with another having increased dimensions but the same geometry of carboxylate units, reticular MOFs can be obtained.104 MIL-101(Cr) is frequently used as an acid catalyst in aqueous solutions. It comprises two zeotypic mesopores of 29 and 34 Å diameter and a surface area of 4100 m2/g and is highly stable in catalytic reactions.105
The clusters of the framework act as nanosized oxides, and the surrounding linkers work as antennae.106 MOFs with transition metals such as Co, Cr, and Fe act as oxidation catalysts.107 MOF provides different active sites such as metal nodes with exchangeable coordination positions that are unconnected to linkers, modified ligands with attached active sites, or inclusion within the void volume of the active species utilized as oxidation catalysts. Depending on the type of oxidation, these catalysts attain a different level of efficiency.108
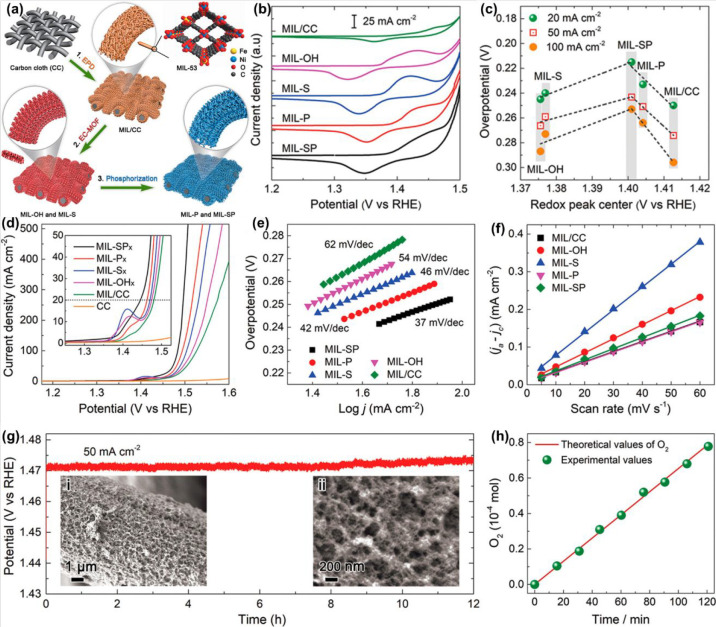
Wen et al.117 proposed the synergistic coupling of anionic ligands to optimize the electronic and catalytic characteristics of MOF-altered oxygen-evolving catalysts. The synthesis of NiFe-doped oxygen evolution reaction (OER) materials with four ligands is shown in Figure 9a. Cyclic voltammogram (CV) graphs attributed to the Ni2+/Ni3+ couple within Figure 9b show that all materials exhibit two oxidation peaks and one prominent anodic peak (VR). In Figure 9c, a volcano-shaped arc was developed by Vredox of the various catalyst scales with their OER performances. IR-corrected OER polarization arcs within Figure 9d indicate that MIL-SP displays the most useful OER performance on overpotentials (η) of just 215, 242, and 253 mV and exhibits valuable current densities of 20, 50, and 100 mA cm–2 correspondingly. Further, MIL-SP displays the lower Tafel plot (37 mV/dec, Figure 9e), revealing exceptional OER kinetics. The O2 grown from the MIL-SP materials was estimated by amount through a gas chromatography study (Figure 9f). The almost equal portions of experimentally evaluated and theoretically measured O2 show an about 100% faradaic efficiency toward the H2O-splitting reaction. Furthermore, for 12 h, MIL-SP may maintain its OER performance while on a fixed current of 50 mA cm–2. Fascinatingly, MIL-SP morphology is rebuilt during the catalytic process within 3D-linked systems constructed through ca. 10 nm nanoparticles (insets i and ii within Figure 9g). To clarify the outcome of morphological characteristics upon the materials’ OER performance, the ECSAs were estimated through varying sweep rate CV trials (Figure 9h).
Figure 9.
(a) Graphical description of the NiFe-based oxygen-evolving standard materials synthesis method from (Ni, Fe)-MIL-53 predecessors. EPD, electrophoretic deposition; EC-MOF, electrochemical conversion of MOFs. (b) CV curves illustrating the Ni2+/Ni3+ redox heights for individual materials were documented at a sweep speed of 10 mV s–1. (c) The relationship between the weighted center and catalytic performance Ni2+/Ni3+ redox elevations. (d) OER polarization arcs of the various specimens at a sweep speed of 2 mV s–1. (e) Interrelated Tafel slops. (f) At a fixed current density of 10 mA cm–2, the time for MIL-SP versus O2 measured experimentally (green sphere) as well as theoretically (red solid) for 2 h is described. (g) Solidity trial for 12 h of MIL-SP at a fixed current density of 50 mA cm–2. The inset illustrates the SEM pictures of MIL-SP after a 12 h OER process. (h) Capacitive current density distinctions (Δj = ja – jc) are plotted as a part of the sweep speeds. Reprinted with permission from ref (117). Copyright 2019 American Chemical Society.
4. Mechanism of MOF-Based Materials in the Removal of Pollutants from Water
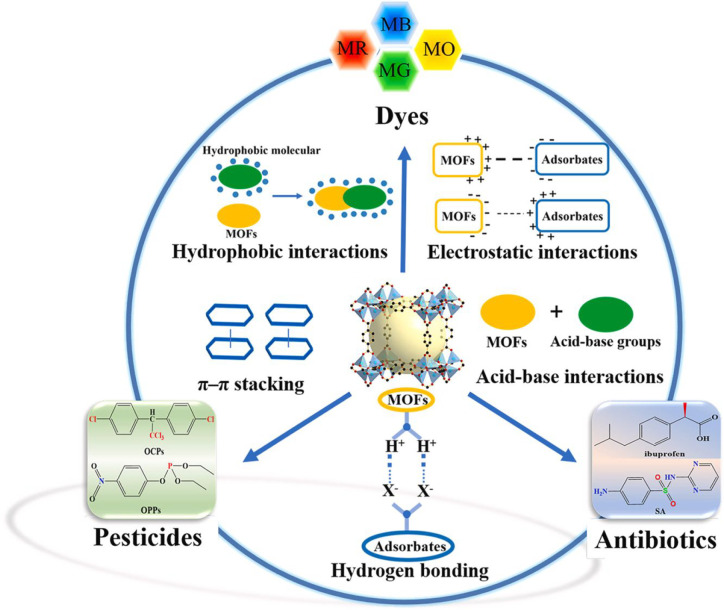
The mechanism of the removal of toxic elements utilizing MOFs most commonly uses π–π interactions, ion exchange, H-bonding, acid–base interactions, and electrostatic interactions.118 Electrostatic interactions are crucial in adsorption processes between surface charges on pollutants (adsorbates) and oppositely charged MOFs (adsorbents). Net surface charges lead to protonation and deprotonation, which favors the electrostatic interactions between the MOFs and pollutants.85 Further, the interactions between hydrogen atoms in N–H, F–H, and O–H bonds and lone pairs of electronegative atoms are known as H-bonding. Studies have revealed that MOFs contain −OH groups and make H-bonds with adsorbates. Acid–base interactions are other mechanisms for the adsorptive removal of MOFs. For instance, Hasan et al.119 removed naproxen and clofibric acids from an aqueous solution using MIL-101 functionalized with acidic (−SO3H) and basic (−NH2) groups. The results revealed that acid–base interactions were dominant and performed better for eliminating acids than did bare MIL-101. Adsorptive mechanisms of MOFs in removing contaminants from wastewater are described in Figure 10.85 Relatively higher surface areas of MOFs facilitate the adsorption of pollutants. Adsorption capacity depends on the characteristics of MOFs as well as on the contaminants. The dominant interactions significantly influence the adsorption mechanism. Therefore, the actual adsorption mechanism is complex, and further investigations are required for exact predictions.
Figure 10.
Adsorptive mechanisms of the MOFs. Reprinted with permission from ref (85). Copyright 2021 Elsevier.
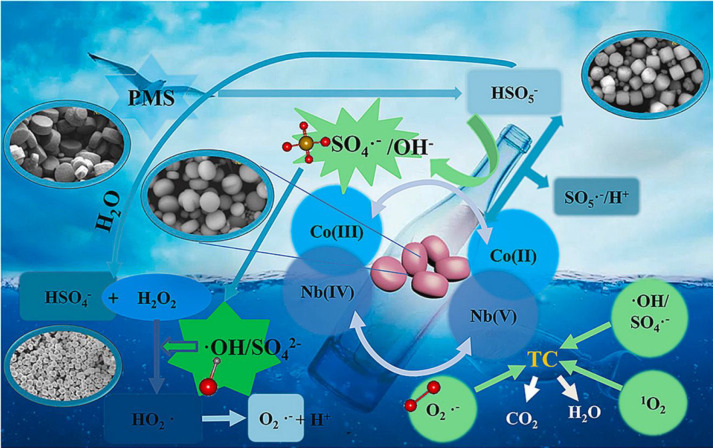
In the adsorption mechanism, toxic pollutants only transfer to the surface of MOFs, and their desorption may result in secondary contaminants.120 Therefore, for the degradation of pollutants from wastewater, catalysts, oxidizing agents, and some reactants are utilized by sewage treatment plants and factories.121−123 Over the past few years, MOFs have attracted much attention for degrading contaminants from wastewater. In catalytic processes, MOFs are combined with highly reactive species such as sulfate radicals (SO4•–) and hydrogen peroxide (H2O2) to promote the oxidation of pollutants into less toxic products.124−126 NbCo-MOF was synthesized to remove tetracycline from wastewater via SO4•– oxidation. The catalytic mechanism of the removal of tetracycline is shown in Figure 11. The observed removal efficiency was 99.7% within 30 min.127 Because of the unique structures of MOFs, closer contacts are provided between pollutants and active sites of MOFs, which enhance the reactions between them. MOFs have several advantages, such as high flexibility, highly selective and relatively higher degradation due to rational design, and different pore structures over other catalysts.
Figure 11.
Schematic illustration of the catalytic mechanism of NbCo-MOF. Reprinted with permission from ref (127). Copyright 2022 Elsevier.
5. Application of Metal–Organic Framework-Based Materials for Wastewater Treatment
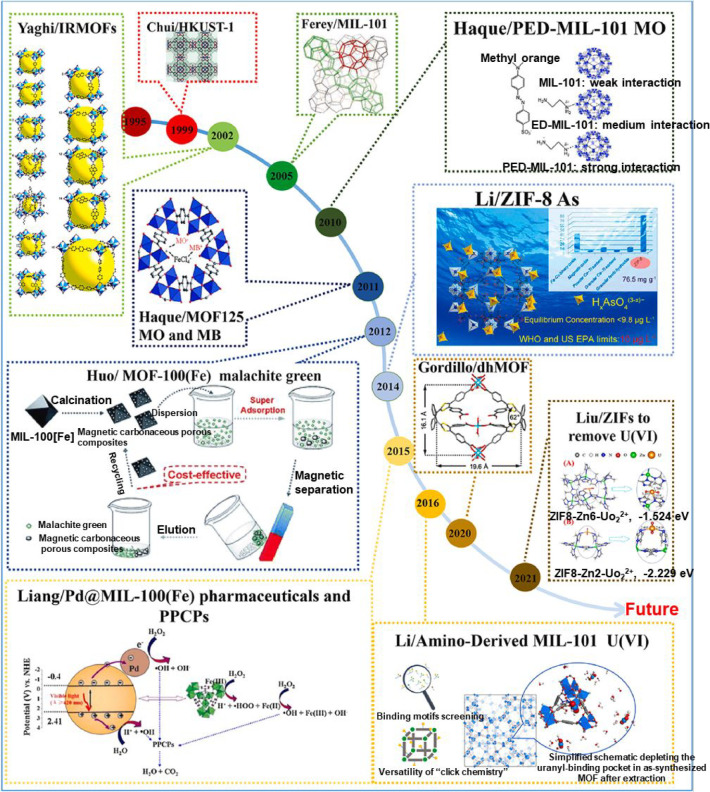
Natural water sources are polluted by many harmful chemicals released from industrial, agricultural, medicinal, and domestic manure that harshly damage the ecological system. MOF-derived materials can be utilized as an adsorbent and catalyst for various processes to eliminate contamination from wastewater due to a higher surface area and tunable pore size. Because of the outstanding separation capability, flexible and controllable size, and composition, MOF-based materials act as scavengers in eliminating heavy metals, organic dyes, pesticides, and other contaminants from wastewater. Therefore, one can say that MOF-based materials are admirable precursors for wastewater treatment in academic research and industrial applications. Figure 12 showcases the applications of MOFs toward the elimination of various contaminants from water from the past few years to the present.128 This section provides information regarding the use of MOF-based materials for wastewater treatment.
Figure 12.
Applications of MOFs toward the elimination of various contaminants from water from the past few years to the present. Reproduced with permission from ref (128). Copyright 2021 Elsevier.
5.1. Removal of Antibiotics
Antibiotics are chemical substances that are generally used in medical treatment and aquaculture. Only 30% of the antibiotic can be absorbed by animals and human beings, and the rest is excreted, say, by urine, which contaminates water sources. In addition, these pharmaceutical industries emit a large amount of antibiotics that severely affect water treatment and are hazardous to the ecosystem.129,130 Many MOFs-based materials have been reported for the elimination of antibiotics through adsorption, catalysis, and photodegradation.131 Xia et al.110 designed Zr-MOFs for the adsorptive removal of tetracycline (TC). Three types of Zr-MOFs were obtained, UiO-66, NU-1000, and MOF-525. For the former two, the adsorption equilibrium was reached within 40 min, while for the latter it was reached within 120 min. The adsorptive capacity of TC on UiO-66 was 145 mg/g, on NU-1000 was 356 mg/g, and on MOF-525 was 807 mg/g. In terms of capacity and adsorption rate, the best performances were given by MOF-525 and NU-1000, respectively. The pore features and topology of MOFs significantly impacted the adsorption execution. The cells whose size matched nicely with TC allowed MOF-525 to obtain the highest adsorption quantity per surface area among the MOFs we inspected. The correct topology of NU-1000 contributed to its high adsorption speed. Figure 13 represents the (a) MOF-based adsorbent, (b) photocatalysts,131 and (c) framework structures of MOFs.110 Another team of researchers132 synthesized MOF-based material Fe3(hexaiminotriphenylene)2 for the removal of TC. For the synthesis, the team made a solution of 25 mL of distilled water with 0.36 mmol of FeCl3 and added this solution at room temperature to another solution containing water and 2,3,6,7,10,11-hexaiminotriphenylene (HITP)·6HCl. Further, 14 M ammonia solution was added. Dark solid precipitates were obtained, which were continuously stirred for 3–4 h. The supernatant then was removed, and the remaining solid was stirred with acetone and water for 24 h. Finally, centrifugation of the resulting powder was done and dried at 60 °C in a vacuum. The authors observed that the MOF-based material showed a removal percentage of 62.7% within 30 min and showed excellent catalytic performance of 97.7%.
Figure 13.
(a) MOF-doped adsorbents. (b) Photocatalysts. Reprinted with permission from ref (131). Copyright 2021 Elsevier. (c) Framework assemblies of MOFs. Reprinted with permission from ref (110). Copyright 2021 Elsevier.
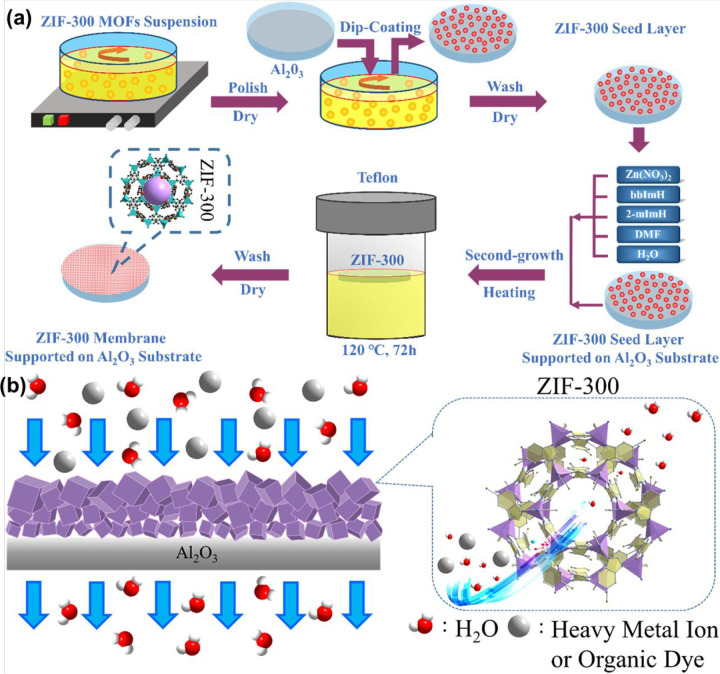
For the elimination of quinolones in wastewater, bimetallic magnetic FexMny catalysts were synthesized by Li et al.133 via the impregnation method. The formed MOF-based material exhibits good porous structure with a high surface area of 122.5 m2/g. Researchers observed that, within 30 min without utilizing any kind of oxidant, the material can degrade 98.3%, 96.0%, 91.0%, 92.2%, and 93.5% of ciprofloxacin (CIP), ofloxacin (OFL), enrofloxacin (ENR), levofloxacin (LEV), and norfloxacin (NOR), respectively. Figure 14 represents (a) the systematic synthesis procedure of the ZIF-300 membrane and (b) the elimination process of heavy metals via the size exclusion mechanism.134 The fabricated membrane exhibits a 99.21% rejection rate and a water permeance of 39.2 L/m2·h·bar.
Figure 14.
(a) Systematic synthesis procedure of the ZIF-300 membrane. (b) Elimination process of heavy metals via the size exclusion mechanism. Reprinted with permission from ref (134). Copyright 2019 Elsevier.
Table 2 showcases different MOFs-based materials utilized for the effective elimination of antibiotics from wastewater.
Table 2. Different MOFs-Based Materials Utilized for the Elimination of Antibiotics from Wastewatera.
| MOF-based material | pollutant removed | preparation method | adsorbent/catalyst dosage (g/L) | pH | surface area (m2/g) | pore size (nm) | removal efficiency (%) | performance | ref |
|---|---|---|---|---|---|---|---|---|---|
| CFC/UiO-66-NH2/AgI | CIP | solvothermal-chemical sedimentation | 10 mg/L | 4.5–8.5 | 730.8 | 2.78 | 79.6 | shows a broader absorption edge (∼440 nm) | (135) |
| LEV | 84.5 | ||||||||
| FexMny | quinolones | facile impregnation method | 0.007 | 122.5 | 13.7 | 92 | degrades quinolones in 30 min without any oxidant | (133) | |
| copper meso-tetra(4-carboxyphenyl) porphine-MOFs | oxytocin | microwave-assisted hydrothermal method | 0.2 | 5 | 342.72 | 0.256 | 83.6 | displays adsorption capacities of 130, 150, and 50 mg/g for oxytocin, TC, and NOR, respectively | (136) |
| TC | 95 | ||||||||
| NOR | |||||||||
| MIL-101 | TC | solvothermal method | 0.15 | 10.2 | 180.41 | 7.55 | 82.52 | shows pore volume of 0.32 cm3/g | (137) |
| UiO-66-NH2 | NOR | hydrothermal route | 0.1 | 8 | 713.2 | 12 | 91.6 | highest partition coefficient of 20.9 mg/g/μM was observed | (138) |
| Zn3(BTC)2 | OFL | sonochemical method | 10 mg/L | 6.7 | 72 | displays adsorption capacity of 25.3 ± 0.8 mg g–1 | (139) | ||
| Zr/Fe-MOF/GO | TC hydrochloride | 20 mg/L | 1–14 | 97.8 | it follows pseudo first- and second-order kinetics | (140) | |||
| alginate-graphene-ZIF67 | TC | in situ route | 1 | 4 | 138.62 | 15.43 | 99.73 | displays adsorption capacities of 456.62 mg/g | (141) |
| UiO-66-(COOH)2/GO | TC | reflux heating method | 0.5 | 3 | 369.6 | 5.04 | have quantum efficiency of 164.91 mg/g | (142) | |
| UiO-66-NH-BT@g-C3N4 | sulfamethoxazole | step-by-step in situ growth strategy | 5 | 40 | 97.6 | initial concentration of antibiotic was 10 mg/L | (143) |
CFC/UiO-66-NH2/AgI, carbon fiber cloth/amine-functionalized zirconium metal organic framework/silver iodide; Zn3(BTC)2, Zn(II) and benzene-1,3,5-tricarboxylate.
5.2. Removal of Organic Dyes
Dyes, when mixed with water, cause adverse effects on marine life, animals, and human beings. Dyes hardly degrade due to their xenobiotic characteristics and complex structure. To dye cotton and wood, methylene blue has been extensively used, which produced a large amount of colored wastewater.144 Globally, around 10 000 tons of dyes is used by textile industries annually.145,146 Dyes generally have high color intensity and carcinogenic and mutagenic properties that result in the toxicity of the effluents. Dyes have detrimental effects on the renal system, respiratory system, eyes, skin, and liver of humans. Acidic dyes can even cause cancer.
Further, organic dyes have toxic effects on aquaculture. Therefore, the elimination of dyes from wastewater is essential.147,148 Numerous methods have been developed to eliminate dyes from water sources such as adsorption, coagulation, oxidation, and biological degradation. MOF and MOF-based materials have a high potential to remove dyes from wastewater by adsorption and catalysis. Dyes are generally cationic or anionic in nature; examples of former dyes are methylene blue (MB) and rhodamine B (RhB), while Congo red and methyl orange are examples of the latter. MOFs also possess either a positive charge or a negative charge. Positively charged MOFs attract negatively charged dyes, and negatively charged MOFs attract cationic dyes.149 These electrostatic attractions are the driving force for the adsorption mechanism of MOFs toward dyes. Hydrogen bonding, π–π interactions, and physical adsorption are other mechanisms of adsorption of dyes on MOFs. The adsorption mechanisms of dyes on MOF surfaces typically depend on many factors.
5.2.1. Effect of Pore Size
The pore size and geometry of MOF-based nanomaterials are crucial for the adsorptive removal of dyes. Because of a higher surface area, porous MOFs can absorb greater quantities of dyes than can nonporous MOFs. A flexible or adjustable design results in several MOF frameworks with multiple pore sizes, dimensions, and geometries. For instance, MOFs such as IRMOF-01 and PCN-222 have square grid channels; PCN-224 and MOF-74 have hexagonal channels.111,112 If the pore size of MOF-based nanocomposites is larger than the size of the dye molecules, then a considerable amount of dye can be adsorbed on the surface of the MOF. However, if the size of the molecules of dyes is larger as compared to the MOF frameworks, then the adsorption of dye molecules is ruled out within the pores of the MOF.113
5.2.2. Effect of Functional Groups
The adherence of adsorbates is promoted by particular functional groups present on MOF-based nanomaterials such as amino groups interacting with acidic dyes and sulfonic acid interacting with basic dyes and with pore geometries of porous MOFs.99
Dyes can facilely be degraded via the oxidation process. The Fenton advanced oxidation process is considered a potential method for removing dyes.115 Li et al.116 fabricated magnetic porous Fe3O4/carbon octahedra via two-step calcination of Fe-based MOF for the elimination of methylene blue. Within 1 h, this material shows 100% removal efficiency in the presence of H2O2 by a Fenton-like heterogeneous reaction. Table 3 showcases different MOFs-based materials utilized to degrade dyes from wastewater.
Table 3. Different MOFs-Based Materials Utilized for the Elimination of Dyes from Wastewater.
| MOF-based materials | dyes removed | preparation method | initial concentration of dye (mg/L) | surface area (m2/g) | pore size (nm) | removal rate | pH | performance | ref |
|---|---|---|---|---|---|---|---|---|---|
| Ce(III)-doped UiO-67 nanoparticles | Congo red | solvothermal method | >100 | 1911.9 | 80 | displays adsorption capacity of 799.6 mg/g | (150) | ||
| Fe-MIL-88NH2 | Congo red | solvothermal method | 5–60 | 87.2% | reached the equilibrium in 60 min | (151) | |||
| MOF@Ox-cotton hybrids | MB | infrared assisted method | 50 | 55–125 | shows adsorption capacity of 75.46–187.03 mg/g | (152) | |||
| RhB | |||||||||
| Co-MOF | methyl orange | solvothermal method | 30 ppm | 79.56% | 4–5 | displays adsorption capacity of 18.80 mg/g and 4.57 for reactive black and methyl orange, respectively | (153) | ||
| reactive black 5 | |||||||||
| Ce-MOF@Fe3O4@activated carbon | indigo carmine and methylene blue | coprecipitation method | 10 | 99% | 7 | shows maximum adsorption capacity of 85.5 and 84.9 mg/g for indigo carmine and methylene blue | (154) | ||
| Sm-MOF/GO | RhB | in situ method | 10 | 91% | rejection rate was maintained even after continuous 5.5 h filtration | (155) | |||
| Cu-MOFs/Fe3O4 | malachite green | in situ method | 35.4 | 3.5 | 90% | displays adsorption capacity of 113.76 mg/g | (156) | ||
| MOF/porous carbon | MB | one-step carbonization treatment | 200 | 1338 | 3.2 | shows adsorption capacity of 2724 mg/g | (157) | ||
| Zr-sulfonic @MOF | MB | solvothermal method | 20 | 93% | 7 | shows maximum adsorption capacity of 1992 mg/g | (158) | ||
| MIL101-Cr/PANI/Ag | MB | hydrothermal method | 25 | 2861 | 153 | 97% | 12 | displays adsorption capacity of 43.29 mg/g | (159) |
5.3. Removal of Heavy Metals
Heavy metals are detected in traces and have detrimental effects on aquatic life. Commonly present heavy metals in wastewater are Cu, Cd, Zn, Hg, Pb, Ca, and Co. Corroded plumbing systems and cable industries are the major sources of Cu in wastewater. Brass coatings and aerosol deodorants are the sources of Zn contamination, while batteries and alloys are the main sources of Pb.160,161Table 4 showcases different MOFs-based materials used for the elimination of heavy metals from wastewater. An ethylenediamine-functionalized Zr-based MOF was synthesized to effectively absorb heavy metals from wastewater.162 The composite was prepared through the Michael addition reaction and has an adsorption capacity of 243.90 mg/g for Pb2+, 208.33 for Cu2+, and 217.39 for Cd2+. Zeolite imidazolate framework-300 was synthesized for the potential elimination of heavy metals from wastewater.134 The authors synthesized the MOF via the second-growth method and observed a rejection rate (99.2%) for CuSO4 and high water permeance of 39.2 L/m2h·bar. For the adsorptive removal of Pb (II), Shi et al.156 fabricated CuMOFs/Fe3O4 via doping of Fe3O4 nanoparticles on the in situ growth of Cu-MOFs and showed an adsorption capacity of 219 mg/g. The surface of CuMOFs/Fe3O4 was 35.4 m2/g and had a removal efficiency of 96%. Chitosan-MOF composite was prepared for the potential degradation of Cr2+, Cu2+, and Ni2+ from wastewater and at 40 °C had 93.6 mg/g adsorption capacity at pH 2 for Cr (VI), while for Cu2+ and Ni2+ the adsorption capacities at pH 5 were 50.6 and 60 mg/g at 60 and 20 °C, respectively.163 Zayan et al.164 synthesized polypyrrole/aluminum fumarate-MOF by in situ oxidative polymerizations for the effective elimination of Pb from the wastewater stream. The prepared composite had a larger surface area of 809 m2/g and exhibits a removal efficiency of nearly 100% in the pH range from 3 to 7.
Table 4. Different MOFs-Based Materials Used for the Elimination of Heavy Metals from Wastewater.
| MOF-based materials | pollutants removed | adsorbent dosage (g/L) | pH | surface area (m2/g) | adsorption capacity (mg/g) | kinetic mode | preparation method | ref |
|---|---|---|---|---|---|---|---|---|
| Fe/Mg-MIL-88B | As (V) | 7 | 360 | 303.6 | pseudo-second | hydrothermal | (165) | |
| 2D-ZIF-L | As (III) | 0.1 | 10 | 67.02 | 43.74 | pseudo-second | (166) | |
| γ-cyclodextrin MOF-based nanoporous carbon | Cd (II) | 7 | 11.4 | 140.85 | pseudo-second | carbonization | (167) | |
| Fe-gallic acid MOFs | Cr (VI) | 1 | 297.8 | 1709.2 | pseudo-second | hydrothermal | (168) | |
| ZIF-8 | Cr (VI) | 0.020 | 7 | 1281 | 0.15 | green method | (169) | |
| MOF-808-EDTA | Hg (II) | 1 | 1173 | 592 | pseudo-second | solvent-assistant linker exchange | (170) | |
| La (III) | 205 | |||||||
| Pb (II) | 313 | |||||||
| MOF-545 | Pb (II) | 7 | 2129 | 73 | pseudo-second | (171) | ||
| Cu-MOFs/Fe3O4 | Pb (II) | 10 | 35.4 | 219 | pseudo-second | in situ | (156) | |
| cadmium terephthalate-based MOF | Pb (II) | 1.0 g | 5–6 | 434.78 | pseudo-second | ultrasonic | (172) | |
| Cu (II) | 769.23 | |||||||
| mercaptosuccinic@ MOF | Hg (II) | 0.1 | 4 | 1180 | pseudo-second | one-step synthesis | (173) | |
| Pb (II) | 510 | |||||||
| MOF-based | Cr (VI) | 0.005 | 7 | 975 | 91% | Pickering emulsion | (174) | |
| Fe-UiOsomes-Pt motors |
5.4. Removal of Agricultural Pollutants
Because of the surging demand for food globally, these days, pesticides, fertilizers, and herbicides are widely used for the protection and growth of crops to meet the growing demand. Various agricultural chemicals such as β-lactams, organophosphates, and sulphonamide have been found in wastewater effluents and livestock farms. Worldwide, approximately three million tons of pesticides are used in agricultural land annually, nearly 15–20 times higher than in the past 30 years.175,176 These agricultural chemicals have detrimental effects on human health as they can damage the endocrine and nervous systems and can cause irritation and carcinogenic effects.177,178
For the photocatalytic degradation of atrazine (ATZ), TC, and sulfamethazine (SMT), MOF-2/graphitic carbon nitride (g-C3N4) nanosheets were prepared by Wang et al.,179 by the vacuum-assisted self-assembly method. MOF-2 nanosheets were synthesized by utilizing top-down delamination of bulk MOF-2, while g-C3N4 nanosheets were synthesized through the chemical exfoliation of bulk g-C3N4. The as-prepared nanosheets exhibit a maximum removal efficiency of 98%, 95%, and 89% for ATZ, TC, and SMT, respectively, at a permeable flux of 23.6 L m–1 h–1 bar–1. For the adsorptive elimination of imidacloprid from water, calcium fumarate-MOF was developed,180 which shows an adsorption capacity of 476.23 mg/g at pH 6.5 with a 98% removal rate within 70 min.
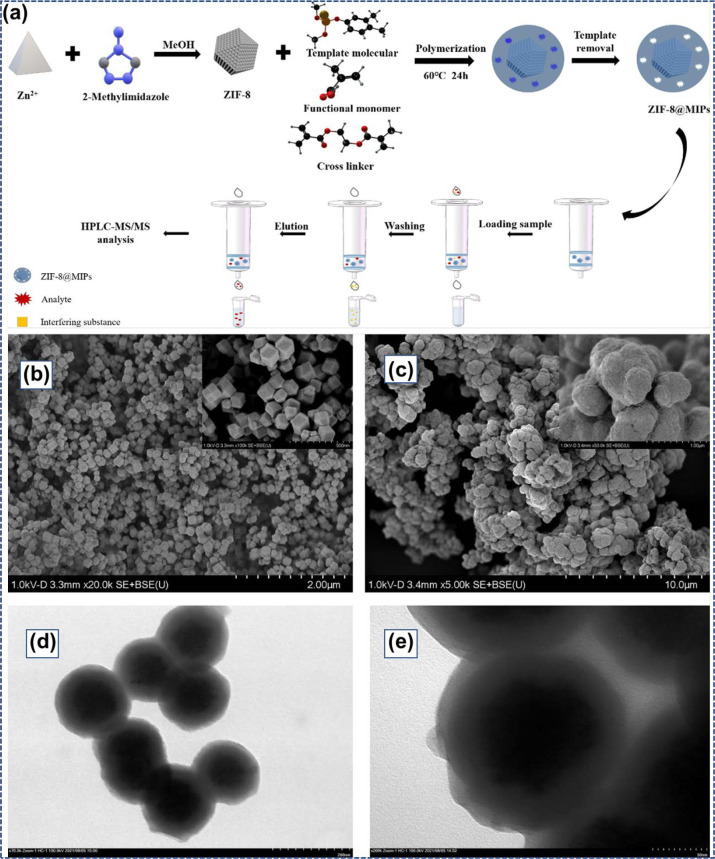
Yang et al.181 synthesized MOF @ molecularly imprinted polymer (ZIF-8@MIPs) adsorbents for the solid-phase extraction of organophosphorus pesticides by agricultural derivatives. Figure 15a shows the graphical illustration for preparing ZIF-8@MIPs via the solid-phase extraction (SPE) method. SEM and TEM characterized the morphological arrangement of the synthesized ZIF-8 and ZIF-8@MIPs. As demonstrated in Figure 15b, ZIF-8 exhibited smooth facades and rhombic dodecahedral shapes with intense sharpness. 100–200 nm was the observed diameter. Corresponding with pristine ZIF-8, the exterior of the mixed composites became brutish with noticeable folds. Successful polymerization of the homogeneous imprinted coating upon the exterior of ZIF-8 was confirmed by the more extensive size of the ZIF-8@MIPs materials (Figure 15c). Also, as shown by typical TEM pictures (Figure 15d and e), the consistency of the MIPs shell with an average of approximately 50 nm was uniformly polymerized on the exterior of the ZIF-8 core to deliver a generally core–shell arrangement. Another organophosphorus pesticide, glyphosate, was removed by the MOF-based material Fe3O4@SiO2@UiO-67.182 The material was synthesized through layer-by-layer assembly, and the material consists of Zr–OH groups that have a high affinity for phosphate-containing groups that enhance the adsorption capacity of the material. The material achieved an adsorption equilibrium within 60 min and exhibited an adsorption capacity of 256.54 mg/g with a lower limit of detection (0.093 mg L–1). Jia et al.183 synthesized MOF-hydrogels (ZIF-8 on Zn2@ sodium alginate) via the layer pillar strategy. This system successfully detected thiophanate-methyl pesticide in real vegetables and fruits within a wide linear range from 10 × 10–6 to 100 × 10–6 in a low detection limit of 0.14 × 10–6. When thiophanate-methyl was sprayed on fruits and vegetables, it produced carbendazim, which was also removed by the prepared material and exhibits an adsorption capacity of 161.8 mg/g. The sensor had a good recyclability with recovery rates in the range of 98.3–102.7%.
Figure 15.
(a) Graphic illustrations of ZIF-8@MIPs synthesis and the SPE method. SEM pictures of the ZIF-8 (b) and ZIF-8@MIPs compounds (c). TEM pictures of ZIF-8@MIPs (d,e). Reprinted with permission from ref (181). Copyright 2022 Elsevier.
In addition to this, a cationic MOFs-based sensor was developed by Wu et al.184 for the detection of six phenoxy carboxylic acid herbicides from water samples. The material was synthesized by soaking Zr-MOFs in the solution of polyvinylidene fluoride followed by functionalization with quaternary amines. The adsorption performance was enhanced by π–π conjugation and cation−π bonding within the detection limit of 0.03–0.059 ng/L and had good recovery rates (80–117%). To remove the insecticide named imidacloprid from water, a team of researchers developed a sensor by utilizing polyethylene terephthalate as a source for the preparation of UiO-66 frameworks.185 The adsorption equilibrium was reached within 60 min, and the sensor exhibits good stability and recyclability. The obtained adsorption capacity was 467.23 mg/g. For the removal of triazole, Cu-based MOF was fabricated by utilizing the Fe3O4-graphene oxide (GO) nanocomposite from the water sample.186 The material exhibits a correlation coefficient of 0.992 and a detection limit of 0.05–0.1.
6. Challenge to Wastewater Treatment and Role of MOFs
For the removal of toxic materials from wastewater resources, numerous techniques such as ion exchange,187 solid-phase extraction,188 electrochemical-based methods,189 and precipitation190 have been utilized over the past couple of years. However, these methods have several demerits, such as complex removal processes and special working conditions. However, due to their simple and straightforward design, user-friendly and cost-effective adsorption techniques are considered an alternative for eliminating contaminants.175,191−194 Many materials, such as activated carbon195 and clay–polymer composites,196 have been extensively utilized as adsorbents to remove toxic particles from water resources. However, limitations such as lower adsorption capacities and tedious separation process limit their effectiveness.197
Because of their attractive properties, such as large surface area and pores and high chemical and solvent stability, MOFs have been explored to remove hazardous particles from wastewater.198,199 Although for widespread applications of MOFs for removing toxic materials, several limitations, such as a more straightforward separable design, have to be overcome. For instance, Abdel-Magied et al.193 synthesized Fe3O4@UiO-66-NH2 by ultrasonication to remove Cd2+ and Pb2+ from an aqueous solution. The obtained maximum adsorption capacity was 714.3 and 833.3 mg/g for Cd2+ and Pb2+, respectively, with excellent reusability.
Kavand et al.200 conducted a study by utilizing activated carbon for the removal of Cd2+ and Pb2+ from wastewater. The adsorption capacities for the former and latter ions were only 9.26 and 9.30 mg/g, respectively. The polymer–clay-based composite was synthesized to remove Pb2+ from the aqueous solution. The maximum adsorption capacity was 21 ± 0.39 mg/g.201 Further, attapulgite clay@carbon was fabricated by Chen et al.202 for the removal of Pb2+ by a one-pot hydrothermal carbonization process. The authors observed a maximum adsorption capacity of 263.83 mg/g. These examples show that the adsorption capacity of MOFs for the removal of heavy metal ions is more than that of other materials. SO3H-UiO-66 (18%) was fabricated by Hasan et al.203 for the adsorptive removal of diclofenac. The authors observed that the adsorption capacity of the as-synthesized material was 263 mg/g, nearly 13 times higher than that of conventional activated carbon (76 mg/g) under similar conditions. The surface area for the MOF-based material was 910 m2/g. A MOF (MIL-53 (Cr)) was fabricated by a team of researchers204 for the effective removal of 2,4-dichlorophenoxyacetic acid from contaminated water. The adsorption capacity of MIL-53 was 556, which is much higher, nearly twice that of activated carbon and zeolite. It removed MB and methyl orange from polluted water by utilizing the adsorption property of MOF-235.2 The authors compared the performance of MOF-235 with that of conventional activated carbon. The adsorption capacity of MOF-235 was 477 and 187 mg/g for methyl orange and MB, respectively, while the adsorption capacity of activated carbon for the former dye was only 11.2 mg/g, and for the latter dye it was 26 mg/g.
7. Advantages of MOF-Based Materials over Other Materials
MOFs can be one of the best supporting active species because they can prevent leaching of homogeneous catalyst either by encapsulating the cavities or by forming covalent bonds with the framework.205
Zeolite-type materials need inorganic or organic templates for their formation, while MOFs do not need these external templates as the solvent itself acts as a template for the formation of MOFs.206
Most metal cations are utilized in the preparation of MOFs, whereas for the formation of other adsorbents, a few cations (Si, Al, and P) can participate in their formation processes.207
Identical ligands and numerous analogues of MOFs can be formed by utilizing different metallic components.208
Isoreticular MOFs can be prepared with the same metal species just by changing the length of the ligands.104
They are highly specific due to easy modification of the pore sizes and surfaces.207
8. Conclusion and Future Prospects
We delivered an inclusive review of recent progress in MOF-based materials for the removal of hazardous pollutants. Because of their different effects and possible applications, MOFs may be valuable to materials for removing contaminants from aqueous media. This Review examined recent investigations on the adsorptive reduction of other pesticides, especially from an aqueous stage employing MOF-based materials. This Review could help scientists to comprehend the existing research scenario on pesticide reduction utilizing MOF-based adsorbents. As summarized, few of the MOF-based adsorbents exhibited performance considerably better than that of traditional adsorbents within the adsorption of different pesticides.
The quest for advanced substances with appropriate characteristics has evolved into a unique approach to reduce the ever-increasing concerns associated with environmental decay. We conclude that the significant adsorption capabilities of MOFs primarily resulted from relations among mark ions and active binding clusters upon the MOFs, jointly with the favorably rated porous network that may be restrained to ease the dispersal of the metal ions. Instructing suitable functional sets within MOFs and adjusting their porosity or incorporating the pores of MOFs with isoreticular or postsynthetic methods have been demonstrated to be efficient techniques for improving their selectivity and adsorption capability for poisonous/radioactive metal ions. MOF-based combinations with universal arrangements, surfaces, and effects have been developed successfully and used in broad areas. Specifically, nanostructured composites from MOFs are nominees for environmental cleaning and monitoring due to their superior preparation and execution.
In the coming days, multiple problems must be examined at the laboratory scale, mainly based on acquiring essential learning of adsorption/catalytic/sensing tools and the affinity between the configuration of MOF byproducts. For practical applications of these nanomaterials in the treatment and management of wastewater, ecofriendly and cost-effective methods should be adopted.
Investigators must concentrate on simplifying preparation methods and optimizing costs while seeking routes to improve resilience, selectivity, and reusability. Thus, attention must be paid to fabricating MOF byproducts with outstanding characteristics to maximize their efficiencies and secure industrial implementations in a challenging environment.
There is still a long path to go in applying MOF materials toward (i) large-scale water processing due to its price; (ii) exploring water-stable MOFs for potential functional applications; (iii) investigations on the consequences of radiation on the strength of MOFs have been lacking, and more details could be alluring; (iv) multiple MOFs emanated from costly ligands, and, therefore, more economical options are of significant need; and (v) the long-term durability of MOFs and resurrection designate a challenge concerning secondary pollution and useful applications. Thus, MOFs resistant to structural degradation caused by moistness, oxidants/reductants, acids/bases, and radiation are selected for prospective investigation. Still, future investigation endeavors are anticipated to promote the possibilities of MOF for practical application.
Finally, MOF-based materials, without any suspicion, have appeared as thrilling advanced composites in environment-based areas, where possibilities and challenges coexist. With maintained steps dedicated to this subject, there is an abundance of room to acquire the actual industrial implementation of MOF-derived materials in the area of eco-friendly remediation and monitoring.
Acknowledgments
We acknowledge the support from the Department of Chemistry and Research & Development Cell of Maharishi Markandeshwar (deemed to be University), Mullana, Ambala, Haryana, India. W.F.A. would like to acknowledge the Taif University TURSP program (TURSP-HC2023/5) for funding. V.K.T. would also like to thank the research support provided by the UKRI via grant no. EP/T024607/1 and the SFC (UIF funding).
Glossary
Abbreviations
- MOFs
metal–organic frameworks
- NMR
nuclear magnetic resonance
- DMF
dimethylformamide
- STLHC
sertraline hydrochloride
- SNDGr
sulfur and nitrogen codoped graphene
- ECSA
electrochemical active surface area
- CV
cyclic voltammetry
- PGE
pencil graphite electrode
- EIS
electrochemical impedance spectroscopy
- LMOF
luminescent MOF
- FTO
fluorine-doped tin dioxide
- AC
activated carbon
- DBA
4-(dodecyloxy)benzoic acid
- SEM
scanning electron microscopy
- spng
sponge-like
- pmg
pomegranate-like
- OER
oxygen evolution reaction
- EPD
electrophoretic deposition
- EC-MOF
electrochemical conversion of metal–organic framework
- TC
tertracyline
- CIP
ciprofloxacin
- OFL
ofloxacin
- ENR
enrofloxacin
- LEV
levofloxacin
- NOR
norfloxacin
- CFC
carbon fiber cloth
- BTC
benzene-1,3,5-tricarboxylate
- MB
methylene blue
- RhB
rhodamine B
- ATZ
atrazine
- SMT
sulfamethazine
- g-C3N4
graphitic carbon nitride
- MIP
molecularly imprinted polymer
- SPE
solid-phase extraction
- TEM
transmission electron microscopy
The authors declare no competing financial interest.
References
- Verma A. Modified Sodium Alginate Hydrogel Composite for Efficient Removal of Malachite Green Dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. 10.1016/j.ijbiomac.2020.01.142. [DOI] [PubMed] [Google Scholar]
- Ates B.; Koytepe S.; Ulu A.; Gurses C.; Thakur V. K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120 (17), 9304–9362. 10.1021/acs.chemrev.9b00553. [DOI] [PubMed] [Google Scholar]
- Sharma B.; Thakur S.; Mamba G.; Prateek; Gupta R. K.; Gupta V. K.; Thakur V. K. Titania Modified Gum Tragacanth Based Hydrogel Nanocomposite for Water Remediation. Journal of Environmental Chemical Engineering 2021, 9, 104608. 10.1016/j.jece.2020.104608. [DOI] [Google Scholar]
- Thakur V. K.; Voicu S. I. Recent Advances in Cellulose and Chitosan Based Membranes for Water Purification: A Concise Review. Carbohydr. Polym. 2016, 146, 148–165. 10.1016/j.carbpol.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Siwal S. S.; Sheoran K.; Mishra K.; Kaur H.; Saini A. K.; Saini V.; Vo D.-V. N.; Nezhad H. Y.; Thakur V. K. Novel synthesis methods and applications of MXene-based nanomaterials (MBNs) for hazardous pollutants degradation: Future perspectives. Chemosphere 2022, 293, 133542. 10.1016/j.chemosphere.2022.133542. [DOI] [PubMed] [Google Scholar]
- Sheoran K.; Kaur H.; Siwal S. S.; Saini A. K.; Vo D.-V. N.; Thakur V. K. Recent advances of carbon-based nanomaterials (CBNMs) for wastewater treatment: Synthesis and application. Chemosphere 2022, 299, 134364. 10.1016/j.chemosphere.2022.134364. [DOI] [PubMed] [Google Scholar]
- Pandey S.; Makhado E.; Kim S.; Kang M. Recent developments of polysaccharide based superabsorbent nanocomposite for organic dye contamination removal from wastewater — A review. Environmental Research 2023, 217, 114909. 10.1016/j.envres.2022.114909. [DOI] [PubMed] [Google Scholar]
- Kumar N.; Gusain R.; Pandey S.; Ray S. S. Hydrogel Nanocomposite Adsorbents and Photocatalysts for Sustainable Water Purification. Advanced Materials Interfaces 2023, n/a (n/a), 2201375. 10.1002/admi.202201375. [DOI] [Google Scholar]
- Rahdar S.; Rahdar A.; Zafar M. N.; Shafqat S. S.; Ahmadi S. Synthesis and characterization of MgO supported Fe-Co-Mn nanoparticles with exceptionally high adsorption capacity for Rhodamine B dye. Journal of Materials Research and Technology 2019, 8 (5), 3800–3810. 10.1016/j.jmrt.2019.06.041. [DOI] [Google Scholar]
- Zhou Y.; Yang Q.; Zhang D.; Gan N.; Li Q.; Cuan J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators, B 2018, 262, 137–143. 10.1016/j.snb.2018.01.218. [DOI] [Google Scholar]
- Imran M.; Das K. R.; Naik M. M. Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Chemosphere 2019, 215, 846–857. 10.1016/j.chemosphere.2018.10.114. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Liu K.; Zhang J.; Liu X.; Yang L.; Wei R.; Wang S.; Zhang D.; Xie S.; Tao F. Antibiotic body burden of elderly Chinese population and health risk assessment: A human biomonitoring-based study. Environ. Pollut. 2020, 256, 113311. 10.1016/j.envpol.2019.113311. [DOI] [PubMed] [Google Scholar]
- Patangia D. V.; Anthony Ryan C.; Dempsey E.; Paul Ross R.; Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11 (1), e1260 10.1002/mbo3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D.; Neogi S.; De S. Adsorptive removal of heavy metals from battery industry effluent using MOF incorporated polymeric beads: A combined experimental and modeling approach. Journal of Hazardous Materials 2021, 403, 123624. 10.1016/j.jhazmat.2020.123624. [DOI] [PubMed] [Google Scholar]
- Thakur V. K.; Thakur M. K. Recent Advances in Green Hydrogels from Lignin: A Review. Int. J. Biol. Macromol. 2015, 72, 834–847. 10.1016/j.ijbiomac.2014.09.044. [DOI] [PubMed] [Google Scholar]
- Thakur S.; Verma A.; Raizada P.; Gunduz O.; Janas D.; Alsanie W. F.; Scarpa F.; Thakur V. K. Bentonite-Based Sodium Alginate/ Dextrin Cross-Linked Poly (Acrylic Acid) Hydrogel Nanohybrids for Facile Removal of Paraquat Herbicide from Aqueous Solutions. Chemosphere 2022, 291, 133002. 10.1016/j.chemosphere.2021.133002. [DOI] [PubMed] [Google Scholar]
- Pasinszki T.; Krebsz M.; Chand D.; Kótai L.; Homonnay Z.; Sajó I. E.; Váczi T. Carbon microspheres decorated with iron sulfide nanoparticles for mercury(II) removal from water. J. Mater. Sci. 2020, 55 (4), 1425–1435. 10.1007/s10853-019-04032-3. [DOI] [Google Scholar]
- Mondol M. M. H.; Jhung S. H. Adsorptive removal of pesticides from water with metal-organic framework-based materials. Chemical Engineering Journal 2021, 421, 129688. 10.1016/j.cej.2021.129688. [DOI] [Google Scholar]
- Rana A. K.; Frollini E.; Thakur V. K. Cellulose Nanocrystals: Pretreatments, Preparation Strategies, and Surface Functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. 10.1016/j.ijbiomac.2021.05.119. [DOI] [PubMed] [Google Scholar]
- Keyikoglu R.; Khataee A.; Lin H.; Orooji Y. Vanadium (V)-doped ZnFe layered double hydroxide for enhanced sonocatalytic degradation of pymetrozine. Chemical Engineering Journal 2022, 434, 134730. 10.1016/j.cej.2022.134730. [DOI] [Google Scholar]
- Patial S.; Raizada P.; Hasija V.; Singh P.; Thakur V. K.; Nguyen V.-H. Recent Advances in Photocatalytic Multivariate Metal Organic Frameworks-Based Nanostructures toward Renewable Energy and the Removal of Environmental Pollutants. Materials Today Energy 2021, 19, 100589. 10.1016/j.mtener.2020.100589. [DOI] [Google Scholar]
- Karaman C.; Karaman O.; Show P.-L.; Orooji Y.; Karimi-Maleh H. Utilization of a double-cross-linked amino-functionalized three-dimensional graphene networks as a monolithic adsorbent for methyl orange removal: Equilibrium, kinetics, thermodynamics and artificial neural network modeling. Environmental Research 2022, 207, 112156. 10.1016/j.envres.2021.112156. [DOI] [PubMed] [Google Scholar]
- Rodríguez Couto S. Dye removal by immobilised fungi. Biotechnology Advances 2009, 27 (3), 227–235. 10.1016/j.biotechadv.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Rajendran S.; Priya T. A. K.; Khoo K. S.; Hoang T. K. A.; Ng H.-S.; Munawaroh H. S. H.; Karaman C.; Orooji Y.; Show P. L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. 10.1016/j.chemosphere.2021.132369. [DOI] [PubMed] [Google Scholar]
- Ghalkhani M.; Zare N.; Karimi F.; Karaman C.; Alizadeh M.; Vasseghian Y. Recent advances in Ponceau dyes monitoring as food colorant substances by electrochemical sensors and developed procedures for their removal from real samples. Food Chem. Toxicol. 2022, 161, 112830. 10.1016/j.fct.2022.112830. [DOI] [PubMed] [Google Scholar]
- Xu G.-R.; An Z.-H.; Xu K.; Liu Q.; Das R.; Zhao H.-L. Metal organic framework (MOF)-based micro/nanoscaled materials for heavy metal ions removal: The cutting-edge study on designs, synthesis, and applications. Coord. Chem. Rev. 2021, 427, 213554. 10.1016/j.ccr.2020.213554. [DOI] [Google Scholar]
- Cai G.; Yan P.; Zhang L.; Zhou H.-C.; Jiang H.-L. Metal-Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121 (20), 12278–12326. 10.1021/acs.chemrev.1c00243. [DOI] [PubMed] [Google Scholar]
- Fang X.; Gan L.; Wang L.; Gong H.; Xu L.; Wu Y.; Lu H.; Han S.; Cui J.; Xia C. Enhanced degradation of bisphenol A by mixed ZIF derived CoZn oxide encapsulated N-doped carbon via peroxymonosulfate activation: The importance of N doping amount. Journal of Hazardous Materials 2021, 419, 126363. 10.1016/j.jhazmat.2021.126363. [DOI] [PubMed] [Google Scholar]
- Xin X.; Song Y.; Guo S.; Zhang Y.; Wang B.; Yu J.; Li X. In-situ growth of high-content 1T phase MoS2 confined in the CuS nanoframe for efficient photocatalytic hydrogen evolution. Applied Catalysis B: Environmental 2020, 269, 118773. 10.1016/j.apcatb.2020.118773. [DOI] [Google Scholar]
- Li J.; Wang H.; Yuan X.; Zhang J.; Chew J. W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. 10.1016/j.ccr.2019.213116. [DOI] [Google Scholar]
- Guo L.; Wang Y.; Yan M.; Li X.; Jiang X.; Wang M.; Wang Q.; Wang X.; Hao Y. Fabrication of Ce-doped DUT-52 as a sorbent for dispersive solid phase extraction of estrogens in human urine samples. Analytical Methods 2022, 14 (32), 3094–3102. 10.1039/D2AY00795A. [DOI] [PubMed] [Google Scholar]
- Öhrström L.; Amombo Noa F. M. An improved water-harvesting cycle. Science 2021, 374 (6566), 402–402. 10.1126/science.abm1854. [DOI] [PubMed] [Google Scholar]
- Valizadeh B.; Nguyen T. N.; Kampouri S.; Sun D. T.; Mensi M. D.; Stylianou K.; Smit B.; Queen W. L. A novel integrated Cr(vi) adsorption-photoreduction system using MOF@polymer composite beads. Journal of Materials Chemistry A 2020, 8 (19), 9629–9637. 10.1039/D0TA01046D. [DOI] [Google Scholar]
- Yang F.; Du M.; Yin K.; Qiu Z.; Zhao J.; Liu C.; Zhang G.; Gao Y.; Pang H. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18 (11), 2105715. 10.1002/smll.202105715. [DOI] [PubMed] [Google Scholar]
- Fu L.; Wang S.; Lin G.; Zhang L.; Liu Q.; Fang J.; Wei C.; Liu G. Post-functionalization of UiO-66-NH2 by 2,5-Dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(II) in water. Journal of Hazardous Materials 2019, 368, 42–51. 10.1016/j.jhazmat.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Lei S.; Chang L.-M.; Gu Z.-G.; Zhang J. A metal-porphyrinic framework film as an efficient optical limiting layer in an electro-optical switchable device. Chem. Commun. 2021, 57 (79), 10166–10169. 10.1039/D1CC04513J. [DOI] [PubMed] [Google Scholar]
- Yuan S.; Feng L.; Wang K.; Pang J.; Bosch M.; Lollar C.; Sun Y.; Qin J.; Yang X.; Zhang P.; Wang Q.; Zou L.; Zhang Y.; Zhang L.; Fang Y.; Li J.; Zhou H.-C. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30 (37), 1704303. 10.1002/adma.201704303. [DOI] [PubMed] [Google Scholar]
- Wang C.; Liu X.; Keser Demir N.; Chen J. P.; Li K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45 (18), 5107–5134. 10.1039/C6CS00362A. [DOI] [PubMed] [Google Scholar]
- Burtch N. C.; Jasuja H.; Walton K. S. Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 2014, 114 (20), 10575–10612. 10.1021/cr5002589. [DOI] [PubMed] [Google Scholar]
- Ahmadijokani F.; Molavi H.; Bahi A.; Fernández R.; Alaee P.; Wu S.; Wuttke S.; Ko F.; Arjmand M. Metal-Organic Frameworks and Electrospinning: A Happy Marriage for Wastewater Treatment. Adv. Funct. Mater. 2022, n/a (n/a), 2207723. 10.1002/adfm.202207723. [DOI] [Google Scholar]
- Howarth A. J.; Liu Y.; Li P.; Li Z.; Wang T. C.; Hupp J. T.; Farha O. K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nature Reviews Materials 2016, 1 (3), 15018. 10.1038/natrevmats.2015.18. [DOI] [Google Scholar]
- Li S.; Huo F. Metal-organic framework composites: from fundamentals to applications. Nanoscale 2015, 7 (17), 7482–7501. 10.1039/C5NR00518C. [DOI] [PubMed] [Google Scholar]
- Falcaro P.; Ricco R.; Yazdi A.; Imaz I.; Furukawa S.; Maspoch D.; Ameloot R.; Evans J. D.; Doonan C. J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. 10.1016/j.ccr.2015.08.002. [DOI] [Google Scholar]
- Liu Y.; Liu Z.; Huang D.; Cheng M.; Zeng G.; Lai C.; Zhang C.; Zhou C.; Wang W.; Jiang D.; Wang H.; Shao B. Metal or metal-containing nanoparticle@MOF nanocomposites as a promising type of photocatalyst. Coord. Chem. Rev. 2019, 388, 63–78. 10.1016/j.ccr.2019.02.031. [DOI] [Google Scholar]
- Velásquez-Hernández M. d. J.; Linares-Moreau M.; Astria E.; Carraro F.; Alyami M. Z.; Khashab N. M.; Sumby C. J.; Doonan C. J.; Falcaro P. Towards applications of bioentities@MOFs in biomedicine. Coord. Chem. Rev. 2021, 429, 213651. 10.1016/j.ccr.2020.213651. [DOI] [Google Scholar]
- Zheng X.; Wang L.; Pei Q.; He S.; Liu S.; Xie Z. Metal-Organic Framework@Porous Organic Polymer Nanocomposite for Photodynamic Therapy. Chem. Mater. 2017, 29 (5), 2374–2381. 10.1021/acs.chemmater.7b00228. [DOI] [Google Scholar]
- Wang S.; McGuirk C. M.; d’Aquino A.; Mason J. A.; Mirkin C. A. Metal-Organic Framework Nanoparticles. Adv. Mater. 2018, 30 (37), 1800202. 10.1002/adma.201800202. [DOI] [PubMed] [Google Scholar]
- Chen L.-W.; Hao Y.-C.; Guo Y.; Zhang Q.; Li J.; Gao W.-Y.; Ren L.; Su X.; Hu L.; Zhang N.; Li S.; Feng X.; Gu L.; Zhang Y.-W.; Yin A.-X.; Wang B. Metal-Organic Framework Membranes Encapsulating Gold Nanoparticles for Direct Plasmonic Photocatalytic Nitrogen Fixation. J. Am. Chem. Soc. 2021, 143 (15), 5727–5736. 10.1021/jacs.0c13342. [DOI] [PubMed] [Google Scholar]
- Yu S.; Pang H.; Huang S.; Tang H.; Wang S.; Qiu M.; Chen Z.; Yang H.; Song G.; Fu D.; Hu B.; Wang X. Recent advances in metal-organic framework membranes for water treatment: A review. Science of The Total Environment 2021, 800, 149662. 10.1016/j.scitotenv.2021.149662. [DOI] [PubMed] [Google Scholar]
- Wyszogrodzka G.; Marszałek B.; Gil B.; Dorożyński P. Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discovery Today 2016, 21 (6), 1009–1018. 10.1016/j.drudis.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Lu G.; Li S.; Guo Z.; Farha O. K.; Hauser B. G.; Qi X.; Wang Y.; Wang X.; Han S.; Liu X.; DuChene J. S.; Zhang H.; Zhang Q.; Chen X.; Ma J.; Loo S. C. J.; Wei W. D.; Yang Y.; Hupp J. T.; Huo F. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4 (4), 310–316. 10.1038/nchem.1272. [DOI] [PubMed] [Google Scholar]
- Liu H.; Chang L.; Bai C.; Chen L.; Luque R.; Li Y. Controllable Encapsulation of “Clean” Metal Clusters within MOFs through Kinetic Modulation: Towards Advanced Heterogeneous Nanocatalysts. Angew. Chem., Int. Ed. 2016, 55 (16), 5019–5023. 10.1002/anie.201511009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D. K.; Bhadra B. N.; Jhung S. H. Adsorptive removal of hazardous organics from water and fuel with functionalized metal-organic frameworks: Contribution of functional groups. Journal of Hazardous Materials 2021, 403, 123655. 10.1016/j.jhazmat.2020.123655. [DOI] [PubMed] [Google Scholar]
- Furukawa S.; Reboul J.; Diring S.; Sumida K.; Kitagawa S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43 (16), 5700–5734. 10.1039/C4CS00106K. [DOI] [PubMed] [Google Scholar]
- Czaja A. U.; Trukhan N.; Müller U. Industrial applications of metal-organic frameworks. Chem. Soc. Rev. 2009, 38 (5), 1284–1293. 10.1039/b804680h. [DOI] [PubMed] [Google Scholar]
- Rubio-Martinez M.; Avci-Camur C.; Thornton A. W.; Imaz I.; Maspoch D.; Hill M. R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46 (11), 3453–3480. 10.1039/C7CS00109F. [DOI] [PubMed] [Google Scholar]
- Saeed T.; Naeem A.; Ud Din I.; Alotaibi M. A.; Alharthi A. I.; Wali Khan I.; Huma Khan N.; Malik T. Structure, nomenclature and viable synthesis of micro/nanoscale metal organic frameworks and their remarkable applications in adsorption of organic pollutants. Microchemical Journal 2020, 159, 105579. 10.1016/j.microc.2020.105579. [DOI] [Google Scholar]
- Lee Y.-R.; Kim J.; Ahn W.-S. Synthesis of metal-organic frameworks: A mini review. Korean Journal of Chemical Engineering 2013, 30 (9), 1667–1680. 10.1007/s11814-013-0140-6. [DOI] [Google Scholar]
- Huang K.; Xu Y.; Wang L.; Wu D. Heterogeneous catalytic wet peroxide oxidation of simulated phenol wastewater by copper metal-organic frameworks. RSC Adv. 2015, 5 (41), 32795–32803. 10.1039/C5RA01707F. [DOI] [Google Scholar]
- Wang H.; Yuan X.; Wu Y.; Zeng G.; Chen X.; Leng L.; Wu Z.; Jiang L.; Li H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. Journal of Hazardous Materials 2015, 286, 187–194. 10.1016/j.jhazmat.2014.11.039. [DOI] [PubMed] [Google Scholar]
- Nguyen H.-T. T.; Tran K.-N. T.; Van Tan L.; Tran V. A.; Doan V.-D.; Lee T.; Nguyen T. D. Microwave-assisted solvothermal synthesis of bimetallic metal-organic framework for efficient photodegradation of organic dyes. Mater. Chem. Phys. 2021, 272, 125040. 10.1016/j.matchemphys.2021.125040. [DOI] [Google Scholar]
- Millange F.; El Osta R.; Medina M. E.; Walton R. I. A time-resolved diffraction study of a window of stability in the synthesis of a copper carboxylate metal-organic framework. CrystEngComm 2011, 13 (1), 103–108. 10.1039/C0CE00530D. [DOI] [Google Scholar]
- Biemmi E.; Christian S.; Stock N.; Bein T. High-throughput screening of synthesis parameters in the formation of the metal-organic frameworks MOF-5 and HKUST-1. Microporous Mesoporous Mater. 2009, 117 (1), 111–117. 10.1016/j.micromeso.2008.06.040. [DOI] [Google Scholar]
- Younis S. A.; Bhardwaj N.; Bhardwaj S. K.; Kim K.-H.; Deep A. Rare earth metal-organic frameworks (RE-MOFs): Synthesis, properties, and biomedical applications. Coord. Chem. Rev. 2021, 429, 213620. 10.1016/j.ccr.2020.213620. [DOI] [Google Scholar]
- Zhang X.-l.; Li S.-m.; Chen S.; Feng F.; Bai J.-q.; Li J.-r. Ammoniated MOF-74(Zn) derivatives as luminescent sensor for highly selective detection of tetrabromobisphenol A. Ecotoxicology and Environmental Safety 2020, 187, 109821. 10.1016/j.ecoenv.2019.109821. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Xu S.; Cheng H.; Liu W.; Chen F.; Liu X.; Liu J.; Chen S.; Hu C. Oriented growth of polyaniline nanofiber arrays onto the glass and flexible substrates using a facile method. Appl. Surf. Sci. 2018, 428, 315–321. 10.1016/j.apsusc.2017.09.087. [DOI] [Google Scholar]
- Zhang X.; Wan K.; Subramanian P.; Xu M.; Luo J.; Fransaer J. J. J. o. M. C. A. Electrochemical deposition of metal-organic framework films and their applications. 2020, 8 (16), 7569–7587. 10.1039/D0TA00406E. [DOI] [Google Scholar]
- Stassen I.; Styles M.; Van Assche T.; Campagnol N.; Fransaer J.; Denayer J.; Tan J.-C.; Falcaro P.; De Vos D.; Ameloot R. Electrochemical Film Deposition of the Zirconium Metal-Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap. Chem. Mater. 2015, 27 (5), 1801–1807. 10.1021/cm504806p. [DOI] [Google Scholar]
- Shi E.; Zou X.; Liu J.; Lin H.; Zhang F.; Shi S.; Liu F.; Zhu G.; Qu F. Electrochemical fabrication of copper-containing metal-organic framework films as amperometric detectors for bromate determination. Dalton Transactions 2016, 45 (18), 7728–7736. 10.1039/C5DT04229A. [DOI] [PubMed] [Google Scholar]
- Li M.; Dincă M. Selective formation of biphasic thin films of metal-organic frameworks by potential-controlled cathodic electrodeposition. Chemical Science 2014, 5 (1), 107–111. 10.1039/C3SC51815A. [DOI] [Google Scholar]
- Hod I.; Bury W.; Karlin D. M.; Deria P.; Kung C.-W.; Katz M. J.; So M.; Klahr B.; Jin D.; Chung Y.-W.; Odom T. W.; Farha O. K.; Hupp J. T. Directed Growth of Electroactive Metal-Organic Framework Thin Films Using Electrophoretic Deposition. Adv. Mater. 2014, 26 (36), 6295–6300. 10.1002/adma.201401940. [DOI] [PubMed] [Google Scholar]
- M. V V.; Nageswaran G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167 (15), 155527. 10.1149/1945-7111/abc6c6. [DOI] [Google Scholar]
- Habibi B.; Pashazadeh S.; Saghatforoush L. A.; Pashazadeh A. Direct electrochemical synthesis of the copper based metal-organic framework on/in the heteroatoms doped graphene/pencil graphite electrode: Highly sensitive and selective electrochemical sensor for sertraline hydrochloride. J. Electroanal. Chem. 2021, 888, 115210. 10.1016/j.jelechem.2021.115210. [DOI] [Google Scholar]
- Zhou S.; Shekhah O.; Jia J.; Czaban-Jóźwiak J.; Bhatt P. M.; Ramírez A.; Gascon J.; Eddaoudi M. Electrochemical synthesis of continuous metal-organic framework membranes for separation of hydrocarbons. Nature Energy 2021, 6 (9), 882–891. 10.1038/s41560-021-00881-y. [DOI] [Google Scholar]
- Thomas-Hillman I.; Laybourn A.; Dodds C.; Kingman S. W. Realising the environmental benefits of metal-organic frameworks: recent advances in microwave synthesis. Journal of Materials Chemistry A 2018, 6 (25), 11564–11581. 10.1039/C8TA02919A. [DOI] [Google Scholar]
- Laybourn A.; Katrib J.; Ferrari-John R. S.; Morris C. G.; Yang S.; Udoudo O.; Easun T. L.; Dodds C.; Champness N. R.; Kingman S. W.; Schröder M. Metal-organic frameworks in seconds via selective microwave heating. Journal of Materials Chemistry A 2017, 5 (16), 7333–7338. 10.1039/C7TA01493G. [DOI] [Google Scholar]
- Bromberg L.; Diao Y.; Wu H.; Speakman S. A.; Hatton T. A. Chromium(III) Terephthalate Metal Organic Framework (MIL-101): HF-Free Synthesis, Structure, Polyoxometalate Composites, and Catalytic Properties. Chem. Mater. 2012, 24 (9), 1664–1675. 10.1021/cm2034382. [DOI] [Google Scholar]
- Xu Y.-P.; Tian Z.-J.; Wang S.-J.; Hu Y.; Wang L.; Wang B.-C.; Ma Y.-C.; Hou L.; Yu J.-Y.; Lin L.-W. Microwave-Enhanced Ionothermal Synthesis of Aluminophosphate Molecular Sieves. Angew. Chem., Int. Ed. 2006, 45 (24), 3965–3970. 10.1002/anie.200600054. [DOI] [PubMed] [Google Scholar]
- C S.; Wei F.; Chen H.; Chen D.; Ding B. Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater. Green Processing and Synthesis 2021, 10 (1), 125–133. 10.1515/gps-2021-0020. [DOI] [Google Scholar]
- Solís R. R.; Gómez-Avilés A.; Belver C.; Rodriguez J. J.; Bedia J. Microwave-assisted synthesis of NH2-MIL-125(Ti) for the solar photocatalytic degradation of aqueous emerging pollutants in batch and continuous tests. Journal of Environmental Chemical Engineering 2021, 9 (5), 106230. 10.1016/j.jece.2021.106230. [DOI] [Google Scholar]
- Li W.-J.; Feng J.-F.; Lin Z.-J.; Yang Y.-L.; Yang Y.; Wang X.-S.; Gao S.-Y.; Cao R. Patterned growth of luminescent metal-organic framework films: a versatile electrochemically-assisted microwave deposition method. Chem. Commun. 2016, 52 (20), 3951–3954. 10.1039/C6CC00519E. [DOI] [PubMed] [Google Scholar]
- Kim J.; Yang S.-T.; Choi S. B.; Sim J.; Kim J.; Ahn W.-S. Control of catenation in CuTATB-n metal-organic frameworks by sonochemical synthesis and its effect on CO2 adsorption. J. Mater. Chem. 2011, 21 (9), 3070–3076. 10.1039/c0jm03318a. [DOI] [Google Scholar]
- Jung D.-W.; Yang D.-A.; Kim J.; Kim J.; Ahn W.-S. Facile synthesis of MOF-177 by a sonochemical method using 1-methyl-2-pyrrolidinone as a solvent. Dalton Transactions 2010, 39 (11), 2883–2887. 10.1039/b925088c. [DOI] [PubMed] [Google Scholar]
- Cho H.-Y.; Kim J.; Kim S.-N.; Ahn W.-S. High yield 1-L scale synthesis of ZIF-8 via a sonochemical route. Microporous Mesoporous Mater. 2013, 169, 180–184. 10.1016/j.micromeso.2012.11.012. [DOI] [Google Scholar]
- Tchinsa A.; Hossain M. F.; Wang T.; Zhou Y. Removal of organic pollutants from aqueous solution using metal organic frameworks (MOFs)-based adsorbents: A review. Chemosphere 2021, 284, 131393. 10.1016/j.chemosphere.2021.131393. [DOI] [PubMed] [Google Scholar]
- Vaitsis C.; Sourkouni G.; Argirusis C.. Sonochemical synthesis of MOFs. In Metal-Organic Frameworks for Biomedical Applications; Mozafari M., Ed.; Woodhead Publishing: Cambridge, 2020; Chapter 11, pp 223–244. [Google Scholar]
- Chen D.; Zhao J.; Zhang P.; Dai S. Mechanochemical synthesis of metal-organic frameworks. Polyhedron 2019, 162, 59–64. 10.1016/j.poly.2019.01.024. [DOI] [Google Scholar]
- Głowniak S.; Szczęśniak B.; Choma J.; Jaroniec M. Mechanochemistry: Toward green synthesis of metal-organic frameworks. Mater. Today 2021, 46, 109–124. 10.1016/j.mattod.2021.01.008. [DOI] [Google Scholar]
- Taheri M.; Enge T. G.; Tsuzuki T. Water stability of cobalt doped ZIF-8: a quantitative study using optical analyses. Materials Today Chemistry 2020, 16, 100231. 10.1016/j.mtchem.2019.100231. [DOI] [Google Scholar]
- Huang Y.-H.; Lo W.-S.; Kuo Y.-W.; Chen W.-J.; Lin C.-H.; Shieh F.-K. Green and rapid synthesis of zirconium metal-organic frameworks via mechanochemistry: UiO-66 analog nanocrystals obtained in one hundred seconds. Chem. Commun. 2017, 53 (43), 5818–5821. 10.1039/C7CC03105J. [DOI] [PubMed] [Google Scholar]
- Garzón-Tovar L.; Cano-Sarabia M.; Carné-Sánchez A.; Carbonell C.; Imaz I.; Maspoch D. A spray-drying continuous-flow method for simultaneous synthesis and shaping of microspherical high nuclearity MOF beads. Reaction Chemistry & Engineering 2016, 1 (5), 533–539. 10.1039/C6RE00065G. [DOI] [Google Scholar]
- Avci-Camur C.; Troyano J.; Pérez-Carvajal J.; Legrand A.; Farrusseng D.; Imaz I.; Maspoch D. Aqueous production of spherical Zr-MOF beads via continuous-flow spray-drying. Green Chem. 2018, 20 (4), 873–878. 10.1039/C7GC03132G. [DOI] [Google Scholar]
- Gandara-Loe J.; Souza B. E.; Missyul A.; Giraldo G.; Tan J. C.; Silvestre-Albero J. MOF-Based Polymeric Nanocomposite Films as Potential Materials for Drug Delivery Devices in Ocular Therapeutics. ACS Appl. Mater. Interfaces 2020, 12 (27), 30189–30197. 10.1021/acsami.0c07517. [DOI] [PubMed] [Google Scholar]
- Zhao D.; Zhang W.; Yu S.; Xia S.-L.; Liu Y.-N.; Yang G.-J. Application of MOF-based nanotherapeutics in light-mediated cancer diagnosis and therapy. J. Nanobiotechnol. 2022, 20 (1), 421. 10.1186/s12951-022-01631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G. E.; Marin R.; Carneiro Neto A. N.; Botas A. M. P.; Ovens J.; Kitos A. A.; Bernini M. C.; Carlos L. D.; Soler-Illia G. J. A. A.; Murugesu M. Tunable Energy-Transfer Process in Heterometallic MOF Materials Based on 2,6-Naphthalenedicarboxylate: Solid-State Lighting and Near-Infrared Luminescence Thermometry. Chem. Mater. 2020, 32 (17), 7458–7468. 10.1021/acs.chemmater.0c02480. [DOI] [Google Scholar]
- Shen Y.; Tissot A.; Serre C. Recent progress on MOF-based optical sensors for VOC sensing. Chemical Science 2022, 13 (47), 13978–14007. 10.1039/D2SC04314A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G.-L.; Jiang X.-L.; Xu H.; Zhao B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17 (22), 2005327. 10.1002/smll.202005327. [DOI] [PubMed] [Google Scholar]
- Song Y.; Xu M.; Li Z.; He L.; Hu M.; He L.; Zhang Z.; Du M. A bimetallic CoNi-based metal-organic framework as efficient platform for label-free impedimetric sensing toward hazardous substances. Sens. Actuators, B 2020, 311, 127927. 10.1016/j.snb.2020.127927. [DOI] [Google Scholar]
- Parmar B.; Bisht K. K.; Rajput G.; Suresh E. Recent advances in metal-organic frameworks as adsorbent materials for hazardous dye molecules. Dalton Transactions 2021, 50 (9), 3083–3108. 10.1039/D0DT03824E. [DOI] [PubMed] [Google Scholar]
- Freund R.; Zaremba O.; Arnauts G.; Ameloot R.; Skorupskii G.; Dincă M.; Bavykina A.; Gascon J.; Ejsmont A.; Goscianska J.; Kalmutzki M.; Lächelt U.; Ploetz E.; Diercks C. S.; Wuttke S. The Current Status of MOF and COF Applications. Angew. Chem., Int. Ed. 2021, 60 (45), 23975–24001. 10.1002/anie.202106259. [DOI] [PubMed] [Google Scholar]
- Abbasi Z.; Cseri L.; Zhang X.; Ladewig B. P.; Wang H.. Metal-Organic Frameworks (MOFs) and MOF-Derived Porous Carbon Materials for Sustainable Adsorptive Wastewater Treatment. In Sustainable Nanoscale Engineering; Szekely G., Livingston A., Eds.; Elsevier: New York, 2020; Chapter 7, pp 163–194. [Google Scholar]
- Linder-Patton O. M.; Rogers B. T.; Sumida K. Impact of Higher-Order Structuralization on the Adsorptive Properties of Metal-Organic Frameworks. Chemistry - An Asian Journal 2018, 13 (16), 1979–1991. 10.1002/asia.201800403. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy A.; Asiri A. M.; Garcia H. Catalysis by metal-organic frameworks in water. Chem. Commun. 2014, 50 (85), 12800–12814. 10.1039/C4CC04387A. [DOI] [PubMed] [Google Scholar]
- Eddaoudi M.; Kim J.; Rosi N.; Vodak D.; Wachter J.; O’Keeffe M.; Yaghi Omar M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295 (5554), 469–472. 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- Akiyama G.; Matsuda R.; Sato H.; Takata M.; Kitagawa S. Cellulose Hydrolysis by a New Porous Coordination Polymer Decorated with Sulfonic Acid Functional Groups. Adv. Mater. 2011, 23 (29), 3294–3297. 10.1002/adma.201101356. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Sun D.; Chen Y.; Huang R.; Ding Z.; Fu X.; Li Z. An Amine-Functionalized Titanium Metal-Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chem., Int. Ed. 2012, 51 (14), 3364–3367. 10.1002/anie.201108357. [DOI] [PubMed] [Google Scholar]
- Valvekens P.; Vermoortele F.; De Vos D. Metal-organic frameworks as catalysts: the role of metal active sites. Catalysis Science & Technology 2013, 3 (6), 1435–1445. 10.1039/c3cy20813c. [DOI] [Google Scholar]
- Dhakshinamoorthy A.; Asiri A. M.; Garcia H. Metal-Organic Frameworks as Catalysts for Oxidation Reactions. Chem.—Eur. J. 2016, 22 (24), 8012–8024. 10.1002/chem.201505141. [DOI] [PubMed] [Google Scholar]
- Liu X.; Kirlikovali K. O.; Chen Z.; Ma K.; Idrees K. B.; Cao R.; Zhang X.; Islamoglu T.; Liu Y.; Farha O. K. Small Molecules, Big Effects: Tuning Adsorption and Catalytic Properties of Metal-Organic Frameworks. Chem. Mater. 2021, 33 (4), 1444–1454. 10.1021/acs.chemmater.0c04675. [DOI] [Google Scholar]
- Xia J.; Gao Y.; Yu G. Tetracycline removal from aqueous solution using zirconium-based metal-organic frameworks (Zr-MOFs) with different pore size and topology: Adsorption isotherm, kinetic and mechanism studies. J. Colloid Interface Sci. 2021, 590, 495–505. 10.1016/j.jcis.2021.01.046. [DOI] [PubMed] [Google Scholar]
- Millward A. R.; Yaghi O. M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127 (51), 17998–17999. 10.1021/ja0570032. [DOI] [PubMed] [Google Scholar]
- Feng D.; Gu Z.-Y.; Chen Y.-P.; Park J.; Wei Z.; Sun Y.; Bosch M.; Yuan S.; Zhou H.-C. A Highly Stable Porphyrinic Zirconium Metal-Organic Framework with shp-a Topology. J. Am. Chem. Soc. 2014, 136 (51), 17714–17717. 10.1021/ja510525s. [DOI] [PubMed] [Google Scholar]
- Rojas S.; Horcajada P. Metal-Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120 (16), 8378–8415. 10.1021/acs.chemrev.9b00797. [DOI] [PubMed] [Google Scholar]
- Cui X.; Chen K.; Xing H.; Yang Q.; Krishna R.; Bao Z.; Wu H.; Zhou W.; Dong X.; Han Y.; Li B.; Ren Q.; Zaworotko M. J.; Chen B. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353 (6295), 141–144. 10.1126/science.aaf2458. [DOI] [PubMed] [Google Scholar]
- Du J.; Bao J.; Fu X.; Lu C.; Kim S. H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Applied Catalysis B: Environmental 2016, 184, 132–141. 10.1016/j.apcatb.2015.11.015. [DOI] [Google Scholar]
- Li W.; Wu X.; Li S.; Tang W.; Chen Y. Magnetic porous Fe3O4/carbon octahedra derived from iron-based metal-organic framework as heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2018, 436, 252–262. 10.1016/j.apsusc.2017.11.151. [DOI] [Google Scholar]
- He W.; Gao H.-M.; Shimoni R.; Lu Z.-Y.; Hod I. Synergistic Coupling of Anionic Ligands To Optimize the Electronic and Catalytic Properties of Metal-Organic Framework-Converted Oxygen-Evolving Catalysts. ACS Applied Energy Materials 2019, 2 (3), 2138–2148. 10.1021/acsaem.8b02160. [DOI] [Google Scholar]
- Kobielska P. A.; Howarth A. J.; Farha O. K.; Nayak S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. 10.1016/j.ccr.2017.12.010. [DOI] [Google Scholar]
- Hasan Z.; Choi E.-J.; Jhung S. H. Adsorption of naproxen and clofibric acid over a metal-organic framework MIL-101 functionalized with acidic and basic groups. Chemical Engineering Journal 2013, 219, 537–544. 10.1016/j.cej.2013.01.002. [DOI] [Google Scholar]
- Yan C.; Jin J.; Wang J.; Zhang F.; Tian Y.; Liu C.; Zhang F.; Cao L.; Zhou Y.; Han Q. Metal-organic frameworks (MOFs) for the efficient removal of contaminants from water: Underlying mechanisms, recent advances, challenges, and future prospects. Coord. Chem. Rev. 2022, 468, 214595. 10.1016/j.ccr.2022.214595. [DOI] [Google Scholar]
- Han Q.; He C.; Zhao M.; Qi B.; Niu J.; Duan C. Engineering Chiral Polyoxometalate Hybrid Metal-Organic Frameworks for Asymmetric Dihydroxylation of Olefins. J. Am. Chem. Soc. 2013, 135 (28), 10186–10189. 10.1021/ja401758c. [DOI] [PubMed] [Google Scholar]
- Li J.; He J.; Si C.; Li M.; Han Q.; Wang Z.; Zhao J. Special-selective C-H oxidation of toluene to benzaldehyde by a hybrid polyoxometalate photocatalyst including a rare [P6W48Fe6O180]30- anion. J. Catal. 2020, 392, 244–253. 10.1016/j.jcat.2020.10.013. [DOI] [Google Scholar]
- Siwal S. S.; Kaur H.; Deng R.; Zhang Q. A review on electrochemical techniques for metal recovery from waste resources. Current Opinion in Green and Sustainable Chemistry 2023, 39, 100722. 10.1016/j.cogsc.2022.100722. [DOI] [Google Scholar]
- Zhu L.; Liu X.-Q.; Jiang H.-L.; Sun L.-B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117 (12), 8129–8176. 10.1021/acs.chemrev.7b00091. [DOI] [PubMed] [Google Scholar]
- Sharma V. K.; Zhao J.; Hidaka H. Mechanism of photocatalytic oxidation of amino acids: Hammett correlations. Catal. Today 2014, 224, 263–268. 10.1016/j.cattod.2013.12.045. [DOI] [Google Scholar]
- Siwal S. S.; Kaur H.; Saini A. K.; Thakur V. K. Recent Progress in Carbon Dots-Based Materials for Electrochemical Energy Storage Toward Environmental Sustainability. Advanced Energy and Sustainability Research 2022, n/a (n/a), 2200062. 10.1002/aesr.202200062. [DOI] [Google Scholar]
- Li Z.; Ning S.; Zhu H.; Wang X.; Yin X.; Fujita T.; Wei Y. Novel NbCo-MOF as an advanced peroxymonosulfate catalyst for organic pollutants removal: Growth, performance and mechanism study. Chemosphere 2022, 288, 132600. 10.1016/j.chemosphere.2021.132600. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Wang J.; Zhang Y.; Ma J.; Huang L.; Yu S.; Chen L.; Song G.; Qiu M.; Wang X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. 10.1016/j.envpol.2021.118076. [DOI] [PubMed] [Google Scholar]
- Kaur H.; Siwal S. S.; Saini R. V.; Singh N.; Thakur V. K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanoscience Au 2023, 3, 1. 10.1021/acsnanoscienceau.2c00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H.; Thakur V. K.; Siwal S. S. Recent advancements in graphdiyne-based nano-materials for biomedical applications. Materials Today: Proceedings 2022, 56, 112–120. 10.1016/j.matpr.2021.12.355. [DOI] [Google Scholar]
- Du C.; Zhang Z.; Yu G.; Wu H.; Chen H.; Zhou L.; Zhang Y.; Su Y.; Tan S.; Yang L.; Song J.; Wang S. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 2021, 272, 129501. 10.1016/j.chemosphere.2020.129501. [DOI] [PubMed] [Google Scholar]
- Xing D.; Cui Z.; Liu Y.; Wang Z.; Wang P.; Zheng Z.; Cheng H.; Dai Y.; Huang B. Two-dimensional π-d conjugated metal-organic framework Fe3(hexaiminotriphenylene)2 as a photo-Fenton like catalyst for highly efficient degradation of antibiotics. Applied Catalysis B: Environmental 2021, 290, 120029. 10.1016/j.apcatb.2021.120029. [DOI] [Google Scholar]
- Li X.; Yang Z.; Hu D.; Wang A.; Chen Y.; Huang Y.; Zhang M.; Yuan H.; Yan K. Bimetallic FexMny catalysts derived from metal organic frameworks for efficient photocatalytic removal of quinolones without oxidant. Environmental Science: Nano 2021, 8 (9), 2595–2606. 10.1039/D1EN00237F. [DOI] [Google Scholar]
- Yuan J.; Hung W.-S.; Zhu H.; Guan K.; Ji Y.; Mao Y.; Liu G.; Lee K.-R.; Jin W. Fabrication of ZIF-300 membrane and its application for efficient removal of heavy metal ions from wastewater. J. Membr. Sci. 2019, 572, 20–27. 10.1016/j.memsci.2018.10.080. [DOI] [Google Scholar]
- Qian T.; Zhang Y.; Cai J.; Cao W.; Liu T.; Chen Z.; Liu J.; Li F.; Zhang L. Decoration of amine functionalized zirconium metal organic framework/silver iodide heterojunction on carbon fiber cloth as a filter- membrane-shaped photocatalyst for degrading antibiotics. J. Colloid Interface Sci. 2021, 603, 582–593. 10.1016/j.jcis.2021.06.112. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Li S.; Zhao Z.; Su Y.; Long Y.; Zheng Z.; Cui D.; Liu Y.; Wang C.; Zhang X.; Zhang Z. Microwave-assisted hydrothermal assembly of 2D copper-porphyrin metal-organic frameworks for the removal of dyes and antibiotics from water. Environmental Science and Pollution Research 2020, 27 (31), 39186–39197. 10.1007/s11356-020-09865-z. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Yang H.; Kang L.; Gao Z.; Ren F. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(II)/ Fe(III) cycle under visible light irradiation. Applied Catalysis B: Environmental 2020, 263, 118282. 10.1016/j.apcatb.2019.118282. [DOI] [Google Scholar]
- Fang X.; Wu S.; Wu Y.; Yang W.; Li Y.; He J.; Hong P.; Nie M.; Xie C.; Wu Z.; Zhang K.; Kong L.; Liu J. High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: Dynamics, thermodynamics, and mechanisms. Appl. Surf. Sci. 2020, 518, 146226. 10.1016/j.apsusc.2020.146226. [DOI] [Google Scholar]
- Capsoni D.; Guerra G.; Puscalau C.; Maraschi F.; Bruni G.; Monteforte F.; Profumo A.; Sturini M. Zinc Based Metal-Organic Frameworks as Ofloxacin Adsorbents in Polluted Waters: ZIF-8 vs. Zn3(BTC)2. International Journal of Environmental Research and Public Health 2021, 18 (4), 1433. 10.3390/ijerph18041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F.; Ren Q.; Zhang H.; Yang L.; Chen H.; Liang Z.; Chen D. Removal of tetracycline hydrochloride from wastewater by Zr/Fe-MOFs/GO composites. RSC Adv. 2021, 11 (17), 9977–9984. 10.1039/D1RA01027A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y.; Zhuang Y.; Han K.; Shi B. Enhanced tetracycline adsorption using alginate-graphene-ZIF67 aerogel. Colloids Surf., A 2020, 588, 124360. 10.1016/j.colsurfa.2019.124360. [DOI] [Google Scholar]
- Wang K.; Wu J.; Zhu M.; Zheng Y.-Z.; Tao X. Highly effective pH-universal removal of tetracycline hydrochloride antibiotics by UiO-66-(COOH)2/GO metal-organic framework composites. J. Solid State Chem. 2020, 284, 121200. 10.1016/j.jssc.2020.121200. [DOI] [Google Scholar]
- Lv S.-W.; Liu J.-M.; Li C.-Y.; Zhao N.; Wang Z.-H.; Wang S. In situ growth of benzothiadiazole functionalized UiO-66-NH2 on carboxyl modified g-C3N4 for enhanced photocatalytic degradation of sulfamethoxazole under visible light. Catalysis Science & Technology 2020, 10 (14), 4703–4711. 10.1039/D0CY01019G. [DOI] [Google Scholar]
- Zhao X.; Liu S.; Tang Z.; Niu H.; Cai Y.; Meng W.; Wu F.; Giesy J. P. Synthesis of magnetic metal-organic framework (MOF) for efficient removal of organic dyes from water. Sci. Rep. 2015, 5 (1), 11849. 10.1038/srep11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan A.; Kumar P. S.; Yaashikaa P. R.; Karishma S.; Jeevanantham S.; Swetha S. Mixed biosorbent of agro waste and bacterial biomass for the separation of Pb(II) ions from water system. Chemosphere 2021, 277, 130236. 10.1016/j.chemosphere.2021.130236. [DOI] [PubMed] [Google Scholar]
- Siwal S. S.; Zhang Q.; Saini A. K.; Gupta V. K.; Roberts D.; Saini V.; Coulon F.; Pareek B.; Thakur V. K. Recent advances in bio-electrochemical system analysis in biorefineries. Journal of Environmental Chemical Engineering 2021, 9 (5), 105982. 10.1016/j.jece.2021.105982. [DOI] [Google Scholar]
- Uddin M. J.; Ampiaw R. E.; Lee W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. 10.1016/j.chemosphere.2021.131314. [DOI] [PubMed] [Google Scholar]
- Siwal S. S.; Thakur V. K.; Zhang Q.. Bio-electrochemical systems for sustainable energy production and environmental prospects. In Functionalized Nanomaterials Based Devices for Environmental Applications; Hussain C. M., Shukla S. K., Joshi G. M., Eds.; Elsevier: New York, 2021; pp 275–301. [Google Scholar]
- Tong M.; Liu D.; Yang Q.; Devautour-Vinot S.; Maurin G.; Zhong C. Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids. Journal of Materials Chemistry A 2013, 1 (30), 8534–8537. 10.1039/c3ta11807j. [DOI] [Google Scholar]
- Yang J.-M.; Yang B.-C.; Zhang Y.; Yang R.-N.; Ji S.-S.; Wang Q.; Quan S.; Zhang R.-Z. Rapid adsorptive removal of cationic and anionic dyes from aqueous solution by a Ce(III)-doped Zr-based metal-organic framework. Microporous Mesoporous Mater. 2020, 292, 109764. 10.1016/j.micromeso.2019.109764. [DOI] [Google Scholar]
- Fu Q.; Lou J.; Zhang R.; Peng L.; Zhou S.; Yan W.; Mo C.; Luo J. Highly effective and fast removal of Congo red from wastewater with metal-organic framework Fe-MIL-88NH2. J. Solid State Chem. 2021, 294, 121836. 10.1016/j.jssc.2020.121836. [DOI] [Google Scholar]
- Abdelhameed R. M.; Emam H. E. Modulation of metal organic framework hybrid cotton for efficient sweeping of dyes and pesticides from wastewater. Sustainable Materials and Technologies 2022, 31, e00366 10.1016/j.susmat.2021.e00366. [DOI] [Google Scholar]
- Kirandeep; Sushila; Sharma A.; Sahoo S. C.; Kumar G.; Mehta S. K.; Kataria R. Synthesis and characterization of 1D-Co/Zn MOFs having potential for efficient dye adsorption from wastewater. J. Mol. Struct. 2021, 1226, 129327. 10.1016/j.molstruc.2020.129327. [DOI] [Google Scholar]
- Paz R.; Viltres H.; Gupta N. K.; Leyva C. Fabrication of magnetic cerium-organic framework-activated carbon composite for charged dye removal from aqueous solutions. J. Mol. Liq. 2021, 337, 116578. 10.1016/j.molliq.2021.116578. [DOI] [Google Scholar]
- Yang G.; Zhang D.; Zhu G.; Zhou T.; Song M.; Qu L.; Xiong K.; Li H. A Sm-MOF/GO nanocomposite membrane for efficient organic dye removal from wastewater. RSC Adv. 2020, 10 (14), 8540–8547. 10.1039/D0RA01110J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.; Xu C.; Guan H.; Li L.; Fan L.; Wang Y.; Liu L.; Meng Q.; Zhang R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surf., A 2018, 539, 382–390. 10.1016/j.colsurfa.2017.12.043. [DOI] [Google Scholar]
- Karimi H.; Heidari M. A.; Emrooz H. B. M.; Shokouhimehr M. Carbonization temperature effects on adsorption performance of metal-organic framework derived nanoporous carbon for removal of methylene blue from wastewater; experimental and spectrometry study. Diamond Relat. Mater. 2020, 108, 107999. 10.1016/j.diamond.2020.107999. [DOI] [Google Scholar]
- Bui T. T.; Nguyen L. T.; Pham N. P.; Tran C. C.; Nguyen L. T.; Nguyen T. A.; Nguyen H. N.; Nguyen M. V. J. R. A. A new approach for ultra-high adsorption of cationic methylene blue in a Zr-sulfonic-based metal-organic framework. 2021, 11 (58), 36626–36635. 10.1039/D1RA06405C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami K.; Beram S. M.; Bayat P.; Siadatnasab F.; Ramezanpour A. A novel nanohybrid based on metal-organic framework MIL101-Cr/PANI/Ag for the adsorption of cationic methylene blue dye from aqueous solution. J. Mol. Struct. 2022, 1247, 131352. 10.1016/j.molstruc.2021.131352. [DOI] [Google Scholar]
- Qasem N. A. A.; Mohammed R. H.; Lawal D. U. Removal of heavy metal ions from wastewater: a comprehensive and critical review. npj Clean Water 2021, 4 (1), 36. 10.1038/s41545-021-00127-0. [DOI] [Google Scholar]
- Singh N.; Poonia T.; Siwal S. S.; Srivastav A. L.; Sharma H. K.; Mittal S. K.. Challenges of water contamination in urban areas. In Current Directions in Water Scarcity Research; Srivastav A. L., Madhav S., Bhardwaj A. K., Valsami-Jones E., Eds.; Elsevier: New York, 2022; Vol. 6, Chapter 9, pp 173–202. [Google Scholar]
- Ahmadijokani F.; Tajahmadi S.; Bahi A.; Molavi H.; Rezakazemi M.; Ko F.; Aminabhavi T. M.; Arjmand M. Ethylenediamine-functionalized Zr-based MOF for efficient removal of heavy metal ions from water. Chemosphere 2021, 264, 128466. 10.1016/j.chemosphere.2020.128466. [DOI] [PubMed] [Google Scholar]
- Wang K.; Tao X.; Xu J.; Yin N. Novel Chitosan-MOF Composite Adsorbent for the Removal of Heavy Metal Ions. Chem. Lett. 2016, 45 (12), 1365–1368. 10.1246/cl.160718. [DOI] [Google Scholar]
- Zayan S.; Elshazly A.; Elkady M. In Situ Polymerization of Polypyrrole @ Aluminum Fumarate Metal-Organic Framework Hybrid Nanocomposites for the Application of Wastewater Treatment. Polymers 2020, 12 (8), 1764. 10.3390/polym12081764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.; Xie D.; Wang Y.; Qin W.; Zhang H.; Wang G.; Zhang Y.; Zhao H. Facile fabrication of composition-tunable Fe/Mg bimetal-organic frameworks for exceptional arsenate removal. Chemical Engineering Journal 2019, 357, 579–588. 10.1016/j.cej.2018.09.174. [DOI] [Google Scholar]
- Nasir A. M.; Md Nordin N. A. H.; Goh P. S.; Ismail A. F. Application of two-dimensional leaf-shaped zeolitic imidazolate framework (2D ZIF-L) as arsenite adsorbent: Kinetic, isotherm and mechanism. J. Mol. Liq. 2018, 250, 269–277. 10.1016/j.molliq.2017.12.005. [DOI] [Google Scholar]
- Liu C.; Wang P.; Liu X.; Yi X.; Liu D.; Zhou Z. Ultrafast Removal of Cadmium(II) by Green Cyclodextrin Metal-Organic-Framework-Based Nanoporous Carbon: Adsorption Mechanism and Application. Chemistry - An Asian Journal 2019, 14 (2), 261–268. 10.1002/asia.201801431. [DOI] [PubMed] [Google Scholar]
- Niu H.; Zheng Y.; Wang S.; He S.; Cai Y. Stable hierarchical microspheres of 1D Fe-gallic acid MOFs for fast and efficient Cr(vi) elimination by a combination of reduction, metal substitution and coprecipitation. Journal of Materials Chemistry A 2017, 5 (32), 16600–16604. 10.1039/C7TA04006G. [DOI] [Google Scholar]
- Niknam Shahrak M.; Ghahramaninezhad M.; Eydifarash M. Zeolitic imidazolate framework-8 for efficient adsorption and removal of Cr(VI) ions from aqueous solution. Environmental Science and Pollution Research 2017, 24 (10), 9624–9634. 10.1007/s11356-017-8577-5. [DOI] [PubMed] [Google Scholar]
- Peng Y.; Huang H.; Zhang Y.; Kang C.; Chen S.; Song L.; Liu D.; Zhong C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9 (1), 187. 10.1038/s41467-017-02600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokalıoğlu Ş.; Yavuz E.; Demir S.; Patat Ş. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. 10.1016/j.foodchem.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Ghaedi A. M.; Panahimehr M.; Nejad A. R. S.; Hosseini S. J.; Vafaei A.; Baneshi M. M. Factorial experimental design for the optimization of highly selective adsorption removal of lead and copper ions using metal organic framework MOF-2 (Cd). J. Mol. Liq. 2018, 272, 15–26. 10.1016/j.molliq.2018.09.051. [DOI] [Google Scholar]
- Wang C.; Lin G.; Xi Y.; Li X.; Huang Z.; Wang S.; Zhao J.; Zhang L. Development of mercaptosuccinic anchored MOF through one-step preparation to enhance adsorption capacity and selectivity for Hg(II) and Pb(II). J. Mol. Liq. 2020, 317, 113896. 10.1016/j.molliq.2020.113896. [DOI] [Google Scholar]
- Huang H.; Li J.; Yuan M.; Yang H.; Zhao Y.; Ying Y.; Wang S. Large-Scale Self-Assembly of MOFs Colloidosomes for Bubble-Propelled Micromotors and Stirring-Free Environmental Remediation. Angew. Chem., Int. Ed. 2022, 61 (46), e202211163 10.1002/anie.202211163. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Feng M.; Zhang P.; Zhou H.-C.; Sharma V. K.; Ma X. J. A. E.; Engineering T. Metal organic frameworks (MOFs) as photocatalysts for the degradation of agricultural pollutants in water. 2021, 1 (5), 804–826. 10.1021/acsestengg.1c00051. [DOI] [Google Scholar]
- Kaur H.; Siwal S. S.; Chauhan G.; Saini A. K.; Kumari A.; Thakur V. K. Recent advances in electrochemical-based sensors amplified with carbon-based nanomaterials (CNMs) for sensing pharmaceutical and food pollutants. Chemosphere 2022, 304, 135182. 10.1016/j.chemosphere.2022.135182. [DOI] [PubMed] [Google Scholar]
- Sabarwal A.; Kumar K.; Singh R. P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environmental Toxicology and Pharmacology 2018, 63, 103–114. 10.1016/j.etap.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Nicolopoulou-Stamati P.; Maipas S.; Kotampasi C.; Stamatis P.; Hens L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. 2016, 4, 1. 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; He M.; Jiang H.; He H.; Qi J.; Ma J. J. C. E. J. Photocatalytic MOF membranes with two-dimensional heterostructure for the enhanced removal of agricultural pollutants in water. 2022, 435, 133870. 10.1016/j.cej.2021.133870. [DOI] [Google Scholar]
- Singh S.; Kaushal S.; Kaur J.; Kaur G.; Mittal S. K.; Singh P. P. CaFu MOF as an efficient adsorbent for simultaneous removal of imidacloprid pesticide and cadmium ions from wastewater. Chemosphere 2021, 272, 129648. 10.1016/j.chemosphere.2021.129648. [DOI] [PubMed] [Google Scholar]
- Li Y.; Li B.; Qi Y.; Zhang Z.; Cong S.; She Y.; Cao X. Synthesis of metal-organic framework @molecularly imprinted polymer adsorbents for solid phase extraction of organophosphorus pesticides from agricultural products. Journal of Chromatography B 2022, 1188, 123081. 10.1016/j.jchromb.2021.123081. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Wang J.; Chen X.; Yang W.; Pei H.; Hu N.; Li Z.; Suo Y.; Li T.; Wang J. The simultaneous detection and removal of organophosphorus pesticides by a novel Zr-MOF based smart adsorbent. Journal of Materials Chemistry A 2018, 6 (5), 2184–2192. 10.1039/C7TA08399H. [DOI] [Google Scholar]
- Jia W.; Fan R.; Zhang J.; Zhu K.; Gai S.; Yin Y.; Yang Y. Smart MOF-on-MOF Hydrogel as a Simple Rod-shaped Core for Visual Detection and Effective Removal of Pesticides. Small 2022, 18 (19), 2201510. 10.1002/smll.202201510. [DOI] [PubMed] [Google Scholar]
- Wu G.; Ma J.; Wang S.; Chai H.; Guo L.; Li J.; Ostovan A.; Guan Y.; Chen L. Cationic metal-organic framework based mixed-matrix membrane for extraction of phenoxy carboxylic acid (PCA) herbicides from water samples followed by UHPLC-MS/MS determination. Journal of Hazardous Materials 2020, 394, 122556. 10.1016/j.jhazmat.2020.122556. [DOI] [PubMed] [Google Scholar]
- Semyonov O.; Chaemchuen S.; Ivanov A.; Verpoort F.; Kolska Z.; Syrtanov M.; Svorcik V.; Yusubov M. S.; Lyutakov O.; Guselnikova O.; Postnikov P. S. Smart recycling of PET to sorbents for insecticides through in situ MOF growth. Applied Materials Today 2021, 22, 100910. 10.1016/j.apmt.2020.100910. [DOI] [Google Scholar]
- Liu G.; Li L.; Huang X.; Zheng S.; Xu D.; Xu X.; Zhang Y.; Lin H. Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Microporous Mesoporous Mater. 2018, 270, 258–264. 10.1016/j.micromeso.2018.05.023. [DOI] [Google Scholar]
- Ahmed S.; Chughtai S.; Keane M. A. The removal of cadmium and lead from aqueous solution by ion exchange with Na—Y zeolite. Sep. Purif. Technol. 1998, 13 (1), 57–64. 10.1016/S1383-5866(97)00063-4. [DOI] [Google Scholar]
- Yuan Y.; Wu Y.; Wang H.; Tong Y.; Sheng X.; Sun Y.; Zhou X.; Zhou Q. Simultaneous enrichment and determination of cadmium and mercury ions using magnetic PAMAM dendrimers as the adsorbents for magnetic solid phase extraction coupled with high performance liquid chromatography. Journal of Hazardous Materials 2020, 386, 121658. 10.1016/j.jhazmat.2019.121658. [DOI] [PubMed] [Google Scholar]
- Yang L.; Hu W.; Chang Z.; Liu T.; Fang D.; Shao P.; Shi H.; Luo X. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: Recent advances and future trends. Environ. Int. 2021, 152, 106512. 10.1016/j.envint.2021.106512. [DOI] [PubMed] [Google Scholar]
- Benalia M. C.; Youcef L.; Bouaziz M. G.; Achour S.; Menasra H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arabian Journal for Science and Engineering 2022, 47 (5), 5587–5599. 10.1007/s13369-021-05525-7. [DOI] [Google Scholar]
- Gao Q.; Xu J.; Bu X.-H. Recent advances about metal-organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. 10.1016/j.ccr.2018.03.015. [DOI] [Google Scholar]
- Li J.; Wang X.; Zhao G.; Chen C.; Chai Z.; Alsaedi A.; Hayat T.; Wang X. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47 (7), 2322–2356. 10.1039/C7CS00543A. [DOI] [PubMed] [Google Scholar]
- Abdel-Magied A. F.; Abdelhamid H. N.; Ashour R. M.; Fu L.; Dowaidar M.; Xia W.; Forsberg K. Magnetic metal-organic frameworks for efficient removal of cadmium(II), and lead(II) from aqueous solution. Journal of Environmental Chemical Engineering 2022, 10 (3), 107467. 10.1016/j.jece.2022.107467. [DOI] [Google Scholar]
- Ashour R. M.; El-sayed R.; Abdel-Magied A. F.; Abdel-khalek A. A.; Ali M. M.; Forsberg K.; Uheida A.; Muhammed M.; Dutta J. Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: kinetic and thermodynamic studies. Chemical Engineering Journal 2017, 327, 286–296. 10.1016/j.cej.2017.06.101. [DOI] [Google Scholar]
- Siwal S. S.; Zhang Q.; Devi N.; Thakur K. V. Carbon-Based Polymer Nanocomposite for High-Performance Energy Storage Applications. Polymers 2020, 12 (3), 505. 10.3390/polym12030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K.; Devi N.; Siwal S. S.; Zhang Q.; Alsanie W. F.; Scarpa F.; Thakur V. K. Ionic Liquid-Based Polymer Nanocomposites for Sensors, Energy, Biomedicine, and Environmental Applications: Roadmap to the Future. Advanced Science 2022, n/a (n/a), 2202187. 10.1002/advs.202202187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S.; Sivareddy I.; De S. Adsorptive removal of potentially toxic metals (cadmium, copper, nickel and zinc) by chemically treated laterite: Single and multicomponent batch and column study. Journal of Environmental Chemical Engineering 2017, 5 (4), 3273–3289. 10.1016/j.jece.2017.06.029. [DOI] [Google Scholar]
- Ashour R. M.; Abdel-Magied A. F.; Wu Q.; Olsson R. T.; Forsberg K. Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. 2020, 12 (5), 1104. 10.3390/polym12051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Magied A. F.; Abdelhamid H. N.; Ashour R. M.; Zou X.; Forsberg K. Hierarchical porous zeolitic imidazolate frameworks nanoparticles for efficient adsorption of rare-earth elements. Microporous Mesoporous Mater. 2019, 278, 175–184. 10.1016/j.micromeso.2018.11.022. [DOI] [Google Scholar]
- Kavand M.; Eslami P.; Razeh L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. Journal of Water Process Engineering 2020, 34, 101151. 10.1016/j.jwpe.2020.101151. [DOI] [Google Scholar]
- Unuabonah E. I.; El-Khaiary M. I.; Olu-Owolabi B. I.; Adebowale K. O. Predicting the dynamics and performance of a polymer-clay based composite in a fixed bed system for the removal of lead (II) ion. Chem. Eng. Res. Des. 2012, 90 (8), 1105–1115. 10.1016/j.cherd.2011.11.009. [DOI] [Google Scholar]
- Chen L.-F.; Liang H.-W.; Lu Y.; Cui C.-H.; Yu S.-H. Synthesis of an Attapulgite Clay@Carbon Nanocomposite Adsorbent by a Hydrothermal Carbonization Process and Their Application in the Removal of Toxic Metal Ions from Water. Langmuir 2011, 27 (14), 8998–9004. 10.1021/la2017165. [DOI] [PubMed] [Google Scholar]
- Hasan Z.; Khan N. A.; Jhung S. H. Adsorptive removal of diclofenac sodium from water with Zr-based metal-organic frameworks. Chemical Engineering Journal 2016, 284, 1406–1413. 10.1016/j.cej.2015.08.087. [DOI] [Google Scholar]
- Jung B. K.; Hasan Z.; Jhung S. H. Adsorptive removal of 2,4-dichlorophenoxyacetic acid (2,4-D) from water with a metal-organic framework. Chemical Engineering Journal 2013, 234, 99–105. 10.1016/j.cej.2013.08.110. [DOI] [Google Scholar]
- Nasalevich M. A.; van der Veen M.; Kapteijn F.; Gascon J. Metal-organic frameworks as heterogeneous photocatalysts: advantages and challenges. CrystEngComm 2014, 16 (23), 4919–4926. 10.1039/C4CE00032C. [DOI] [Google Scholar]
- Férey G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 2008, 37 (1), 191–214. 10.1039/B618320B. [DOI] [PubMed] [Google Scholar]
- Khan N. A.; Hasan Z.; Jhung S. H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. Journal of Hazardous Materials 2013, 244–245, 444–456. 10.1016/j.jhazmat.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Jhung S. H.; Khan N. A.; Hasan Z. Analogous porous metal-organic frameworks: synthesis, stability and application in adsorption. CrystEngComm 2012, 14 (21), 7099–7109. 10.1039/c2ce25760b. [DOI] [Google Scholar]