Abstract
Thirty percent of psoriasis patients develop psoriatic arthritis (PsA), nevertheless the mechanism remains unknown. Endogenous GU-rich miRNAs activate endosomal TLR7 that plays a critical role in autoimmune diseases. We found that endogenous TLR7 ligands, miR-29 and miR-Let7b, were markedly increased in PsA compared to osteoarthritis (OA) synovial fluid (SF)s. We showed that intradermal (i.d.) miR-Let7b injection promoted skin inflammation, which was characterized by amplified Th1 cells, CD68+M1 macrophages and transcriptional upregulation of glycolytic mediators, GLUT1, C-MYC and HIF1α. Expansion of skin Th1 cells driven by miR-Let7b was also linked to elevated M1-associated IRFs. Interestingly, i.d. miR-Let7b administration exacerbated suboptimal joint inflammation along with metabolic reconfiguration of the PsA-like preclinical model. Moreover, TLR7 agonist, R837, potentiated metabolic reprogramming and expression of IL-1β, IL-6 and IL-12 in murine macrophages, enabling myeloid-to-T cell crosstalk. Consistently, treatment with glycolytic inhibitors, 2-DG and/or HIF1αi, reversed R837-induced metabolic remodeling and disrupted the TLR7-driven inflammatory phenotype in myeloid and lymphoid cells. Similar to miR-Let7b, R837 also differentiates progenitor cells into mature osteoclasts, primarily through RANKL induction. Taken together, this study indicates that TLR7-instigated metabolic rewiring of macrophages and their cross-regulation of T cells connects skin immunopathology to joint inflammation.
Keywords: TLR7, psoriatic arthritis (PsA), miRNA, glycolysis, macrophages
Graphical Abstract
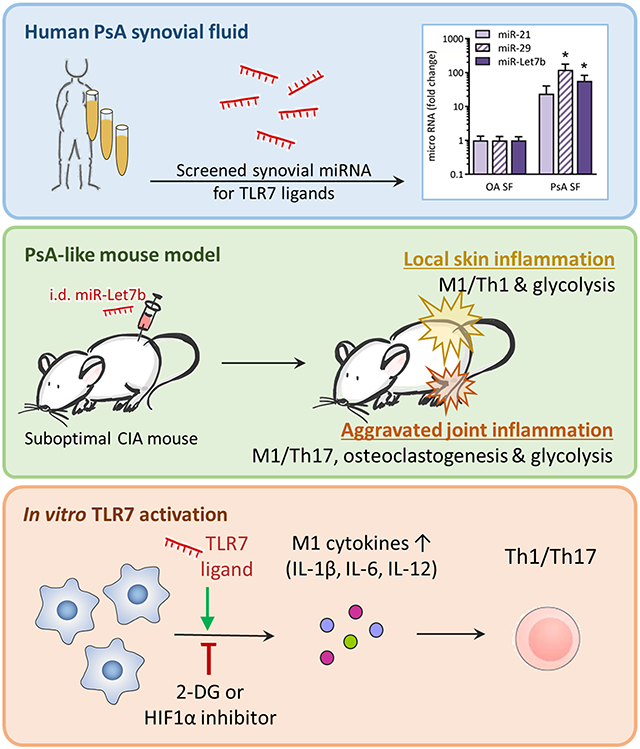
INTRODUCTION
Toll-like receptors (TLRs) are key regulators of inflammation and autoimmunity [1]. GU-rich microRNAs (miRNAs), activating endosomal TLR7, modulate gene expression and play an important role in rheumatologic disorders [2, 3]. Elevated levels of endogenous TLR7 ligands are detected in psoriasis (PsO). Particularly miR-21 is highly upregulated in blood and skin lesions of PsO patients [4]. A causal role was suggested for miR-21, as blockade of its function reduced psoriatic gene expression and epidermal thickening in preclinical models [5]. In the human psoriatic skin graft model, the benefit of miR-21 inhibition was comparable to that of TNFα inhibitor, Etanercept [5]. TLR7 activation by imiquimod (IMQ/R837) also promotes psoriasis-like dermatitis in mice [6, 7].
Psoriasis is often associated with comorbidities. Notably, about 30% of patients develop psoriatic arthritis (PsA), a seronegative spondyloarthritis that mostly develops years after disease onset. miRNAs were proposed as novel biomarkers for metabolic and inflammatory disorders such as PsO and PsA, having both diagnostic and predictive values [8]. Distinct miRNAs have been associated with either PsO, manifesting only at the dermal level, or PsA, suggesting that these differentially expressed miRNAs play a crucial role in disease progression and development of articular symptoms. Despite its widely accepted inflammatory function, one publication has shown circulating miR-Let7b levels negatively correlate with arthritic joint involvement in psoriasis [8]. This study has not characterized miR-Let7b’s mechanism of action and contradicts our findings as well as earlier reports [2].
Key modulators of glycolysis, glucose transporter (GLUT1) and glycolytic enzyme (HK2), are implicated in autoimmunity [9, 10]. TLR7-activated AKT/mTOR/IRF4 signaling stimulates glucose uptake and glycolysis in CD8+ T cells [11]. However, the impact of TLR7-induced metabolic rewiring of other immune cells was not examined. Macrophages undergo metabolic reconfiguration to accommodate increased bioenergetics demands that accompany the inflammatory response [12-14]. Inhibition of glucose metabolism was proposed as a novel PsO treatment strategy [15, 16]. In the PsA, a metabolic switch in endothelial cells was facilitated by GLUT1, PFKFB3 and PKM2 upregulation [17]. Nevertheless, immunometabolic regulation of other cells in PsA remains underexplored.
In this study, we found that GU-rich miR-29 and miR-Let7b are abundantly expressed in PsA SF. In arthritic mice, i.d. miR-Let7b injection aggravated disease activity. Exacerbation of joint inflammation by i.d. miR-Let7b was accompanied by hypermetabolic activity of inflammatory cells. To substantiate these preclinical observations, we report that murine MΦs and T cells activated by R837 displayed a heightened inflammatory phenotype, dependent on myeloid glycolytic remodeling. Our results suggest that TLR7 ligands trigger unique crosstalk between the skin and joint immunometabolism. Therefore, treatments that dysregulate TLR7 signaling could provide a promising strategy to disconnect the skin pathology from joint inflammation.
RESULTS & DISCUSSION
TLR7 ligands are elevated in PsA SF
To elucidate the importance of TLR7 ligands in PsA, miR-Let7b, miR-21 and miR-29 expression was evaluated in PsA and OA (Fig. 1A). miR-29 and miR-Let7b were significantly elevated in PsA compared to OA SF (121-57x respectively). Although miR-21 levels followed a similar trend (24-fold; p=0.09), a statistically significant difference was not observed.
Figure 1. TLR7 ligands in PsA patients and preclinical models.
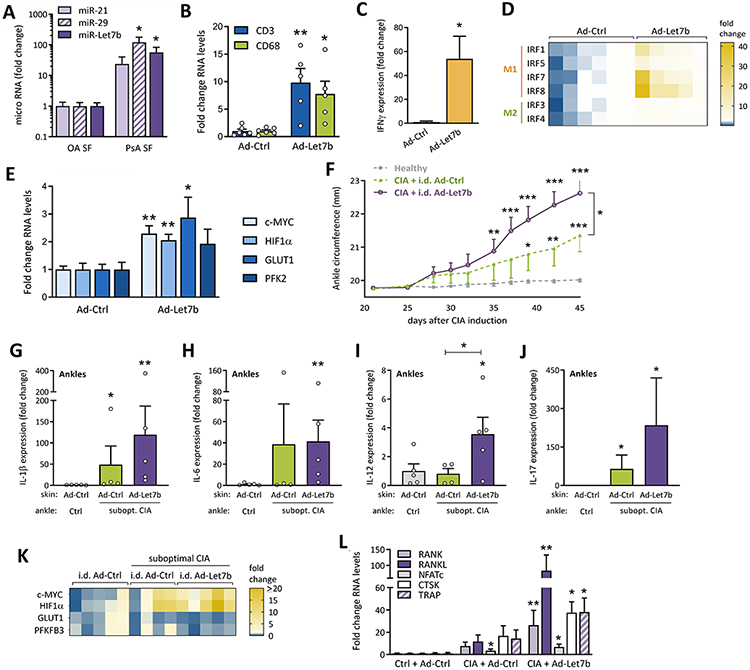
(A) miRNA levels of TLR7 ligands in OA and PsA SF were determined by qRT-PCR, normalized against miR-U6 (n=8 or 14 independent donors for OA or PsA; samples independently collected over time, results confirmed in 2 independent runs). Data were analyzed using a one-tailed unpaired t-test with Welch’s correction. (B-E) Using qRT-PCR, we analyzed gene expression in skin of mice injected i.d. on days 1, 8 and 15 with Ad-Ctrl (n=6 mice) or Ad-Let7b (n=5 mice) and sacrificed on day 17, statistically analyzed by 2-tailed unpaired t-test (all mice treated and analyzed independently). (F-L) On day 1, mice were control-treated or suboptimal CIA was initiated. The low-grade arthritic phenotype was boosted on day 21. Mice with or without suboptimal CIA were injected i.d. on days 25, 32 and 39 with Ad-Ctrl or Ad-Let7b. (F) Changes in ankle circumference (c = 2π * √[(a2+b2)/2] in which a and b are the anterior-posterior and lateral diameter measured by caliper) were monitored among different treatment groups (healthy: n=10, CIA+Ad-Ctrl: n=8, CIA+Ad-Let7b: n=10 independent ankles in the representative experiment), starting at day 21, before skin treatment, until day 45. Measurements were compared using a two-tailed unpaired t-test. (G-L) At day 45, joints were harvested and expression of interleukins (IL-1β, IL-6, IL-12 and IL-17; G-J; one-tailed Mann-Whitney test), glycolytic genes (K) and osteoclastic mediators were determined by qRT-PCR (L; unpaired t-test) (G-I, K-L: n=4-5 independently treated and processed ankles; J: n=9 (CIA+Ad-Ctrl) to 12 (Ctrl and CIA+Ad-Let7b) ankles pooled from two experiments). Data are presented as mean ± SEM. Except for miRNA, all qRT-PCR results represented in Figure 1 were normalized against GAPDH. *p<0.5, **p<0.01
Dermal miR-Let7b expression exacerbates suboptimal CIA
Skin miR-Let7b expression promoted dermal accumulation of CD3+T cells and CD68+MΦs, reflected by increased biomarkers of these cell types (Fig. 1B). Consistent with the expanded CD3+T cell population, skin IFNγ expression was amplified in i.d. Ad-Let7b injection compared to the control group (Ad-Ctrl) (Fig. 1C). Next, we evaluated IFNγ responsive factors (IRFs) which are known to polarize MΦs. While miR-Let7b had a modest effect on M2-associated IRF3 and IRF4, transcription of M1-linked IRFs was highly upregulated. Transcription of skin IRF1, IRF5 and IRF8 levels were 7-, 4- and 17x upregulated in Ad-Let7b compared to Ad-Ctrl (Fig. 1D). Following Ad-Let7b injection, IRF7 levels were expanded 12x although statistically insignificant (p=0.059). Consistent with PsO clinical presentation [18], skin TLR7 activation elevated GLUT1 C-MYC and hypoxia-inducible factor 1α (HIF1α) transcription levels (Fig. 1E). Collectively, the dermal miR-Let7b injection could amplify M1-induced IRFs to promote inflammation and hypermetabolic activity.
Since dermal TLR7 activation triggers PsO-like pathology [6, 7], we asked if TLR7 ligands could mimic more complex skin-to-joint cross-regulation observed in PsA. When suboptimal collagen-induced arthritis (CIA) mice were i.d. treated with miR-Let7b, joint swelling was markedly expanded on day 35-45 relative to non-arthritic mice (Fig. 1F). These mice exhibited more severe joint swelling than the suboptimal CIA group i.d. injected with Ad-Ctrl starting on day 39. Changes in ankle circumference were about 2-fold greater in miR-Let7b-treated mice relative to CIA+Ad-Ctrl (+1.34mm) (Fig. 1F).
miRNAs can drive autoimmune pathology by cultivating excessive levels of inflammatory cytokines. Endogenous TLR7 ligands were shown to upregulate TNFα, IFNα and IL-6 in RA and systemic lupus erythematosus [19]. In PsO, miR-21 increases skin IL-23 and IL-17 expression [19]. Accordingly, qRT-PCR analysis reveals a more robust inflammatory profile in suboptimal CIA+Ad-Let7b compared to control-treated mice. Notably, only in combination with cutaneous miR-Let7b overexpression, does suboptimal CIA expand joint IL-6 and IL-12 levels (41-4x respectively; Figs. 1H-I). Intriguingly, joint IL-1β expression is elevated in the suboptimal CIA, regardless of skin treatment (Fig. 1G). In line with the upregulation of T cell polarizing cytokines, a trend towards higher joint IL-17 expression was observed in CIA following dermal miR-Let7b administration (7 of 12 joints) relative to non-arthritic mice (2 of 12 joints). Our findings suggest that dermal miR-Let7b potentiate joint inflammation primarily via MΦs.
PsA-like phenotype is characterized by glycolytic and osteoclastic gene expression
Consistent with the inflammatory response observed in the PsA-like model, HIF1α and C-MYC were upregulated in CIA ankles following dermal miR-Let7b expression, compared to non-arthritic mice (14- and 7-fold, respectively) (Fig. 1K). In contrast, transcription of glycolytic modulators GLUT1 and PFKFB3 was unaltered. Of note, in the absence of CIA, i.d. miR-Let7b had no impact on either inflammation or metabolism (supplementary Figs. S1A-B).
In PsA, myeloid progenitor cells can be remodeled into bone-eroding osteoclasts in part via RANKL/RANK pathway [8, 20]. Dermal miR-Let7b expression potentiated the expression of osteoclastic markers including RANK, NFATc, CTSK and TRAP in CIA ankles (Fig. 1L). Particularly, RANKL expression was amplified in CIA mice that received i.d. miR-Let7b injection compared to Ad-Ctrl-treated CIA and non-arthritic mice (7- and 84-fold respectively).
TLR7 activation in murine myeloid cells aggravates an inflammatory phenotype
Data from this preclinical PsA-like model indicate that skin TLR7 ligation upregulates Th17-promoting cytokines in arthritic joints. To examine the involvement of MΦs in TLR7-driven pathogenesis, murine progenitor cells were untreated or stimulated with R837. Stimulation with R837 resulted in higher expression of IL-1β, IL-6 and IL-12 (381, 340 and 105x, respectively), while IL-23 was unchanged (Fig. 2A). R837-activated MΦs undergo metabolic remodeling towards glycolysis, as reflected by the elevated HIF1α and GLUT1 expression (Fig. 2B). HIF1α regulates the expression of various glycolytic enzymes, whereas GLUT1 tightly modulates glucose uptake. Hence, both facilitate efficient glycolytic metabolism [21]. Flow cytometry analysis reveals that R837 activation upregulates the MFI and percentage of F4/80+CD80+ M1 MΦs which is reversed by 2-DG or HIF1α inhibitor (i) treatment (Figs. 2C-E). Additionally, R837 amplifies the production of the classic M1 cytokine, TNFα, which is abrogated by 2-DG treatment (supplementary Fig. S1C). These data underline that R837/TLR7 expands M1 polarization along with their glycolytic activity. Nevertheless, a limitation of the study is the instability of synthetic and the adenoviral miR-Let7b in the in vitro studies.
Figure 2. Impact of TLR7 activation on MΦ and T cell metabolic activity.
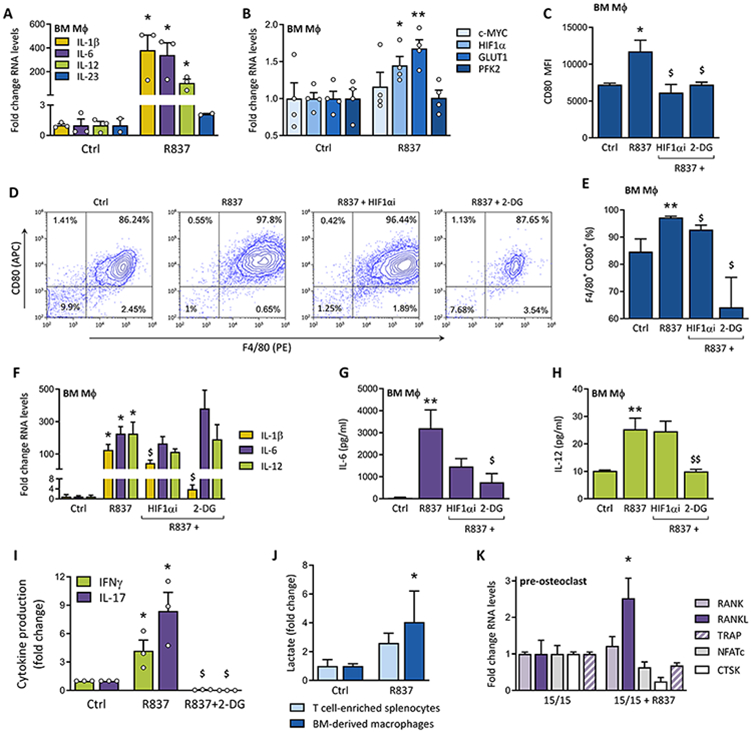
We evaluated inflammatory (A; n=2-3; experimental conditions repeated in triplicate) and glycolytic (B; n=4; cells for 4 independent experiments obtained from 4 donor mice) effects of TLR7 activation on R837-stimulated (1μg/ml) murine MΦs, by evaluating the expression of T cell-polarizing interleukins (IL-1β, IL-6, IL-12 and IL-23) and metabolic genes (C-MYC, HIF1α, GLUT1, PFK2) by qRT-PCR. (C) M1 polarization was evaluated by flow cytometry, quantifying MFI of CD80 staining on MΦs stimulated with R837 (2μg/ml) +/− glycolysis inhibitors (2μM HIF1αi or 5mM 2-DG) (n=4, cells obtained from 4 donor mice for 4 independent experiments). (D-E) Contour plots for 1 representative F4/80 and CD80 staining out of 4 are shown, as well as the average percentage of double-positive F4/80+CD80+ MΦs under different experimental conditions (n=4 mice and representative of four independent experiments). (F-H) The impact of glycolysis inhibition (2μM HIF1αi or 5mM 2-DG) on cytokine expression or production was quantified by qRT-PCR (F; IL-1β, IL-6 and IL-12 expression; n=3; cells obtained from 3 donor mice for 3 experiments) or ELISA (G-H; IL-6 and IL-12 protein levels; n=5; cells obtained from 5 donor mice for 5 experiments). (I) IFNγ and IL-17 protein concentrations in the conditioned media of control- or R837-stimulated (2μg/ml) MΦs-splenocyte co-cultures, +/− 2-DG, were measured by ELISA to assess Th1 and Th17 polarization, respectively (n=3 donor mice for 3 experiments). (J) The concentration of L-lactate in the conditioned media of separately cultured murine MΦs and T cell-enriched splenocytes (control or 2μg/ml R837; 24h) was determined colorimetrically (n=6; cells obtained from 6 different mice for 6 experiments; Mann-Whitney test). (K) Pretreated BM pre-osteoclasts, co-cultured with splenocytes, were stimulated with R837 (1μg/ml) to measure R837-induced expression of osteoclastic genes by qRT-PCR (n=6; cells for 6 independent experiments obtained from 6 mice). (E) Data are represented as the mean ± SEM. All qRT-PCR results represented in Figure 2 were normalized against β-Actin. * p<0.5, *** p<0.001 (compared to Ctrl or 15/15); $ p<0.5 (compared to R837)
R837-induced IL-1β expression was also reduced by HIF1αi and 2-DG (Fig. 2F). Similarly, we substantiated that 2-DG, but not HIF1αi therapy, can diminish the R837-stimulated IL-6 and IL-12 production (Figs. 2G-H). Accordingly, R837 heightened IFNγ (Th1) and IL-17 (Th17) secretion from bone marrow (BM) MΦ-splenocyte co-culture, while 2-DG treatment curtailed this mechanism of function (Fig. 2I). However, our experimental design doesn't exclude the possibility of 2-DG abrogating anti-CD3 and anti-CD28-potentiated IFNγ and IL-17 secretion.
These findings authenticate that R837 reprograms glycolytic MΦs to elicit Th1/Th17 cell polarization. Unlike myeloid cells, R837 did not change C-MYC, HIF1α, GLUT1 and PFKFB3 expression or its end-product, L-lactate, in T cell-enriched splenocytes (supplementary Fig. S1D and Fig. 2J). We are hereby the first to uncover a link between PsA and MΦ glycolysis. Interestingly, C-MYC expression in murine MΦs was unaffected by R837 stimulation, despite the increase in joint C-MYC detected in PsA-like mice (Figs. 1K and 2B). These data suggest that C-MYC upregulation in arthritic ankles did not originate from MΦs or T cells, but from other cells such as fibroblasts or endothelial cells, which may similarly undergo metabolic reprogramming. Our data also suggest that HIF1α blockade insufficiently reverses inflammation, while glucose uptake controlled by GLUT1 is a critical gate-keeper in glycolysis-driven inflammation.
TLR7-induced MΦ-to-T cell crosstalk upregulates RANKL expression in glycolytic pre-osteoclasts
In PsA-like mouse joints, expression of osteoclastic RANKL, RANK, NFATc, CTSK and TRAP was upregulated. While R837 stimulated RANKL expression in co-cultures of murine pre-osteoclasts and splenocytes (2.5x; Fig. 2K), RANK, NFATc, CTSK and TRAP transcription were unaffected. In the presence of R837, these pre-osteoclast cultures also secreted significantly higher levels of L-lactate, but not pyruvate (supplementary Fig. S1E). Taken together, our data suggest that progenitor cells are rewired by TLR7 into inflammatory cells.
CONCLUDING REMARKS
Taken together, this study reveals that PsA SFs are enriched with endogenous TLR7 ligands, like miR-29 and miR-Let7b, that activate inflammation and osteoclastogenesis, in part by rewiring MΦ immunometabolism. TLR7-induced cytokine production indirectly expands Th1/Th17 cell polarization. Dermal administration of miR-Let7b in low-grade arthritic mice results in earlier onset and more severe disease (Fig. 3). Biologics that target cytokines such as TNFα have greatly improved the quality of life for many PsA patients. Nevertheless, a considerable number of patients remain unresponsive to current standard-of-care therapies. Alternatives have aimed at blocking IFNγ and IL-17 pathways to halt PsA progression [22]. Given the impact of TLR7 ligands on MΦ-to-T cell crosstalk, we postulate that TLR7 or its ligands are equally formidable candidates for therapy. Amplified TLR7 activity in PsA cultivates glycolytic MΦs that promote Th17 polarization. Taken together, TLR7 binding to macrophages and their cross-regulation of T cells provides insight into the skin and joint immunopathogenic changes (Fig. 3).
Figure 3. miR-Let7b/TLR7 triggers skin-to-joint crosstalk.
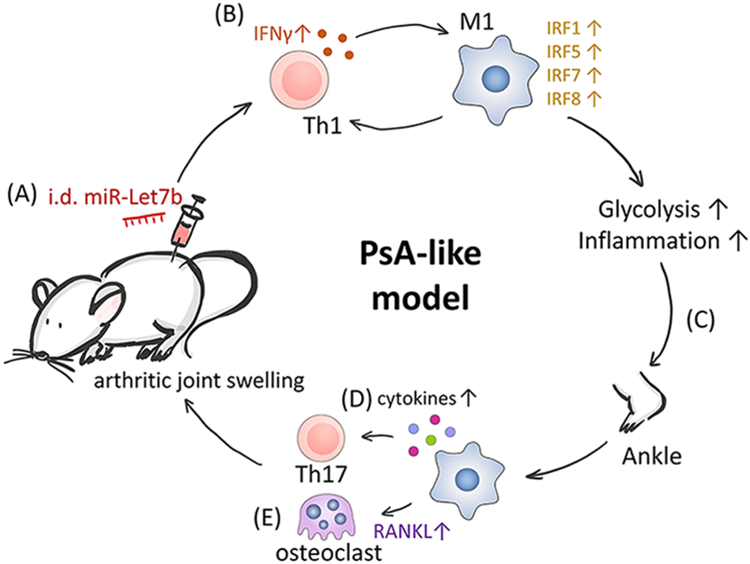
The immunometabolic impact of TLR7 activation and its potential to trigger skin-to-joint crosstalk was summarized schematically. Intradermal miR-Let7b injection (A) expands skin Th1 cells (IFNγ), CD68+M1 MΦs and M1-associated IRFs (B). Glycolytic skin inflammation signals to joints (C) and triggers synovial MΦs to produce Th17-polarizing cytokines (D). Increased RANKL expression differentiates joint progenitors into mature osteoclasts (E). Finally, Th17-driven inflammation and joint erosion establish a PsA-like phenotype.
MATERIALS & METHODS
Patient samples
The protocol was approved by UIC IRB office. SFs were donated following informed written consent. Blood-free SF samples were collected in Na-heparin-coated BD vacutainers. Samples were centrifuged (2000 rpm; 10 min) and cell-free supernatants were removed aseptically.
miRNA quantification
RNA was purified using 5xTRIzol (Life Technologies). TaqMan microRNA Reverse Transcription kit (Applied Biosystems) was used to transcribe isolated miRNA, adding targeted primers for miR-Let7b, miR-21, miR-29 and miR-U6 (housekeeping miRNA). Reverse transcription and qRT-PCR primers were provided by ThermoFisher.
PsA animal model
Preclinical studies were approved by the UIC Animal Care and Use Committee. For suboptimal CIA induction, DBA/1J mice were immunized at 8 weeks (day 0) with 100μl of collagen (Chondrex, 2mg/ml) plus CFA (1mg/ml; 1:1 dilution) and on day 21 with collagen (2mg/ml) plus IFA [23]. On day 23, the hair on the back of mice was removed to allow for skin treatment at day 25. Mice received i.d. injection of control (Ad-Ctrl; Welgen Inc.) or miR-Let7b (Ad-Let7b; 3*109vp; Welgen Inc.). Adenovirus injection was repeated on days 32 and 39. Joint circumference was monitored from days 21-45, thereafter joints were harvested for qRT-PCR. In an independent experiment, non-arthritic mice were injected i.d. with either Ad-Ctrl or Ad-Let7b on days 1, 8 and 15. On day 17, mice were sacrificed and skin around the injection site (+/− 25mm2) was harvested for RNA isolation.
Cell isolation and differentiation
Mouse BM cells and splenocytes were freshly isolated for cell culture experiments. Detailed methods are described in supplementary materials.
qRT-PCR
RNA from cell cultures and ankles was isolated using TRIzol. The high-capacity cDNA reverse transcription kit (Applied Biosystems) was used to obtain cDNA. We used Taqman gene expression master mix (Applied Biosystems) and Taqman primer/probe assays supplied by Integrated DNA Technologies. Fold change in expression was calculated based on the 2−ΔΔCt method using GAPDH (Fig. 1) or β-Actin (Fig. 2) as housekeeping gene [24].
Flow cytometry
Flow cytometry was performed on BM-derived MΦs stained with anti-mouse F4/80 PE and anti-mouse CD80 APC (eBioscience) and evaluated using a Cytoflex S Flow Cytometer (Beckman). Gating was performed as demonstrated in supplementary Fig. S2. Authors have followed the guidelines for the use of flow cytometry in immunological studies [25].
Metabolite quantification
We evaluated pyruvate and L-lactate levels using colorimetric assays (MAK332 and MAK329; Sigma-Aldrich) according to the manufacturer’s instructions.
Statistical analysis
ANOVA was used to validate the statistical significance between multiple groups. Differences between two groups were evaluated by unpaired t-test unless otherwise specified in figure legends. Statistical significance was defined as p < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by awards from the Department of Veteran’s Affairs MERIT Award BX002286, the National Institutes of Health NIH AI147697, AR056099 and AR065778 and the National Psoriasis Foundation (NPF), Pfizer Investigator-Initiated Research (IIR) Program and Chicago Biomedical Consortium (CBC) Accelerator Award.
ABBREVIATIONS
- Ad-Ctrl
Empty control adenoviral vector
- Ad-Let7b
Recombinant adenovirus expressing miR-Let7b
- CIA
Collagen-induced arthritis
- GLUT1
Glucose transporter 1
- HIF1α
Hypoxia-inducible factor 1α
- HIF1αi
HIF1α inhibitor
- i.d.
Intradermal
- IL
Interleukin
- IRF
IFNγ responsive factor
- miRNA
microRNA
- MΦ
Macrophage
- OA
Osteoarthritis
- PsA
Psoriatic arthritis
- PsO
Psoriasis
- RA
Rheumatoid arthritis
- SF
Synovial fluid
- Th1
T helper cell expressing IFNγ
- Th17
T helper cell expressing IL-17
- TLR
Toll-like receptor
- 2-DG
2-Deoxy-D-glucose
Footnotes
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Data availability statement
The data that support the findings of this study are presented in this paper. No shared databases were used or created.
REFERENCES
- 1.Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA and Shahrara S, TLRs, future potential therapeutic targets for RA. Autoimmun Rev 2017. 16: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SJ, Chen Z, Essani AB, Elshabrawy HA, Volin MV, Volkov S, Swedler W, Arami S, Sweiss N and Shahrara S, Identification of a Novel Toll-like Receptor 7 Endogenous Ligand in Rheumatoid Arthritis Synovial Fluid That Can Provoke Arthritic Joint Inflammation. Arthritis Rheumatol 2016. 68: 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamberlain ND, Kim SJ, Vila OM, Volin MV, Volkov S, Pope RM, Arami S, Mandelin AM 2nd, and Shahrara S, Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNFalpha in monocytes. Annals of the Rheumatic Diseases. 2013. 72: 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masalha M, Sidi Y and Avni D, The contribution of feedback loops between miRNAs, cytokines and growth factors to the pathogenesis of psoriasis. Exp Dermatol 2018. 27: 603–610. [DOI] [PubMed] [Google Scholar]
- 5.Guinea-Viniegra J, Jimenez M, Schonthaler HB, Navarro R, Delgado Y, Concha-Garzon MJ, Tschachler E, Obad S, Dauden E and Wagner EF, Targeting miR-21 to treat psoriasis. Sci Transl Med 2014. 6: 225re221. [DOI] [PubMed] [Google Scholar]
- 6.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP and Lubberts E, Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009. 182: 5836–5845. [DOI] [PubMed] [Google Scholar]
- 7.Flutter B and Nestle FO, TLRs to cytokines: mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol 2013. 43: 3138–3146. [DOI] [PubMed] [Google Scholar]
- 8.Pasquali L, Svedbom A, Srivastava A, Rosen E, Lindqvist U, Stahle M, Pivarcsi A and Sonkoly E, Circulating microRNAs in extracellular vesicles as potential biomarkers for psoriatic arthritis in patients with psoriasis. J Eur Acad Dermatol Venereol 2020. [DOI] [PubMed] [Google Scholar]
- 9.Wasik F, Jedrzejak J and Miklaszewska M, Granulocyte pyruvate kinase in psoriasis vulgaris and psoriasis arthropathica. Br J Dermatol 1987. 116: 9–14. [DOI] [PubMed] [Google Scholar]
- 10.Zezina E, Sercan-Alp O, Herrmann M and Biesemann N, Glucose transporter 1 in rheumatoid arthritis and autoimmunity. Wiley Interdiscip Rev Syst Biol Med 2020: e1483. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Yan Y, Liu J, Huang X, Zhang X, Kirschning C, Xu HC, Lang PA, Dittmer U, Zhang E and Lu M, Toll-Like Receptor 7 Activation Enhances CD8+ T Cell Effector Functions by Promoting Cellular Glycolysis. Front Immunol 2019. 10: 2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon BR, Oh YJ, Kang SW, Lee EB and Lee WW, Role of SLC7A5 in Metabolic Reprogramming of Human Monocyte/Macrophage Immune Responses. Front Immunol 2018. 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe R, Hilhorst M, Zhang H, Zeisbrich M, Berry GJ, Wallis BB, Harrison DG, Giacomini JC, Goronzy JJ and Weyand CM, Glucose metabolism controls disease-specific signatures of macrophage effector functions. JCI Insight 2018. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyand CM, Zeisbrich M and Goronzy JJ, Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Curr Opin Immunol 2017. 46: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SY, Heo MJ, Lee C, Choi YM, An IS, Bae S, An S and Jung JH, 2-deoxy-d-glucose Ameliorates Animal Models of Dermatitis. Biomedicines 2020. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makuch S, Wozniak M, Krawczyk M, Pastuch-Gawolek G, Szeja W and Agrawal S, Glycoconjugation as a promising treatment strategy for psoriasis. J Pharmacol Exp Ther 2020. [DOI] [PubMed] [Google Scholar]
- 17.Wade SM, Ohnesorge N, McLoughlin H, Biniecka M, Carter SP, Trenkman M, Cunningham CC, McGarry T, Canavan M, Kennedy BN, Veale DJ and Fearon U, Dysregulated miR-125a promotes angiogenesis through enhanced glycolysis. EBioMedicine 2019. 47: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Chen J, Zeng W, Wu X, Chen M and Chen X, Membrane-enriched solute carrier family 2 member 1 (SLC2A1/GLUT1) in psoriatic keratinocytes confers sensitivity to 2-deoxy-D-glucose (2-DG) treatment. Exp Dermatol 2019. 28: 198–201. [DOI] [PubMed] [Google Scholar]
- 19.Salvi V, Gianello V, Tiberio L, Sozzani S and Bosisio D, Cytokine Targeting by miRNAs in Autoimmune Diseases. Front Immunol 2019. 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JH, Lee NK and Lee SY, Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol Cells 2017. 40: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Liu H, Lian G, Zhang SY, Wang X and Jiang C, HIF1alpha-Induced Glycolysis Metabolism Is Essential to the Activation of Inflammatory Macrophages. Mediators Inflamm 2017. 2017: 9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelosi A, Lunardi C, Fiore PF, Tinazzi E, Patuzzo G, Argentino G, Moretta F, Puccetti A and Dolcino M, MicroRNA Expression Profiling in Psoriatic Arthritis. Biomed Res Int 2018. 2018: 7305380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM 2nd, Agrawal H, Matsui M, Yoshimoto T and Shahrara S, Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum 2011. 63: 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 25.Cossarizza A, Chang HD, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace WW, Aghaeepour N, Akdis M, Allez M, Almeida LN, Alvisi G, Anderson G, Andra I, Annunziato F, Anselmo A, Bacher P, Baldari CT, Bari S, Barnaba V, Barros-Martins J, Battistini L, Bauer W, Baumgart S, Baumgarth N, Baumjohann D, Baying B, Bebawy M, Becher B, Beisker W, Benes V, Beyaert R, Blanco A, Boardman DA, Bogdan C, Borger JG, Borsellino G, Boulais PE, Bradford JA, Brenner D, Brinkman RR, Brooks AES, Busch DH, Buscher M, Bushnell TP, Calzetti F, Cameron G, Cammarata I, Cao X, Cardell SL, Casola S, Cassatella MA, Cavani A, Celada A, Chatenoud L, Chattopadhyay PK, Chow S, Christakou E, Cicin-Sain L, Clerici M, Colombo FS, Cook L, Cooke A, Cooper AM, Corbett AJ, Cosma A, Cosmi L, Coulie PG, Cumano A, Cvetkovic L, Dang VD, Dang-Heine C, Davey MS, Davies D, De Biasi S, Del Zotto G, Dela Cruz GV, Delacher M, Della Bella S, Dellabona P, Deniz G, Dessing M, Di Santo JP, Diefenbach A, Dieli F, Dolf A, Dorner T, Dress RJ, Dudziak D, Dustin M, Dutertre CA, Ebner F, Eckle SBG, Edinger M, Eede P, Ehrhardt GRA, Eich M, Engel P, Engelhardt B, Erdei A, Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol 2019. 49: 1457–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are presented in this paper. No shared databases were used or created.


