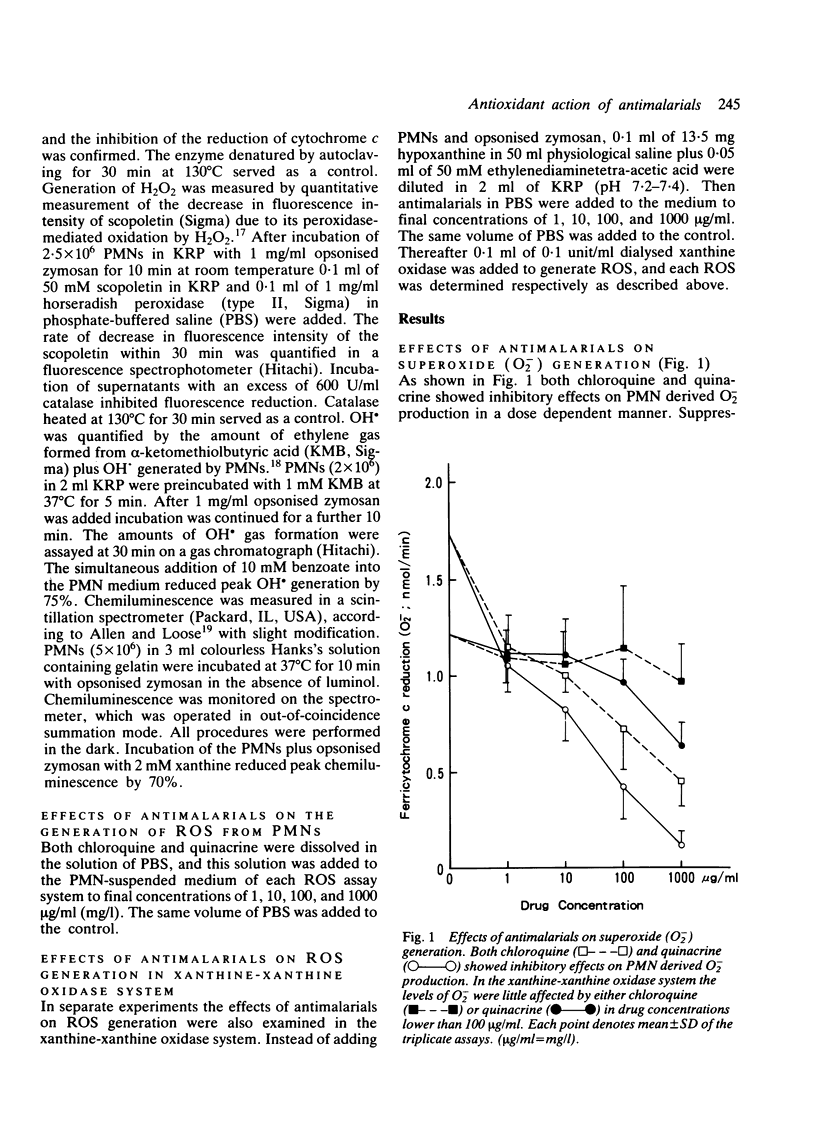
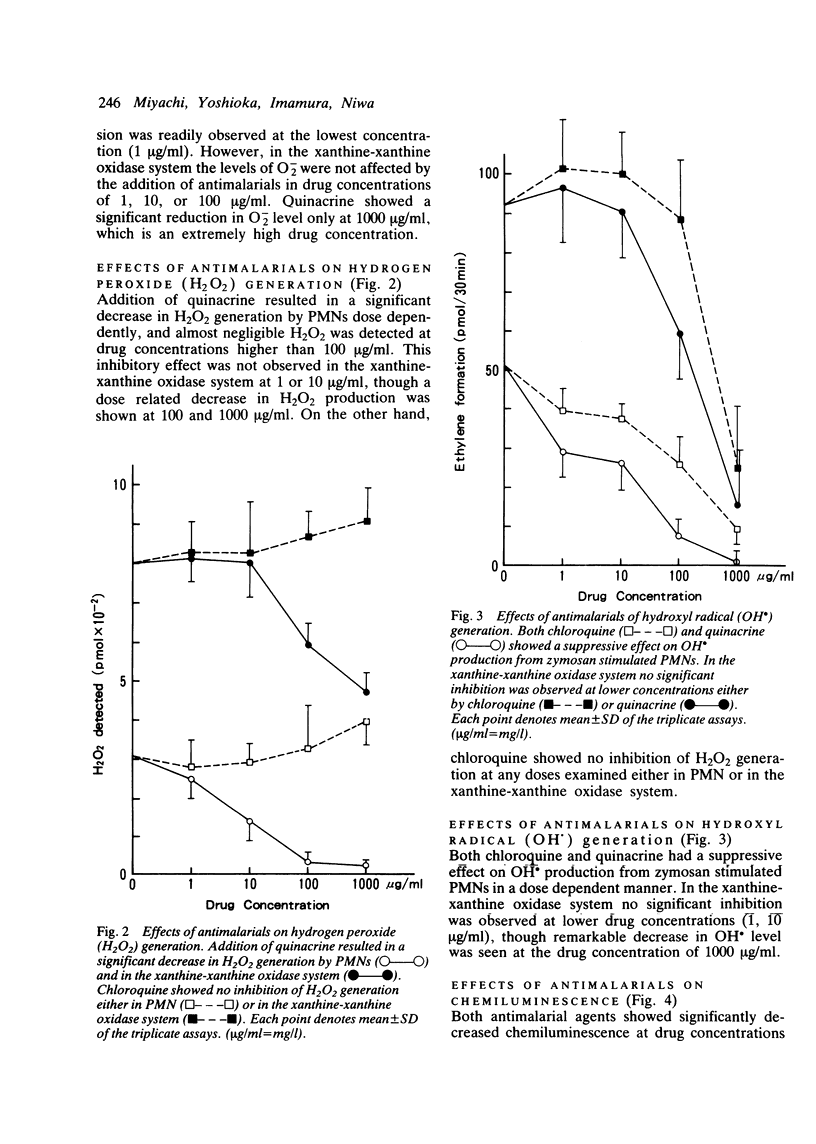
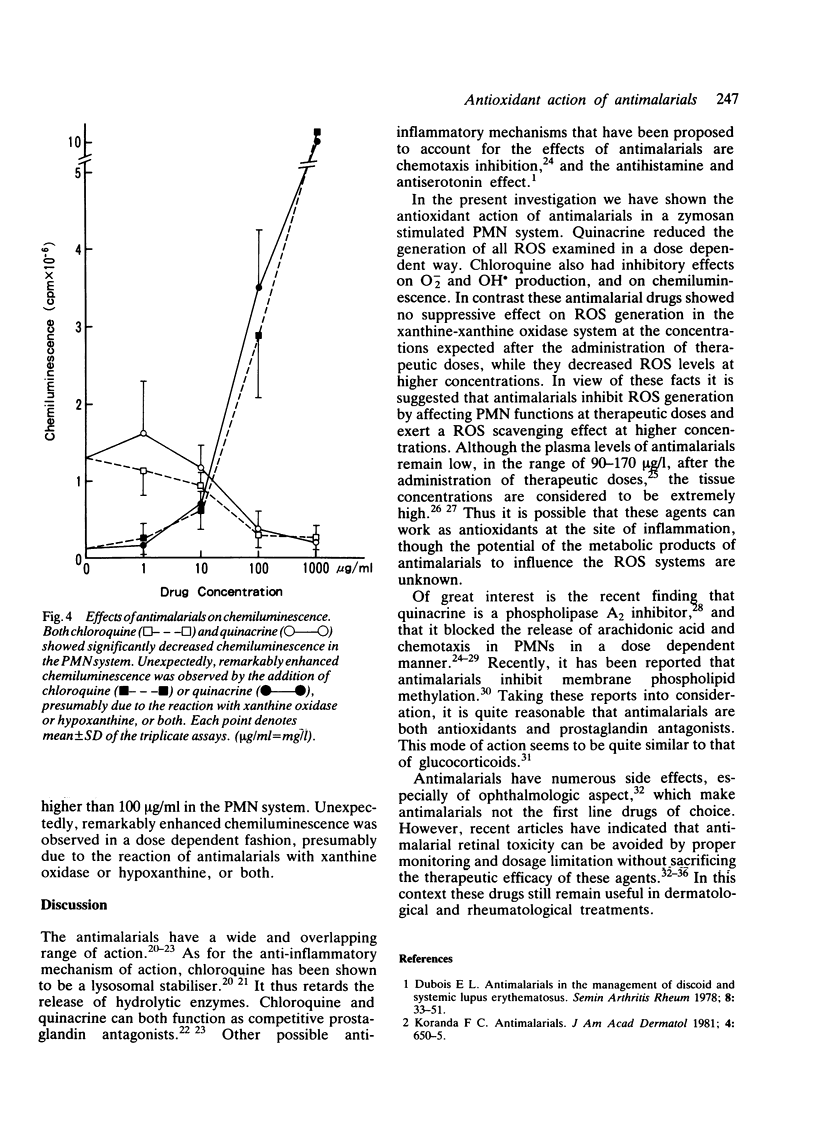
Abstract
The effects of antimalarials, chloroquine and quinacrine, on the generation of reactive oxygen species were examined both in polymorphonuclear leucocytes and in the xanthine-xanthine oxidase system. Antimalarials showed inhibitory effects on the production of reactive oxygen species probably by affecting cell functions, such as membrane phospholipid methylation. It is suggested that antimalarial agents can work as antioxidants at the site of inflammation protecting against auto-oxidative tissue damage with resultant anti-inflammatory effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Alving A. S., Eichelberger L., Craige B., Jones R., Whorton C. M., Pullman T. N. STUDIES ON THE CHRONIC TOXICITY OF CHLOROQUINE (SN-7618). J Clin Invest. 1948 May;27(3 Pt 2):60–65. doi: 10.1172/JCI101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coblyn J. S., Taylor P. Treatment of chronic Lyme arthritis with hydroxychloroquine. Arthritis Rheum. 1981 Dec;24(12):1567–1569. doi: 10.1002/art.1780241217. [DOI] [PubMed] [Google Scholar]
- Dubois E. L. Antimalarials in the management of discoid and systemic lupus erythematosus. Semin Arthritis Rheum. 1978 Aug;8(1):33–51. doi: 10.1016/0049-0172(78)90033-1. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrobin D. F., Manku M. S., Karmazyn M., Ally A. I., Morgan R. O., Karmali R. A. Quinacrine is a prostaglandin antagonist. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1188–1193. doi: 10.1016/0006-291x(77)90981-0. [DOI] [PubMed] [Google Scholar]
- Isaacson D., Elgart M., Turner M. L. Anti-malarials in dermatology. Int J Dermatol. 1982 Sep;21(7):379–395. doi: 10.1111/j.1365-4362.1982.tb03155.x. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Rosen H. Ethylene formation by polymorphonuclear leukocytes. Role of myeloperoxidase. J Exp Med. 1978 Aug 1;148(2):490–506. doi: 10.1084/jem.148.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koranda F. C. Antimalarials. J Am Acad Dermatol. 1981 Jun;4(6):650–655. doi: 10.1016/s0190-9622(81)70065-3. [DOI] [PubMed] [Google Scholar]
- Lie S. O., Schofield B. Inactivation of lysosomal function in normal cultured human fibroblasts by chloroquine. Biochem Pharmacol. 1973 Dec 1;22(23):3109–3114. doi: 10.1016/0006-2952(73)90197-4. [DOI] [PubMed] [Google Scholar]
- MCCHESNEY E. W., NACHOD F. C., TAINTER M. L. Rationale for the treatment of lupus erythematosus with antimalarials. J Invest Dermatol. 1957 Aug;29(2):97–104. doi: 10.1038/jid.1957.76. [DOI] [PubMed] [Google Scholar]
- Manku M. S., Horrobin D. F. Chloroquine, quinine, procaine, quinidine, tricyclic antidepressants, and methylxanthines as prostaglandin agonists and antagonists. Lancet. 1976 Nov 20;2(7995):1115–1117. doi: 10.1016/s0140-6736(76)91090-4. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Niwa Y. Effects of potassium iodide, colchicine and dapsone on the generation of polymorphonuclear leukocyte-derived oxygen intermediates. Br J Dermatol. 1982 Aug;107(2):209–214. doi: 10.1111/j.1365-2133.1982.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Niwa Y. Effects of psoriatic sera on the generation of oxygen intermediates by normal polymorphonuclear leucocytes. Arch Dermatol Res. 1983;275(1):23–26. doi: 10.1007/BF00516550. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Ozaki M., Uchida K., Niwa Y. Effects of thalidomide on the generation of oxygen intermediates by zymosan-stimulated normal polymorphonuclear leukocytes. Arch Dermatol Res. 1982;274(3-4):363–367. doi: 10.1007/BF00403742. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yanase K., Imamura S., Niwa Y. Increased hydroxyl radical generation by normal polymorphonuclear leukocytes incubated in sera from patients with leukocytoclastic vasculitis. Arch Dermatol Res. 1982;274(1-2):65–71. doi: 10.1007/BF00510359. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Miyake S., Sakane T., Shingu M., Yokoyama M. Auto-oxidative damage in Behçet's disease--endothelial cell damage following the elevated oxygen radicals generated by stimulated neutrophils. Clin Exp Immunol. 1982 Jul;49(1):247–255. [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Miyachi Y. Dissociation of the inhibitory effect of dapsone on the generation of oxygen intermediates--in comparison with that of colchicine and various scavengers. Biochem Pharmacol. 1984 Aug 1;33(15):2355–2360. doi: 10.1016/0006-2952(84)90705-6. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Shingu M., Miyachi Y. Role of stimulated neutrophils from patients with systemic lupus erythematosus in tissue injury, with special reference to serum factors and increased active oxygen species generated by neutrophils. Inflammation. 1985 Jun;9(2):163–172. doi: 10.1007/BF00917588. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Shingu M., Yokoyama M. M. Effect of stimulated neutrophils from the synovial fluid of patients with rheumatoid arthritis on lymphocytes--a possible role of increased oxygen radicals generated by the neutrophils. J Clin Immunol. 1983 Jul;3(3):228–240. doi: 10.1007/BF00915347. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Shingu M., Yokoyama M. Role of stimulated neutrophils from patients with systemic lupus erythematosus in disturbed immunoreactivity, with special reference to increased oxygen intermediates generated by the neutrophils. J Clin Lab Immunol. 1984 May;14(1):35–43. [PubMed] [Google Scholar]
- Niwa Y., Sohmiya K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984 Jan;104(1):56–60. doi: 10.1016/s0022-3476(84)80589-2. [DOI] [PubMed] [Google Scholar]
- Olansky A. J. Antimalarials and ophthalmologic safety. J Am Acad Dermatol. 1982 Jan;6(1):19–23. doi: 10.1016/s0190-9622(82)70002-7. [DOI] [PubMed] [Google Scholar]
- Portnoy J. Z., Callen J. P. Ophthalmologic aspects of chloroquine and hydroxychloroquine therapy. Int J Dermatol. 1983 Jun;22(5):273–278. doi: 10.1111/j.1365-4362.1983.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynes R. I., Krohel G., Falbo A., Reinecke R. D., Wolfe B., Bartholomew L. E. Ophthalmologic safety of long-term hydroxychloroquine treatment. Arthritis Rheum. 1979 Aug;22(8):832–836. doi: 10.1002/art.1780220805. [DOI] [PubMed] [Google Scholar]
- SHAFFER B., CAHN M. M., LEVY E. J. Absorption of antimalarial drugs in human skin; spectroscopic and chemical analysis in epidermis and corium. J Invest Dermatol. 1958 Jun;30(6):341–345. doi: 10.1038/jid.1958.63. [DOI] [PubMed] [Google Scholar]
- Skosey J. L., Chow D., Damgaard E., Sorensen L. B. Effect of cytochalasin B on response of human polymorphonuclear leukocytes to zymosan. J Cell Biol. 1973 Apr;57(1):237–240. doi: 10.1083/jcb.57.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum L., Tuffanelli D. L. Antimalarial agents. Chloroquine, hydroxychloroquine, and quinacrine. Arch Dermatol. 1980 May;116(5):587–591. doi: 10.1001/archderm.116.5.587. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Lysosomes. N Engl J Med. 1965 Nov 18;273(21):1143–concl. doi: 10.1056/NEJM196511182732107. [DOI] [PubMed] [Google Scholar]
- Yorio T., Bentley P. J. Phospholipase A and the mechanism of action of aldosterone. Nature. 1978 Jan 5;271(5640):79–81. doi: 10.1038/271079a0. [DOI] [PubMed] [Google Scholar]
- Zuehlke R. L., Lillis P. J., Tice A. Antimalarial therapy for lupus erythematosus: an apparent advantage of quinacrine. Int J Dermatol. 1981 Jan-Feb;20(1):57–61. doi: 10.1111/j.1365-4362.1981.tb05295.x. [DOI] [PubMed] [Google Scholar]


