INTRODUCTION: PRIMER ON OPIOID DOSE DISORDER
In Greek mythology, Hypnos is the God of Sleep, and he is often depicted floating through the air spreading poppies or opium elixir to induce sleep. His son, Morpheus, is the Greek God of Dreams and from whose name the word morphine is derived. These ancient mythological inferences suggest a strong relationship between sleep and opioids, but it is only recently in the context of the opioid use disorder (OUD) epidemic that this relationship has begun to receive significant scientific inquiry. Examining this relationship provides the opportunity to significantly improve symptoms among patients with OUD and potentially improve long-terms outcomes from this disorder.
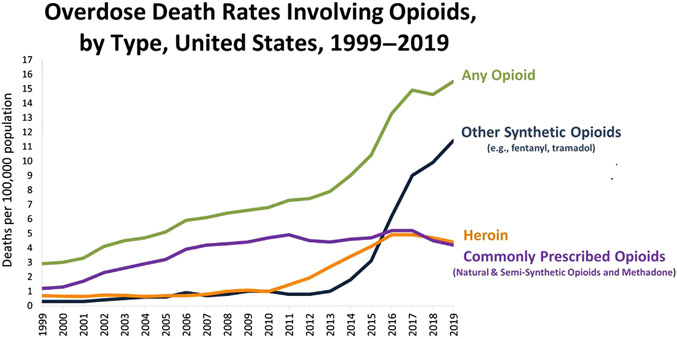
The Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM V) defines the presence and severity of substance use disorder based on several key criteria including tolerance, withdrawal, increasing use, loss of control over use, and craving in combination with social or functional impairment. OUD is a subset of substance use disorder and a chronic and relapsing brain disorder characterized by loss of control over opioid use and deficits in cognitive function, mood, pain perception, and autonomic activity. OUD affects more than 3 million US citizens, 16 million individuals worldwide, and causes one overdose death every 20 minutes.1 In the 1990s, health care providers increased opioid prescribing in response to the "pain as fifth vital sign" campaign, a downplay of the abuse potential of opioids, and aggressive marketing of drugs such as oxycodone hydrochloride. However, opioids are indeed highly vulnerable to abuse and, due to their central nervous depressant effects, accidental death. Subsequently, there have been 3 “waves” contributing to the epidemic increase in opioid overdose death (Fig. 1): wave 1: increase in prescription opioid overdose deaths that started in 1990s; wave 2: increase in heroin overdose deaths that started in 2010; and wave 3: increase in synthetic opioid overdose deaths that started in 2013.2 These progressive waves have in part been related to access (in the case of heroin) and the potency, speed, and intensity of the pleasure (in the case of synthetic opioids such as fentanyl). Most opioid-related deaths occur during sleep due to respiratory failure, where opioid-induced respiratory depression, sleep-related loss of respiratory drive, and loss of protective mechanisms that control breathing co-occur.
Fig. 1.
The 3 epidemiologic “waves” of overdose deaths involving opioids: prescribed opioids, heroin, and synthetic opioids. (Data from CDC/NCHS, National Vital Statistics System, Mortality. CDC WONDER, Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://wonder.cdc.gov/.)
The endogenous opioid system is believed to significantly contribute to the development of OUD. The endogenous opioid system plays a central role in the regulation of mood and well-being consisting of 3 G protein–coupled receptors, mu, delta, and kappa, which are stimulated by a family of endogenous opioid peptides.3 Opioid receptors can also be activated exogenously by alkaloid opiates, the prototype of which is morphine. The finding that morphine’s analgesic and addictive properties are abolished in mice lacking the mu receptor4 points to the mu-opioid receptor as a key molecular player in the therapeutic effects of opioids and in OUD.
Effective evidence-based frontline treatments exist in the form of Food and Drug Administration (FDA)-approved medications for OUD (MOUD). These include methadone (full mu-opioid receptor agonist), buprenorphine (partial mu-opioid agonist), and naltrexone (long-acting mu-opioid antagonist).5 These treatments work by preventing withdrawal, relieving craving, and blocking or attenuating the euphoric effect of exogenous opioids; this has the effect of shifting the patient from “high” or “sick” functional states to a “straight” functional state. These treatments help decrease illicit drug use, treatment attrition, and disease transmission and improve social functioning. However, there is significant variability in treatment responses. Relapse rates are high (even in treatment) and are associated with lack of retention in treatment and a continued cycle of setbacks and return to use, risk for injection-related infectious complications, overdose, and death.6,7
Basic Concepts of Sleep and the Construct of Sleep Deficiency
One of the basic concepts of sleep is termed the “2-process model.” This model is a conceptual model of sleep regulation that posits that 2 constituent processes—(1) a sleep-wake–dependent homeostatic Process S (sleep drive) and (2) Process C (circadian drive)—generate the timing of sleep and wakefulness. The sleep drive is low on waking and continues to increase throughout wakefulness. Normally, this drive peaks just before habitual bedtime. In parallel, the circadian drive is low on wakening and also increases during the day in a manner that balances the sleep drive. However, just before habitual bedtime there is a sharp decrease in the circadian alerting signal, which creates maximal sleep pressure due to an unopposed, high level of sleep drive and potentiates the onset of sleep. The circadian system remains quiescent throughout the biological night, which is defined as the time from melatonin onset to offset. In fact, it is this lack of arousal from the circadian system that allows sleep to continue and be of the longest duration and highest quality during biological night.8,9 Circadian entrainment (ie, alignment between biological time and solar day-night) is continuously adjusted by external cues of light, eating, physical activity, social interactions, and so on such that, in the case of healthy sleep, biological night occurs during solar night.
There are several key functions of sleep and circadian rhythmicity including hormone secretion and metabolic regulation, supporting immune function, energy conservation (particularly in the brain), replenishment of brain macromolecules, removal of neurotoxic waste via the glymphatic system, cognitive function and memory consolidation, mood regulation, brain plasticity, performance, and recovery.10
The American Academy of Sleep Medicine recommends at least 7 hours of sleep daily for adults, based on evidence linking sleep duration to health outcomes.11 In contrast to a focus on individual sleep disorders (eg, sleep apnea), sleep deficiency, as defined by the National Institutes of Health (NIH), is a broader construct that includes insufficient sleep duration (sleep deprivation), sleep out of sync with the body’s circadian rhythm (noncircadian sleep), not getting all the different types of sleep that the body needs (impaired sleep architecture), and poor sleep quality. Common symptoms of sleep deficiency include insomnia (difficulty initiating or maintaining sleep), hypersomnia (excessive daytime sleepiness), or even parasomnia (abnormal behaviors during sleep).
Sleep Deficiency Across the Opiod Use Disorder Trajectory
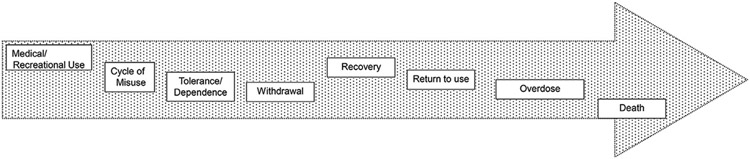
To achieve better outcomes for individuals who are maintained on MOUD, there is a critical need to identify novel strategies and new approaches to complement or even enhance MOUD programs and foster skills necessary for long-term recovery. One promising strategy is to identify and target a neurobiological system that may be linked to OUD relapse, namely the sleep and circadian system. Sleep deficiency is an important correlate of OUD. It is associated with overlapping cognitive deficits in executive function and reward processing.12,13 Sleep is increasingly being recognized as a key determinant of brain health, as it is an important factor in several physical and mental health disorders.14,15 A growing scientific consensus has identified sleep deficiency as a critical component of OUD, both during the active disease state and during recovery. A 2018 FDA public meeting that included patients with OUD identified sleep disturbance as a primary contributor to relapse and treatment attrition.16 More recently, NIH committed 25 million to fund a research program entitled “Sleep dysfunction as a core feature of opioid use disorder and recovery” as part of the Helping End Addiction Long-term (HEAL) initiative.17 Sleep deficiency accompanies OUD across the trajectory of this disorder from initial medical or recreational use through misuse, addiction, recovery, setbacks, return to use, overdose, and death (Fig. 2). Data have also emerged that disrupted circadian rhythms are linked to increasing susceptibility to addiction. For example, there is an association between delayed sleep phase chronotype and addiction vulnerability.13,18 Delayed sleep-phase syndrome is a delay in the major sleep period accompanied by alertness in the evening, sleep-onset insomnia, morning sleepiness, and difficulty awakening from sleep. A form of noncircadian sleep termed “social jet lag” has been coined to describe the circadian desynchrony resulting from large time differences in social (weekend) and academic/work schedules (week-days). Social jet leg is the difference between the average midpoint of sleep on workdays versus the average midpoint of sleep on free days, and this form of circadian desynchrony is associated with increased addiction among adolescents.19
Fig. 2.
Opioid use disorder trajectory.
Sleep disturbance is common and often severe during opioid withdrawal. Withdrawal occurs because taking opioids over a long period can lead to tolerance and dependence. A person who depends on opioids will experience symptoms of withdrawal should they reduce or suddenly stop taking opioids. Signs of withdrawal are similar for all opioids and can include nausea, vomiting, diarrhea, insomnia, anxiety, tachycardia, hypertension, muscle and bone pain, hyperthermia, sweating, and chills.20 Persons with OUD undergoing supervised withdrawal report significant sleep disturbances including increased sleep-onset latency, reduced total sleep time, and poor sleep quality.21 This reduced sleep quality often persists into the postwithdrawal period and has been linked to increased drug craving.22
Sleep disturbance remains a major concern for persons on MOUD.23 Many patients report significant sleep disturbance on entering MOUD recovery programs24 and continue to report poor sleep quality during treatment.25 As with withdrawal, persons in early stages of abstinence who are craving opiates have increased sleep disturbance.26 In one study of patients undergoing MOUD, 90% of patients experienced poor sleep quality defined as a Pittsburgh Sleep Quality Index greater than or equal to 5, nearly half had excessive daytime sleepiness defined as and Epworth Sleepiness Score greater than or equal to 10,25 and 41% were found to be at high risk for sleep apnea.
MECHANISMS OF SLEEP DEFICIENCY LEADING TO OPIOD USE DISORDER
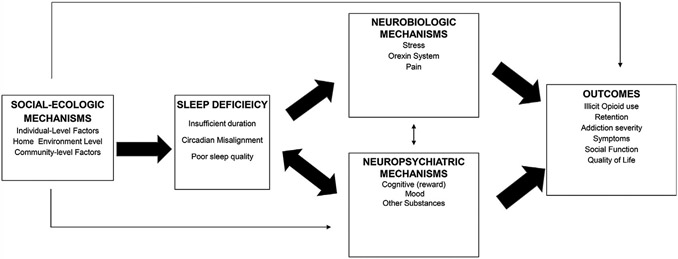
Whether sleep deficiency contributes to OUD relapse is the focus of several ongoing mechanistic studies, observational cohorts, and mechanistic clinical trials studies funded by the NIH Helping to End Addiction Long-term (HEAL) initiative. Importantly, there are several plausible mechanisms whereby sleep deficiency may lead to worse OUD outcomes. Here, the “bio-psycho-social” model of cause holds very well for how sleep deficiency may affect and contribute to OUD. Fig. 3 illustrates that sleep deficiency may contribute to OUD outcomes through its influence on a range of neurobiological mechanisms linked to addiction, such as chronic stress, involvement of the orexin/hypocretin neurotransmitter system, and pain. On the other hand, sleep deficiency among patients with OUD may lead to neuropsychiatric mechanisms such as cognitive control, reward dysregulation, negative affect, as well as the influence other substance use (eg, nicotine, stimulants, alcohol) that may increase the likelihood of illicit opioid use. Finally, social-ecologic factors (at the individual, home-environment, and community level) that often accompany OUD may predispose to the development of sleep deficiency and subsequently influence outcomes. These mechanistic pathways are described later in further detail.
Fig. 3.
An organizing framework for the neurobiologic, neuropsychiatric, and social-ecologic mechanisms for sleep deficiency influencing outcomes along the trajectory of opioid use disorder.
Neurobiologic Mechanisms: Stress, the Orexin System, and Pain
Sleep deficiency activates both immediate short-term stress pathways that lead to the generation of catecholamines as well as prolonged stress pathways, leading to the generation of mineralocorticoids and glucocorticoids. Periods of sleep deficiency lead to heightened blood pressure27 and elevated cortisol28 and promote sympathetic activation.29 Both acute and chronic stress are associated with the onset and progression30 of OUD, and chronic stress and stress reactivity contributes to setbacks and return to use in patients with addiction,31 particularly early in recovery.32 Thus, evidence points toward a bidirectional (and mutually enforcing) relationship between sleep deficiency and chronic stress among patients with OUD.
Orexin (a.k.a., hypocretin)-producing neurons, which are primarily located in the lateral hypothalamus, project to several subcortical and brainstem regions. Deficiency of orexin causes type I narcolepsy. Sleep and circadian rhythmicity provide inputs to the orexin system. Increased orexin signaling causes arousal, sleep disturbances, and stress activation. Orexin neurons also project to reward-associated brain regions. Orexin neurons are responsible for regulating wakefulness/ arousal, diurnal neuroendocrine stress signaling, food, drink, sexual behavior, and even drug consumption.33-36 Evidence from preclinical models of OUD indicates that increased orexin signaling contributes to arousal and stress reactivity (one of the neurobiological hallmarks of OUD) and orexin receptor antagonists (commonly prescribed for insomnia) attenuate opioid withdrawal symptoms.35-37 Thus, this system may be an important target in OUD. In 2018, the National Institute of Drug Abuse listed the orexin system as part of its “Ten Most Wanted” medication development priorities in response to the opioid crisis.
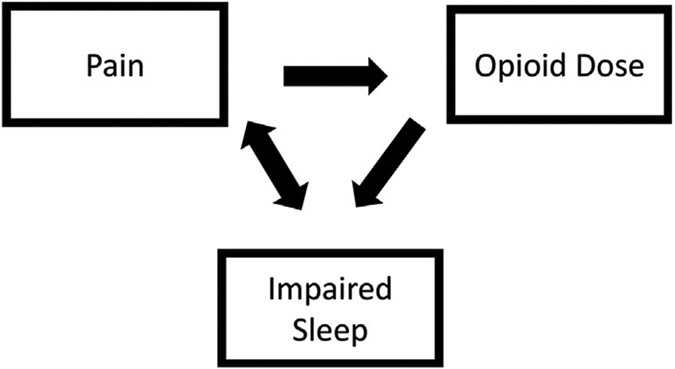
Pain disrupts sleep. Furthermore, sleep deficiency produces “hyperalgesia” (increased pain sensitivity to noxious stimuli) in healthy subjects and clinical samples.38 Relatedly, acute and chronic opioid use impairs sleep (Table 1), and this may lead to a vicious cycle of opioid dose escalation, whereby opioids impair sleep, which lowers the pain threshold and leads to greater opioid use and worsened pain (Fig. 4).38 Importantly, there may be important sex differences in these relationships. Experimental research indicates that women experience pain differently from men.39 Women may be more likely to take prescription opioids without a prescription to cope with pain and are more likely to misuse prescription opioids to self-treat other problems such as anxiety.40 Women have an earlier age of initiation of substance use and a more rapid progression to drug involvement and dependence than men.41
Table 1.
Acute and chronic effects of opioids on sleep
| Acute Use | Chronic Use | |
|---|---|---|
| REM latency | ← | ← |
| % REM sleep | ← | ← |
| % Light (N1/N2) sleep | ← | ← |
| % Deep (N3) | ← | ← |
| Total sleep time | Unchanged | Unchanged |
| Number of arousals/sleep disturbances | ← | ← |
Abbreviation: REM, rapid eye movement.
Fig. 4.
Pain, opioid, sleep-deficiency cycle.
Neuropsychiatric Mechanisms: Cognitive Mechanisms, Mood, and Other Substances
Repeated drug exposure can affect circuits in the prefrontal cortex (regulates executive function), extended amygdala (regulates reward/antireward), and basal ganglia (responsible for incentive salience).42 These 3 brain regions and circuits correspond to a 3-stage cycle of addiction of binge intoxication, withdrawal, and preoccupation anticipation (craving). Importantly, these cognitive domains are impaired in addictive disorders and are also affected by sleep deficiency forming, a feed-forward allostatic framework.43
Among adults with mood disorders, sleep deficiency compromises health and may contribute to substance use comorbidity and suicidality.44 Certain psychological states have been associated with increased opiate craving, including low positive affect and high negative affect. Increases in negative emotional responses to various stimuli and overall self-reported dysphoria are common in individuals with addictive disorders, and the reduction in negative affect (eg, self-medication) has long been held up as a primary driver for the consumption of addictive substances mapping to the extended amygdala.12,42 In addition, anhedonia is thought to partially mediate the relationship between sleep quality and opiate craving postwithdrawal.22 This evidence has triggered a shift away from viewing sleep deficiency as an epiphenomenon to an important but underrecognized mechanism in the multifactorial cause and maintenance of the various mood disorders. In contrast to the current rates of depressive and anxiety disorders in the general population (2%–5% and 6%–10%),45 between 4% and 24% of treatment-seeking individuals with OUD meet current criteria for a depressive disorder and between 5% and 17% meet criteria for an anxiety disorder.46-49 Ongoing mood disorders are important to monitor, as they are associated with continued substance use, poorer retention, lower quality of life, and suicidality.50-54
Other substance use is common in OUD and may also contribute to sleep deficiency.55-57 For example, the rate of cigarette smoking among individuals with OUD far exceeds that of the general population.58 Nicotine lengthens sleep-onset latency and decreases total sleep duration, particularly during deeper sleep stages.57 Likewise, alcohol and cocaine use disorders are highly comorbid in OUD59 and are associated with poorer OUD treatment outcomes.60 Alcohol, a depressant, may promote the initiation of sleep and maintenance of sleep during the first half of the sleep period (eg, decreased sleep onset latency), but it can be disruptive during the second half of sleep (eg, increased wake time after sleep onset, decreased slow wave sleep)61,62 and contribute to sleep-disordered breathing (SDB).63
Social-Ecologic Mechanisms Contributing to Sleep Deficiency: the Role of Individual-Level, Home/Family Environment, and Community Factors
Social-ecologic factors often accompany OUD and may predispose to the development of sleep deficiency and subsequently influence OUD outcomes. Individual psychosocial experiences and perceptions contribute to sleep deficiency. Stressful life events are powerful risk factors for both sleep deficiency64 and drug use.65 For example, adverse childhood experiences (ACEs), stressful traumatic life events that occur during the first 18 years of life (emotional, physical, or sexual abuse; emotional or physical neglect; or other forms of family dysfunction), are pervasive and significant public health problems that consistently contribute to drug use later in life.65 Furthermore, ACEs contribute to many attributes of sleep deficiency66 and specific sleep disorders, including sleep apnea, nightmare distress, and psychiatric sleep disorders.67 ACEs and other stressful experiences also contribute to posttraumatic stress disorder (PTSD), a condition experienced by about 33% of people with OUD that may have a negative effect on adherence to MOUD.68 Sleep deficiency is also a common risk factor and perpetuating factor for PTSD, with as many as 80% to 90% experiencing insomnia and nightmares; SDB, periodic limb movement disorder, and parasomnias are also common.69
OUD and its treatment and sleep deficiency occur within the context of the family. Social support from family and others contributes to successful MOUD70 and good sleep quality. Sleep also occurs within the context of the family, with each member of the family influencing the sleep of the other.71 For example, being married and having a close family relationship were associated with lower levels of drug use and successful treatment,72 whereas married couples had better sleep than others in the United States73 and single marital status had a negative impact on sleep quality.74 In one study, women who reported marital happiness had better sleep than others,75 whereas marital conflict was a risk factor for poor sleep. Notably, the quality of attachment and family support76 were associated with better sleep quality. In contrast, family conflict during childhood contributed to the development of insomnia in adults,67 and household chaos and disturbing behaviors of family members also predicted poor sleep.71
Physical characteristics of the home environment are well-known proximal contributors to poor sleep. Factors such as bright light and noisy environments, the presence of other people in the bedroom, and comfortable bedding are traditional factors that are often the focus of behavioral sleep “hygiene” intervention. Exposure to electronic “screen time” near bedtime is increasingly a focus of concern because of the contributions of blue light to wakefulness and hyperarousal.77 In addition, housing instability (ie, recent, past, or potential for homelessness) and housing type (eg, house, apartment, trailer) will be addressed because housing type is a risk factor for poorer sleep that is also often associated with economic adversity.78
Community-level factors can be either risk factors or protective factors for sleep. Both social and physical characteristics of the neighborhood in which one lives contribute to sleep deficiency.79 Social factors including social ties and social cohesion in the community seem to be protective factors that are closely tied with sleep.80 On the other hand, social fragmentation and neighborhood disadvantage (a factor that is often associated with racial disparities in sleep), contributed to wake after sleep onset.81 Adverse neighborhood environments were associated with shorter sleep duration,82 whereas neighborhoods characterized by more social disorder, low social cohesion, and lower safety were associated with shorter sleep duration after controlling for socioeconomic status and the physical environment.83 Positive aspects of the physical neighborhood environment, such as those associated with good health (eg, walkability and green space, lower population density, lower noise levels), are associated with improved sleep.84 A recent review indicated the promise of addressing environmental characteristics, such as walkability and green space on sleep.79 Taken together, these findings suggest the critical importance of understanding the individual, family, and social context for sleep deficiency.
Mechanisms of Opioid Use Disorder Leading to Sleep Deficiency
Direct effects of opioids on sleep
Information on the differential effects of opioid use on key sleep parameters in different populations including potential acute versus chronic differences is covered in detail elsewhere85-87 and is summarized in Table 1.
Sleep deficiency affects most patients in methadone treatment programs.25 Importantly, in one prospective cohort study, receiving methadone, MOUD treatment had no significant effect on sleep disturbance. In the final multivariate model, younger age, pain, high nicotine dependence, and suicide risk were all associated with sleep disturbance.88
In this context, it is important to note that improvements in sleep architecture may occur with chronic opioid use89; opioids can improve sleep quality and increase sleep time in people with chronic nonmalignant pain.87,90 Given the evidence for a bidirectional relationship between sleep and pain, whereby poor sleep worsens pain perception and vice versa (see Fig. 4),38,91 opioids may improve sleep, at least in part, via reductions in pain. Given that strategies to improve sleep can reduce pain,92 evaluation of the effects of opioids on sleep may be an important consideration in the clinical management of pain in people taking opioids.
Effects of Opioids on Sleep-Disordered Breathing: Ataxic Breathing, Central Apnea, and Obstructive Sleep Apnea
There are 3 basic components to the ventilatory control system: the controller (cerebral cortex, pons, medulla), the sensors (carotid, aortic bodies), and effectors (diaphragm, accessory muscles of respiration). Most opioid-related deaths occur during sleep; this is, in part, mediated by the sleep-related loss of wakefulness respiratory drive, decreased O2 and CO2 chemosensitivity, and decreased tone in the muscles of the upper airway pharyngeal dilator muscles and ventilatory pump effector muscles. Respiratory depression is further amplified by the addition of opioids, which results in the suppression of protective mechanisms that control breathing, leading to respiratory failure and death.
The combined effects of sleep and opioids on the ventilatory control system also contribute to several different forms of sleep apnea, including ataxic breathing (termed Biot respiration), central sleep apnea, and obstructive sleep apnea (OSA), which may further impair sleep quality.93 Similar to high-altitude exposure, whereby everyone will eventually develop SDB if they go high enough,93 the same is likely true for SDB and opioids.
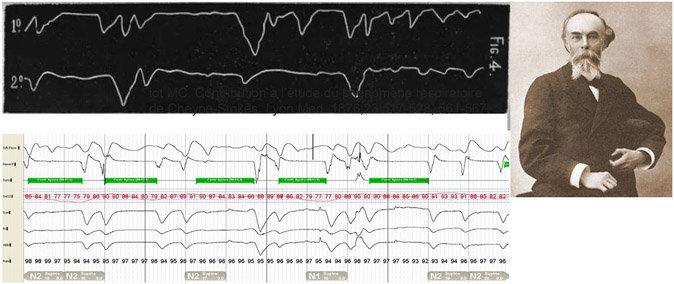
Breathing can slow and become irregular, leading to hypercapnia and hypoxia with high doses of opioids.93,94 An ataxic breathing pattern, termed Biot respiration after Dr Camille Biot (Fig. 5), with irregular variation tidal volumes and rhythm, bradypnea, oxygen desaturations, and arousals has been described in association with both acute neurologic disease as well as opioid use.95,96
Fig. 5.
A current tracing compared with an original tracing from Biot’s Respiration: Ataxic Breathing with Opioid Use. (From Camille Biot. Contribution a l’étude du phénomène respiratoire de Cheyne-Stokes. Lyon Med. 1876;23:517-528.)
The presence and severity and opioid-induced central sleep apnea vary due to multiple factors including the dose of opioid, wakefulness-to-sleep transitions, and sleep stage. Opioids cause central respiratory depression and central sleep apnea in a dose-dependent manner.97,98 Loss of the wakefulness drive to breathe, which itself can predispose to central apnea at sleep onset,93,99 may become magnified when combined with opioid-induced central respiratory depression via activation of mu-opioid receptors.100
On polysomnography, central apneic events are distinguished from obstructive apneic events by absent airflow in the context of no respiratory effort. However, there is overlap in the pathophysiology of obstructive and central sleep apnea. For example, centrally mediated loss of respiratory drive not only causes central apnea but it also contributes to upper airway closure due to concurrent central reductions in the neural drive to the pharyngeal dilators muscles.101 Thus, unstable control of breathing is not only a contributor to central apnea but is also a feature of OSA for at least 30% of patients.102 There are several key physiologic traits that contribute to OSA pathophysiology.102-105 Impaired pharyngeal anatomy, or a collapsible upper airway, is the pathophysiological trait common to all patients with OSA. Stable upper airways require a more negative critical closing pressure (Pcrit), whereas highly collapsible upper airways have a more positive Pcrit. Thus, the magnitude of anatomic impairment varies markedly between individuals.102,103 Importantly, almost 60% of people with OSA also have one or more nonanatomical physiologic traits that contribute to their OSA.102,103 These traits include (1) inadequate upper-airway dilator muscle activity during sleep, (2) unstable control of breathing/excessive sensitivity to minor changes in CO2 (high loop gain), and (3) a low respiratory arousal threshold (waking up too easily to minor airway narrowing), which prevents deeper more stable sleep.102,103 Limited data exist regarding the impact of escalating doses of opioids on these nonanatomic physiologic traits of OSA. Overall, the spectrum of variables that likely affect the effects of opioids on breathing stability during sleep include the dose of opioids, pharyngeal anatomy, nonanatomic physiologic traits of sleep apnea, pharmacokinetics of opioids, baseline sleep deficiency, comorbidities, and body habitus and position.
Targeting Sleep Deficiency to Improve Opiod Use Disorder: Behavioral, Positive Airway Pressure, and Pharmacologic Interventions
Behavioral
Cognitive-behavioral therapy for insomnia (CBTi) is the first-line treatment of insomnia and can be delivered face-to-face, via telehealth, or web-based platforms. It involves (1) behavioral techniques (sleep restriction, stimulus control) designed to target excessive time in bed, irregular sleep schedules, sleep incompatible activities, and hyperarousal; (2) cognitive techniques (cognitive therapy, paradoxic intention) designed to target unrealistic sleep expectations, misconceptions about sleep, sleep-related worries, and poor coping skills; and (3) educational techniques (sleep hygiene education, sleep information) designed to target inadequate sleep hygiene. There is a real need to conduct intervention trials to determine whether CBTi helps to consolidate sleep among patients with OUD and sleep deficiency and explore its impact on key outcomes. Importantly, this therapy does not involve the use of prescription sedative-hypnotics that have abuse potential. Furthermore, CBTi may have overlapping benefits in other domains including the treatment of anxiety and pain.
Positive airway pressure therapy
Given the dose-response relationship between opioids and SDB,97,98 dose reduction is highly effective in reducing SDB severity and, thus, should be prioritized.93,106-108 Few studies have systematically investigated positive airway pressure (PAP) therapy for people with opioid-induced SDB. The existing data indicate variable success with PAP and adaptive servo-ventilation (ASV) approaches.87,106,109-114 Variable responses likely reflect, at least in part, the different manifestations of opioid-induced SDB (ie, central vs obstructive). ASV may be effective in treating opioid-induced central sleep apea.109,111 Effective treatment of SDB with PAP may improve health and well-being in this population.115
Pharmacologic
Given concerns for abuse with benzodiazepines, previous pharmacologic trials targeting sleep consolidation and insomnia among patients with OUD have largely focused on nonbenzodiazepine medications. To date, we are aware of only 3 published trials. The first was a randomized, double-blind, placebo-controlled trial that compared trazadone with placebo with 6 months of follow-up in 137 patients recruited from methadone maintenance programs. Trazadone did not improve subjective or objective sleep nor did it significantly increase or decrease illicit drug use relative to placebo.116 A more recent pilot trial of 10 methadone maintenance patients compared mirtazapine (30 mg) versus zolpidem sustained release (12.5 mg) versus mirtazapine (30 mg) + zolpidem (10 mg) versus placebo using a within-subject, cross-over design with a 1-week washout between drugs. The mirtazapine arm alone improved total sleep time (23 minutes), sleep latency (23 minutes), and sleep efficiency (3%), surpassing all the other regimens.117 Finally, a randomized, double-blind, placebo-controlled trial of 54 patients receiving methadone maintenance comparing melatonin, 10 mg, with placebo for 3 months resulted in significant improvement in subjective sleep quality, depression symptoms, and anxiety symptoms versus placebo.118
Given the overlapping effects of modulating sleep and opioid withdrawal symptoms of the orexin (hypocretin) system described earlier (see discussion neurobiological mechanisms), there is considerable interest in examining the impact of antagonists or negative modulators of the orexin neuropeptides on sleep deficiency and other outcomes among patients with OUD. Currently, 2 FDA-approved dual orexin receptor antagonists (DORAs) already exist and are FDA approved for the treatment of insomnia: suvorexant (Belsomra) and another recent DORA, lemborexant. In addition to insomnia therapeutic benefits, there is preclinical evidence that DORAs reduce opioid withdrawal and drug seeking35,36; this may, in part, be mediated through normalizing sleep disturbances present in 75% of patients with OUD, as sleep disturbances can worsen OUD outcomes.24 Given that these are schedule IV controlled substances, this drug needs to be rigorously tested in clinical trials, several of which are currently underway: NCT03412591, NCT03897062, NCT03789214, NCT03937986, NCT03789214, NCT03657355.
To date, the effects of the opioid receptor antagonist naloxone on sleep apnea have been mixed,119,120 and respiratory drive suppression and depression of the hypoglossal nerve activity remains a critical problem. The use of ampakines, modulators of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) glutamatergic receptors that mediate the excitatory transmission of respiratory centers and the cranial nerve XII (hypoglossal nerve), may counteract mu-opioid receptor–mediated depression; this offers the promise that it may be possible to maintain analgesia while preventing the unwanted respiratory depression that accompanies opioids.121-125 Intranasal leptin can augment hypercapnic and hypoxic sensitivity, prevent opioid-induced SDB, and has been demonstrated to improve survival after overdose in mouse models. Thus, this therapy has the potential to be of benefit in opioid-induced SDB, particularly those with major hypoventilation.126,127 However, this work has not yet been translated to humans. New emerging pharmacotherapies to treat OSA have recently reported both using metabolic modulation and targeting the nonanatomic physiologic traits.128-131 However, these approaches may be beneficial for opioid-related SDB, but this requires further investigation in this target population.
SUMMARY
OUD is a chronic and relapsing brain disease characterized by loss of control over opioid use. Sleep deficiency is present in greater than 75% of patients with OUD. The focus of this article is to highlight bidirectional mechanisms between OUD and sleep deficiency and point toward promising therapeutic targets. Behavioral, pharmacologic, and PAP interventions targeting sleep deficiency may ultimately help promote long-term, healthy recovery among patients with OUD.
CLINICS CARE POINTS.
OUD is a subset of substance use disorder and a chronic and relapsing brain disorder characterized by loss of control over opioid use and deficits in cognitive function, mood, pain perception, and autonomic activity.
Relapse rates are high (even in treatment) and are associated with lack of retention in treatment and a continued cycle of setbacks and return to use, risk for injection-related infectious complications, overdose, and death. To achieve better outcomes for individuals who are maintained on MOUD, there is a critical need to identify novel strategies and new approaches to complement or even enhance MOUD programs and foster skills necessary for long-term recovery. One promising strategy is to identify and target a neurobiological system that may be linked to OUD relapse, namely the sleep and circadian system.
Sleep deficiency accompanies OUD across the trajectory of this disorder from initial medical or recreational use through misuse, addiction, recovery, setbacks, return to use, overdose, and death.
There are bidirectional (and mutually reinforcing) mechanisms between sleep deficiency and OUD.
There are behavioral, PAP, and pharmacologic interventions targeting sleep deficiency that may help to improve symptoms and outcomes among patients with OUD; these include CBTi, ASV, mirtazapine, melatonin, and DORAs.
Ampakines and intranasal leptin are experimental therapies that offer promise in reducing the unwanted respiratory depression associated with opioids.
KEY POINTS.
Opioid use disorder (OUD) is a chronic and relapsing brain disease characterized by loss of control over opioid use and impairments in cognitive function, mood, pain perception, and autonomic activity.
Sleep deficiency, a term that encompasses insufficient or disrupted sleep due to multiple potential causes, including circadian disruption, and poor sleep quality, is present in greater than 75% of patients with OUD.
This article focuses on existing bidirectional mechanisms between OUD and sleep deficiency and points toward promising therapeutic targets.
Behavioral, pharmacologic, and positive airway pressure interventions targeting sleep deficiency may ultimately help promote long-term, healthy recovery among patients with OUD.
DISCLOSURE
Agency: NIH/NHLBII.D.#: U01 HL150596Title: The Collaboration Linking Opioid Use Disorder and Sleep (CLOUDS”) Study.
H.K. Yaggi is supported by U01 HL150596, R01 NR018335, K24 HL132093.
REFERENCES
- 1.Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep 2018;67(5152):1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020, NCHS Data Brief 2021;427:1–8. [PubMed] [Google Scholar]
- 3.Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol 1995;15(6):615–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthes HW, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 1996;383(6603):819–23. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies-tackling the opioid-overdose epidemic. N Engl J Med 2014;370(22):2063–6. [DOI] [PubMed] [Google Scholar]
- 6.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci 1995;15(5 Pt 1):3526–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depner CM, Melanson EL, McHill AW, et al. Mis-timed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Natl Acad Sci U S A 2018;115(23):E5390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kryger MH, Roth T, Goldstein CA. Principles and practice of sleep medicine. Philadelphia: Elsevier; 2021. [Google Scholar]
- 11.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep 2015;38(6):843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwako LE, Momenan R, Litten RZ, et al. Addictions neuroclinical assessment: a neuroscience-based framework for addictive disorders. Biol Psychiatry 2016;80(3):179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulick D, Gamsby JJ. Racing the clock: the role of circadian rhythmicity in addiction across the lifespan. Pharmacol Ther 2018;188:124–39. [DOI] [PubMed] [Google Scholar]
- 14.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353(19):2034–41. [DOI] [PubMed] [Google Scholar]
- 15.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry 2005;66(10):1254–69. [DOI] [PubMed] [Google Scholar]
- 16.Administration FaD. Public meeting on patient-focused drug development for chronic pain. 2018. Available at: https://www.fda.gov/drugs/newsevents-human-drugs/public-meeting-patient-focused-drug-developmentchronic-pain.
- 17.Laposky AD. Sleep dysfunction as a core feature of opioid use disorder and recvoery. 2020. Available at: https://heal.nih.gov/research/new-strategies/sleep-dysfunction. [Google Scholar]
- 18.Murray G, Nicholas CL, Kleiman J, et al. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion 2009;9(5):705–16. [DOI] [PubMed] [Google Scholar]
- 19.Wittmann M, Dinich J, Merrow M, et al. Social jetlag: misalignment of biological and social time. Chronobiol Int 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 20.Shara B, Bruner A, Basrnett GF M. Opioid use diosorders. Child Adolesc Psychiatr Clin N Am 2016;25(3):473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyefeso A, Sedgwick P, Ghodse H. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug Alcohol Depend 1997;48(1):9–16. [DOI] [PubMed] [Google Scholar]
- 22.Lydon-Staley DM, Cleveland HH, Huhn AS, et al. Daily sleep quality affects drug craving, partially through indirect associations with positive affect, in patients in treatment for nonmedical use of prescription drugs. Addict Behav 2017;65:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finan PH, Mun CJ, Epstein DH, et al. Multimodal assessment of sleep in men and women during treatment for opioid use disorder. Drug Alcohol Depend 2020;207:107698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartwell EE, Pfeifer JG, McCauley JL, et al. Sleep disturbances and pain among individuals with prescription opioid dependence. Addict Behav 2014;39(10):1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldassarri SR, Beitel M, Zinchuk A, et al. Correlates of sleep quality and excessive daytime sleepiness in people with opioid use disorder receiving methadone treatment. Sleep Breath 2020;24(4):1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiluk BD, Yip SW, DeVito EE, et al. Anhedonia as a key clinical feature in the maintenance and treatment of opioid use disorder. Clin Psychol Sci 2019;7(6):1190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calhoun DA, Harding SM. Sleep and hypertension. Chest 2010;138(2):434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leproult R, Copinschi G, Buxton O, et al. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997;20(10):865–70. [PubMed] [Google Scholar]
- 29.Irwin M, Thompson J, Miller C, et al. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab 1999;84(6):1979–85. [DOI] [PubMed] [Google Scholar]
- 30.Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res 2010;1314:219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha R The role of stress in addiction relapse. Curr Psychiatry Rep 2007;9(5):388–95. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF. A role for brain stress systems in addiction. Neuron 2008;59(1):11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A 1999;96(2):748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998;92(4):573–85. [DOI] [PubMed] [Google Scholar]
- 35.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci 2006;29(10):571–7. [DOI] [PubMed] [Google Scholar]
- 36.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 2005;437(7058):556–9. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Fardon R, Zorrilla EP, Ciccocioppo R, et al. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res 2010;1314:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kundermann B, Krieg JC, Schreiber W, et al. The effect of sleep deprivation on pain. Pain Res Manag 2004;9(1):25–32. [DOI] [PubMed] [Google Scholar]
- 39.Riley JL 3rd, Robinson ME, Wise EA, et al. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 1998;74(2–3):181–7. [DOI] [PubMed] [Google Scholar]
- 40.McHugh RK, Devito EE, Dodd D, et al. Gender differences in a clinical trial for prescription opioid dependence. J Subst Abuse Treat 2013;45(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unger A, Jung E, Winklbaur B, et al. Gender issues in the pharmacotherapy of opioid-addicted women: buprenorphine. J Addict Dis 2010;29(2):217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkow ND, Jones EB, Einstein EB, et al. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry 2019;76(2):208–16. [DOI] [PubMed] [Google Scholar]
- 43.Koob GF, Colrain IM. Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology 2020;45(1):141–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adrien J Neurobiological bases for the relation between sleep and depression. Sleep Med Rev 2002;6(5):341–51. [PubMed] [Google Scholar]
- 45.Andrade L, Caraveo-Anduaga J, Berglund P, et al. Cross-national comparisons of the prevalences and correlates of mental disorders. Bull World Health Organ 2000;78:413–25. [PMC free article] [PubMed] [Google Scholar]
- 46.Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry 1997;54(1):71–80. [DOI] [PubMed] [Google Scholar]
- 47.Kidorf M, Disney ER, King VL, et al. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend 2004;74(2):115–22. [DOI] [PubMed] [Google Scholar]
- 48.Abbott PJ, Weller SB, Walker SR. Psychiatric disorders of opioid addicts entering treatment. J Addict Dis 1995;13(3):1–11. [DOI] [PubMed] [Google Scholar]
- 49.Kidorf M, Solazzo S, Yan H, et al. Psychiatric and substance use comorbidity in treatment-seeking injection opioid users referred from syringe exchange. J Dual Diagn 2018;1–8. 10.1080/15504263.15502018.11510148. [DOI] [PubMed] [Google Scholar]
- 50.Anand D, Paquette C, Bartuska A, et al. Substance type moderates the longitudinal association between depression and substance use from pretreatment through a 1-year follow-up. Drug and Alcohol Dependence 2019. 10.1016/j.drugalcdep.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rounsaville BJ, Weissman MM, Crits-Christoph K, et al. Diagnosis and symptoms of depression in opiate addicts: course and relationship to treatment outcome. Arch Gen Psychiatry 1982;39(2):151–6. [DOI] [PubMed] [Google Scholar]
- 52.Teoh Bing Fei J, Yee A, Habil MHB. Psychiatric comorbidity among patients on methadone maintenance therapy and its influence on quality of life. Am J Addict 2016;25(1):49–55. [DOI] [PubMed] [Google Scholar]
- 53.Fatséas M, Denis C, Lavie E, et al. Relationship between anxiety disorders and opiate dependence—a systematic review of the literature: implications for diagnosis and treatment. J Substance Abuse Treat 2010;38(3):220–30. [DOI] [PubMed] [Google Scholar]
- 54.Timko C, Schultz NR, Cucciare MA, et al. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict diSeases 2016;35(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips BA, Danner FJ. Cigarette smoking and sleep disturbance. Arch Intern Med 1995;155(7):734–7. [PubMed] [Google Scholar]
- 56.Wetter DW, Young TB. The relation between cigarette smoking and sleep disturbance. Prev Med 1994;23(3):328–34. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Samet J, Caffo B, et al. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol 2006;164(6):529–37. [DOI] [PubMed] [Google Scholar]
- 58.Parker MA, Streck JM, Sigmon SC. Associations between opioid and nicotine dependence in nationally representative samples of United States adult daily smokers. Drug Alcohol Depend 2018;186:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witkiewitz K, Vowles KE. Alcohol and opioid use, Co-Use, and chronic pain in the context of the opioid epidemic: a critical review. Alcohol Clin Exp Res 2018;42(3):478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhingra L, Perlman DC, Masson C, et al. Longitudinal analysis of pain and illicit drug use behaviors in outpatients on methadone maintenance. Drug Alcohol Depend 2015;149:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebrahim IO, Shapiro CM, Williams AJ, et al. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res 2013;37(4):539–49. [DOI] [PubMed] [Google Scholar]
- 62.Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health 2001;25(2):101–9. [PMC free article] [PubMed] [Google Scholar]
- 63.Burgos-Sanchez C, Jones NN, Avillion M, et al. Impact of alcohol consumption on snoring and sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2020;163(6):1078–86. [DOI] [PubMed] [Google Scholar]
- 64.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med 2000;62(2):227–30. [DOI] [PubMed] [Google Scholar]
- 65.Douglas KR, Chan G, Gelernter J, et al. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict Behav 2010;35(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chapman DP, Wheaton AG, Anda RF, et al. Adverse childhood experiences and sleep disturbances in adults. Sleep Med 2011;12(8):773–9. [DOI] [PubMed] [Google Scholar]
- 67.Kajeepeta S, Gelaye B, Jackson CL, et al. Adverse childhood experiences are associated with adult sleep disorders: a systematic review. Sleep Med 2015;16(3):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ecker AH, Hundt N. Posttraumatic stress disorder in opioid agonist therapy: a review. Psychol Trauma 2018;10(6):636–42. [DOI] [PubMed] [Google Scholar]
- 69.Koffel E, Khawaja IS, Germain A. Sleep disturbances in posttraumatic stress disorder: updated review and implications for treatment. Psychiatr Ann 2016;46(3):173–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavaiola AA, Fulmer BA, Stout D. The impact of social support and attachment style on quality of life and readiness to change in a sample of individuals receiving medication-assisted treatment for opioid dependence. Subst Abus 2015;36(2):183–91. [DOI] [PubMed] [Google Scholar]
- 71.Spilsbury JC, Patel SR, Morris N, et al. Household chaos and sleep-disturbing behavior of family members: results of a pilot study of African American early adolescents. Sleep Health 2017;3(2):84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinz AJ, Wu J, Witkiewitz K, et al. Marriage and relationship closeness as predictors of cocaine and heroin use. Addict Behav 2009;34(3):258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grandner MA, Patel NP, Gehrman PR, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med 2010;11(5):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sekine M, Chandola T, Martikainen P, et al. Work and family characteristics as determinants of socioeconomic and sex inequalities in sleep: the Japanese Civil Servants Study. Sleep 2006;29(2):206–16. [DOI] [PubMed] [Google Scholar]
- 75.Troxel WM, Buysse DJ, Hall M, et al. Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. Behav Sleep Med 2009;7(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kent de Grey RG, Uchino BN, Pietromonaco PR, et al. Strained bedfellows: an actor-partner analysis of spousal attachment insecurity and sleep quality. Ann Behav Med 2019;53(2):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heo JY, Kim K, Fava M, et al. Effects of smartphone use with and without blue light at night in healthy adults: a randomized, double-blind, cross-over, placebo-controlled comparison. J Psychiatr Res 2017;87:61–70. [DOI] [PubMed] [Google Scholar]
- 78.Johnson DA, Billings ME, Hale L. Environmental determinants of insufficient sleep and sleep disorders: implications for population health. Curr Epidemiol Rep 2018;5(2):61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunter JC, Hayden KM. The association of sleep with neighborhood physical and social environment. Public Health 2018;162:126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson DA, Lisabeth L, Hickson D, et al. The social patterning of sleep in african Americans: associations of socioeconomic position and neighborhood characteristics with sleep in the jackson heart study. Sleep 2016;39(9):1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pabayo R, Molnar BE, Street N, et al. The relationship between social fragmentation and sleep among adolescents living in Boston, Massachusetts. J Public Health (Oxf) 2014;36(4):587–98. [DOI] [PubMed] [Google Scholar]
- 82.Billings ME, Johnson DA, Simonelli G, et al. Neighborhood walking environment and activity level are associated with OSA: the multi-ethnic study of atherosclerosis. Chest 2016;150(5):1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Desantis AS, Diez Roux AV, Moore K, et al. Associations of neighborhood characteristics with sleep timing and quality: the Multi-Ethnic Study of Atherosclerosis. Sleep 2013;36(10):1543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson BS, Malecki KM, Peppard PE, et al. Exposure to neighborhood green space and sleep: evidence from the Survey of the Health of Wisconsin. Sleep Health 2018;4(5):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev 2007;11(1):35–46. [DOI] [PubMed] [Google Scholar]
- 86.Shaw IR, Lavigne G, Mayer P, et al. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep 2005;28(6):677–82. [DOI] [PubMed] [Google Scholar]
- 87.Cutrufello NJ, lanus VD, Rowley JA. Opioids and sleep. Curr Opin Pulm Med 2020;26(6):634–41. [DOI] [PubMed] [Google Scholar]
- 88.Nordmann S, Lions C, Vilotitch A, et al. A prospective, longitudinal study of sleep disturbance and comorbidity in opiate dependence (the ANRS Methaville study). Psychopharmacology (Berl) 2016;233(7):1203–13. [DOI] [PubMed] [Google Scholar]
- 89.Kay DC. Human sleep during chronic morphine intoxication. Psychopharmacologia 1975;44(2):117–24. [DOI] [PubMed] [Google Scholar]
- 90.Rosenthal M, Moore P, Groves E, et al. Sleep improves when patients with chronic OA pain are managed with morning dosing of once a day extended-release morphine sulfate (AVINZA): findings from a pilot study. J Opioid Manag 2007;3(3):145–54. [DOI] [PubMed] [Google Scholar]
- 91.Alsaadi SM, McAuley JH, Hush JM, et al. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain 2014;30(9):755–65. [DOI] [PubMed] [Google Scholar]
- 92.O’Hagan ET, Hubscher M, Miller CB, et al. Zolpidem reduces pain intensity postoperatively: a systematic review and meta-analysis of the effect of hypnotic medicines on post-operative pain intensity. Syst Rev 2020;9(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eckert DJ, Jordan AS, Merchia P, et al. Central sleep apnea: pathophysiology and treatment. Chest 2007;131(2):595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leino K, Mildh L, Lertola K, et al. Time course of changes in breathing pattern in morphine- and oxycodone-induced respiratory depression. Anaesthesia 1999;54(9):835–40. [DOI] [PubMed] [Google Scholar]
- 95.Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med 2007;3(5):455–61. [PMC free article] [PubMed] [Google Scholar]
- 96.Biot C, Contribution a L’etude du Phenomene respiratorire de Cheyne-Stokes, Lyon Med, 187623517–28. [Google Scholar]
- 97.Correa D, Farney RJ, Chung F, et al. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg 2015;120(6):1273–85. [DOI] [PubMed] [Google Scholar]
- 98.Rose AR, Catcheside PG, McEvoy RD, et al. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. J Clin Sleep Med 2014;10(8):847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eckert DJ, Butler JE. Respiratory physiology: understanding the control of ventilation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th edition. Philadelphia, PA: Elsevier Saunders; 2016. p. 167–73. [Google Scholar]
- 100.Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des 2012;18(37):5994–6004. [DOI] [PubMed] [Google Scholar]
- 101.Badr MS, Toiber F, Skatrud JB, et al. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995;78(5):1806–15. [DOI] [PubMed] [Google Scholar]
- 102.Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eckert DJ. Phenotypic approaches to obstructive sleep apnoea - new pathways for targeted therapy. Sleep Med Rev 2018;37:45–59. [DOI] [PubMed] [Google Scholar]
- 104.Altree TJ, Chung F, Chan MTV, et al. Vulnerability to postoperative complications in obstructive sleep apnea: importance of phenotypes. Anesth Analg 2021;132(5):1328–37. [DOI] [PubMed] [Google Scholar]
- 105.Dutta R, Delaney G, Toson B, et al. A Novel model to estimate key obstructive sleep apnea endotypes from standard polysomnography and clinical data and their contribution to obstructive sleep apnea severity. Ann Am Thorac Soc 2021;18(4):656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wasef S, Mir S, Ryan C, et al. Treatment for patients with sleep apnea on opioids for chronic pain: results of the OpSafe trial. J Clin Sleep Med 2021;17(4):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Ryswyk E, Antic NA. Opioids and sleep-disordered breathing. Chest 2016;150(4):934–44. [DOI] [PubMed] [Google Scholar]
- 108.Javaheri S, Patel S. Opioids cause central and complex sleep apnea in humans and reversal with discontinuation: a plea for detoxification. J Clin Sleep Med 2017;13(6):829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shapiro CM, Chung SA, Wylie PE, et al. Home-use servo-ventilation therapy in chronic pain patients with central sleep apnea: initial and 3-month follow-up. Sleep Breath 2015;19(4):1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farney RJ, McDonald AM, Boyle KM, et al. Sleep disordered breathing in patients receiving therapy with buprenorphine/naloxone. Eur Respir J 2013;42(2):394–403. [DOI] [PubMed] [Google Scholar]
- 111.Javaheri S, Harris N, Howard J, et al. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med 2014;10(6):637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chowdhuri S, Ghabsha A, Sinha P, et al. Treatment of central sleep apnea in U.S. veterans. J Clin Sleep Med 2012;8(5):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Farney RJ, Walker JM, Boyle KM, et al. Adaptive servoventilation (ASV) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med 2008;4(4):311–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Cao M, Cardell CY, Willes L, et al. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med 2014;10(8):855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosen IM, Aurora RN, Kirsch DB, et al. Chronic opioid therapy and sleep: an american academy of sleep medicine position statement. J Clin Sleep Med 2019;15(11):1671–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stein MD, Kurth ME, Sharkey KM, et al. Trazodone for sleep disturbance during methadone maintenance: a double-blind, placebo-controlled trial. Drug Alcohol Depend 2012;120(1–3):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stein MD, Kurth ME, Anderson BJ, et al. A pilot crossover trial of sleep medications for sleep-disturbed methadone maintenance patients. J Addict Med 2020;14(2):126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghaderi A, Banafshe HR, Mirhosseini N, et al. The effects of melatonin supplementation on mental health, metabolic and genetic profiles in patients under methadone maintenance treatment. Addict Biol 2019;24(4):754–64. [DOI] [PubMed] [Google Scholar]
- 119.Atkinson RL, Suratt PM, Wilhoit SC, et al. Naloxone improves sleep apnea in obese humans. Int J Obes 1985;9(4):233–9. [PubMed] [Google Scholar]
- 120.Guilleminault C, Hayes B. Naloxone, theophylline, bromocriptine, and obstructive sleep apnea. Negative results. Bull Eur Physiopathol Respir 1983;19(6):632–4. [PubMed] [Google Scholar]
- 121.Lorier AR, Funk GD, Greer JJ. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS One 2010;5(1):e8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dahan A, van der Schrier R, Smith T, et al. Averting opioid-induced respiratory depression without affecting analgesia. Anesthesiology 2018;128(5):1027–37. [DOI] [PubMed] [Google Scholar]
- 123.Ren J, Poon BY, Tang Y, et al. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med 2006;174(12):1384–91. [DOI] [PubMed] [Google Scholar]
- 124.Zaig S, da Silveira Scarpellini C, Montandon G. Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish. Elife 2021;10:e63407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montandon G, Slutsky AS. Solving the opioid crisis: respiratory depression by opioids as critical End point. Chest 2019;156(4):653–8. [DOI] [PubMed] [Google Scholar]
- 126.Freire C, Pho H, Kim LJ, et al. Intranasal leptin prevents opioid-induced sleep-disordered breathing in obese mice. Am J Respir Cell Mol Biol 2020;63(4):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freire C, Pho H, Bevans-Fonti S, et al. Intranasal leptin improves survival after opioid overdose in a mouse model. J Transl Med 2021;19(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taranto-Montemurro L, Messineo L, Sands SA, et al. The Combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med 2019;199(10):1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim R, Messineo L, Grunstein RR, et al. The noradrenergic agent reboxetine plus the antimuscarinic hyoscine butylbromide reduces sleep apnoea severity: a double-blind, placebo-controlled, randomised crossover trial. J Physiol 2021. [DOI] [PubMed] [Google Scholar]
- 130.Aishah A, Lim R, Sands SA, et al. Different antimuscarinics when combined with atomoxetine have differential effects on obstructive sleep apnea severity J Appl Physiol (1985) 2021;130(5):1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neeland IJ, Eliasson B, Kasai T, et al. The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2020;43(12):3007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]