Abstract
Glycosylation is a sophisticated informational system that controls specific biological functions at both the cellular and organismal level. Dysregulation of glycosylation may underlie some of the most complex and common diseases of the modern era. In the past 5 years, microRNA have come to the forefront as a critical regulator of the glycome. Herein we review the current literature on miRNA regulation of glycosylation and how this work may point to a new way to identify the biological importance of glycosylation enzymes.
Keywords: microRNA, glycomics, glycan, carbohydrate, miR, sugar, glycosylation
INTRODUCTION
Glycosylation is increasingly being revealed as a sophisticated informational system that underlies important biological functions at both the cellular and organismal level1. Glycans, aka carbohydrates or oligosaccharides, are the products of multiple glycosyltransferases and glycosidases working in a coordinated manner to synthesize structures appended to proteins and/or lipids. The importance of glycosylation is perhaps most apparent from the ever increasing number of genetic disorders and genome-wide association studies (GWAS) that point to glycosylation enzymes as causative agents of disease. In recent work, Joshi et al found that glycosylation enzymes implicated in complex diseases by GWAS are highly regulated, arguing that precise control over specific glycans is necessary2. However, how nature keeps these low abundance transcripts tightly controlled is unclear.
In the past 5 years, microRNAs have come to the forefront as critical regulators of the glycome3–5. microRNAs (miRNAs, miRs) are small, non-coding RNAs that bind to messenger RNAs (mRNAs) and regulate mRNA translation into proteins. miRs exit the nucleus as hairpin pre-miRs, which are then cut into 2 mature miRs, the 5p miR (which comes from the 5’ end, miR-5p) and the 3p miR (which is derived from the 3’ end, miR-3p, Figure 1). These miRs can have distinct mRNA targets within the cell. A mature miR is then loaded into a RISC complex where it binds the mRNA. The binding is often, but not exclusively, to the 3’-untranslated region (3’-UTR) and leads to translational repression. In many cases, mRNA degradation also occurs. A single miR can have hundreds of targets. If a glycosylation enzyme or other glycan related protein (e.g. transporters, metabolic enzymes, etc.; with glycosylation enzymes, collectively known as glycogenes) is regulated by a miR, a loss of the concomitant sugar epitope is then observed.
Figure 1.
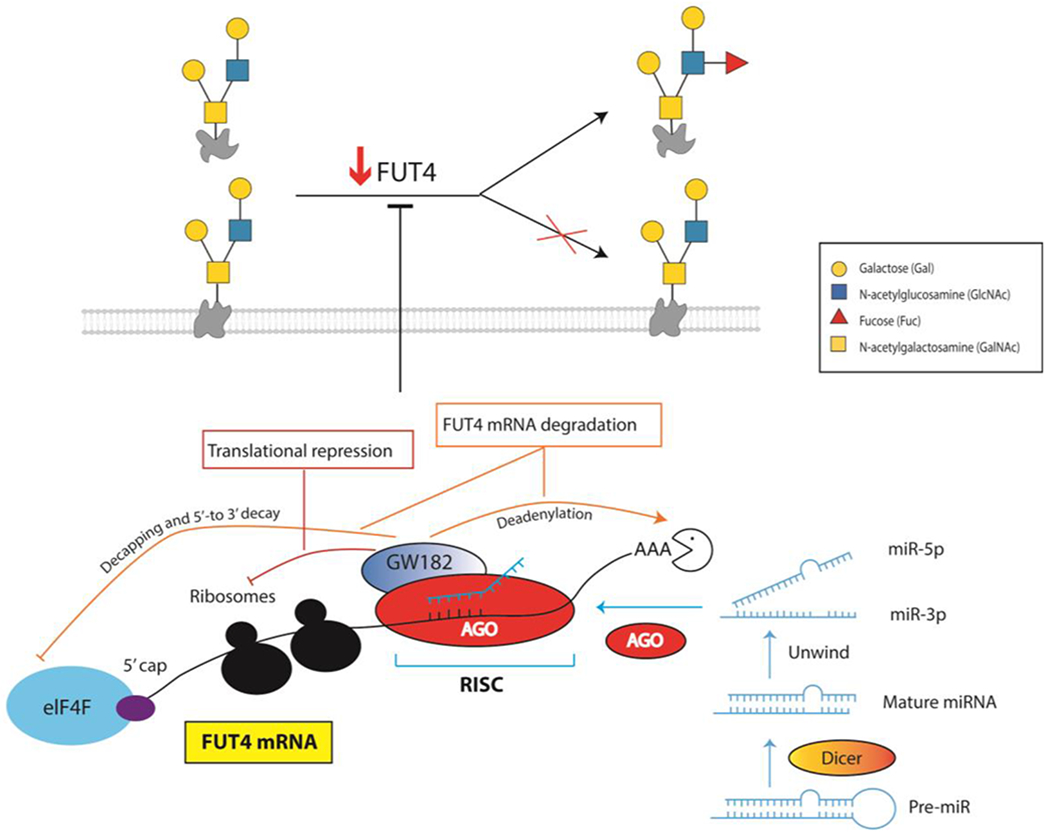
miRs are loaded into RISC complexes and inhibit protein expression through translational repression or mRNA degradation. This impacts glycosylation through repression of glycogenes such as FUT4. Lowered expression of the biosynthetic enzyme would shift the expression of the corresponding glycan epitope, as shown above.
Unlike transcriptional regulators, the role of miRs is not to turn a gene on or off but rather to tune protein expression. Thus, miRs provide a mechanism to maintain tight control of protein levels within a specific window6. This control is dependent on the precise mRNA transcript. Many genes have several transcripts that differ only in their 3’-UTRs and lead to identical proteins. Because networks of miRs act in concert, the biosynthesis of a glycan epitope may be regulated by multiple miRs acting to tune the expression of several glycogenes simultaneously (Figure 2). The predicted glycogene targets of miRs are unevenly distributed. An analysis of miR:mRNA interactions predicted by the miRANDA algorithm identified some glycogene transcripts as highly-regulated, with multiple miR target sites, while others had few predicted sites5. Several glycogenes known to be involved in complex diseases (e.g. FUT87–9, GALNT710–19, and GALNT120–22) were predicted to have highly-regulated transcripts, implying that miRs may play a direct role in dysregulation of these enzymes5. Enzymes previously believed to be “functionally redundant” such as the 20-member GALNT family, show large differences in potential miR regulation, implying that they control different biology. This argument against “redundancy” from the regulatory perspective, is borne out by new work showing that the GALNTs do indeed have distinct functions 23, 24.
Figure 2.
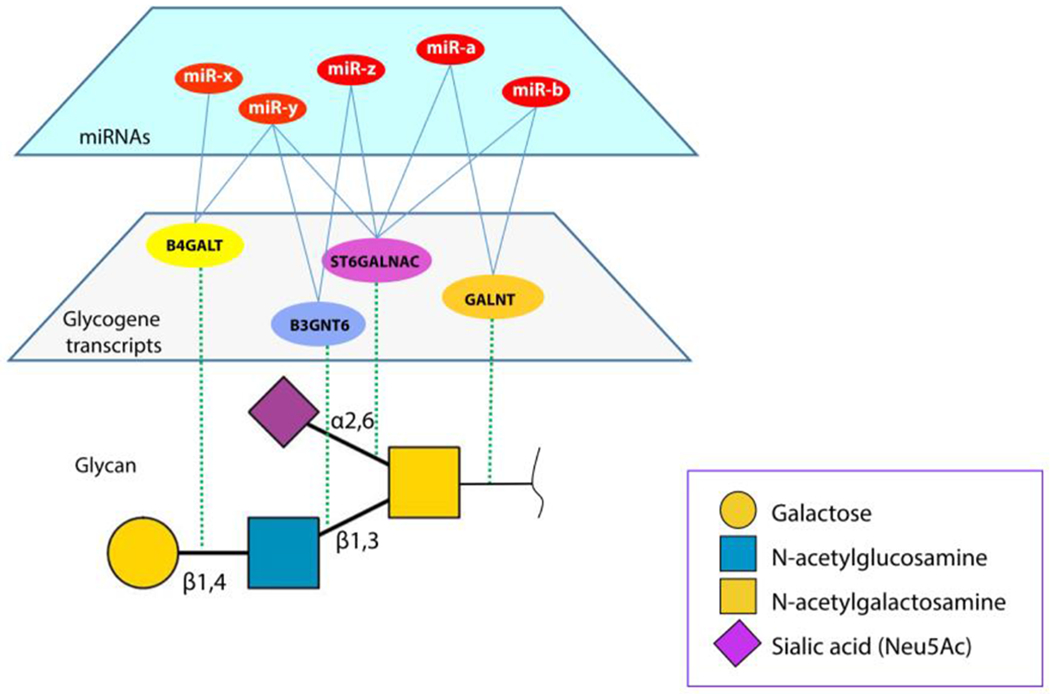
miRs can regulate multiple glycogenes in a network, modulating glycan structures.
MICRORNAS ARE CRITICAL REGULATORS OF THE GLYCOME
A study in Caenorhabditis elegans (C. elegans) by Han and coworkers in 2009 was one of the earliest to show glycosylation as a major target of miRs25. In this work, they characterized the miRs found in RISC complexes during worm development. They found that miR targets were enriched in signaling proteins, while housekeeping genes were under-represented. In addition, gene transcripts involved in glycosylation pathways were highly enriched in the pool of strong miR targets. They demonstrated that appending the 3’-UTR of one of the enriched glycogenes, sqv-3, was enough to repress expression of GFP in larval stages where this transcript was observed in the RISC complexes. To our knowledge, this work was the first example of glycogene regulation by a miR and supported a major role for miRs in the regulation of glycan biosynthesis.
In 2014, work from our laboratory directly demonstrated a critical role for miRs in the regulation of glycosylation in human cells4. Using bioinformatic methods we integrated miR profiles of the NCI-60, a 59 human cancer cell line panel, with glycomic analysis obtained using our lectin microarray approach26, 27. We identified multiple miRs that correlated with specific glycosylation patterns. These miRs directly targeted the transcripts of glycogenes underlying the observed glycans and were able to alter the glycosylation of cells. Our work underscored the important role of miRs in controlling the glycome. At the time of this publication in 2014, only 10 glycogenes were known targets of miRs. In the past 5 years, there has been an explosion of interest in miR regulation of glycogenes and over 80 glycogenes are currently known miR targets (Table 1). Herein, we discuss select examples of glycome regulation by miRs and the potential of miRs to identify the biological functions of specific glycosylation motifs.
Table 1.
List of known miR regulators for human glycogenes organized by pathway. The HUGO Genome Nomenclature Committee (HGNC) symbol is given for each gene along with the nomenclature used in the accompanying literature cited. For the miRs, Designations for -5p and- 3p are noted where specified in reference.
| Pathway | Gene Symbol (HGNC) | Alternative symbols used in literature | miRNAs |
|---|---|---|---|
| O-GlcNAc | OGT | O-GLCNAC, HRNT1, MGC22921, FLJ23071, OGT1 | hsa-miR-485-5p36, 39, hsa-miR-10135, hsa-miR-48337, hsa-miR-200a/200b-3p38, hsa-miR-24-140, hsa-miR-42442, hsa-miR-423-5p44, hsa-miR-746 |
| OGA | MGEA5, MEA5, NCOAT | hsa-miR-53947 | |
| N-linked pathway | |||
| Glycosyltransferases | RPN2 | SWP1, RPNII, RIBIIR, RPN-II | hsa-miR-12876, hsa-miR-37877 |
| ALG3 | NOT56L, Not56, CDGS4, D16Ertd36e | hsa-miR-34278 | |
| ALG12 | ECM39, CDG1G | hsa-miR-147a79 | |
| ALG13 | GLT28D1, CXorf45, CDG1S | hsa-miR-34a80 | |
| FUT8 | hsa-miR-1229, hsa-miR-34a9, hsa-miR-26a53, has-miR-26b53, hsa-miR-146a8, hsa-miR-198 69 | ||
| MGAT3 | GNT-III | hsa-miR-23a 81 | |
| MGAT4A | GnT-Iva, GnT-4a | hsa-miR-42442, hsa-let-7c50 | |
| Glycosidases | EDEM1 | KIAA0212, EDEM | hsa-miR-211 82, hsa-miR-581 83, 84, hsa-miR-204 83, 84 |
| MAN1A2 | MAN1B | hsa-miR-30c, hsa-miR-3614 | |
| MAN1B1 | hsa-miR-125b85, 86 | ||
| MANEA | FLJ12838 | hsa-miR-120287 | |
| O-linked pathway | |||
| Initiation | GALNT1 | GalNAc-T1 | hsa-miR-216b21, hsa-miR-30b/30d10, hsa-miR-10a88, hsa-miR-12920 |
| GALNT2 | GalNAc-T2 | hsa-let-7b89 | |
| GALNT3 | GalNAc-T3, HHS, HFTC | hsa-miR-26a90, hsa-miR-17-3p and hsa-miR-22191 | |
| GALNT4 | GalNAc-T4 | hsa-miR-426292, hsa-miR-993, hsa-miR-36594 | |
| GALNT5 | GalNAc-T5 | hsa-miR-196b-5p95 | |
| GALNT7 | GalNAc-T7 | hsa-miR-15418, hsa-miR-21411, 17, hsa-miR-30a-5p16, hsa-miR-49414, 15, hsa-miR-34a/c13, hsa-miR-17-3p/5p12, hsa-miR-21411, 17,hsa-miR-30b/30d10, hsa-miR-378 10–19 | |
| GALNT10 | GalNAc-T10 | hsa-miR-12296 | |
| GALNT13 | GalNAc-T13, KIAA1918 | hsa-miR-42442 |
|
| GALNT14 | GalNAc-T14, FLJ12691 | hsa-miR-125a97 | |
| TMTC2 | DKFZp762A217 | hsa-miR-14298 | |
| POGLUT1 | KDELCL1, MDS010, MDS010, MGC32995, 9630046K23Rik, MDSRP, hCLP46, Rumi | hsa-miR-13499, hsa-miR-14299, 100 | |
| Elongation and Branching | B3GAT3 | GlcAT-I | hsa-miR-23b101 |
| B3GLCT | B3GALTL | hsa-miR-200b, hsa-miR-200c, hsa-miR-4293 | |
| B3GNT5 | B3GN-T5, beta3Gn-T5 | hsa-miR-203102 | |
| C1GALT1 | C1GALT, T-synthase | hsa-miR-148b103 | |
| C1GALT1C1 | COSMC, C1GALT2 | hsa-miR-320104, hsa-miR-155105, hsa-miR-374b106 | |
| GCNT2 | NACGT1, II, GCNT5, CCAT, IGNT, NAGCT1, bA421M1.1, bA360O19.2, ULG3 | hsa-miR-199a/b-5p 107 | |
| GCNT3 | C2GnT-M, C2/4GnT, C2GnT2 | hsa-miR-302b-3p 108, hsa-miR-15b 109 | |
| LFNG | SCDO3 | hsa-miR-200f 110, hsa-miR-125a-5p 111, 112, hsa-miR-146a 113 | |
| Capping | |||
| PolyLacNAc | B3GALT5 | beta3Gal-T5, B3GalT-V, GLCT5, B3T5 | hsa-miR-203114 |
| B4GALT1 | GGTB2 | hsa-miR-124-3p115 | |
| Sialylation | ST3GAL3 | ST3Gal III, SIAT6, MRT12 | hsa-miR-200a116 |
| ST3GAL4 | STZ, SAT3, FLJ11867, CGS23, SIAT4, NANTA3, SIAT4C | hsa-miR-200a116, hsa-miR-370113 | |
| ST3GAL5 | SIAT9, ST3GalV, SIATGM3S | hsa-miR-26a117, hsa-miR-548l117, hsa-miR-34a117, hsa-miR-200b3, hsa-miR-200c3, hsa-miR-4293 | |
| ST3GAL6 | SIAT10, ST3GALVI | hsa-miR-26a118, 119 | |
| ST6GAL1 | SIAT1, ST6Gal I | hsa-miR-9120 | |
| ST6GALNAC1 | SIAT7A, ST6GalNAcI | hsa-miR-30d-5p121 | |
| ST6GALNAC2 | SIAT7, SIAT7B, SIATL1 | hsa-miR-182122, 123, hsa-miR-135b122, 123 | |
| ST6GALNAC4 | SIAT7D, ST6GALNACIV, SIAT3C | hsa-miR-4299124 | |
| ST6GALNAC5 | SIAT7E, MGC3184, ST6GalNAcV | hsa-miR-200b, hsa-miR-200c, hsa-miR-4293 | |
| ST8SIA1 | SIAT8, SIAT8A | hsa-miR-33a,hsa- let-7e125 | |
| ST8SIA2 | SIAT8B, STX, ST8SIA-II, HsT19690 | hsa-miR-3099126 | |
| ST8SIA4 | SIAT8D, ST8Sia IV | hsa-miR-26a/26b127, hsa-miR-146a/146b128, hsa-miR-181c128 | |
| Fucosylation | FUT1 | H, HSC | hsa-miR-140-5p129, hsa-miR-149 129, hsa-miR-34a 130 |
| FUT2 | SE, sej, Se2, SEC2 | hsa-miR-15b 131 | |
| FUT4 | CD15, FUC-TIV, FCT3A, ELFT | hsa-miR-125a-5p 129, hsa-miR-26a/26b 53, 56, hsa-miR-200c 55, hsa-miR-200b 55, hsa-miR-493-5p 52, hsa-miR-224-3p 51 | |
| FUT5 | FUC-TV | hsa-miR-125a-3p 132 | |
| FUT6 | FT1A, FCT3A, FucT-VI, FLJ40754 | hsa-miR-326 133, hsa-miR-125a-3p 132, hsa-miR-106b 133 | |
| FUT8* | See above in N-linked pathway | ||
| GAG related enzymes | |||
| Chondroitin Sulfate Synthetases | CHSY1 | KIAA0990, CSS1 |
has-miR-194, hsa-miR-515134 |
| CHPF | CSS2, CHSY2 | has-miR-194, hsa-miR-515134 | |
| CHSY3 | CSS3, CHSY-2 | has-miR-194, hsa-miR-515134 | |
| Glucuronyl acid epimerase | GLCE | KIAA0836, HSEPI | hsa-miR-218135 |
| Sulfotransferases/sulfatases | CHST3 | C6ST, C6ST1 | hsa-miR-513a-5p 136 |
| HS3ST2 | 3OST2 | hsa-miR-100 137 | |
| HS6ST2 | hsa-miR-141-3p, hsa-miR-145-5p 138 | ||
| NDST1 | HSST, NST1 | hsa-miR-149139, hsa-miR-24139, hsa-miR-191139 | |
| SULF1 | KIAA1077, SULF-1, hSulf-1 | hsa-miR-21140 | |
|
Hyaluronan synthetases |
HAS1 | HAS | hsa-miR-125a141, hsa-miR-214141 |
| HAS2 | hsa-miR-410 (up-regulating) 142, hsa-miR-7143, hsa-miR-26b144, hsa-miR-378144, hsa-miR-23a-3p 145, hsa-miR-424/424*146, hsa-miR-23147, hsa-miR-574147, hsa-miR-101-3p148 | ||
| HAS3 | hsa-miR-26a-5p149, hsa-miR-29a-3p150 | ||
| Others | |||
|
Glycosidases |
FUCA2 | MGC1314, dJ20N2.5 | hsa-miR-145151, hsa-miR-200b, hsa-miR-200c, hsa-miR-429 4 |
| GALC | hsa-miR-140-5p152 | ||
| GBA | GLUC, GBA1 | hsa-miR-22-3p153 | |
| NEU1 | NEU | hsa-miR-125b154 | |
| HEXB | hsa-miR-207, hsa-miR-352 155 | ||
|
Nucleotide Sugar Metabolism |
PMM2 | CDG1, CDGS, CDG1a, PMI, PMI1 | hsa-miR-451a156, 157 |
| TSTA3 | FX, P35B, SDR4E1 | hsa-miR-125a-5p, hsa-miR-125b158 | |
| CMAHP | CMAH | hsa-miR-155-5p, hsa-miR-425-5p, hsa-miR-15a-5p, hsa-miR-503-5p, hsa-miR-16-5p, hsa-miR-29a-3p, and hsa-miR-29b-3p159 | |
| UAP1 | SPAG2, AGX1, AgX | hsa-miR-224-5p 160 | |
|
Nucleotide sugar transporters
|
SLC35B2 | PAPST1, UGTrel4 | hsa-miR-22161 |
| SLC35B4 | FLJ14697, YEA4 | hsa-miR-1764, hsa-miR-1700162 | |
| SLC35F5 | FLJ22004 | hsa-miR-369-3p 163 | |
| UDP-Glucuronyltransferases (involved in Drug Metabolism) | UGT2B15 | UGT2B8 | hsa-miR-331-5p164, 165, hsa-miR-376c 166, hsa-miR-770-5p 165, hsa-miR-103b 165, hsa-miR-3924 165, hsa-miR-376b-3p 165, hsa-miR-455-5p, 165 hsa-miR-605 165, hsa-miR-624-3p 165, hsa-miR-4712-5p 165, hsa-miR-3675-3p 165, hsa-miR-6500-5p 165, hsa-miR-548as-3p 165, hsa-miR-4292 165 |
| UGT2B17 | hsa-miR-376c 166 | ||
| UGT2B7 | UGT2B9 | hsa-miR-1293, hsa-miR-3664-3p, hsa-miR-4317, hsa-miR-513c-3p, hsa-miR-4483, and hsa-miR-142-3p 164, 167 | |
|
Other Glycosylation Related Proteins |
COG6 | COD2, KIAA1134 | hsa-miR-1 168 |
| KL (Klotho) | hsa-miR-34a169, hsa-miR-199b-5p170, 171, hsa-miR-504172, hsa-miR-339172, hsa-miR-556173 | ||
| SPOCK1 | TIC1, SPOCK, testican-1 | hsa-miR-150-3p/5p 174, 175, hsa-miR-129-5p 174, hsa-miR-585 175 | |
| SPOCK3 | testican-3 | hsa-miR-145176 |
MICRORNA REGULATION OF O-GLCNAC
Unlike canonical O-glycosylation, O-GlcNAc is a dynamic glycan modification found on cytoplasmic, mitochondrial and nuclear proteins28. This modification is controlled by two enzymes, O-GlcNAc transferase (OGT) and the glycosidase, OGA. O-GlcNAc modifies a diverse set of proteins including histones, transcription factors and signaling proteins and is involved in many biological processes from cell cycle to Alzheimer’s29–31 to obesity32, 33. OGT expression is tightly controlled at both protein and mRNA levels34 and is predicted to be highly-regulated by miRs5. There are 11 known mRNA transcripts for OGT, and 3 protein isoforms. To date, work has focused on the most abundant OGT transcript (ID ENST00000373719.8) an mRNA of the nucleocytoplamic proteoform. In recent years, 10 miR:mRNA interactions have been identified for this transcript of OGT, regulating the enzyme in a variety of diseases from cancer to cardiovascular disease 35–46. Several of the miRs identified to hit OGT directly impact cell proliferation and are involved in cancer progression. Examples include miR-483 in gastric cancer and miR-485-5p in esophageal and colorectal cancers36, 37. Other identified functions of miRs targeting OGT include reducing tumor angiogenesis (miR-746), inhibiting cell invasion (miR-2441), and modulating glucose-induced inflammation (miR-200a/b38).
In contrast to OGT, OGA is not predicted to be a highly regulated gene. To date, only one miR:mRNA interaction has been identified for this glycogene. miR-539 is up-regulated in the failing heart, and targets OGA, increasing O-GlcNAcylation during heart failure47. O-GlcNAcylation is highly dynamic in the heart, as are the transcript levels of both OGT and OGA. miR-24, which is involved in cardiovascular function has also been shown to modulate OGT48, although not in the context of cardiovascular disease. Currently we still have a limited understanding of the regulation of OGT and OGA, but it is increasingly clear that miRs may play a strong role in the dynamic expression of these enzymes and the resulting O-GlcNAcylation levels. Given the critical importance of O-GlcNAcylation to a wide variety of diseases, miR regulation of these enzymes warrants a more thorough examination.
MICRORNA REGULATION OF N-LINKED GLYCOSYLATION PATHWAY
The N-linked biosynthetic pathway begins with the biosynthesis of Glc3Man9GlcNAc2-dolichol primarily by the ALG genes. Bioinformatic analysis of these genes show 2, ALG13 and ALG9, are predicted to be highly-regulated. ALG13, a known epilepsy-related gene49, is targeted by miR-34a in neuroblastoma cells. Currently there are no known miR targets for ALG9. The Glc3Man9GlcNAc2 moiety is subsequently transferred onto asparagines in an Asn-X-Ser/Thr motif, where X is any amino acid except proline (Pro), the glucoses are removed by glucosidases as part of the protein quality control system to form the Man9GlcNAc2 structure (Man9). Mannosidases then trim the subsequent high mannose structures culminating in Man5GlcNAc2 (Man5). The mannosidases MANEA, MAN1A1 and MAN1A2 are highly regulated by miRs based on prediction5. Of these genes, miR regulation has been found for MANEA and MAN1A2. Interestingly, the high mannose epitope, levels of which are controlled by these genes, is emerging as a signaling molecule in infection and inflammation49.
Man5 is further elaborated and trimmed by a variety of enzymes to make hybrid and complex N-glycans. Only select enzymes within this pathway are predicted to be highly-regulated by miRs, including MGAT2, MGAT4A and FUT8 (which controls core fucosylation). Currently miR:mRNA interactions have been validated for MGAT4A42, 50 and FUT851–57. MGAT4A catalyzes the transfer of GlcNAc to the biantennary (or tri-antennary) core structure of N-linked oligosaccharides to form a β1-4 linkage on the Manα1,3 arm (Figure 3). In recent work, miR-424 was shown to directly repress MGAT4A expression42. This miR inhibits cell cycle progression and was thought to mediate that effect through repression of CCDN1 and CDC25A, known cell cycle promoters. Downregulation of MGAT4A expression was also sufficient to inhibit cell cycle progression, indicating that this substructure is essential to intra- and inter-cellular interactions that maintain cell growth. Core fucosylation, which is controlled by FUT8, is dysregulated in many diseases including melanoma metastasis7, lung adenocarcinoma58, liver cancer8, 9 and emphysema59–63. Increasingly, this modification is found to have a direct role in promoting disease59, 60, 64–68. Currently there are five miRs known to target FUT8, all of which have roles in tumor progression8, 9, 69. Again, our knowledge of the miR regulation of the N-glycan pathway is woefully incomplete, but a picture is emerging in which glycogenes that are highly regulated are often involved in complex disease states, in line with the hypothesis of Joshi et al2.
Figure 3.
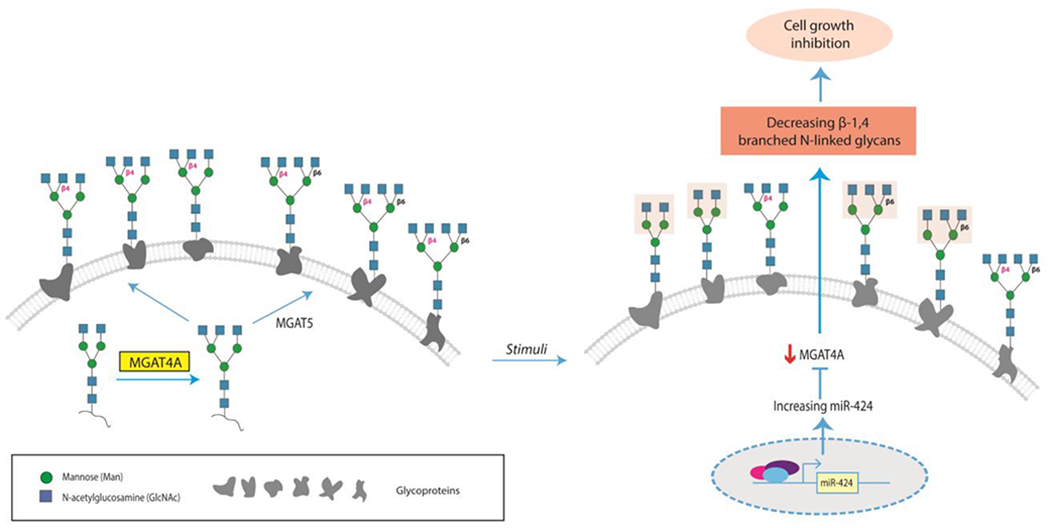
Regulation of MGAT4A, and corresponding β-1,4 branching, by miR-424-5p as an example of how miR modulation of glycan structures can drive a biological state (cell growth) 42. Structures showing loss of β-1,4 branching are highlighted.
MICRORNA PROXY HYPOTHESIS AND APPLICATION TO GLYCOSYLATION
In one of the earliest examples of glycan regulation by miRs, Hernando and coworkers identified the GALNT7 as a target for miR-30d, a microRNA that promoted melanoma metastasis in patients and mouse models. Downregulation of GALNT7 was found to phenocopy miR-30d, increasing metastasis as a result of inhibiting O-glycosylation10. This showcases a common theme in miR biology, namely that downregulation of the targets of a miR phenocopies the effects of miR expression. This observation led us to propose the microRNA proxy hypothesis. Our hypothesis states that the regulation of protein expression by changes in the expression levels of miRs identifies proteins holding a privileged position in driving the underlying biology. In other words, if a miR drives a specific biological phenotype, such as migration or metastasis, the targets of that miR will drive the same biological phenotype. Thus, miRs can be used to identify (by proxy) the biological functions of specific glycosylation enzymes (or other proteins). We first formulated and tested this powerful hypothesis in a publication in 20153. In that work, we examined the targets of miR-200b-3p, a miR that controls epithelial to mesenchymal transition (EMT). This miR is high in epithelial cells and low in mesenchymal cells. We identified 5 targets of miR-200b-3p and tested 3 of them to see whether inhibiting the expression of these enzymes would phenocopy overexpression of the miR. In all 3 cases (B3GLCT, ST3GAL5 and ST6GALNAC5), mesenchymal cells reverted to an epithelial state upon repression of these glycosylation enzymes. This phenotype was not transduced by repression of the transcription factor ZEB1, another target of miR-200b-3p commonly thought to be responsible for the EMT phenotype. Instead knockdown of all 3 glycogenes caused increases in ZEB1 levels, arguing that inhibiting glycosylation can alter EMT independent of the transcription factor. This provides evidence that miRs target key hubs driving the biological phenotypes that they regulate, in line with our hypothesis. A further example of using a known miR phenotype to identify glycan function comes from the aforementioned MGAT4A work42. Here, a role in cell cycle was found.
To date, all of the work examining our hypothesis has focused on single miRs. This is because currently the prediction algorithms for miR:mRNA interactions are inaccurate70, 71. This inaccuracy may stem from the use of transcriptional data in creating the algorithms. Although commonly used as a metric of protein abundance, transcriptional data is not accurate to the proteome 72. A recent analysis of the agreement between protein expression and mRNA expression levels using data from the Human Proteome Map and Genotype-Tissue expression project found strong concordance in the expression levels for only 6.1% of genes73. For low abundance proteins, such as glycogenes, it is known that transcription levels are not accurate to protein abundance 74. All miR interactions impact translation, and at best only ~80% of interactions impact the transcriptome75. This may be lower for low abundance genes, where transcriptional data is inherently more noisy. Thus, reliance on the transcriptome may bias current algorithms. At present, studies into miR:mRNA interactions requires that each interaction be validated by luciferase assay. If one were to study multiple miRs that co-regulate a biological phenotype, this would then require tens to hundreds of luciferase assays to validate interactions and identify a common target set. Improving the prediction algorithms for miR regulation of glycogenes, and other gene sets, is crucial for testing our hypothesis and truly understanding miR regulation of protein expression.
CONCLUSIONS
Glycosylation enzymes that are more tightly regulated appear to be more prevalent in controlling underlying complex disease states. microRNA is an emerging regulator of glycosylation, helping to provide fine tuning for the low abundance glycan biosynthetic enzymes. Given the emerging importance of miRs in disease and their potential to identify genes that underlie specific biological function, it is clear that more attention should be paid to miR:glycogene interactions.
ACKNOWLEDGMENT
This work was supported by the NIH Common Fund (NIH Grant; U01CA221229)
Funding Sources
This work was supported by the NIH Common Fund (NIH Grant; U01CA221229)
ABBREVIATIONS
- EMT
epithelial-mesesnchymal transition
- Fuc
fucose
- Gal
galactose
- GWAS
genome-wide association study
- Glc
glucose
- GFP
green fluorescent protein
- Man
mannose
- miRNA, miR
microRNA
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- OGA
O-GlcNAcase
- OGT
O-GlcNAcyltransferase
- RISC
RNA induced silencing complex
- Neu5Ac
sialic acid
- UTR
un-translated region
REFERENCES
- [1].Varki A, Kannagi R, Toole B, and Stanley P (2015) Glycosylation Changes in Cancer, In Essentials of Glycobiology (Varki A rd, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, and Seeberger PH, Eds.), pp 597–609, Cold Spring Harbor; (NY: ). [Google Scholar]
- [2].Joshi HJ, Hansen L, Narimatsu Y, Freeze HH, Henrissat B, Bennett E, Wandall HH, Clausen H, and Schjoldager KT (2018) Glycosyltransferase genes that cause monogenic congenital disorders of glycosylation are distinct from glycosyltransferase genes associated with complex diseases, Glycobiology 28, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kurcon T, Liu Z, Paradkar AV, Vaiana CA, Koppolu S, Agrawal P, and Mahal LK (2015) miRNA proxy approach reveals hidden functions of glycosylation, Proc. Natl. Acad. Sci. U. S. A 112, 7327–7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Agrawal P, Kurcon T, Pilobello KT, Rakus JF, Koppolu S, Liu Z, Batista BS, Eng WS, Hsu KL, Liang Y, and Mahal LK (2014) Mapping posttranscriptional regulation of the human glycome uncovers microRNA defining the glycocode, Proc. Natl. Acad. Sci. U. S. A 111, 4338–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kasper BT, Koppolu S, and Mahal LK (2014) Insights into miRNA regulation of the human glycome, Biochem. Biophys. Res. Commun 445, 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmiedel JM, Klemm SL, Zheng Y, Sahay A, Bluthgen N, Marks DS, and van Oudenaarden A (2015) Gene expression. MicroRNA control of protein expression noise, Science 348, 128–132. [DOI] [PubMed] [Google Scholar]
- [7].Agrawal P, Fontanals-Cirera B, Sokolova E, Jacob S, Vaiana CA, Argibay D, Davalos V, McDermott M, Nayak S, Darvishian F, Castillo M, Ueberheide B, Osman I, Fenyo D, Mahal LK, and Hernando E (2017) A Systems Biology Approach Identifies FUT8 as a Driver of Melanoma Metastasis, Cancer Cell 31, 804–819 e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheng L, Gao S, Song X, Dong W, Zhou H, Zhao L, and Jia L (2016) Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs, Oncotarget 7, 61199–61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bernardi C, Soffientini U, Piacente F, and Tonetti MG (2013) Effects of microRNAs on fucosyltransferase 8 (FUT8) expression in hepatocarcinoma cells, PLoS One 8, e76540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, Rakus JF, Dankert JF, Shang S, Kerbel RS, Bhardwaj N, Shao Y, Darvishian F, Zavadil J, Erlebacher A, Mahal LK, Osman I, and Hernando E (2011) miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis, Cancer Cell 20, 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peng RQ, Wan HY, Li HF, Liu M, Li X, and Tang H (2012) MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7, J. Biol. Chem 287, 14301–14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shan SW, Fang L, Shatseva T, Rutnam ZJ, Yang X, Du W, Lu WY, Xuan JW, Deng Z, and Yang BB (2013) Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways, J. Cell Sci 126, 1517–1530. [DOI] [PubMed] [Google Scholar]
- [13].Li W, Ma H, and Sun J (2014) MicroRNA34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression, Mol Med Rep 9, 1293–1298. [DOI] [PubMed] [Google Scholar]
- [14].Duan HF, Li XQ, Hu HY, Li YC, Cai Z, Mei XS, Yu P, Nie LP, Zhang W, Yu ZD, and Nie GH (2015) Functional elucidation of miR-494 in the tumorigenesis of nasopharyngeal carcinoma, Tumour Biol. 36, 6679–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nie GH, Luo L, Duan HF, Li XQ, Yin MJ, Li Z, and Zhang W (2016) GALNT7, a target of miR-494, participates in the oncogenesis of nasopharyngeal carcinoma, Tumour Biol. 37, 4559–4567. [DOI] [PubMed] [Google Scholar]
- [16].Li Y, Li Y, Chen D, Jin L, Su Z, Liu J, Duan H, Li X, Qi Z, Shi M, Ni L, Yang S, Gui Y, Mao X, Chen Y, and Lai Y (2016) miR30a5p in the tumorigenesis of renal cell carcinoma: A tumor suppressive microRNA, Mol Med Rep 13, 4085–4094. [DOI] [PubMed] [Google Scholar]
- [17].Lu Q, Xu L, Li C, Yuan Y, Huang S, and Chen H (2016) miR-214 inhibits invasion and migration via downregulating GALNT7 in esophageal squamous cell cancer, Tumour Biol. 37, 14605–14614. [DOI] [PubMed] [Google Scholar]
- [18].Niu JT, Zhang LJ, Huang YW, Li C, Jiang N, and Niu YJ (2018) MiR-154 inhibits the growth of laryngeal squamous cell carcinoma by targeting GALNT7, Biochem. Cell Biol 96, 752–760. [DOI] [PubMed] [Google Scholar]
- [19].Kahai S, Lee SC, Lee DY, Yang J, Li M, Wang CH, Jiang Z, Zhang Y, Peng C, and Yang BB (2009) MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7, PLoS One 4, e7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dyrskjot L, Ostenfeld MS, Bramsen JB, Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL, Andersen CL, Zieger K, Kauppinen S, Ulhoi BP, Kjems J, Borre M, and Orntoft TF (2009) Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro, Cancer Res. 69, 4851–4860. [DOI] [PubMed] [Google Scholar]
- [21].Shan Y, Ma J, Pan Y, Hu J, Liu B, and Jia L (2018) LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1, Cell Death Dis. 9, 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakagawa Y, Nishikimi T, Kuwahara K, Fujishima A, Oka S, Tsutamoto T, Kinoshita H, Nakao K, Cho K, Inazumi H, Okamoto H, Nishida M, Kato T, Fukushima H, Yamashita JK, Wijnen WJ, Creemers EE, Kangawa K, Minamino N, Nakao K, and Kimura T (2017) MiR30-GALNT1/2 Axis-Mediated Glycosylation Contributes to the Increased Secretion of Inactive Human Prohormone for Brain Natriuretic Peptide (proBNP) From Failing Hearts, J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schjoldager KT, Joshi HJ, Kong Y, Goth CK, King SL, Wandall HH, Bennett EP, Vakhrushev SY, and Clausen H (2015) Deconstruction of O-glycosylation--GalNAc-T isoforms direct distinct subsets of the O-glycoproteome, EMBO Rep 16, 1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hintze J, Ye Z, Narimatsu Y, Madsen TD, Joshi HJ, Goth CK, Linstedt A, Bachert C, Mandel U, Bennett EP, Vakhrushev SY, and Schjoldager KT (2018) Probing the contribution of individual polypeptide GalNAc-transferase isoforms to the O-glycoproteome by inducible expression in isogenic cell lines, J. Biol. Chem 293, 19064–19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang L, Hammell M, Kudlow BA, Ambros V, and Han M (2009) Systematic analysis of dynamic miRNA-target interactions during C. elegans development, Development 136, 3043–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pilobello KT, Krishnamoorthy L, Slawek D, and Mahal LK (2005) Development of a lectin microarray for the rapid analysis of protein glycopatterns, Chembiochem 6, 985–989. [DOI] [PubMed] [Google Scholar]
- [27].Pilobello KT, Slawek DE, and Mahal LK (2007) A ratiometric lectin microarray approach to analysis of the dynamic mammalian glycome, Proc. Natl. Acad. Sci. U. S. A 104, 11534–11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bond MR, and Hanover JA (2015) A little sugar goes a long way: the cell biology of O-GlcNAc, J. Cell Biol 208, 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhu Y, Shan X, Yuzwa SA, and Vocadlo DJ (2014) The emerging link between O-GlcNAc and Alzheimer disease, J. Biol. Chem 289, 34472–34481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hart GW (2019) Nutrient regulation of signaling and transcription, J. Biol. Chem 294, 2211–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yuzwa SA, and Vocadlo DJ (2014) O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer’s disease and beyond, Chem. Soc. Rev 43, 6839–6858. [DOI] [PubMed] [Google Scholar]
- [32].Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, and Yang X (2014) O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat, Cell 159, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li MD, Vera NB, Yang Y, Zhang B, Ni W, Ziso-Qejvanaj E, Ding S, Zhang K, Yin R, Wang S, Zhou X, Fang EX, Xu T, Erion DM, and Yang X (2018) Adipocyte OGT governs diet-induced hyperphagia and obesity, Nat Commun 9, 5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qian K, Wang S, Fu M, Zhou J, Singh JP, Li MD, Yang Y, Zhang K, Wu J, Nie Y, Ruan HB, and Yang X (2018) Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer, J. Biol. Chem 293, 13989–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang M, Xu B, Li X, Shang Y, Chu Y, Wang W, Chen D, Wu N, Hu S, Zhang S, Li M, Wu K, Yang X, Liang J, Nie Y, and Fan D (2019) O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit, Oncogene 38, 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Han DL, Wang LL, Zhang GF, Yang WF, Chai J, Lin HM, Fu Z, and Yu JM (2019) MiRNA-485-5p, inhibits esophageal cancer cells proliferation and invasion by down-regulating O-linked N-acetylglucosamine transferase, Eur. Rev. Med. Pharmacol. Sci 23, 2809–2816. [DOI] [PubMed] [Google Scholar]
- [37].Yu FY, Zhou CY, Liu YB, Wang B, Mao L, and Li Y (2018) miR-483 is down-regulated in gastric cancer and suppresses cell proliferation, invasion and protein O-GlcNAcylation by targeting OGT, Neoplasma 65, 406–414. [DOI] [PubMed] [Google Scholar]
- [38].Lo WY, Yang WK, Peng CT, Pai WY, and Wang HJ (2018) MicroRNA-200a/200b Modulate High Glucose-Induced Endothelial Inflammation by Targeting O-linked N-Acetylglucosamine Transferase Expression, Front. Physiol 9, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chai Y, Du Y, Zhang S, Xiao J, Luo Z, He F, and Huang K (2018) MicroRNA-485-5p reduces O-GlcNAcylation of Bmi-1 and inhibits colorectal cancer proliferation, Exp. Cell Res 368, 111–118. [DOI] [PubMed] [Google Scholar]
- [40].Liu Y, Huang H, Liu M, Wu Q, Li W, and Zhang J (2017) MicroRNA-24-1 suppresses mouse hepatoma cell invasion and metastasis via directly targeting O-GlcNAc transferase, Biomed. Pharmacother 91, 731–738. [DOI] [PubMed] [Google Scholar]
- [41].Liu Y, Huang H, Cao Y, Wu Q, Li W, and Zhang J (2017) Suppression of OGT by microRNA24 reduces FOXA1 stability and prevents breast cancer cells invasion, Biochem. Biophys. Res. Commun 487, 755–762. [DOI] [PubMed] [Google Scholar]
- [42].Vaiana CA, Kurcon T, and Mahal LK (2016) MicroRNA-424 Predicts a Role for beta-1,4 Branched Glycosylation in Cell Cycle Progression, J. Biol. Chem 291, 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Murashov AK, Pak ES, Koury M, Ajmera A, Jeyakumar M, Parker M, Williams O, Ding J, Walters D, and Neufer PD (2016) Paternal long-term exercise programs offspring for low energy expenditure and increased risk for obesity in mice, FASEB J 30, 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Luo P, He T, Jiang R, and Li G (2015) MicroRNA-423–5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes, Mol Med Rep 12, 1163–1168. [DOI] [PubMed] [Google Scholar]
- [45].Solary E, Bernard OA, Tefferi A, Fuks F, and Vainchenker W (2014) The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases, Leukemia 28, 485–496. [DOI] [PubMed] [Google Scholar]
- [46].Babae N, Bourajjaj M, Liu Y, Van Beijnum JR, Cerisoli F, Scaria PV, Verheul M, Van Berkel MP, Pieters EH, Van Haastert RJ, Yousefi A, Mastrobattista E, Storm G, Berezikov E, Cuppen E, Woodle M, Schaapveld RQ, Prevost GP, Griffioen AW, Van Noort PI, and Schiffelers RM (2014) Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma, Oncotarget 5, 6687–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Muthusamy S, DeMartino AM, Watson LJ, Brittian KR, Zafir A, Dassanayaka S, Hong KU, and Jones SP (2014) MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression, J. Biol. Chem 289, 29665–29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang D, Hu X, Lee SH, Chen F, Jiang K, Tu Z, Liu Z, Du J, Wang L, Yin C, Liao Y, Shang H, Martin KA, Herzog RI, Young LH, Qian L, Hwa J, and Xiang Y (2018) Diabetes Exacerbates Myocardial Ischemia/Reperfusion Injury by Down-Regulation of MicroRNA and Up-Regulation of O-GlcNAcylation, JACC Basic Transl Sci 3, 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ortega-Moreno L, Giraldez BG, Soto-Insuga V, Losada-Del Pozo R, Rodrigo-Moreno M, Alarcon-Morcillo C, Sanchez-Martin G, Diaz-Gomez E, Guerrero-Lopez R, Serratosa JM, and Grupo Espanol de Genetica de las Epilepsias de la, I. (2017) Molecular diagnosis of patients with epilepsy and developmental delay using a customized panel of epilepsy genes, PLoS One 12, e0188978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Guo Y, Li S, Qu J, Ye L, Wang S, Fan J, Wang Q, and Zhang J (2014) Let-7c inhibits metastatic ability of mouse hepatocarcinoma cells via targeting mannoside acetylglucosaminyltransferase 4 isoenzyme A, Int. J. Biochem. Cell Biol 53, 1–8. [DOI] [PubMed] [Google Scholar]
- [51].Feng X, Zhao L, Gao S, Song X, Dong W, Zhao Y, Zhou H, Cheng L, Miao X, and Jia L (2016) Increased fucosylation has a pivotal role in multidrug resistance of breast cancer cells through miR-224-3p targeting FUT4, Gene 578, 232–241. [DOI] [PubMed] [Google Scholar]
- [52].Zhao L, Feng X, Song X, Zhou H, Zhao Y, Cheng L, and Jia L (2016) miR-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting FUT4, Oncol. Rep 36, 1007–1015. [DOI] [PubMed] [Google Scholar]
- [53].Li Y, Sun Z, Liu B, Shan Y, Zhao L, and Jia L (2017) Tumor-suppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer, Cell Death Dis. 8, e2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zheng Q, Cui X, Zhang D, Yang Y, Yan X, Liu M, Niang B, Aziz F, Liu S, Yan Q, and Liu J (2017) miR-200b inhibits proliferation and metastasis of breast cancer by targeting fucosyltransferase IV and alpha1,3-fucosylated glycans, Oncogenesis 6, e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zheng Q, Zhang D, Yang YU, Cui X, Sun J, Liang C, Qin H, Yang X, Liu S, and Yan Q (2017) MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and alpha1,3-fucosylation, Cell Death Differ. 24, 2161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hu J, Wang Z, Pan Y, Ma J, Miao X, Qi X, Zhou H, and Jia L (2018) MiR-26a and miR-26b mediate osteoarthritis progression by targeting FUT4 via NF-kappaB signaling pathway, Int. J. Biochem. Cell Biol 94, 79–88. [DOI] [PubMed] [Google Scholar]
- [57].Zhang Y, Zhang D, Lv J, Wang S, and Zhang Q (2018) MiR-125a-5p suppresses bladder cancer progression through targeting FUT4, Biomed. Pharmacother 108, 1039–1047. [DOI] [PubMed] [Google Scholar]
- [58].Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Yang PC, Hsiao M, Hsu TL, and Wong CH (2013) Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer, Proc. Natl. Acad. Sci. U. S. A 110, 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang X, Fukuda T, Li W, Gao CX, Kondo A, Matsumoto A, Miyoshi E, Taniguchi N, and Gu J (2009) Requirement of Fut8 for the expression of vascular endothelial growth factor receptor-2: a new mechanism for the emphysema-like changes observed in Fut8-deficient mice, J. Biochem 145, 643–651. [DOI] [PubMed] [Google Scholar]
- [60].Kamio K, Yoshida T, Gao C, Ishii T, Ota F, Motegi T, Kobayashi S, Fujinawa R, Ohtsubo K, Kitazume S, Angata T, Azuma A, Gemma A, Nishimura M, Betsuyaku T, Kida K, and Taniguchi N (2012) alpha1,6-Fucosyltransferase (Fut8) is implicated in vulnerability to elastase-induced emphysema in mice and a possible non-invasive predictive marker for disease progression and exacerbations in chronic obstructive pulmonary disease (COPD), Biochem. Biophys. Res. Commun 424, 112–117. [DOI] [PubMed] [Google Scholar]
- [61].Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, Fujinawa R, Korekane H, Yamaguchi Y, Wada Y, and Taniguchi N (2014) The absence of core fucose up-regulates GnT-III and Wnt target genes: a possible mechanism for an adaptive response in terms of glycan function, J. Biol. Chem 289, 11704–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Iijima J, Kobayashi S, Kitazume S, Kizuka Y, Fujinawa R, Korekane H, Shibata T, Saitoh SI, Akashi-Takamura S, Miyake K, Miyoshi E, and Taniguchi N (2017) Core fucose is critical for CD14-dependent Toll-like receptor 4 signaling, Glycobiology 27, 1006–1015. [DOI] [PubMed] [Google Scholar]
- [63].Wang X, Gu J, Miyoshi E, Honke K, and Taniguchi N (2006) Phenotype changes of Fut8 knockout mouse: core fucosylation is crucial for the function of growth factor receptor(s), Methods Enzymol. 417, 11–22. [DOI] [PubMed] [Google Scholar]
- [64].Li S, Liu XY, Pan Q, Wu J, Liu ZH, Wang Y, Liu M, and Zhang XL (2019) Hepatitis C Virus-Induced FUT8 Causes 5-FU Drug Resistance in Human Hepatoma Huh7.5.1 Cells, Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Noda M, Okayama H, Kofunato Y, Chida S, Saito K, Tada T, Ashizawa M, Nakajima T, Aoto K, Kikuchi T, Sakamoto W, Endo H, Fujita S, Saito M, Momma T, Ohki S, and Kono K (2018) Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer, PLoS One 13, e0200315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fujii H, Shinzaki S, Iijima H, Wakamatsu K, Iwamoto C, Sobajima T, Kuwahara R, Hiyama S, Hayashi Y, Takamatsu S, Uozumi N, Kamada Y, Tsujii M, Taniguchi N, Takehara T, and Miyoshi E (2016) Core Fucosylation on T Cells, Required for Activation of T-Cell Receptor Signaling and Induction of Colitis in Mice, Is Increased in Patients With Inflammatory Bowel Disease, Gastroenterology 150, 1620–1632. [DOI] [PubMed] [Google Scholar]
- [67].Yue L, Han C, Li Z, Li X, Liu D, Liu S, and Yu H (2016) Fucosyltransferase 8 expression in breast cancer patients: A high throughput tissue microarray analysis, Histol. Histopathol 31, 547–555. [DOI] [PubMed] [Google Scholar]
- [68].Gu W, Fukuda T, Isaji T, Hang Q, Lee HH, Sakai S, Morise J, Mitoma J, Higashi H, Taniguchi N, Yawo H, Oka S, and Gu J (2015) Loss of alpha1,6-Fucosyltransferase Decreases Hippocampal Long Term Potentiation: IMPLICATIONS FOR CORE FUCOSYLATION IN THE REGULATION OF AMPA RECEPTOR HETEROMERIZATION AND CELLULAR SIGNALING, J. Biol. Chem 290, 17566–17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wang M, Wang J, Kong X, Chen H, Wang Y, Qin M, Lin Y, Chen H, Xu J, Hong J, Chen YX, Zou W, and Fang JY (2014) MiR-198 represses tumor growth and metastasis in colorectal cancer by targeting fucosyl transferase 8, Sci. Rep 4, 6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou P, Xu W, Peng X, Luo Z, Xing Q, Chen X, Hou C, Liang W, Zhou J, Wu X, Songyang Z, and Jiang S (2013) Large-scale screens of miRNA-mRNA interactions unveiled that the 3’UTR of a gene is targeted by multiple miRNAs, PLoS One 8, e68204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wolter JM, Kotagama K, Pierre-Bez AC, Firago M, and Mangone M (2014) 3’LIFE: a functional assay to detect miRNA targets in high-throughput, Nucleic Acids Res. 42, e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vogel C, and Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses, Nat Rev Genet 13, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kosti I, Jain N, Aran D, Butte AJ, and Sirota M (2016) Cross-tissue Analysis of Gene and Protein Expression in Normal and Cancer Tissues, Sci. Rep 6, 24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ostlund G, and Sonnhammer EL (2012) Quality criteria for finding genes with high mRNA-protein expression correlation and coexpression correlation, Gene 497, 228–236. [DOI] [PubMed] [Google Scholar]
- [75].Eichhorn SW, Guo H, McGeary SE, Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villen J, and Bartel DP (2014) mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues, Mol. Cell 56, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ghahramani Seno MM, Gwadry FG, Hu P, and Scherer SW (2013) Neuregulin 1-alpha regulates phosphorylation, acetylation, and alternative splicing in lymphoblastoid cells, Genome 56, 619–625. [DOI] [PubMed] [Google Scholar]
- [77].Chan JK, Kiet TK, Blansit K, Ramasubbaiah R, Hilton JF, Kapp DS, and Matei D (2014) MiR-378 as a biomarker for response to anti-angiogenic treatment in ovarian cancer, Gynecol. Oncol 133, 568–574. [DOI] [PubMed] [Google Scholar]
- [78].Liu B, Ma X, Liu Q, Xiao Y, Pan S, and Jia L (2018) Aberrant mannosylation profile and FTX/miR-342/ALG3-axis contribute to development of drug resistance in acute myeloid leukemia, Cell Death Dis. 9, 688. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [79].Tatura R, Buchholz M, Dickson DW, van Swieten J, McLean C, Hoglinger G, and Muller U (2016) microRNA profiling: increased expression of miR-147a and miR-518e in progressive supranuclear palsy (PSP), Neurogenetics 17, 165–171. [DOI] [PubMed] [Google Scholar]
- [80].De Antonellis P, Carotenuto M, Vandenbussche J, De Vita G, Ferrucci V, Medaglia C, Boffa I, Galiero A, Di Somma S, Magliulo D, Aiese N, Alonzi A, Spano D, Liguori L, Chiarolla C, Verrico A, Schulte JH, Mestdagh P, Vandesompele J, Gevaert K, and Zollo M (2014) Early targets of miR-34a in neuroblastoma, Mol. Cell. Proteomics 13, 2114–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Huang H, Liu Y, Yu P, Qu J, Guo Y, Li W, Wang S, and Zhang J (2018) MiR-23a transcriptional activated by Runx2 increases metastatic potential of mouse hepatoma cell via directly targeting Mgat3, Sci. Rep 8, 7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang YQ, Ren YF, Song YJ, Xue YF, Zhang XJ, Cao ST, Deng ZJ, Wu J, Chen L, Li G, Shi KQ, Chen YP, Ren H, Huang AL, and Tang KF (2014) MicroRNA-581 promotes hepatitis B virus surface antigen expression by targeting Dicer and EDEM1, Carcinogenesis 35, 2127–2133. [DOI] [PubMed] [Google Scholar]
- [83].Li G, Luna C, Qiu J, Epstein DL, and Gonzalez P (2011) Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells, Invest. Ophthalmol. Vis. Sci 52, 2999–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vitiello M, Tuccoli A, D’Aurizio R, Sarti S, Giannecchini L, Lubrano S, Marranci A, Evangelista M, Peppicelli S, Ippolito C, Barravecchia I, Guzzolino E, Montagnani V, Gowen M, Mercoledi E, Mercatanti A, Comelli L, Gurrieri S, Wu LW, Ope O, Flaherty K, Boland GM, Hammond MR, Kwong L, Chiariello M, Stecca B, Zhang G, Salvetti A, Angeloni D, Pitto L, Calorini L, Chiorino G, Pellegrini M, Herlyn M, Osman I, and Poliseno L (2017) Context-dependent miR-204 and miR-211 affect the biological properties of amelanotic and melanotic melanoma cells, Oncotarget 8, 25395–25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lim SM, Park SH, Lee JH, Kim SH, Kim JY, Min JK, Lee GM, and Kim YG (2018) Differential expression of microRNAs in recombinant Chinese hamster ovary cells treated with sodium butyrate using digital RNA counting, J. Biotechnol 283, 37–42. [DOI] [PubMed] [Google Scholar]
- [86].Pan S, Cheng X, Chen H, Castro PD, Ittmann MM, Hutson AW, Zapata SK, and Sifers RN (2013) ERManI is a target of miR-125b and promotes transformation phenotypes in hepatocellular carcinoma (HCC), PLoS One 8, e72829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Adhikari S, and Mandal P (2019) Integrated analysis of global gene and microRNA expression profiling associated with aplastic anaemia, Life Sci. 228, 47–52. [DOI] [PubMed] [Google Scholar]
- [88].Bhise NS, Chauhan L, Shin M, Cao X, Pounds S, Lamba V, and Lamba JK (2015) MicroRNA-mRNA Pairs Associated with Outcome in AML: From In Vitro Cell-Based Studies to AML Patients, Front. Pharmacol 6, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Serino G, Sallustio F, Curci C, Cox SN, Pesce F, De Palma G, and Schena FP (2015) Role of let-7b in the regulation of N-acetylgalactosaminyltransferase 2 in IgA nephropathy, Nephrol. Dial. Transplant 30, 1132–1139. [DOI] [PubMed] [Google Scholar]
- [90].Liu B, Pan S, Xiao Y, Liu Q, Xu J, and Jia L (2018) LINC01296/miR-26a/GALNT3 axis contributes to colorectal cancer progression by regulating O-glycosylated MUC1 via PI3K/AKT pathway, J. Exp. Clin. Cancer Res 37, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nakamura S, Horie M, Daidoji T, Honda T, Yasugi M, Kuno A, Komori T, Okuzaki D, Narimatsu H, Nakaya T, and Tomonaga K (2016) Influenza A Virus-Induced Expression of a GalNAc Transferase, GALNT3, via MicroRNAs Is Required for Enhanced Viral Replication, J. Virol 90, 1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Qu JJ, Qu XY, and Zhou DZ (2017) miR4262 inhibits colon cancer cell proliferation via targeting of GALNT4, Mol Med Rep 16, 3731–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu Y, Liu H, Yang L, Wu Q, Liu W, Fu Q, Zhang W, Zhang H, Xu J, and Gu J (2017) Loss of N-Acetylgalactosaminyltransferase-4 Orchestrates Oncogenic MicroRNA-9 in Hepatocellular Carcinoma, J. Biol. Chem 292, 3186–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhang J, Zhang Z, Wang Q, Xing XJ, and Zhao Y (2016) Overexpression of microRNA-365 inhibits breast cancer cell growth and chemo-resistance through GALNT4, Eur. Rev. Med. Pharmacol. Sci 20, 4710–4718. [PubMed] [Google Scholar]
- [95].Stiegelbauer V, Vychytilova-Faltejskova P, Karbiener M, Pehserl AM, Reicher A, Resel M, Heitzer E, Ivan C, Bullock M, Ling H, Deutsch A, Wulf-Goldenberg A, Adiprasito JB, Stoeger H, Haybaeck J, Svoboda M, Stotz M, Hoefler G, Slaby O, Calin GA, Gerger A, and Pichler M (2017) miR-196b-5p Regulates Colorectal Cancer Cell Migration and Metastases through Interaction with HOXB7 and GALNT5, Clin. Cancer Res 23, 5255–5266. [DOI] [PubMed] [Google Scholar]
- [96].Wu Q, Liu HO, Liu YD, Liu WS, Pan D, Zhang WJ, Yang L, Fu Q, Xu JJ, and Gu JX (2015) Decreased expression of hepatocyte nuclear factor 4alpha (Hnf4alpha)/microRNA-122 (miR-122) axis in hepatitis B virus-associated hepatocellular carcinoma enhances potential oncogenic GALNT10 protein activity, J. Biol. Chem 290, 1170–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yang J, Li G, and Zhang K (2016) MiR-125a regulates ovarian cancer proliferation and invasion by repressing GALNT14 expression, Biomed. Pharmacother 80, 381–387. [DOI] [PubMed] [Google Scholar]
- [98].Liu X, Chen J, Guan T, Yao H, Zhang W, Guan Z, and Wang Y (2019) miRNAs and target genes in the blood as biomarkers for the early diagnosis of Parkinson’s disease, BMC Syst. Biol 13, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Gao Y, Liu T, and Huang Y (2015) MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins, FEBS Lett. 589, 207–214. [DOI] [PubMed] [Google Scholar]
- [100].Thapa I, Fox HS, and Bastola D (2015) Coexpression Network Analysis of miRNA-142 Overexpression in Neuronal Cells, Biomed Res Int 2015, 921517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Metzler-Guillemain C, Victorero G, Lepoivre C, Bergon A, Yammine M, Perrin J, Sari-Minodier I, Boulanger N, Rihet P, and Nguyen C (2015) Sperm mRNAs and microRNAs as candidate markers for the impact of toxicants on human spermatogenesis: an application to tobacco smoking, Syst. Biol. Reprod. Med 61, 139–149. [DOI] [PubMed] [Google Scholar]
- [102].Wang R, Fang J, Ma H, Feng L, Lian M, Yang F, Wang H, Wang Q, and Chen X (2015) Effect of microRNA-203 on tumor growth in human hypopharyngeal squamous cell carcinoma, Mol. Cell. Biochem 405, 97–104. [DOI] [PubMed] [Google Scholar]
- [103].Serino G, Sallustio F, Cox SN, Pesce F, and Schena FP (2012) Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy, J. Am. Soc. Nephrol 23, 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li C, Shi J, and Zhao Y (2018) MiR-320 promotes B cell proliferation and the production of aberrant glycosylated IgA1 in IgA nephropathy, J. Cell. Biochem 119, 4607–4614. [DOI] [PubMed] [Google Scholar]
- [105].Yang L, Zhang X, Peng W, Wei M, and Qin W (2017) MicroRNA-155-induced T lymphocyte subgroup drifting in IgA nephropathy, Int. Urol. Nephrol 49, 353–361. [DOI] [PubMed] [Google Scholar]
- [106].Hu S, Bao H, Xu X, Zhou X, Qin W, Zeng C, and Liu Z (2015) Increased miR-374b promotes cell proliferation and the production of aberrant glycosylated IgA1 in B cells of IgA nephropathy, FEBS Lett. 589, 4019–4025. [DOI] [PubMed] [Google Scholar]
- [107].Chao CC, Wu PH, Huang HC, Chung HY, Chou YC, Cai BH, and Kannagi R (2017) Downregulation of miR-199a/b-5p is associated with GCNT2 induction upon epithelial-mesenchymal transition in colon cancer, FEBS Lett. 591, 1902–1917. [DOI] [PubMed] [Google Scholar]
- [108].Li Q, Ran P, Zhang X, Guo X, Yuan Y, Dong T, Zhu B, Zheng S, and Xiao C (2018) Downregulation of N-Acetylglucosaminyltransferase GCNT3 by miR-302b-3p Decreases Non-Small Cell Lung Cancer (NSCLC) Cell Proliferation, Migration and Invasion, Cell. Physiol. Biochem 50, 987–1004. [DOI] [PubMed] [Google Scholar]
- [109].Gonzalez-Vallinas M, Molina S, Vicente G, Zarza V, Martin-Hernandez R, Garcia-Risco MR, Fornari T, Reglero G, and Ramirez de Molina A (2014) Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer, PLoS One 9, e98556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].He XJ, Xiao Y, Zhang Q, Ma LP, Li N, and Yang J (2013) Detection and functional annotation of misregulated microRNAs in the brain of the Ts65Dn mouse model of Down syndrome, Chin. Med. J. (Engl.) 126, 108–113. [PubMed] [Google Scholar]
- [111].Riley MF, Bochter MS, Wahi K, Nuovo GJ, and Cole SE (2013) Mir-125a-5p-mediated regulation of Lfng is essential for the avian segmentation clock, Dev. Cell 24, 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wahi K, Friesen S, Coppola V, and Cole SE (2017) Putative binding sites for mir-125 family miRNAs in the mouse Lfng 3’UTR affect transcript expression in the segmentation clock, but mir-125a-5p is dispensable for normal somitogenesis, Dev. Dyn 246, 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Raimo M, Orso F, Grassi E, Cimino D, Penna E, De Pitta C, Stadler MB, Primo L, Calautti E, Quaglino P, Provero P, and Taverna D (2016) miR-146a Exerts Differential Effects on Melanoma Growth and Metastatization, Mol. Cancer Res 14, 548–562. [DOI] [PubMed] [Google Scholar]
- [114].Wang L, Wei Z, Wu K, Dai W, Zhang C, Peng J, and He Y (2018) Long noncoding RNA B3GALT5-AS1 suppresses colon cancer liver metastasis via repressing microRNA-203, Aging (Albany N. Y.) 10, 3662–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu YX, Wang L, Liu WJ, Zhang HT, Xue JH, Zhang ZW, and Gao CJ (2016) MiR-124–3p/B4GALT1 axis plays an important role in SOCS3-regulated growth and chemo-sensitivity of CML, J. Hematol. Oncol 9, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Teruel R, Martinez-Martinez I, Guerrero JA, Gonzalez-Conejero R, de la Morena-Barrio ME, Salloum-Asfar S, Arroyo AB, Aguila S, Garcia-Barbera N, Minano A, Vicente V, Corral J, and Martinez C (2013) Control of post-translational modifications in antithrombin during murine post-natal development by miR-200a, J. Biomed. Sci 20, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cai H, Zhou H, Miao Y, Li N, Zhao L, and Jia L (2017) MiRNA expression profiles reveal the involvement of miR-26a, miR-548l and miR-34a in hepatocellular carcinoma progression through regulation of ST3GAL5, Lab. Invest 97, 530–542. [DOI] [PubMed] [Google Scholar]
- [118].Tonevitsky AG, Maltseva DV, Abbasi A, Samatov TR, Sakharov DA, Shkurnikov MU, Lebedev AE, Galatenko VV, Grigoriev AI, and Northoff H (2013) Dynamically regulated miRNA-mRNA networks revealed by exercise, BMC Physiol. 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sun M, Zhao X, Liang L, Pan X, Lv H, and Zhao Y (2017) Sialyltransferase ST3GAL6 mediates the effect of microRNA-26a on cell growth, migration, and invasion in hepatocellular carcinoma through the protein kinase B/mammalian target of rapamycin pathway, Cancer Sci. 108, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Han Y, Liu Y, Fu X, Zhang Q, Huang H, Zhang C, Li W, and Zhang J (2018) miR-9 inhibits the metastatic ability of hepatocellular carcinoma via targeting beta galactoside alpha-2,6-sialyltransferase 1, J. Physiol. Biochem 74, 491–501. [DOI] [PubMed] [Google Scholar]
- [121].Gao L, He RQ, Wu HY, Zhang TT, Liang HW, Ye ZH, Li ZY, Xie TT, Shi Q, Ma J, Hu XH, and Chen G (2018) Expression Signature and Role of miR-30d-5p in Non-Small Cell Lung Cancer: a Comprehensive Study Based on in Silico Analysis of Public Databases and in Vitro Experiments, Cell. Physiol. Biochem 50, 1964–1987. [DOI] [PubMed] [Google Scholar]
- [122].Liu B, Liu Y, Zhao L, Pan Y, Shan Y, Li Y, and Jia L (2017) Upregulation of microRNA-135b and microRNA-182 promotes chemoresistance of colorectal cancer by targeting ST6GALNAC2 via PI3K/AKT pathway, Mol. Carcinog 56, 2669–2680. [DOI] [PubMed] [Google Scholar]
- [123].Jia L, Luo S, Ren X, Li Y, Hu J, Liu B, Zhao L, Shan Y, and Zhou H (2017) miR-182 and miR-135b Mediate the Tumorigenesis and Invasiveness of Colorectal Cancer Cells via Targeting ST6GALNAC2 and PI3K/AKT Pathway, Dig. Dis. Sci 62, 3447–3459. [DOI] [PubMed] [Google Scholar]
- [124].Miao X, Jia L, Zhou H, Song X, Zhou M, Xu J, Zhao L, Feng X, and Zhao Y (2016) miR-4299 mediates the invasive properties and tumorigenicity of human follicular thyroid carcinoma by targeting ST6GALNAC4, IUBMB Life 68, 136–144. [DOI] [PubMed] [Google Scholar]
- [125].Shan Y, Liu Y, Zhao L, Liu B, Li Y, and Jia L (2017) MicroRNA-33a and let-7e inhibit human colorectal cancer progression by targeting ST8SIA1, Int. J. Biochem. Cell Biol 90, 48–58. [DOI] [PubMed] [Google Scholar]
- [126].Liu QY, Miao Y, Wang XH, Wang P, Cheng ZC, and Qian TM (2019) Increased levels of miR-3099 induced by peripheral nerve injury promote Schwann cell proliferation and migration, Neural Regen Res 14, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ma X, Dong W, Su Z, Zhao L, Miao Y, Li N, Zhou H, and Jia L (2016) Functional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8SIA4, Cell Death Dis. 7, e2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhao L, Li Y, Song X, Zhou H, Li N, Miao Y, and Jia L (2016) Upregulation of miR-181c inhibits chemoresistance by targeting ST8SIA4 in chronic myelocytic leukemia, Oncotarget 7, 60074–60086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wang Z, Hu J, Pan Y, Shan Y, Jiang L, Qi X, and Jia L (2018) miR-140-5p/miR-149 Affects Chondrocyte Proliferation, Apoptosis, and Autophagy by Targeting FUT1 in Osteoarthritis, Inflammation 41, 959–971. [DOI] [PubMed] [Google Scholar]
- [130].Wang Y, Chen J, Chen X, Jiang F, Sun Y, Pan Y, Zhang W, and Zhang J (2017) MiR-34a suppresses HNSCC growth through modulating cell cycle arrest and senescence, Neoplasma 64, 543–553. [DOI] [PubMed] [Google Scholar]
- [131].Wu CS, Yen CJ, Chou RH, Chen JN, Huang WC, Wu CY, and Yu YL (2014) Downregulation of microRNA-15b by hepatitis B virus X enhances hepatocellular carcinoma proliferation via fucosyltransferase 2-induced Globo H expression, Int. J. Cancer 134, 1638–1647. [DOI] [PubMed] [Google Scholar]
- [132].Liang L, Gao C, Li Y, Sun M, Xu J, Li H, Jia L, and Zhao Y (2017) miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway, Cell Death Dis. 8, e2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Li N, Liu Y, Miao Y, Zhao L, Zhou H, and Jia L (2016) MicroRNA-106b targets FUT6 to promote cell migration, invasion, and proliferation in human breast cancer, IUBMB Life 68, 764–775. [DOI] [PubMed] [Google Scholar]
- [134].Hu B, Xu C, Tian Y, Shi C, Zhang Y, Deng L, Zhou H, Cao P, Chen H, and Yuan W (2017) Inflammatory microRNA-194 and -515 attenuate the biosynthesis of chondroitin sulfate during human intervertebral disc degeneration, Oncotarget 8, 49303–49317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Prudnikova TY, Mostovich LA, Kashuba VI, Ernberg I, Zabarovsky ER, and Grigorieva EV (2012) miRNA-218 contributes to the regulation of D-glucuronyl C5-epimerase expression in normal and tumor breast tissues, Epigenetics 7, 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Song YQ, Karasugi T, Cheung KM, Chiba K, Ho DW, Miyake A, Kao PY, Sze KL, Yee A, Takahashi A, Kawaguchi Y, Mikami Y, Matsumoto M, Togawa D, Kanayama M, Shi D, Dai J, Jiang Q, Wu C, Tian W, Wang N, Leong JC, Luk KD, Yip SP, Cherny SS, Wang J, Mundlos S, Kelempisioti A, Eskola PJ, Mannikko M, Makela P, Karppinen J, Jarvelin MR, O’Reilly PF, Kubo M, Kimura T, Kubo T, Toyama Y, Mizuta H, Cheah KS, Tsunoda T, Sham PC, Ikegawa S, and Chan D (2013) Lumbar disc degeneration is linked to a carbohydrate sulfotransferase 3 variant, J. Clin. Invest 123, 4909–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yang G, Gong Y, Wang Q, Wang Y, and Zhang X (2015) The role of miR-100-mediated Notch pathway in apoptosis of gastric tumor cells, Cell. Signal 27, 1087–1101. [DOI] [PubMed] [Google Scholar]
- [138].Liep J, Kilic E, Meyer HA, Busch J, Jung K, and Rabien A (2016) Cooperative Effect of miR-141–3p and miR-145–5p in the Regulation of Targets in Clear Cell Renal Cell Carcinoma, PLoS One 11, e0157801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Shi X, Su S, Long J, Mei B, and Chen Y (2011) MicroRNA-191 targets N-deacetylase/N-sulfotransferase 1 and promotes cell growth in human gastric carcinoma cell line MGC803, Acta Biochim Biophys Sin (Shanghai) 43, 849–856. [DOI] [PubMed] [Google Scholar]
- [140].Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, Zeng Y, Sun B, Qian H, Chen L, Wu M, Su C, and Chen J (2013) MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways, Cancer Lett. 337, 226–236. [DOI] [PubMed] [Google Scholar]
- [141].Yang L, Zhang S, Guo K, Huang H, Qi S, Yao J, and Zhang Z (2019) miR-125a restrains cell migration and invasion by targeting STAT3 in gastric cancer cells, Onco Targets Ther. 12, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [142].Wang G, Zhao W, Gao X, Zhang D, Li Y, Zhang Y, and Li W (2017) HNF1AAS1 promotes growth and metastasis of esophageal squamous cell carcinoma by sponging miR214 to upregulate the expression of SOX-4, Int. J. Oncol 51, 657–667. [DOI] [PubMed] [Google Scholar]
- [143].Midgley AC, Morris G, Phillips AO, and Steadman R (2016) 17beta-estradiol ameliorates age-associated loss of fibroblast function by attenuating IFN-gamma/STAT1-dependent miR-7 upregulation, Aging Cell 15, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Pan B, Toms D, Shen W, and Li J (2015) MicroRNA-378 regulates oocyte maturation via the suppression of aromatase in porcine cumulus cells, Am. J. Physiol. Endocrinol. Metab 308, E525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Rock K, Tigges J, Sass S, Schutze A, Florea AM, Fender AC, Theis FJ, Krutmann J, Boege F, Fritsche E, Reifenberger G, and Fischer JW (2015) miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: possible implication for skin aging, J. Invest. Dermatol 135, 369–377. [DOI] [PubMed] [Google Scholar]
- [146].Zhang J, Chang JJ, Xu F, Ma XJ, Wu Y, Li WC, Wang HJ, Huang GY, and Ma D (2013) MicroRNA deregulation in right ventricular outflow tract myocardium in nonsyndromic tetralogy of fallot, Can. J. Cardiol 29, 1695–1703. [DOI] [PubMed] [Google Scholar]
- [147].Lagendijk AK, Goumans MJ, Burkhard SB, and Bakkers J (2011) MicroRNA-23 restricts cardiac valve formation by inhibiting Has2 and extracellular hyaluronic acid production, Circ. Res 109, 649–657. [DOI] [PubMed] [Google Scholar]
- [148].Pan B, Toms D, and Li J (2018) MicroRNA-574 suppresses oocyte maturation via targeting hyaluronan synthase 2 in porcine cumulus cells, Am. J. Physiol. Cell Physiol 314, C268–C277. [DOI] [PubMed] [Google Scholar]
- [149].Li HM, Xiao YJ, Min ZS, and Tan C (2019) Identification and interaction analysis of key genes and microRNAs in atopic dermatitis by bioinformatics analysis, Clin. Exp. Dermatol 44, 257–264. [DOI] [PubMed] [Google Scholar]
- [150].Bai F, Jiu M, You Y, Feng Y, Xin R, Liu X, Mo L, and Nie Y (2018) miR29a3p represses proliferation and metastasis of gastric cancer cells via attenuating HAS3 levels, Mol Med Rep 17, 8145–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Pashaei E, Guzel E, Ozgurses ME, Demirel G, Aydin N, and Ozen M (2016) A Meta-Analysis: Identification of Common Mir-145 Target Genes that have Similar Behavior in Different GEO Datasets, PLoS One 11, e0161491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Barter MJ, Tselepi M, Gomez R, Woods S, Hui W, Smith GR, Shanley DP, Clark IM, and Young DA (2015) Genome-Wide MicroRNA and Gene Analysis of Mesenchymal Stem Cell Chondrogenesis Identifies an Essential Role and Multiple Targets for miR-140-5p, Stem Cells 33, 3266–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Straniero L, Rimoldi V, Samarani M, Goldwurm S, Di Fonzo A, Kruger R, Deleidi M, Aureli M, Solda G, Duga S, and Asselta R (2017) The GBAP1 pseudogene acts as a ceRNA for the glucocerebrosidase gene GBA by sponging miR-22-3p, Sci. Rep 7, 12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Chang S, He S, Qiu G, Lu J, Wang J, Liu J, Fan L, Zhao W, and Che X (2016) MicroRNA-125b promotes invasion and metastasis of gastric cancer by targeting STARD13 and NEU1, Tumour Biol. 37, 12141–12151. [DOI] [PubMed] [Google Scholar]
- [155].Tao J, Liu W, Shang G, Zheng Y, Huang J, Lin R, and Chen L (2015) MiR-207/352 regulate lysosomal-associated membrane proteins and enzymes following ischemic stroke, Neuroscience 305, 1–14. [DOI] [PubMed] [Google Scholar]
- [156].Yang C, Ren J, Li B, Zhang D, Ma C, Cheng C, Sun Y, Fu L, and Shi X (2018) Identification of clinical tumor stages related mRNAs and miRNAs in cervical squamous cell carcinoma, Pathol. Res. Pract 214, 1638–1647. [DOI] [PubMed] [Google Scholar]
- [157].Yamada Y, Arai T, Sugawara S, Okato A, Kato M, Kojima S, Yamazaki K, Naya Y, Ichikawa T, and Seki N (2018) Impact of novel oncogenic pathways regulated by antitumor miR-451a in renal cell carcinoma, Cancer Sci. 109, 1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Sun Y, Liu X, Zhang Q, Mao X, Feng L, Su P, Chen H, Guo Y, and Jin F (2016) Oncogenic potential of TSTA3 in breast cancer and its regulation by the tumor suppressors miR-125a-5p and miR-125b, Tumour Biol. 37, 4963–4972. [DOI] [PubMed] [Google Scholar]
- [159].Kwon DN, Chang BS, and Kim JH (2014) MicroRNA dysregulation in liver and pancreas of CMP-Neu5Ac hydroxylase null mice disrupts insulin/PI3K-AKT signaling, Biomed Res Int 2014, 236385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Gan BL, Zhang LJ, Gao L, Ma FC, He RQ, Chen G, Ma J, Zhong JC, and Hu XH (2018) Downregulation of miR2245p in prostate cancer and its relevant molecular mechanism via TCGA, GEO database and in silico analyses, Oncol. Rep 40, 3171–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Xu QF, Pan YW, Li LC, Zhou Z, Huang QL, Pang JC, Zhu XP, Ren Y, Yang H, Ohgaki H, and Lv SQ (2014) MiR-22 is frequently downregulated in medulloblastomas and inhibits cell proliferation via the novel target PAPST1, Brain Pathol. 24, 568–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lim CH, Jeong W, Lim W, Kim J, Song G, and Bazer FW (2012) Differential expression of select members of the SLC family of genes and regulation of expression by microRNAs in the chicken oviduct, Biol. Reprod 87, 145. [DOI] [PubMed] [Google Scholar]
- [163].Hao GJ, Ding YH, Wen H, Li XF, Zhang W, Su HY, Liu DM, and Xie NL (2017) Attenuation of deregulated miR-369–3p expression sensitizes non-small cell lung cancer cells to cisplatin via modulation of the nucleotide sugar transporter SLC35F5, Biochem. Biophys. Res. Commun 488, 501–508. [DOI] [PubMed] [Google Scholar]
- [164].Wijayakumara DD, Mackenzie PI, McKinnon RA, Hu DG, and Meech R (2018) Regulation of UDP-Glucuronosyltransferase 2B15 by miR-331–5p in Prostate Cancer Cells Involves Canonical and Noncanonical Target Sites, J. Pharmacol. Exp. Ther 365, 48–59. [DOI] [PubMed] [Google Scholar]
- [165].Margaillan G, Levesque E, and Guillemette C (2016) Epigenetic regulation of steroid inactivating UDP-glucuronosyltransferases by microRNAs in prostate cancer, J. Steroid Biochem. Mol. Biol 155, 85–93. [DOI] [PubMed] [Google Scholar]
- [166].Wijayakumara DD, Hu DG, Meech R, McKinnon RA, and Mackenzie PI (2015) Regulation of Human UGT2B15 and UGT2B17 by miR-376c in Prostate Cancer Cell Lines, J. Pharmacol. Exp. Ther 354, 417–425. [DOI] [PubMed] [Google Scholar]
- [167].Papageorgiou I, and Court MH (2017) Identification and validation of the microRNA response elements in the 3’-untranslated region of the UDP glucuronosyltransferase (UGT) 2B7 and 2B15 genes by a functional genomics approach, Biochem. Pharmacol 146, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zhang JJ, Wang LN, Feng Y, Zhi H, Ma GS, Ye XZ, Qian SS, and Wang B (2012) [Association study on the microRNA-1 target gene polymorphism and the risk of premature coronary artery disease], Zhonghua Xin Xue Guan Bing Za Zhi 40, 386–391. [PubMed] [Google Scholar]
- [169].Fu T, and Kemper JK (2016) MicroRNA-34a and Impaired FGF19/21 Signaling in Obesity, Vitam. Horm 101, 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kang WL, and Xu GS (2016) Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy, Sci. Rep 6, 19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].He XJ, Ma YY, Yu S, Jiang XT, Lu YD, Tao L, Wang HP, Hu ZM, and Tao HQ (2014) Up-regulated miR-199a-5p in gastric cancer functions as an oncogene and targets klotho, BMC Cancer 14, 218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [172].Jiang B, Gu Y, and Chen Y (2014) Identification of novel predictive markers for the prognosis of pancreatic ductal adenocarcinoma, Cancer Invest. 32, 218–225. [DOI] [PubMed] [Google Scholar]
- [173].Mehi SJ, Maltare A, Abraham CR, and King GD (2014) MicroRNA-339 and microRNA-556 regulate Klotho expression in vitro, Age (Dordr) 36, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Koshizuka K, Hanazawa T, Kikkawa N, Katada K, Okato A, Arai T, Idichi T, Osako Y, Okamoto Y, and Seki N (2018) Antitumor miR-150–5p and miR-150–3p inhibit cancer cell aggressiveness by targeting SPOCK1 in head and neck squamous cell carcinoma, Auris Nasus Larynx 45, 854–865. [DOI] [PubMed] [Google Scholar]
- [175].Osako Y, Seki N, Koshizuka K, Okato A, Idichi T, Arai T, Omoto I, Sasaki K, Uchikado Y, Kita Y, Kurahara H, Maemura K, and Natsugoe S (2017) Regulation of SPOCK1 by dual strands of pre-miR-150 inhibit cancer cell migration and invasion in esophageal squamous cell carcinoma, J. Hum. Genet 62, 935–944. [DOI] [PubMed] [Google Scholar]
- [176].Lee SJ, Kim SJ, Seo HH, Shin SP, Kim D, Park CS, Kim KT, Kim YH, Jeong JS, and Kim IH (2012) Over-expression of miR-145 enhances the effectiveness of HSVtk gene therapy for malignant glioma, Cancer Lett. 320, 72–80. [DOI] [PubMed] [Google Scholar]


