Summary:
SHR-1701, a bispecific fusion protein directed against PD-L1 and TFG-β, demonstrates promising clinical activity in advanced/recurrent cervical cancer; a recent study serves as a proof of principle that the TGF-β pathway may be successfully targeted, and that bispecific antibodies offer a novel therapeutic approach to do so.
In this issue of Clinical Cancer Research, Feng and colleagues report on the safety and efficacy of a bifunctional fusion protein targeting PD-L1 and TGF-β in a phase I expansion cohort of advanced cervical squamous cell carcinoma (SCC) (1). In this study, the investigators demonstrate that SHR-1701 has clinical activity in immunotherapy-naive patients, with an objective response rate (ORR) of 15.6% and a median duration of response that has not yet been reached. Notably, the ORR was similar regardless of PD-L1 expression.
Despite some advances in the management of metastatic cervical cancer, the outcomes of these patients remain poor, even with the incorporation of PD-1/PD-L1 blockade. In the recently published KEYNOTE-826 study, the median overall survival for patients treated with pembrolizumab plus chemotherapy with or without bevacizumab was 24 months (2). Single agent PD-1/PD-L1 blockade has demonstrated modest but durable benefits in advanced cervical cancer (3,4), and pembrolizumab monotherapy is approved for use in PD-L1 positive tumors after progression on platinum-based chemotherapy. There remain many unanswered questions regarding mechanisms of resistance, best combinatorial strategies, and an ongoing search for prognostic biomarkers beyond PD-L expression in this patient population.
The authors’ work builds on prior studies of combinatorial immuno-oncology agents in advanced or recurrent cervical SCC. CheckMate-358 examined several dosing levels and schedules of nivolumab plus the anti-CTLA4 antibody ipilimumab; in patients who had not received prior systemic therapies, the ORR ranged from 32–46%; clinical benefit was seen regardless of PD-L1 status (5). Similarly, the combination of balstilimab (anti-PD-1) and zalifrelimab (anti-CTLA-4) for patients with recurrent or metastatic disease resulted in an ORR of 22%; PD-L1 expression enriched for responders, but responses were seen in PD-L1 negative patients (6).
In studies such as these, the goal is to enhance the anti-tumor immune response by addressing immunosuppressive factors beyond the PD-1/PD-L1 axis (7,8). In solid tumors, the tumor microenvironment (TME) plays a key role in resistance to immune checkpoint inhibitors, including immunosuppressive populations of regulatory T-cells (Tregs), tumor-associated macrophages, and myeloid derived suppressor cells. Moreover, cytokines such as interleukin-10, and TGF-β have been implicated in diminished host antitumor response leading to tumor immune escape (9,10).
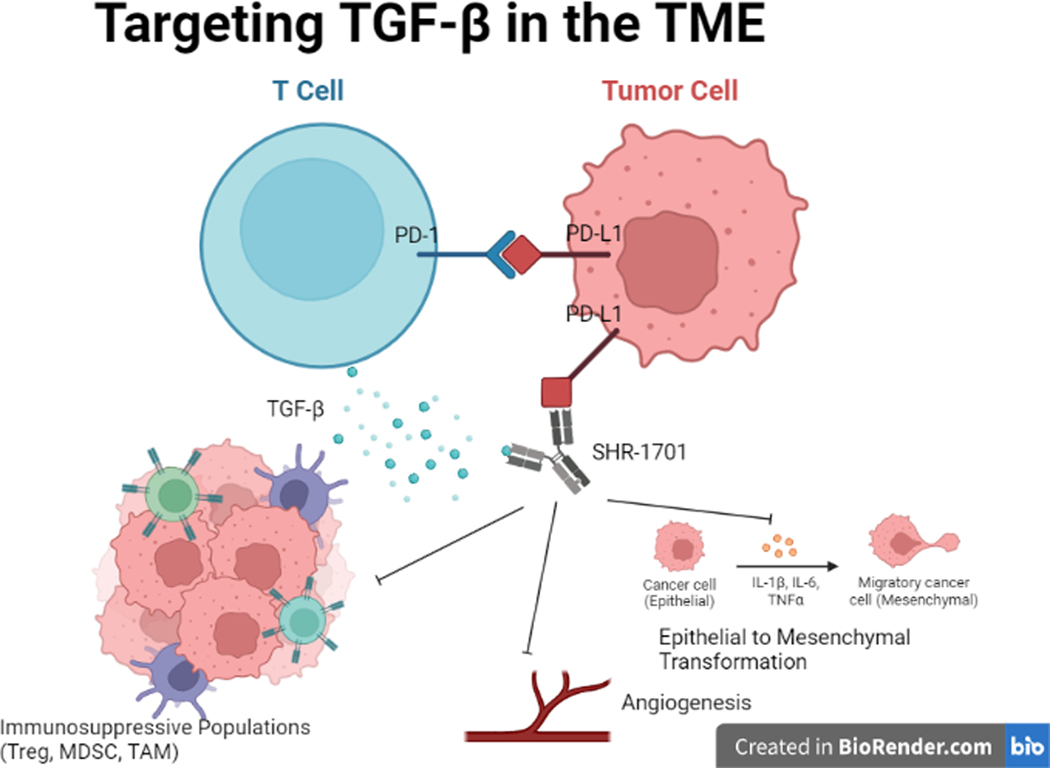
TGF-β plays a particularly important role in the TME due to its twin functions in both normal cellular processes and immunosuppression (Fig. 1). It is an important inhibitor of cell proliferation with roles in apoptosis, angiogenesis, and wound healing. In early neoplastic processes, TGF-β aids in tumor suppression, but can later drive tumor progression and metastasis by disrupting host immune response and inducing tumor epithelial-to-mesenchymal transformation (11). Targeting of TGF-β thereby offers a strategy to remodel the TME and invigorate host response and is a leading candidate for drug development. Its impact has been investigated in both pre-clinical and clinical studies with small molecule inhibitors of the TGF-β receptor and anti-TGF-β monoclonal antibodies. In contrast to the excellent preclinical results, TGF-β blockade has been disappointing in clinical trials, with single agent activity only noted in small subsets of patients (12,13). Thus, there has remained a need for an alternative approach to inhibit TGF-β in the TME.
Figure 1:
Transforming growth factor-β (TGFβ) signaling in tumor cells drives epithelial to mesenchymal cellular transformation, promotes increased angiogenesis, and recruits immunosuppressive populations such as regulatory T cells. By simultaneously targeting PD-L1 expression and TGF-β within the tumor microenvironment, SHR-1701 creates a more favorable pro-inflammatory milieu.
SHR-1701, a bifunctional fusion protein composed of a monoclonal antibody against PD-1 fused with the extracellular membrane of TGF-β receptor II, represents a novel strategy to overcome these proposed mechanisms of immune resistance through targeting non-redundant pathways. In preclinical animal models of murine CMT167 lung tumor cells, SHR-1701 demonstrated both successful inhibition of the TGF-B/TGF-B receptor pathway and preservation of downstream pAkt pathway of PD-1/PD-L1, thus overcoming acquired resistance to anti-PD-1 antibodies in mice with impaired lymphocyte recovery. These findings support the utility of a bifunctional protein in cancer previously exposed to chemotherapy and associated imbalances in CD8+T/Treg cells (14).
Other bifunctional fusion proteins include bintrafusp alfa (M7824) which also consists of a TGF-β ‘trap’ and IgG1 antibody blocking PD-L1, and the anti-TGF-β/PD-L1 bispecific antibody YM101 (15,16). In preclinical trials, bintrafusp alfa demonstrated similar ability as SHR-1701 to decrease Treg activity and reverse the epithelial-to-mesenchymal transformation, thereby improving chemo-sensitivity and augmenting host T-cell response (17). Its clinical activity and safety have been investigated in HPV-associated cancers (18), and in patients with advanced/recurrent cervical cancer, bintrafusp alfa demonstrated an ORR of 28.2% (19). Importantly, preclinical data for SHR-1701, bintrafusp alfa, and YM101 all demonstrate that the bifunctionality of these proteins increase the concentration of TGF-β receptor II in the TME through binding PD-L1. This offers a clear rationale for a single bifunctional molecule versus the use of an anti-PD-L1 monoclonal antibody in combination with the use of an TGF-β inhibitor while also reducing the potential adverse effects of the systemic delivery of a TGF-β antagonist.
Notably, in this study, there were no differences in response observed between patients with and without PD-L1 expression. This finding is consistent with previously published data for bintrafusp alfa in SCC of the head and neck (15), and is in contrast to the FDA approval of pembrolizumab which is limited to those patients with PD-L1 positive disease. While PD-L1 expression lacks both sensitivity and specificity as a biomarker for response to ICI, these data suggest that a bispecific approach may offer an alternative therapeutic tactic for patients with PD-L1 negative disease. The authors also attempt to define a new prognostic biomarker through the measurement of pSMAD2. SMAD proteins play a role in the downstream activation of TGF-B signaling; an elevated pSMAD2 level may predict those with higher response to inhibition of the SMAD-dependent TGF-β pathway. Although limited by the small sample size, the suggestion of an association is hypothesis-generating and warrants further study.
The development of novel immuno-oncology agents, alone or in combination with other systemic, targeted, or biologic agents, represents an exciting step forward in the treatment of cervical cancer. The authors report that SHR-1701 will be investigated in a phase III study comparing its use versus placebo in combination with upfront chemotherapy +/− bevacizumab; similar phase III studies are ongoing with BCD-100 (anti-PD-1) and atezolizumab (anti-PD-L1) (NCT03912415, NCT03556839). AK104, an anti-PD1 and anti-CTLA4 bispecific antibody, already has demonstrated impressive clinical activity and reasonable safety when combined with standard chemotherapy in the upfront setting (20).
In conclusion, SHR-1701 demonstrates anti-tumor activity and safety in patients with recurrent or metastatic cervical cancer after platinum-based chemotherapy. This clinical space is evolving quickly, with the incorporation of single and potentially dual checkpoint blockade into the upfront treatment of advanced or recurrent disease. This trial may serve as proof of principle that the TGF-β pathway may be successfully targeted, and thus a therapeutic target moving forward.
Funding:
The authors are supported in part by a Cancer Center Support Grant of the NIH/NCI (Grant No. P30CA008748).
Footnotes
Conflicts of Interest: C. Friedman reports participation in the scientific advisory boards for Merck (LYNK-002) and Genentech (MyPathway), compensation waived. She reports consulting for Seagen and BMS. She reports institutional research funds from Genentech/Roche, Bristol Myers Squibb, Merck, AstraZeneca, and Daiichi. K. Miller reports no relevant COI.
References:
- 1.Feng J, Tang D, Wang J, Zhou Q, Peng J, Lou H, et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-beta, for recurrent or metastatic cervical cancer: a clinical expansion cohort of phase 1 study. Clin Cancer Res 2022. doi 10.1158/1078-0432.CCR-22-0346. [DOI] [PubMed]
- 2.Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. New England Journal of Medicine 2021;385(20):1856–67 doi 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 3.Naumann RW, Hollebecque A, Meyer T, Devlin M-J, Oaknin A, Kerger J, et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. Journal of Clinical Oncology 2019;37(31):2825–34 doi 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019;37(17):1470–8 doi 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 5.Naumann RW, Oaknin A, Meyer T, Lopez-Picazo JM, Lao C, Bang YJ, et al. LBA62 - Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: Results from CheckMate 358. Annals of Oncology 2019;30:v898–v9 doi 10.1093/annonc/mdz394.059. [DOI] [Google Scholar]
- 6.O’Malley DM, Oaknin A, Monk BJ, Leary A, Selle F, Alexandre J, et al. LBA34 Single-agent anti-PD-1 balstilimab or in combination with anti-CTLA-4 zalifrelimab for recurrent/metastatic (R/M) cervical cancer (CC): Preliminary results of two independent phase II trials. Annals of Oncology 2020;31:S1164–S5 doi 10.1016/j.annonc.2020.08.2264. [DOI] [Google Scholar]
- 7.Reddy OL, Shintaku PI, Moatamed NA. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn Pathol 2017;12(1):45- doi 10.1186/s13000-017-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heeren AM, Punt S, Bleeker MC, Gaarenstroom KN, van der Velden J, Kenter GG, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol 2016;29(7):753–63 doi 10.1038/modpathol.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Liu M, Do MH, Chou C, Stamatiades EG, Nixon BG, et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature 2020;587(7832):121–5 doi 10.1038/s41586-020-2850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang W, O’Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity 2019;50(4):871–91 doi 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Smith AL, Robin TP, Ford HL. Molecular Pathways: Targeting the TGF-β Pathway for Cancer Therapy. Clinical Cancer Research 2012;18(17):4514–21 doi 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira AF, ten Dijke P, Zhu H-J. On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Frontiers in Cell and Developmental Biology 2020;8 doi 10.3389/fcell.2020.00605. [DOI] [PMC free article] [PubMed]
- 13.Kim B-G, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. Journal of Hematology & Oncology 2021;14(1):55 doi 10.1186/s13045-021-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng B, Ding K, Chen P, Ji J, Luo T, Guo X, et al. Anti-PD-L1/TGF-βR fusion protein (SHR-1701) overcomes disrupted lymphocyte recovery-induced resistance to PD-1/PD-L1 inhibitors in lung cancer. Cancer Commun (Lond) 2022;42(1):17–36 doi 10.1002/cac2.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho BC, Daste A, Ravaud A, Salas S, Isambert N, McClay EF, et al. Long-term follow-up of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck (SCCHN). Journal of Clinical Oncology 2021;39(15_suppl):6020- doi 10.1200/JCO.2021.39.15_suppl.6020. [DOI] [Google Scholar]
- 16.Yi M, Zhang J, Li A, Niu M, Yan Y, Jiao Y, et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol 2021;14(1):27- doi 10.1186/s13045-021-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jochems C, Tritsch SR, Pellom ST, Su Z, Soon-Shiong P, Wong HC, et al. Analyses of functions of an anti-PD-L1/TGFβR2 bispecific fusion protein (M7824). Oncotarget 2017;8(43):75217–31 doi 10.18632/oncotarget.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss J, Gatti-Mays ME, Cho BC, Hill A, Salas S, McClay E, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. Journal for immunotherapy of cancer 2020;8(2):e001395 doi 10.1136/jitc-2020-001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss J, Braiteh FS, Calvo E, Miguel MD, Cervantes A, Edenfield WJ, et al. Evaluation of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in cervical cancer: Data from phase 1 and phase 2 studies. Journal of Clinical Oncology 2021;39(15_suppl):5509- doi 10.1200/JCO.2021.39.15_suppl.5509. [DOI] [Google Scholar]
- 20.Wang J, Lou H, Cai H-B, Huang X, Li G, Wang L, et al. A study of AK104 (an anti-PD1 and anti-CTLA4 bispecific antibody) combined with standard therapy for the first-line treatment of persistent, recurrent, or metastatic cervical cancer (R/M CC). Journal of Clinical Oncology 2022;40(16_suppl):106- doi 10.1200/JCO.2022.40.16_suppl.106.34652957 [DOI] [Google Scholar]