Abstract
Background
The primary malaria vector-control interventions, indoor residual spraying and long-lasting insecticidal nets, are effective against indoor biting and resting mosquito species. Consequently, outdoor biting and resting malaria vectors might elude the primary interventions and sustain malaria transmission. Varied vector biting and resting behaviour calls for robust entomological surveillance. This study investigated the bionomics of malaria vectors in rural south-east Zambia, focusing on species composition, their resting and host-seeking behaviour and sporozoite infection rates.
Methods
The study was conducted in Nyimba District, Zambia. Randomly selected households served as sentinel houses for monthly collection of mosquitoes indoors using CDC-light traps (CDC-LTs) and pyrethrum spray catches (PSC), and outdoors using only CDC-LTs for 12 months. Mosquitoes were identified using morphological taxonomic keys. Specimens belonging to the Anopheles gambiae complex and Anopheles funestus group were further identified using molecular techniques. Plasmodium falciparum sporozoite infection was determined using sandwich enzyme-linked immunosorbent assays.
Results
From 304 indoor and 257 outdoor light trap-nights and 420 resting collection, 1409 female Anopheles species mosquitoes were collected and identified morphologically; An. funestus (n = 613; 43.5%), An. gambiae sensu lato (s.l.)(n = 293; 20.8%), Anopheles pretoriensis (n = 282; 20.0%), Anopheles maculipalpis (n = 130; 9.2%), Anopheles rufipes (n = 55; 3.9%), Anopheles coustani s.l. (n = 33; 2.3%), and Anopheles squamosus (n = 3, 0.2%). Anopheles funestus sensu stricto (s.s.) (n = 144; 91.1%) and Anopheles arabiensis (n = 77; 77.0%) were the dominant species within the An. funestus group and An. gambiae complex, respectively. Overall, outdoor CDC-LTs captured more Anopheles mosquitoes (mean = 2.25, 95% CI 1.22–3,28) than indoor CDC-LTs (mean = 2.13, 95% CI 1.54–2.73). Fewer resting mosquitoes were collected with PSC (mean = 0.44, 95% CI 0.24–0.63). Sporozoite infectivity rates for An. funestus, An. arabiensis and An. rufipes were 2.5%, 0.57% and 9.1%, respectively. Indoor entomological inoculation rates (EIRs) for An. funestus s.s, An. arabiensis and An. rufipes were estimated at 4.44, 1.15 and 1.20 infectious bites/person/year respectively. Outdoor EIRs for An. funestus s.s. and An. rufipes at 7.19 and 4.31 infectious bites/person/year, respectively.
Conclusion
The findings of this study suggest that An. rufipes may play an important role in malaria transmission alongside An. funestus s.s. and An. arabiensis in the study location.
Graphical Abstract

Keywords: Anopheles rufipes, Anopheles funestus, Anopheles arabiensis, Vector-control, Entomological inoculation rate, Zambia
Background
Malaria is endemic throughout Zambia, where it continues to be a major public health concern. In 2018, Zambia reported a national average malaria parasite prevalence of 9.1% in children under the age of five years [1, 2]. While this signifies progress compared to previous years (2010: 16.0%, 2012:14.9% and 2015:19.4% [1, 3]), this progress is not uniform across the country. In the southern regions, i.e., Lusaka and Southern provinces, malaria incidences have steadily decreased to less than 1% [1]. However, the disease remains intractable in the northern and eastern regions where parasite prevalence can be as high as 30% in children under the age of five years [1]. This is despite high coverages of primary vector-control interventions, namely indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) [4–8]. The 2018 nationwide malaria indicator survey indicated that in the southern regions, more than 83% of households had at least one LLIN or had received IRS the previous year. Coverages were higher in the northern and eastern regions; approximately 94% of households had at least one LLINs or had received IRS [1, 2].
The high malaria prevalence has been attributed, in part, to the development of insecticide resistance to commonly used insecticides for malaria vector control [4, 8–10]. Resistance to carbamates, pyrethroids and the organochlorine DDT has been reported in multiple sites in Zambia in the primary malaria vectors Anopheles funestus and Anopheles gambiae sensu stricto (s.s.) [9, 11–14]. Insecticide resistance undermines the continued efficacy offered by both LLINs and IRS by reducing mosquito susceptibility to the insecticides used in the two vector-control methods [15]. Further, behavioural resistance, such as outdoor vector biting and resting behaviour to avoid contact with insecticides, such as the increased exophagy observed in An. funestus [16, 17], poses a threat to malaria control and elimination efforts. And whilst increased vector-control interventions have led to a population decline of the primary vectors An. funestus and An. arabiensis [18, 19], this suppression has sometimes led to a proportionally increased role in malaria transmission by secondary vectors, such as Anopheles squamosus and Anopheles coustani s.l. [20–23]. In the Southern and Northern provinces of Zambia, An. coustani s.l. and An. squamosus exhibited anthropophilic tendencies with a high human blood index [23, 24] and were found harbouring malaria parasites [21, 25]. In the Eastern province, Lobo et al. [22], found a larger than expected number of sporozoite infected An. coustani s.l. mosquitoes. As many of the secondary vectors are exophilic and exophagic [26], they may have minimal contact with insecticides sprayed on the inside walls of houses or impregnated in LLINs. Subsequently, An. coustani s.l., An. squamosus or other secondary vectors may evade current vector-control interventions and thus sustain residual malaria transmission after the main endophilic and endophagic vectors have been reduced by IRS and/or LLINs [26, 27].
In recent years, Nyimba district in Eastern province Zambia has benefitted from increased vector-control interventions, primarily IRS and LLINs [13, 28, 29]. The current interventions are primarily intra-domicilliary and target mosquito species that prefer to feed and rest indoors. Thus, malaria vectors which feed, and rest outdoors may elude vector control interventions and be responsible for residual malaria transmission. This phenomenon, therefore, calls for entomological surveillance of all mosquito populations to understand which species might be responsible for transmission and whether, based on their behaviour, they will be sufficiently targeted by current interventions [30]. This study aimed to contribute to the understanding of the species composition of potential malaria vectors and their relative abundance and to determine their sporozoite infectivity and entomological inoculation rates (EIRs) as measures of malaria transmission in rural south-east Zambia and whether they will respond to current interventions.
Methods
Study area
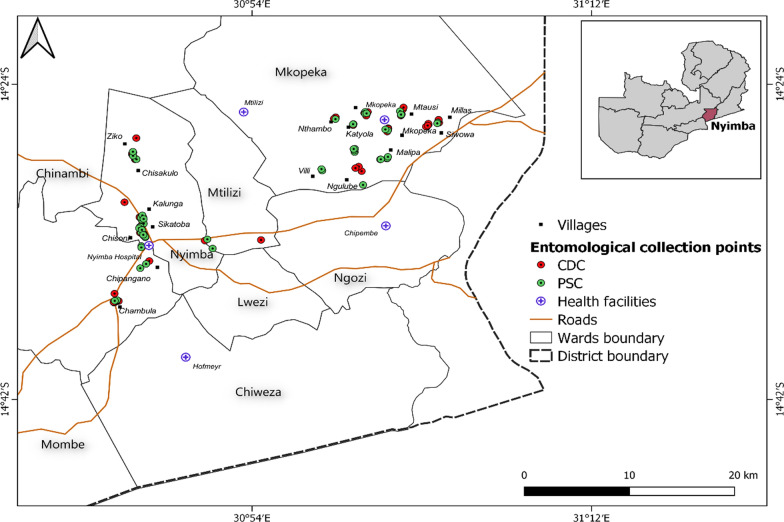
This study was conducted in Nyimba district, located in south-eastern Zambia (Fig. 1) between January-May 2019 and July 2019 to January 2020. Nyimba is predominantly a rural area with an estimated population of 108,637 persons [6]. Geographically, Nyimba district is divided into two parts; the eastern part of the district lies on a plateau whilst the western is in the Luangwa River valley. It shares an international boundary with Mozambique [31]. Nyimba district experiences three distinct seasons. Warm and wet from December to April; cool and dry winter from May to August and, hot and dry from September to November. Malaria transmission is perennial with a reported incidence rate of 467 cases per 1000 persons per year as of 2018 for the entire district [District Health Information System [DHIS]). Malaria transmission peaks after the rainy season between March and May [1].
Fig. 1.
Map of Nyimba district showing the location of households that were used for entomological collection. Insert: Map of Zambia showing the location of Nyimba district
Two neighbouring health facility catchment areas were selected for this study: Mkopeka and Nyimba Urban (Fig. 1). In 2018 Mkopeka and Nyimba Urban had malaria incidence rates of 414 and 161 cases per 1000 persons/year respectively (Nyimba District Medical Office [DMO]). The houses in the study area were largely of two types: traditional mud or fire brick walls and grass thatched roof and mud or fire brick walls with metallic roofs.
IRS is the frontline vector-control intervention with annual spraying done since 2009 [28]. Starting 2014, IRS had been conducted using blanket application of the organophosphate, pirimiphos-methyl (PM) between the years 2013 and 2018 [13, 28, 32]. In this district LLIN distributions were only done in 2014 and 2018 [33]. However, starting 2019, continuous distribution of LLINs through antenatal care (ANC) clinics and school-based distribution continued as per national guidelines. During the study period, no IRS was conducted in the study area.
Adult mosquito collection
Longitudinal mosquito surveys were conducted between January-May 2019 and July 2019 to January 2020. No collections were made in June 2019 due to logistical challenges. Households in Mkopeka and Nyimba Urban were enumerated, mapped and each household individually assigned a unique identification number. From the household list generated, 60 houses were randomly selected to serve as sentinel houses for entomological surveillance. Twenty-five served as sentinel houses for Centre for Disease Control and prevention light traps (CDC-LTs; Model 512, John W Hock, Florida, USA); 10 were in Nyimba Urban and 15 in Mkopeka. Another 35 houses were used for pyrethrum spray catches (PSC); 15 in Nyimba Urban and 20 in Mkopeka [13, 34]. The houses were spread across 20 villages. Each village had a minimum of two sentinel houses, 50 m apart, with one house serving for CDC-LT collections and another serving for PSC collections. At least 15 villages had three houses with two for PSC collections.
Mosquito collections were undertaken both indoors and outdoors using CDC-LTs. On each night of collection, two CDC-LTs were deployed per household; one inside and another outside. For indoor collections, the CDC-LT was set up between 18:00 and 06:00 h by hanging the trap, with its entrance 1.5 m above the floor and about 1.5 m away from the feet of a person sleeping under a treated mosquito net [35]. For outdoor collections, the CDC-LT was hung 5–10 m from where the family would usually sit to eat and/or spend evenings before going to bed. This distance allows for the effective range for CDC-LT whilst preventing inhabitants from acting as unprotected bait [36]. The trap was switched on at 18:00 h and switched off at 06:00 h. Both indoor and outdoor CDC-LTs, collections were made in five nights to complete the 25 houses. For each house, collections were made once per month.
Indoor mosquito resting densities were estimated monthly using pyrethrum spray collections (PSC; Mortein Energy ball®, Reckitt Benckiser) [40]. During each collection, the number of people who slept in the house the previous night and bed net use were made were recorded. PSC collections were made monthly in each of the sentinel houses. Five houses per day were sprayed, requiring 7 days to complete.
Morphological identification of mosquitoes
All collected mosquitoes were morphologically identified [37] and the physiological status of each female was noted as either unfed, fed or gravid. All morphologically identified Anopheles mosquitoes were then individually placed in clearly labelled 1.5 ml microcentrifuge tubes containing silica gel desiccant (Fisher Scientific) and cotton wool and stored for molecular analysis. All culicine mosquitoes were counted and discarded.
DNA extraction and PCR amplification for species identification
DNA was extracted using a modified salt extraction method [38]. Members of the An. funestus group (n = 236; 38.5%) and An. gambiae complex (n = 110; 37.5%) were further identified to sibling species level by polymerase chain reaction (PCR) [39–41]. Specimens that did not amplify on either the Gambiae-PCR or Funestus-PCR were confirmed using the internal transcribed spacer-2 ribosomal-DNA polymerase chain reaction i.e., ITS2 PCR. The ITS2 PCR technique targets the ITS2 region of nuclear ribosomal deoxyribonucleic acid (rDNA) to produce amplicons of varying band sizes depending on the mosquito species [21, 40, 44, 45]. In each month of collection, a subset of between 25–60% of the total collected female mosquitoes per species separated by collection method was targeted for species identification by PCR. In months where less than 10 mosquitoes were collected, all were subjected to species identification through PCR.
Blood meal analysis
Blood meal analysis was performed on blood-fed An. funestus (n = 81), An. gambiae s.l. (n = 33) and An. rufipes (n = 7). PCR analysis was used to detect and identify host blood from 121 mosquito abdomens from which DNA was extracted using the multiplex PCR assay [38] which targeted the cytochrome b region of the hosts mitochondrial DNA [38].
Detection of Plasmodium falciparum infection in mosquitoes
A random subsample, by sampling method and month of collection of female An. funestus (n = 360/613; 58.7%), An. gambiae s.l. (n = 174/293; 59.4%), An. pretoriensis (n = 72/282; 25.5%), An. rufipes (n = 42/55; 76.3%), An. coustani s.l. (n = 18/33; 54.5%) and An. squamosus (n = 3/3; 100%) mosquitoes were tested for P. falciparum circumsporozoite proteins (CSPs) using sandwich enzyme-linked immunosorbent assays (ELISA) [46]. To avoid false CSP positives common in zoophilic species the ELISA lysates were heated [47]. Sporozoite infectivity was determined separately for mosquitoes caught indoors and outdoors.
Statistical analyses
All data were entered and stored into an Excel spreadsheet (Microsoft Office 2018) and exported to open-source statistical software R version 3.51 [48] for analysis. Descriptive statistics namely mean catches per trap per night and proportions of mosquitoes caught per sampling method per catchment area were used to summarize the data. Species-specific mean catches were calculated by dividing the total number of mosquitoes caught by the number of trap-nights. The human blood index (HBI), sporozoite infectivity rate (SIR) and entomological inoculation rate (EIR) were calculated as a measure of malaria transmission intensity using the following formulae.
Human blood index (HBI)
The human blood index (HBI) was calculated as the proportion of mosquitoes fed on human blood meals out of the total mosquitoes that successfully amplified for blood meals [49].
Mixed (human + domestic animal) blood meals were added to the number of human blood meals when calculating the HBI.
Sporozoite infectivity rate (SIR)
Sporozoite infectivity rate (SIR) is defined as the proportion of Anopheles mosquitoes with sporozoites in their salivary glands to the total number of mosquitoes examined for sporozoites [50]. Sporozoite infectivity was determined separately for each species. This was determined using the following formula:
Sporozoite infectivity rates were determined separately for indoor (PSC and CDC-LTs) and outdoor (CDC-LTs only) collection methods and were species-specific. The Pearson’s Chi-square tests were used to evaluate the difference in proportions and infectivity rates at an α = 0.05 level of significance.
Entomological inoculation rate (EIR)
Entomological inoculation rate (EIR) is defined as the number of infectious bites per person per unit time, usually expressed per year or month [51]. Species-specific EIR was calculated based on the mean number of female Anopheles mosquitoes caught per trap/night, without adjusting for room occupancy [10, 50]. Annual EIR was calculated separately for indoors and outdoors using the formula:
For PSC collections, EIRs was calculated using the formula described in [52].
EIR = Human Biting Rate (HBR) x SIR × 365 days where SIR as defined above and the human biting rate as shown below.
Results
Species composition of Anopheles mosquitoes
The sampling design of this study resulted in an overall 304 indoor and 257 outdoor CDC light trap-night collections. Less frequent outdoor CDC-LTs collections were due to the rainy season when heavy rains would interfere with trapping. A total of 420 resting collections were done using the pyrethrum spray catch (PSC) method. The average number of human occupants during PSC collections was three.
A total of 1409 female Anopheles mosquitoes were collectively sampled in 977 collections. Overall, seven species were identified morphologically. The An. funestus group (n = 613; 43.5%) represented the predominant malaria vectors in the study area followed by An. gambiae s.l. (n = 293; 20.8%). Other species were Anopheles pretoriensis (n = 282; 20.0%), Anopheles maculipalpis (n = 130; 9.2%), An. rufipes (n = 55; 3.9%), An. coustani s.l. (n = 33; 2.3%), and An. squamosus (n = 3, 0.2%). Table 1 summarizes the species composition and mean collections per sampling method per night. Only eight male Anopheles mosquitoes were collected: An. gambiae s.l. (n = 3) and An. pretoriensis (n = 5). At the same time 2052 female culicine mosquitoes were collected.
Table 1.
Anopheles species composition and mean collections per sampling method in the study area
| Species | Overall | CDC LT-IN | CDC | PSC | |||
|---|---|---|---|---|---|---|---|
| LT-OUT | |||||||
| N | n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | |
| An. funestus group | 613 | 331 | 1.09 (0.92–1.25) | 140 | 0.55 (0.46- 0.65) | 142 | 0.34 (0.19–0.42) |
| An. gambiae s.l | 293 | 167 | 0.55 (0.38–0.71) | 107 | 0.42 (0.35–0.49) | 19 | 0.04 (0.02–0.06) |
| An. pretoriensis | 282 | 82 | 0.27 (0.15–0.39) | 183 | 0.71 (0.46–0.97) | 17 | 0.04 (0.01–0.07) |
| An. maculipalpis | 130 | 53 | 0.17 (0.06–0.29) | 74 | 0.29 (0.22–0.36) | 3 | 0.01 (0–0.01) |
| An. rufipes | 55 | 6 | 0.03 (0.02–0.04) | 47 | 0.18 (0.14–0.22) | 2 | 0.004 |
| An. coustani | 33 | 8 | 0.03 (0–0.05) | 25 | 0.10 (0.08–0.11) | 0 | 0 |
| An. squamosus | 3 | 1 | 0 | 2 | 0.01 (0–0.02) | 0 | 0 |
CDC Centers for Disease Control and Prevention, LT Light Trap, PSC Pythrerum Spray Catches, IN Indoor OUT Outdoor
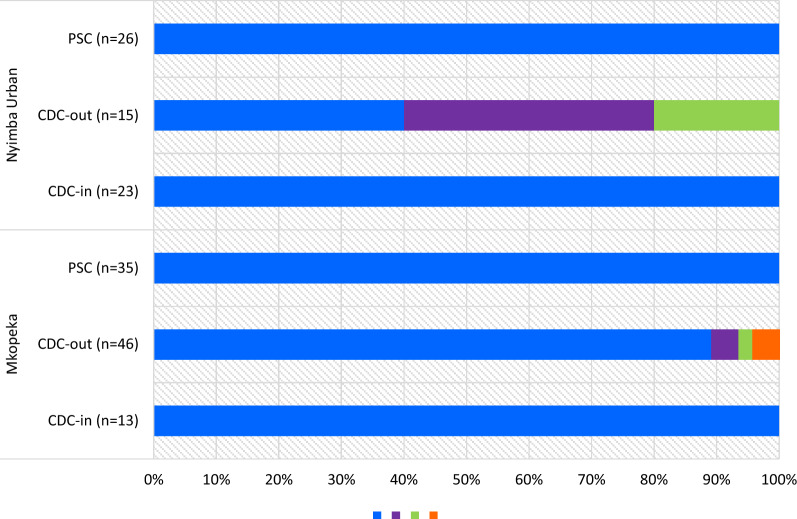
Polymerase chain reaction was performed on a random subsample of 236 (38.5%) of all collected female An. funestus mosquitoes. Of these, 158 specimens successfully amplified. A total of 74 specimens did not amplify and four gave non-specific amplification on the ITS2-PCR (n = 2, 700 base pairs and n = 2, 900 bp). Overall, collections from both sites revealed the predominant species found was An. funestus sensu stricto (s.s.) (n = 144/158; 91.1%); PSC (n = 61/61), indoor CDC-LT (n = 36/36) and outdoor CDC-LT (n = 47/61). There was a significantly higher occurrence of An. funestus s.s. in indoor versus outdoor traps (χ2 = 7.73, df = 1, P = 0.03). Other species identified within the An. funestus group were Anopheles leesoni (n = 8; 5.1%), Anopheles parensis (n = 4; 2.5%) and Anopheles vaneedeni (n = 2; 1.2%). Anopheles leesoni, An. parensis and An. vaneedeni amplified from specimens caught only outdoors. Figure 2 shows the different proportions of species within the An. funestus group per sampling method per site.
Fig. 2.
Proportions of species within the Anopheles funestus group in the two study areas. The numbers in parentheses indicate the total number of specimens that successfully amplified per collection method per study site
Polymerase chain reaction (PCR) was performed on a random subsample of 110 (37.5%) female An. gambiae s.l. mosquitoes. Of these 100 successfully amplified. Eight did not amplify and two gave non-specific amplifications on the ITS2-PCR (n = 2, 280 bp) upon further analyses.
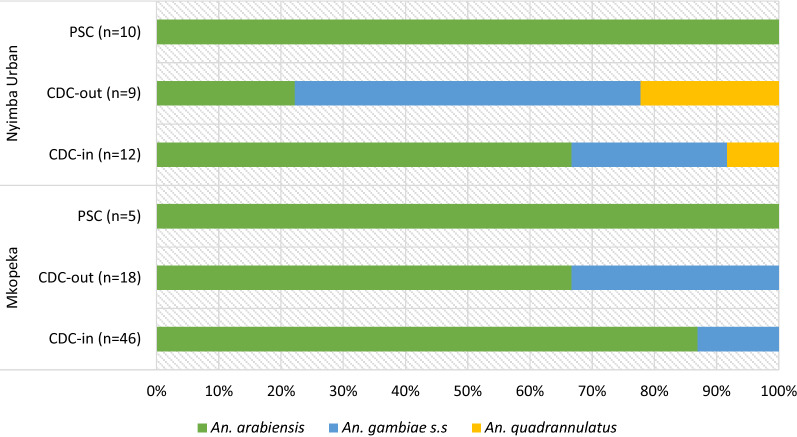
Within the An. gambiae complex, the predominant species was An. arabiensis (n = 77; 77.0%); PSC (n = 15/15), indoor CDC-LT (n = 48/58) and outdoor CDC-LT (n = 14/27). Anopheles gambiae s.s. (n = 20; 20.0%) and Anopheles quadriannulatus (n = 3; 3.0%) were the two other species within this complex in the study area. No An. gambiae s.s. were found in PSC with few occurring in indoor (n = 9/61) and outdoor (n = 11/27) CDC-LT collections. Likewise, no An. quadriannulatus were collected using PSC with few collected in indoor (n = 1/61) and outdoor (n = 2/27) CDC-LT collections. Figure 3 shows species composition and proportions within An. gambiae s.l. per collection method and separated by study site.
Fig. 3.
Proportions of species within the Anopheles gambiae complex in the two study areas. The numbers in parentheses indicate the total number of successfully amplified specimens per collection method per study site
Indoor and outdoor host-seeking and resting collections
Similar numbers of host-seeking Anopheles mosquitoes were trapped with light traps outdoors (mean = 2.25, 95% CI 1.22–3.28) and indoors (mean = 2.13, 95% 1.54–2.73) per trap. Fewer mosquitoes were collected per PSC trap night (mean = 0.44, 95% CI 0.24–0.63).
At the species level, more host-seeking mosquitoes of the An. funestus group were trapped using indoor CDC-LTs (95% CI 0.92–1.25) per night per house than outdoors (mean 0.55; 95% CI 0.46–0.65) (Table1). Indoor resting densities of An. funestus group were slightly lower with a mean of 0.31 (95% CI 0.19–0.42) per house. Only 23.2% of all collected female An. funestus mosquitoes (n = 142/613) were caught resting indoors with most of these blood-fed (n = 123/142, 87.6%).
The mean number of An. gambiae s.l. mosquitoes trapped with indoor CDC-LTs (mean = 0.55, 95% CI 0.38–0.71) per night per house was slightly higher than collected outdoors (mean = 0.42, 95% CI 0.35–0.49) (Table 1). Only 6.5% of all collected female An. gambiae s.l. mosquitoes (n = 19/293) were caught resting indoors with most of these being blood-fed (n = 16/19, 84.2%).
The 503 other anopheline specimens, included the species An. pretoriensis, An. maculipalpis, An. rufipes, An. coustani s.l. and An. squamosus. Most of these were caught outdoors (n = 318/503, 63.2%) rather than indoors (n = 150/503, 29.8%). Taken together, a larger proportion of these specimens were outdoor host-seeking (χ2 = 21.1, df = 4, P < 0.01). Few of the other anopheline specimens were caught resting indoors (n = 22/503, 4.4%) with zero blood-fed.
Blood meal sources
Of the 121 blood-fed mosquitoes analysed, only 18 (14.9%) amplified successfully. Of these, 13 blood meals were from humans and three had mixed human-goat blood meal host (Table 2). The overall human blood index from resting collections and CDC-LT collections both indoors and outdoors was found to be 0.89. Due to the small sample size of mosquitoes that amplified on the blood meal analysis, these results are interpreted with caution.
Table 2.
Blood meal sources of Anopheles mosquitoes per sampling method
| Method | Anopheles species | # analysed | Human | Mixed: human/goat | Dog | Unamplified | Human blood index |
|---|---|---|---|---|---|---|---|
| PSC | An. funestus | 40 | 3 | 1 | 0 | 36 | 1.00 |
| An. gambiae | 14 | 2 | 0 | 0 | 12 | 1.00 | |
| CDC LT indoors | An. funestus | 20 | 3 | 1 | 0 | 16 | 1.00 |
| An. gambiae | 10 | 2 | 0 | 0 | 8 | 1.00 | |
| CDC LT outdoors | An. funestus | 21 | 3 | 1 | 0 | 17 | 1.00 |
| An. gambiae | 9 | 0 | 0 | 0 | 9 | 0.00 | |
| An. rufipes | 7 | 0 | 0 | 2 | 5 | 0.00 | |
| Total | 121 | 13 | 3 | 2 | 103 | 0.89 |
Sporozoite infectivity and entomological inoculation rates
A total of 360 (58.7%) female specimens of the An. funestus group were tested for the presence of P. falciparum circumsporozoite protein (Pf CSP). Of these, nine mosquitoes tested positive for sporozoites giving an overall sporozoite infectivity rate of 2.5%. The nine sporozoite infected mosquitoes came from samples collected in February 2019 (n = 3), March 2019 (n = 2), July 2019 (n = 1) and January 2020 (n = 3). All sporozoite infected mosquitoes were An. funestus s.s. Other species within the An. funestus group, namely An. leesoni, An. parensis and An. vaneendeni tested negative for P. falciparum sporozoites.
A total of 174 (59.4%) female An. gambiae s.l. mosquitoes were tested for the presence of the Pf CSP. One tested positive giving an overall sporozoite infectivity rate of 0.57%. The sporozoite infected mosquito was An. arabiensis trapped in March 2019. The other members within the An. gambiae complex namely, An. gambiae s.s. and An. quadriannulatus tested negative for P. falciparum sporozoites.
Other anopheline mosquitoes, namely An. pretoriensis (n = 70/282; 24.8.0%), An. coustani s.l. (n = 17/33; 51.5%), An. rufipes (n = 33/55; 94%) and An. squamosus (n = 3/3; 100%) were analysed for Pf-CSP. Three An. rufipes specimens tested positive for sporozoites, giving an overall sporozoite infectivity rate of 9.1% for An. rufipes (Table 3). The three sporozoite infected An. rufipes were trapped indoors using CDC-LTs in February 2019 (n = 1) and outdoors using CDC-LTs in March and February 2019 (n = 2) in the Mkopeka study sites. The morphological identification of the An. rufipes mosquitoes was confirmed using the ITS2-PCR, resulting in an amplification of 500 bp. In all the above, heating the ELISA lysate did not change the Pf-CSP positive result.
Table 3.
Annual EIR estimation based on CDC-LT and PSC catches for An. arabiensis, An. funestus s.s and An. rufipes mosquitoes
| Method | Species | # assayed | Sporozoite positive | Proportion of mosquitoes infected (SIR) | EIR (ib/p/yr) |
|---|---|---|---|---|---|
| CDC-LT Indoors | An. funestus group | 179 | 2 | 0.01 | 4.44 |
| An. gambiae s.l | 91 | 1 | 0.01 | 1.15 | |
| An. rufipes | 6 | 1 | 0.17 | 1.20 | |
| CDC-LT Outdoors | An. funestus group | 83 | 3 | 0.04 | 7.19 |
| An. gambiae s.l | 83 | 0 | 0.00 | 0.0 | |
| An. rufipes | 27 | 2 | 0.07 | 4.31 | |
| PSC | An. funestus group | 98 | 4 | 0.05 | 1.19 |
The species-specific estimated indoor and outdoor annual EIR based on CDC-LT catches for An. arabiensis, An. funestus s.s. and An. rufipes mosquitoes is shown in Table 3. Indoor EIRs for An. funestus s.s, and An. arabiensis were estimated at 4.44 and 1.15 infectious bites per person per year (ib/p/y), respectively. Indoor EIR for An. rufipes in the study area was estimated at 1.20 ib/p/y. Outdoor EIR for An. funestus s.s and An. rufipes were estimated at 7.19 and 4.31 ib/p/y, respectively (Table 3). Only An. funestus specimens, collected with PSC, tested positive for sporozoites. Indoor EIRs for An. funestus s.s, collected with PSC, was estimated at 1.19 ib/p/y. However, these results are interpreted with caution due to the extremely low number of blood meals that were amplified in the blood meal analysis.
Discussion
Anopheles funestus group made up the majority of anopheline mosquitoes collected in this study. Species identification by PCR further revealed that this group was predominantly made up of An. funestus s.s. (henceforth simply referred to as An. funestus). This confirms previous reports that describe An. funestus as the main driver of malaria transmission in the study area [22, 28, 53]. Anopheles funestus is historically highly anthropophilic with strong endophagic and endophilic behaviour [54, 55]. Thus, in the absence of insecticide resistance and/or improved formulations of current insecticides, this species may be controlled by LLINs and IRS [55]. This is supported by the fact that the indoor EIR by An. funestus reported in this study (4.4 ib/p/y) was 16 times lower than previously reported in the same location. An EIR of 70.1 ib/p/y was observed between the years 2011–2013 [53]. This decreased EIR may highlight suppression of sporozoite infectivity following increased vector-control interventions, namely LLINs and IRS with pirimiphos-methyl (IRS-PM). These observations are consistent with previous studies conducted in other parts of Zambia which demonstrated the impact of increased IRS-PM and population-wide coverage of LLINs in reducing sporozoite infection rates of An. funestus [11, 19]. Similar findings have been reported in neighbouring Mozambique [56], north-western Tanzania [57] and western Kenya [58]. However, that malaria transmission persists, albeit at low levels, shows that these core interventions cannot be deployed solely.
The persistence of malaria has been associated with behavioral changes observed in anopheline mosquitoes. Findings of this study indicate that An. funestus may also be transmitting malaria outdoors. In this study, An. funestus outdoor EIR, estimated at 7.19 ib/p/y was higher than EIR indoor. The higher outdoor EIR in An. funestus may highlight suppression of the highly endophagic species, thereby increasing the proportions of outdoor host seeking mosquitoes [16, 17]. This behavioural modification may be as result of the increased use of LLINs or IRS in the study area [16, 59]. The outdoor malaria transmission described in this study has implications for malaria control and eradication in Zambia and in sub-Saharan Africa. A recent study shows that a 10% increase in outdoor biting would result in 58.2% increase in malaria cases per year on the African continent, assuming a “perfect scenario” of 100% LLINs coverage and zero insecticide resistance [60]. Outdoor biting vectors, thus pose a significant threat to elimination efforts by sustaining malaria transmission. Subsequently, indoor-vector control interventions such as LLINs and IRS alone may not be enough to eliminate malaria [61, 62].
Secondary vectors may also play a role in continued malaria transmission. In this study sporozoite infected specimens of An. rufipes were found. Similar findings of An. rufipes harbouring sporozoites have been reported in southern Zambia [25], Kenya [63], Cameroon [64–66], Burkina Faso [67] and Nigeria [68]. This study thus incriminates An. rufipes as a potential malaria vector in rural south-east Zambia [69] with estimated EIRs of 1.20 and 4.31 ib/p/y indoors and outdoors, respectively. The estimated EIR for An. rufipes was higher than that of An. arabiensis, indicating the need for further studies to investigate the role of secondary malaria vectors in maintaining malaria transmission [26, 70]. Sporozoite infected An rufipes mosquitoes were collected during the peak malaria season in Zambia, between February and April [1, 70] when vectors were most abundant. That this species is largely zoophilic and exophagic [25] makes it a threat to achieving malaria elimination as it may evade indoor-centric vector-control interventions [26].
Anopheles gambiae s.l., which was primarily An. arabiensis, confirming previous results [71], was found with lower sporozoite infectivity when compared to An. rufipes. Thus, in Nyimba district, An. arabiensis may be considered a vector of secondary importance when compared to An. funestus and An. rufipes. This study also confirms previous observations that in cases where An. arabiensis and An. funestus occur in sympatry, the latter appears to be the more competent malaria vector [55, 72, 73]. Nonetheless, that An. arabiensis was found in both indoor and outdoor traps suggest that it can forage both indoors and outdoors thereby making it less amenable to the traditional indoor-based vector-control interventions [19, 74].
The mosquito community in this study included diverse species. Within the An. funestus group, were found An. leesoni, An. parensis and An. vaneedeni- largely zoophilic species [27] all of which tested negative for malaria parasites. Similarly, other members of the An. gambiae complex, namely, An. quadriannulatus and An. gambiae s.s. also tested negative for malaria parasites. However, Lobo et al. [22] found sporozoite infected An. quadriannulatus, An. pretoriensis and An. coustani from the same study locations. Thus, in this region of Zambia, the vector population plasticity, species diversity and co-occurrence of both primary and secondary vectors with different behaviours, may sustain malaria transmission and calls for more integrated vector-control approaches. Future research should determine the bionomics, morphology, and breeding habitats of potential secondary vectors for a comprehensive understanding of their roles in malaria transmission [21–27]. Additionally, the period (less than a year) and geographical scope of sampling was not extensive and may explain some of the low vector densities observed in this study. More sampling sites are required to establish malaria transmission by An. rufipes and other potential secondary vectors. A further limitation of this study was the lack of amplification of some specimens for PCR species identification. This may be attributed to specimen degradation or morphological misidentification, attributed to damaged mosquito specimens. This is common with CDC-LT collections [22]. This calls for improvement in and coupling of morphological identifications with molecular methods of identification. Furthermore, molecular identification was not performed beyond the ITS2 PCR. A two-step procedure for species identification was carried out; first morphological identifications based on morphological keys [37] similar to methods used by Tabue et al.[64] and Awono-Ambene et al. [65]. Second, confirmation of the identification using the ITS2 PCR to ensure that the specimens identified as An. rufipes were indeed such. Additional molecular identifications- perhaps by ITS2 gene sequencing to adequately incriminate and identify vectors of malaria [22, 27] should be included in future research.
Findings of this study are limited by several factors. An extremely small number of samples amplified for the blood-meal analyses. Several re-runs were made without success. This might be due to storage conditions. Possibly, DNA of the blood meal host may have been degraded since specimens were stored for several months on silica gel before molecular analysis. Further, mosquitoes may have had incomplete blood meals or the blood meal may have been digested resulting in degradation of host DNA [75]. The successful identification of blood meal hosts by PCR depends on the quality and quantity of the host´s DNA contained in the abdomen of mosquitoes [75]. Yet another possiblity is that mosquitoes fed on hosts other than those included in the primer set e.g., avian-specific primers. Further investigations in blood meal studies in Zambia to document the range of blood meal hosts of malaria vectors are strongly recommended.
Conclusion
This study confirms earlier reports that An. rufipes might be involved in malaria transmission in rural south-east Zambia. Whilst for long, the species has been considered of secondary importance in Zambia due to its largely zoophilic, exophilic and exophagic tendencies, recent successes in vector control require a new evaluation of the remaining vectors. Based on these findings, increased routine entomological surveillance and Plasmodium sporozoite infectivity screening for all potential malaria vectors is recommended. Additionally, vector-control interventions should be diversified to include outdoor interventions for improved control and efforts towards malaria elimination.
Acknowledgements
The authors are grateful to the communities in Nyimba Urban and Mkopeka in whose households collections were made. We thank the Ministry of Health through the National Malaria Elimination Centre (NMEC), Nyimba District Medical Office and traditional leadership in Nyimba Urban and Mkopeka for their co-operation and support. We thank Limonthy Simubali and Twig Mudenda from Macha Research Trust for their help on the molecular analysis.We thank Emily Kimathi for her help with the map and Dr. Cheryl Tosh for editing.
Abbreviations
- LLINs
Long-lasting insecticidal nets
- IRS
Indoor residual spraying
- IRS-PM
Indoor residual spraying with pirimiphos-methyl
- DMO
District medical office
- CDC LTs
Centres for disease control and prevention light traps
- PSC
Pyrethrum spray catches
- PCR
Polymerase chain reaction
- CSP
Circumsporozoite protein
- ELISA
Enzyme-linked immunosorbent assays
- SIR
Sporozoite infectivity rate
- EIR
Entomological inoculation rates
Author contributions
CMM, UF, EC and FM conceived the study and wrote the main study protocol. KS, CdJ, POS, UF and CMM designed this study. KS, MM, AS, FM, POS and BH supervised the study data collections. KS and AS performed the molecular analysis. KS performed data analysis. KS wrote the initial draft of the manuscript, which was revised by CMM, UF, FM, CdJ, POS, TEN and BH. All authors read and approved the final manuscript.
Funding
The authors gratefully acknowledge the financial support for this research by the following organizations and agencies: Global Environmental Fund (GEF) through United Nations Environmental Programme (UNEP) and the WHO-Africa Regional Office (WHO-AFRO) (Grant number: 4668); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors. Kochelani Saili was supported by a German Academic Exchange Service (DAAD) In-Region Postgraduate Scholarship and a University of Pretoria doctoral bursary.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol and informed consent forms were reviewed and approved by the ERES Converges IRB Zambia (Reference: 2018-Oct-007 and 2020-Jul-018), the National Research Health Authority (Ref: NHRA00002/23/04/2021 and Health Researcher Registration #: NHRAR-R-119/27/05/2022) and the research ethics committee of the University of Pretoria (Ref: 242/2020). Written permission to undertake the study was obtained from the Ministry of Health through the National Malaria Elimination Centre (NMEC) and Nyimba District Medical office. Local and traditional leadership were also informed about the purposes of the study. Participation in the study was voluntary, and informed consent was obtained from household heads and every participant above the age of 18 years. Verbal consent was obtained from household heads before routine mosquito collections.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Republic of Zambia MoH. 2018 Zambia National Malaria Indicator Survey 2018. Lusaka, Zambia.
- 2.WHO . World malaria report 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 3.Republic of Zambia MoH. Zambia National Malaria Indicator Survey, 2015. Lusaka, Zambia, 2015.
- 4.Mukonka VM, Chanda E, Haque U, Kamuliwo M, Mushinge G, Chileshe J, et al. High burden of malaria following scale-up of control interventions in Nchelenge District, Luapula Province. Zambia Malar J. 2014;13:153. doi: 10.1186/1475-2875-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawa M, Hangoma P, Morse AP, Michelo C. Investigating the upsurge of malaria prevalence in Zambia between 2010 and 2015: a decomposition of determinants. Malar J. 2019;18:61. doi: 10.1186/s12936-019-2698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamainza B, Moonga H, Sikaala CH, Kamuliwo M, Bennett A, Eisele TP, et al. Monitoring, characterization and control of chronic, symptomatic malaria infections in rural Zambia through monthly household visits by paid community health workers. Malar J. 2014;13:128. doi: 10.1186/1475-2875-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hast MA, Chaponda M, Muleba M, Kabuya J-B, Lupiya J, Kobayashi T, et al. The impact of three years of targeted IRS with pirimiphos-methyl on malaria parasite prevalence in a high-transmission area of northern Zambia. Am J Epidemiol. 2019;188:2120–2130. doi: 10.1093/aje/kwz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steketee RW, Miller JM, Kawesha EC. Implications of the MDA trial in Southern Province, Zambia, for malaria control and elimination. Am J Trop Med Hyg. 2020;103:98–101. doi: 10.4269/ajtmh.19-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanda E, Hemingway J, Kleinschmidt I, Rehman AM, Ramdeen V, Phiri FN, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS ONE. 2011;6:e24336. doi: 10.1371/journal.pone.0024336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson JC, Pinchoff J, Muleba M, Lupiya J, Chilusu H, Mwelwa I, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: implications for vector control. Parasit Vectors. 2016;9:510. doi: 10.1186/s13071-016-1786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanda J, Saili K, Phiri F, Stevenson JC, Mwenda M, Chishimba S, et al. Pyrethroid and carbamate resistance in Anopheles funestus Giles along Lake Kariba in southern Zambia. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.19-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi KS, Christian R, Nardini L, Wood OR, Agubuzo E, Muleba M, et al. Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasit Vectors. 2014;7:464. doi: 10.1186/s13071-014-0464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamainza B, Sikaala CH, Moonga HB, Chanda J, Chinula D, Mwenda M, et al. Incremental impact upon malaria transmission of supplementing pyrethroid-impregnated long-lasting insecticidal nets with indoor residual spraying using pyrethroids or the organophosphate, pirimiphos methyl. Malar J. 2016;15:100. doi: 10.1186/s12936-016-1143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomsen EK, Strode C, Hemmings K, Hughes AJ, Chanda E, Musapa M, et al. Underpinning sustainable vector control through informed insecticide resistance management. PLoS ONE. 2014;9:e99822. doi: 10.1371/journal.pone.0099822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Seyoum A, Sikaala CH, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M, et al. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley. South-east Zambia Parasit Vectors. 2012;5:101. doi: 10.1186/1756-3305-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ippolito MM, Gebhardt ME, Ferriss E, Schue JL, Kobayashi T, Chaponda M, et al. Scientific findings of the Southern and Central Africa International center of excellence for malaria research: ten years of malaria control impact assessments in hypo-, meso-, and holoendemic transmission zones in Zambia and Zimbabwe. Am J Trop Med Hyg. 2022;107:55–67. doi: 10.4269/ajtmh.21-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hast MA, Stevenson JC, Muleba M, Chaponda M, Kabuya J-B, Mulenga M, et al. The impact of three years of targeted indoor residual spraying with pirimiphos-methyl on household vector abundance in a high malaria transmission area of northern Zambia. Am J Trop Med Hyg. 2021;104:683–694. doi: 10.4269/ajtmh.20-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanda E, Phiri FN, Chanda J, Ramdeen V, Kamu-Liwo M, Baboo KS. Impact of entomological interventions on malaria vector bionomics in low transmission settings in Zambia. J Public Health Epidemiol. 2012;4:189–196. doi: 10.5897/JPHE12.038. [DOI] [Google Scholar]
- 20.Hoffman JE, Ciubotariu II, Simubali L, Mudenda T, Moss WJ, Carpi G, et al. Phylogenetic complexity of morphologically identified Anopheles squamosus in Southern Zambia. Insects. 2021;12:146. doi: 10.3390/insects12020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson JC, Simubali L, Mbambara S, Musonda M, Mweetwa S, Mudenda T, et al. Detection of Plasmodium falciparum infection in Anopheles squamosus (Diptera: Culicidae) in an area targeted for malaria elimination. Southern Zambia J Med Entomol. 2016;53:1482–1487. doi: 10.1093/jme/tjw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo NF, St Laurent B, Sikaala CH, Hamainza B, Chanda J, Chinula D, et al. Unexpected diversity of Anopheles species in Eastern Zambia: implications for evaluating vector behavior and interventions using molecular tools. Sci Rep. 2015;5:17952. doi: 10.1038/srep17952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha. Zambia. Vector Borne Zoonotic Dis. 2011;11:1173–9. doi: 10.1089/vbz.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciubotariu II, Jones CM, Kobayashi T, Bobanga T, Muleba M, Pringle JC, et al. Genetic diversity of Anopheles coustani (Diptera: Culicidae) in malaria transmission foci in Southern and Central Africa. J Med Entomol. 2020;57:1782–1792. doi: 10.1093/jme/tjaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebhardt ME, Searle KM, Kobayashi T, Shields TM, Hamapumbu H, Simubali L, et al. Understudied Anophelines contribute to malaria transmission in a low-transmission setting in the Choma District, Southern Province. Zambia Am J Trop Med Hyg. 2022;106:1406–1413. doi: 10.4269/ajtmh.21-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afrane YA, Bonizzoni M, Yan G. Secondary malaria vectors of sub-Saharan Africa: threat to malaria elimination on the continent. In: Rodriguez-Morales AJ, editor. Current Topics in Malaria. IntechOpen; 2016. pp. 473–490. [Google Scholar]
- 27.Stevenson JC, Norris DE. Implicating cryptic and novel anophelines as malaria vectors in Africa. Insects. 2016;8:1. doi: 10.3390/insects8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen DA, Martin A, Pollard D, Nielsen CF, Hamainza B, Burns M, et al. Leveraging risk maps of malaria vector abundance to guide control efforts reduces malaria incidence in Eastern Province. Zambia Sci Rep. 2020;10:10307. doi: 10.1038/s41598-020-66968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chizema-Kawesha E, Miller JM, Steketee RW, Mukonka VM, Mukuka C, Mohamed AD, et al. Scaling up malaria control in Zambia: progress and impact 2005–2008. Am J Trop Med Hyg. 2010;83:480–488. doi: 10.4269/ajtmh.2010.10-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conn JE, Norris DE, Donnelly MJ, Beebe NW, Burkot TR, Coulibaly MB, et al. Entomological monitoring and evaluation: diverse transmission settings of ICEMR projects will require local and regional malaria elimination strategies. Am J Trop Med Hyg. 2015;93:28–41. doi: 10.4269/ajtmh.15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumbo DJ, Mumba KY, Kaliwile MM, Moombe KB, Mfuni TI. 2016. Agrarian changes in the Nyimba District of Zambia. In: Deakin EL, Kshatriya M, Sunderland TC (Eds), Agrarian change in tropical landscapes Center for International Forestry Research.
- 32.Chinula D, Hamainza B, Chizema E, Kavishe DR, Sikaala CH, Killeen GF. Proportional decline of Anopheles quadriannulatus and increased contribution of An. arabiensis to the An. gambiae complex following introduction of indoor residual spraying with pirimiphos-methyl: an observational, retrospective secondary analysis of pre-existing data from south-east Zambia. Parasit Vectors. 2018;11:544. doi: 10.1186/s13071-018-3121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masaninga F, Mukumbuta N, Ndhlovu K, Hamainza B, Wamulume P, Chanda E, et al. Insecticide-treated nets mass distribution campaign: benefits and lessons in Zambia. Malar J. 2018;17:173. doi: 10.1186/s12936-018-2314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malaria Elimination Initiative . Entomological surveillance planning tool. San Francisco: The Global Health Group, University of California; 2020. [Google Scholar]
- 35.Mboera L, Kihonda J, Braks M, Knols B. Influence of centers for disease control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am J Trop Med Hyg. 1998;59:595–596. doi: 10.4269/ajtmh.1998.59.595. [DOI] [PubMed] [Google Scholar]
- 36.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillies M, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;5:1–143. [Google Scholar]
- 38.Kent RJ, Norris DE. Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005;73:336–342. doi: 10.4269/ajtmh.2005.73.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 40.Koekemoer LL, Lochouarn L, Hunt RH, Coetzee M. Single-strand conformation polymorphism analysis for identification of four members of the Anopheles funestus (Diptera: Culicidae) group. J Med Entomol. 1999;36:125–130. doi: 10.1093/jmedent/36.2.125. [DOI] [PubMed] [Google Scholar]
- 41.MR4. Methods in Anopheles Research- 4th Edn. BEI Resources. 2014.
- 42.Irish SR, Kyalo D, Snow RW, Coetzee M. Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical Region and associated islands. Zootaxa. 2020 doi: 10.11646/zootaxa.4747.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nkya TE, Fillinger U, Sangoro OP, Marubu R, Chanda E, Mutero CM. Six decades of malaria vector control in southern Africa: a review of the entomological evidence-base. Malar J. 2022;21:279. doi: 10.1186/s12936-022-04292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohuet A, Simard F, Toto J-C, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg. 2003;69:200–205. doi: 10.4269/ajtmh.2003.69.200. [DOI] [PubMed] [Google Scholar]
- 45.Spillings BL, Brooke BD, Koekemoer LL, Chiphwanya J, Coetzee M, Hunt RH. A new species concealed by Anopheles funestus Giles, a major malaria vector in Africa. Am J Trop Med Hyg. 2009;81:510–515. doi: 10.4269/ajtmh.2009.81.510. [DOI] [PubMed] [Google Scholar]
- 46.Wirtz R, Zavala F, Charoenvit Y, Campbell G, Burkot T, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 47.Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195. doi: 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. URL http://www.rstudio.com/..
- 49.Pappa V, Reddy M, Overgaard HJ, Abaga S, Caccone A. Estimation of the human blood index in malaria mosquito vectors in Equatorial Guinea after indoor antivector interventions. Am J Trop Med Hyg. 2011;84:298. doi: 10.4269/ajtmh.2011.10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drakeley C, Schellenberg D, Kihonda J, Sousa C, Arez A, Lopes D, et al. An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8:767–774. doi: 10.1046/j.1365-3156.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 51.Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, Wamai P, et al. Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol. 2014;43:200–206. doi: 10.1093/jmedent/43.2.200. [DOI] [PubMed] [Google Scholar]
- 52.WHO . Malaria entomology and vector control. Geneva: World Health Organization; 2013. [Google Scholar]
- 53.Sikaala CH, Chinula D, Chanda J, Hamainza B, Mwenda M, Mukali I, et al. A cost-effective, community-based, mosquito-trapping scheme that captures spatial and temporal heterogeneities of malaria transmission in rural Zambia. Malar J. 2014;13:225. doi: 10.1186/1475-2875-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahamba NF, Finda M, Ngowo HS, Msugupakulya BJ, Baldini F, Koekemoer LL, et al. Using ecological observations to improve malaria control in areas where Anopheles funestus is the dominant vector. Malar J. 2022;21:158. doi: 10.1186/s12936-022-04198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagman JM, Varela K, Zulliger R, Saifodine A, Muthoni R, Magesa S, et al. Reduced exposure to malaria vectors following indoor residual spraying of pirimiphos-methyl in a high-burden district of rural Mozambique with high ownership of long-lasting insecticidal nets: entomological surveillance results from a cluster-randomized trial. Malar J. 2021;20:54. doi: 10.1186/s12936-021-03583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kakilla C, Manjurano A, Nelwin K, Martin J, Mashauri F, Kinung’hi SM, et al. Malaria vector species composition and entomological indices following indoor residual spraying in regions bordering Lake Victoria. Tanzania Malar J. 2020;19:383. doi: 10.1186/s12936-020-03452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abong’o B, Gimnig JE, Torr SJ, Longman B, Omoke D, Muchoki M, et al. Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci Rep. 2020;10:4518. doi: 10.1038/s41598-020-61350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes: new insights into malaria vectors. IntechOpen; 2013. [Google Scholar]
- 60.Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez MH. Residual malaria: limitations of current vector control strategies to eliminate transmission in residual foci. J Infect Dis. 2021;223:S55–S60. doi: 10.1093/infdis/jiaa582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loha E, Deressa W, Gari T, Balkew M, Kenea O, Solomon T, et al. Long-lasting insecticidal nets and indoor residual spraying may not be sufficient to eliminate malaria in a low malaria incidence area: results from a cluster randomized controlled trial in Ethiopia. Malar J. 2019;18:141. doi: 10.1186/s12936-019-2775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong D, Hemming-Schroeder E, Wang X, Kibret S, Zhou G, Atieli H, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci Rep. 2020;10:16139. doi: 10.1038/s41598-020-73073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabue RN, Awono-Ambene P, Etang J, Atangana J, Antonio-Nkondjio C, Toto JC, et al. Role of Anopheles (Cellia) rufipes (Gough, 1910) and other local anophelines in human malaria transmission in the northern savannah of Cameroon: a cross-sectional survey. Parasit Vectors. 2017;10:22. doi: 10.1186/s13071-016-1933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awono-Ambene PH, Etang J, Antonio-Nkondjio C, Ndo C, Eyisap WE, Piameu MC, et al. The bionomics of the malaria vector Anopheles rufipes Gough, 1910 and its susceptibility to deltamethrin insecticide in North Cameroon. Parasit Vectors. 2018;11:253. doi: 10.1186/s13071-018-2809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekoko WE, Awono-Ambene P, Bigoga J, Mandeng S, Piameu M, Nvondo N, et al. Patterns of anopheline feeding/resting behaviour and Plasmodium infections in North Cameroon, 2011–2014: implications for malaria control. Parasit Vectors. 2019;12:297. doi: 10.1186/s13071-019-3552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Da D, Mouline K, Awono-Ambene H. Anopheles rufipes remains a potential malaria vector after the first detection of infected specimens in 1960 in Burkina Faso. J Infect Dis Ther. 2013;1:112. [Google Scholar]
- 68.Gelfand H. Natural malaria infection in Anopheles rufipes (Gough) J Trop Med Hyg. 1947;50:159–160. [PubMed] [Google Scholar]
- 69.Graumans W, Jacobs E, Bousema T, Sinnis P. When is a Plasmodium-infected mosquito an infectious mosquito? Trends Parasitol. 2020;36:705–716. doi: 10.1016/j.pt.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masaninga F, Chanda E, Chanda-Kapata P, Hamainza B, Masendu HT, Kamuliwo M, et al. Review of the malaria epidemiology and trends in Zambia. Asian Pacific J Trop Biomed. 2013;3:89–94. doi: 10.1016/S2221-1691(13)60030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jumbam DT, Stevenson JC, Matoba J, Grieco JP, Ahern LN, Hamainza B, et al. Knowledge, attitudes and practices assessment of malaria interventions in rural Zambia. BMC Public Health. 2020;20:216. doi: 10.1186/s12889-020-8235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okumu F, Finda M. Key characteristics of residual malaria transmission in two districts in south-eastern Tanzania - implications for improved control. J Infect Dis. 2021;223:S143–S154. doi: 10.1093/infdis/jiaa653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south–eastern Tanzania. PLoS ONE. 2017;12:e0177807. doi: 10.1371/journal.pone.0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perugini E, Guelbeogo WM, Calzetta M, Manzi S, Virgillito C, Caputo B, et al. Behavioural plasticity of Anopheles coluzzii and Anopheles arabiensis undermines LLIN community protective effect in a Sudanese-savannah village in Burkina Faso. Parasit Vectors. 2020;13:277. doi: 10.1186/s13071-020-04142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martínez-de la Puente J, Ruiz S, Soriguer R, Figuerola J. Effect of blood meal digestion and DNA extraction protocol on the success of blood meal source determination in the malaria vector Anopheles atroparvus. Malar J. 2013;12:109. doi: 10.1186/1475-2875-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.