Abstract
Necrotizing enterocolitis (NEC) is the leading cause of death and disability from gastrointestinal disease in premature infants. The mortality of patients with NEC is approximately 30%, a figure that has not changed in many decades, reflecting the need for a greater understanding of its pathogenesis. Progress towards understanding the cellular and molecular mechanisms underlying NEC requires the study of highly translational animal models. Such animal models must mimic the biology and physiology of premature infants, while still allowing for safe experimental manipulation of environmental and microbial factors thought to be associated with the risk and severity of NEC. Findings from animal models have yielded insights into the interactions between the host, the colonizing microbes, and the innate immune receptor Toll-like Receptor 4 (TLR4) in driving disease development. This review discusses the relative strengths and weaknesses of available in vivo, in vitro, and NEC-in-a-dish models of this disease. We also highlight the unique contributions that each model has made to our understanding of the complex interactions between enterocytes, microbiota, and immune cells in the pathogenesis of NEC. The overall purpose of this review is to provide a menu of options regarding currently available animal models of NEC, while in parallel hopefully reducing the potential uncertainty and confusion regarding NEC models to assist those who wish to enter this field from other disciplines.
Keywords: NEC, Necrotizing Enterocolitis, Models of Necrotizing Enterocolitis
Introduction
It is widely recognized that the leading cause of death from gastrointestinal disease in premature infants is necrotizing enterocolitis (NEC), a statistic that has changed little since the disease was described some 40 years ago.1,2 The fact that NEC remains a condition with such remarkable morbidity and mortality despite decades of experience in its management underscores the need for additional, detailed studies of its pathogenesis – studies that will involve, in part, highly relevant animal models. This review will highlight our understanding of the animal models that are available to study NEC, with an emphasis on their advantages and disadvantages. We will also review the recently described “NEC-in-a-dish” models, which use human intestinal cells exposed to NEC’s microbial and environmental conditions to reproduce the relevant biochemical pathways in the actual disease. The overall purpose of this review is to provide a menu of options regarding currently available animal models of NEC, while in parallel hopefully reducing the potential uncertainty and confusion regarding NEC models to assist those who wish to enter this field from other disciplines.
Risk factors and clinical manifestations for NEC in the design of animal models
The ability to recapitulate NEC in animal models is predicated upon a clear understanding of the relevant risk factors for disease development and an appreciation of the various forms of presentation of this complex disease. Risk factors for NEC and its clinical manifestations are reviewed below.
Risk factors for NEC
The typical NEC patient is a premature infant, typically several weeks of age, who has recently received formula feeds.3,4 The contribution of formula feeding to the induction of NEC has been shown in several large series.5,6 It is highlighted by the consistent finding that over 90% of infants who develop NEC are enterally fed, and those infants who receive infant formula develop NEC more frequently than those who are fed breast milk.7,8 Additional risk factors for NEC include the recent administration of blood transfusions,9 use of H2 blockers,10–12 broad-spectrum antibiotics,13–15 chorioamnionitis,16 bacterial colonization of the GI tract with dysbiotic bacteria,17 congenital heart disease,18,19 remote infection,20,21 or sibling with NEC.21 By contrast, protective factors for NEC include the administration of breast milk,5,6 the use of probiotics,22–24 and strategies that minimize exposure to the key risk factors described above. An understanding of these risk factors is critical in the development of clinically relevant animal models. For instance, the combined administration of infant formula and intestinal microbes can induce NEC in rodents and piglets. In immature mice, transfusion of red blood cells can cause NEC-like lesions25.
Clinical presentation of NEC
The development of accurate and valuable models for the study of NEC must account for the recognition that the presentation of this disease can be quite variable. For instance, its presentation in a very low birth weight premature infant (where NEC typically takes several weeks to develop) may be quite different from its presentation in a neonate born closer to term, who may have, where the disease onset is usually much sooner. To clarify our understanding of how NEC can present clinically, we have recently described five clinical presentations of the disease, as follows:26
“Textbook” NEC – this is the presentation of NEC that perhaps first comes to mind when the diagnosis of NEC is considered. “Textbook NEC” refers to the presentation in which a premature infant who is predominantly formula-fed develops abdominal distention and bloody stools, and which is associated with the presence of a characteristic finding on abdominal plain films termed pneumatosis intestinalis, which refers to the presence of gas within the wall of the bowel. After an initial period of treatment consisting of cessation of all feeds, nasogastric decompression, resuscitation, and administration of broad-spectrum antibiotics, patients may either recover (in 50% of cases) or progress towards the development of intestinal necrosis and intestinal perforation27, as revealed by the presence of peritonitis and the finding of pneumoperitoneum on abdominal films. Abdominal exploration then reveals the presence of patchy necrosis involving regions of the small and large intestine28, and overall survival is determined by a host of factors, including the reversibility of the accompanying septic process and presence of comorbidities.
Persistent NEC without free air – this presentation refers to the infant who develops NEC as above (presence of pneumatosis intestinalis) and fails to improve clinically but does not demonstrate obvious intestinal perforation. In the absence of clear improvement, exploratory laparotomy may reveal patchy necrosis and evidence of acute or indolent intestinal perforations.
Portal venous gas and abdominal tenderness – This presentation refers to the child with abdominal findings of air within the portal system, which generally suggests significant intestinal necrosis in the setting of abdominal tenderness.
Staccato NEC – this presentation refers to the child with NEC who is initially relatively stable yet rapidly develops deterioration characterized by overwhelming sepsis accompanied by clinical and radiographic evidence of NEC that evolves over hours.
NEC totalis – the child with NEC totalis exhibits extensive necrosis that involves nearly all the small and large intestines. While NEC totalis may be suspected on abdominal X-rays based upon the extent of pneumatosis intestinalis, the diagnosis of NEC totalis is often only made at laparotomy.
It is obvious that no single animal model can reliably mimic all these five presentations of NEC. Indeed, no human presents with all 5, although there is significant overlap between presentations (i.e., presentations 1 and 2 overlap to a degree, as do 3 and 4). Given the clinical complexity of NEC, it may be helpful to recognize that there is not one “best” NEC model. Rather, the choice of animal model should be based upon the specific question to be answered (for example, the investigation of a particular pathway that may be implicated in disease pathogenesis versus the assessment of a potential treatment), the desired phenotype (patchy versus diffuse intestinal necrosis) and the resources available (see Table 1).
Table 1.
Models of NEC and associated phenotypes. Most significant resource requirements and some significant aspects of NEC pathogenesis learned from these models are listed.
| Animal Model | Phenotype | Resources | Examples of specific questions answered with each model |
|---|---|---|---|
| Rat | • Sloughing of epithelial cells at tips of villi • Attenuation of normal developmental increase in heart rate variability • Increased serum levels of proinflammatory cytokines (e.g., IL-1B, IL-6) |
• Low cost • Easy breeding • Limited availability of transgenic models |
• Role of probiotics in NEC prevention93 • Therapeutic applications of anti-TNF-alpha antibody94 • Protective effect of antioxidants95 • Correlation of intestinal fatty acid binding protein on timing of NEC onset96 • Role of protein kinase A inhibitor as a potential therapy in experimental NEC 97 |
| Mouse | • Ileum is scattered with patchy lesions of injury • Tissue destruction ranging from mild destruction to the tips of villi to transmural necrosis • Upregulation of inflammatory factors and antimicrobial peptides (e.g., IL1-B, Cxcl2, Reg3g) |
• Low cost • Easy breeding • Able to create transgenic models |
• The role of TLR4 in the pathogenesis of NEC30 • Therapeutic potential of short-chain fatty acids in alleviating intestinal inflammation in NEC98 • Impact of human milk oligosaccharides on experimental NEC 99 |
| Piglet | • Severe abdominal distension • Hemorrhagic discoloration and sloughing throughout small and large bowel • Necrosis and sloughing of epithelium |
• High cost • Limited availability of transgenic models |
• Role of nitric oxide production in the pathogenesis of NEC100 • Diagnostic applications of transabdominal near infra-red spectroscopy101 • Impact of NEC on hippocampal development 98 • Impact of abrupt versus gradual advancement of enteral feeds on the incidence of NEC81 • Potential of probiotics in lowering the incidence of NEC 102 |
Mouse models of NEC
Mouse models are some of the earliest29,30 and most frequently utilized models to study NEC. In general terms, as with other diseases, mouse models offer the opportunity for genetic modification to test disease pathogenesis and have the advantage of being relatively inexpensive to purchase and house compared with other animals.31 While individual models vary somewhat, the typical mouse models are performed on mice within the first week or so of life and utilize a combination of gavage with infant formula, brief exposure to hypoxia and/or hypothermia, and the addition of either bacterial lipopolysaccharide or bacteria obtained from human infants with clinical NEC.32 The complexity of the mouse model also gives rise to its inherent disadvantages: mouse models of NEC require regular feeding and a team of investigators trained to administer formula by gavage to tiny mice. The model typically lasts 4 or 5 days, upon which a vast majority of mice will display the patchy intestinal edema, pneumatosis intestinalis, and necrosis that characterizes human NEC.33
The mouse NEC model performed in our laboratory occurs as follows:34–40 Pups are separated from their mother on day seven after delivery and housed in a temperature-controlled (37oC) neonatal incubator to maintain homeostasis. The pups are fed by gavage formula five times daily using a 22ga feeding catheter and colonized with enteric bacteria isolated from stool obtained from infants with NEC. Mice are subjected to brief periods of hypoxia twice daily (10 min at 95% N2, 5% O2). On day 5 of the model, mouse pups are euthanized by decapitation with surgical scissors. We typically sample the intestine at a fixed point in all cases to avoid sampling bias, mainly 1cm from the terminal ileum. Tissues are obtained for PCR analysis, histology, and western blot. A typical study will involve fifteen to twenty p7 mice per experimental group, and expected mortality is approximately 20%, with a disease penetrance of over 80%.
This model has been utilized with modification by several investigators, including Besner et al.,41 who added hypothermia, and Pierro et al.,42 who combined hypoxia with LPS administration. Maheshwari et al., on the other hand, employed asphyxia (100% nitrogen gas) and cold stress (4°C for 10 min) twice daily43 and have also utilized a blood transfusion model to induce NEC in a mouse correlate of this important risk factor in pathogenesis25. Genetic modifications used include the innate immune receptor TLR4,44 IL-18,45 eNOS46 and others. Further, Ginzel et al. have utilized the mucosal irritant DSS to induce NEC-like lesions in the small and large bowel in the absence of hypoxia and hypothermia.47 McElroy et al. have used both chemical and genetic ablation of Paneth cells in mice at P14-P16 days of age, resulting in NEC-like intestinal injury and a shift toward Enterobacteriaceae species.48,49 These studies reveal the utility of mouse models for NEC and the various modifications that can be used to achieve a reliable platform for understanding pathogenesis.
Several approaches to validating the mouse and human NEC models have been performed. First, the newborn mouse shares features consistent with a 28-week infant, i.e., minimal subcutaneous fat, eyes closed most of the time, poor thermoregulation, and immature gut motility.50 Second, the newborn mouse with NEC exhibits a pattern of gene expression in component immune cells within the small intestine that resembles the human infant with NEC.51 Further, the intestinal microbiome seen in mice with NEC parallels that in humans.46,52 Most importantly, biochemical and genetic pathways that play causative roles in mouse NEC are also observed in the human disease. Among these are pathways involving TLR4, IgA, EGF, and HMGB1.38,53–56 To this end, agents that prevent or ameliorate NEC in mouse models have shown promise against human disease in clinical studies46 or ex vivo studies with human tissue.33 Taken together, despite the potential drawbacks of the mouse model based on its small size and incomplete overlap between mouse and human immune cells, mouse NEC models have emerged as essential tools in studying NEC pathogenesis.
Rat models
Rats were the first animal used to create an experimental model of NEC. In 1974 and 1975, Barlow and Santulli reported that a NEC-like histopathological phenotype could be achieved in prematurely delivered pups by gavage feeding formula and treating them with intermittent episodes of cold or hypoxia57,58. Several researchers have successfully adapted this model to study specific variables thought to play a role in the risk and severity of NEC. In 1994, Caplan et al. reported that the introduction of bacteria into the formula led to NEC, thus revealing a role for bacterial colonization in NEC development59. Additional laboratories have made further adaptations to the rat NEC model to study the effects of colonization with different species of bacteria found in the stool of infants with necrotizing enterocolitis, including Cronobacter sakazakii60, Klebiella61,62, and Clostridia63–67. LPS has also been used to increase the extent of intestinal injury in the rat model68. In one iteration of the rat model, Zani et al. induced NEC by administering 4 mg/kg/day of LPS obtained from E. coli mixed with formula to e21.5 pups beginning on the fifth day of life.69 Similar to other animal models of NEC, rat models have been shown to exhibit morphological changes in the intestinal epithelium similar to those seen in patients with acute NEC, and iNOS mRNA upregulation, enterocyte apoptosis, and decreased IL-12 production in the intestinal epithelium have been implicated at important aspects of NEC pathophysiology70.
The advantages of using rats in NEC research include their low cost and relative tolerance of the model, while disadvantages center primarily on the inability to manipulate the genome to interrogate specific pathways involved in disease development. Rat models of NEC may have essential roles in pharmacokinetics studies and safety and efficacy studies of potential NEC therapies, as reported by several groups71–75.
Piglet models
Piglets offer an attractive model for studying NEC because of their anatomical, physiological, and developmental similarities to the gastrointestinal tract of humans.76 The premature piglet model, in particular, offers specific advantages in that the model is performed on animals weighing between 1000–1300g, which approximates the size of the human infant with NEC. The model’s technical details, as described by Sangild et al., include delivery at 90% term, which results in a natural period of hypoxia and hypothermia77, and subsequently formula fed to induce intestinal injury.77 Sangild et al. also used TPN to induce the intestinal changes seen in the human disease.78
The piglet model as performed in our lab makes use of timed-pregnant White Yorkshire sows whose piglets are delivered prematurely via cesarean section at 95% gestation. NEC is induced by feeding 20 mL/kg of formula supplemented with enteric bacteria obtained from infants with surgical NEC five times daily. Piglets are housed in an incubator for the duration of the model and euthanized with intracardiac potassium chloride injection following anesthesia with ketamine. The piglet NEC model has been used to understand early NEC detection methods, potential therapies, and preventative strategies. For instance, Zamora, Burrin, and Chen employed this model to develop non-invasive methods for early detection of NEC using near-infrared spectroscopy.79 Other researchers, including Burrin et al. and Buddington et al., used the piglet model to systematically evaluate the impact of various feeding protocols on the incidence of NEC-like pathology and the effects of lactose versus maltodextrin content in formula.80,81 Utilizing this model, Puiman et al. found that formula feeding, as opposed to feeding with bovine colostrum, was associated with decreased gut protein synthesis and lower levels of MUC2, the predominant secretory mucin in the human intestinal tract.82 The model has elucidated findings including reactive oxygen species83, platelet-activating factor,84 intramural intestinal pH,85 and the role of T-cell mediated mucosal immunity.86 Our lab has used the piglet model to demonstrate the effects of human milk oligosaccharides87 and bacterial DNA1 as potential NEC therapy.
While the piglet model closely mimics human NEC since the piglets used are premature and are close in size to newborn infants, there are significant drawbacks. These include the high cost associated with model development, the large team needed to care for a litter of piglets around the clock, and the facilities required to care for a large sow and her offspring. Moreover, the piglet genome is difficult to manipulate, thus limiting the applicability for pathogenesis studies. As with the rat models, the piglet models may be most effective in pharmacologic and drug safety studies and pre-clinical evaluation of specific nutrient components relevant to premature newborn health.
NEC-in-a-DISH models
Organoid models are three-dimensional cultures of cells that recapitulate some functional aspects of whole organs.88 These in vitro systems can be derived from progenitor and stem cells from both mouse and human tissue.88 Intestinal organoids can be applied to NEC research by representing a functional intestine-in-a-dish.89 To accomplish this, organoids may be obtained from the differentiation of iPSCs, or enteroids may be cultured ex vivo from the intestinal crypts of experimental animals or patients. While in culture, these organ units are exposed to NEC bacteria and hypoxia, critical components of the NEC model. Importantly, organoids exposed to hypoxia or NEC bacteria alone show no overt changes in structure or gross cell morphology. However, those exposed to a combination of these conditions display distorted architecture, an increase in inflammatory cytokine up-regulation, and reduced proliferation,56 consistent with the structural and functional changes observed in human NEC. This model, termed “NEC-in-a-dish”, has been used to assess biological features relevant to NEC, including barrier function, immune response, and transcriptional profiles in intestinal organoids.56,90 Furthermore, they have been used to confirm the importance of TLR4 activation in human tissue, elucidate the role of milk oligosaccharides, and discover novel cellular pathways involved in NEC, such as necroptosis.44,56
Organoid NEC-in-a-DISH models offer significant advantages compared to in vivo models for future research. These systems are now developed with standardized protocols and are scalable for large-scale approaches such as drug and therapy screening. NEC-in-a-dish models can also be derived from fresh or frozen tissue and be grown from human tissue, leading to conclusions more relevant to human disease.48,90 Organoid models derived from patient samples have the same genetic background as the patient, allowing for the assessment of precision treatments specific to each patient.48 Finally, organoid NEC-in-a-dish models are low cost, allowing for widespread adoption in various workflows. However, NEC-in-a-dish models also have limitations. Notably, current organoid approaches lack the immune component of the intestine, which is implicated in NEC development.91 As an isolated system, there is also a lack of circulating factors that contribute to NEC.48 Further developments to NEC-in-a-dish models, including the use of co-culture systems with isolated primary circulating and intestinal epithelial lymphocytes, may remedy these issues and lead to further understanding of NEC pathogenesis.
Conclusion
NEC remains a leading cause of morbidity and mortality among premature infants.92 Over the last several decades, many experimental models have been developed to enhance our understanding of this disease. Because multiple risk factors are involved in the pathophysiology of NEC, modeling this disease has often proven to be beyond the scope of studies in traditional single-cell cultures. To this end, animal models, and “NEC-in-a-dish” models all play a crucial role in modeling and deciphering the biological pathways and potential therapeutic avenues in NEC. Recent discoveries utilizing various models in parallel underscore the importance of understanding each model’s distinct advantages, drawbacks, and applicability.
Figure 1.
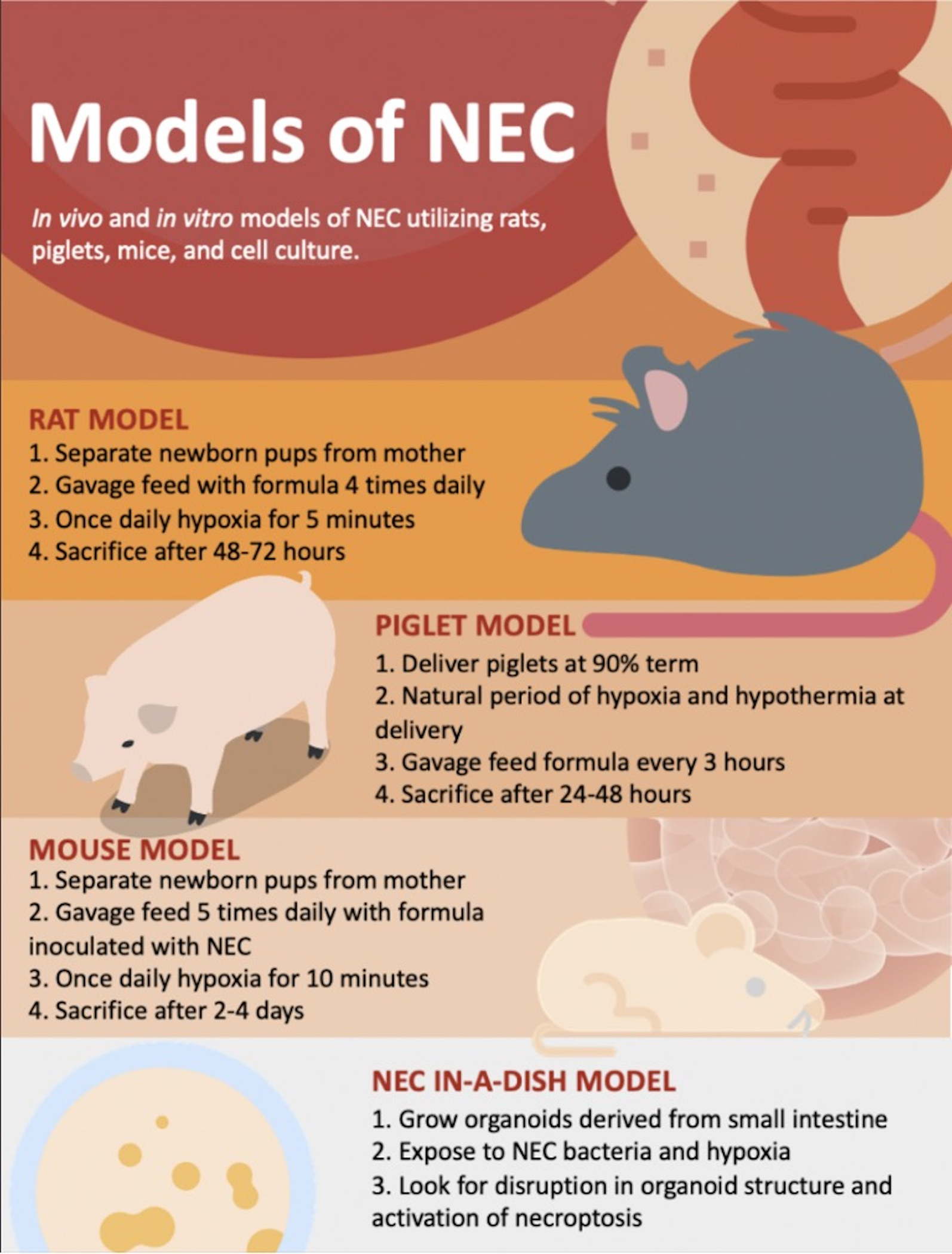
Experimental models have served a critical role in the study of necrotizing enterocolitis pathogenesis. In-vivo models involve exposing young rats, piglets or mice to hypoxia and bacteria to recapitulate conditions involved in NEC. More novel in-vitro models have made use of organoids growing in culture under similar conditions.
References
- 1.Sodhi CP, Ahmad R, Jia H, et al. The administration of amnion-derived multipotent cell secretome ST266 protects against necrotizing enterocolitis in mice and piglets. Am J Physiol Gastrointest Liver Physiol. Jul 12 2022;doi: 10.1152/ajpgi.00364.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berken JA, Chang J. Neurologic consequences of neonatal necrotizing enterocolitis. Dev Neurosci Jun 13 2022;doi: 10.1159/000525378 [DOI] [PubMed] [Google Scholar]
- 3.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. The Lancet 1990. [DOI] [PubMed] [Google Scholar]
- 4.Altobelli E, Angeletti PM, Verrotti A, Petrocelli R. The Impact of Human Milk on Necrotizing Enterocolitis: A Systematic Review and Meta-Analysis. Nutrients. May 06 2020;12(5)doi: 10.3390/nu12051322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowning R, Radmacher P, Lewis S, Serke L, Pettit N, Adamkin DH. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J Perinatol. Mar 2016;36(3):221–4. doi: 10.1038/jp.2015.179 [DOI] [PubMed] [Google Scholar]
- 6.Hair AB, Peluso AM, Hawthorne KM, et al. Beyond Necrotizing Enterocolitis Prevention: Improving Outcomes with an Exclusive Human Milk-Based Diet. Breastfeed Med. Mar 2016;11(2):70–4. doi: 10.1089/bfm.2015.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan M, Henderson G, McGuire W. Enteral feeding for very low birth weight infants: reducing the risk of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. Mar 2008;93(2):F162–6. doi: 10.1136/adc.2007.115824 [DOI] [PubMed] [Google Scholar]
- 8.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care. Apr 2012;12(2):77–87; quiz 88–9. doi: 10.1097/ANC.0b013e31824cee94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay S, Zupancic JA, Flannery DD, Kirpalani H, Dukhovny D. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin Perinatol. 02 2017;41(1):80–91. doi: 10.1053/j.semperi.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 10.Gupta RW, Tran L, Norori J, et al. Histamine-2 receptor blockers alter the fecal microbiota in premature infants. J Pediatr Gastroenterol Nutr. Apr 2013;56(4):397–400. doi: 10.1097/MPG.0b013e318282a8c2 [DOI] [PubMed] [Google Scholar]
- 11.Terrin G, Passariello A, De Curtis M, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics 2012. [DOI] [PubMed] [Google Scholar]
- 12.Guillet R, Stoll BJ, Cotten CM, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2006. p. e137–e142. [DOI] [PubMed] [Google Scholar]
- 13.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. Jan 2009;123(1):58–66. doi: 10.1542/peds.2007-3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. Sep 2011;159(3):392–7. doi: 10.1016/j.jpeds.2011.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. Nov 2011;159(5):720–5. doi: 10.1016/j.jpeds.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr. Feb 2013;162(2):236–42.e2. doi: 10.1016/j.jpeds.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 17.Pammi M, Cope J, Tarr PI, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. J Pediatr Gastroenterol Nutr. Apr 2009;48(4):437–42. doi: 10.1097/mpg.0b013e31817b6dbe [DOI] [PubMed] [Google Scholar]
- 19.Lu Q, Cheng S, Zhou M, Yu J. Risk Factors for Necrotizing Enterocolitis in Neonates: A Retrospective Case-Control Study. Pediatr Neonatol. 04 2017;58(2):165–170. doi: 10.1016/j.pedneo.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 20.Reducing Incidence of Necrotizing Enterocolitis, (2017). [DOI] [PubMed]
- 21.Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. Sep 2015;78(3):232–8. doi: 10.1038/pr.2015.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aceti A, Gori D, Barone G, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: systematic review and meta-analysis. Ital J Pediatr. Nov 14 2015;41:89. doi: 10.1186/s13052-015-0199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butel MJ, Roland N, Hibert A, et al. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. May 1998;47(5):391–9. doi: 10.1099/00222615-47-5-391 [DOI] [PubMed] [Google Scholar]
- 24.Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology 1999. [DOI] [PubMed] [Google Scholar]
- 25.MohanKumar K, Namachivayam K, Song T, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nature communications: Nat Commun; 2019. p. 3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackam DJ. Surgery for Necrotizing Enterocolitis: Indications, Techniques, and Outcomes. In: Hackam DJ, ed. Necrotizing Enterocolitis: Pathogenesis, Diagnosis, and Treatment. First edition ed. Taylor & Francis; 2021:chap 10. [Google Scholar]
- 27.Mara K, Clark R, Carey WA. Necrotizing Enterocolitis in Very Low Birth Weight Neonates: A Natural History Study. Am J Perinatol. May 12 2022;doi: 10.1055/a-1851-1692 [DOI] [PubMed] [Google Scholar]
- 28.Thakkar HS, Lakhoo K. The surgical management of necrotising enterocolitis (NEC). Early Human Development 2016. [DOI] [PubMed] [Google Scholar]
- 29.Jilling T, Simon D, Lu J, et al. The Roles of Bacteria and TLR4 in Rat and Murine Models of Necrotizing Enterocolitis. The Journal of Immunology 2006. p. 3273–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neal MD, Leaphart C, Levy R, et al. Enterocyte TLR4 Mediates Phagocytosis and Translocation of Bacteria Across the Intestinal Barrier. The Journal of Immunology 2006. [DOI] [PubMed] [Google Scholar]
- 31.Nolan LS, Wynn JL, Good M. Exploring Clinically-Relevant Experimental Models of Neonatal Shock and Necrotizing Enterocolitis. Shock. 05 2020;53(5):596–604. doi: 10.1097/SHK.0000000000001507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu P, Sodhi CP, Jia H, et al. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: Pathophysiology, translational relevance, and challenges. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2014/April/26 ed2014. p. G917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neal MD, Jia H, Eyer B, et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE. 2013/June/19 ed2013. p. e65779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afrazi A, Branca MF, Sodhi CP, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. Apr 4 2014;289(14):9584–99. doi: 10.1074/jbc.M113.526517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leaphart CL, Cavallo J, Gribar SC, et al. A Critical Role for TLR4 in the Pathogenesis of Necrotizing Enterocolitis by Modulating Intestinal Injury and Repair. The Journal of Immunology: American Association of Immunologists; 2007. p. 4808–4820. [DOI] [PubMed] [Google Scholar]
- 36.Gribar SC, Sodhi CP, Richardson WM, et al. Reciprocal Expression and Signaling of TLR4 and TLR9 in the Pathogenesis and Treatment of Necrotizing Enterocolitis. The Journal of Immunology 2009. p. 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leaphart CL, Qureshi F, Cetin S, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. Jun 2007;132(7):2395–411. doi: 10.1053/j.gastro.2007.03.029 [DOI] [PubMed] [Google Scholar]
- 38.Sodhi CP, Shi XH, Richardson WM, et al. Toll-Like Receptor-4 Inhibits Enterocyte Proliferation via Impaired β-Catenin Signaling in Necrotizing Enterocolitis. Gastroenterology 2010. p. 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai S, Sodhi C, Cetin S, et al. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of toll-like receptor-4 and increased cell-matrix adhesiveness. Journal of Biological Chemistry 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sodhi CP, Neal MD, Siggers R, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012/July/17 ed2012. p. 708–18 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. Apr 2006;41(4):742–7; discussion 742–7. doi: 10.1016/j.jpedsurg.2005.12.020 [DOI] [PubMed] [Google Scholar]
- 42.Zani A, Zani-Ruttenstock E, Peyvandi F, Lee C, Li B, Pierro A. A spectrum of intestinal injury models in neonatal mice. Pediatr Surg Int. Jan 2016;32(1):65–70. doi: 10.1007/s00383-015-3813-x [DOI] [PubMed] [Google Scholar]
- 43.Maynard AA, Dvorak K, Khailova L, et al. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. American Journal of Physiology - Gastrointestinal and Liver Physiology 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodhi CP, Neal MD, Siggers R, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. Sep 2012;143(3):708–718.e5. doi: 10.1053/j.gastro.2012.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halpern MD, Khailova L, Molla-Hosseini D, et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol. Jan 2008;294(1):G20–6. doi: 10.1152/ajpgi.00168.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Good M, Sodhi CP, Yamaguchi Y, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. British Journal of Nutrition 2016. p. 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginzel M, Feng X, Kuebler JF, et al. Dextran sodium sulfate (DSS) induces necrotizing enterocolitis-like lesions in neonatal mice. PLoS One. 2017;12(8):e0182732. doi: 10.1371/journal.pone.0182732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovler ML, Sodhi CP, Hackam DJ. Precision-based modeling approaches for necrotizing enterocolitis. Dis Model Mech. June 24 2020;13(6)doi: 10.1242/dmm.044388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lueschow SR, Stumphy J, Gong H, et al. Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis. PLoS One. 2018;13(10):e0204967. doi: 10.1371/journal.pone.0204967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy R, Martin-Fairey C, Sojka DK, et al. Mouse models of preterm birth: suggested assessment and reporting guidelines. Biol Reprod. November 01 2018;99(5):922–937. doi: 10.1093/biolre/ioy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho SX, Rudloff I, Lao JC, et al. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nature Communications 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner BB, Deych E, Zhou Y, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. May 7 2016;387(10031):1928–1936. doi: 10.1016/S0140-6736(16)00081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. Sep 01 2006;177(5):3273–82. doi: 10.4049/jimmunol.177.5.3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gopalakrishna KP, Macadangdang BR, Rogers MB, et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat Med Jul 2019;25(7):1110–1115. doi: 10.1038/s41591-019-0480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Good M, Siggers RH, Sodhi CP, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proceedings of the National Academy of Sciences of the United States of America 2012. p. 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werts AD, Fulton WB, Ladd MR, et al. A Novel Role for Necroptosis in the Pathogenesis of Necrotizing Enterocolitis. Cell Mol Gastroenterol Hepatol. 2020;9(3):403–423. doi: 10.1016/j.jcmgh.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barlow B, Santulli T, Heird W, Pitt J, Blanc W, Schullinger J. An experimental study of acute neonatal enterocolitis—the importance of breast milk. Journal of Pediatric Surgery. 1974;9(5) [DOI] [PubMed] [Google Scholar]
- 58.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. May 1975;77(5):687–90. [PubMed] [Google Scholar]
- 59.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994 Nov-Dec 1994;14(6):1017–28. doi: 10.3109/15513819409037698 [DOI] [PubMed] [Google Scholar]
- 60.Hunter CJ, Singamsetty VK, Chokshi NK, et al. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. Aug 15 2008;198(4):586–93. doi: 10.1086/590186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olm MR, Bhattacharya N, Crits-Christoph A, et al. Necrotizing enterocolitis is preceded by increased gut bacterial replication,. Sci Adv. 12 2019;5(12):eaax5727. doi: 10.1126/sciadv.aax5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C, Sherman MP, Prince LS, et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. Jul 2012;5(4):522–32. doi: 10.1242/dmm.009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cassir N, Benamar S, Khalil JB, et al. Clostridium butyricum Strains and Dysbiosis Linked to Necrotizing Enterocolitis in Preterm Neonates. Clin Infect Dis. Oct 01 2015;61(7):1107–15. doi: 10.1093/cid/civ468 [DOI] [PubMed] [Google Scholar]
- 64.Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev Mar 2012;88 Suppl 1:S41–9. doi: 10.1016/j.earlhumdev.2011.12.027 [DOI] [PubMed] [Google Scholar]
- 65.Schönherr-Hellec S, Klein GL, Delannoy J, et al. Clostridial Strain-Specific Characteristics Associated with Necrotizing Enterocolitis. Appl Environ Microbiol. April 01 2018;84(7)doi: 10.1128/AEM.02428-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.La Rosa PS, Warner BB, Zhou Y, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A. Aug 26 2014;111(34):12522–7. doi: 10.1073/pnas.1409497111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vágnerová I, Kohnová I, Saitz J, Urbanová K. [Clostridium difficile as a potential pathogen in preterm neonates]. Klin Mikrobiol Infekc Lek. Feb 2009;15(1):22–5. [PubMed] [Google Scholar]
- 68.Chan KL, Ho JC, Chan KW, Tam PK. A study of gut immunity to enteral endotoxin in rats of different ages: a possible cause for necrotizing enterocolitis. J Pediatr Surg. Oct 2002;37(10):1435–40. doi: 10.1053/jpsu.2002.35407 [DOI] [PubMed] [Google Scholar]
- 69.Zani A, Eaton S, Leon FF, et al. Captopril reduces the severity of bowel damage in a neonatal rat model of necrotizing enterocolitis. J Pediatr Surg. Feb 2008;43(2):308–14. doi: 10.1016/j.jpedsurg.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 70.Nadler EP, Dickinson E, Knisely A, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. Jul 2000;92(1):71–7. doi: 10.1006/jsre.2000.5877 [DOI] [PubMed] [Google Scholar]
- 71.Li B, Wu RY, Horne RG, et al. Human Milk Oligosaccharides Protect against Necrotizing Enterocolitis by Activating Intestinal Cell Differentiation. Mol Nutr Food Res. November 2020;64(21):e2000519. doi: 10.1002/mnfr.202000519 [DOI] [PubMed] [Google Scholar]
- 72.Wu RY, Li B, Koike Y, et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol Nutr Food Res. February 2019;63(3):e1800658. doi: 10.1002/mnfr.201800658 [DOI] [PubMed] [Google Scholar]
- 73.Zeng R, Wang J, Zhuo Z, Luo Y, Sha W, Chen H. Stem cells and exosomes: promising candidates for necrotizing enterocolitis therapy. Stem Cell Res Ther. June 05 2021;12(1):323. doi: 10.1186/s13287-021-02389-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCulloh CJ, Olson JK, Wang Y, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. Jun 2018;53(6):1215–1220. doi: 10.1016/j.jpedsurg.2018.02.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li B, Lee C, O’Connell JS, et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. September 14 2020;11(9):750. doi: 10.1038/s41419-020-02964-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu P, Sodhi CP, Jia H, et al. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. Jun 1 2014;306(11):G917–28. doi: 10.1152/ajpgi.00422.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. May 2006;130(6):1776–92. doi: 10.1053/j.gastro.2006.02.026 [DOI] [PubMed] [Google Scholar]
- 78.Sangild PT, Petersen YM, Schmidt M, et al. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr. 12 2002;132(12):3786–94. doi: 10.1093/jn/132.9.2673 [DOI] [PubMed] [Google Scholar]
- 79.Gay AN, Lazar DA, Stoll B, et al. Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J Pediatr Surg. Jun 2011;46(6):1034–40. doi: 10.1016/j.jpedsurg.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buddington RK, Davis SL, Buddington KK. The Risk of Necrotizing Enterocolitis Differs Among Preterm Pigs Fed Formulas With Either Lactose or Maltodextrin. J Pediatr Gastroenterol Nutr. 03 2018;66(3):e61–e66. doi: 10.1097/MPG.0000000000001707 [DOI] [PubMed] [Google Scholar]
- 81.Ghoneim N, Bauchart-Thevret C, Oosterloo B, et al. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS One. 2014;9(9):e106888. doi: 10.1371/journal.pone.0106888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puiman PJ, Jensen M, Stoll B, et al. Intestinal threonine utilization for protein and mucin synthesis is decreased in formula-fed preterm pigs. J Nutr. Jul 2011;141(7):1306–11. doi: 10.3945/jn.110.135145 [DOI] [PubMed] [Google Scholar]
- 83.Koivusalo A, Kauppinen H, Anttila A, et al. Intraluminal casein model of necrotizing enterocolitis for assessment of mucosal destruction, bacterial translocation, and the effects of allopurinol and N-acetylcysteine. Pediatr Surg Int. Dec 2002;18(8):712–7. doi: 10.1007/s00383-002-0871-7 [DOI] [PubMed] [Google Scholar]
- 84.Ewer AK, Al-Salti W, Coney AM, Marshall JM, Ramani P, Booth IW. The role of platelet activating factor in a neonatal piglet model of necrotising enterocolitis. Gut. Feb 2004;53(2):207–13. doi: 10.1136/gut.2003.024521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koivusalo A, Kauppinen H, Anttila A, Heikkilä P, Rintala R, Lindahl H. Rectosigmoid pHi monitoring during experimental necrotizing enterocolitis. J Pediatr Surg. Oct 2000;35(10):1462–7. doi: 10.1053/jpsu.2000.16415 [DOI] [PubMed] [Google Scholar]
- 86.Anttila A, Kauppinen H, Koivusalo A, Heikkila P, Savilahti E, Rintala R. T-cell-mediated mucosal immunity is attenuated in experimental necrotizing enterocolitis. Pediatr Surg Int. Jul 2003;19(5):326–30. doi: 10.1007/s00383-003-1004-7 [DOI] [PubMed] [Google Scholar]
- 87.Sodhi CP, Wipf P, Yamaguchi Y, et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr Res. 01 2021;89(1):91–101. doi: 10.1038/s41390-020-0852-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6(5):402–420. doi: 10.1038/s41578-021-00279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ares GJ, Buonpane C, Yuan C, Wood D, Hunter CJ. A Novel Human Epithelial Enteroid Model of Necrotizing Enterocolitis. J Vis Exp April 10 2019;(146)doi: 10.3791/59194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li B, Lee C, Cadete M, Miyake H, Lee D, Pierro A. Neonatal intestinal organoids as an ex vivo approach to study early intestinal epithelial disorders. Pediatr Surg Int. Jan 2019;35(1):3–7. doi: 10.1007/s00383-018-4369-3 [DOI] [PubMed] [Google Scholar]
- 91.Egan CE, Sodhi CP, Good M, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. Feb 2016;126(2):495–508. doi: 10.1172/JCI83356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han SM, Hong CR, Knell J, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J Pediatr Surg. Jun 2020;55(6):998–1001. doi: 10.1016/j.jpedsurg.2020.02.046 [DOI] [PubMed] [Google Scholar]
- 93.Khailova L, Dvorak K, Arganbright KM, et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. Nov 2009;297(5):G940–9. doi: 10.1152/ajpgi.00141.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Halpern MD, Clark JA, Saunders TA, et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. Am J Physiol Gastrointest Liver Physiol. Apr 2006;290(4):G757–64. doi: 10.1152/ajpgi.00408.2005 [DOI] [PubMed] [Google Scholar]
- 95.Ozdemir R, Yurttutan S, Sarı FN, et al. Antioxidant effects of N-acetylcysteine in a neonatal rat model of necrotizing enterocolitis. J Pediatr Surg. Sep 2012;47(9):1652–7. doi: 10.1016/j.jpedsurg.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 96.Simões AL, Figueira RL, Gonçalves FL, et al. Temporal profile of intestinal tissue expression of intestinal fatty acid-binding protein in a rat model of necrotizing enterocolitis. Clinics (Sao Paulo). Jul 2016;71(7):412–9. doi: 10.6061/clinics/2016(07)10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blackwood BP, Wood DR, Yuan C, et al. A Role for cAMP and Protein Kinase A in Experimental Necrotizing Enterocolitis. Am J Pathol. Feb 2017;187(2):401–417. doi: 10.1016/j.ajpath.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun Q, Ji YC, Wang ZL, et al. Sodium Butyrate Alleviates Intestinal Inflammation in Mice with Necrotizing Enterocolitis. Mediators Inflamm. 2021;2021:6259381. doi: 10.1155/2021/6259381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Autran CA, Schoterman MH, Jantscher-Krenn E, Kamerling JP, Bode L. Sialylated galacto-oligosaccharides and 2’-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br J Nutr. July 2016;116(2):294–9. doi: 10.1017/S0007114516002038 [DOI] [PubMed] [Google Scholar]
- 100.Robinson JL, Smith VA, Stoll B, et al. Prematurity reduces citrulline-arginine-nitric oxide production and precedes the onset of necrotizing enterocolitis in piglets. Am J Physiol Gastrointest Liver Physiol. October 01 2018;315(4):G638–G649. doi: 10.1152/ajpgi.00198.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zamora IJ, Stoll B, Ethun CG, et al. Low Abdominal NIRS Values and Elevated Plasma Intestinal Fatty Acid-Binding Protein in a Premature Piglet Model of Necrotizing Enterocolitis. PLoS One. 2015;10(6):e0125437. doi: 10.1371/journal.pone.0125437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lanik W, Cliff L, Nolan L, Good M. Microfluidic Device Facilitates Novel In Vitro Modeling of Human Neonatal Necrotizing Enterocolitis-on-a-Chip. bioRxiv. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]


