Abstract
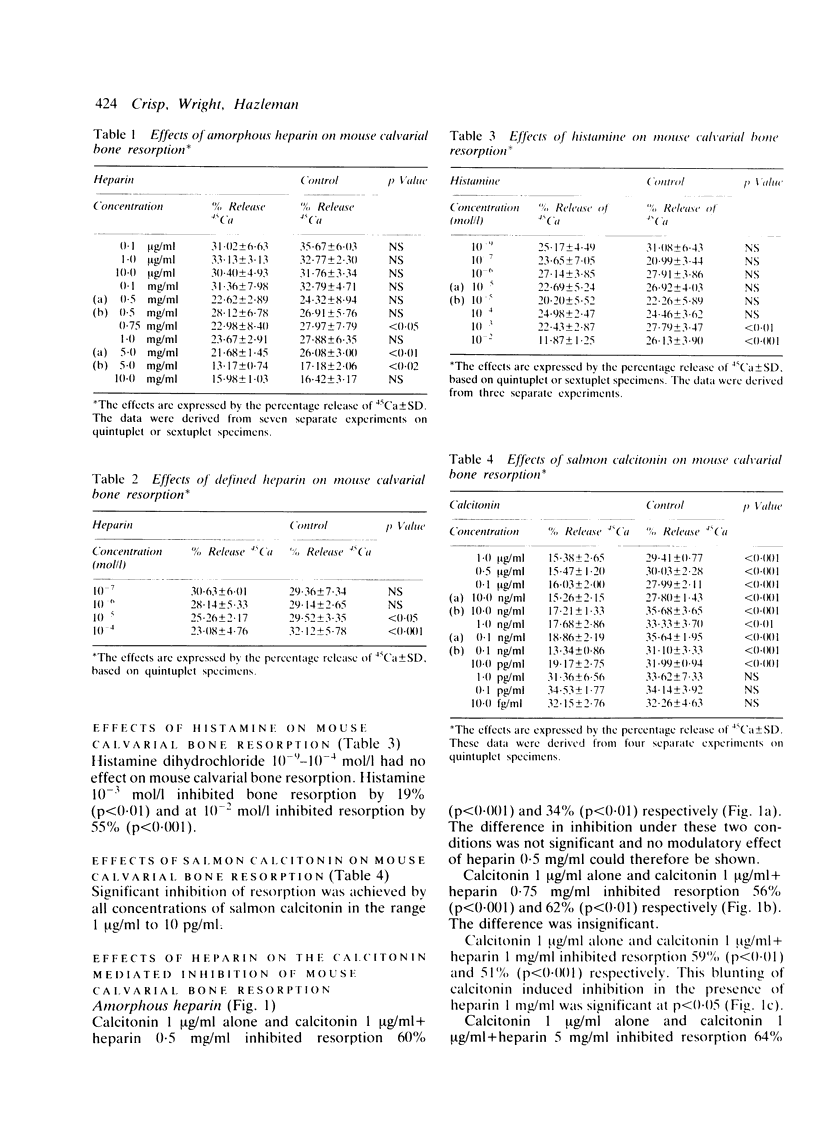
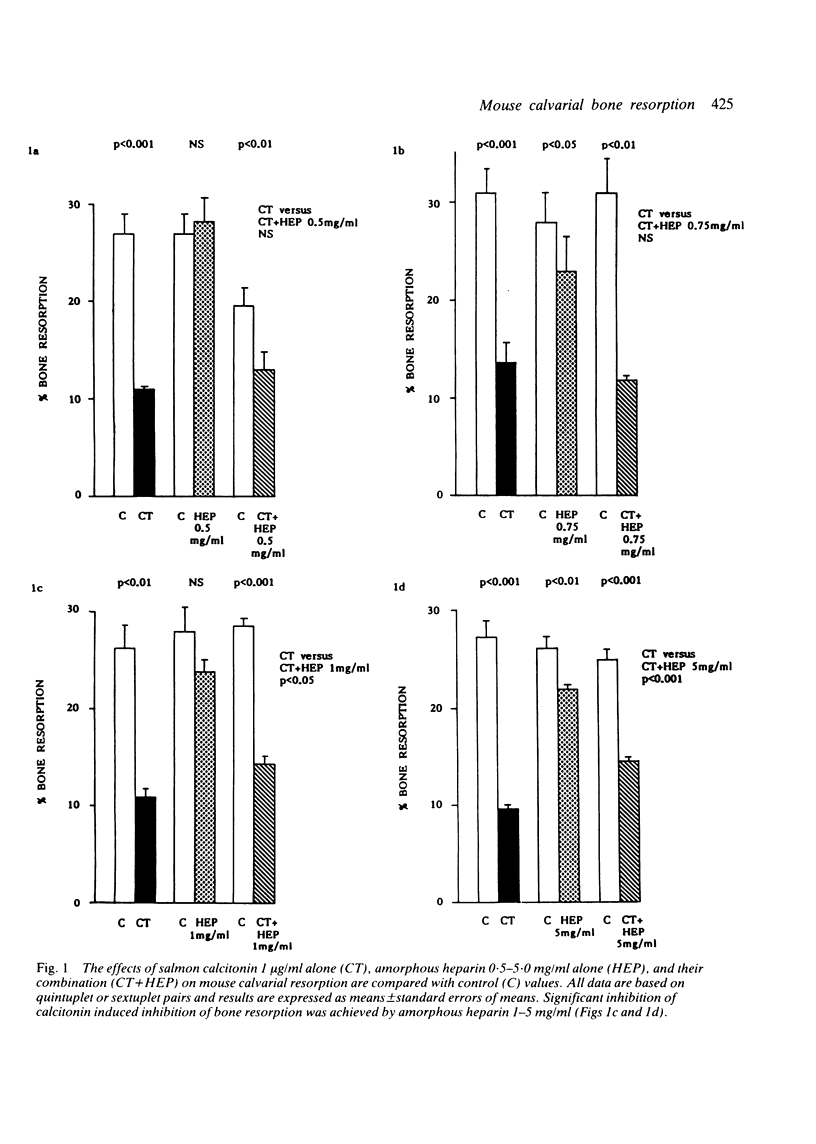
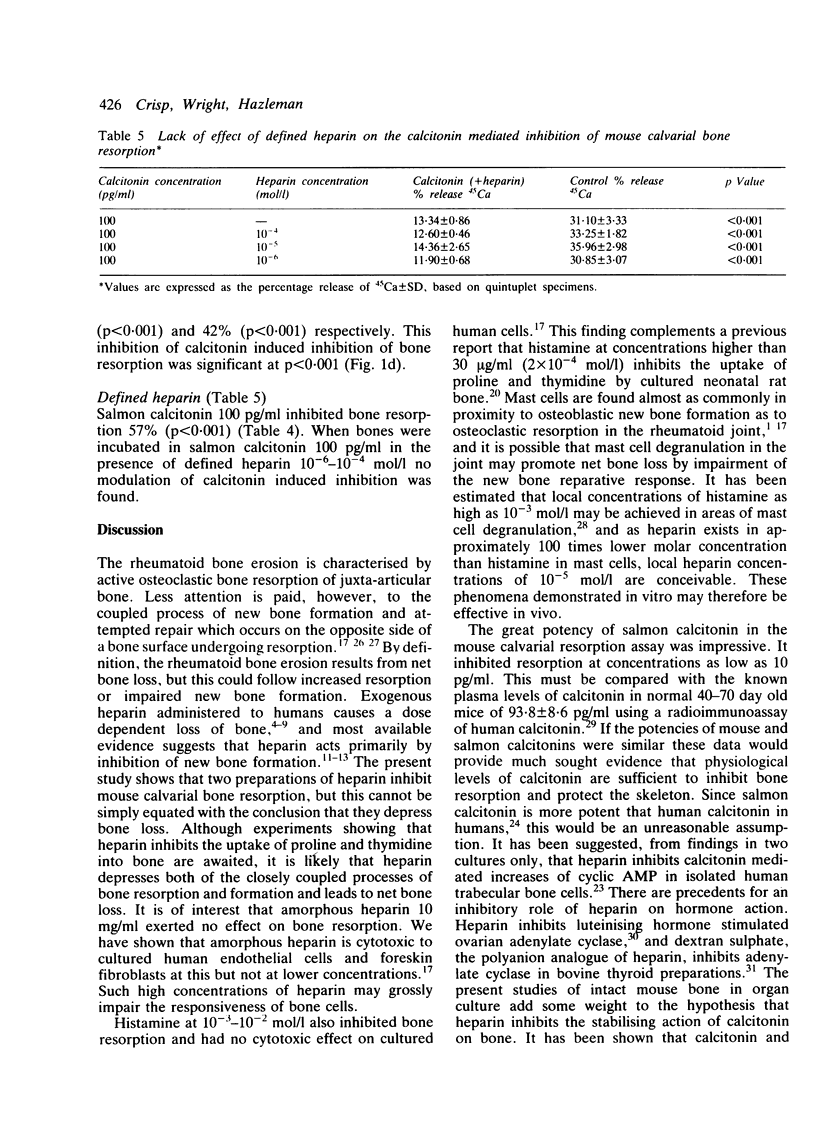
A quantitative mouse calvarial bone resorption assay was employed to investigate the effects of the mast cell products, heparin and histamine, and of salmon calcitonin. 'Amorphous' heparin, containing a range of molecular weight fractions, inhibited resorption by 15-20% at concentrations of 0.75-5.0 mg/ml. A 'defined' heparin species of mol.wt 13 500 inhibited resorption by 14-28% at 10(-5)-10(-4) mol/l. Histamine inhibited resorption by 19-55% at 10(-3)-10(-2) mol/l. It is proposed that heparin and histamine depress coupled bone resorption and formation and may lead to net loss of bone. Salmon calcitonin inhibited resorption at concentrations as low as 10 pg/ml. 'Amorphous' (but not 'defined') heparin blunted calcitonin induced inhibition of bone resorption and may derepress osteoclasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin L. A., Heath H., 3rd Calcitonin: physiology and pathophysiology. N Engl J Med. 1981 Jan 29;304(5):269–278. doi: 10.1056/NEJM198101293040505. [DOI] [PubMed] [Google Scholar]
- Avioli L. V. Heparin-induced osteopenia: an appraisal. Adv Exp Med Biol. 1975;52:375–387. doi: 10.1007/978-1-4684-0946-8_33. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Crisp A. J., Chapman C. M., Kirkham S. E., Schiller A. L., Krane S. M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984 Aug;27(8):845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- Crisp A. J., Roelke M. S., Goldring S. R., Krane S. M. Heparin modulates intracellular cyclic AMP in human trabecular bone cells and adherent rheumatoid synovial cells. Ann Rheum Dis. 1984 Aug;43(4):628–634. doi: 10.1136/ard.43.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp A. J. Studies of histamine in the rheumatoid joint. Rheumatol Int. 1984;4(3):125–128. doi: 10.1007/BF00541181. [DOI] [PubMed] [Google Scholar]
- Ellis H. A., Peart K. M. The effects of heparin and dextran sulphate on cultured mouse limb bones. Br J Exp Pathol. 1970 Feb;51(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- GOLDHABER P. HEPARIN ENHANCEMENT OF FACTORS STIMULATING BONE RESORPTION IN TISSUE CULTURE. Science. 1965 Jan 22;147(3656):407–408. doi: 10.1126/science.147.3656.407. [DOI] [PubMed] [Google Scholar]
- GRIFFITH G. C., NICHOLS G., Jr, ASHER J. D., FLANAGAN B. HEPARIN OSTEOPOROSIS. JAMA. 1965 Jul 12;193:91–94. doi: 10.1001/jama.1965.03090020005001. [DOI] [PubMed] [Google Scholar]
- Glowacki J. The effects of heparin and protamine on resorption of bone particles. Life Sci. 1983 Sep 12;33(11):1019–1024. doi: 10.1016/0024-3205(83)90655-0. [DOI] [PubMed] [Google Scholar]
- KINGERY F. A. OSTEOPOROSIS, FRACTURES, AND HEPARIN THERAPY. JAMA. 1965 Jul 12;193:152–152. [PubMed] [Google Scholar]
- Kent G. N., Cohn D. V. Blood levels of calcitonin in microphthalmic (mi/mi) osteopetrotic mouse cannot account for the resistance of bone to this hormone. Metab Bone Dis Relat Res. 1981;3(2):151–153. doi: 10.1016/0221-8747(81)90034-5. [DOI] [PubMed] [Google Scholar]
- Lenaers-Claeys G., Vaes G. Collagenase, procollagenase and bone resorption. Effects of heparin, parathyroid hormone and calcitonin. Biochim Biophys Acta. 1979 May 16;584(3):375–388. doi: 10.1016/0304-4165(79)90114-4. [DOI] [PubMed] [Google Scholar]
- Lippman M. M., Mathews M. B. Heparins: varying effects on cell proliferation in vitro and lack of correlation with anticoagulant activity. Fed Proc. 1977 Jan;36(1):55–59. [PubMed] [Google Scholar]
- Metcalfe D. D., Lewis R. A., Silbert J. E., Rosenberg R. D., Wasserman S. I., Austen K. F. Isolation and characterization of heparin from human lung. J Clin Invest. 1979 Dec;64(6):1537–1543. doi: 10.1172/JCI109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe D. D., Soter N. A., Wasserman S. I., Austen K. F. Identification of sulfated mucopolysaccharides including heparin in the lesional skin of a patient with mastocytosis. J Invest Dermatol. 1980 Apr;74(4):210–215. doi: 10.1111/1523-1747.ep12541737. [DOI] [PubMed] [Google Scholar]
- Norton L. A., Proffit W. R., Moore R. R. Inhibition of bone growth in vitro by endotoxin: histamine effect. Nature. 1969 Feb 1;221(5179):469–471. doi: 10.1038/221469a0. [DOI] [PubMed] [Google Scholar]
- STINCHFIELD F. E., SANKARAN B., SAMILSON R. The effect of anticoagulant therapy on bone repair. J Bone Joint Surg Am. 1956 Apr;38-A(2):270–282. [PubMed] [Google Scholar]
- Sackler J. P., Liu L. Heparin-induced osteoporosis. Br J Radiol. 1973 Jul;46(547):548–550. doi: 10.1259/0007-1285-46-547-548. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Amsterdam A. Heparin: a potent inhibitor of ovarian luteinizing hormone-sensitive adenylate cyclase. FEBS Lett. 1977 Nov 15;83(2):263–266. doi: 10.1016/0014-5793(77)81019-3. [DOI] [PubMed] [Google Scholar]
- Squires J. W., Pinch L. W. Heparin-induced spinal fractures. JAMA. 1979 Jun 1;241(22):2417–2418. [PubMed] [Google Scholar]
- Straus A. H., Nader H. B., Dietrich C. P. Absence of heparin or heparin-like compounds in mast-cell-free tissues and animals. Biochim Biophys Acta. 1982 Aug 27;717(3):478–485. doi: 10.1016/0304-4165(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Charge effects in the activation of adenylate cyclase. J Biol Chem. 1975 Sep 10;250(17):6897–6903. [PubMed] [Google Scholar]
- de Swiet M., Ward P. D., Fidler J., Horsman A., Katz D., Letsky E., Peacock M., Wise P. H. Prolonged heparin therapy in pregnancy causes bone demineralization. Br J Obstet Gynaecol. 1983 Dec;90(12):1129–1134. doi: 10.1111/j.1471-0528.1983.tb06459.x. [DOI] [PubMed] [Google Scholar]


