Abstract
There is a public concern that COVID-19 vaccination and SARS-CoV-2 antibodies (Abs) negatively affect male fertility. However, the evidence for the presence of SARS-CoV-2 Abs in seminal plasma (SP) is lacking. We examined whether Abs were detectable in SP after COVID-19 vaccination in 86 men using a direct Ab measurement and by quantification of their neutralizing activity. The results show the presence of SARS-CoV-2 Abs in SP, with a strong correlation to the serum Abs, increasing with the number of vaccinations. Furthermore, the Ab titers are correlating with the neutralization activity. The SARS-CoV-2 vaccination parameters showed no association with the markers of sperm quality. In conclusion, this study indicates substantial levels of Abs in SP after COVID-19 vaccination that correlate with serum Ab titers but do not associate with sperm quality.
Keywords: COVID-19, Fertility, Men's health, Reproduction
Graphical abstract
Introduction
There is a public concern about COVID-19 vaccination negatively affecting male fertility [1]. In the case of a SARS-CoV-2 infection, the notion was supported in post-mortem analyses involving autopsy [2] and in clinical trials with patients with COVID-19 [3]. The abnormalities in seminal plasma (SP) appeared to correlate with the concentration of serum Abs to SARS-CoV-2 after infection [4]. However, the potential presence of vaccination-induced SARS-CoV-2 Ab in SP has not yet been reported, and there are no data indicating a potential relevance of such Ab to poor sperm quality. In healthy men, the blood-testis barrier (BTB) separates the basal and apical compartments of the seminiferous epithelium and protects developing germ cells from potentially harmful constituents circulating in blood. SARS-CoV-2 has been shown to overcome this barrier, likely due to the high local expression of angiotensin-converting enzyme 2 (ACE2) [5] and downregulation of a number of proteins, leading to an impairment of spermatogenesis and sperm motility [2]. Our aim was to investigate whether Abs to SARS-CoV-2 are directly detectable in SP and whether their titers are associated with serum concentrations and parameters of sperm quality.
Methods
Study design
In this observational case-control study, vaccination-induced Abs and their neutralization activity against Spike-Protein were analyzed in paired serum and SP samples from 86 adult men (n = 43 vaccinated; n = 43 nonvaccinated). The analysis of the SARS-CoV-2 vaccination parameters was carried out by researchers blinded to the clinical information in a laboratory in Berlin, Germany. The samples and fertility data were collected by the fertility center Das Kinderwunsch Institut Schenk GmbH (Dobl, Austria) and stored under standardized conditions at Biobank Graz, Medical University of Graz, Austria (Cohort 5001_12, KIWI Collection) [6]. The recommendations of the Declaration of Helsinki were followed, all participants gave written informed consent before analysis, and the protocol had been approved by the ethics committee of the Medical University of Graz, Austria (approval number: 34-186ex21/22; 23-Feb-2022).
Quantification of SARS-CoV-2 Abs and their neutralization activity
Ab titers were determined with a sensitive binding assay using the S1 domain of SARS-CoV-2 fused to firefly luciferase. Briefly, serum and SP were incubated with a fusion protein containing secreted firefly luciferase in frame to the S1 protein of SARS-CoV-2. The samples were incubated overnight at 4°C, and the immune complexes formed (SARS-CoV-2 Ab bound to luciferase-S1 fusion protein) were precipitated with Protein A-Sepharose, washed, and analyzed for luciferase activity in a luminometer. Luminescence corresponding to SARS-CoV-2 Ab concentration in the original sample was recorded as relative light units and analyzed in relation to negative control signals. The results were quantified as binding index (fold over control). Inter- and intra-assay coefficient-of-variation using a positive sample as the standard were below 15% and 11%, respectively.
To confirm the validity, the neutralizing activity to the binding of spike protein to ACE2 (Spike Protein Inhibition Assay, product code: DKO205/RUO, ids Holdings PLC) was determined [7]. Briefly, the neutralizing Abs against SARS-CoV-2 were measured in serum and SP by a competitive method. The interference of the recombinant spike protein with the SARS-CoV-2 receptor ACE2 was quantified. The measurement range extends from 0% to 100%. Inter- and intra-assay CV using a control sample as the standard were below 10% and 20%, respectively.
Determination of sperm quality parameters
The sperm quality parameters were determined at the fertility center. Briefly, men were instructed to abstain for 2-7 days before semen collection. The samples were collected at home and transported immediately to the clinics or donated directly in the clinics and processed within 20 minutes of reception. The native semen samples were analyzed macroscopically and microscopically; the morphology was assessed using the Sperm Morpho Slide (Vitromed GmbH, Langenfeld, Germany), and a spermiogram was recorded according to the current World Health Organization guidelines by a validated methodology [8].
Statistics
The distribution of numerical variables was investigated using the Shapiro-Wilk test. Pairwise comparisons were conducted using the Wilcoxon rank-sum test, and categorical variables were compared using Fisher's exact test and Pearson chi-squared test to detect the differences in serum and SP markers. Spearman rank correlation was used to detect correlations between continuous variables. All statistical analyses were two-sided, and P-values below 0.05 were classified as statistically significant. Statistical analyses were performed using R on RStudio (version 1.02.5042).
Results
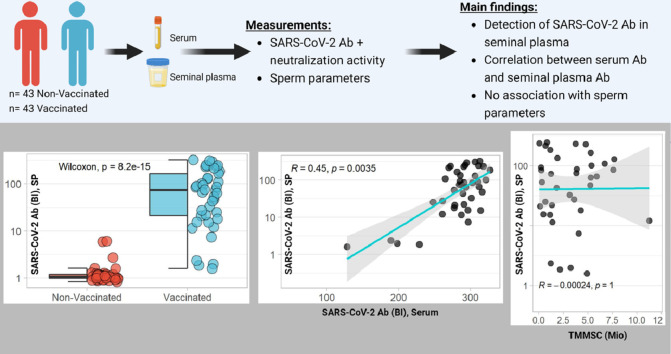
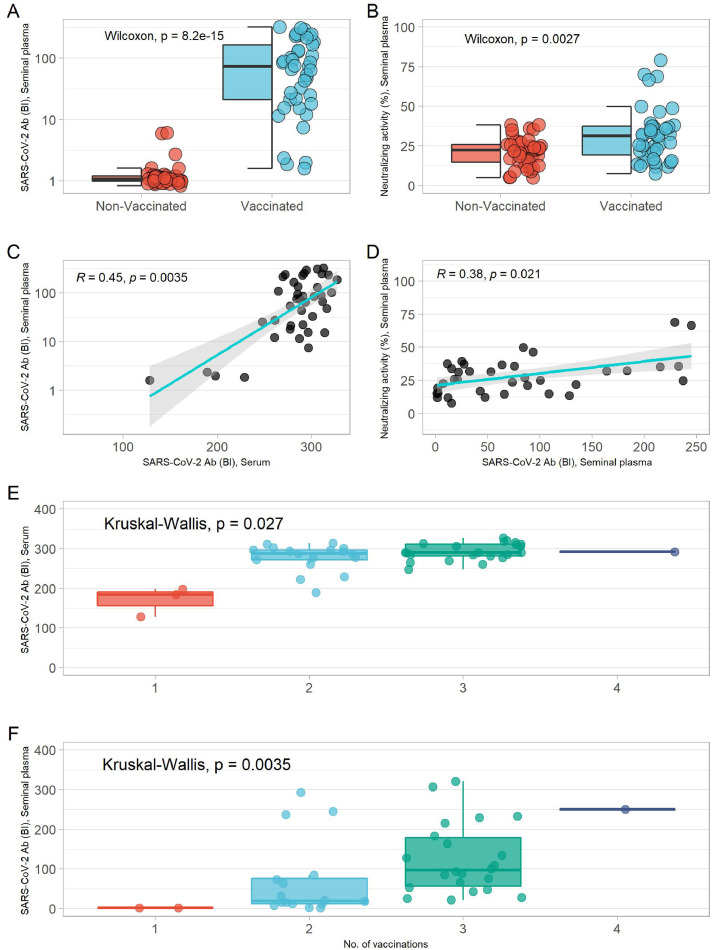
The descriptive patient data and variables of sperm quality were compared between vaccinated and nonvaccinated subjects (Supplementary Table 1). The groups did not differ in age (P = 0.41), body mass index (P = 0.95), or smoking habits (P = 0.52). The overall median of age and body mass index was at 38.2 years and 25.4 kg/m² (Supplementary Table 1). On average, the vaccinated males had higher concentrations of SARS-CoV-2 Ab in serum and SP than unvaccinated participants (Supplementary Figure 1a, Figure 1 a). Neutralizing activity was higher in the serum and SP samples of vaccinated men than the nonvaccinated participants (Supplementary Figure 1b, Figure 1b).
Figure 1.
SARS-CoV-2 antibodies and their neutralizing activity in serum and SP. (a) Comparison of SARS-CoV-2 antibody concentrations in SP of vaccinated and nonvaccinated subjects. (b) Comparison of neutralizing activity in SP of vaccinated and nonvaccinated subjects. Wilcoxon-Rank-sum test was used to calculate the P-value. (c) Correlation of SP SARS-CoV-2 antibodies and serum SARS-CoV-2 antibodies. (d) Correlation of neutralizing activity and SARS-CoV-2 antibodies in SP. Correlations were analyzed using the Spearman` rank test. (e) Comparison of serum SARS-CoV-2 antibody titers based on number of vaccinations. (f) Comparison of SP SARS-CoV-2 antibody titers based on number of vaccinations. Kruskal-Wallis test was used to calculate the P-value for difference.
BI, binding index; SP, seminal plasma.
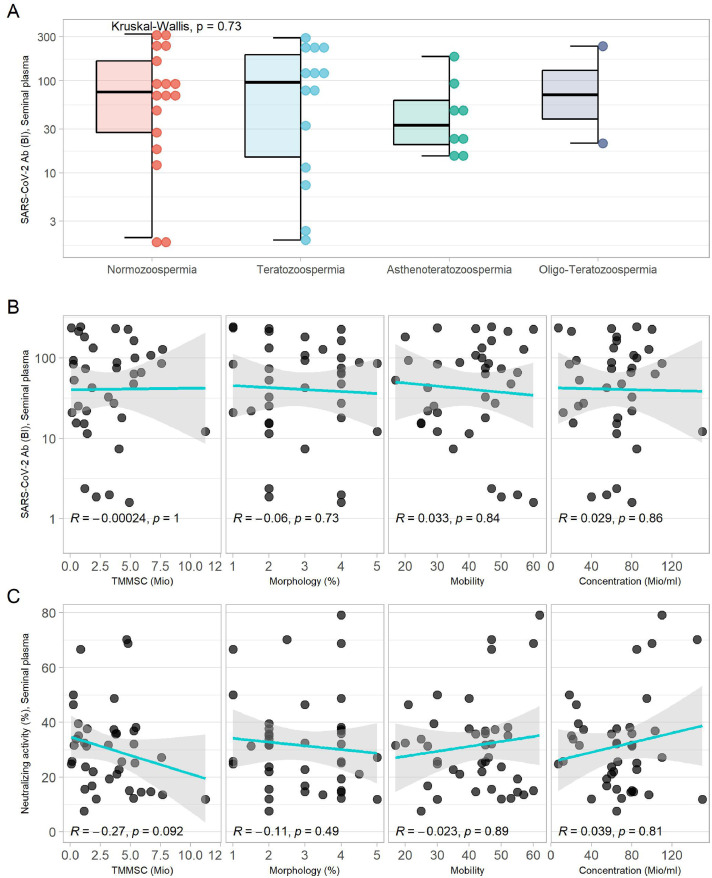
Serum and SP Ab titers to SARS-CoV-2 correlated positively with the neutralizing activity of the serum and SP (Supplementary Figure 1c, Figure 1d). A significant correlation was observed between SARS-CoV-2 Ab titers in serum and in SP (Figure 1c). SARS-CoV-2 Ab titers in serum and SP increased with number of vaccinations (Figure 1e,f), independent of the vaccine type or vaccine combination used (Supplementary Figure 1a,b). SARS-CoV-2 Abs were detectable in all groups of sperm pathology and showed no significant difference in titers (Figure 2 a). There was no significant correlation of SARS-CoV-2 Ab titers in SP and their neutralizing activity with sperm concentration, morphology, motility, or total motile morphologically normal sperm count (Figure 2b,c).
Figure 2.
SARS-CoV-2 antibody titers in SP and their association to sperm parameters. (a) SARS-CoV-2 antibody titers in SP according to sperm pathology. Kruskal-Wallis test was used to calculate the P-value for difference. (b and c) Correlation of the SARS-CoV-2 antibodies and neutralizing activity in SP with different sperm parameters. Correlations were analyzed using the Spearman rank test.
BI, binding index; SP, seminal plasma; TMMSC, total motile morphologically normal sperm count.
Discussion
This study describes the detection of SARS-CoV-2 Abs in SP and their neutralizing activity. Furthermore, the potential association of the SARS-CoV-2 vaccination parameters to sperm parameters was analyzed. The Abs in SP correlated significantly to serum Ab, and sperm pathology was not associated with the presence of Abs, indicating the passage of Abs through the BTB in fertile and nonfertile men. The SP Ab titers increased with the number of vaccinations, similar to serum Ab and irrespective of vaccine or vaccine combination chosen, suggesting a general positive association between serum and SP Ab. In serum and SP, Ab concentration and neutralizing activity showed a significant correlation. An analysis of SP Abs to SARS-CoV-2 with different sperm parameters yielded no significant associations, which was in agreement with studies analyzing serum Ab and sperm parameters after vaccination [9,10], independent of vaccine (messenger RNA or viral vector) or combinations thereof [11].
The direct detection of SARS-CoV-2 Abs in SP constitutes a relevant finding regarding the current concerns of a potential interaction of SARS-CoV-2 vaccination with male fertility and reproduction. The lack of associations between Ab titers in SP with any of the established sperm quality parameters provides further assurance for the vaccine safety regarding fertility. However, the consistent and concordant presence of the Abs in the blood and SP, irrespective of vaccination choice or underlying pathology, challenges our understanding of the role of the BTB for protecting sperm from humoral immune responses due to vaccination, infections, or autoimmune diseases. The current and highly variant immune status concerning SARS-CoV-2 provides a particular wide and promising research opportunity to better characterize the general mechanisms of immunoglobulin passage into the SP. The absence of associations between SARS-CoV-2 Abs in the SP with sperm parameters may be due to a lack of relevant autoantigens in SP, but this assuring finding should not be extrapolated to all infection- and immunization-induced Abs or pathogenic autoantibodies.
The strength of our study is the direct detection of SARS-CoV-2 Abs in the paired samples of serum and SP, with no apparent effects on sperm quality. The limitations of the study include the single time point of the analysis without further follow-up, the differences in vaccination mode between participants, and the lack of information on vaccination side effects.
In conclusion, this study detected vaccine-induced Abs to SARS-CoV-2 in the SP that do not appear to affect sperm quality.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This study was funded by the German Federal Ministry for Economic Affairs and Energy (BMWi, ZIM program, project #KK5051601BM0 to LS).
Acknowledgments
The authors would like to acknowledge the study participants and physicians collecting the samples. The authors thank their colleagues Gabriele Boehm, Vartitér Seher, and Anja Fischbach for helpful technical support in the laboratory analyses.
Author contributions
L. Schomburg had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Chillon, Schomburg, Schenk. Acquisition, analysis, or interpretation of data: Chillon, Demircan, Weiss, Schomburg. Drafting of the manuscript: Chillon, Demircan, Weiss, Schomburg. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Chillon, Demircan. Obtained funding: Schomburg. Administrative, technical, or material support: Weiss, Minich. Supervision: Schomburg, Schenk, Weiss.
Data sharing policy
Anonymized data will be made available upon reasonable request from the corresponding author.
Role of the funder/sponsor
The funder had no role in study design, data analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.03.018.
Appendix. Supplementary materials
References
- 1.Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013–1015. doi: 10.1001/jama.2022.2404. [DOI] [PubMed] [Google Scholar]
- 2.Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184 doi: 10.1016/j.cell.2021.01.004. 775–791.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalmedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donders GGG, Bosmans E, Reumers J, Donders F, Jonckheere J, Salembier G, et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril. 2022;117:287–296. doi: 10.1016/j.fertnstert.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghpanah A, Masjedi F, Alborzi S, Hosseinpour A, Dehghani A, Malekmakan L, et al. Potential mechanisms of SARS-CoV-2 action on male gonadal function and fertility: current status and future prospects. Andrologia. 2021;53:e13883. doi: 10.1111/and.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenk M, Huppertz B, Obermayer-Pietsch B, Kastelic D, Hörmann-Kröpfl M, Weiss G. Biobanking of different body fluids within the frame of IVF-a standard operating procedure to improve reproductive biology research. J Assist Reprod Genet. 2017;34:283–290. doi: 10.1007/s10815-016-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chillon TS, Maares M, Demircan K, Hackler J, Sun Q, Heller RA, et al. Serum free zinc is associated with vaccination response to SARS-CoV-2. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.906551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . 6th ed. World Health Organization; Geneva: 2021. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 9.Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326:273–274. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wesselink AK, Hatch EE, Rothman KJ, Wang TR, Willis MD, Yland J, et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol. 2022;191:1383–1395. doi: 10.1093/aje/kwac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massarotti C, Stigliani S, Maccarini E, Bovis F, Ferraro MF, Gazzo I, et al. mRNA and viral vector COVID-19 vaccines do not affect male fertility: a prospective study. World J Mens Health. 2022;40:561–569. doi: 10.5534/wjmh.220055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.