Abstract
Natural killer (NK) cells have critical roles in the innate immunosurveillance of cancer and viral infections. They are ‘first responders’ that can spontaneously recognize abnormal cells in the body, rapidly eliminate them through focused cytotoxicity mechanisms, and potently produce proinflammatory cytokines and chemokines that recruit and activate other immune cells to initiate an adaptive response. From the initial discovery of the diverse cell surface receptors on NK cells to the characterization of regulatory events controlling their development and function, our understanding of the basic biology of NK cells has improved dramatically in the past three decades. This advanced knowledge has revealed increased mechanistic complexity, which has opened the doors to the development of a plethora of exciting new therapeutics that can effectively manipulate and target NK cell functional responses, particularly in cancer patients. This translational progression of the field is well underway, as modern medicine is learning to effectively exploit the beneficial innate effector responses of NK cells to effectively combat cancer. Here, we summarize the basic mechanisms regulating NK cell biology, review a wide variety of drugs, cytokines, and antibodies currently being developed and employed to stimulate NK cell responses, and briefly outline newly evolving NK cell adoptive transfer approaches to treat cancer.
Natural killer (NK) cells are innate lymphocytes with unique capacity to rapidly kill infected, transformed, allogeneic, or stressed cells without prior encounter. T and B cells are essential mediators of durable immune responses to infections and cancer and for decades have shared center stage in the debate of immune relevance. Interest in NK cells has continued to blossom, however, since it has become clear that they perform as both direct and supporting actors in inflammatory responses. Whereas T and B cells generate adaptive immunity toward ‘non-self’ foreign antigens through the expression of antigen receptors, NK cells can identify and rapidly attack stressed cells, notably tumour cells, that have downregulated class I major histocompatibility complex (MHC-I) expression, which was termed ‘missing self’ recognition1. Importantly, NK cells can also swiftly generate potent cytokine production, particularly the secretion of interferon (IFN)-γ, tumour necrosis factor (TNF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and chemokines that recruit other immune cells and promote T and B cells to generate a robust secondary adaptive response2,3. In addition, NK cells can be triggered by cells targeted by immunoglobulin g (IgG) opsonization to mediate antibody-dependent cellular cytotoxicity (ADCC)2. Although originally named for their capacity to spontaneously eradicate aberrant cells, NK cells have safety features, as they rarely elicit autoimmunity and can actually promote immune homeostasis to counteract autoimmune disease3. In addition, a subset of long-lived NK cells can exhibit memory-like recall responses, which is characteristic of adaptive immunity4. Thus, NK cells have numerous beneficial attributes, making them attractive targets for immunotherapeutics that can harness their potent antitumour effector mechanisms.
Substantial evidence supports a crucial role for NK cells in routine surveillance against cancer. For example, an eleven-year study found higher incidence of cancer development in individuals with low peripheral NK cell cytotoxicity responses5. Furthermore, patients with congenital NK cell deficiencies experience increased incidence of malignancies, although most of these are driven by a concomitant increase in tumour-driving Epstein Barr virus or human papillomavirus infections6. Given their prevalence in peripheral blood, NK cells are most effective in directly counteracting haematopoietic cancers, including acute myeloid leukemia (AML), multiple myeloma (MM), non-Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL)7,8. They also have an important role in eradicating metastasizing tumour cells, as illustrated in recent noteworthy studies that demonstrated selective elimination of single breast tumour cells in circulation by NK cells as opposed to circulating clusters of cancer cells that are more resistant, and preferential NK cell-mediated attack of SOX2high regenerative-lineage metastatic lung cancer cells, while SOX9high cells escape9,10. Furthermore, preneoplastic senescent cells are targeted by NK cells, thereby potentially removing these cells that might otherwise progress to carcinomas11. In addition to direct tumour cell cytolysis, potent production of IFN-γ by NK cells can induce expression of a wide array of interferon response genes to elicit inflammatory antitumour effects12.
An exciting era of translational research has led to the rapid development of numerous immunotherapeutic strategies to boost antitumour responses by NK cells. In this article, we review this trajectory by first summarizing the basic foundations of NK cell biology and how NK cells can become functionally exhausted within the immunosuppressive tumour microenvironment (TME). Next, we outline therapeutic agents that augment NK cell-mediated antitumour responses, ranging from current drugs and other innate immune stimulants under exploration to cytokines as well as antibodies that activate NK cells through blocking inhibitory checkpoint receptors or targeting ADCC responses. Finally, we briefly describe current strategies of adoptive NK cell therapy, including arming adoptively transferred NK cells with chimeric antigen receptors (CARs) to enhance antitumour specificity.
The biology of human NK cells
What defines a NK cell?
To understand how NK cells can be manipulated therapeutically, we must first comprehend how they are hard wired. NK cells are the predominant member of group 1 innate lymphoid cells (ILC1), and they are characterized by the production of the type 1 cytokines, IFN-γ and TNF. The ILC1 family also includes a minor population of tissue resident intraepithelial cells that produce type 1 cytokines too, but also express both natural cytotoxicity triggering receptor 2 (NCR2, also known as NKp44) and integrin alpha-E (also known as CD103), unlike conventional NK cells13. NK cells constitute 5–15% of peripheral blood lymphocytes and are readily found in bone marrow, spleen, liver, lung, and other tissues, but are rare in lymph nodes2,4,14. The classic surface marker profile defining human NK cells in peripheral blood is CD3−CD56+, although expression of NKp80 (encoded by KLRF1) or CD7 can differentiate them from CD56+ myeloid cells4,15. Two major subsets of circulating human NK cells are distinguished by expression levels of CD56, namely CD56bright NK cells, which are immature and constitute 5–10% of peripheral blood NK cells, and CD56dim NK cells, which are more mature and mediate robust serial cytotoxicity of aberrant cells4,14. CD56bright cells can extravasate from the circulation into tissues and lymph nodes through expression of L-selectin (also known as CD62L) and C-C chemokine receptor type 7 (CCR7) and potently produce cytokines, but exhibit limited cytotoxicity responses2,4,14. CD56dim cells can also produce cytokines, but are highly cytolytic toward susceptible target cells and can mediate ADCC, owing to expression of IgG Fc region receptor III-A (FcγRIIIa, also known as CD16)2,4. Importantly, the majority of tissue-resident NK cells are CD56bright and often express CD69 and CXCR6 in lymph node, spleen, bone marrow, tonsil, and liver16,17. Furthermore, upon stimulation with IL-2 or IL-15, both CD56bright and CD56dim NK cells express high levels of CD56 and become highly cytolytic18. Recent high resolution molecular studies have defined more intricate subsets of NK cells in a variety of tissues beyond the simple CD56dim versus CD56bright delineation, which was established based on studies in peripheral blood13,16,19–21.
NK cells develop from bone marrow-derived progenitor cells in a thymus-independent manner22. In contrast to T and B cells, which express diverse antigen receptors generated by gene rearrangements mediated by recombination activating genes (RAG1/2), NK cells express a broad array of germline-encoded receptors2,4. IL-15 strongly stimulates NK cell proliferation, activation, and cytotoxicity and is essential for normal NK cell development and survival23,24. IL-2 can substitute for IL-15 to support NK cell proliferation ex vivo (as receptors for IL-2 and IL-15 (IL-2R and IL-15R) share common β (CD122) and γ chains (CD132) but is dispensable in vivo, since NK cells develop and function normally in IL-2-deficient mice14,25.
How do receptors regulate NK function?
NK cells are regulated by a dynamic balance between signals transduced by a diverse set of activating and inhibitory receptors. NK cells explore their environment through physical interactions with adjacent cells, whereby NK receptors engage with ligands on other cells at contact interfaces called immune synapses. Most healthy cells display ubiquitous MHC-I expression, also known as class I human leukocyte antigens (HLA), which thereby elicits dominant inhibitory signaling, disengagement, and tolerance by NK cells26. Virus-infected and tumour cells often downregulate MHC-I2,27, however, thereby escaping recognition by CD8+ cytolytic T lymphocytes (CTL), since CTL rely upon an antigen receptor that detects antigenic peptides presented by MHC-I. By contrast, loss of this MHC-I inhibitory receptor ligand and/or upregulation of activating receptor ligands tips the balance toward activation in the NK cell to trigger spontaneous attack2. An activating immune synapse initiates strong adhesion and focused exocytosis of cytolytic granules to release perforins that form membrane pores, and granzymes that induce apoptosis of the target cell26. Focused degranulation prevents damage to healthy adjacent bystander cells26. NK cells can also mediate delayed killing by expressing TNF ligand superfamily member 6 (also known as Fas ligand, FASL, or CD95L) or TNFSF10 (also known as TRAIL or CD253), to induce target cell apoptosis2,3.
Fundamental signaling in NK cells involves activating receptor-induced stimulation of protein tyrosine kinases (especially Syk and ZAP-70) and inhibitory receptors that counteract tyrosine phosphorylation events by recruiting and activating protein tyrosine phosphatases (especially SHP-1 (also known as PTPN6) and SHP-2 (also known as PTPN11)26. The major activating and inhibitory receptors on human NK cells and their ligands are summarized in Table 1. The primary inhibitory receptors that recognize MHC-I are killer cell Ig-like receptors (KIRs, which detect the classical MHC-I molecules: HLA-A, HLA -B, and HLA-C), NKG2A (which heterodimerizes with CD94 and binds the non-classical MHC-I: HLA-E), and leukocyte immunoglobulin-like receptor subfamily B member 1 (LILRB-1, also known as ILT-2 or CD85j, which has broad specificity to classical and non-classical MHC-I)2,28,29. Activating receptors include the NCRs, (namely NKp30, NKp44, and NKp46, which recognize various ligands on tumour and stressed cells), CD16, NKG2D (which binds MICA/MICB and ULBP family stress molecules), DNAM-1 (also known as CD226, and which engages with Poliovirus receptor PVR and nectin-2, also known as CD155 and CD112, respectively), and signaling lymphocytic activation molecule (SLAM) family receptors (especially 2B4, which recognizes CD48, and SLAMF7, which is a self-ligand receptor)2,4,30. Some of these receptors are linked to dimers of transmembrane adaptors (TYRO protein tyrosine kinase-binding protein, also known as DAP-12, TCR-ζ, or FcR-γ, which contain immunoreceptor tyrosine-based activating motifs (ITAMs) for robust activation or DAP-10, which is co-stimulatory), whereas others contain cytoplasmic tyrosine motifs for intracellular signaling26. Several adhesion molecules on NK cells are also critical for assembling activating immune synapses with target cells, especially integrin alpha-L (also known as LFA-1)26. Importantly, subsets of T cells readily express many of these NK cell receptors, so therapeutic targeting of them will also impact some T cells.
Table 1 |.
Human NK cell receptors and reported ligands
| Receptors | Functions | Ligands |
|---|---|---|
| NKG2D | activating | MICA, MICB, ULBP1–6 |
| DNAM-1 | activating | Nectin-2 (CD112), PVR (CD155) |
| FcγRIIIa (also known as CD16) | activating | Fc domains of IgG1 and IgG3 |
| NKp80 | activating | AICL |
| NKG2C/CD94 | activating | HLA-E |
| SLAMF7 | activating | SLAMF7 |
| KIR2DS1 | activating | Group 2 HLA-C |
| KIR2DS4 | activating | Some HLA-C, HLA-A*11, HLA-F |
| KIR3DS1 | activating | HLA-Bw4, HLA-F |
| TM1GD2 (CD28H) | activating | HHLA2 |
| NKp30 (NCR3) | activating | B7-H6, HLA-B associated transcript 3 (BAT3)/ Bcl2-associated anthogene 6 (BAG6) (soluble/exosomes), galectin-3, heparan sulfate proteoglycans |
| NKp46 (NCR1) | activating | hemagglutinins, heparan sulfate proteoglycans, vimentin, complement factor P (CFP; properdin, soluble) |
| NKp44 (NCR2) | activating | 21spe-MLL5 (splice variant of MLL5), heparan sulfate proteoglycans, PDGF-DD (soluble), some HLA-DP, hemagglutinins, Nidogen-1, Dengue virus E protein |
| inhibitory | PCNA, Nidogen-1 (soluble) | |
| 2B4 | activating/ inhibitory | CD48 |
| KIR2DL1 | inhibitory | Group 2 HLA-C |
| KIR2DL2, KIR2DL3 | inhibitory | Group 1 HLA-C, some group 2 HLA-C, some HLA-B |
| KIR2DL5 | inhibitory | PVR |
| KIR3DL1 | inhibitory | HLA-Bw4 containing alleles of HLA-B and HLA-A |
| KIR3DL2 | inhibitory | some HLA-A (such as A*03 and A*11), HLA-F |
| KIR3DL3 | inhibitory | HHLA2 |
| NKG2A/CD94 | inhibitory | HLA-E |
| NKR-P1A | inhibitory | CLEC2D (LLT1) |
| LILRB1 (ILT2, LIR1) | inhibitory | HLA-A, HLA-B, HLA-C, HLA-G, HLA-F, S100A9 |
| KLRG1 | inhibitory | E-cadherin, N- cadherin, and R-cadherin |
| CD96 (TACTILE) | inhibitory | Nectin-1 (CD111), PVR |
| TIGIT | inhibitory | Nectin-2, PVR |
| PVRIG | inhibitory | Nectin-2 |
| TIM-3 | inhibitory | Galectin-9, CEACAM-1, phosphatidyl serine, HMGB1 |
| LAG-3 | inhibitory | MHC class II, galectin-3, LSECtin (liver sinusoidal endothelial cell lectin), FGL1 |
| PD-1 | inhibitory | PD-L1, PD-L2 |
Abbreviations: AICL, activation-induced C-type lectin; LAG-3; PVR, PD-1, PD-L1, PVRIG ; TIGIT; TIM-3
NK cells also express structurally-related families of receptors that contain both activating and inhibitory members, some of which interact with the same ligands. For example, KIRs have distinct members (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL5, and KIR3DL1, KIR3DL2 and KIR3DL3) with long cytoplasmic domains (L) that contain immunoreceptor tyrosine-based inhibitory motifs (ITIMs) to mediate inhibitory function. Alternatively, other KIRs (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5 and KIR3DS1) have short (S) cytoplasmic domains lacking the ITIM, but associate with DAP12 to transduce activation signals28. Similarly, whereas NKG2A is an inhibitory receptor containing ITIMs, NKG2C is an activating receptor lacking ITIMs and associating with DAP1226. Additionally, NCR family members have differentially spliced gene products, some that encode classic activating receptors, and others that encode suppressive forms30. The complexities and expression conditions for differentially spliced NCRs are still under investigation. Another mixed function NK receptor family consists of the co-stimulatory DNAM-1 and suppressive CD96 (also known as TACTILE), TIGIT, and PVRIG (also known as D112R)31,32, all of which share ligands upregulated on various tumours (Table 1).
KIRs diversify, tolerize, and mature
KIRs are highly polymorphic and provide the human NK cell repertoire with extensive diversity, through three strategic attributes.28 First, each person is capable of inheriting a different haplotype of the 14 available KIR family member genes (Figure 1A). This varied gene content can include almost entirely inhibitory KIRs (haplotype A), or a more diverse repertoire enriched with more activating KIRs (haplotype B). Second, individual KIRs recognize distinct subsets of the classical MHC-I ligands (Figure 1B). The inhibitory KIRs have co-evolved with MHC-I in primates to detect HLA-C via KIR2DL1, KIR2DL2, or KIR2DL3, subsets of HLA-B and HLA-A (those containing the Bw4 epitope) by KIR3DL1, and certain HLA-A (particularly HLA-A3 and -A11) by KIR3DL2. Importantly, since KIR and HLA gene loci are located on separate chromosomes, they are differentially inherited, and some KIR are inherited and expressed, yet lack an available cognate ‘self’ MHC-I ligand. Third, the available KIR genes are randomly expressed on individual NK cells to generate a diverse repertoire. Owing to this variegated KIR expression, the loss of one allele of MHC-I could make a tumour cell susceptible to a subset of NK cells that expressed only the KIR that has lost its cognate ligand (Figure 1C).
Figure 1. Killer Cell Ig-like Receptors (KIRs) provide diversity, tolerance, and education to human NK cells.
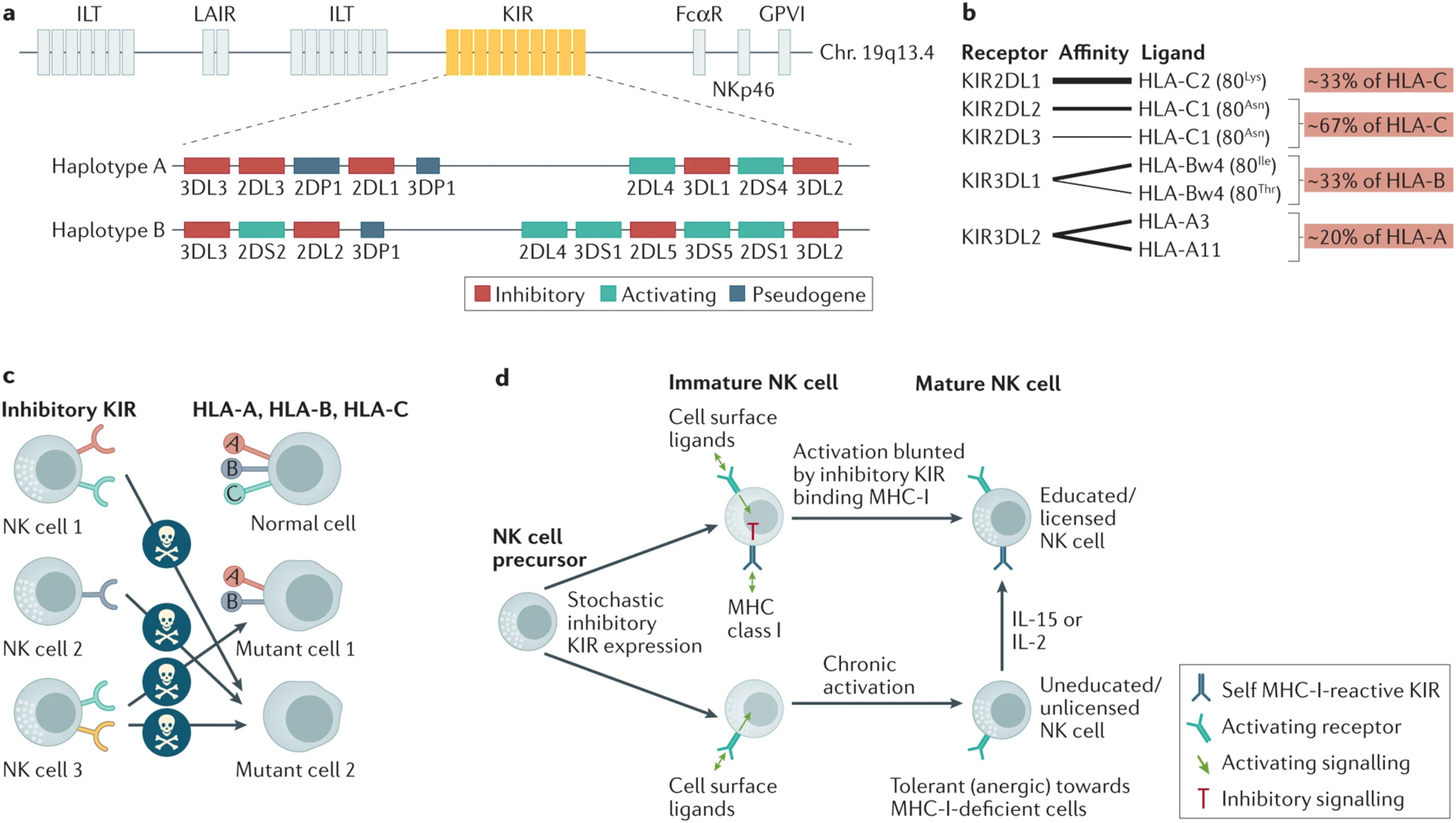
a. Humans inherit different numbers and combinations (haplotypes) of the 14 available killer cell inhibitory receptor (KIR) genes, with only two examples shown. The KIR genes are inherited in close proximity in a locus on chromosome 19. Haplotype A contains combinations of mostly inhibitory KIRs and only two activating KIRs (usually KIR2DL4 and KIR2DS4), while haplotype B contains more activating KIRs. b. Different inhibitory KIRs recognize subsets of the available alleles of the class I human leukocyte antigen complex (HLA). Relative receptor-ligand affinities are represented by thickness of the interlinking lines. Class I HLA subgroups are indicated with their general frequency in the human population. HLA-C alleles can be divided into C1 and C2 subgroups according to either asparagine or lysine at position 80, respectively, and HLA-B alleles can be divided into Bw4 or Bw6 subgroups. c. KIRs are expressed on NK cells in a variegated manner, forming a repertoire that can be tolerized and educated by interaction of at least one KIR with the endogenous ‘self’ class I HLA. A subset of NK cell clones could recognize a cell that has lost only one class I HLA allele (mutant cell #1), whereas if an abnormal cell loses all class I HLA (mutant cell #2), it has lost ligands for all KIR and is susceptible to all NK cells. d. KIR-mediated education during NK cell development. KIRs are stochastically expressed on individual NK cell clones during development. If a KIR recognizes ‘self’ class I HLA (top), it inhibits activation signaling and promotes maturation to a fully competent NK cell (educated), but if no KIR is expressed that recognizes ‘self’ class I HLA, chronic activation signaling results in an hyporesponsive NK cell (uneducated; bottom). Exposure to IL-15 or IL-2 can stimulate an uneducated NK cell into a competent, educated state. Figure 1B has been adapted with permission from REF.252 and figure 1D has been adapted with permission from REF.2.
In view of the variegated expression of KIRs and other receptors, mass cytometry analysis of receptors on peripheral blood NK cells in adult healthy donors revealed a repertoire of between 6,000 and 30,000 distinct NK cell ‘clones’ within each donor, as defined by distinct combinations of expressed receptors33,34. Owing to this diversity, a subset of NK cell clones would potentially have an optimal receptor expression phenotype to respond to the unique ligand expression characteristics of a particular tumour cell. Once an NK cell clone has been activated toward a compromised target cell, it can proliferate and terminally differentiate to generate long-lived ‘memory’ NK cells capable of mounting a more rapid secondary response, thereby providing an adaptive immune response35. A prominent example is the expansion of NKG2C+ CD57+ NK cells in response to human cytomegalovirus infection, which are also referred to as ‘adaptive-like’ NK cells that are long-lived and exhibit strong antitumour responsiveness21,36,37.
The inheritance of certain haplotypes of KIR and MHC-I ligands has been associated with susceptibility to diseases, including psoriatic arthritis, viral infections, cancer, and pre-eclampsia in pregnant women38. Furthermore, inhibitory KIR/MHC-I mismatch has been shown to be beneficial under defined conditions of haematopoietic stem cell transplantation and adoptive NK cell therapies39.
Although inhibitory KIRs and NKG2A have key roles in enabling NK cells to tolerize healthy MHC-I-expressing cells, they also have a second critical role in instructing NK cells to be functionally competent during their developmental maturation. During normal development, early stage NK cell precursors stochastically express individual KIR genes in ‘sequential acquisition’ until one inhibitory receptor recognizes a ‘self’ MHC-I molecule to effectively transduce a negative signal and restrict expression of additional inhibitory receptor genes40. This process generates a repertoire of NK cells, each of which permanently expresses a defined combination of inhibitory receptors. Successful engagement of an inhibitory KIR or NKG2A with MHC-I ligand promotes maturation to a functionally responsive NK cell through a process that has been called ‘education’ or ‘licensing’ (Figure 1D). Educated mature NK cells are subsequently capable of being activated upon encounter of an MHC-I-deficient target cell41,42. By contrast, a small subset of NK cells never successfully expresses an inhibitory receptor that can recognize endogenous MHC-I, and these remain hyporesponsive. NK cells in patients who are deficient for antigen peptide transporter 1 (TAP1), who lack normal MHC-I expression, are similarly hyporesponsive43. Unlicensed or uneducated NK cells can, however, be ‘primed’ to achieve a functional state if cultured with IL-2 or IL-15, which suggests they may become activated under certain conditions in vivo and may have a lower threshold of activation toward MHC-I-expressing tumours44. Education is also evident in patients after receiving MHC-I-mismatched cord blood transplants, as the most responsive NK cells that develop in these patients express a KIR for which both donor and recipient express the cognate ligand45. If, however, the patient lacks the cognate MHC-I ligand for a particular inhibitory KIR, the NK cells expressing that KIR lose some responsiveness45.
As an overview, maturation, survival and functionality of NK cells strongly depends on suppression of activation signaling mediated by tonic inhibitory receptors, which starkly contrasts with the primary need in T and B cells for antigen receptor-mediated activation signaling to support the same processes. In a striking example of this fundamental signaling dichotomy, mice deficient in the activation signaling protein B-cell adaptor for phosphoinositide 3-kinase (BCAP, encoded by PIK3AP1) have B cells that are less mature, less functional, and more susceptible to apoptosis, whereas NK cells that develop in the same mouse have the exact opposite phenotype46.
NK Cells in the TME
While haematopoietic tumours in peripheral blood are readily accessible to NK cells, solid tumours are more challenging to reach and infiltrate. Nonetheless, increased influx and activation of NK cells in solid tumours has been associated with longer overall survival in a variety of cancers47. To reach solid tumours, NK cells must extravasate from the blood and traverse the extracellular matrix and tumour stroma. The expression of L-selectin allows immature CD56bright NK cells to contact high endothelial venules and leave the vasculature for secondary lymphoid organs, and CCR7 expression also draws them toward C-C motif chemokine 21 (CCL21) expressed on these lymphatic endothelial cells and toward CCL19 and CCL21 in T cell zones of lymph nodes48,49. NK cells can degrade and thereby traverse the extracellular matrix through expression of matrix metalloproteinases, urokinase plasminogen activator, and serine dipeptidylpeptidase IV50. In contrast to those found in blood, NK cells in most normal peripheral tissues are mainly CD56bright, and this subset is even more enriched in tumours51. Once in a tumour, however, NK cell responsiveness is often hindered by the immunosuppressive TME.
The immunosuppressive TME can drive NK cells into an exhausted state. Tumour growth factor beta (TGF-β) is readily expressed in most TMEs and is an important suppressor of NK cells through a variety of mechanisms52,53. TGF-β drives differentiation of NK cells into intraepithelial ILC1, which are less capable of mediating antitumour cytolytic responses, are retained in tissue upon upregulation of integrin alpha-1 (also known as CD49a) and CD103, and lose expression of eomesodermin homolog (EOMES), the loss of which is a sign of NK cell exhaustion52,54,55. The TME is also hypoxic, which can downregulate expression of NCRs and NKG2D and reduce natural cytotoxicity by NK cells56. Hypoxia also induces hypoxia-inducible factor 1α (HIF-1α) expression in tumour-infiltrating NK cells, which suppresses their antitumour and IFN-γ production responses57, and deletion or pharmacological inhibition of HIF-1α substantially potentiates NK cell antitumour responses57. Dysfunctional NK cells in liver cancer exhibit mitochondrial fragmentation, which is associated with lower oxidative phosphorylation, increased reactive oxygen species (ROS), and a hypoxic gene expression signature that contributes to poor antitumour response and increased apoptosis58. Also, NK cell dysfunction in the TME is driven by oxidative stress-induced suppression of glucose metabolism29. Additional suppressive mediators in the TME that can inhibit NK cell function include prostaglandin E259, 5’-deoxy-5’-methylthioadenosine60, indoleamine 2,3-dioxygenase (IDO)-mediated catabolism of tryptophan to L-kynurenine61, lactate-mediated acidification causing mitochondrial distress62, and adenosine produced from ATP by the ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, also known as CD39) and 5’-nucleotidase (also known as CD73) ectoenzymes63, as well as limited glucose availability to support glycolysis by NK cells64. Furthermore, expression of members of the a disintegrin and metalloproteinase (ADAM) family within the TME can cleave and release soluble forms of ligands for NKG2D and NKp30, thereby limiting NK cell recognition and activation65. Given the harsh environment in the TME, successful pharmacological potentiation of NK cell responses in tumours will likely require multiple approaches to overcome these many immunosuppressive barriers.
Agents enhancing NK cell function
Immunomodulatory imide drugs
Immunomodulatory imide drugs (IMiDs) are a class of drugs containing an imide group that enhance the function of lymphocytes. The IMiDs include thalidomide and the related compounds, lenalidomide and pomalidomide, which are used to treat MM, myelodysplastic syndrome (MDS) and some lymphomas66. Pomalidomide has higher potency and shows efficacy in patients who are resistant to lenalidomide and thalidomide67. At least part of the immunotherapeutic benefit of IMiDs is believed to result from enhanced NK cell activity. One of the primary mechanisms by which IMiDs activate NK cells is through inducing IL-2 production by T cells. Lenalidomide, however, can also directly enhance NK cell responses by lowering their activation threshold for CD16- and NKG2D-mediated stimulation of IFN-γ production68. In addition, IMiDs can make malignant plasma cells more susceptible to NK cells by increasing their expression of the NKG2D and DNAM-1 receptor ligands, MICA and PVR, respectively69.
A primary molecular mechanism of IMiD action involves interaction with cereblon (CRBN), which forms a complex with cullin-4A (CUL4A), DNA damage binding protein-1 (DDB1), and E3 ubiquitin-protein ligase RBX1 (also known as ROC1) to mediate E3 ubiquitin ligase activity70–72. Thalidomide, lenalidomide and pomalidomide have similar binding modes and affinities for CRBN, and this binding promotes CRBN to more efficiently interact with the transcription factors Ikaros (IKZF1) and Aiolos (IKZF3) and induce their targeted ubiquitination and proteolytic degradation71–73. IKZF1 and IKZF3 normally suppress IL2 mRNA expression in T cells, and their degradation is responsible for the increased secretion of IL-2 in lenalidomide-treated T cells71.
Pomalidomide can also directly bind and activate tyrosine-protein kinase ZAP-70 in T cells and NK cells, and kinase activation was independent of CRBN74. In addition, pomalidomide can induce granzyme-B expression in NK cells, and this increase depends on ZAP-70 activation74. Therefore, IMiDs can enhance NK and T cell function by ZAP-70-mediated and CRBN-mediated mechanisms and offer potential to restore the activity of NK cells in the immunosuppressive TME. Additional next-generation CRBN-binding drugs are also under development with improved capacity to degrade IKZF1 and IKZF2 ubiquitylation targets73.
Proteasome inhibitors
In vitro treatment of tumour cell lines with the proteasome inhibitor bortezomib upregulates expression of ligands for NKG2D and DNAM-1 substantially, increasing their susceptibility to NK cell-mediated cytotoxicity75–77. MICA upregulation by bortezomib is dependent on the ATM/CHK2 DNA damage response pathway78. To further increase susceptibility to NK cells, bortezomib also downregulates MHC-I expression on MM cells treated overnight in vitro or when assayed in blood from patients 48 hours after a single dose79.
STING agonists
The cyclic GMP-AMP synthase (cGAS)–stimulator of interferon genes protein (STING) pathway has a key role in innate immune sensing of aberrant cytosolic DNA from viral or bacterial pathogens. Activation of this pathway generates type I interferons (IFN-α and IFN-β) and proinflammatory cytokines and chemokines80,81. cGAS converts double-stranded viral DNA into 2′,3′-cyclic guanosine monophosphate–adenosine monophosphate (cGAMP)80, which binds to STING in the endoplasmic reticulum membrane80. The cGAMP-STING complex recruits protein kinase TBK1 and activates interferon regulatory factor 3 (IRF3) and nuclear factor kappa B (NFκB) transcription factors80. In mice, tumour cells were found to produce cGAMP, which activated STING in neighboring myeloid cells to produce IFN-β that subsequently activated NK cells to mediate an antitumour immune response82.
STING agonists, such as cyclic dinucleotides (CDN), can stimulate CD8+ T cells and NK cells83. CDN treatment slowed tumour growth in a wide variety of mouse cancer models and this effect was independent of CD8+ T cells84. NK-mediated antitumour effects were dependent on type I IFNs produced by dendritic cells (DCs), macrophages, monocytes, and endothelial cells84. These cells can have a pivotal role in the antitumour effect of intratumoral CDN injection83,85,86. The type I IFNs produced in response to CDN treatment induced IL-15Rα and IL-15 production by DCs, which resulted in systemic activation of NK cells84. Several clinical trials investigating different STING agonists to treat lymphomas are currently ongoing (e.g. NCT04144140 and NCT04609579).
Cytokines to stimulate NK cells
A number of cytokines have an effect on NK cell function and are being investigated for stimulating NK cells in patients using various forms of the activating cytokines or antagonists of an inhibitory cytokine. The general impacts and active clinical trials of these are listed in Table 2.
Table 2.
Clinical trials involving cytokines that have important roles in NK cell biology
| Cytokine agonist or antagonist | Active clinical trials |
|---|---|
| IL-2 (and agonists ALKS 4230, F42K) | NCT02799095, NCT03861793, NCT04144517, NCT04592653, NCT04830124, NCT05092360 |
| IL-12 (fusions with antibodies, encoding genetic material, and controlled release) | NCT02062827, NCT02483312, NCT03132675, NCT03567720, NCT03823131, NCT04095689, NCT04370587, NCT04471987, NCT04526730, NCT04756505, NCT04911166 |
| IL-15 (hetIL-15, N-803) | NCT01898793, NCT02138734, NCT02452268, NCT02465957, NCT02523469, NCT02782546, NCT02890758, NCT02989844, NCT0322825, NCT03228667, NCT03387111, NCT03520686, NCT03563157, NCT04261439, NCT04290546, NCT04390399, NCT04847466, NCT04898543, NCT04927884, NCT05096663 |
| IL-18 | NCT04787042 |
| TGF- β Inhibitors (fresolimumab, galunisertib) | NCT01582269, NCT01682187, NCT02452008, NCT02581787, NCT02672475, NCT02688712, NCT03206177, NCT04605562 |
IL-2 and IL-15
IL-2 has long been known to directly stimulate the proliferation and activation of T and NK cells87,88. Originally, stimulation of human peripheral blood mononuclear cells with IL-2 was found to expand cytotoxic cells, which were named lymphocyte-activated killer (LAK) cells87–89. Subsequent work found that tumour destruction by LAK cells was predominantly mediated by NK cells90. IL-2 is still commonly used for ex vivo NK cell stimulation, but therapeutic use is limited by common toxicity, which ranges from flu-like symptoms to severe capillary leak syndrome with high doses91. Moreover, IL-2 induces the proliferation and activation of highly suppressive regulatory T (Treg) cells expressing inducible T-cell costimulator (ICOS)92. Various groups are developing modernized variations of IL-2 with improved toxicity profiles, such as ALKS 4230 and F42K-modified IL-2, which induce greater activation and expansion of NK cells with reduced expansion of Treg cells92,93.
The most promising therapeutic substitute for IL-2 is IL-15. Importantly, IL-15 stimulates NK and CD8+ T cells, but not Treg cells94. As mentioned before, both IL-2 and IL-15 receptors share common receptor elements, namely β-chain and common γ-chain heterodimers, that are expressed on NK cells. IL-2R also utilizes the IL-2Rα chain (CD25) for high affinity binding, whereas IL-15R uses the IL-15Rα chain (CD215) for high affinity IL-15 binding. The primary method of IL-15 signaling in NK cells involves ‘transpresentation’ of the cytokine in a bioactive complex with IL-15Rα expressed on the surface of another cell, often DCs and monocytes, as well as in a soluble paracrine complex in serum95,96.
In vitro studies have shown that IL-15 can potentiate the function of NK cells exhausted by the TME. Treatment of dysfunctional tumour-derived NK cells with IL-15 under hypoxic conditions restored mitochondrial integrity, increased expression of granzyme B, reduced apoptosis, and enhanced cytotoxicity and IFN-γ production58. Treatment with IL-15 also restored the function of NK cells exposed to H2O2-mediated oxidative stress by upregulating expression and activity of the anti-oxidant, thioredoxin-1, to increase cell surface thiol levels and reduce accumulation of ROS97.
Owing to strong impact on NK and cytotoxic T cells, several recombinant forms of IL-15 have been engineered for clinical use. Subcutaneous dosing of recombinant IL-15 stimulated activation and robust expansion of NK and CD8+ T cells, and was well tolerated in patients, although serious cytokine release syndrome was observed with high dosage in patients that had received lymphodepletion and adoptive transfer therapies98,99. More recent innovations involve engineered fusion complexes of recombinant IL-15 and IL-15Rα. One heterodimeric fusion (hetIL-15; NIZ985) packaged in extracellular vesicles is currently in phase 1/1b clinical trials100. In preclinical studies, hetIL-15 slowed tumour growth and increased tumour infiltration with NK cells and CD8+ T cells with increased expression of IFN-γ, cytotoxic granule components, and anti-apoptotic BCL-2101. Another promising IL-15 ‘superagonist’ is N-803 (formerly called ALT-803), which consists of recombinant IL-15 containing an N72D mutation that increases affinity to a dimeric IL-15Rα sushi domain-IgG1 fusion protein to facilitate transpresentation and prolong half-life in blood102. In preclinical studies, N-803 induced tumour regression in mouse models and prolonged survival compare to unconjugated IL-15103,104. Subcutaneous administration of N-803 in patients with leukaemia or lymphoma was safe, stimulated expansion of NK cells and CD8+ T cells, but not of Treg cells, induced expression of NK cell receptors, and showed some indications of clinical efficacy105,106. Early injections induced transient increase in serum IL-6 and IFN-γ associated with minor flu-like symptoms105,106. N-803 is currently being tested in numerous clinical trials (Table 2). Caution must be stressed in treating patients with IL-15, however, since long term exposure has been shown to cause NK cell dysfunction through metabolic and epigenetic reprogramming mechanisms107,108.
IL-12
IL-12 is a heterodimeric pro-inflammatory cytokine primarily produced by antigen-presenting cells, especially DCs and macrophages that stimulates the recruitment, effector functions, and IFN-γ production of CD8+ T cells and NK cells109. In addition to direct activation of NK cells, IL-12 stimulates T helper 1 (TH1) cells to produce IFN-γ and IL-2, which subsequently can stimulate NK cells to produce more IFN-γ, perforin, and granzyme B110.
Despite these positive effects on NK cell function, systemic administration of IL-12 causes numerous adverse side effects, including flu-like symptoms (fever, fatigue, headache, chills, and arthromyalgia) toxicity to liver (increased transaminases, hyperbilirubinemia, and hypoalbuminemia) and bone marrow (neutropenia and thrombocytopenia)111. Owing to these adverse effects, maximum tolerated doses have shown limited anti-cancer efficacy, so alternative methods of IL-12 delivery are being developed.
Alternative IL-12 delivery strategies can be divided into three groups: fusion molecules of IL-12 with tumour binding antibodies, introducing genetic material encoding IL-12, and controlled release of recombinant IL-12 from a delivery system, such as nanoparticles112. In mouse models immunocytokine constructs fusing IL-12 protein to targeting antibody domains can activate T cells and NK cells more efficiently at tumour sites than soluble IL-12113,114. Direct intratumoural delivery of DNA or RNA encoding IL-12 into tumour has been explored. A recent phase II clinical trial testing electroporation of accessible melanomas with the IL12-encoding plasmid tavokinogene telseplasmid (tavo) in combination with the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab showed increased NK cell-associated gene expression in tumours and enhanced antitumour immune responses115. Engineered viruses expressing IL-12 are also being tested, with several clinical trials ongoing (Table 2). Nanoparticle approaches are also being tested to deliver IL-12112. Another promising strategy is intratumoural injection of IL-12 expressed on the surface of exosomes, which increased tumour infiltrating NK and CD8+ T cells in mouse studies and kept the cytokine localized to the injection site in mice and monkeys to reduce systemic toxicity116. Clinical trials delivering IL-12 with many of these technologies are still pending, but promising preclinical studies indicate that localized delivery of the cytokine will reduce adverse effects substantially.
IL-18
IL-18 is a proinflammatory member of the IL-1 cytokine family produced by a wide variety of cells, including myeloid cells, intestinal epithelial cells, keratinocytes, and endothelial cells117. It is produced as pro-IL-18 and converted into mature cytokine by caspase 1-mediated cleavage through activation of the inflammasome pathway, whereupon it binds a heterodimeric receptor composed of IL-18Rα and IL-18Rβ.118 IL-18 (especially in combination with IL-12) induces FASL expression in NK cells and potentiates production of IFN-γ and TNF118. Moreover, IL-18 induces proliferation of NK cells and expression of the CCR7 chemokine receptor for CCL21, which promotes migration to secondary lymphoid organs118. In mice, the combination of IL-18 antibodies against PD-1 and cytotoxic T-lymphocyte protein 4 (CTLA4) is much more effective than the antibodies alone, and the response was significantly abrogated upon depletion of NK or CD8+ T cells119.
Owing to these favourable functions, IL-18 is being investigated in the clinic, with initial clinical trials showing tolerable toxicity120. Phase I trials testing the combination of IL-18 and anti-CD20 antibodies for treatment of B-cell lymphomas showed increased IFN-γ in serum and objective responses in some patients who were previously refractory to anti-CD20 antibodies alone121,122. Nonetheless, despite the relatively low toxicity profile, few clinical trials have studied IL-18 infusions to treat cancer, since efficacy has been limited. Impediments to the positive therapeutic impact of IL-18 on NK cells are the endogenous immune proteins interleukin-1 receptor 8 long isoform (IL-1R8, which suppresses IL-18 receptor function) and IL-18 binding protein (IL-18BP, which inactivates IL-18 in serum)123,124. A modified form of IL-18 that is unable to bind to IL-18BP, but still stimulates the IL-18 receptor is a promising alternative currently in clinical trial (NCT04787042)125.
IL-21
IL-21 is produced by TH17 cells and DCs, and signals through a heterodimeric receptor composed of IL-12Rα and γc, which is the common γ chain also shared by IL-2R and IL-15R118. IL-21 enhances the maturation and proliferation of NK cells and potentiates their cytotoxic activity by increasing expression of perforin and granzyme B126, as well as upregulation of NKp30 and NK cell receptor 2B4127. By contrast, however, culture of human primary NK and CD8+ T cells with IL-21 and IL-2 significantly decreases cell surface expression of NKG2D, compared to IL-2 alone, which parallels reduced DAP10 transcription127. The combination of IL-21 with other cytokines such as IL-2, IL-15 and IL-18 increases NK cell proliferation, cytotoxicity and IFN-γ production128,129. IL-21 infusions were safe and well tolerated in clinical trials, although clinical efficacy of the cytokine alone was limited, which has prompted testing in combination therapies130. It should also be cautioned that IL-21 is a growth factor for B cells and can thereby promote the proliferation of some B cell neoplasms, such as MM, Burkitt lymphoma, and Hodgkin lymphoma130.
TGF-β inhibitors
TGF-β strongly inhibits NK cell functions and promotes NK cells to differentiate into intraepithelial ILC1, which are tissue resident and less cytolytic toward tumours52,54,55. TGF-β is often expressed within the TME, so a strategy to inhibit TGF-β in patients has potential to restore NK cell function and suppress tumour growth.
Approaches to inhibit TGF-β that have been tested in clinical and pre-clinical studies include neutralizing antibodies, ligand traps, and receptor kinase inhibitors131. The neutralizing monoclonal antibody (mAb) anti-TGF-β1/TGF-β2 fresolimumab (GC1008) has been tested in several clinical trials and was well tolerated, but exhibited only modest responses and elicited reversable cutaneous keratoacanthomas, hyperkeratosis, and carcinomas132. A clinical trial of fresolimumab with local hypofractionated radiation in patients with metastatic breast cancer found increased circulating NK cells and CD8+ effector and central memory T cells, but surprisingly also increased Treg cells, demonstrating broad disruption of T cell homeostasis133. Galunisertib (LY2157299) is a small molecule inhibitor of TGF-β receptor complex TGFβRI kinase that was found to improve survival of neuroblastoma-bearing NSG mice treated with an ADCC-inducing antibody and adoptively transferred NK cells134. Treatment of patients with liver and pancreatic cancer with galunisertib was well tolerated and showed improved overall survival, although NK cells were not analyzed in treated patients135,136. Although TGF-β targeting agents show promising potential, their effects on NK cell activity and functions have been limited in patients.
Antibodies to boost NK cell function
Checkpoint blocking antibodies
Since NK cell functional responses are determined by the integration of activating and inhibitory receptor signaling, targeting inhibitory receptors with blocking antibodies offers an enticing strategy to potentiate the NK cell antitumour response in cancer patients (Figure 2). Below, we summarize the major checkpoint targeted therapies impacting NK cells that are currently undergoing clinical trials.
Figure 2. Antibodies in NK cell immunotherapy.
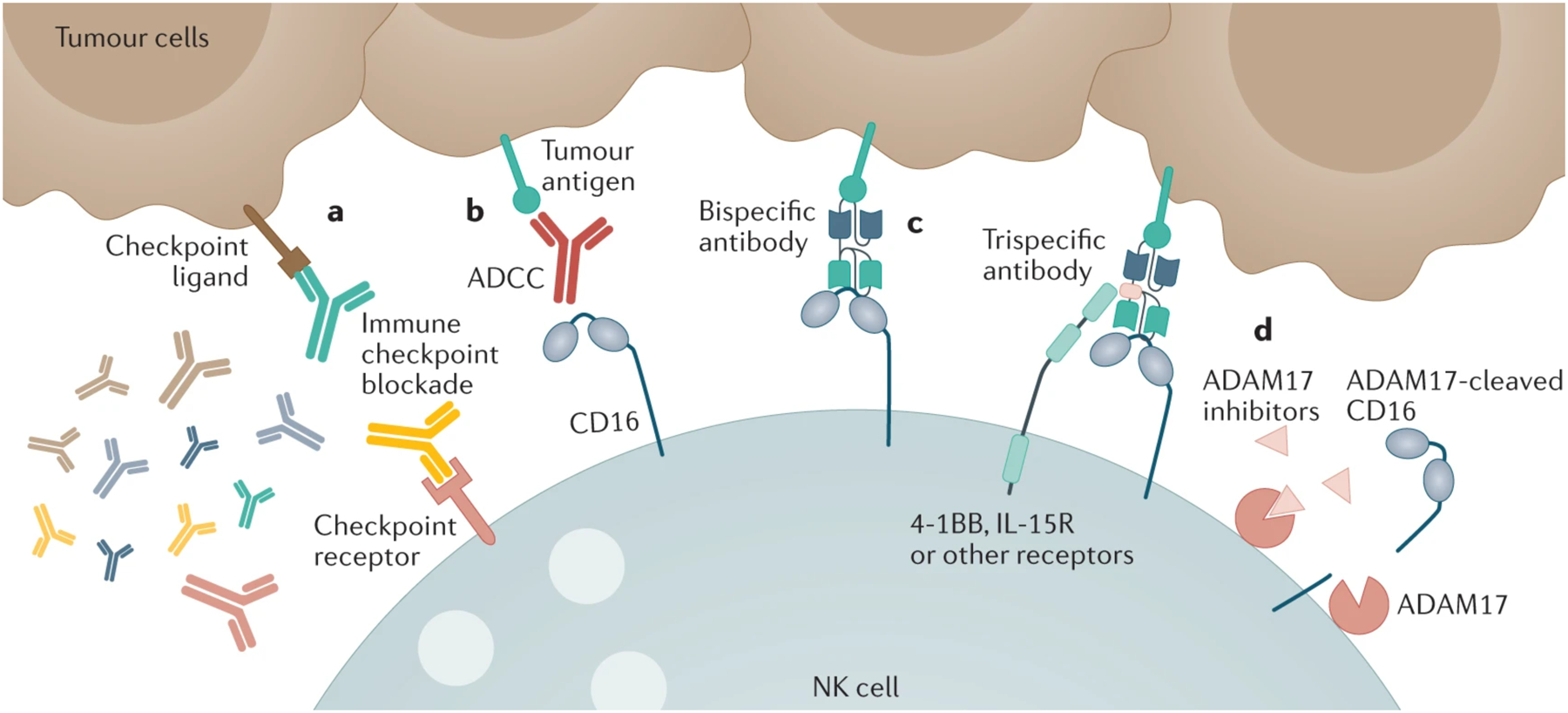
Antibodies can be used to enhance NK cell function in several different ways. a. Immune checkpoint blockade can be used to prevent the engagement of checkpoint inhibitory receptors on NK cells with their ligands on tumours. b. Antibodies targeting tumour antigens can be engaged via their Fc domains with CD16 on the surface of NK cells, triggering ADCC. c. Next generation bi- and tri-specific antibody constructs have also been designed that more efficiently engage with CD16 (and/or other activating receptors) than classic antitumour monoclonal antibodies. d. Finally, it is being investigated whether inhibitors of the metalloprotease ADAM17 can enhance ADCC by preventing the cleavage of CD16. The diversity of therapeutic strategies on display means they can often be used in tandem to activate NK cells through the interaction of multiple signaling pathways, although targeting the NK cell to the tumour is critical.
PD-1, PD-L1, and CTLA-4 inhibitors
Immune checkpoint blockade targeting PD-1, its ligand PD-L1, or CTLA4 has established a revolutionary breakthrough for overcoming T cell exhaustion to reinvigorate antitumour responsiveness. Characterization of immune changes in response to these treatments indicate that NK cells can play an ancillary part to support antitumour responses during T cell-directed checkpoint blockade137,138. Although the direct impact of PD-1/PD-L1 blockade is less obvious for NK cells than T cells, preclinical studies have shown that it can enhance ADCC-induced antitumour function139,140. Also, ADCC-induced IFN-γ production by NK cells can promote expression of PD-L1 on the surface of tumour cells, thereby indicating a synergistic value in adding PD-1 blockade to ADCC immunotherapy138,141. A subset of NK cells can express PD-1142, including low-level expression on resting NK cells and a single chain Fv of PD-1 antibody was recently shown to increase their responsiveness toward PD-L1-expressing target cells143, which suggests that NK cells can also be a direct target for stimulation by PD-1 blockade. The low levels of PD-1 detected on the surface of NK cells, however, has raised doubts of their inhibitory potential when engaged with PD-L1 on tumours144. In fact, it has been suggested that PD-1 expression on NK cells may be passively acquired from other cells through trogocytosis144, which is a mechanism by which NK cells can acquire a variety of transmembrane proteins145,146. In contrast, many NK cells reportedly express PD-1 mRNA transcripts and protein in the cytoplasm, suggesting that certain TMEs may be able to induce upregulation of PD-1 on the NK cell surface147. Accordingly, in mouse models of cytomegalovirus infection, the production of glucocorticoids resulted in upregulation of PD-1 surface expression on mouse NK cells148. Interestingly, corticosterone upregulated PD-1 on mouse NK cells when cultured with IL-15 and IL-18, but further addition of IL-12 abrogated this effect148. Alternatively, an intriguing study found that PD-L1 is upregulated on activated NK cells, and binding of PD-L1 blocking antibodies to these NK cells stimulates their cytolytic and cytokine responses to PD-L1− tumour cells149,150. The authors propose that this mechanism could explain the effectiveness of PD-L1 antibody therapies in patients with PD-L1− tumours.
Whereas CTLA-4 blockade has generally been studied within the context of T cells, several studies suggest that NK cells can contribute to positive outcomes in patients with melanoma treated with the anti-CTLA-4 mAb, ipilimumab151,152. Sanseviero et al. observed in mouse tumour models treated with CTLA-4 blockade that NK cells assisted in tumour control by clearing anti-CTLA-4 opsonized Treg cells, which has implications for human therapy as well, since ipilumumab is an IgG1 that can mediate ADCC152. Also, intracellular CTLA-4 protein was found in NK cells derived from human lung tumour tissue and in human NK cells stimulated with cytokines153,154. It is unclear, however, if CTLA-4 blockade can directly impact NK cell-mediated tumour clearance in view of its low surface expression, similar to PD-1155.
KIRs and NKG2A inhibitors
The first immune checkpoint blocking antibody developed to stimulate NK cell responses was the KIR antibody, lirilumab (BMS-986015). Lirilumab is a fully human IgG4 that targets KIRs with two Ig-like domains, specifically inhibitory KIR2DL1, KIR2DL2, and KIR2DL3, as well as activating KIR2DS1 and KIR2DS2156. Therefore, it has broad specificity for the HLA-C-specific family of KIR2D receptors. Promising preclinical evidence supported the potential for use of lirilumab in patients157. In the first results of a Phase II clinical trial studying lirilumab as a monotherapy for treating AML, however, there was no measurable benefit compared with placebo (NCT01687387). After this setback, lirilumab trials have focused on combination therapies, most notably with the PD-1 antibody, nivolumab. Currently only one basket clinical trial (NCT02813135) is actively recruiting paediatric patients with refractory or recurrent malignancies to receive lirilumab in combination with nivolumab.
Another NK cell checkpoint blocking antibody is monalizumab (IPH2201), which is a humanized IgG4 blocking NKG2A. NKG2A is prominently found on solid tumour-infiltrating NK cells51,158, whereas its ligand HLA-E is also upregulated in many different types of tumours, most notably in ovarian cancer, breast cancers, non-small cell lung cancer (NSCLC), and head and neck cancer159,160. Similar to lirilumab, phase II results of monalizumab suggested limited effectiveness as a monotherapy161. Several clinical trials of monalizumab in combination with other agents are currently active (NCT04590963, NCT02643550, NCT04307329, NCT02671435). It should be noted that a Phase I/II trial studying the combination of monalizumab and the tyrosine-protein kinase BTK inhibitor ibrutinib to treat CLL was recently terminated owing to serious adverse effects (NCT02557516). However, ibrutinib is known to interact with many off-target kinases and has been shown to disrupt NK cytotoxicity in patients162, so the adverse results in this combination may not reflect the safety and efficacy of monalizumab treatment in other contexts.
We have previously reported that a cocktail of antibodies blocking NKG2A and all KIRs was necessary to significantly enhance in vitro ADCC responses by primary human NK cells163. Therefore, combination therapy with both monalizumab and lirilumab may be worth testing in clinical trials to achieve an NK cell activation threshold more effectively than with either antibody alone, particularly in combination with an additional ADCC-inducing antitumour antibody.
Caution should be exercised in KIR and NKG2A antibody blocking therapies, however, owing to the potential risk of disrupting NK cell functional responsiveness. As previously discussed, inhibitory receptors not only tolerize mature NK cells from attacking normal cells but are also critical in educating the developing NK cells to become fully functional. Accordingly, lirilumab blockade of KIR2D receptors in patients suppressed the responsiveness of the KIR2D-expressing NK cells in peripheral blood, and the authors concluded that this was due to disrupting the normal education process, thereby reverting the NK cells to a hyporesponsive state that would serve as poor effectors for tumour clearance164. This does not entirely rule out checkpoint blockade as a therapeutic option to enhance antitumour responses by NK cells, but it may not be effective as a long term therapy and may require short-term or pulsed treatment regimens to periodically boost NK cell responses.
TIGIT, TIM3, and LAG3 inhibitors
There is potential for expanding checkpoint blocking therapy in NK cells by exploring checkpoint receptors that have already been established in T cells. Some of these receptors, such as TIGIT, hepatitis A virus cellular receptor 2 (also known as TIM-3), and lymphocyte activation gene 3 protein (LAG-3) are also expressed on peripheral and tumour infiltrating NK cells, and can be further induced with in vitro activation stimuli (for example with IL-15 or IL-12)108,165,166. How these receptors impact NK cell effector responses is being characterized in preclinical models. TIGIT blockade promotes antitumour responses by NK cells in multiple mouse models and reverses exhaustion in NK cells derived from patients with colon cancer.167 Blockade of TIM-3 in vitro can also reverse exhaustion in NK cells derived from patients with metastatic melanoma and bladder cancer, promoting proliferation, increased IFN-γ production, and increased cytotoxicity168,169. Finally, therapies that pair IL-12 and/or IL-15 stimulation of NK cells with TIGIT and/or LAG-3 blockade in mouse tumour models have shown promise165,166. TIGIT, TIM-3, and LAG-3 blocking antibodies are already in clinical trials for T cell targeting cancer therapies170. This offers opportunities to examine their impacts on NK cell responses in patients, including whether blocking TIGIT and/or TIM-3 may impact NK cell education171.
Additional checkpoints on NK Cells
Additional receptor–ligand pairs have been identified as promising checkpoint blockade targets on NK cells, namely NKp44 and proliferating cell nuclear antigen (PCNA), KIR3DL3 and HHLA2, and SIRPα and CD47. Several years ago, NKp44 was shown to mediate inhibitory signaling in NK cells when engaged with PCNA as a ligand on the surface of target cells172. This result was puzzling, since NKp44 was originally considered an activating receptor linked to DAP12, and PCNA was considered a nuclear protein173,174. However, the cytoplasmic domain of NKp44 contains an ITIM, which was required to transduce the inhibitory signaling. In addition, PCNA was shown to move to the cell surface and localize at the immune synapse when engaged with NKp44-expressing NK cells172. The same group recently developed an antibody against a domain on PCNA that could be exposed on tumour cell surfaces and blocked the NKp44 interaction to effectively potentiated NK cell-mediated responses to several PCNA-expressing tumour cells175. The work demonstrates that PCNA does indeed reach the cell surface and can be targeted with specific antibodies to overcome the NKp44/PCNA inhibitory axis.
HHLA2 is a ligand for the inhibitory KIR3DL3 and could therefore be another possible target for checkpoint blockade to boost NK cell responses. HHLA2 expression was found in different regions of tumours to those expressing PD-L1176,177, although a recent meta-analysis of HHLA2 expression in a variety of cancers suggests that it may be difficult to draw conclusions yet on its prognostic significance178. KIR3DL3 is highly polymorphic, but has been minimally detected on NK cells to date179,180, although it is unclear if expression is induced by the TME. As another complication, HHLA2 is also the ligand for the NK activating receptor transmembrane and immunoglobulin domain-containing protein 2 (TMIGD2)181,182. Nonetheless, some HHLA2 antibodies can block the KIR3DL3 interaction without inhibiting TMIGD2-binding, which indicates the potential to develop antibodies that selectively block the inhibitory pathway183. Although it is unclear if the KIR3DL3–HHLA2 inhibitory axis will prove to be a viable target cancer therapy, it is worth further exploration.
CD47 is a widely expressed tumour surface antigen that is known to block phagocytosis of tumour cells by engaging with the macrophage receptor SIRPα184. SIRPα is also expressed on the surface of NK cells, where it inhibits natural cytotoxicity against mouse MHC-I− CD47+ tumour cells185. Checkpoint blockade therapeutics targeting the CD47 axis are currently being studied in clinical trials for its effects on antigen presenting cells, but NK cell function may be enhanced as well (NCT02663518, NCT04588324).
Antibodies that induce ADCC responses
A hallmark of NK cell effector function is the ability to kill IgG1- or IgG3-opsonized target cells by ADCC through CD16 (Figure 2). A variety of humanized mAbs have thus emerged that exploit this form of NK cell cytotoxicity by targeting tumour antigens in a variety of cancers. Many of these antibodies are currently in clinical trials, and a summarized list of several of these, their cognate antigens, prospective tumour targets being evaluated, and stage of testing can be found in Table 3.
Table 3.
Select ADCC-inducing antibodies undergoing clinical trials.
| Target | Antibody | Type | Status, selected active clinical trials |
|---|---|---|---|
| HER2 | Trastuzumab | Humanized IgG1 | FDA approved: breast cancer and gastric/GEJ cancer |
| Margetuximab | Fc modified, chimeric IgG1191 | FDA approved: breast cancer Phase II/III: Gastric/GEJ cancer (NCT04082364) |
|
| CD20 | Rituximab | Chimeric IgG1 | FDA approved: NHL and CLL Phase II: ALL (NCT02199184) |
| Ofatumumab | Human IgG1 | FDA approved: CLL Phase II: NHL, ALL (NCT02199184) |
|
| Obinutuzumab | Afucosylated, Fc modified, humanized IgG1250 | FDA approved: CLL and FL Phase II: NHL (NCT03198026), hairy cell leukaemia (NCT03410875) |
|
| Ublituximab | Low fucose, chimeric IgG1250 | Phase III: CLL (NCT02612311) Phase II: NHL (NCT02793583) |
|
| CD19 | Tafasitamab | Fc modified, humanized IgG1251 | FDA approved: R/R DLBCL Phase I: NHL (NCT04661007) |
| EGFR | Cetuximab | Chimeric IgG1 | FDA approved: head and neck cancers, colorectal cancer Phase II: NSCLC (NCT04648189) |
| GD2 | Dinutuximab | Chimeric IgG1 | FDA approved: neuroblastoma Phase I: High risk and R/R neuroblastoma (NCT02573896) |
| PD-L1 | Avelumab | Human IgG1 | FDA approved: Merkel cell carcinoma, urothelial carcinoma and renal cell carcinoma Phase I/II: Gestational trophoblastic neoplasia (NCT04396223), squamous cell anal carcinoma (NCT03944252) |
| SLAMF7 | Elotuzumab | Humanized IgG1 | FDA approved: MM Phase II: R/R MM (NCT03361306) |
| CD38 | Daratumumab | Human IgG1 | FDA approved: MM Phase III: SMM (NCT03937635) Phase II: R/R MM (NCT03841565) |
| Isatuximab | Chimeric IgG1 | FDA approved: MM Phase III: SMM (NCT04270409) Phase II: R/R MM (NCT04287855) |
Abbreviations: ALL, acute lymphocytic leukaemia; CLL, chronic lymphocytic leukaemia CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma, FL, follicular lymphoma; GEJ, gastroesophageal junction; NHL, non-Hodgkin lymphoma; MM, multiple myeloma; NSCLC, non-small cell lung cancer; R/R, relapsed/refractory; SMM, smoldering multiple myeloma
A common polymorphism is found in CD16 that significantly affects affinity for Fc and can influence ADCC responses by NK cells. Specifically, the polymorphism at position 158 of phenylalanine (158F) or valine (158V) results in CD16 with low or increased affinity for Fc, respectively186. The ADCC-inducing antibodies trastuzumab (anti-HER2) and rituximab (anti-CD20) both have limitations in response rate when used to treat patients that express the low affinity 158F variant on NK cells, which is somewhat more prevalent in humans than the higher affinity variant 158V187–190. Accordingly, many current generation ADCC-inducing antibodies are Fc-modified to improve CD16 binding and serum retention. For example, the next generation HER2 antibody margetuximab recently approved for HER2+ breast cancer treatment (NCT02492711) that has five amino acid substitutions in Fc to increase binding to low affinity CD16191. Most of these engineered mAbs are also synthesized in a mammalian culture system that prevents the Fc region of the antibody from fucosylation, which also enhances binding to CD16. Further information on current design strategies of antibody engineering can be found in a recent review192.
Similarly, CD20-directed antibodies analogous to rituximab have gone through several iterations of improved design to treat B cell malignancies, such as obinutuzumab and ublituximab. Obinutuzumab generally provides better outcomes than rituximab, but some comparative clinical trials have found higher incidence of adverse effects from obinutuzumab193,194, thereby prompting caution. Ublituximab seems to lack the toxicity of obinutuzumab, and has shown a very promising response rate in combination with the PI3K-δ inhibitor umbralisib across patients with NHL and small lymphocytic lymphoma (SLL) or CLL195. Importantly, mechanisms other than ADCC may contribute to the antitumour response by anti-CD20 antibodies in vivo, such as activating complement (rituximab, ofatumumab, ublituximab), inducing direct cell lysis (obinutuzumab), or initiating antibody-dependent cellular phagocytosis by CD16-expressing macrophages196,197. From the perspective of NK-based therapy, it is critical to consider the pleiotropic effects potentially induced by any IgG1- and IgG3-based antibody in addition to stimulating ADCC.
Treatment of MM has also benefited from ADCC-inducing antibodies, such as elotuzumab (anti-SLAMF7) and daratumumab (anti-CD38). CD38 and SLAMF7 are highly expressed on myeloma cells, but also on NK cells. Elotuzumab efficacy in patients with myeloma requires combination therapy with bortezomib, lenalidomide, or pomalidomide, all of which stimulate NK cell function198. This strongly suggests that NK cells are crucial in antitumour responses by elotuzumab. Interestingly, although elotuzumab triggers strong ADCC, it can also enhance cytotoxicity through direct co-stimulation of SLAMF7 on the NK cell surface and promote homotypic SLAMF7–SLAMF7 interactions between NK and tumour cells199,200. When given in combination with pomalidomide and dexamethasone, elotuzumab has shown a 53% overall response rate in MM (compared with a 26% response rate with the pomalidomide + dexamethasone combination alone)201. Daratumumab is effective as a single agent, but the combination of daratumumab, pomalidomide, and dexamethasone resulted in a 69% overall response rate in an ongoing MM trial, as compared to 46% for pomalidomide + dexamethasone alone202. However, daratumumab can also induce NK cell fratricide, which has not been observed with elotuzumab treatment, but may limit daratumumab effectiveness over multiple doses203.
Several other antibodies previously used to enhance NK cytotoxicity indirectly or through blockade can also function through ADCC. Avelumab is an IgG1 antibody against PD-L1 that can induce ADCC in addition to checkpoint blockade204,205, which makes it unique compared to other PD-L1 antibodies that are IgG4 or Fc-mutated to reduce binding to FcγRs and thereby limit immune cell activation and increase available levels of circulating antibody. Other antibodies are targeting MICA, which can be shed by some tumours, and this soluble form can suppress NK cells by engaging with NKG2D. MICA antibodies can stabilize MICA expression on tumour cells206, and they also enhance NK cytotoxicity through ADCC, offering a new option for clinical utility207,208.
Finally, strategies to stabilize surface expression of CD16 on NK cells and target antigen on tumour cells are under investigation to improve ADCC responses. ADAM17 is a metalloprotease expressed by NK cells that can cleave CD16 from the NK cell surface209. ADAM17 inhibitors can enhance ADCC responses by NK cells in vitro209, and promising early results were released from a phase II clinical trial using an ADAM17 inhibitor in combination with rituximab to treat DLBCL (NCT02141451). There is reason for skepticism as to whether ADAM17 inhibitors will effectively enhance ADCC in vivo, however, since CD16 cleavage by ADAM17 is important for the natural release of NK cells after ADCC of a target cell, so blocking this cleavage could limit engagement with further target cells210. Also, preclinical studies in mice showed that treatment with compounds that block antibody-mediated endocytosis of tumour antigens (Dyngo4a and the approved drug prochlorperazine) significantly improved surface retention of bound ADCC-inducing antibodies and antitumour responses, which required intact Fc and NK cells211.
Bispecific and trispecific antibodies
Bispecific and trispecific antibodies, which can simultaneously engage with NK activating receptors and tumor antigens, have shown promise in pre-clinical studies and early clinical trials. These molecules have been engineered to engage with CD16 and one or multiple other targets on the tumour cell and NK cell, bringing both into proximity and simultaneously promoting more efficient and/or sustained NK-mediated cytotoxicity (schematics in Figures 2 and 3). Unlike normal mAbs, these constructs can be designed for binding multiple types of tumour antigens or NK receptors simultaneously and can be made with CD16 engaging Fv fragments instead of Fc for stronger and equal binding affinity for 158V and 158F polymorphisms and fewer Fc-mediated off-target interactions (such as becoming fixed through complement or binding other Fc receptors). Success has been achieved with similar systems that direct T cell cytotoxicity, specifically through engaging CD3, and some of these drugs have already received FDA approval212. Despite these successes, similar NK cell-specific constructs that trigger CD16 directly can ensure activation of the cytolytic CD56dim NK cells and overcome the low affinity Fc binding of the 158F CD16 polymorphism, whereas constructs that engage CD3 not only stimulate the CD8+ cytotoxic T cell population, but also CD4+ TH and Treg cells.
Figure 3. Different strategies for producing bi- or tri-specific antibody constructs.
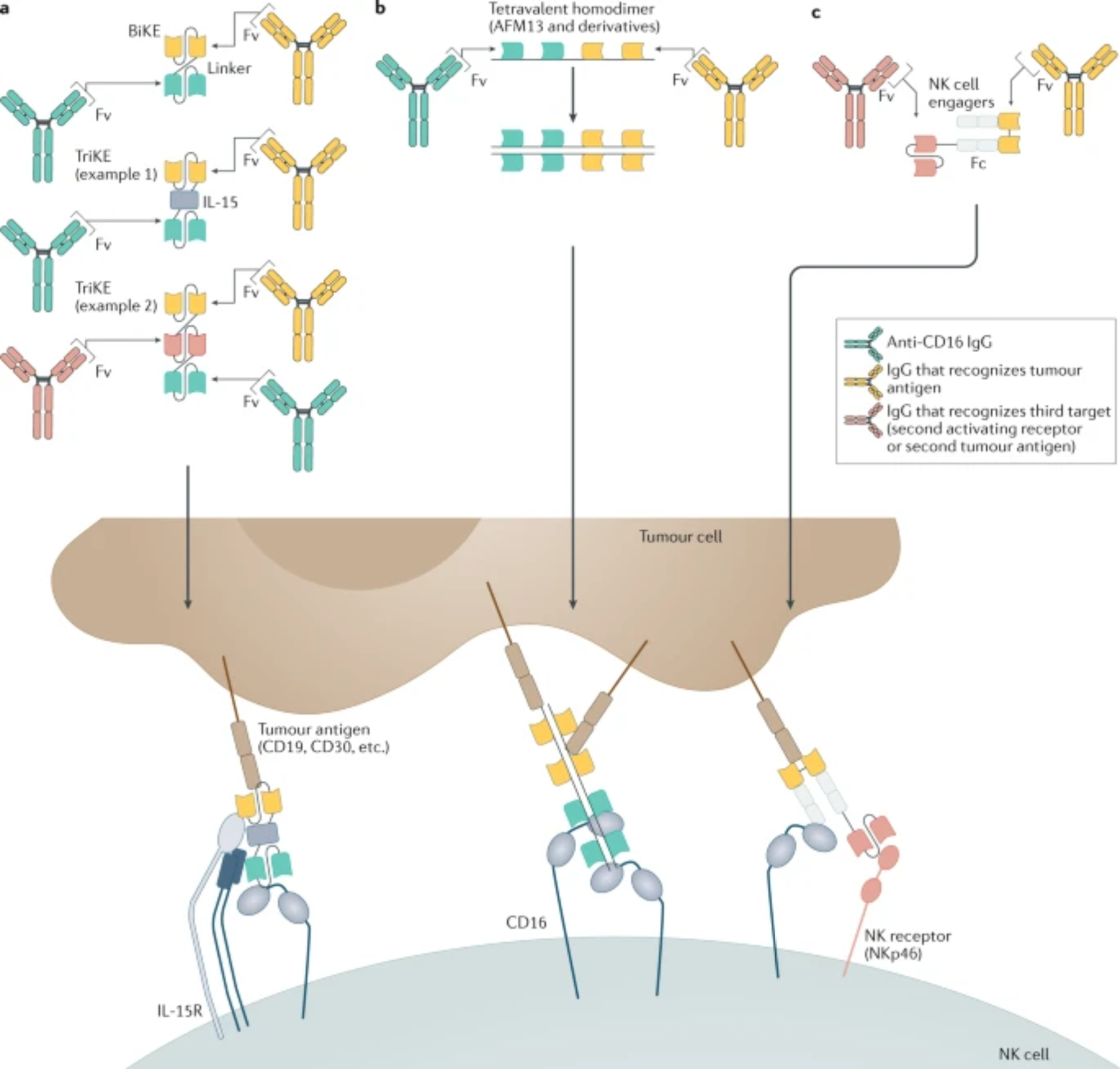
Many modern antibody constructs do not retain Fc domains and instead engage with CD16 through Fv domains. a. BiKEs and TriKEs are made from various combinations of two or three (respective) Fv fragments from mAbs or ligand molecules linked in a single polypeptide chain, which target some combination of tumor antigen, CD16 and another NK activating receptor. A common TriKE design includes an IL-15 moiety for better stimulation of NK cell activation, survival, and proliferation. b. Other constructs contain multiple Fv domains that form homodimers or homomultimers. Of this category AFM13, a bispecific construct consisting of two anti-CD30 and two anti-CD16 Fv domains, has shown promising results in clinical trials. c. A new innovative type of construct, the NK cell engager, is a complex consisting of different polypeptides that include both Fv and Fc domains. The Fc region of the construct can be modified for efficient CD16 engagement and optimally spaced Fv that optimally engages a NK cell activating receptor (e.g. NKp46) for further potentiation of NK cell stimulation, as well as one tumour antigen.
The CD16 bispecific antibody that has progressed furthest in the clinic is AFM13, which targets CD30213, a surface antigen expressed in B and T cell lymphomas. AFM13 has been well tolerated in Phase I and II trials in the treatment of various lymphomas213 and has been granted orphan drug designation for the treatment of peripheral T cell lymphoma. As of 2021, several active clinical trials employ AFM13 for treatment of refracted or relapsed peripheral T cell lymphoma as a monotherapy (NCT04101331) or to treat HL and NHL in conjunction with chemotherapy (NCT04074746)214. AFM24 is another tetravalent bispecific like AFM13, but it targets epidermal growth factor receptor (EGFR) instead of CD30, and it is currently in Phase I/II for the treatment of metastatic colorectal cancer and NSCLC (NCT04259450).
Various other anti-CD16 bispecific antibody systems have succeeded at enhancing tumour cell killing by NK cells, such as homodimeric tribodies with two HER2 or CD20-binding domains215,216 or the single chain recombinant Fv Bispecific Killer Engagers (BiKEs), which were developed to engage a variety of antigens, including HER2, CD20, and CD30217. Tri-specific killer engagers (TriKEs) incorporate two Fv domains targeting a tumour antigen and CD16 with intervening IL-15 that enhances NK cell activity (Figure 3). TriKEs in preclinical development target CD133 to eradicate cancer stem cells218, CD19 to eliminate Burkitt lymphoma and CLL tumour cells219, or CLEC12A to eliminate AML cells220, to name a few examples. A CD33 TriKE is in Phase I/II clinical trials as a monotherapy for the treatment of myelodysplastic syndromes, refractory/relapsed AML or advanced-stage systemic mastocytosis (NCT03214666).
Additional advanced trispecific antibodies are also being engineered to enhance NK cell-mediated antitumour responses. NK cell engagers are innovative trispecific constructs that bind tumor antigen, CD16, and NKp46 or NKp30 and have shown impressive preclinical activity221. Another approach combines a CD16/cetuximab bispecific antibody with an Fv domain to bind CD137, as engaging CD137 enhances response to cetuximab222,223. Altogether, the potential for engaging multiple NK cell receptors simultaneously makes trispecific antibody constructs an exciting frontier in NK cell immunotherapy, although establishing which activating receptors to engage for optimal boost of antitumour responses will take further research.
Adoptive NK cell therapies
Adoptive transfer of NK cells has come of age with the recent success of chimeric antigen receptor (CAR)-bearing NK cell and cytokine-stimulated NK cell therapies224. Most NK-based adoptive transfer therapies harvest NK cells from various sources, enhance their function ex vivo through a variety of methods, then transfuse them into a cancer patient. A recent review has detailed these exciting advances224, so we will only briefly summarize some of the most impactful advances and future directions under development. Active clinical trials are listed in Table 4.
Table 4.
Ongoing clinical trials with engineered NK cells
| Modification | Phase | Malignancy | Combination Treatments | Clinicaltrial.gov Designation |
|---|---|---|---|---|
| NKG2D ligand CAR | Phase I | R/R AML, R/R MDS | Monotherapy | NCT04623944 |
| HER2 CAR | Phase I | Glioblastoma | Monotherapy | NCT03383978 |
| 158V non-cleavable CD16 | Phase I | B cell lymphoma, AML, solid tumors | CD20 or PD-L1 mAbs, chemotherapy, IL-2 | NCT04023071, NCT04551885 |
| 158V non-cleavable CD16, CD38 KO, contiguously active IL-15R | Phase I | Multiple Myeloma, AML, monocytic leukemia | Daratumumab or elotuzumab, chemotherapy | NCT04614636, NCT04714372 |
| CD19 CAR, 158V non-cleavable CD16, contiguously active IL-15R | Phase I | B cell lymphoma, CLL | Monotherapy or CD20 mAbs | NCT04245722 |
| CD19 CAR / IL-15 production | Phase I/II | B cell malignancies | Chemotherapy | NCT03056339 |
| BCMA CAR | Phase I/II | Multiple myeloma | Monotherapy | NCT03940833 |
| ROBO1 CAR | Phase I/II | ROBO1 positive tumours | Monotherapy | NCT03940820, NCT03931720 |
| Pancreatic cancer | Monotherapy | NCT03941457 | ||
| PD-L1 CAR, 158V CD16, IL-2 production | Phase II | Various | Chemotherapy, aldoxorubicin HCl, IL-15 superagonist | NCT04390399, NCT03228667, NCT04847466 |
| CD5 CAR, IL-15 production | Phase I/II | R/R Hematological malignancies | Chemotherapy | NCT05110742 |
Abbreviations: AML, BCMA, CLL; ICB, immune checkpoint blockade; MDS, PD-L1, ROBO1, Roundabout homolog 1; R/R, relapsed/refractory
Both autologous and allogeneic NK cells can be sources for adoptive transfer, and whereas autologous NK cells avoid risk of a graft versus host response, KIR mismatch in adoptive transfer of allogeneic haploidentical NK cells can be useful in treating AML, which is sensitive to the NK cell-mediated graft versus tumour response39,225,226. This strategy has been further refined through the in vitro differentiation of allogeneic NK cells from induced pluripotent stem cells227,228. Allogeneic NK cells can also be derived from sources rich in NK cells, such as cord blood. There is some evidence to suggest that cord blood-derived NK cells are easier to grow and stimulate in culture, but NK cells derived from adult peripheral blood are more naturally cytolytic229,230. As the field develops, certain therapeutic strategies may benefit from taking NK cells from distinct sources. In addition, infusions of an ex vivo expanded autologous NK cell product, ACP-001, has been recently approved by the FDA for orphan drug designation to treat MM (NCT04558853).
A straightforward approach to enhance NK cell function has involved ex vivo stimulation with cytokines before transfusion, historically using IL-2231, which has fallen out of favour owing to adverse effects. In vitro stimulation of NK cells with the combination of IL-12/IL-15/IL-18 generates highly functional long-lived ‘memory-like’ NK cells for adoptive transfer in both preclinical and clinical studies224. These ‘memory-like’ NK cells are less sensitive to KIR inhibition and a phase I trial showed encouraging antitumour responses in patients with AML232,233. IL-15, IL-12, and IL-18 have recently been shown to stimulate an ER stress response pathway in NK cells to promote MYC-dependent proliferation and oxidative phosphorylation in the mitochondria associated with enhanced antitumour responses234. Another recent study used an interesting approach of pre-loading ‘memory-like’ NK cells with the tetravalent anti-CD16/CD30 bispecific antibody AFM13, which binds to NK cells with high affinity through two CD16 targeting Fv domains213, to facilitate potent responses toward CD30+ lymphomas upon adoptive transfer into a mouse xenograph model235. Highly functional and long-lived NK cells can also be stimulated and expanded in vitro through transgenic feeder cell lines that express membrane-bound IL-15, IL-21, and/or other stimulatory molecules236,237. Interestingly, in NK cells expanded ex vivo in the presence of IL-21-mediated STAT3 signaling, glycolysis is upregulated and oxidative phosphorylation is downregulated (Warburg effect), similar to tumour cells29. This reprogramming not only made the NK cells more adaptable to nutrient-deprived metabolic conditions in the TME, but also remarkably enhanced their tumour killing capacity over time when exposed to the TME29.
As an exciting next generation approach, NK cell adoptive therapies have employed CAR–bearing NK cells. CAR-T cells that recognize CD19 have been approved by the FDA for treating B cell NHL, and cord blood-derived NK cells transduced to express similar CD19-targeting CAR have shown impressive early results in clinical trials for treating B cell lymphomas and leukaemias238. Importantly, the therapy did not induce cytokine storm or graft versus host toxicity, and some NK cells persisted for over one year238. Other antigen targets for CARs in NK cells are also at varying stages of clinical trials (Table 4). As a whole, CAR-NK cells offer several advantages over CAR-T cells, including lower risk of cytokine release syndrome, which is likely due to their lack of IL-6 production and less enduring production of TNF239, and a lack of autocrine growth signaling that would perpetuate long-term NK cell survival beyond required antitumour need (such as IL-2 production by T cells that could enable their long-term persistence). Additionally, NK cells that lose the transgenic CAR can still recognize and kill through their endogenously expressed activating receptors. CAR on NK cells can also overcome inhibitory KIR and NKG2A receptors182,240. Many CAR-NK cells are derived from sources previously mentioned238,239, but increased demand for easily modified ‘off the shelf’ NK cell platforms has included development of CAR-expressing NK cell lines, such as NK-92241–243. Although the durability of these NK cellular therapies is still under investigation, many have been shown to be safe and effective.
In addition to introducing CARs, additional genetic modifications are being explored to enhance NK cells for adoptive transfer, many of which are in clinical trials (Table 4). Better tumour infiltration has been demonstrated in preclinical studies through transgenic expression of chemokine or adhesion receptors, such as CXCR3 in myeloma models or CCR7 in lymphoma models244,245. ADCC-based therapies may also use NK cells expressing high affinity 158V CD16 (including CD16 modified to prevent proteolysis by ADAM17) in combination with an ADCC-inducing antibody246,247, which are currently undergoing early clinical trials (NCT04023071, NCT04551885, NCT03853317). Finally, CRISPR/Cas9 gene editing methods are being used to remove negative regulators, such as the IL-15R — a member of the suppressor of cytokine signaling (SOCS) family (CIS) — from iPSC-derived NK cells248. Gene editing has also been used to remove CD38 from NK cells and circumvent daratumumab-induced fratricide249. The introduction of transgenes, such as CARs, and use of modern gene editing technologies to modify NK cells offer extensive possibilities for building better NK cells for adoptive immunotherapy to treat both haematopoietic and solid tumours.
Conclusions and future directions
After decades of research that informed the basic biology of NK cells, the field has recently evolved to make rapid advances in developing effective immunotherapies that exploit these cells to treat cancer. Cytokines and numerous other factors can be used to broadly potentiate cytotoxicity and cytokine responses by NK cells and are being tested in the clinic. In particular, superagonist forms of IL-15 offer substantial opportunities to induce the activation, proliferation, and survival of NK cells in patients. Antibody-mediated therapies have evolved substantially to reduce NK cell activation threshold by blocking immune checkpoint receptors and to facilitate targeted attack upon bridging tumour surface markers with CD16 and other activating receptors. Adoptive NK cell therapies have also made exciting advances and have proven to be safe and effective in recent clinical trials. Although the durability of these responses is still under investigation, genetic manipulation offers unlimited opportunities to further optimize NK cellular therapies by introducing CARs and targeting receptors and removing inhibitory molecules. The lack of serious cytokine release syndrome or autoimmune responses in these adoptive therapies clearly demonstrates that many inhibitory and exhaustion mechanisms are restraining NK cells from damaging healthy tissues, which offers profound advantages over T cell-mediated approaches.
In fact, this field is still in its infancy, and the future looks bright for development of new ways to further enhance the NK cell immunotherapy platform. Important goals will be to improve NK cell targeting of solid tumours and enhance their activation, cytolytic capacity, and survival upon reaching the harsh immunosuppressive TME. Because their activation is orchestrated by a wide array of cell surface receptors, therapeutic agents that target these receptors to engage with tumour cell surfaces is key. CARs, as well as advanced antibody engineering and other multimeric receptor engagement technologies provide extensive opportunities to optimally trigger NK cell activation in contact with tumour cells. NK cell-focused combination therapies should provide the next wave of clinical advances in finding the appropriate balance of therapeutics that improves their targeted activation while effectively overcoming their embedded restraint mechanisms.
Acknowledgements
The authors appreciate funding from a Health Research Formula Fund grant from the Commonwealth of Pennsylvania (CURE; to K.S.C.), NIH grant AI148117 (K.S.C. and Y. Sykulev), NCI NIH Training Grant T32 CA9035 (N.A.M.), and NCI Comprehensive Cancer Center Support Grant CA06927 (FCCC).
Potential Competing Interests
K.S.C. has received research support from Janssen Pharma, Genentech, Horizon Pharma, ImmunityBio, and Immune Oncology Biosciences, consulting fees from Immunitas and Tavotec, and has patents with ImmunityBio.
Glossary terms:
- Opsonization
The binding of antibodies to the surface of a pathogen or tumour cell, which are recognized by Fc receptors on phagocytes to facilitate antibody dependent cellular phagocytosis or by CD16 on NK cells to trigger ADCC.
- Tolerize
The process by which normal cells induce tolerance toward NK cell-mediated attack by expressing MHC-I molecules that engage inhibitory receptors, such as KIR.
- Fucosylation
The process of covalently attaching the sugar fucose to IgG molecules as a post-translational event, which reduces the affinity of IgG1 or IgG3 to bind CD16 and thereby decreases their potency in stimulating ADCC by NK cells.
- Fratricide
Classically defined as killing one’s brother, this term has been used to define the process by which NK cells sometimes aberrantly attack and kill each other.
- Feeder cell lines
Cell lines that are used to stimulate the ex vivo proliferation of NK cells and are generally chosen for low surface expression of MHC-I and/or engineered to express surface bound cytokines or ligands for NK cell activating receptors.
References
- 1.Ljunggren H-G & Kärre K In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990). [DOI] [PubMed] [Google Scholar]
- 2.Campbell KS & Hasegawa J Natural killer cell biology: an update and future directions. J Allergy Clin Immunol 132, 536–544, doi: 10.1016/j.jaci.2013.07.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zitti B & Bryceson YT Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev 42, 37–46, doi: 10.1016/j.cytogfr.2018.08.001 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Freud AG, Mundy-Bosse BL, Yu J & Caligiuri MA The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 47, 820–833, doi: 10.1016/j.immuni.2017.10.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai K, Matsuyama S, Miyake S, Suga K & Nakachi K Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799, doi: 10.1016/S0140-6736(00)03231-1 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Orange JS Natural killer cell deficiency. J Allergy Clin Immunol 132, 515–525, doi: 10.1016/j.jaci.2013.07.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacFarlane AW 4th et al. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Oncoimmunology 6, e1330235, doi: 10.1080/2162402X.2017.1330235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazina T et al. Alterations of NK Cell Phenotype in the Disease Course of Multiple Myeloma. Cancers (Basel) 13, doi: 10.3390/cancers13020226 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laughney AM et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat Med 26, 259–269, doi: 10.1038/s41591-019-0750-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors found metastatasizing lung adenocarcinoma cells that express high levels of the transcription factor SOX9 (aveolar epithelial progenitor state) can better evade NK cell-mediated killing, due to increased MHC-I expression, whereas SOX2high cells (regenerative state) are more sensitive to NK cell attack, through downregulation of MHC-I and upregulation of activating receptor ligands.
- 10.Lo HC et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nature Cancer 1, 709–722, doi: 10.1038/s43018-020-0068-9 (2020). [DOI] [PubMed] [Google Scholar]; This work found that individual circulating tumour cells with mesenchymal characteristics are more sensitive to NK cell-mediated elimination, as compared to polyclonal clusters of circulating tumour cells that are more likely to escape, due to more epithelial phenotype, lower expression of NK cell activating ligands, and higher expression of adhesion molecules.
- 11.Krizhanovsky V et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667, doi: 10.1016/j.cell.2008.06.049 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivashkiv LB IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol 18, 545–558, doi: 10.1038/s41577-018-0029-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins PL et al. Gene Regulatory Programs Conferring Phenotypic Identities to Human NK Cells. Cell 176, 348–360 e312, doi: 10.1016/j.cell.2018.11.045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehniger TA et al. CD56bright Natural Killer Cells are Present in Human Lymph Nodes and are Activated by T cell Derived IL-2: a Potential New Link between Adaptive and Innate Immunity. Blood 100, 1935–1947 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Milush JM et al. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood 114, 4823–4831, doi: 10.1182/blood-2009-04-216374 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogra P et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 180, 749–763 e713, doi: 10.1016/j.cell.2020.01.022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melsen JE, Lugthart G, Lankester AC & Schilham MW Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front Immunol 7, 262, doi: 10.3389/fimmu.2016.00262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper MA, Fehniger TA & Caligiuri MA The biology of human natural killer-cell subsets. Trends Immunol 22, 633–640. (2001). [DOI] [PubMed] [Google Scholar]
- 19.Crinier A et al. High-Dimensional Single-Cell Analysis Identifies Organ-Specific Signatures and Conserved NK Cell Subsets in Humans and Mice. Immunity 49, 971–986 e975, doi: 10.1016/j.immuni.2018.09.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yudanin NA et al. Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity. Immunity 50, 505–519 e504, doi: 10.1016/j.immuni.2019.01.012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownlie D et al. Expansions of adaptive-like NK cells with a tissue-resident phenotype in human lung and blood. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2016580118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freud AG & Caligiuri MA Human natural killer cell development. Immunol. Rev 214, 56–72 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Carson WE et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest 99, 937–943 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper MA et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100, 3633–3638, doi: 10.1182/blood-2001-12-0293 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Vosshenrich CA et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol 174, 1213–1221 (2005). [DOI] [PubMed] [Google Scholar]
- 26.MacFarlane AW 4th & Campbell KS Signal transduction in natural killer cells. Curr Top Microbiol Immunol 298, 23–57 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Dhatchinamoorthy K, Colbert JD & Rock KL Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol 12, 636568, doi: 10.3389/fimmu.2021.636568 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell KS & Purdy AK Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 132, 315–325, doi: 10.1111/j.1365-2567.2010.03398.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poznanski SM et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab, doi: 10.1016/j.cmet.2021.03.023 (2021). [DOI] [PubMed] [Google Scholar]; Oxidative stress in the tumor microenvironment (TME) impairs NK cell glucose metabolism and disrupts their function, but this study found expansion of NK cells with IL-21 or treatment with an activator of the Nrf2 antioxidant pathway promote Warburg-like metabolism with increased glycolysis and reduced oxidative phosphorylation, resulting in substantially improved resistance to the oxidative stressed and nutrient-deprived TME and enhanced antitumour responses.
- 30.Pazina T, Shemesh A, Brusilovsky M, Porgador A & Campbell KS Regulation of the Functions of Natural Cytotoxicity Receptors by Interactions with Diverse Ligands and Alterations in Splice Variant Expression. Front Immunol 8, 369, doi: 10.3389/fimmu.2017.00369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorvel L & Olive D Targeting the “PVR-TIGIT axis” with immune checkpoint therapies. F1000Res 9, doi: 10.12688/f1000research.22877.1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J et al. PVRIG is a novel NK cell immune checkpoint receptor in acute myeloid leukemia. Haematologica Online ahead of print, doi: 10.3324/haematol.2020.258574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horowitz A et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 5, 208ra145, doi: 10.1126/scitranslmed.3006702 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was the first to apply mass cytometry to examine expression of 35 receptors on peripheral blood NK cells in healthy donors, including monozygotic twins, and found incredibly high diversity with up to 30,000 different NK cell “clones” within an individual, based on unique receptor expression phenotypes.
- 34.Strauss-Albee DM et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med 7, 297ra115, doi: 10.1126/scitranslmed.aac5722 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerwenka A & Lanier LL Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol 16, 112–123, doi: 10.1038/nri.2015.9 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Foley B et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 119, 2665–2674, doi: 10.1182/blood-2011-10-386995 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Verges S et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A 108, 14725–14732, doi: 10.1073/pnas.1110900108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulkarni S, Martin MP & Carrington M The Yin and Yang of HLA and KIR in human disease. Semin Immunol 20, 343–352, doi: 10.1016/j.smim.2008.06.003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggeri L et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Schönberg K, Sribar M, Enczmann J, Fischer JC & Uhrberg M Analyses of HLA-C–specific KIR repertoires in donors with group A and B haplotypes suggest a ligand-instructed model of NK cell receptor acquisition. Blood 117, 98–107, doi: 10.1182/blood-2010-03-273656 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Anfossi N et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Kim S et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A 105, 3053–3058 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furukawa H et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I). Hum Immunol 60, 32–40, doi:S0198885998000974 [pii] (1999). [DOI] [PubMed] [Google Scholar]
- 44.Kim S et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Boudreau JE et al. Cell-Extrinsic MHC Class I Molecule Engagement Augments Human NK Cell Education Programmed by Cell-Intrinsic MHC Class I. Immunity 45, 280–291, doi: 10.1016/j.immuni.2016.07.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacFarlane AW 4th et al. Enhanced NK-cell development and function in BCAP-deficient mice. Blood 112, 131–140, doi:blood-2007-08-107847 [pii] 10.1182/blood-2007-08-107847 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; B-cell adaptor for phosphatidylinositol 3-kinase (BCAP) facilitates activation signaling and BCAP-deficient mice have defects in B cell development and function, but this study found that these mice paradoxically exhibit enhanced NK cell development to a more terminally mature and functional state, indicating that loss of activation signaling through BCAP promotes NK cell maturation and function.
- 47.Bald T, Krummel MF, Smyth MJ & Barry KC The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol 21, 835–847, doi: 10.1038/s41590-020-0728-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper MA et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97, 3146–3151 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Hauser MA & Legler DF Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J Leukoc Biol 99, 869–882, doi: 10.1189/jlb.2MR0815-380R (2016). [DOI] [PubMed] [Google Scholar]
- 50.Edsparr K, Basse PH, Goldfarb RH & Albertsson P Matrix metalloproteinases in cytotoxic lymphocytes impact on tumour infiltration and immunomodulation. Cancer Microenviron 4, 351–360, doi: 10.1007/s12307-010-0057-0 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrega P et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol 192, 3805–3815, doi: 10.4049/jimmunol.1301889 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Fionda C et al. Hitting More Birds with a Stone: Impact of TGF-beta on ILC Activity in Cancer. J Clin Med 9, doi: 10.3390/jcm9010143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slattery K & Gardiner CM NK Cell Metabolism and TGFbeta - Implications for Immunotherapy. Front Immunol 10, 2915, doi: 10.3389/fimmu.2019.02915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Y et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 18, 1004–1015, doi: 10.1038/ni.3800 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Cortez VS et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat Immunol 18, 995–1003, doi: 10.1038/ni.3809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balsamo M et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol 43, 2756–2764, doi: 10.1002/eji.201343448 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Ni J et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1alpha Unleashes NK Cell Activity. Immunity 52, 1075–1087 e1078, doi: 10.1016/j.immuni.2020.05.001 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Zheng X et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol 20, 1656–1667, doi: 10.1038/s41590-019-0511-1 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Bonavita E et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity 53, 1215–1229 e1218, doi: 10.1016/j.immuni.2020.10.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobs B et al. The Oncometabolite 5’-Deoxy-5’-Methylthioadenosine Blocks Multiple Signaling Pathways of NK Cell Activation. Front Immunol 11, 2128, doi: 10.3389/fimmu.2020.02128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Della Chiesa M et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125, doi: 10.1182/blood-2006-03-006700 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Harmon C et al. Lactate-Mediated Acidification of Tumor Microenvironment Induces Apoptosis of Liver-Resident NK Cells in Colorectal Liver Metastasis. Cancer Immunol Res 7, 335–346, doi: 10.1158/2326-6066.CIR-18-0481 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Chambers AM et al. Adenosinergic Signaling Alters Natural Killer Cell Functional Responses. Front Immunol 9, 2533, doi: 10.3389/fimmu.2018.02533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Brien KL & Finlay DK Immunometabolism and natural killer cell responses. Nat Rev Immunol 19, 282–290, doi: 10.1038/s41577-019-0139-2 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Molfetta R, Zingoni A, Santoni A & Paolini R Post-translational Mechanisms Regulating NK Cell Activating Receptors and Their Ligands in Cancer: Potential Targets for Therapeutic Intervention. Front Immunol 10, 2557, doi: 10.3389/fimmu.2019.02557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan B & Lentzsch S The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol Ther 136, 56–68, doi: 10.1016/j.pharmthera.2012.07.004 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Lacy MQ et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia 24, 1934–1939, doi: 10.1038/leu.2010.190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagrue K, Carisey A, Morgan DJ, Chopra R & Davis DM Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood 126, 50–60, doi: 10.1182/blood-2015-01-625004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fionda C et al. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget 6, 23609–23630, doi: 10.18632/oncotarget.4603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito T et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350, doi: 10.1126/science.1177319 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Kronke J et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305, doi: 10.1126/science.1244851 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu G et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343, 305–309, doi: 10.1126/science.1244917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Asatsuma-Okumura T, Ito T & Handa H Molecular mechanisms of cereblon-based drugs. Pharmacol Ther 202, 132–139, doi: 10.1016/j.pharmthera.2019.06.004 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Hideshima T et al. Immunomodulatory drugs activate NK cells via both Zap-70 and cereblon-dependent pathways. Leukemia, doi: 10.1038/s41375-020-0809-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Armeanu S et al. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin Cancer Res 14, 3520–3528, doi: 10.1158/1078-0432.CCR-07-4744 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Niu C et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and gammadelta T cell-mediated lysis in multiple myeloma. Oncotarget 8, 5954–5964, doi: 10.18632/oncotarget.13979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gras Navarro A et al. Pretreatment of Glioblastoma with Bortezomib Potentiates Natural Killer Cell Cytotoxicity through TRAIL/DR5 Mediated Apoptosis and Prolongs Animal Survival. Cancers (Basel) 11, doi: 10.3390/cancers11070996 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jinushi M et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 105, 1285–1290, doi: 10.1073/pnas.0711293105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi J et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood 111, 1309–1317, doi: 10.1182/blood-2007-03-078535 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Q, Sun L & Chen ZJ Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 17, 1142–1149, doi: 10.1038/ni.3558 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Ishikawa H, Ma Z & Barber GN STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792, doi: 10.1038/nature08476 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marcus A et al. Tumor-Derived cGAMP Triggers a STING-Mediated Interferon Response in Non-tumor Cells to Activate the NK Cell Response. Immunity 49, 754–763 e754, doi: 10.1016/j.immuni.2018.09.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivick KE et al. Magnitude of Therapeutic STING Activation Determines CD8(+) T Cell-Mediated Anti-tumor Immunity. Cell Rep 29, 785–789, doi: 10.1016/j.celrep.2019.09.089 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Nicolai CJ et al. NK cells mediate clearance of CD8(+) T cell-resistant tumors in response to STING agonists. Sci Immunol 5, doi: 10.1126/sciimmunol.aaz2738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; Injection of a variety of CTL-resistant tumours in mice with a cyclic dinucleotide STING agonist in this study substantially enhanced antitumour responses by NK cells toward the injected tumors, as well as non-injected tumors, by promoting type I interferon production and stimulating IL-15 production by dendritic cells.
- 85.Francica BJ et al. TNFalpha and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors. Cancer Immunol Res 6, 422–433, doi: 10.1158/2326-6066.CIR-17-0263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demaria O et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A 112, 15408–15413, doi: 10.1073/pnas.1512832112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lotze MT, Grimm EA, Mazumder A, Strausser JL & Rosenberg SA Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res 41, 4420–4425 (1981). [PubMed] [Google Scholar]
- 88.Grimm EA, Mazumder A, Zhang HZ & Rosenberg SA Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 155, 1823–1841, doi: 10.1084/jem.155.6.1823 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pisani RJ, Krco CJ, Wold LE & McKean DJ Lymphokine-activated killer (LAK) cell activity in tumor-infiltrating lymphocytes from non-small cell lung cancer. Am J Clin Pathol 92, 435–446, doi: 10.1093/ajcp/92.4.435 (1989). [DOI] [PubMed] [Google Scholar]
- 90.Phillips JH, Gemlo BT, Myers WW, Rayner AA & Lanier LL In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J Clin Oncol 5, 1933–1941, doi: 10.1200/JCO.1987.5.12.1933 (1987). [DOI] [PubMed] [Google Scholar]
- 91.Wu Y et al. Natural killer cells as a double-edged sword in cancer immunotherapy: A comprehensive review from cytokine therapy to adoptive cell immunotherapy. Pharmacol Res 155, 104691, doi: 10.1016/j.phrs.2020.104691 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Sim GC et al. IL2 Variant Circumvents ICOS+ Regulatory T-cell Expansion and Promotes NK Cell Activation. Cancer Immunol Res 4, 983–994, doi: 10.1158/2326-6066.Cir-15-0195 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Lopes JE et al. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. Journal for ImmunoTherapy of Cancer 8, e000673, doi: 10.1136/jitc-2020-000673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waldmann TA The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6, 595–601, doi: 10.1038/nri1901 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Bergamaschi C et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood 120, e1–8, doi: 10.1182/blood-2011-10-384362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi H et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105, 721–727, doi: 10.1182/blood-2003-12-4187 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Yang Y et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. J Clin Invest 130, 5508–5522, doi: 10.1172/JCI137585 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooley S et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv 3, 1970–1980, doi: 10.1182/bloodadvances.2018028332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller JS et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin Cancer Res 24, 1525–1535, doi: 10.1158/1078-0432.CCR-17-2451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watson DC et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles 7, 1442088, doi: 10.1080/20013078.2018.1442088 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergamaschi C et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-gamma, CXCL9 and CXCL10. J Immunother Cancer 8, doi: 10.1136/jitc-2020-000599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Han KP et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 56, 804–810, doi: 10.1016/j.cyto.2011.09.028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu W et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res 73, 3075–3086, doi: 10.1158/0008-5472.CAN-12-2357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim PS et al. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 7, 16130–16145, doi: 10.18632/oncotarget.7470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wrangle JM et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 19, 694–704, doi: 10.1016/S1470-2045(18)30148-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romee R et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527, doi: 10.1182/blood-2017-12-823757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Felices M et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 3, doi: 10.1172/jci.insight.96219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merino A et al. Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J Clin Invest 129, 3770–3785, doi: 10.1172/JCI125916 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirlekar B & Pylayeva-Gupta Y IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers (Basel) 13, doi: 10.3390/cancers13020167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zundler S & Neurath MF Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev 26, 559–568, doi: 10.1016/j.cytogfr.2015.07.003 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Lasek W, Zagozdzon R & Jakobisiak M Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother 63, 419–435, doi: 10.1007/s00262-014-1523-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen KG et al. Localized Interleukin-12 for Cancer Immunotherapy. Front Immunol 11, 575597, doi: 10.3389/fimmu.2020.575597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gillies SD et al. Antibody-IL-12 fusion proteins are effective in SCID mouse models of prostate and colon carcinoma metastases. J Immunol 160, 6195–6203 (1998). [PubMed] [Google Scholar]
- 114.Peng LS, Penichet ML, Dela Cruz JS, Sampogna SL & Morrison SL Mechanism of antitumor activity of a single-chain interleukin-12 IgG3 antibody fusion protein (mscIL-12.her2.IgG3). J Interferon Cytokine Res 21, 709–720, doi: 10.1089/107999001753124444 (2001). [DOI] [PubMed] [Google Scholar]
- 115.Algazi AP et al. Phase II Trial of IL-12 Plasmid Transfection and PD-1 Blockade in Immunologically Quiescent Melanoma. Clin Cancer Res 26, 2827–2837, doi: 10.1158/1078-0432.CCR-19-2217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis ND et al. Exosome Surface Display of IL12 Results in Tumor-Retained Pharmacology with Superior Potency and Limited Systemic Exposure Compared with Recombinant IL12. Mol Cancer Ther 20, 523–534, doi: 10.1158/1535-7163.MCT-20-0484 (2021). [DOI] [PubMed] [Google Scholar]
- 117.Kaplanski G Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev 281, 138–153, doi: 10.1111/imr.12616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Konjevic GM, Vuletic AM, Mirjacic Martinovic KM, Larsen AK & Jurisic VB The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 117, 30–40, doi: 10.1016/j.cyto.2019.02.001 (2019). [DOI] [PubMed] [Google Scholar]
- 119.Ma Z et al. Augmentation of Immune Checkpoint Cancer Immunotherapy with IL18. Clin Cancer Res 22, 2969–2980, doi: 10.1158/1078-0432.CCR-15-1655 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Robertson MJ et al. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin Cancer Res 14, 3462–3469, doi: 10.1158/1078-0432.CCR-07-4740 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robertson MJ et al. A dose-escalation study of recombinant human interleukin-18 in combination with rituximab in patients with non-Hodgkin lymphoma. J Immunother 36, 331–341, doi: 10.1097/CJI.0b013e31829d7e2e (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Robertson MJ et al. A Dose-escalation Study of Recombinant Human Interleukin-18 in Combination With Ofatumumab After Autologous Peripheral Blood Stem Cell Transplantation for Lymphoma. J Immunother 41, 151–157, doi: 10.1097/CJI.0000000000000220 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fabbi M, Carbotti G & Ferrini S Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol 97, 665–675, doi: 10.1189/jlb.5RU0714-360RR (2015). [DOI] [PubMed] [Google Scholar]
- 124.Molgora M et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 551, 110–114, doi: 10.1038/nature24293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou T et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614, doi: 10.1038/s41586-020-2422-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors engineered a novel form of IL-18 that can stimulate potent antitumour responses by NK and CD8+ T cells, but is incapable of interacting with the negative regulator, IL-18BP, which is produced in many cancers, binds to IL-18 with high affinity to prevent its biological activity, and has previously been found to limit efficacy of IL-18 therapies in cancer patients.
- 126.Skak K, Frederiksen KS & Lundsgaard D Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 123, 575–583, doi: 10.1111/j.1365-2567.2007.02730.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burgess SJ, Marusina AI, Pathmanathan I, Borrego F & Coligan JE IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J Immunol 176, 1490–1497, doi: 10.4049/jimmunol.176.3.1490 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Strengell M et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol 170, 5464–5469, doi: 10.4049/jimmunol.170.11.5464 (2003). [DOI] [PubMed] [Google Scholar]
- 129.French AR, Holroyd EB, Yang L, Kim S & Yokoyama WM IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine 35, 229–234, doi: 10.1016/j.cyto.2006.08.006 (2006). [DOI] [PubMed] [Google Scholar]
- 130.Bhatt S, Sarosiek KA & Lossos IS Interleukin 21 - its potential role in the therapy of B-cell lymphomas. Leuk Lymphoma 58, 17–29, doi: 10.1080/10428194.2016.1201568 (2017). [DOI] [PubMed] [Google Scholar]
- 131.Huynh LK, Hipolito CJ & Ten Dijke P A Perspective on the Development of TGF-beta Inhibitors for Cancer Treatment. Biomolecules 9, doi: 10.3390/biom9110743 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morris JC et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 9, e90353, doi: 10.1371/journal.pone.0090353 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Formenti SC et al. Focal Irradiation and Systemic TGFbeta Blockade in Metastatic Breast Cancer. Clin Cancer Res 24, 2493–2504, doi: 10.1158/1078-0432.CCR-17-3322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tran HC et al. TGFbetaR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (ch14.18) with Natural Killer Cells. Clin Cancer Res 23, 804–813, doi: 10.1158/1078-0432.CCR-16-1743 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faivre S et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int 39, 1468–1477, doi: 10.1111/liv.14113 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Melisi D et al. TGFbeta receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol 83, 975–991, doi: 10.1007/s00280-019-03807-4 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Barry KC et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med 24, 1178–1191, doi: 10.1038/s41591-018-0085-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zemek RM et al. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Science Translational Medicine 11, eaav7816, doi: 10.1126/scitranslmed.aav7816 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Bezman NA et al. PD-1 blockade enhances elotuzumab efficacy in mouse tumor models. Blood Adv 1, 753–765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siebert N, Zumpe M, Jüttner M, Troschke-Meurer S & Lode HN PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD(2) antibody ch14.18/CHO. Oncoimmunology 6, e1343775, doi: 10.1080/2162402x.2017.1343775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamashita K et al. Trastuzumab upregulates programmed death ligand-1 expression through interaction with NK cells in gastric cancer. British Journal of Cancer 124, 595–603, doi: 10.1038/s41416-020-01138-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pesce S et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol 139, 335–346.e333, doi: 10.1016/j.jaci.2016.04.025 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Davis Z et al. Low-density PD-1 expression on resting human natural killer cells is functional and upregulated after transplantation. Blood Adv 5, 1069–1080, doi: 10.1182/bloodadvances.2019001110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Judge SJ et al. Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J Clin Invest 130, 3051–3068, doi: 10.1172/jci133353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caumartin J et al. Trogocytosis-based generation of suppressive NK cells. EMBO J 26, 1423–1433, doi: 10.1038/sj.emboj.7601570 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakamura K et al. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc Natl Acad Sci U S A 110, 9421–9426, doi: 10.1073/pnas.1300140110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mariotti FR et al. PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology 8, 1557030, doi: 10.1080/2162402x.2018.1557030 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Quatrini L et al. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol 19, 954–962, doi: 10.1038/s41590-018-0185-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oyer JL, Gitto SB, Altomare DA & Copik AJ PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology 7, e1509819, doi: 10.1080/2162402x.2018.1509819 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dong W et al. The Mechanism of Anti-PD-L1 Antibody Efficacy against PD-L1-Negative Tumors Identifies NK Cells Expressing PD-L1 as a Cytolytic Effector. Cancer discovery 9, 1422–1437, doi: 10.1158/2159-8290.cd-18-1259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that direct contact with susceptible tumours can promote PD-L1 expression on NK cells as a marker of their activation, including in AML patients, and an anti-PD-L1 antibody can surprisingly stimulate antitumour responses by these PD-L1+ NK cells via the induction of p38 signaling.
- 151.Tallerico R et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology 6, e1261242, doi: 10.1080/2162402X.2016.1261242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanseviero E et al. Anti–CTLA-4 Activates Intratumoral NK Cells and Combined with IL15/IL15Rα Complexes Enhances Tumor Control. Cancer immunology research 7, 1371–1380, doi: 10.1158/2326-6066.cir-18-0386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Russick J et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J Immunother Cancer 8, e001054, doi: 10.1136/jitc-2020-001054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lougaris V et al. CTLA-4 regulates human Natural Killer cell effector functions. Clinical Immunology 194, 43–45, doi: 10.1016/j.clim.2018.06.010 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Lanuza PM et al. Recalling the Biological Significance of Immune Checkpoints on NK Cells: A Chance to Overcome LAG3, PD1, and CTLA4 Inhibitory Pathways by Adoptive NK Cell Transfer? Frontiers in Immunology 10, doi: 10.3389/fimmu.2019.03010 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romagne F et al. Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677, doi:blood-2009-02-206532 [pii] 10.1182/blood-2009-02-206532 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kohrt HE et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 123, 678–686, doi: 10.1182/blood-2013-08-519199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mamessier E et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. The Journal of Clinical Investigation 121, 3609–3622, doi: 10.1172/JCI45816 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Borst L, van der Burg SH & van Hall T The NKG2A–HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment. Clinical Cancer Research 26, 5549–5556, doi: 10.1158/1078-0432.ccr-19-2095 (2020). [DOI] [PubMed] [Google Scholar]
- 160.van Montfoort N et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell 175, 1744–1755 e1715, doi: 10.1016/j.cell.2018.10.028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Galot R et al. A phase II study of monalizumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: The I1 cohort of the EORTC-HNCG-1559 UPSTREAM trial. Eur J Cancer 158, 17–26, doi: 10.1016/j.ejca.2021.09.003 (2021). [DOI] [PubMed] [Google Scholar]
- 162.Thijs WHF et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 105, e76–e79, doi: 10.3324/haematol.2019.220590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Binyamin L et al. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 180, 6392–6401 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carlsten M et al. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin Cancer Res 22, 5211–5222, doi: 10.1158/1078-0432.ccr-16-1108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; To follow up a clinical trial of single agent lirilumab in multiple myeloma patients, Carlsten et al. found a significant reduction in expression of KIR2D on NK and T cells of treated patients, and the remaining KIR2D+ NK cells were found to be hyporesponsive toward MHC-I-deficient tumour target cells, suggesting that blocking KIR2D by lirilumab can disrupt the education/licensing process
- 165.Chauvin J-M et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma. Clinical Cancer Research 26, 5520–5533, doi: 10.1158/1078-0432.ccr-20-0575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohs I et al. Restoration of Natural Killer Cell Antimetastatic Activity by IL12 and Checkpoint Blockade. Cancer Research 77, 7059–7071, doi: 10.1158/0008-5472.can-17-1032 (2017). [DOI] [PubMed] [Google Scholar]
- 167.Zhang Q et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature Immunology 19, 723–732, doi: 10.1038/s41590-018-0132-0 (2018). [DOI] [PubMed] [Google Scholar]
- 168.da Silva IP et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2, 410–422, doi: 10.1158/2326-6066.cir-13-0171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Farkas AM et al. Abstract 4745: Tim-3 and TIGIT mark NK and T cells susceptible to effector dysfunction in human bladder cancer. Cancer Research 78, 4745, doi: 10.1158/1538-7445.AM2018-4745 (2018).29930101 [DOI] [Google Scholar]
- 170.Davar D & Zarour HM Immunological Targets for Immunotherapy: Inhibitory T Cell Receptors. Methods Mol Biol 2055, 23–60, doi: 10.1007/978-1-4939-9773-2_2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.He Y et al. Contribution of inhibitory receptor TIGIT to NK cell education. Journal of Autoimmunity 81, 1–12, doi: 10.1016/j.jaut.2017.04.001 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Rosental B et al. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J Immunol 187, 5693–5702, doi: 10.4049/jimmunol.1102267 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cantoni C et al. NKp44, a triggering receptor involved in tumor cell lysis by activating human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med 189, 787–796 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gonzalez-Magana A & Blanco FJ Human PCNA Structure, Function and Interactions. Biomolecules 10, doi: 10.3390/biom10040570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kundu K et al. Inhibition of the NKp44-PCNA Immune Checkpoint Using a mAb to PCNA. Cancer Immunol Res 7, 1120–1134, doi: 10.1158/2326-6066.CIR-19-0023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; A monoclonal antibody was developed that interacts with a portion of PCNA that is exposed on tumour cell surfaces, which disrupts binding to the NK cell receptor NKp44 and prevents inhibitory signaling, and the antibody substantially potentiated NK cell responses toward PCNA+ tumour cells, both in vitro and in mouse models.
- 176.Cheng H et al. Wide Expression and Significance of Alternative Immune Checkpoint Molecules, B7x and HHLA2, in PD-L1–Negative Human Lung Cancers. Clinical Cancer Research 24, 1954–1964, doi: 10.1158/1078-0432.ccr-17-2924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jing CY et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer 7, 77, doi: 10.1186/s40425-019-0554-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang B, Ran Z, Liu M & Ou Y Prognostic Significance of Potential Immune Checkpoint Member HHLA2 in Human Tumors: A Comprehensive Analysis. Frontiers in Immunology 10, doi: 10.3389/fimmu.2019.01573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Trundley AE et al. Molecular characterization of KIR3DL3. Immunogenetics 57, 904–916, doi: 10.1007/s00251-005-0060-7 (2006). [DOI] [PubMed] [Google Scholar]
- 180.Leaton LA et al. Conservation, Extensive Heterozygosity, and Convergence of Signaling Potential All Indicate a Critical Role for KIR3DL3 in Higher Primates. Front Immunol 10, 24, doi: 10.3389/fimmu.2019.00024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Janakiram M et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res 21, 2359–2366, doi: 10.1158/1078-0432.CCR-14-1495 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhuang X & Long EO CD28 Homolog Is a Strong Activator of Natural Killer Cells for Lysis of B7H7(+) Tumor Cells. Cancer Immunol Res 7, 939–951, doi: 10.1158/2326-6066.CIR-18-0733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhatt RS et al. KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunology Research, canimm.0315.2020, doi: 10.1158/2326-6066.cir-20-0315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Veillette A & Chen J SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends in immunology 39, 173–184, doi: 10.1016/j.it.2017.12.005 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Deuse T et al. The SIRPα-CD47 immune checkpoint in NK cells. The Journal of experimental medicine 218, doi: 10.1084/jem.20200839 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Temming AR et al. Functional Attributes of Antibodies, Effector Cells, and Target Cells Affecting NK Cell–Mediated Antibody-Dependent Cellular Cytotoxicity. The Journal of Immunology 203, 3126–3135, doi: 10.4049/jimmunol.1900985 (2019). [DOI] [PubMed] [Google Scholar]
- 187.Veeramani S et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 118, 3347–3349, doi: 10.1182/blood-2011-05-351411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Treon SP et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenström’s macroglobulinemia. J Clin Oncol 23, 474–481, doi: 10.1200/jco.2005.06.059 (2005). [DOI] [PubMed] [Google Scholar]
- 189.Musolino A et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 26, 1789–1796, doi: 10.1200/jco.2007.14.8957 (2008). [DOI] [PubMed] [Google Scholar]
- 190.Cartron G et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99, 754–758 (2002). [DOI] [PubMed] [Google Scholar]
- 191.Bang YJ et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 28, 855–861, doi: 10.1093/annonc/mdx002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chenoweth AM, Wines BD, Anania JC & Mark Hogarth P Harnessing the immune system via FcγR function in immune therapy: a pathway to next-gen mAbs. Immunology & Cell Biology 98, 287–304, doi: 10.1111/imcb.12326 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vitolo U et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology 35, 3529–3537, doi: 10.1200/jco.2017.73.3402 (2017). [DOI] [PubMed] [Google Scholar]
- 194.Marcus R et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 377, 1331–1344, doi: 10.1056/NEJMoa1614598 (2017). [DOI] [PubMed] [Google Scholar]
- 195.Lunning M et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 134, 1811–1820, doi: 10.1182/blood.2019002118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kumar A, Planchais C, Fronzes R, Mouquet H & Reyes N Binding mechanisms of therapeutic antibodies to human CD20. Science 369, 793–799, doi: 10.1126/science.abb8008 (2020). [DOI] [PubMed] [Google Scholar]
- 197.Chan HT et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res 63, 5480–5489 (2003). [PubMed] [Google Scholar]
- 198.Campbell KS, Cohen AD & Pazina T Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma. Front Immunol 9, 2551, doi: 10.3389/fimmu.2018.02551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pazina T et al. The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and –independent mechanisms. Oncoimmunology 6, e1339853, doi: 10.1080/2162402X.2017.1339853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pazina T et al. Enhanced SLAMF7 Homotypic Interactions by Elotuzumab Improves NK Cell Killing of Multiple Myeloma. Cancer Immunol Res 7, 1633–1646, doi: 10.1158/2326-6066.CIR-18-0579 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dimopoulos MA et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. New England Journal of Medicine 379, 1811–1822, doi: 10.1056/NEJMoa1805762 (2018). [DOI] [PubMed] [Google Scholar]
- 202.Dimopoulos MA et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol 22, 801–812, doi: 10.1016/S1470-2045(21)00128-5 (2021). [DOI] [PubMed] [Google Scholar]
- 203.Casneuf T et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv 1, 2105–2114, doi: 10.1182/bloodadvances.2017006866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Boyerinas B et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunology Research 3, 1148–1157, doi: 10.1158/2326-6066.cir-15-0059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Juliá EP, Amante A, Pampena MB, Mordoh J & Levy EM Avelumab, an IgG1 anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production Against Triple Negative Breast Cancer Cells. Front Immunol 9, 2140, doi: 10.3389/fimmu.2018.02140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ferrari de Andrade L et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell–driven tumor immunity. Science 359, 1537–1542, doi: 10.1126/science.aao0505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Du C et al. MICA immune complex formed with alpha 3 domain-specific antibody activates human NK cells in a Fc-dependent manner. Journal for ImmunoTherapy of Cancer 7, 207, doi: 10.1186/s40425-019-0687-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kaur K, Safaie T, Ko MW, Wang Y & Jewett A ADCC against MICA/B Is Mediated against Differentiated Oral and Pancreatic and Not Stem-Like/Poorly Differentiated Tumors by the NK Cells; Loss in Cancer Patients due to Down-Modulation of CD16 Receptor. Cancers (Basel) 13, doi: 10.3390/cancers13020239 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Romee R et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 121, 3599–3608, doi: 10.1182/blood-2012-04-425397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Srpan K et al. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. The Journal of cell biology 217, 3267–3283, doi: 10.1083/jcb.201712085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chew HY et al. Endocytosis Inhibition in Humans to Improve Responses to ADCC-Mediating Antibodies. Cell 180, 895–914 e827, doi: 10.1016/j.cell.2020.02.019 (2020). [DOI] [PubMed] [Google Scholar]
- 212.Goebeler M-E & Bargou RC T cell-engaging therapies — BiTEs and beyond. Nature Reviews Clinical Oncology 17, 418–434, doi: 10.1038/s41571-020-0347-5 (2020). [DOI] [PubMed] [Google Scholar]
- 213.Ellwanger K et al. Redirected optimized cell killing (ROCK®): A highly versatile multispecific fit-for-purpose antibody platform for engaging innate immunity. mAbs 11, 899–918, doi: 10.1080/19420862.2019.1616506 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dahlén E, Veitonmäki N & Norlén P Bispecific antibodies in cancer immunotherapy. Therapeutic Advances in Vaccines and Immunotherapy 6, 3–17, doi: 10.1177/2515135518763280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Oberg HH et al. Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing γδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Frontiers in Immunology 9, doi: 10.3389/fimmu.2018.00814 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Glorius P et al. The novel tribody [(CD20)2xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia 27, 190–201, doi: 10.1038/leu.2012.150 (2013). [DOI] [PubMed] [Google Scholar]
- 217.Davis ZB, Vallera DA, Miller JS & Felices M Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Seminars in Immunology 31, 64–75, doi: 10.1016/j.smim.2017.07.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schmohl JU et al. Engineering of Anti-CD133 Trispecific Molecule Capable of Inducing NK Expansion and Driving Antibody-Dependent Cell-Mediated Cytotoxicity. Cancer research and treatment : official journal of Korean Cancer Association 49, 1140–1152, doi: 10.4143/crt.2016.491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Felices M et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Advances 3, 897–907, doi: 10.1182/bloodadvances.2018029371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Arvindam US et al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia, doi: 10.1038/s41375-020-01065-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gauthier L et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 177, 1701–1713.e1716, doi: 10.1016/j.cell.2019.04.041 (2019). [DOI] [PubMed] [Google Scholar]; Gauthier et al. have systematically engineered trispecific antibodies, which they call NK cell engagers, that consist of a tumour surface antigen binding element along with optimally spaced interaction sites for NKp46 and CD16 to trigger highly potent NK cell-mediated antitumour responses in vitro and in mouse models.
- 222.Au KM, Park SI & Wang AZ Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Science Advances 6, eaba8564, doi: 10.1126/sciadv.aba8564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Srivastava RM et al. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 707–716, doi: 10.1158/1078-0432.CCR-16-0879 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Myers JA & Miller JS Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol 18, 85–100, doi: 10.1038/s41571-020-0426-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cooley S et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 116, 2411–2419, doi: 10.1182/blood-2010-05-283051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Venstrom JM et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med 367, 805–816, doi: 10.1056/NEJMoa1200503 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Knorr DA et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem cells translational medicine 2, 274–283, doi: 10.5966/sctm.2012-0084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hermanson DL et al. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem cells (Dayton, Ohio) 34, 93–101, doi: 10.1002/stem.2230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Shereck E et al. Immunophenotypic, cytotoxic, proteomic and genomic characterization of human cord blood vs. peripheral blood CD56(Dim) NK cells. Innate Immun 25, 294–304, doi: 10.1177/1753425919846584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Herrera L et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci Rep 9, 18729, doi: 10.1038/s41598-019-55239-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rosenberg SA et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313, 1485–1492, doi: 10.1056/nejm198512053132327 (1985). [DOI] [PubMed] [Google Scholar]
- 232.Ewen EM, Pahl JHW, Miller M, Watzl C & Cerwenka A KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur J Immunol 48, 355–365, doi: 10.1002/eji.201747128 (2018). [DOI] [PubMed] [Google Scholar]
- 233.Romee R et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 8, 357ra123, doi: 10.1126/scitranslmed.aaf2341 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report that culture in the combination of IL-12, IL-15, and IL-18 induces expansion of ‘memory-like’ NK cells that exhibit robust IFN-γ and cytotoxicity responses toward primary AML cells in vitro and in xenograft mice that are independent of KIR inhibition, and a small clinical trial showed promising antileukemia responses after adoptive transfer of memory-like NK cells into AML patients, resulting in clinical responses in five of nine evaluable patients.
- 234.Dong H et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat Immunol 20, 865–878, doi: 10.1038/s41590-019-0388-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kerbauy LN et al. Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood-Derived NK Cells Facilitates CAR-like Responses Against CD30(+) Malignancies. Clin Cancer Res 27, 3744–3756, doi: 10.1158/1078-0432.CCR-21-0164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; AFM13, the tetravalent anti-CD16/CD30 bispecific antibody currently in clinical trials for CD30+ malignancies, is shown to have specific applications for adoptive transfer therapy; first, for enhanced cytotoxicity when paired with cytokine-induced ‘memory-like’ NK cells, and second, for its high avidity to CD16, which allows it to be pre-loaded onto NK cells prior to adoptive transfer.
- 236.Kweon S et al. Expansion of Human NK Cells Using K562 Cells Expressing OX40 Ligand and Short Exposure to IL-21. Front Immunol 10, 879, doi: 10.3389/fimmu.2019.00879 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fujisaki H et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 69, 4010–4017, doi: 10.1158/0008-5472.Can-08-3712 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu E et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med 382, 545–553, doi: 10.1056/NEJMoa1910607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report describes a clinical trial in which patients with B-cell tumours were treated with MHC-I-mismatched NK cells bearing an anti-CD19 chimeric antigen receptor (CAR), IL-15, and an inducible caspase 9 suicide gene, and eight of eleven patients experienced rapid clinical responses and long-term survival of CAR-bearing NK cells without cytokine release syndrome or other autoimmune toxicities.
- 239.Li Y, Hermanson DL, Moriarity BS & Kaufman DS Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell stem cell 23, 181–192.e185, doi: 10.1016/j.stem.2018.06.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Oei VYS et al. Intrinsic Functional Potential of NK-Cell Subsets Constrains Retargeting Driven by Chimeric Antigen Receptors. Cancer Immunol Res 6, 467–480, doi: 10.1158/2326-6066.cir-17-0207 (2018). [DOI] [PubMed] [Google Scholar]
- 241.Jochems C et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 7, 86359–86373, doi: 10.18632/oncotarget.13411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mitwasi N et al. “UniCAR”-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci Rep 10, 2141, doi: 10.1038/s41598-020-59082-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Williams BA et al. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 8, 89256–89268, doi: 10.18632/oncotarget.19204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bonanni V, Antonangeli F, Santoni A & Bernardini G Targeting of CXCR3 improves anti-myeloma efficacy of adoptively transferred activated natural killer cells. Journal for immunotherapy of cancer 7, 290–290, doi: 10.1186/s40425-019-0751-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Carlsten M et al. Efficient mRNA-Based Genetic Engineering of Human NK Cells with High-Affinity CD16 and CCR7 Augments Rituximab-Induced ADCC against Lymphoma and Targets NK Cell Migration toward the Lymph Node-Associated Chemokine CCL19. Front Immunol 7, 105, doi: 10.3389/fimmu.2016.00105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jing Y et al. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One 10, e0121788, doi: 10.1371/journal.pone.0121788 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhu H et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 135, 399–410, doi: 10.1182/blood.2019000621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhu H et al. Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-tumor Activity. Cell stem cell 27, 224–237.e226, doi: 10.1016/j.stem.2020.05.008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors generated iPSC-derived NK cells expressing a modified CD16 that was high-affinity (158V) and ADAM17 cleavage resistant (S197P), which are noteworthy for their more robust ADCC response and more stable CD16 expression than unmodified iPSC-derived NK cells.
- 249.Naeimi Kararoudi M et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood, doi: 10.1182/blood.2020006200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.van der Horst HJ, Nijhof IS, Mutis T & Chamuleau ME D. Fc-Engineered Antibodies with Enhanced Fc-Effector Function for the Treatment of B-Cell Malignancies. Cancers (Basel) 12, doi: 10.3390/cancers12103041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Horton HM et al. Potent In vitro and In vivo Activity of an Fc-Engineered Anti-CD19 Monoclonal Antibody against Lymphoma and Leukemia. Cancer Research 68, 8049–8057, doi: 10.1158/0008-5472.can-08-2268 (2008). [DOI] [PubMed] [Google Scholar]
- 252.Purdy AK & Campbell KS Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR). Cancer Biol Ther 8, 2211–2220, doi:10455 [pii] (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


