Abstract
The intervertebral disc and cartilage are physiologically hypoxic and rely on HIF transcription-factors to mediate cellular responses to changes in oxygen tension. During homeostatic development, oxygen-dependent prolyl hydroxylases (PHDs), circadian clock proteins, and metabolic intermediates control HIF-1/2 activity in these tissues. Mechanistically, HIF-1 is the master regulator of glycolytic flux and cytosolic lactate levels. HIF-1 also regulates mitochondrial metabolism by promoting TCA cycle activity and inhibiting oxidative phosphorylation, while controlling mitochondrial health through modulation of the mitophagic pathway. Accumulation of metabolic intermediates from HIF-dependent processes, including H+/lactate and CO2, contribute to intracellular pH regulation in the disc and cartilage. To prevent changes in disc cell pH that could lead to death, HIF-1 orchestrates a bicarbonate buffering system, controlled by carbonic anhydrases 9/12 (CA9/12), sodium bicarbonate cotransporters (NBCs), and an intracellular H+/lactate efflux mechanism, facilitated by the lactate transporter, monocarboxylate transporter 4 (MCT4). In contrast to HIF-1, the role of HIF-2 remains elusive: in disorders of disc and cartilage, its function has been linked to both anabolic and catabolic pathways. The current knowledge of hypoxic cell metabolism and regulation of HIF-1 activity provides a strong basis for the development of future therapies designed to treat the ravages of disc degeneration.
Introduction
Within the last century, Nobel Prizes have been awarded for discoveries underlying oxygen-sensing and cellular respiration. The 2019 Nobel Prize in Physiology and Medicine awarded to Drs. Kaelin, Ratcliffe, and Semenza recognized the importance of understanding how oxygen regulates cellular metabolism in various tissue types and organisms throughout their lifespans. The foundation for this work was based on studies by the 1931 Nobel laureate, Otto Warburg, who discovered the enzymatic basis of cellular respiration, from which has emerged an appreciation of the role of oxidative metabolism in health and disease. The combined efforts of these Nobel laureates and other scientists delineated how intracellular oxygen levels are directly coupled to changes in gene expression through the regulation of Hypoxia Inducible Factor (HIF) and its degradation by the von Hippel- Lindau (VHL) axis1–4.
While most cell types utilize the local O2 tension in the mitochondrial electron transport chain (ETC) to generate ATP, hypoxic cells adapt to the decreased O2 availability by inducing HIF-dependent transcription of genes necessary for survival5. The HIF-family comprises three heterodimeric transcription factors composed of an O2-regulated subunit (HIF-1α, HIF-2α, or HIF-3α) dimerized with a constitutively expressed subunit (HIF-1β)4. The α-subunits contain a basic-helix-loop-helix (bHLH) domain, two PER–ARNT-SIM (PAS) homology domains, a PAS-associated COOH-terminal (PAC) domain, an oxygen-dependent (ODD domain) with an associated NH2-terminal transactivation domain (N-TAD) and C-terminus transactivation domain (C-TAD). In normoxic cell types, prolyl hydroxylase domain enzymes (PHDs) utilize O2, α-ketoglutarate, and co-substrate, L-ascorbate, to hydroxylate proline residues in the ODD domain of the HIF-α subunit. Proline hydroxylation results in interaction with VHL/E3-ubiquitin ligase and targets HIF-α for proteasomal degradation1,6. However, PHD-mediated hydroxylation is substrate inhibited in hypoxic cells. In this way, low O2 tension causes HIF-α accumulation, subsequent heterodimerization, and transcriptional activation of HIF targets.
Much of our understanding of HIF function stems from studies of HIF-1α and HIF-2α. These isoforms are structurally similar and regulate transcription through hypoxia-response elements (HRE) contained within target gene loci7. Hypoxia-responsive genes are implicated in the physiological regulation of the developing embryo and adult tissues, as well as in the pathogenesis of arthritis, inflammation, and cancer. Numerous studies evaluating tissue-specific expression, temporal induction, and phenotypes following gene deletion or mutation confirm that the isoforms are functionally non-redundant8. HIF-1α is ubiquitously expressed and considered a master regulator of metabolic reprogramming, cell-cycle regulation, angiogenesis, and tumorigenesis9. HIF-2α is notably expressed in endothelial tissues and bone marrow macrophages where it exerts a larger role in angiogenic signaling, guidance cues, and extracellular matrix remodeling9,10.
To those studying the biology and pathologies afflicting skeletal tissues, mounting evidence demonstrates that HIF function is required for intervertebral disc integrity 11–15, fetal growth plate development 16, and endochondral ossification 17,18, while dysregulation of HIF signaling is an early impetus for degenerative cascades (See Box 1). To date, there are no cures for disc degeneration, osteoarthritis (OA), or rheumatoid arthritis although they are the most common joint diseases. Low back pain (LBP) and neck pain, specifically, are the first and fourth leading causes of chronic disability in the United States and contribute significantly to the global disease burden19. The common risk factor for LBP is intervertebral disc degeneration, a multifactorial pathology characterized by progressive loss of extracellular matrix components and increased intradiscal acidosis, both of which increase susceptibility to herniation, fissuring of the endplate cartilage, a raised immune response, and thus, discogenic pain 20–25. The genetic link between HIFs and disc degeneration and OA are two-fold. Single nucleotide polymorphisms (SNPs) in HIF-1/2 correlate with the susceptibility and severity of lumbar disc degeneration and OA in single-ethnicity association studies18,26. Second, the regulation of a myriad of hypoxia-responsive signaling pathways, including those required for matrix remodeling and cellular metabolism, evidence genetic disarray during disc degeneration and arthritis. In this review, we present the argument that HIF1/2 are non-canonically regulated in the hypoxic intervertebral disc, whereby they are distinctly required for the regulation of cellular metabolism, pH regulation, and cell survival pathways. From this perspective, HIF transcription factors provide an important opportunity for the development of therapeutic targets for degenerative skeletal diseases.
Box 1: Diverging Roles of HIF-1 and HIF-2 in Disc and Cartilage
HIF-1α is considered the guardian of hypoxic cells. Functionally, HIF-1α mediates NP cell survival57 and chondrocyte growth and development110, while the HIF-1α-β-catenin interaction prevents the pathological loss of articular cartilage in OA111. In contrast, the role of HIF-2α in the intervertebral disc has not been characterized in vivo, yet it has been shown that HIF-2α expression promotes both matrix anabolism and catabolism in articular chondrocytes 17,18,112. Two studies in 2010 reported that HIF-2α is a central mediator of endochondral ossification and may induce development of the OA phenotype. Specifically, HIF-2α is elevated in human OA tissue and upregulates Col10a1, Mmp13, and Vegfa gene expression in mouse models of OA. Further analysis demonstrated that HIF-2α controls the IL1β-induced expression of MMPs, ADAMTS4, NOS2, and PTGS2; the exogenous introduction of HIF-2α to mouse and rabbit knees induced cartilage destruction. Collectively, these studies showed HIF-2α is a key participant in the induction of OA. However, this pathogenic role of HIF-2α was confounded by the findings by Thoms et al. that HIF-2 through regulation of Sox9, promoted extracellular matrix production and cartilage repair 112. HIF-1α and HIF-2α also show diverging roles in the fetal growth plate and limb bud. HIF-1α is indispensable for chondrocyte survival and differentiation in the hypoxic regions of the fetal growth plate 110 and the limb bud mesenchymal cells 113,114. In contrast, Araldi and colleagues demonstrated that the HIF-2α contribution to growth plate development is minor 115. Loss of HIF-2α in the limb bud mesenchyme caused a modest delay in endochondral bone development which was linked to impaired differentiation of chondroprogenitor cells into hypertrophic chondrocytes115. These studies underscore divergent functions of HIF-1α and HIF-2α in skeletal tissues, and demonstrate that HIF-2α function differs between articular and growth plate chondrocytes.
Box 2: Lactate Regulation of Transcription
There is growing evidence to suggest that metabolic intermediates can directly link cellular metabolism to physiological functions, such as cell proliferation and survival, through alterations in the epigenetic landscape. The enriched biological processes in the NP cells of MCT4 null mice were in fact driven by changes in histone genes and DNA binding proteins11. The major pathways associated with these genes were Nucleosome Assembly, Regulation of Epigenetic Gene Expression, and Negative Regulation of Cell Proliferation. Based on these findings, we contend that buildup of lactic acid directly affects gene transcription in NP cells11. In fact, many essential glycolytic and mitochondrial enzymes moonlight in the nucleus where they are involved in DNA binding and the generation of intermediates that regulate gene transcription 116–119. Phosphorylation of Tyr238 on LDH promotes nuclear translocation, whereby nuclear produced lactate inhibits HDACs and increases gene transcription 120,121. Most encouraging, Zhang et al. recently detailed a process called “lactylation”: a lactate-derived epigenetic modification of 28 distinct histone lysine residues which directly stimulates gene transcription according to a “lactate clock” 122. We therefore propose that elevated intradiscal lactate levels may contribute to disc degeneration through a direct and cumulative effect on the transcriptional and epigenetic regulation in NP cells. Likewise, the metabolic regulation of epigenetics in chondrocytes is an interesting area for future study.
Box 3: Clinical Development of HIF/PHD Inhibitors
Pharmacological prolyl hydroxylase inhibitors (PHIs) and HIF inhibitors were initially developed for treatment of renal anemia and cancer therapy 123–125. The question remains as to which of these inhibitors would be most suitable for regeneration of hypoxic skeletal tissues where HIF-1α signaling is considered a hallmark of healthy tissue, and degeneration is correlated with loss of HIF function. PHIs may be the preferred therapeutic candidate for the following reasons: PHIs induce a transient increase in HIF-regulated gene expression 125 and various clinical studies demonstrated the efficacy of PHIs for the treatment of renal anemia124. Promising results from phase III clinical trials make a strong argument for the future testing of PHIs in disc and cartilage. PHIs with PHD2 specificity may be capable of elevating and/or stabilizing HIF-1α expression in NP cells and articular chondrocytes, thus upregulating HIF target gene expression (i.e. VEGF, CA12, MCT4) in degenerated discs and OA.
On the other hand, HIF-2α has been associated with both agonistic and antagonistic pathways in cartilage. In some instances, inhibitors that block HIF-2 dimerization126, may be useful to reduce the negative effects of HIF-2 on matrix catabolism and the development of OA. There are several highly selective HIF-2 inhibitors in phase II clinical trials for the treatment of VHL-associated renal cell carcinoma (RCC): for example, novel compounds, PT2385 and PT2977, inhibit HIF-2 dimerization and DNA binding123,127. A similar compound, PT2399, has shown even greater effectiveness for treating RCC in a xenograft platform128. It is important to investigate if such HIF-2α inhibitors would exert positive effects on the fate of chondrocytes in OA.
The Hypoxic Intervertebral Disc Niche
The intervertebral disc and adjoining superior and inferior vertebrae form the functional spinal motion segment capable of polyaxial movements and withstanding compressive and tensile loads. It can be argued that the motion segment is a diarthrodial joint with two articulating hyaline cartilaginous-endplates (CEP), surrounding an inner nucleus pulposus (NP), rich in chondroitin-sulfate proteoglycans which give the disc its characteristic swelling properties21,27. The NP is encapsulated by a fibrocartilaginous annulus fibrosus (AF) composed of concentric Collagen I lamellae. The local blood supply in the subchondral bone reaches the bony-endplate (BEP) and outer layers of the CEP and AF, but does not infiltrate the inner AF nor the innermost NP28. Due to these anatomical constraints, the intervertebral disc is considered to be the largest avascular organ in vertebrates. As a consequence of avascularity, NP cells experience hypoxia 29–31.
Pathways Regulating HIF in the Disc
Over the past two decades, several studies highlighted the unique regulation of HIF activity in NP cells. That is, HIF-1/2 isoforms are robustly expressed under normoxia and their levels, to a large extent, are insensitive to oxemic tension 29,30. Furthermore, HIFs and their target genes are regulated by the peripheral circadian clock32,33. The novel HIF signature in NP cells is therefore attributed to both oxygen-dependent and -independent mechanisms of regulation and sensitive to the disc’s diurnal loading cycles (Fig. 1).
Figure 1. Regulation of HIF-1α in hypoxic NP cells.

A) Schematic of the intervertebral disc tissue compartments and vasculature. The absence of vasculature in disc compartments makes the NP tissue physiologically hypoxic resulting in robust HIF-1α expression. B) Oxygen dependent mechanisms of HIF-α regulation. In the presence of sufficient O2, PHD2 hydroxylates proline residues in the ODD of HIF-1α targeting it for VHL-mediated polyubiquitination and 26S proteasomal degradation. PHD2 function can be blocked by two mechanisms: 1) Lactate accumulation generates metabolic intermediates, including pyruvate and succinate, which compete with the PHD2 substrate, 2-OG, and inhibit PHD activity. 2) Class I and II HDACs directly inhibit HIF-PHD2 axis. Unlike PHD2, PHD3 serves as a cofactor for transcriptional activation of C-TAD dependent target genes. In NP cells, HIF-1 function is refractory to FIH mediated inhibition. C). Oxygen-independent mechanisms of HIF-α regulation. HIF-1α can be targeted for 26S degradation by HSP70 possibly through displacement of HSP90. In NP cells, HIF-1α is a circadian clock-regulated gene. BMAL1 and RORα synergize to upregulate N-TAD and C-TAD dependent target genes, without evidence of direct binding to HIF-α. HDAC6 is shown to recruit HSP90 as a cofactor to upregulate HIF target gene expression, whereas CCN2 was reported to block HIF-1α cofactor binding and diminish its activity.
O2-dependent/ independent Mechanisms
The peculiarities in oxygen-dependent HIF stability and signaling in NP cells are largely due to non-canonical regulation of HIF-1α and HIF-2α by PHDs. 26S proteasomal degradation of HIF-1α in NP cells is mediated by PHD2, but not by PHD1 or PHD331,34. Noteworthy, PHD enzymatic function in NP cells is not limited by substrate availability e.g. α-ketoglutarate, a topic considered further in the review. Surprisingly, proteasomal degradation of HIF-2α is independent of PHD activity in NP cells31,34 and is an avenue for future investigations. In contrast to NP cells, HIF-1/2α degradation in chondrocytes proceeds through proline hydroxylation, suggesting that the regulation of HIF-α in NP cells is cell-type specific31,35. PHDs are known transcriptional targets of HIFs; together HIF-α isoforms and PHD enzymes are involved in a reciprocally dependent regulatory loop. In NP cells, PHD3 expression is robustly increased under hypoxia and promoter/enhancer activity is regulated by HIF-1/2α34. Although PHD3 modulation has little effect on HIF-1α protein levels, PHD3 enhances HIF-1α transcriptional activity in NP cells31,34. That is, PHD3 is a HIF-1α cofactor required for transcriptional activation of a subset of HIF-1α C-TAD-dependent genes36. Middle-aged PHD3 null mice showed disc degeneration and a significantly decreased expression of HIF-1α C-TAD targets, VEGF, GLUT1, and LDHA36. Unlike other cells, where PHD3 functions as a HIF-1α coactivator through a PKM2-JMJD5 axis,37 manipulation of PKM2 and JMJD5 levels in NP cells had no effect on PHD3-dependent HIF-1α activity or target gene activation36,38.
The published work on HIF-PHDs has provided a clear indication that the degradation of HIF-1/2α in NP cells is also prominently controlled by oxygen-independent pathways, e.g. lysosomal autophagic degradation31. Heat shock proteins are specifically implicated in such oxygen-independent cellular adaptations to their hypoxic niche39. This is apparent in NP cells where Hsp70 promotes the proteasomal degradation of HIF-1α, yet HIFs reciprocally suppress Hsp70 transcription40. Furthermore, Schoepflin et al. showed that HDAC6 was required for the recruitment of Hsp90, a cofactor necessary for HIF-1α mediated transcription, thereby acting as a positive regulator of HIF-1α41. Moreover, Class I and IIa HDACs are involved in HIF-1α stabilization through modulation of the HIF-PHD2 axis41. A second oxygen-independent regulatory loop in NP cells links HIF-1α and connective tissue growth factor, CCN242. Unlike heat shock proteins, CCN2 diminishes HIF-1 target gene expression and blocks HIF-1α from recruiting additional coactivators. The further details of the mechanism of action of CCN2 on HIF function remain to be elucidated, but it is not likely to be involved in HIF degradation. Finally, it is important to note that in many cell types, HIF-1α activity is regulated by modulating its interaction with p300/CBP through hydroxylation of a conserved asparagine residue in the C-TAD domain of HIF-1α by factor inhibiting HIF-1(FIH), an asparaginyl hydroxylase43. However, FIH was dispensable in NP cells and did not regulate canonical HIF-1 targets44. Interestingly, studies showed a lack of HIF-related phenotypes in FIH null mice while implicating FIH in processes related to glucose and fatty acid metabolism. This observation raised questions about the in vivo relevance of this post-translational modification on HIF activity45.
Circadian Rhythm
Given that the intervertebral disc is hypoxic, it is logical to focus on HIF regulation in response to oxygen availability. However, from an anatomical and functional perspective, the disc is also characterized by kinesiological factors, in other words, by diurnal patterns of cyclical loading during active hours and unloading during inactive hours. Joint tissues in general are influenced by daily rest-active cycles which are indirectly controlled by the peripheral circadian clock46; conversely, the Clock gene is mechano-sensitive, implying that daily activity could manipulate the joint circadian rhythm47. Intriguingly, several studies have shown that the cellular hypoxic response and circadian clock are linked by a synergistic cross-talk, utilizing HIF-1 as the central mediator48–50. Briefly, HIF-1α is an E-box regulated gene under the transcriptional control of the circadian clock genes, Clock/BMAL1, meanwhile period circadian clock 2 (PER2), recruits HIF-1α to HRE motifs on target genes48,49. Downstream, HIF-1α reciprocally modifies the expression of circadian clock genes, including, PER2, effectively dampening circadian rhythm49. As such, the hypoxic-induction of HIF-1α signaling can cause tissue-specific circadian misalignments50. Importantly, both HIF-1 and canonical clock genes were found to be dysregulated during cartilage degeneration in human OA51,52. Furthermore, the master clock gene, ARNTL/BMAL1, has now been implicated in maintenance of articular cartilage integrity. Conditional loss of BMAL1 in chondrocytes leads to progressive degeneration of the articular cartilage in mouse knee joints, and correlates with loss of cartilage circadian rhythm53. It is therefore not surprising that NP cell transcriptome is dependent on circadian rhythm genes CLOCK, BMAL1, and RORα32,33. In fact, Dudek and colleagues discovered 607 rhythmic genes in the disc, which account for 3.5% of the disc transcriptome33. The importance of rhythmic gene regulation was confirmed in the disc using BMAL1 knock-out mice. These mice showed diverse degenerative changes in the disc which correlated with the discovery of a regulatory loop in NP cells, whereby HIF-1α and HIF target gene expression are regulated by BMAL1 and RORα32. It is plausible that loss of HIF-1α signaling may account for some of the degenerative changes observed in the BMAL1 knock-out mice.
Hypoxic Regulation of Cell Survival and Autophagic Pathways
Concerning the function of HIF-α isoforms, HIF-1α in particular, regulates the expression of many genes critical to the survival of NP cells- i.e. plasma-membrane glucose transporters and glycolytic enzymes30. For glycolysis to occur, glucose must passively diffuse from the vertebral capillaries, through the CEP and dense proteoglycan matrix of the NP compartment to reach resident NP cells at the center of the disc54. In an environment where glucose and oxygen concentrations are limiting and lactic acid concentrations are relatively high55, maintaining glycolytic flux and nutrient-metabolite balance is critical for NP survival 30,56. Importantly, two mouse models of notochord-specific HIF-1α conditional deletion driven by constitutive Foxa2-Cre and Shh-Cre exhibited severe disc degeneration that is likely instigated by metabolic failure of NP cells57,58. In the first model, driven by Foxa2-Cre, the null mice presented with reduced size of the NP compartment at E15.5, followed by a massive cell death at birth, and postnatal disappearance and remodeling of the NP compartment57. It is hypothesized that mutant NP cells died due to metabolic failure. This was evidenced by significant loss of the HIF-1α target gene, PGK1, which catalyzes a reversible conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate and phosphorylates ADP, at the substrate level, to ATP. Accordingly, loss of PGK function would result in blockage of glycolytic flux and contribute to cell death in the mutant NP. The second mouse model of NP-specific HIF-1α deletion, driven by a Shh-Cre driver, showed increased cell death and disc degeneration by 6 and 12-weeks of age, respectively58. Mutant discs also showed lower levels of Acan, Col2a1, and Vegf, which may also have contributed to cell death. An additional mouse model of spontaneous, early-onset disc degeneration was recently characterized20,59. The inbred SM/J mouse strain, known to have poor cartilage regenerative properties, showed early signs of disc degeneration characterized by loss of NP cells and the presence of cells with hypertrophic chondrocyte-like characteristics. In this model, diminished expression of HIF-1α target gene, Vegf, correlated with increased cell death. Related to this observation, a pro-survival role of VEGF-A in NP cells was reported by Fujita et al60.
It is also known that hypoxia and HIF-1α modulate important survival and adaptive pathways, including autophagy, ER stress, and mitophagy61,62. The hypoxic induction of autophagy is often mediated by the HIF-1 induction of Bcl-2/E1B 19 kDa-interacting protein 3 (BNIP3/BNIP3L)63, whereas, the HIF-independent autophagic response is signaled through a nutritional stress response via AMPK-mTOR and the unfolded protein response (UPR) pathways. It has been shown that NP cells adapt to their hypoxic niche through the modulation of autophagy and ER stress, whereby hypoxia increases autophagic flux and lowers the ER stress burden15,61,64. However, the hypoxic induction of autophagy in NP cells is regulated in a non-canonical manner that is independent of both HIF-1α and MTOR signaling. While inhibition of non-canonical autophagic flux had no effect on glycolytic metabolism, long-term inhibition compromised NP cell survival. This suggests that autophagy plays a unique, non-metabolic role in hypoxic NP cells that should be further investigated. On the other hand, HIF-1α is directly linked to attenuating ER stress and modulating the NP cell secretome64. Dysregulation of autophagic and ER stress-related pathways may explain the increased UPR and decreased ECM integrity of aged and degenerated discs61,64.
As NP cells reside in a physiologically hypoxic environment and utilize glycolysis for energy production, a prevalent notion was that the mitochondria would play a minor biological role in disc physiology. Very recently, Madhu and colleagues used a mito-QC mouse model to demonstrate that, in fact, NP cells contain numerous well-networked, tubular, and hypoxia-responsive mitochondria12. Specifically, hypoxia and HIF-1α govern mitochondrial morphology, composition, and mass in NP cells. These researchers leveraged the finding that NP cells have functional, HIF-dependent mitochondria to study mitochondrial dynamics. They noted that mitophagy and mitochondrial fragmentation are regulated by HIF-1α through modulation of BNIP3 and DRP1/OPA1, respectively. While the hypoxic induction of mitophagy normally requires the HIF-1α-BNIP3 axis, when HIF-1α is silenced there is compensation through NIX and non-receptor-mediated pathways. Although research into mitochondrial dynamics in NP cells is at an early stage, each discovery has the potential to change how researchers in the field consider the role of mitochondria within a hypoxic niche. In the following section that deals with the functional importance of the mitochondrial TCA cycle in NP cell metabolism, we consider the hypothesis that dysregulation of the mitophagic pathway compromises NP cell survival.
Hypoxic Regulation of Cell Metabolism
For many decades, the most critical research questions concerned mechanisms of disc cell survival and function in their physiologically hypoxic and acidic milieu. Until recently, research in this area was limited to understanding basal nutrient-metabolite concentrations in the disc, solute transport dynamics through the disc matrix, and the effects of dynamic niche conditions on cell viability in culture systems55,56,65,66. However, a series of recent publications showed how hypoxic signaling and HIF-1 function control the complex metabolic systems in the disc. In the following sections, we summarize how HIF-1α regulates the overall biosynthetic capacity of NP cells by modulating both glycolytic and mitochondrial metabolism.
Regulation of Glycolysis and TCA Cycle
NP cells generate ~75% of their ATP through glycolysis30. HIF-1α regulates glycolytic flux through the transcriptional regulation of glucose transporters and glycolytic enzymes29,30,67,68. In contrast, the mechanisms by which flux through the TCA cycle is controlled in the hypoxic NP, or why this cycle is important for cell survival, is still unresolved. Two milestone studies recently explored the complex interplay between oxygen availability, HIF-1 function, and metabolic flux in NP cells12,13. Hypoxia increases the overall concentration of glycolytic pathway intermediates and decreases the concentration of TCA cycle intermediates in NP cells12. These studies suggest that HIF-1α regulates these specific reactions in novel ways. For example, loss of HIF-1α in hypoxia leads to an increase in the concentration of initial glycolytic intermediates, glucose and glucose-6-phosphate, but a decrease in middle- and late-stage glycolytic intermediates, DHAP and pyruvate12. Surprisingly, the concentration of the TCA intermediates citrate, succinate, fumarate, malate, and oxaloacetate were also reduced, suggesting that although hypoxia downregulates the TCA cycle, HIF-1α either directly or indirectly maintains TCA cycle flux. This leads to the question of whether the decreased flux through glycolysis in HIF-1α silenced cells reduces TCA cycle flux, or if HIF-1α dysregulates specific reactions within these linked pathways. It turns out, loss of HIF-1α promotes the redirection of TCA flux towards glutamate production, through glutamate dehydrogenase (GDH). When reconciled with metabolic profiling data, it is clear that increased flux to glutamate is geared to maintain the NAD+/NADH redox ratio in HIF-1α silenced cells, and as a result there is reduced flux to succinate, effectively slowing the generation of 4-carbon intermediates of the TCA cycle. Accordingly, HIF-1α regulates TCA cycle flux, in addition to glycolytic flux, in order to maintain redox homeostasis in NP cells.
How is it possible to reconcile the observation that HIF-1α positively regulates pyruvate entry into the TCA cycle in hypoxic NP cells, with the observation that HIF trans-activates pyruvate dehydrogenase kinase 1 (PDK1) in other cell types69. PDK1 inactivates pyruvate dehydrogenase (PDH), effectively promoting the reduction of pyruvate to lactate rather than oxidation to acetyl-CoA. In fact, HIF regulation of PDK1 has been considered a “metabolic switch” towards glycolytic metabolism that is unique to hypoxic cells. Perhaps, in NP cells, glycolytic metabolism is only suitable for the generation of ATP. The TCA cycle, on the other hand, may be required for the generation of anabolic intermediates needed for critical protein, lipoprotein, and proteoglycan synthesis21,70,71. If this is the case, then a strict metabolic switch blocking pyruvate entry into the TCA cycle would be detrimental to NP cell survival.
Regulation of the Mitochondrial ETC
As was discussed earlier, there has also been some controversy regarding the contribution of mitochondria to biosynthetic flux and ATP production in NP cells. While HIF-1α positively regulates TCA flux, the question was raised concerning its role in mitochondrial respiration and ETC function. Initial studies of mitochondrial activity were performed under normoxic conditions in order to assess whether NP cell mitochondria had functional ETC in the presence of available O213. Silencing HIF-1α in NP cells under normoxic culture conditions significantly decreased the extracellular acidification rate (ECAR), however, this was reversed by blocking mitochondrial ETC function with antimycin A13. HIF-1α silencing also increased mitochondrial oxygen consumption rate (OCR) in culture conditions where oxygen was readily available. Under these conditions, mitochondrial OCR increased in a dose-dependent manner when treated with mitochondrial uncoupler, FCCP, ranging from 400 to 1000 nM. Normoxic culture conditions are not physiological, nonetheless, these results show that NP cells possess sufficient metabolic plasticity, are capable of oxidative metabolism, and have reserve cytochrome capacity that can be tapped into under specific circumstances, such as when HIF-1α signaling is compromised. It is important to note that neither sustained O2 availability nor re-oxygenation after hypoxic culture are sufficient to upregulate OCR in NP cells when HIF-1α is expressed12,13.
How can we reconcile that HIF-1α simultaneously upregulates TCA cycle flux and down-regulates ETC function in mitochondria? A recent study by Madhu et al. showed that hypoxia decreases the expression of ETC complexes and cytochrome C in NP cells12. It is also conceivable that while the TCA cycle is required to generate metabolic intermediates, including CO2, and to maintain redox balance in hypoxic NP cells, the O2-dependent flow of electrons through the cytochromes may be redundant if ATP generation through glycolysis is sufficient.
We can learn about NP cell metabolism from insights of other glycolytic cells. In fact, it is known that mitochondria are uncoupled in hypoxic growth plate chondrocytes. This uncoupled state is mediated by a HIF-1α-dependent protonophore, mitochondrial uncoupling protein 3 (UCP3), modulating ATP synthesis by facilitating H+ transport across the inner mitochondrial membrane72. In chondrocytes, mitochondrial uncoupling is necessary to limit O2 utilization and maintain mitochondrial membrane potential and autophagic flux, rather than for the common physiologic role of thermogenesis. Supporting these ideas, Yao and colleagues recently demonstrated that HIF-1α suppresses mitochondrial respiration in the developing growth plate to prevent anoxia16. The findings support the conclusion that mitochondrial respiration is detrimental to fetal chondrocytes and HIF-1α signaling is protective during development. Accordingly, future studies into the relationship between TCA cycle and ETC are warranted in NP cells, as the two mitochondrial pathways may be similarly uncoupled.
Regulation of Lactate/H+ Efflux
It is undisputed that HIF-1α controls glycolytic flux through the transcriptional activation of many enzymes at the beginning, middle, and late stages of glycolysis. However, Silagi and colleagues made the observation that HIF-1α regulates glycolytic flux, in part, by maintaining H+/lactate efflux from NP cells at the final stage of glycolysis11. Specifically, HIF-1α upregulates the SLC16A3 gene, encoding the coupled H+/lactate transporter, MCT4. The MCTs are a family of 14 lactate, pyruvate, and ketone body transporters with distinct tissue-specific function and localization73,74. It is thought that MCT4 is adapted for lactate efflux in glycolytic cells due to the relatively low Km for L-lactate (28 mM) over pyruvate (150 mM) avoiding pyruvate export from the cell and maintaining cytosolic redox 75,76. The hypoxic induction of SLC16A3 transcription is mediated by HIF-1 binding to an intronic enhancer in NP cells, rather than to a previously reported HRE in the proximal promoter77. Strikingly, acute inhibition of MCT4 down-regulated glycolysis and increased TCA cycle flux- essentially rewiring NP cell metabolism. Such a metabolic switch was particularly striking as MCT4, unlike HIF-1α, is not a transcription factor or a major regulator of glycolytic metabolism. This raised two distinct possibilities. First, by oxidizing lactate into pyruvate, NP cells may prevent cytotoxic acidosis from intracellular H+/lactate. Second, reducing equivalents generated from the increased TCA cycle flux and glutamate production may be required to maintain the redox state, which is disturbed by feed-back inhibition of lactate on LDH activity. The fact that acute MCT inhibition does not alter the NAD+/NADH ratio suggests this may be the case.
We propose that the metabolic plasticity of NP cells enables them to withstand short-term MCT inhibition by upregulating TCA cycle flux and maintaining redox ratios; however, it is evident that long-term inhibition of MCT4 compromised NP cell viability likely due to cytosolic acidification and failure to maintain high TCA flux. MCT4 silencing in vivo correlated with decreased nucleosome assembly and epigenetic programming in degenerated NP tissue (See Box 2). Studies clearly showed that loss of MCT4 in mice recapitulates the major pathoanatomical hallmarks of human disc degeneration, including loss of cellular phenotypic markers and matrix integrity78,79. Based on results of in vivo studies, we hypothesize that loss of MCT4 expression contributes in a significant manner to the cascade of events directly linked to human disc degeneration.
Lactate as a Signaling Molecule
In addition to controlling metabolic flux and intracellular pH, high lactate levels act as a hypoxia mimetic factor by instigating the biosynthesis of TCA cycle intermediates that functionally compete with the TCA-cycle intermediate and cofactor, α-ketoglutarate, that is necessary for HIF-1α hydroxylation and degradation by PHDs 80,81. Mechanistically, hydroxylation of HIF-1α is catalyzed by Fe(II)-dependent PHD dioxygenases, which use O2 and α-ketoglutarate as substrates for HIF hydroxylation in a reaction that produces CO2 and succinate 6. The PHDs have a tight affinity for α-ketoglutarate, with a Km of 1–2 μM for PHD1/2 and 12 μM for PHD382. However, PHD hydroxylase function can be competitively inhibited by TCA-cycle intermediates succinate and fumarate with Ki values of 50–80 μM and 350–460 μM, respectively82. Crystallographic studies demonstrate that succinate and fumarate competitively inhibit α-ketoglutarate-dependent dioxygenases by directly binding to Lys-214 and Tyr-145 residues in the substrate binding pocket 83. Binding of similar substrates to this site, such as succinate and fumarate, blocks the necessary ligation of 2-oxo from α-ketoglutarate to the Asp-201 residue in the active site, followed by oxidative decarboxylation and succinate formation 83. Other studies have shown that both lactate and pyruvate are capable of inhibiting PHD activity as well. Some reports suggest that the conversion of lactate to pyruvate by LDHs increases the concentration of TCA cycle intermediates, succinate and fumarate, which in turn inhibit PHD activity 81. It is also possible that pyruvate may also function as a competitive structural mimic of α-ketoglutarate and is capable of blocking PHD function independent of its metabolism into TCA cycle intermediates 84.
Studies in NP cells show a dynamic relationship between metabolic flux and HIF-1α activity is mediated by cytosolic lactate levels 11. On one hand, intracellular lactate accumulation increases HIF-1α activity, on the other hand, MCT4 transcriptional-activation is regulated through a newly discovered HIF-1-sensitive intronic enhancer. This observation suggests that a positive feedback loop exists between hypoxia-inducible MCT4 function and HIF-1α stability in NP cells, and that this loop is modulated by intracellular lactate levels.
Hypoxic Regulation of Intracellular pH
A consequence of glycolysis in hypoxic tissues is lactate accumulation and acidosis, unless pH is properly maintained. In NP cells, it has been shown that acidic pH (~6.5) exacerbates the breakdown of extracellular matrix proteins and decreases glycolytic flux56,71,85,86. As a result, glycolytic cells recruit a robust network of enzymes for intracellular pH regulation, many of which are controlled by HIF-1α87. One mechanism to regulate intracellular pH is mediated by proton extrusion: NP cells express both Na+/H+ exchangers (NHEs) and H+-ATPases (V-ATPases). Recent work by Silagi et al. has expanded our knowledge of pH control by NP cells in relationship to metabolism to include HIF-1α dependent CO2/HCO3- recycling by carbonic anhydrase (CA)9/1213 (Fig. 2).
Figure 2. HIF-1α -dependent metabolic and pH regulatory pathways in NP cells.
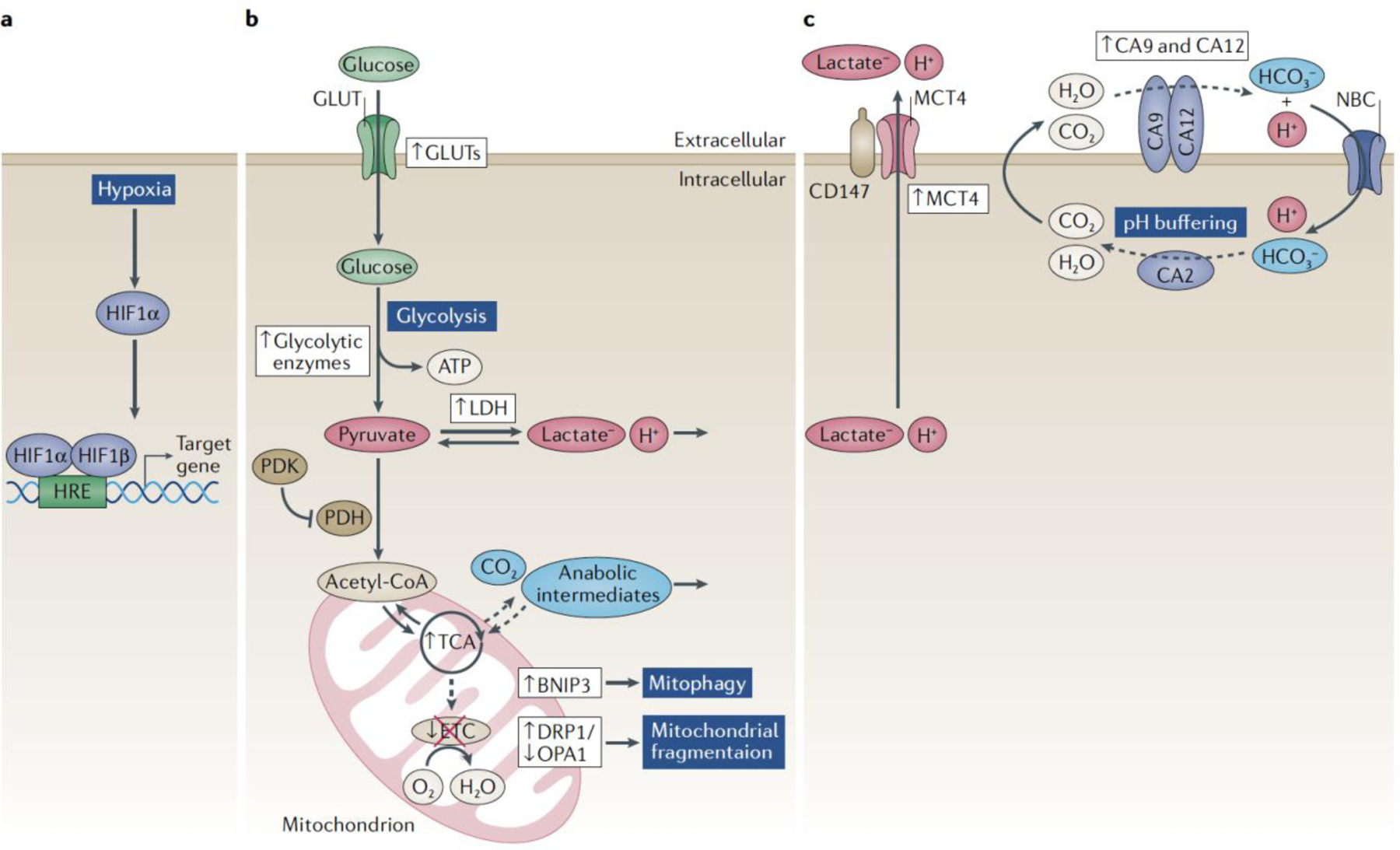
In the hypoxic NP cell, HIF-1α transcriptionally regulates many genes involved with glycolysis and pH regulation; HIF targets are shown in violet boxes, arrows denote up- or down- regulation. HIF-1α promotes glycolytic flux and lactate generation by controlling glucose import through GLUT1 and upregulating glycolytic enzymes. MCT4 facilitates the export of H+/lactate, in order to maintain intracellular pH and the perpetuation of pyruvate reduction. HIF-1α also modulates pyruvate entry into the mitochondrial TCA cycle through PDH-PDK1 axis, an area ripe for future investigations in disc cells. Although TCA cycle function is preserved in the NP, mitochondrial ETC is inhibited by hypoxia; arrows denote up- or down-regulation of the pathways. In order to maintain healthy mitochondrial activity, hypoxia and HIF-1α modulates autophagic and mitophagic pathways; HIF-targets shown in green boxes; arrows denote up- or down-regulation. Overall, to tightly control the intracellular pH in glycolytic NP cells, HIF-1α orchestrates a HCO3- buffering system, governed by CA9/12 and NBCs, and fueled by recycled and TCA-cycle derived CO2.
The CAs are a family of 16 proteins which catalyze the reversible hydration of CO2 to produce equal parts HCO3- and H+ units at an exceedingly efficient rate of up to 106 s−1 at 37oC 88. However, the specific function of the different CA isoforms is not redundant; their functional relevance is determined by cellular localization and the directionality of the enzymatic reaction i.e. favoring production of HCO3-/H+ or CO2/H2O. In fact, contrary to the general understanding, rates of CO2 hydration by CAs are substantial enough to contribute up to 100% of total extracellular proton production, a metric often erroneously associated solely with glycolytic H+/lactate production89.
In NP cells, HIF-1α binds to the conserved HREs closest to the transcriptional start sites on Car9 and Car12 promoters and induces their expression under hypoxia13. Mechanistically, these studies demonstrated that CA9/12 catalyze the hydration of CO2 (recycled and/or generated by TCA cycle) to HCO3- and H+ ions in the pericellular space of NP cells13. The extracellularly produced HCO3- ions are shuttled into the cytosol by sodium-bicarbonate cotransporters (NBCs) in order to buffer intracellular pH, in a reaction that regenerates CO2 and H2O inside the NP cell via the ubiquitously expressed CA2 isoform. This mechanism of pH regulation is dubbed the “bicarbonate transport metabolon”90. Furthermore, inhibition of CA9/12 results in a striking decrease in extracellular H+ production. However, CA inhibition has no effect on select pathways regulating or regulated by glycolytic flux; i.e. CA inhibition did not alter extracellular lactate concentrations, HIF-1α activity, or MCT4 levels. If such a high concentration of extracellular H+ units is directly generated by the CA9/12 reaction, then what is there fate? We hypothesize that protons will transit the pericellular space and diffuse out of the disc, or alternatively be used as a form of energy currency. That is, extracellularly facing CA9/12 may act as H+ donors for nearby membrane associated co-transporters (such as MCTs) that also function in the complex network of pH sensors91,92.
An additional nuance was added to this system by Pan et al. who discovered that CA12 expression was simultaneously controlled by the RNA-binding protein, HuR. This discovery established an additional mechanism to explain how CA12 maintains intracellular pH levels in NP cells. Despite that HIF-1α mRNA is an HuR binding target,14 HuR upregulates CA12 expression independently of HIF-1α signaling. Importantly, CA12 expression is upregulated in degenerated human discs through a HIF-1α-PHD-dependent mechanism93. This suggests that perhaps CA12 expression is increased to compensate for lost enzymatic activity, or that CA12 is simply required to resist further acidification in degenerated discs.
In addition to CAs, our studies demonstrate that MCT4 is responsible for the facilitated co-transport of H+/lactate out of the NP cell in order to maintain intracellular pH11. While CAs buffer pH via a HCO3- transport metabolon that involves multiple proteins and available CO2 and HCO3- stores, MCT4 simply expedites H+ extrusion. This mechanism of H+ extrusion is similar to the method of pH regulation by the transporters identified in NP cells by Urban and colleagues, the Na+/H+ exchangers and H+-ATPases 94. Furthermore, acute inhibition of MCT4 function does not affect intracellular pH in NP cells to the same extent as inhibition of CA9/12, possibly due to rapid utilization of lactate by the TCA cycle11. Accordingly, as MCT4 regulates intracellular pH in a manner that involves a critical metabolite, lactate, its expression alters NP cell metabolism in a way that is not observed with CA9/12.
Metabolism in the Aging Disc
Aging is one of the important risk factors for disc degeneration and affects NP and AF cell bioenergetics95. Hartman et al. have reported that with age, NP cells lose both glycolytic and mitochondrial function evidenced by decreased glycolytic and mitochondrial reserve capacity, and maximum aerobic capacity95. These decreases correlate with loss of matrix synthesis- an energy demanding process. On the other hand, aging does not change mitochondrial respiration in AF cells, however it does cause an increase in glycolytic flux. In addition to these mechanistic findings, a recent study by Novais et al. demonstrates a strong clinical link between cellular metabolism and disc degeneration96. Their work clearly shows that many aspects of metabolism, including glucose homeostasis, carbohydrate homeostasis, lipid metabolism, and phosphate metabolic processes are modulated in two separate aging mouse models. Taken together, these studies show that, HIF-1α aside, the straight-forward study of metabolic pathways and processes may further illuminate our understanding of the aging disc.
HIF/Hypoxia in Tissue Regeneration
Earlier studies lend strong support to the long-standing hypothesis that cells of the NP and cartilage have adapted to their adverse hypoxic, acidic, and nutrient-limiting environments. In vitro studies confirm that the NP niche-conditions lead to a reduction in stem and progenitor cell proliferation, promote chondrogenic differentiation, decrease matrix biosynthesis, and enhance cell death 97,98. As discussed previously, the limited nutrient supply in the NP compartment is a result of tissue avascularity, and thus sole reliance on diffusion for metabolite transport and maintenance of cellular metabolism, impacts cell survival in degenerating discs55. With this in mind, researchers must acknowledge the importance of survival factors when developing strategies for disc and cartilage regeneration using biological therapies. Three commonly investigated regenerative therapies include the implantation of differentiated cells or stem-cells to produce healthy matrix molecules99, the implantation of whole, tissue-engineered scaffolds seeded with cells100,101, or altering the activity of degenerated cells using gene therapy or intradiscal injection of therapeutic growth factors102,103. Cumulatively, these strategies rely on the fact that the implanted or regenerated cells survive and remain biosynthetically active despite diminished solute uptake through degenerated CEPs104. A recent study from the Dolor et al. demonstrates utility of improving nutrient diffusion into human discs by treating the CEPs with MMP8 105. However, the concentration of advanced glycation end-products in the CEP significantly affects the efficacy of matrix perturbation with MMPs and the subsequent capacity for nutrient uptake into the disc.
Considering that the native progenitor cells are scarce in the disc and cartilage and declines with age106, researchers have differentiated human pluripotent stem cells into notochord-like and NP-like cells, often leveraging hypoxic culture conditions to prime cell metabolism or push them towards a desired lineage101,107,108. However, the likelihood that these new cells survive is dependent on the maintenance of the local nutrient supply. For example, implantation of highly active stem cells or growth factors that increase rates of biosynthesis and proliferation may be counterproductive in that they alter the delicate nutrient-metabolite balance in the already degenerated tissues 101. Therefore, pairing therapeutic approaches with inhibitors of cytokine production, that are known to increase nutrient consumption may be useful 24,109. In addition to biological therapies, the use of pharmacological PHD and HIF inhibitors that are currently being evaluated in phase II and III clinical trials for the treatment of anemia and hypoxic cancers may be of value for the regeneration of both intervertebral disc and cartilage tissue (See Box 3).
Conclusion
The work presented provides an explanation of mechanisms by which cells adapted to the loss of vascularity and low oxygen tension within the disc. Adaption is mediated by the transcription factor HIF-1 which is involved with more than simply energy metabolism. By adjusting cellular energy metabolism to the low oxygen tension, HIF-1 controls, directly or indirectly, intracellular pH and cellular processes that range from matrix biosynthesis to epigenetic programming (Fig. 3). The impact of dysregulation of HIF signaling on downstream targets controlling cellular metabolism is key to understanding the pathogenesis of skeletal diseases and disorders, including but not limited to degenerative disc disease and OA.
Figure 3. Pathological link between loss of HIF-1α function and intervertebral disc degeneration.
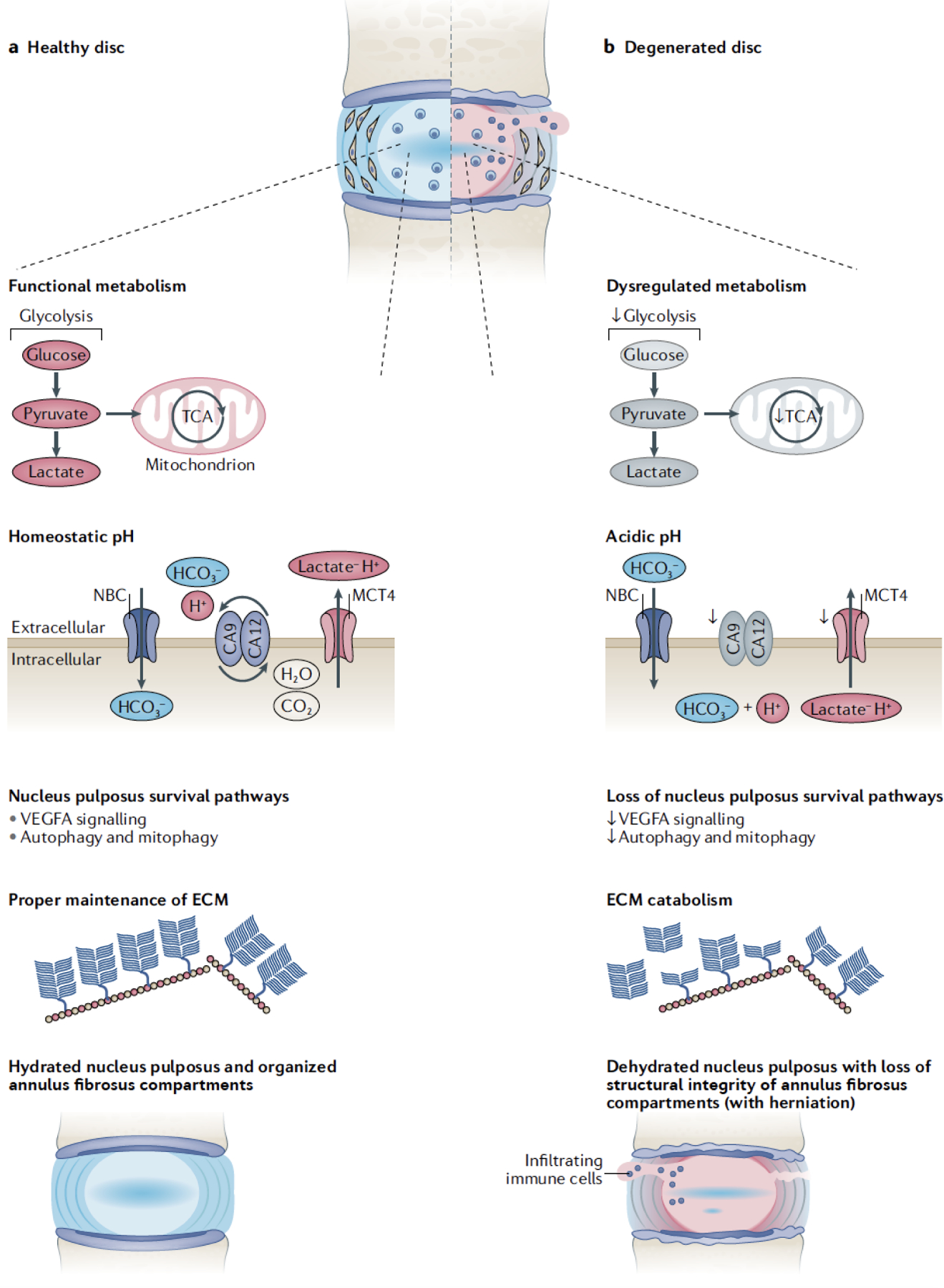
A) Schematic of a healthy intervertebral disc. Healthy NP cells are characterized by functional glycolytic and TCA cycle flux. They possess multiple pathways to buffer intracellular H+ production and maintain homeostatic pHi, including H+/lactate extrusion by MCT4 and HCO3- buffering by the CA9/CA12/NBC axis. Functional NP tissue compartments are maintained by hypoxia and HIF-dependent survival pathways- i.e. VEGFA signaling, autophagy, and mitophagy. Healthy NP tissue possess a chondroitin-sulfate proteoglycan-rich ECM which are responsible for the disc’s biomechanical function. B) Degenerated intervertebral discs. This phenotype recapitulates the fate of discs lacking HIF function and activity. Loss of HIF-1α signaling diminishes target gene expression required for cell metabolism and intracellular pH buffering. Dysregulation of the critical NP cell survival pathways and acidosis results in NP cell death and increased matrix breakdown. Compromised ECM and diminished biomechanical function makes the tissue susceptible to herniations, immune cell activation and pain.
Key Points.
HIF-1/2 are uniquely regulated by both oxygen-dependent and oxygen-independent mechanisms involving PHDs and circadian clock genes.
Disc cells possess functional mitochondria and, in NP cells, mitochondria undergo HIF-dependent mitophagy and fragmentation regulated by BNIP3 and the DRP1-OPA1 axis.
HIF-1 maintains glycolytic and TCA cycle flux while simultaneously inhibiting oxidative phosphorylation in NP cells.
HIF-1 controls intracellular H+/lactate levels via MCT4. Conversely, accumulated lactate is capable of stabilizing HIF protein by inhibiting PHD function as well as controlling transcriptional programs via histone lactylation.
In addition to the well-studied H+-extrusion mechanisms, intracellular pH in NP cells is maintained by a HIF-dependent bicarbonate buffering mechanism controlled by CA9/12, NBCs, and CO2.
Loss of control of HIF-1 and HIF-dependent metabolic pathways involving PHD3, MCT4, and CA12 lead to intervertebral disc degeneration, while loss of HIF-2 function is implicated in OA.
Footnotes
Competing Interests: None to disclose for all authors.
References
- 1.Kaelin WG The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nature Reviews Cancer vol. 8 865–873 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Shen C & Kaelin WG The VHL/HIF axis in clear cell renal carcinoma. Seminars in Cancer Biology vol. 23 18–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schödel J & Ratcliffe PJ Mechanisms of hypoxia signalling: new implications for nephrology. Nature Reviews Nephrology vol. 15 641–659 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schito L & Semenza GL Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends in Cancer vol. 2 758–770 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Yang M, Su H, Soga T, Kranc KR & Pollard PJ Prolyl hydroxylase domain enzymes: important regulators of cancer metabolism. Hypoxia (Auckland, N.Z.) 2, 127–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengler VL, Galbraith MD & Espinosa JM Transcriptional regulation by hypoxia inducible factors. Critical Reviews in Biochemistry and Molecular Biology vol. 49 1–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratcliffe PJ HIF-1 and HIF-2: Working alone or together in hypoxia? Journal of Clinical Investigation vol. 117 862–865 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downes NL, Laham-Karam N, Kaikkonen MU & Ylä-Herttuala S Differential but Complementary HIF1α and HIF2α Transcriptional Regulation. Mol. Ther 26, 1735–1745 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talks KL et al. The expression and distribution of the hypoxia-inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol 157, 411–421 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silagi ES et al. Lactate Efflux From Intervertebral Disc Cells Is Required for Maintenance of Spine Health. J. Bone Miner. Res 35, 550–570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhu V et al. Hypoxic regulation of mitochondrial metabolism and mitophagy in nucleus pulposus cells is dependent on HIF‐1α ‐BNIP3 axis. J. Bone Miner. Res (2020) doi: 10.1002/jbmr.4019. [DOI] [PMC free article] [PubMed]
- 13.Silagi ES et al. Bicarbonate Recycling by HIF-1-Dependent Carbonic Anhydrase Isoforms 9 and 12 Is Critical in Maintaining Intracellular pH and Viability of Nucleus Pulposus Cells. J. Bone Miner. Res 33, 338–355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan H et al. RNA binding protein HuR regulates extracellular matrix gene expression and pH homeostasis independent of controlling HIF-1α signaling in nucleus pulposus cells. Matrix Biol 77, 23–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi H et al. Hypoxia promotes noncanonical autophagy in nucleus pulposus cells independent of MTOR and HIF1A signaling. Autophagy 12, 1631–1646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao Q et al. Suppressing Mitochondrial Respiration Is Critical for Hypoxia Tolerance in the Fetal Growth Plate. Dev. Cell 49, 748–763 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S et al. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med 16, 687–694 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Saito T et al. Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat. Med 16, 678–687 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Murray CJL The State of US Health, 1990–2010. JAMA 310, 591 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H et al. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol 70, 102–122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silagi ES, Shapiro IM & Risbud MV Glycosaminoglycan synthesis in the nucleus pulposus: Dysregulation and the pathogenesis of disc degeneration. Matrix Biology 71–72, 368–379 (2018). [DOI] [PMC free article] [PubMed]
- 22.Le Maitre CL, Pockert AP, Buttle DJ, Freemont AJ & Hoyland JA Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans 35, 652–5 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Gorth DJ, Shapiro IM & Risbud MV Transgenic mice overexpressing human TNF-α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis 10, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risbud MV & Shapiro IM Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol 10, 44–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachemson A Intradiscal Measurements of pH in Patients with Lumbar Rhizopathies. Acta Orthop. Scand 40, 23–42 (1969). [DOI] [PubMed] [Google Scholar]
- 26.Lin WP et al. Polymorphism in the Hypoxia-Inducible Factor 1alpha Gene May Confer Susceptibility to LDD in Chinese Cohort. PLoS One 8, 2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tessier S et al. TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression. Matrix Biol 87, 94–111 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudert M & Tillmann B Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop. Scand 64, 37 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Risbud MV et al. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J. Cell. Biochem 98, 152–159 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Agrawal A et al. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am. J. Physiol. Cell Physiol 293, C621–C631 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Fujita N, Chiba K, Shapiro IM & Risbud MV HIF-1alpha and HIF-2alpha degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J Bone Min. Res 27, 401–412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suyama K et al. Circadian factors BMAL1 and RORα control HIF-1α transcriptional activity in nucleus pulposus cells: Implications in maintenance of intervertebral disc health. Oncotarget 7, 23056–71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudek M et al. The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. Ann. Rheum. Dis 76, 576–584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita N et al. Expression of Prolyl Hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: Distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia. J. Biol. Chem 287, 16975–16986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thoms BL & Murphy CL Inhibition of hypoxia-inducible factor-targeting Prolyl Hydroxylase Domain-containing Protein 2 (PHD2) enhances matrix synthesis by human chondrocytes. J. Biol. Chem 285, 20472–20480 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoepflin ZR, Silagi ES, Shapiro IM & Risbud MV PHD3 is a transcriptional coactivator of HIF-1a in nucleus pulposus cells independent of the PKM2-JMJD5 axis. FASEB J 31, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HJ et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc. Natl. Acad. Sci. U. S. A 111, 279–284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo W et al. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but not HIF-2α. J. Biol. Chem 285, 3651–3663 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogate SS, Fujita N, Skubutyte R, Shapiro IM & Risbud MV Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: Role of Hsp70 in HIF-1α degradation. J. Bone Miner. Res 27, 1106–1117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoepflin ZR, Shapiro IM & Risbud MV Class I and IIa HDACs Mediate HIF-1α Stability through PHD2-Dependent Mechanism while HDAC6, a Class IIb Member, Promotes HIF-1α Transcriptional Activity in Nucleus Pulposus Cells of the Intervertebral Disc. J. Bone Miner. Res 31, 1287–99 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran CM et al. Hypoxia-inducible factor (HIF)-1α and CCN2 form a regulatory circuit in hypoxic nucleus pulposus cells: CCN2 suppresses HIF-1α level and transcriptional activity. J. Biol. Chem 288, 12654–12666 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lando D et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 16, 1466–1471 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirose Y et al. FIH-1-Mint3 axis does not control HIF-1a transcriptional activity in nucleus pulposus cells. J. Biol. Chem 289, 20594–20605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang N et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab 11, 364–378 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berenbaum F & Meng QJ The brain-joint axis in osteoarthritis: Nerves, circadian clocks and beyond. Nature Reviews Rheumatology vol. 12 508–516 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Kanbe K, Inoue K, Xiang C & Chen Q Identification of clock as a mechanosensitive gene by large-scale DNA microarray analysis: Downregulation in osteoarthritic cartilage. Mod. Rheumatol 16, 131–136 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi M et al. A circadian clock gene, PER2, activates HIF-1 as an effector molecule for recruitment of HIF-1α to promoter regions of its downstream genes. FEBS J 284, 3804–3816 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Wu Y et al. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab 25, 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Manella G et al. Hypoxia induces a time- And tissue-specific response that elicits intertissue circadian clock misalignment. Proc. Natl. Acad. Sci. U. S. A 117, 779–786 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bass J Circadian topology of metabolism. Nature vol. 491 348–356 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Fisch KM et al. Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthr. Cartil 26, 1531–1538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudek M et al. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Invest 126, 365–376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grunhagen T, Shirazi-Adl A, Fairbank JCT & Urban JPG Intervertebral Disk Nutrition: A Review of Factors Influencing Concentrations of Nutrients and Metabolites. Orthop. Clin. North Am 42, 465–477 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Huang YC, Urban JPG & Luk KDK Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol 10, 561–566 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Bibby SRS, Jones D.a , Ripley RM. & Urban JPG. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila. Pa. 1976) 30, 487–496 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Merceron C et al. Loss of HIF-1alpha in the notochord results in cell death and complete disappearance of the nucleus pulposus. PLoS One 9, e110768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu WJ et al. SHH-dependent knockout of HIF-1 alpha accelerates the degenerative process in mouse intervertebral disc. Int. J. Immunopathol. Pharmacol 26, 601–609 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y et al. Early onset of disc degeneration in SM/J mice is associated with changes in ion transport systems and fibrotic events. Matrix Biol 70, 123–139 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Fujita N et al. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem. Biophys. Res. Commun 372, 367–372 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Madhu V, Guntur AR & Risbud MV Role of autophagy in intervertebral disc and cartilage function: implications in health and disease. Matrix Biol (2020) doi: 10.1016/j.matbio.2020.12.002. [DOI] [PMC free article] [PubMed]
- 62.Hu S et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis 11, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazure NM Atypical BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia Metabolic Pathways and Redox Homeostasis in Cancer View project (2009) doi: 10.4161/auto.9042. [DOI] [PubMed]
- 64.Novais EJ et al. Hypoxia and Hypoxia-Inducible Factor-1α Regulate Endoplasmic Reticulum Stress in Nucleus Pulposus Cells. Am. J. Pathol (2020) doi: 10.1016/j.ajpath.2020.11.012. [DOI] [PMC free article] [PubMed]
- 65.Bibby SRS & Urban JPG Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J 13, 694–701 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartels EM, Fairbank JC, Winlove CP & Urban JP Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila. Pa. 1976) 23, 1–7; discussion 8 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Richardson SM, Knowles R, Tyler J, Mobasheri A & Hoyland JA Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1alpha in normal and degenerate human intervertebral disc. Histochem. Cell Biol 129, 503–11 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Papandreou I, Cairns RA, Fontana L, Lim AL & Denko NC HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3, 187–97 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Kim JW, Tchernyshyov I, Semenza GL & Dang CV HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3, 177–185 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Ishihara H & Urban JP Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J. Orthop. Res 17, 829–35 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Ohshima H & Urban JP The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine vol. 17 1079–82 (1992). [DOI] [PubMed] [Google Scholar]
- 72.Watanabe H, Bohensky J, Freeman T, Srinivas V & Shapiro IM Hypoxic induction of UCP3 in the growth plate: UCP3 suppresses chondrocyte autophagy. J. Cell. Physiol 216, 419–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adijanto J & Philp NJ The SLC16A family of monocarboxylate transporters (MCTs)-physiology and function in cellular metabolism, pH homeostasis, and fluid transport. Curr Top Membr 70, 275–311 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Philp NJ, Yoon H & Grollman EF Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am. J. Physiol. Integr. Comp. Physiol 274, R1824–8 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Dimmer KS, Friedrich B, Lang F, Deitmer JW & Bröer S The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J 350 Pt 1, 219–27 (2000). [PMC free article] [PubMed] [Google Scholar]
- 76.Halestrap AP The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 64, 1–9 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Ullah MS, Davies AJ & Halestrap AP The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J. Biol. Chem 281, 9030–9037 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Roberts S, Evans H, Trivedi J & Menage J Histology and pathology of the human intervertebral disc. J. Bone Joint Surg. Am 88 Suppl 2, 10–14 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Thompson JP et al. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila. Pa. 1976) 15, 411–5 (1990). [DOI] [PubMed] [Google Scholar]
- 80.Lu H, Forbes RA & Verma A Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem 277, 23111–23115 (2002). [DOI] [PubMed] [Google Scholar]
- 81.De Saedeleer CJ et al. Lactate Activates HIF-1 in Oxidative but Not in Warburg-Phenotype Human Tumor Cells. PLoS One 7, e46571 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koivunen P et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem 282, 4524–32 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Hewitson KS et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem 282, 3293–301 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Lu H et al. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J. Biol. Chem 280, 41928–39 (2005). [DOI] [PubMed] [Google Scholar]
- 85.Razaq S, Wilkins RJ & Urban JPG The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur. Spine J 12, 341–349 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gilbert HTJ, Hodson N, Baird P, Richardson SM & Hoyland JA Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel −3 as a potential therapeutic target. Sci. Rep 6, 37360 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deitmer JW, Theparambil SM, Ruminot I & Becker HM The role of membrane acid/base transporters and carbonic anhydrases for cellular pH and metabolic processes. Front. Neurosci 8, 430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maren TH Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol. Rev 47, 595–781 (1967). [DOI] [PubMed] [Google Scholar]
- 89.Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG & Brand MD The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta - Bioenerg 1847, 171–181 (2015). [DOI] [PubMed] [Google Scholar]
- 90.McMurtrie HL et al. The bicarbonate transport metabolon. J. Enzyme Inhib. Med. Chem 19, 231–6 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Jamali S et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep 5, 13605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stridh MH et al. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J. Physiol 590, 2333–51 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S et al. PHD/HIF-1 upregulates CA12 to protect against degenerative disc disease: a human sample, in vitro and ex vivo study. Lab. Invest (2016) doi: 10.1038/labinvest.2016.32. [DOI] [PubMed]
- 94.Razaq S, Urban JP & Wilkins RJ Regulation of intracellular pH by bovine intervertebral disc cells. Cell. Physiol. Biochem 10, 109–15 (2000). [DOI] [PubMed] [Google Scholar]
- 95.Hartman R et al. Age-dependent changes in intervertebral disc cell mitochondria and bioenergetics. Eur. Cells Mater 36, 171–183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Novais EJ et al. Comparison of inbred mouse strains shows diverse phenotypic outcomes of intervertebral disc aging. Aging Cell 19, 213148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horner HA & Urban JPG 2001 Volvo award winner in basic science studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine (Phila. Pa. 1976) 26, 2543–2549 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Huang YC, Leung VYL, Lu WW & Luk KDK The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J 13, 352–362 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Nomura T, Mochida J, Okuma M, Nishimura K & Sakabe K Nucleus pulposus allograft retards intervertebral disc degeneration. Clin. Orthop. Relat. Res 389, 94–101 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Bowles RD, Gebhard HH, Härtl R & Bonassar LJ Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc. Natl. Acad. Sci 108, 13106–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sakai D & Andersson GBJ Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol 11, 243–256 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Bae WC & Masuda K Emerging Technologies for Molecular Therapy for Intervertebral Disk Degeneration. Orthop. Clin. North Am 42, 585–601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woods BI, Vo N, Sowa G & Kang JD Gene Therapy for Intervertebral Disk Degeneration. Orthop. Clin. North Am 42, 563–574 (2011). [DOI] [PubMed] [Google Scholar]
- 104.Wong J et al. Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy. Osteoarthr. Cartil 27, 956–964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dolor A et al. Matrix modification for enhancing the transport properties of the human cartilage endplate to improve disc nutrition. PLoS One 14, e0215218–e0215218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sakai D et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun 3, 1–11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thorpe AA, Boyes VL, Sammon C & Le Maitre CL Thermally triggered injectable hydrogel, which induces mesenchymal stem cell differentiation to nucleus pulposus cells: Potential for regeneration of the intervertebral disc. Acta Biomater 36, 99–111 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep 30, 2791–2806.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 109.Stefanovic‐Racic M, Stadler J, Georgescu HI & Evans CH Nitric oxide and energy production in articular chondrocytes. J. Cell. Physiol 159, 274–280 (1994). [DOI] [PubMed] [Google Scholar]
- 110.Schipani E et al. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev 15, 2865–2876 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bouaziz W et al. Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. U. S. A 113, 5453–5458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thoms BL, Dudek KA, Lafont JE & Murphy CL Hypoxia Promotes the Production and Inhibits the Destruction of Human Articular Cartilage. Arthritis Rheum 65, 13021312 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Amarilio R et al. HIF1α regulation of Sox9 in necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development 134, 3917–3928 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Provot S et al. Hif-1α regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol 177, 451–464 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Araldi E, Khatri R, Giaccia AJ, Simon MC & Schipani E Lack of HIF-2α in limb bud mesenchyme causes a modest and transient delay of endochondral bone development. Nat. Med 17, 25–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boukouris AE, Zervopoulos SD & Michelakis ED Metabolic Enzymes Moonlighting in the Nucleus: Metabolic Regulation of Gene Transcription. Trends Biochem. Sci 41, 712–730 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Castello A, Hentze MW & Preiss T Metabolic Enzymes Enjoying New Partnerships as RNA-Binding Proteins. Trends Endocrinol. Metab 26, 746–757 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao X et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun 7, 11960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao S et al. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep 17, 1037–1052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Castonguay Z, Auger C, Thomas SC, Chahma M & Appanna VD Nuclear lactate dehydrogenase modulates histone modification in human hepatocytes. Biochem. Biophys. Res. Commun 454, 172–7 (2014). [DOI] [PubMed] [Google Scholar]
- 121.Latham T et al. Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res 40, 4794–803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang D et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fallah J & Rini BI HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep 21, 6 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Haase VH Therapeutic targeting of the HIF oxygen-sensing pathway: Lessons learned from clinical studies. Exp. Cell Res 356, 160–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernhardt WM et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J. Am. Soc. Nephrol 21, 2151–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scheuermann TH et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol 9, 271–276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Courtney KD et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2a antagonist in patients with previously treated advanced clear cell renal cell carcinoma. in Journal of Clinical Oncology vol. 36 867–874 (American Society of Clinical Oncology, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen W et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


