Abstract
Tobramycin shows synergistic antibacterial activity with colistin and can reduce the toxic effects of colistin. In this study, we developed dry powder formulations that contain colistin and tobramycin by spray drying. Two combination formulations, with 1:1 or 1:5 molar ratios of colistin and tobramycin, had fine particle fractions (FPF) of approximately 85%, which was significantly higher than that of the spray dried tobramycin (approximately 45%). FPF values of the tobramycin formulation increased significantly to approximately 60% when stored for four weeks at both 20% and 55% RH. In contrast, FPF values of the combination formulations and spray dried colistin remained stable at both humidity levels of 20% and 55% RH for four weeks. The superior aerosol performance and aerosolization stability of the combination formulations were attributed to enrichment of colistin on the co-spray dried particle surface, as supported by the XPS, SEM and surface energy data. An interesting finding was that addition of hygroscopic colistin prevented moisture-induced agglomeration of the spray dried particles at mild humidity conditions, which could be due to surfactant-like assembly of colistin molecules during spray drying leading to a relatively hydrophobic particle surface.
Keywords: dry powder inhalation, respiratory infections, combination antibiotics, storage stability
INTRODUCTION
Polymyxins are a group of last-line antibiotics against multi-drug resistant Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae [1]. Parenteral polymyxin administration has poor efficacy against lung infections caused by these bacteria mainly due to low drug concentrations at the site of infection [2–4]. Moreover, the parenteral dose of polymyxins cannot be much increased because of its dose-dependent nephrotoxicity in up to 60% patients [1, 5]. In contrast, inhalation can deliver high drug doses directly to the lungs, which increases efficacy and minimizes systemic toxicity [6–9].
In clinical studies, inhalation of polymyxins often causes coughing and bronchospasm at high pulmonary doses [7]. A recent study also showed that polymyxins may cause concentration- and time-dependent toxicity in human lung epithelial A549 cells and can activate multiple apoptosis pathways [10]. We have discovered that a combination of polymyxins and aminoglycosides (e.g. tobramycin) can reduce polymyxin toxicity to lung epithelial cells in mice [11]. In addition, combination therapies using colistin (Col) and tobramycin (Tob) have shown superior antibacterial activity compared to the monotherapy [12–15]. Herrmann et al. (2010) reported that combination therapy of colistin and tobramycin showed enhanced killing of P. aeruginosa in an in vitro biofilm model, a rat lung infection model and CF patients[12]. Kashyap et al. also discovered that combination of colistin and tobramycin inhibited A. baumannii persister cells by membrane hyperpolarization and down-regulation of efflux pumps [13]. Therefore, we aimed to develop inhalable combination formulations of polymyxins and tobramycin.
There are several methods to administer antibiotics via the pulmonary route. Antibiotic solutions or suspensions can be nebulized and inhaled by patients, which is commonly used as a complementary treatment for respiratory infections [16]. However, nebulization requires bulky equipment and long administration time, both of which are inconvenient for patients [17]. On the other hand, dry powder inhalation (DPI) is convenient for patients because the device is usually portable and easy to use. Dry powders generally have superior chemical stability than drug solutions or suspensions with the capability to deliver high drug doses [18–20]. Two major techniques are used to produce dry powder formulations: jet milling and spray drying [21]. Jet milled powders often have high surface energy and high static charge, which lead to poor dispersibility [21]. Spray dried powders can be more dispersible by engineering particles with desirable size, morphology and surface chemistry [22, 23], which have been applied to develop DPIs of colistin [24] and tobramycin [25, 26]. In addition, spray drying can incorporate two or more drugs into a single particle, which maximizes synergistic bioactivities [27–30]. In this study, we aimed to develop DPI formulations of colistin and tobramycin combination using spray drying and determine their aerosol performance and aerosolization stability during storage at mild humidity conditions.
MATERIALS AND METHODS
Materials
Colistin sulfate and tobramycin sulfate were obtained from BetaPharma Co. Ltd (Wujiang City, JiangSu Province, China). Both free base and sulfate salt of tobramycin have been previously used to developed DPI formulations [31–33] and the latter was used in this study. Acetonitrile (HPLC grade) and sodium sulfate (ACS grade) were obtained from Fisher Chemicals (Pittsburgh, PA, USA) and used for HPLC analysis. The chemicals used for inverse gas chromatography were obtained from several manufacturers: Heptane and Octane (extra pure grade) from Acros Organics (Pittsburgh, PA, USA), n-hexane and dichloromethane (Suprasolv® grade) from MilliporeSigma (Burlington, MA, USA), nonane and ethyl acetate (GC grade) from Sigma-Aldrich (St. Louis, MO, USA) and, decane and acetone from Fisher Chemicals (Pittsburgh, PA, USA).
Spray Drying
A BÜCHI B-290 spray dryer (BÜCHI Labortechnik AG, Flawil, Switzerland) with a two-fluid nozzle (diameter 0.7 mm) was used to produce powder formulations. The spray drying parameters were chosen based on previous studies [29, 34]. Feed solutions were prepared in purified water with a total solid content of 21.7 mg/mL. The feed solutions of combination formulations were obtained by mixing solutions of colistin and tobramycin at molar ratios of 1:1 and 1:5 (equivalent mass ratios of 7:3 and 3:7 respectively). These ratios were chosen because colistin and tobramycin at molar ratio 1:5 led to a significantly reduced toxicity to lung epithelial A549 cells [11]. The feed solutions were atomized into the drying chamber at 2 mL/min and dried using hot air at 120 ± 5°C that was aspirated at 35 m3/h. The air temperature at the chamber outlet was approximately 68 ± 3°C. The atomizing air flow rate was 0.7 m3/h.
Particle Size Distribution (PSD)
Particle size distribution of spray dried formulations was measured using a Malvern Mastersizer 3000 (Malvern Instruments, Worcestershire, UK). Sample feed rate was set to 50–60%, which maintained laser obscuration between 2% and 6%. Powders were dispersed by an Aero S dispersion unit using compressed air (4 bars) and passed through an optical measurement cell. The background signal was measured for 10 s and the sample signal was measured for 5 s. The software calculated percentile diameters based on volume distribution, namely D10, D50, and D90, for each sample. Span, which is a ratio of (D90 - D10) to D50, was used to measure the breadth of size distributions.
Scanning Electron Microscopy (SEM)
Particle morphology of the spray dried formulations was examined using a scanning electron microscope (NOVA nanoSEM, FEI Company, Hillsboro, Oregon, USA). A thin layer of powder sample was applied on a sample stub via an adhesive carbon tape. Excessive particles were removed using pressurized air. Then, the particles were coated with a thin platinum film by sputtering at 40 mA for 1 min (208 HR, Cressington Sputter Coater, England, UK). The coated particles were imaged using the electron microscope at 5 kV acceleration voltage.
Powder X-Ray Diffraction (PXRD)
A Rigaku Smartlab™ diffractometer (Rigaku Americas, The Woodlands, TX) with a Cu-Kα radiation source and a D/tex ultra-detector was used to measure powder X-ray diffraction pattern. The diffraction patterns were recorded over a 2θ range of 5 – 40° at a scan rate of 5°/min. The radiation source was operated at 40 kV voltage and 44 mA current.
Drug Analysis
Colistin was quantified using an established high-performance liquid chromatography (HPLC) method [35]. The analysis was performed on an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA) using an Eclipse Plus C18 column (5 μm, 150 × 4.6 mm, Agilent, Waldbronn, Germany). The column temperature was not controlled so it was similar to room temperature (~22 ± 3 °C). An absorbance wavelength of 214 nm was used to detect colistin. A solvent mixture of 76% v/v 30 mM sodium sulfate solution (adjusted to pH 2.5 with H3PO4), and 24% v/v acetonitrile was used as the mobile phase with a flow rate of 1.0 mL/min. The sample injection volume was 30 μL.
Tobramycin was quantified by HPLC using an Eclipse Plus C18 column (5 μm, 50 × 4.6 mm, Agilent, Waldbronn, Germany) [36]. The column temperature was maintained at 65°C. A 0.1M disodium tetraborate solution was adjusted to pH 9.0 using 0.1M phosphoric acid to prepare a disodium tetraborate buffer. A 20:20:60 (v/v) mixture of methanol, disodium tetraborate buffer (0.1 M, pH = 9.0), and water was prepared, and 1 g/L sodium octane sulfonate was added to make the mobile phase for tobramycin analysis. The flow rate of the mobile phase was 1.0 mL/min and the sample injection volume was 80 μL.
Content Uniformity
The content uniformity of tobramycin and colistin in the spray dried combination formulations was determined based on the United States Pharmacopeia standard on dosage form uniformity [37]. Around 10 mg of a formulation was accurately weighed and dissolved in 25 mL of Milli-Q water. The colistin and tobramycin content of the solution was assayed using HPLC. Ten replicate solutions were assayed for each formulation (n = 10). Drug content (%) was calculated using the following equation.
| Equation 1: |
Based on the United States Pharmacopeia standard [37], acceptance value () was calculated by the following equation:
| Equation 2: |
where is the reference value, is the mean drug content (%), is acceptability constant (k = 2.4 for n = 10), and is the standard deviation of drug content. If is less than 15%, the content uniformity is deemed acceptable.
X-Ray Photoelectron Spectroscopy
X-Ray Photoelectron Spectroscopy (XPS) is an analytical technique that utilizes the photoelectric effect of X-rays to characterize surface chemistry of a solid sample [38]. Based on the X-ray energy and the electron emission kinetic energy, the binding energy of an electron to its parent atom can be calculated [39]. Electron binding energy is specific to different atoms and their chemical states, which can be exploited to detect distinct compounds present on a surface [40].
We used a Kratos Axis Ultra DLD spectrometer (Kratos Analytical, Manchester, UK) with a monochromic Al Kα radiation (1486.6 eV) source to study the formulations. Two levels of pass energy (PE) were used: 20 eV for high-resolution spectra, and 160 eV for survey spectra. The instrument resolution was approximately 0.35 eV when the pass energy was 20 eV. A commercial Kratos charge neutralizer prevented inhomogeneous electric charge in non-conducting powders and helped achieve higher resolution. Powder samples were attached to a stainless-steel sample holder using a double-sided Cu tape. The instrument energy scale was calibrated using Au 4f7/2 at 84.0 eV and Cu 2p3/2 at 932.67 eV. Binding energy values refer to the Fermi edge.
Before data analysis, the C-C component of the C 1s binding energy peak was set to 284.8 eV. The XPS data was analyzed with CasaXPS software. The background was removed using the Tougaard algorithm. The model binding energy peaks of pure compounds were fitted to the sample spectra to calculate their atomic composition in the sample. The atomic composition in the near-surface region was estimated based on the corresponding Scofield atomic sensitivity factors and the inelastic mean free path of photoelectrons. The data analysis assumed a homogeneous mixture of the elements across the probed depth (~10 nm).
In vitro Aerosolization Performance
Next-Generation Impactor (NGI, Copley, Nottingham, UK) without the pre-separator was used to determine in vitro aerosolization behavior of the spray dried formulations. The dispersion of each formulation was measured using four replicates of ~20 mg each. For each measurement, two size-3 hydroxypropyl methylcellulose capsules (Qualicaps, Whitsett, NC) with 10 mg sample each, were consecutively fired through a low-resistance RS01 DPI device for dispersion (Plastiape S.p.A., Osnago, Italy). During dispersion, four liters of air was drawn through the inhaler by a vacuum pump at a flow rate of 100 L/min, which generated approximately 4 kPa pressure drop across the device. The dispersed powders were collected from the capsule, device, NGI throat, and all the NGI stages using water, and quantified using the established HPLC analysis. The percentage of recovered drug that exited the device was defined as emitted dose (ED). Fine particle fraction (FPF) was calculated as the percentage of the total recovered drug with aerodynamic particle diameters below 5 μm. The emitted fine particle fraction (E-FPF) was calculated as the percentage of emitted particles with aerodynamic diameters below 5 μm.
Dynamic Vapor Sorption
Dynamic vapor sorption instrument (DVS Intrinsic, Surface Measurement Systems Ltd., London UK) was employed to determine moisture sorption behavior of the spray dried formulations. Approximately 8 mg of each formulation was loaded on the sample pan and equilibrated at 0% RH and 25°C. The sample was then exposed to two cycles of an adsorption-desorption program: 0–90% RH with 10% steps at 25°C. The associated moisture uptake was measured from weight change. For each step, equilibration condition was set to a percent weight change less than 0.002 %/min and the maximum allowed duration was 360 min. The results were presented as a moisture sorption isotherm, which is a plot of percent moisture sorption relative to sample dry mass vs. relative humidity.
Storage Stability of Formulations
Morphological and solid-state stability of spray dried formulations were assessed over 4 weeks of storage at room temperature (~22 ± 3 °C) under two relative humidity conditions: 20 and 55%. The degree of particle agglomeration was determined using SEM imaging and particle size distribution measurement. Particle sizes were obtained at two dispersion pressures - 0.5 bar (low) and 4.0 bar (high) – to indirectly determine the strength of particle agglomeration. Powder X-ray diffraction patterns were used to determine crystallinity during storage. The effect of storage on in vitro aerosolization performance was measured using NGI.
Surface Energy Measurement by Inverse Gas Chromatography
Surface energy of the spray dried formulations was determined using Inverse Gas Chromatography (Surface Measurement Systems Ltd., London UK) at a range of finite probe concentrations [29]. To prepare the powder column, approximately 70 mg of formulation powder was filled into a pre-silanized glass column (flanked by glass wool) with 4 mm internal diameter and 300 mm length, and consolidated with gentle tapping. Helium was used as carrier gas at 25°C and at a flow rate of 10 mL/min. Before each analysis, the column was conditioned at 0% RH and 30°C for 1 hour. Vapors of various probe compounds were passed through the column at a series of concentrations (p/po = 0.05 – 0.4) and the respective retention times were used to calculate surface energy. The probes used to calculate dispersive component of surface energy were decane, nonane, octane, heptane, and hexane. Dichloromethane, ethyl acetate and acetone were used to determine the specific surface energy. A flame ionization detector determined the overall retention time. Net retention time was determined by subtracting from the overall retention time the ‘dead volume’ of the column which was measured using methane gas. The surface energy calculations were performed by the standard instrument analysis software 1.4.2.0 (SMS, London, UK). The retention time parameter was set to peak max time. Schultz method was used for dispersive surface energy calculation, and is based on a linear equation relating it to the free energy of adsorption as shown [41, 42]:
| Equation 3: |
Here is the gas constant, is the temperature, is the net retention volume, denotes the dispersive component of surface free energy of the solid, denotes the dispersive component of surface free energy of the probe molecule, denotes the area occupied by one adsorbing molecule, is the Avogadro number and is a constant. was determined from the slope of a vs. line.
The specific surface energy was calculated via Lewis acid and Lewis base components. Interaction of the column with monopolar probes dichloromethane (acidic) and ethyl acetate (basic) was studied at different coverages to determine the acid and basic components, respectively. At each coverage, the specific component of the free energy of adsorption for each polar probe was determined as the vertical deviation from the alkane line plotted for Schultz calculation of dispersive energy. The acid and base surface energies of the solid were then obtained using Good-van Oss theory [43, 44]:
| Equation 4: |
DM denotes dichloromethane, and denote specific acidic and basic surface energy, is the Avogadro number, and is the area occupied by a dichloromethane molecule. The basic component was considered 0. Inserting values of from Della Volpe Lexis acid/base scale and yielded . Similarly, was obtained using a similar equation for ethyl acetate, which was assumed to have a negligible acid surface energy. The specific surface energy was obtained using the following relation:
| Equation 5: |
The total surface energy was determined by addition of the dispersive and specific surface energies:
| Equation 6: |
Statistical Analysis
Analysis of variance (ANOVA) was performed for determining significant difference between groups (α = 0.05). For multiple comparisons, Tukey’s studentized range test was used (α = 0.05).
RESULTS
Particle Morphology and Size
Spray dried (SD) colistin particles were mainly dimpled, with some being smooth and hollow. In contrast, the SD tobramycin particles were spherical with rougher surfaces (Figure 1). The morphology of SD combination formulations was between that of SD colistin and SD tobramycin particles, and showed both dimpled and rough surfaces. The particles of all formulations appeared less than 5 μm in the SEM images. As measured by laser diffraction, the median diameters or D50 values of all SD formulations were in the range of 2.2 – 2.5 μm, and their span values were in the range of 1.7 – 2 (Table 1).
Fig. 1.
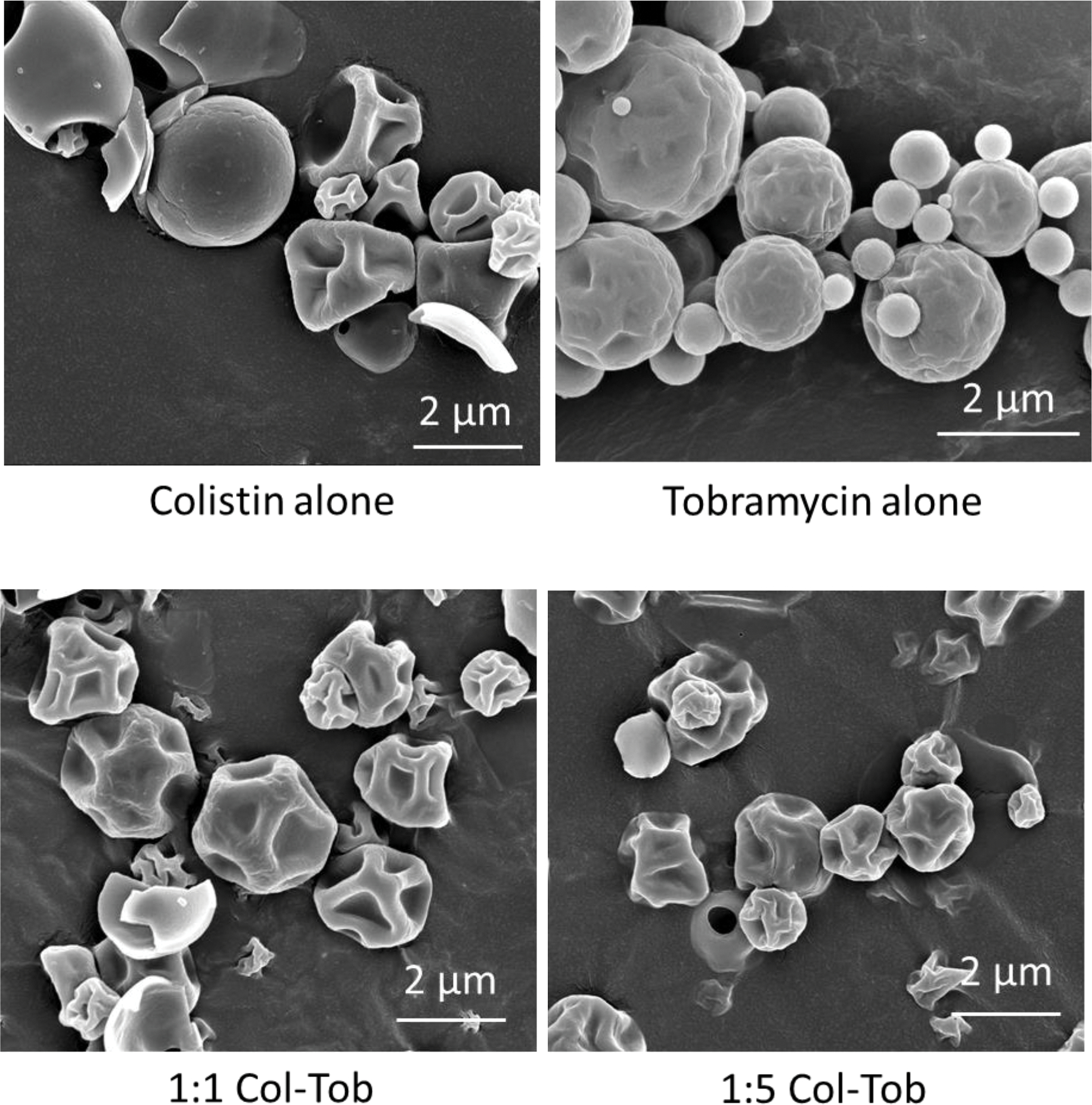
Representative scanning electron microscopy images of the spray dried formulations
Table 1.
Particle sizes of the spray dried powder formulations (n=3). Data is presented as mean ± standard deviation
| Formulation | D10 (μm) | D50 (μm) | D90 (μm) | Span |
|---|---|---|---|---|
|
| ||||
| Colistin alone | 1.2 ± 0.0 | 2.4 ± 0.0 | 5.2 ± 0.3 | 1.7 ± 0.1 |
| 1:1 Col a -Tob b | 1.1 ± 0.0 | 2.3 ± 0.0 | 5.0 ± 0.1 | 1.7 ± 0.0 |
| 1:5 Col-Tob | 1.1 ± 0.0 | 2.3 ± 0.0 | 5.4 ± 0.1 | 1.9 ± 0.0 |
| Tobramycin alone | 1.0 ± 0.0 | 2.3 ± 0.1 | 5.4 ± 0.4 | 2.0 ± 0.1 |
Col – colistin
Tob – tobramycin
Content Uniformity
For all spray dried combination formulations, the acceptance values were below 15%, which indicates acceptable content uniformity (Table 2).
Table 2.
Content uniformity of tobramycin and colistin in the spray dried combination formulations (n=10)
| Formulation | Drug | Drug content (%) | Acceptance value | |
|---|---|---|---|---|
| X c | s d | |||
|
| ||||
| 1:1 Col a -Tob b | Colistin | 99.6 | 2.6 | 6.1 |
| Tobramycin | 101.2 | 1.9 | 4.6 | |
| 1:5 Col-Tob | Colistin | 98.7 | 2.0 | 4.9 |
| Tobramycin | 99.7 | 2.4 | 5.9 | |
Col – colistin
Tob – tobramycin
X – mean drug content
s - standard deviation of drug content
Crystallinity
The patterns of all spray dried formulations exhibited no sharp peaks, which suggests a mainly amorphous state (Figure 2).
Fig. 2.
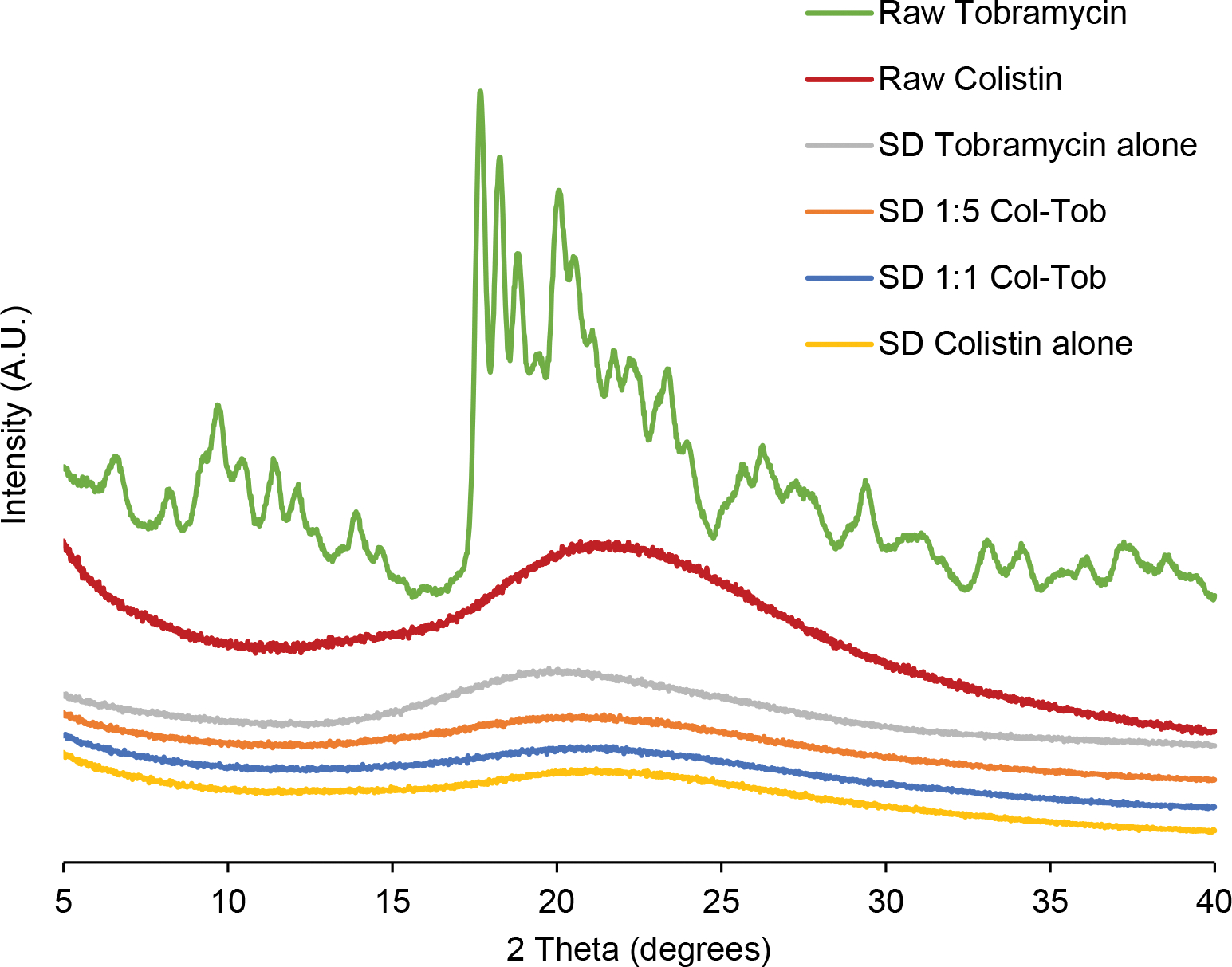
X-ray diffraction patterns of the spray dried (SD) formulations and raw drug powders
Dynamic Vapor Sorption
The SD tobramycin adsorbed more moisture than the SD colistin up to 50% relative humidity (RH) (Figure 3); SD tobramycin adsorbed 14.6% by weight moisture at 50% RH, whereas colistin adsorbed approximately 12.1%. The adsorption by SD 1:1 Col-Tob was lower than SD colistin and that by SD 1:5 Col-Tob was slightly higher up to 50% RH.
Fig. 3.
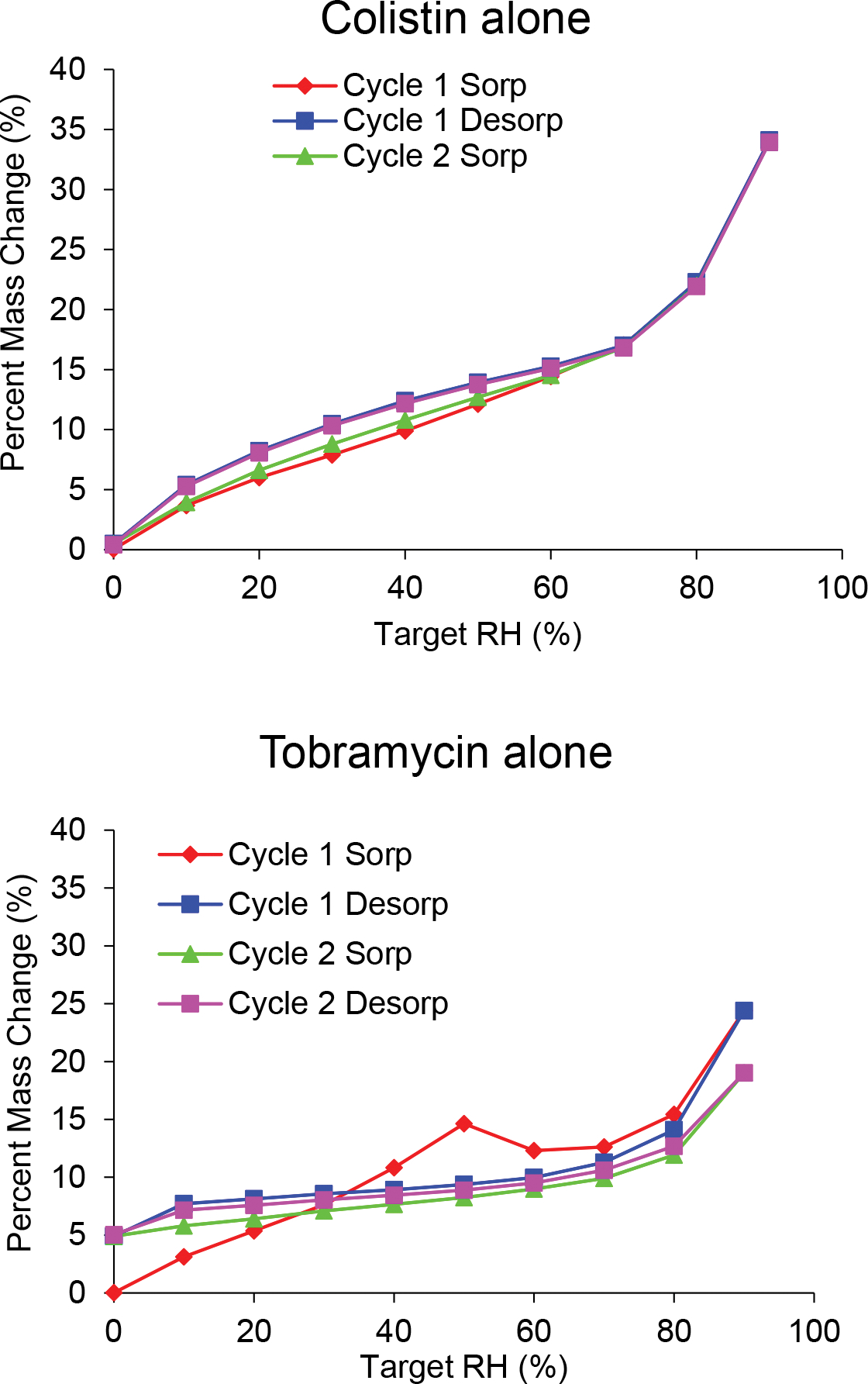
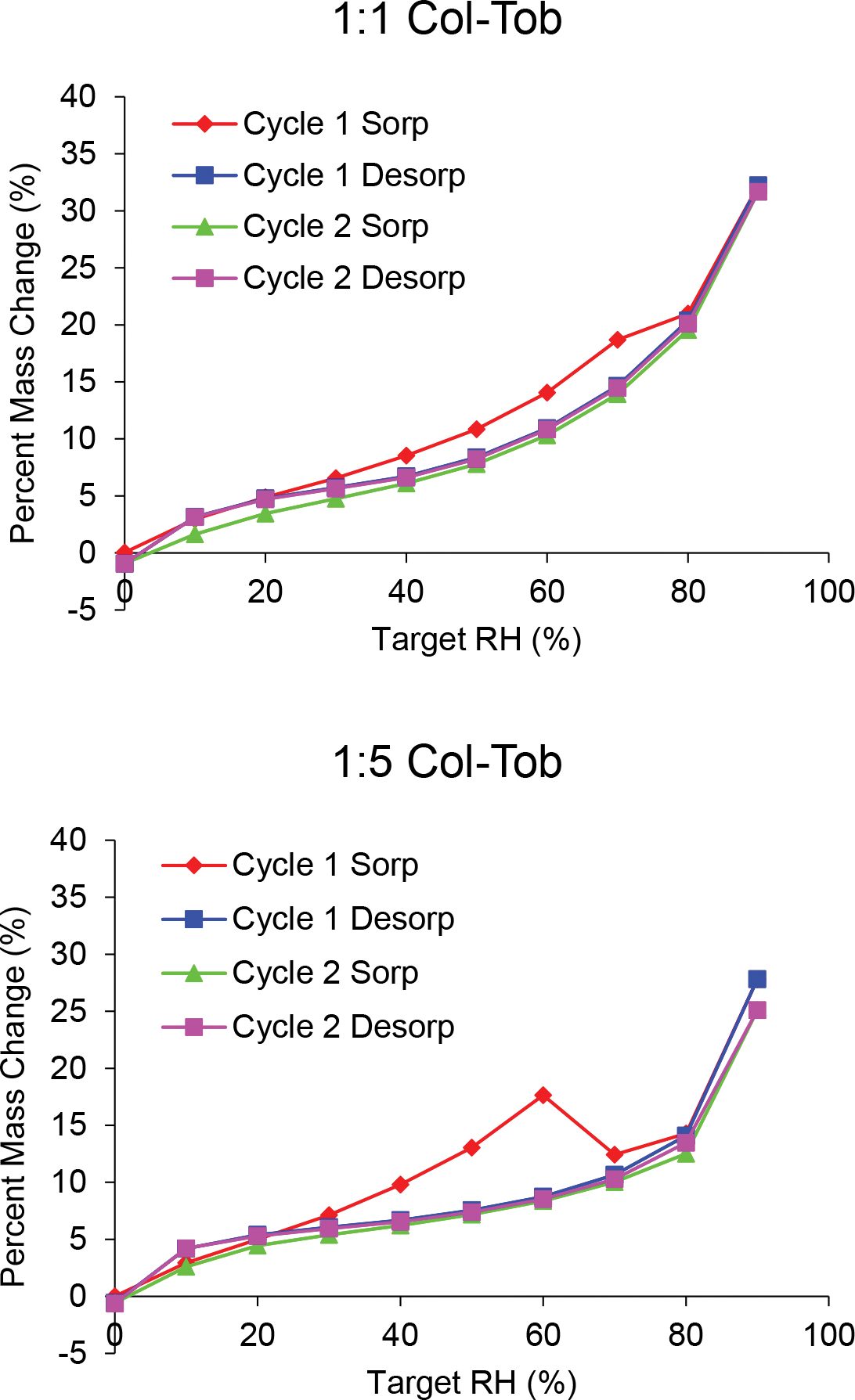
Water vapor sorption isotherms of the spray dried formulations measured over two consecutive adsorption-desorption cycles.
Moisture content of the SD tobramycin formulation at 60% RH was lower than that at 50% RH, which suggests that SD tobramycin crystallizes between 50% and 60% RH (Figure 3). This change was confirmed by a second adsorption-desorption cycle, which showed no such drop in moisture content between 50 and 60% RH and involved an overall lower moisture uptake with increase in RH. Formation of a tobramycin hydrate is indicated by a moisture retention of 5% at the end of the first desorption. Crystal peaks were also observed in the X-ray diffraction pattern obtained after one cycle of the water sorption experiment (Figure 4). Similar moisture sorption behavior and X-ray diffraction patterns indicate that tobramycin in both SD combination formulations crystallized due to water sorption. However, the combinations differed in the RH range in which crystallization was induced: 1:1 combination crystallized in 70–80% RH and 1:5 combination crystallized in 60–70% RH. The RH value at which crystallization occurred for different formulations can be seen in the percent mass change profiles (Supplementary Figure S-1). Presence of colistin to the two SD combination formulations seemed to postpone moisture-driven crystallization of tobramycin to higher RH. In contrast, the SD colistin formulation did not show any sign of crystal formation in either DVS isotherms or X-ray diffraction pattern.
Fig. 4.
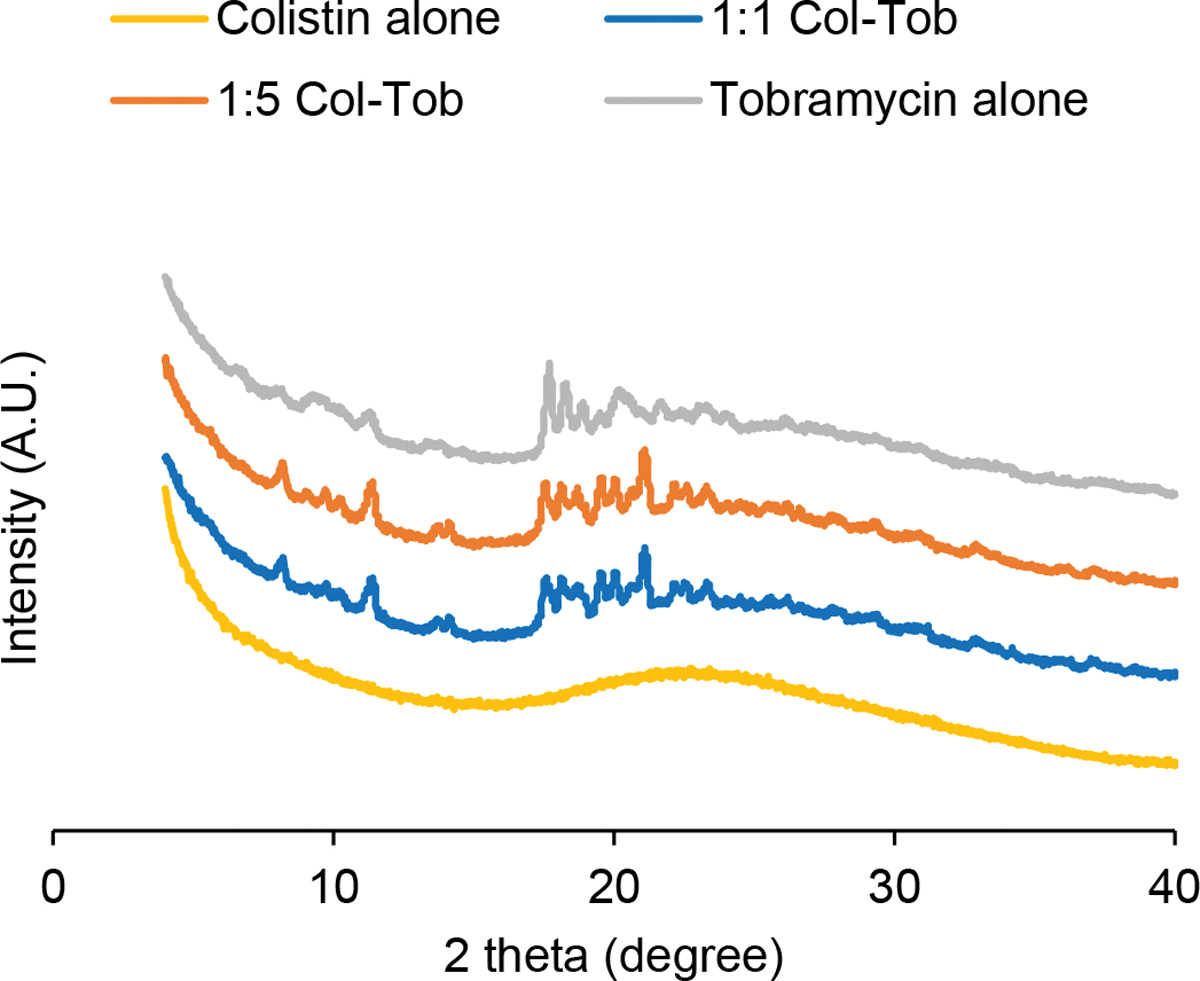
X-ray diffraction patterns of the spray dried formulations subjected to one DVS sorption cycle
X-Ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy was used to determine the surface composition of colistin and tobramycin in the spray dried combination particles. The analysis provided the proportion of carbon atoms on the particle surface that belong to colistin or tobramycin molecules. These values were converted into molar proportions of the two molecules on the surface, which were then used to calculate the weight proportions. For each formulation, the experimental weight proportion of colistin on the surface was compared to the theoretical weight proportion value (Table 3). This theoretical value was the ratio of colistin calculated by the ratio of drugs in the formulation. In both SD combination formulations, the particle surface showed a significantly higher weight proportion of colistin than the theoretical composition. This shows that colistin molecules enrich the particle surface of SD combination formulations.
Table 3.
Percent weight and moles of colistin on the surface of spray dried combination particles determined by X-ray photoelectron spectroscopy (n=3 or 4). Data is presented as mean ± standard deviation
| Formulation | Percentage weight fraction of colistin | Percentage molar fraction of colistin | ||
|---|---|---|---|---|
| Theoretical | Experimental | Theoretical | Experimental | |
|
| ||||
| Colistin alone | 100.0 | 99.6 ± 0.6 | 100.0 | 99.2 ± 1.1 |
| 1:1 Col a -Tob b | 69.0 | 88.6 ± 5.0 | 50.0 | 78.0 ± 7.3 |
| 1:5 Col-Tob | 31.0 | 76.8 ± 1.3 | 16.7 | 59.7 ± 1.5 |
| Tobramycin alone | 0.0 | 0.0 ± 0.1 | 0.0 | 0.0 ± 0.0 |
Col – colistin
Tob – tobramycin
Surface Energy by Inverse Gas Chromatography
SD tobramycin had a higher dispersive (non-polar) surface energy than SD colistin and SD combination formulations at fractional coverages between 0.05 to 0.4 (Figure 5). The SD 1:1 combination formulation showed a dispersive energy profile similar to SD colistin formulation, and both showed generally lower values than SD 1:5 formulation. These data indicate that the addition of colistin to tobramycin in SD formulations reduces their dispersive surface energy.
Fig. 5.
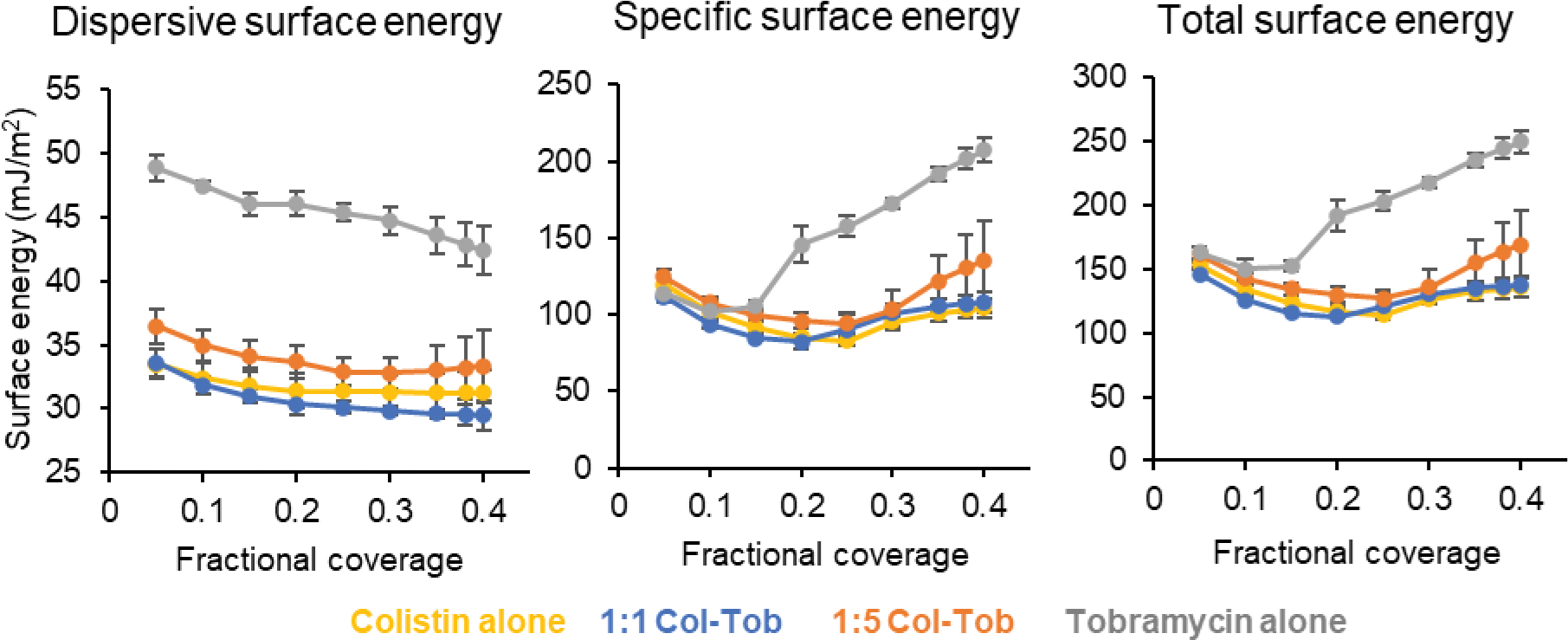
Dispersive, specific, and total surface energy data of the spray dried formulations. Error bars show standard deviations (n=4)
Specific or polar surface energy of SD tobramycin formulation was similar to SD colistin and SD combination formulations at low coverages (<0.2) but was significantly higher at coverages above 0.2 (Figure 5). The specific surface energy values were similar for SD 1:1 combination, SD 1:5 combination and the SD colistin formulation.
The SD tobramycin formulation had a significantly higher total surface energy than SD combinations and SD colistin formulation at higher coverage values (Figure 5). The total surface energy values for SD colistin formulation, SD 1:1 combination and SD 1:5 combination were similar. Overall, the SD combination formulations show a significantly lower potential for surface interactions than SD tobramycin formulation, which may translate to better particle aerosolization.
In Vitro Aerosolization Performance
The ED, FPF and E-FPF values of SD colistin and SD combination formulations were significantly higher than those of SD tobramycin formulation (p < 0.05) (Table 4). For each SD combination formulation, colistin and tobramycin showed similar ED, FPF and E-FPF values, which suggested a homogenous deposition. This can be attributed to a uniform distribution of the two drugs in the formulation, which is supported by the content uniformity results.
Table 4.
Emitted dose, fine particle fraction, emitted fine particle fraction of spray dried formulations (mean ± standard deviation, n=3)
| Formulation | Drug | Emitted Dose (%) | Fine Particle Fraction (%) | Emitted Fine Particle Fraction (%) |
|---|---|---|---|---|
|
| ||||
| Colistin alone | Colistin | 88.9 ± 1.7 | 79.9 ± 2.4 | 89.9 ± 1.0 |
| 1:1 Col a -Tob b | Colistin | 90.8 ± 1.1 | 85.2 ± 0.9 * | 93.9 ± 3.2 * |
| Tobramycin | 89.5 ± 0.5 # | 83.29 ± 1.3 # | 93.1 ± 0.7 # | |
| 1:5 Col-Tob | Colistin | 90.6 ± 0.5 | 84.8 ± 2.8 | 93.6 ± 2.7 * |
| Tobramycin | 89.6 ± 0.8 # | 82.7 ± 0.7 # | 92.3 ± 1.0 # | |
| Tobramycin alone | Tobramycin | 56.0 ± 3.3 | 44.7 ± 2.4 | 79.9 ± 1.6 |
indicates significant difference compared to the spray dried colistin alone, p < 0.05.
indicates significant difference compared to the spray dried tobramycin alone, p < 0.05.
Col – colistin
Tob – tobramycin
Formulation Stability
Crystallinity
Adsorption of moisture by an amorphous solid from the environment may promote crystallization and significantly affect its aerosolization performance [30, 45]. Therefore, we measured X-ray diffraction patterns of the spray dried formulations during their storage at 20 and 55% RH. We observed no crystal peaks in the diffraction patterns of any spray dried formulation for at least four weeks at both 20 and 55%RH (Figure 6).
Fig. 6.
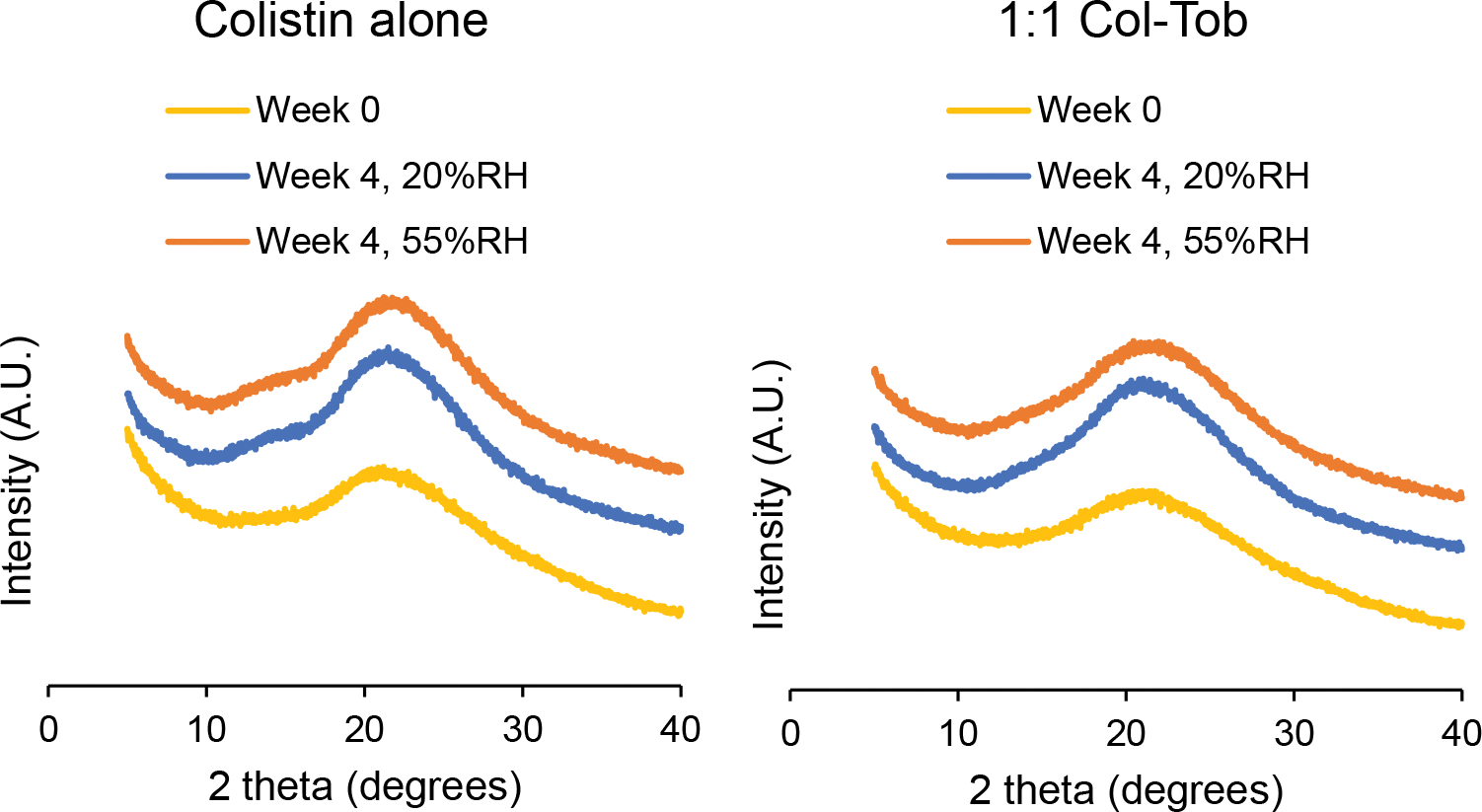
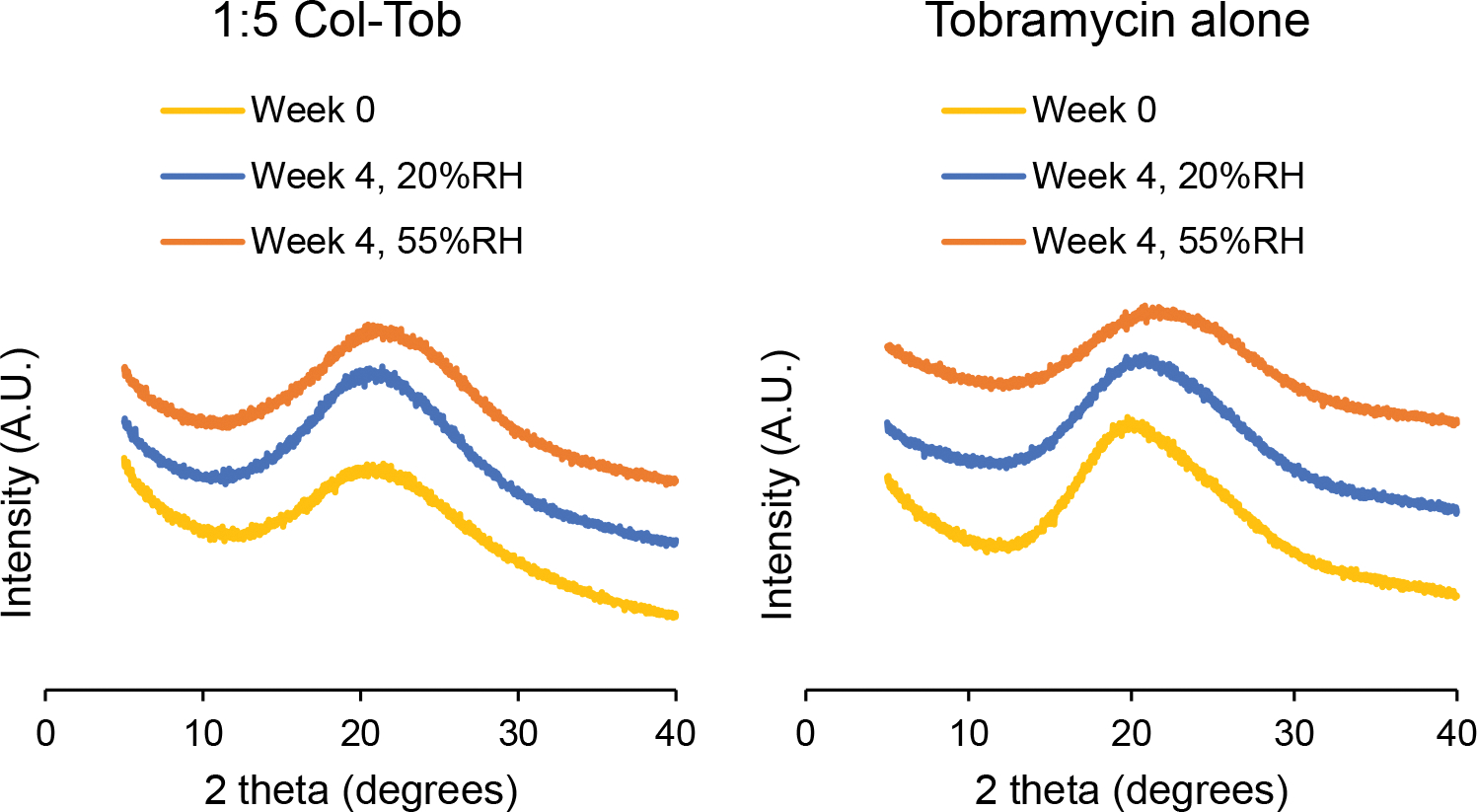
X-ray diffraction patterns of the spray dried formulations right after preparation, and after storage at 20% or 55% RH for 4 weeks
Morphology
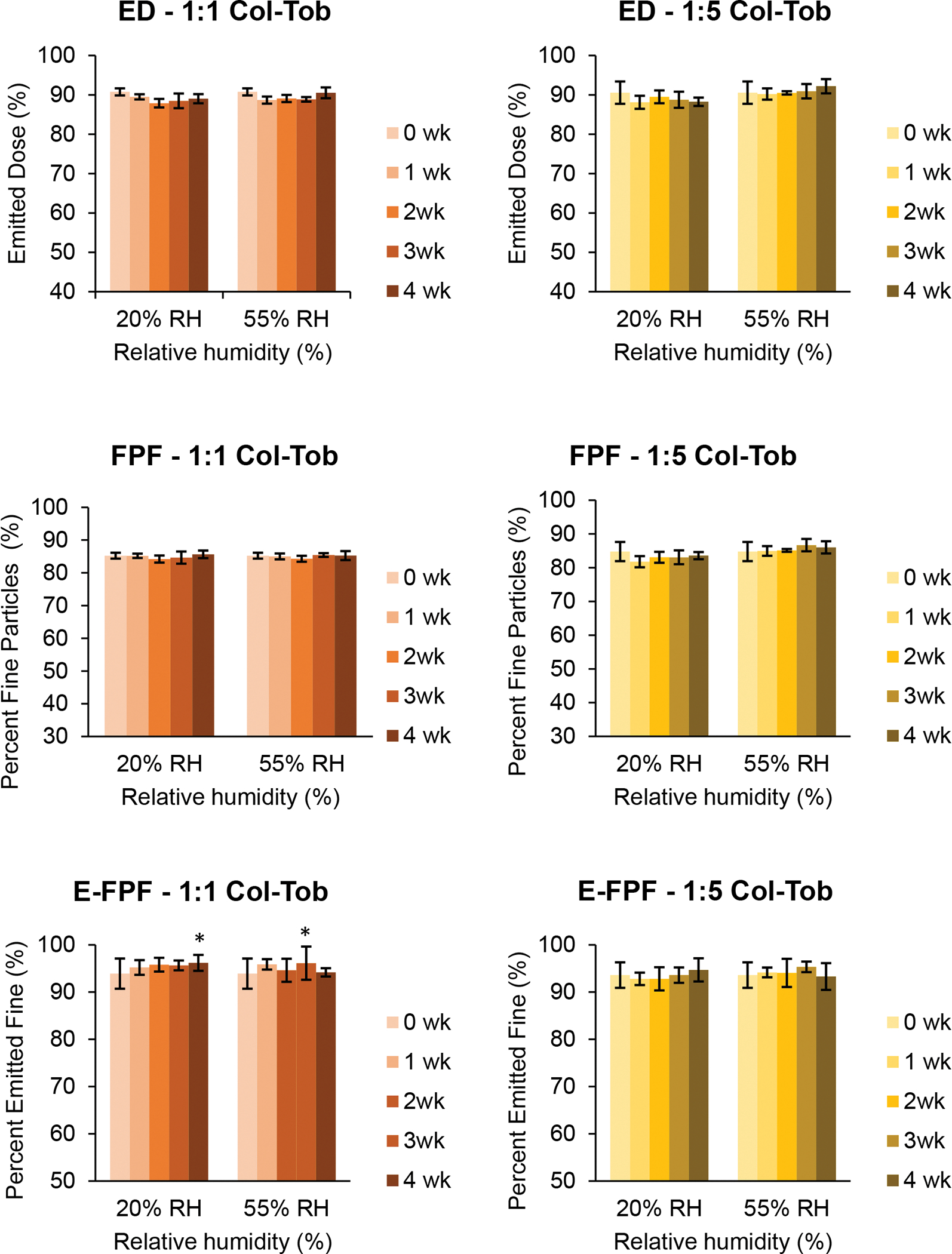
The particles of SD tobramycin fused within two weeks of storage at both humidity conditions, which indicates capillary condensation of water on the surface of the particles (Figure 7). These changes were more prominent at 55% RH than at 20% RH. However, the particles of SD colistin and both SD combination formulations showed no such morphological changes during four weeks of storage (Figures 8–10).
Fig. 7.
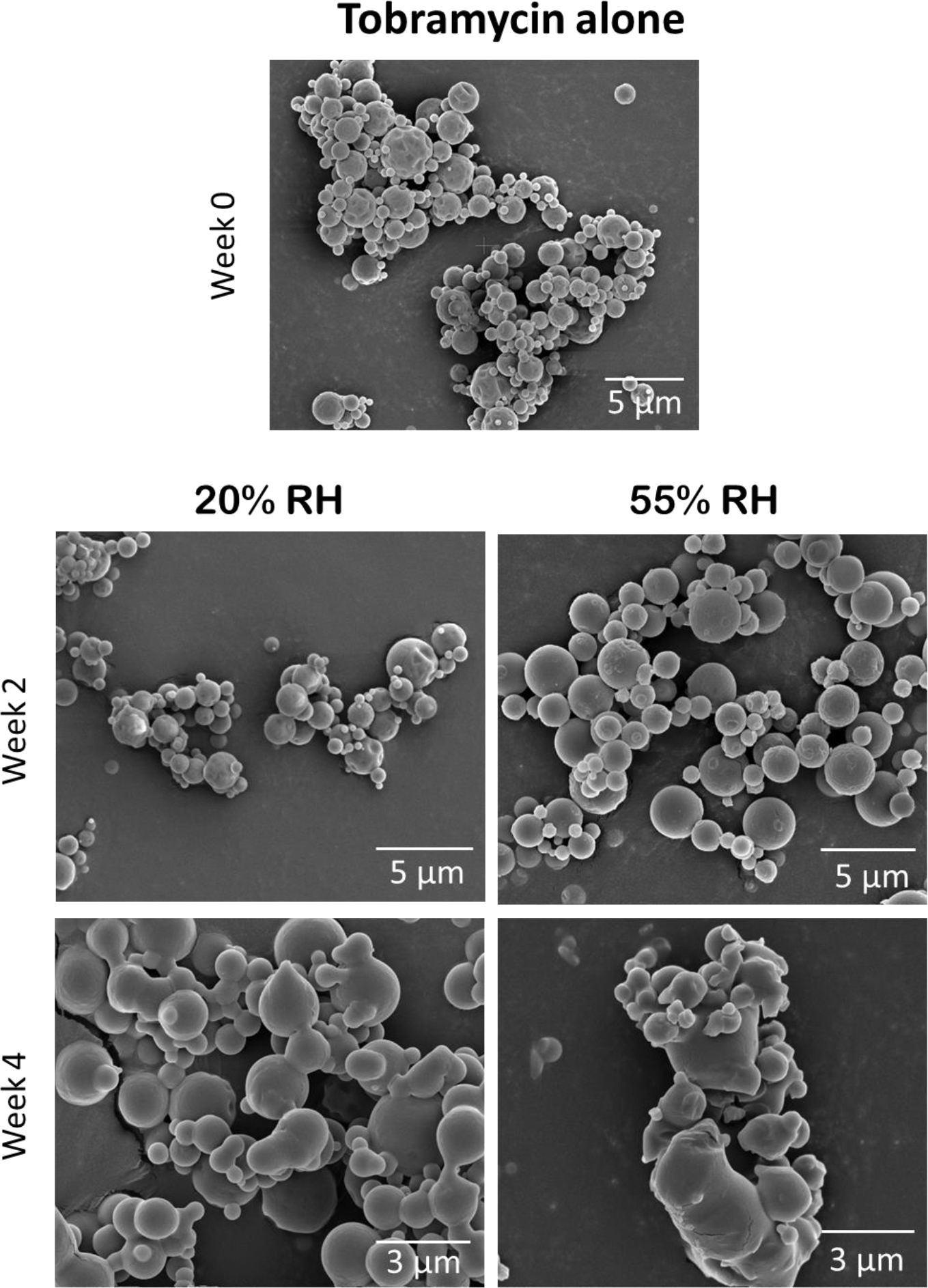
Representative SEM images of the SD tobramycin formulation at the start and after 2 and 4 weeks of storage at 20 and 55% RH
Fig. 8.

Representative SEM images of SD colistin formulation at the start and after 2 and 4 weeks of storage at 20 and 55% RH
Fig. 10.
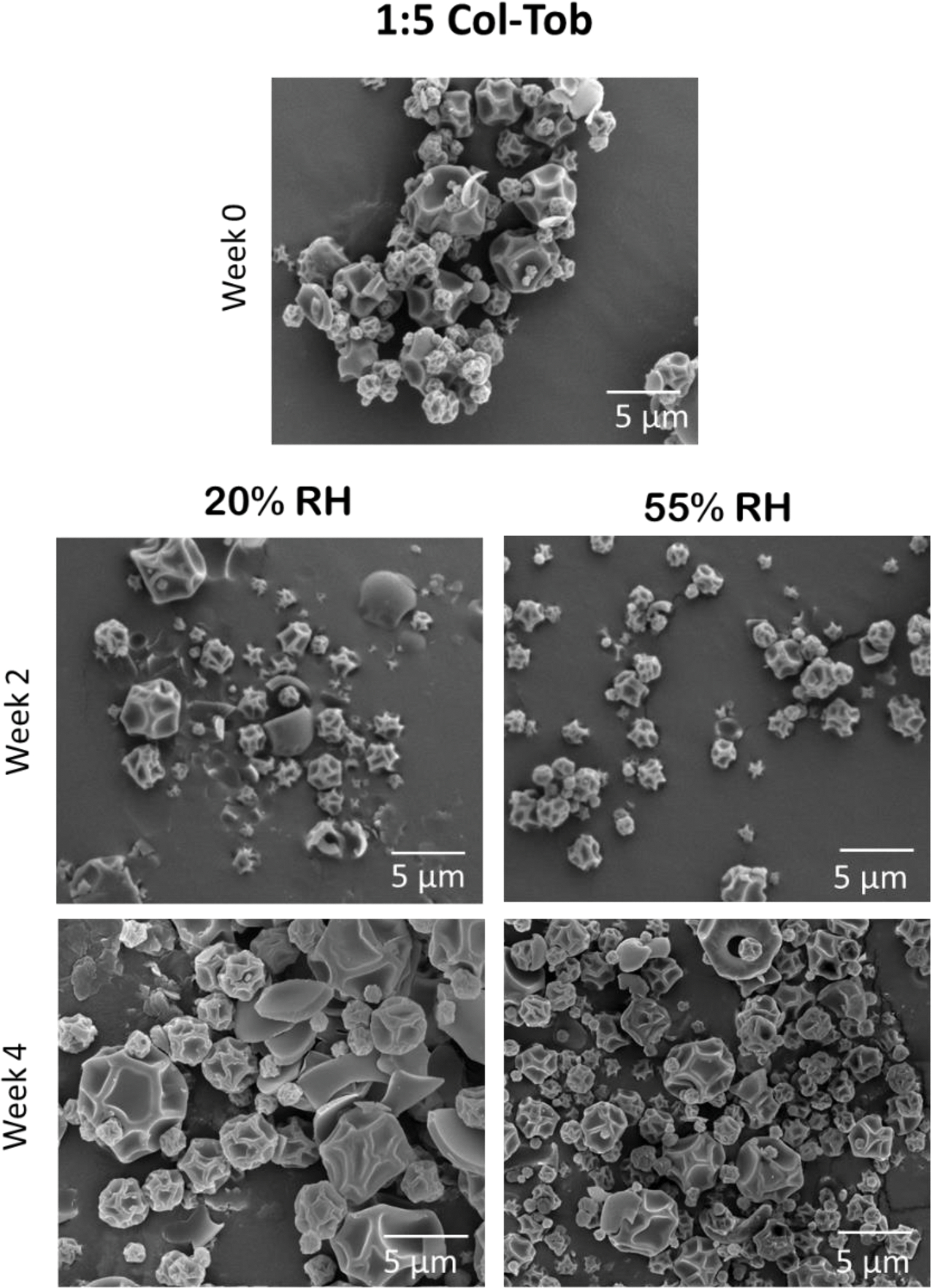
Representative SEM images of SD 1:5 combination formulation at the start and after 2 and 4 weeks of storage at 20 and 55% RH
Particle Agglomeration
Moisture adsorption by solid particles can accelerate particle agglomeration due to change in surface energy and the introduction of capillary forces [46]. When particle size was measured using a dispersion pressure of 4 bar, the percentile particle size D50 of SD tobramycin increased dramatically after two and four weeks at 55% RH, which indicates strong agglomeration (Table 6). However, the SD colistin and both SD combination formulations showed no significant change in particle size at 55% RH and maintained D50 values below 5 μm. At 20% RH, we observed no significant agglomeration in any formulation (Table 6).
Table 6.
Change in particle size (D50) of spray dried formulations at 20% and 55% RH measured during storage using 4.0 bar dispersion pressure. Data is presented as mean ± standard deviation (n=3 or 4)
| Formulation | D50 measured at 4.0 bar dispersion pressure (μm) |
|||||
|---|---|---|---|---|---|---|
| 20% RH | 55% RH | |||||
| 0 wk | 2 wk | 4 wk | 0 wk | 2 wk | 4 wk | |
|
| ||||||
| Colistin alone | 2.21 ± 0.02 | 2.14 ± 0.07 | 2.14 ± 0.01 | 2.21 ± 0.02 | 2.16 ± 0.01 | 2.14 ± 0.01 * |
| 1:1 Col-Tob | 2.08 ± 0.03 | 2.02 ± 0.01 * | 2.00 ± 0.02 * | 2.08 ± 0.03 | 2.09 ± 0.00 | 2.06 ± 0.01 * |
| 1:5 Col-Tob | 1.95 ± 0.01 | 1.94 ± 0.01 | 1.94 ± 0.01 | 1.95 ± 0.01 | 1.96 ± 0.02 | 1.94 ± 0.03 * |
| Tobramycin alone | 1.79 ± 0.03 | 1.87 ± 0.01 * | 1.89 ± 0.00 * | 1.79 ± 0.03 | 2.63 ± 0.02 | 57.4 ± 7.02 * |
indicates significant difference with corresponding wk 0 value, p < 0.05
Col – colistin
Tob – tobramycin
When particle sizes were measured using a dispersion pressure of 0.5 bar, all formulations showed higher percentile sizes relative to corresponding values measured at 4 bars (Table 5). This is understandable because the shear forces would be weaker at a lower dispersion pressure and may not completely break-up weakly agglomerated particles. Conversely, this difference revealed a weaker form of agglomeration among spray dried particles. At 55% RH storage, particle size increased in all formulations, indicating weak agglomeration (Table 5). Interestingly, as shown by D50 values, the degree of agglomeration increased with the amount of tobramycin in the formulation. At 20% RH storage, all formulations except the SD tobramycin showed stable particle size distributions with time (Table 5).
Table 5.
Change in particle size (D50) of spray dried formulations at 20% and 55% RH measured during storage using 0.5 bar dispersion pressure. Data is presented as mean ± standard deviation (n=3 or 4)
| Formulation | D50 measured at 0.5 bar dispersion pressure (μm) |
|||||
|---|---|---|---|---|---|---|
| 20% RH | 55% RH | |||||
| 0 wk | 2 wk | 4 wk | 0 wk | 2 wk | 4 wk | |
|
| ||||||
| Colistin alone | 2.50 ± 0.07 | 2.52 ± 0.02 | 2.59 ± 0.02 | 2.50 ± 0.07 | 2.93 ± 0.22 | 2.92 ± 0.02 * |
| 1:1 Col a -Tob b | 2.59 ± 0.06 | 2.46 ± 0.03 | 2.55 ± 0.04 | 2.59 ± 0.06 | 2.84 ± 0.04 | 2.99 ± 0.01 * |
| 1:5 Col-Tob | 2.47 ± 0.02 | 2.34 ± 0.04 * | 2.36 ± 0.03 * | 2.47 ± 0.02 | 3.20 ± 0.09 | 3.76 ± 0.13 * |
| Tobramycin alone | 30.2 ± 2.88 | 38.7 ± 0.85 | 78.7 ± 5.98 * | 30.2 ± 2.88 | 51.3 ± 1.58 * | 110 ± 11.0 * |
indicates significant difference with corresponding wk 0 value, p < 0.05
Col – colistin
Tob – tobramycin
In vitro Aerosolization
The emitted dose (ED), fine particle fraction (FPF) and emitted fine particle fraction (E-FPF) of SD colistin were all at relatively high levels and did not change significantly after four weeks at 20% and 55% RH conditions (Figure 11), indicating a stable aerosolization performance at the mild humidity conditions.
Fig. 11.
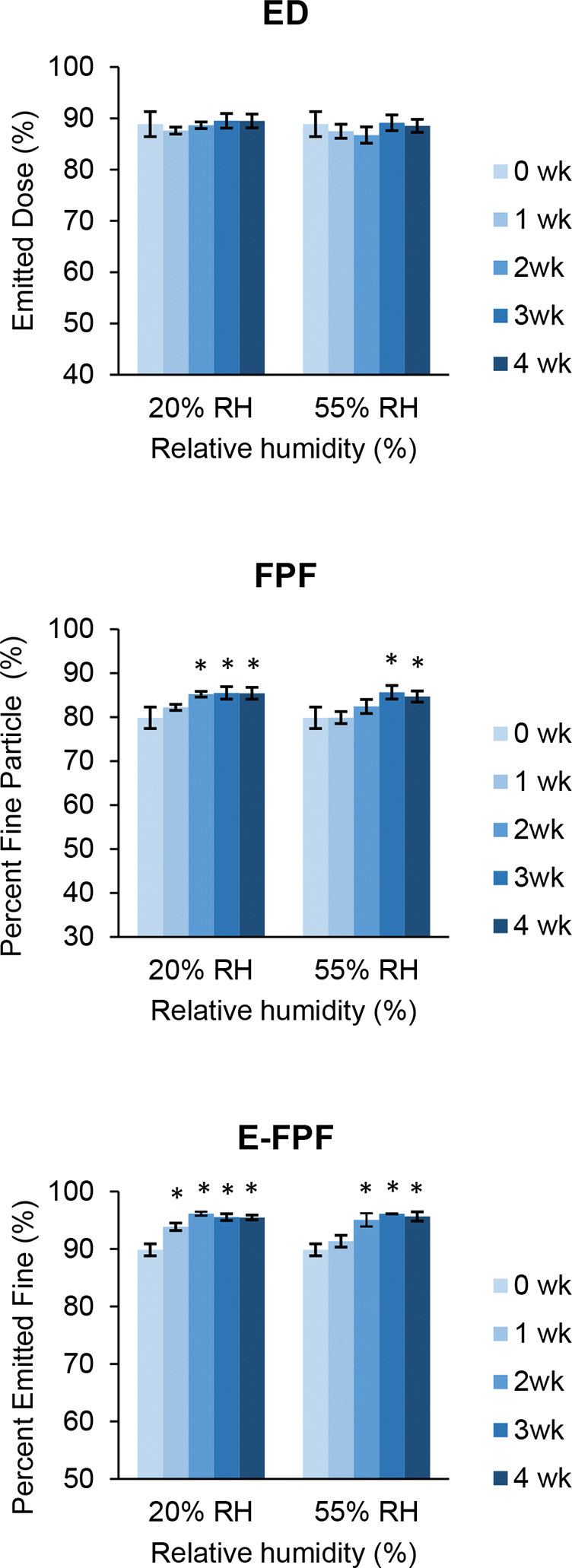
Changes in emitted dose (ED), fine particle fraction (FPF), and emitted fine particle fraction (E-FPF) of spray dried colistin during storage at 20 and 55% RH over 4 weeks. Error bars show standard deviation (n=4). Bars marked with * are significantly different (p < 0.05) from corresponding values at week 0
ED of the SD tobramycin increased steadily with storage time at 20% RH (Figure 12). In contrast, the ED jumped after one week at 55% RH and stabilized for the next three weeks. The increase in ED could be due to particle agglomeration as shown in Tables 5 and 6, which can reduce the effect of adhesive interaction with the inhaler and capsule, thus improving flowability [47]. The increase in FPF of SD tobramycin at both conditions seems to be related to the trend of ED: as the dose emitted from the inhaler increased, the FPF also increased (Figure 12). However, the final FPF after four weeks at 55% RH was similar to that at 20% RH, despite a significantly higher ED at 55% RH. At 20% RH, the E-FPF of SD tobramycin remained unchanged for 3 weeks and increased after the fourth week (Figure 12). In contrast, the E-FPF at 55% RH gradually decreased.
Fig. 12.
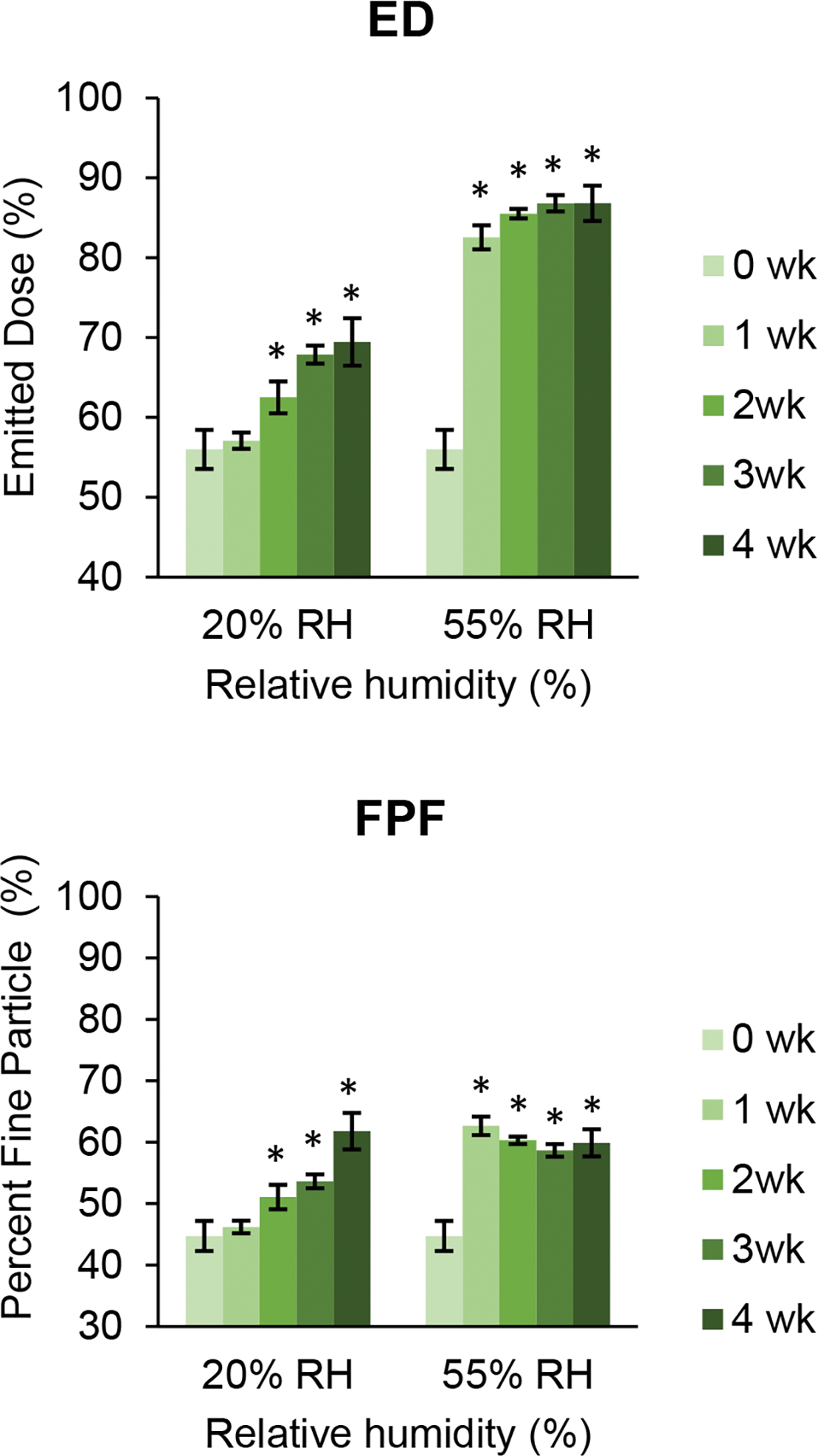
Changes in emitted dose (ED), fine particle fraction (FPF), and emitted fine particle fraction (E-FPF) of spray dried tobramycin during storage at 20 and 55% RH over 4 weeks. Error bars show standard deviations (n=4). Bars marked with * are significantly different (p < 0.05) from corresponding values at week 0
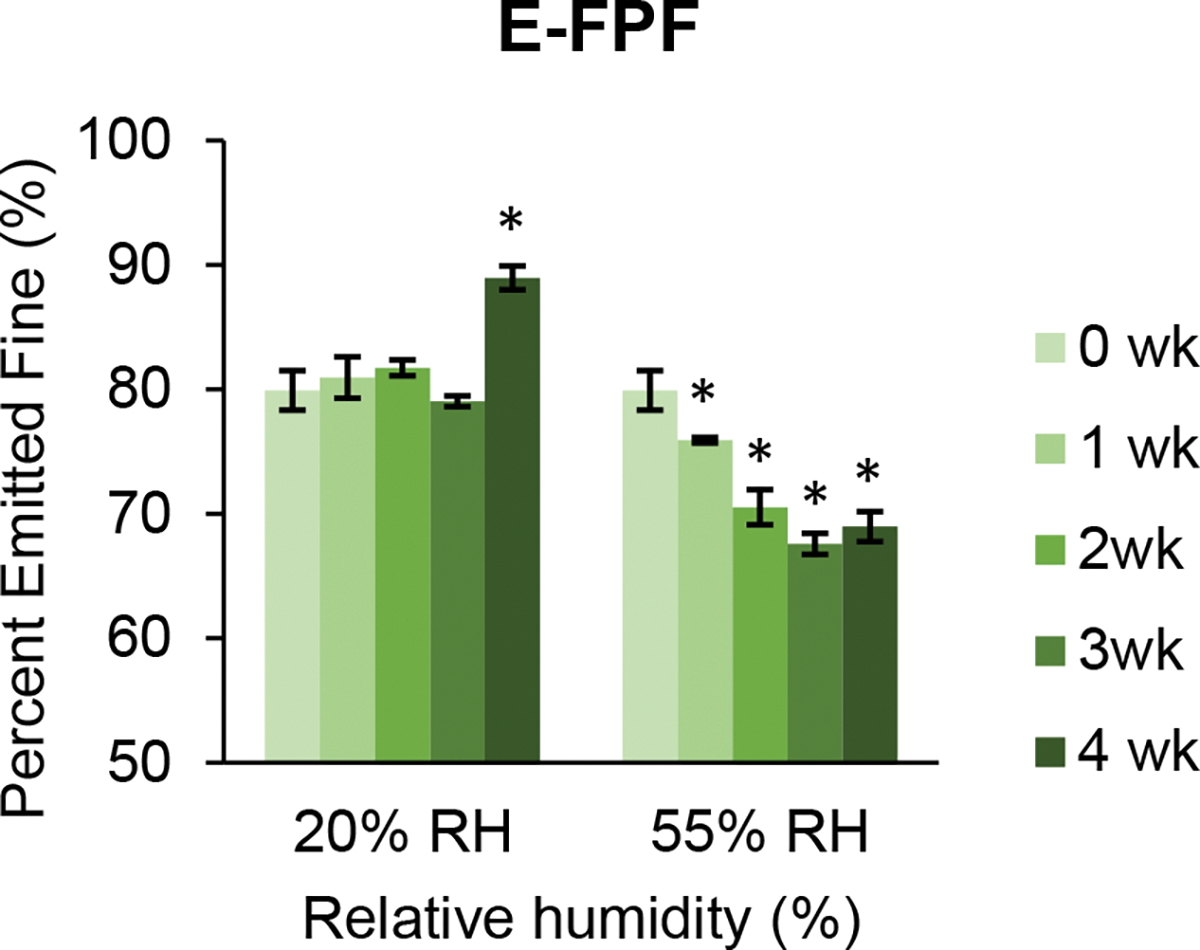
The aerosolization performance of SD 1:1 and 1:5 combination formulations remained relatively high and stable for four weeks of storage at both 20 and 55% RH (Figure 13). The aerosolization data for tobramycin and colistin components of these formulations were equivalent, suggesting a highly homogenous distribution of two drugs (Supplementary Figures S-3 and S-4). Interestingly, the aerosolization characteristics of the SD combination formulations resembled that of the SD colistin formulation. This is especially noteworthy for the SD 1:5 combination formulation, in which colistin is present in a lower molar proportion.
Fig. 13.
Changes in emitted dose (ED), fine particle fraction (FPF), and emitted fine particle fraction (E-FPF) of colistin in spray dried combination formulations during storage at 20 and 55% RH over 4 weeks: 1:1 Col-Tob (left) and 1:5 Col-Tob (right). Error bars show standard deviations (n=4). Bars marked with * are significantly different (p < 0.05) from corresponding values at week 0
DISCUSSION
The rationale to develop combination formulations of colistin and tobramycin is based on reduced pulmonary toxicity and enhanced antimicrobial activities [11]. The tobramycin DPI (TOBI® Podhaler®) is administered at 112 mg twice daily [48]; while the colistin DPI (Colobreathe®) is administered at 125 mg colistimethate sodium twice daily [49]. We chose the molar ratio of colistin : tobramycin = 1:5 as a starting point based on our toxicity data [11]. Our earlier toxicity data showed that tobramycin significantly inhibited polymyxin-induced toxicity in human lung epithelial A549 cells (i.e. increased cell viability) [11]. Tobramycin (5.0 mM) significantly increased the cell viability from approximately 40% by 1.0 mM polymyxin B to approximately 60% by the combination (p<0.0001) [11].In the present study, combination formulations of colistin and tobramycin were produced using spray drying. The optimal ratio of polymyxin : tobramycin for future clinical studies may need further optimization based on PK/PD data.
Both combination formulations showed equivalent aerosol performance to spray dried (SD) colistin, and markedly higher emitted doses and fine particle fractions than SD tobramycin formulation. Such an improved aerosol performance could be attributed to a significantly lower surface energy and a dimpled particle surface. The surface energy of powders directly affects interactions between particles and interactions with other surfaces, which is important for powder aerosolization [50]. The total surface energy of SD tobramycin was significantly higher than that of SD colistin and SD combination formulations. This may lead to stronger cohesive interactions between the particles of SD tobramycin, resulting in stronger agglomeration and reduced fine particle fraction. These SD tobramycin particles may also tend to adhere more to capsules and inhalers, resulting in a lower emitted dose. The morphology of a particle also affects its capacity for surface interaction. The colistin-containing SD particles were dimpled, while the SD tobramycin particles were relatively smooth and spherical. Dimpled particles may have reduced interparticle contact area than smooth particles, which could contribute to the better aerosol performance [51, 52].
We hypothesized that the equivalence of aerosol performance, surface energy and particle morphology between the SD combination formulations and the SD colistin formulation was attributable to enriched colistin on the composite particle surface. Due to the surfactant-like nature of the colistin molecule, during spray drying it tends to migrate to the surface and orient its hydrophobic portion towards the water-air interface [53, 54]. Such enrichment of colistin was verified on the composite particle surface by XPS data: the SD 1:5 formulation contained 31% (w/w) colistin overall, but its particle surface (an approximately 10 nm deep layer) contained 76.8 ± 1.3% (w/w) colistin. This showed that colistin molecules dominantly affected the particle morphology, surface energy and aerosol performance of the combination through surface enrichment.
Another benefit of colistin enrichment on the SD combination particle surface was improved aerosolization stability during storage at two mild humidity conditions: 20% and 55% RH. The SD colistin and SD combination formulations showed stable aerosol performance over a duration of four weeks at both humidity conditions. However, the aerosol behavior of SD tobramycin shifted significantly over storage: emitted dose and fine particle fraction increased significantly. This shift could be due to extensive particle agglomeration, which was demonstrated by particle size analysis and SEM images. Particle agglomeration can reduce the effect of adhesive interaction with the inhaler and capsule, and therefore improve flowability [47]. It was interesting to observe that in contrast to SD tobramycin, SD colistin and SD combination formulations showed negligible moisture-related agglomeration at the mild humidity conditions. This is despite the fact that the SD colistin and SD combination formulations adsorbed similar albeit slight lower amount of water relative to SD tobramycin during DVS analyses. The excessive moisture adsorption in SD tobramycin could be due to its higher specific surface energy values (i.e. higher polarity, as shown by IGC analysis) and higher hydrophilicity. As discussed above, SD colistin and combinations possess lower surface hydrophilicity due to the orientation of colistin molecules on the spray dried particle surface [25]. This is also supported by the observation that SD colistin particles temporarily float on water surface and resists wetting. In this way, the colistin molecules on the particle surface of SD combination formulations resisted excessive moisture adsorption up to 55% RH, resulting in negligible moisture-driven agglomeration. However, previous studies with SD colistin showed that, when relative humidity exceeds a threshold (approximately 70% RH), moisture condenses on the particles, leading to liquid bridge formation between the particles and agglomeration [24]. Moreover, the aerosol performance of SD colistin decreased significantly when stored at 75% RH. In future studies, non-hygroscopic excipients such as leucine may be incorporated to provide further moisture protection [30, 55]. Overall, the SD combination formulations showed excellent stability of aerosolization up to 55% RH, which may be attributed to their advantageous surface properties.
CONCLUSIONS
Combination formulations of colistin and tobramycin prepared by spray drying showed significantly higher in vitro aerosol performance and improved aerosolization stability than spray dried tobramycin during storage at mild humidity conditions of 20% and 55% RH. The improved aerosol performance and aerosolization stability were attributed to the enrichment of colistin on the co-spray dried particle surface, as supported by X-ray photoelectron spectroscopy, scanning electron microscopy and surface energy data. Interestingly, hygroscopic colistin prevented moisture-induced particle agglomeration at 20 and 55% RH when co-spray dried with tobramycin. Such an effect of colistin is due to its self-assembling behavior during spray drying that leads to a relatively hydrophobic particle surface. The spray dried combination formulations of colistin and tobramycin developed in this study are promising to treat lung infections caused by multi-drug resistant Gram-negative bacteria.
Supplementary Material
Fig. 9.
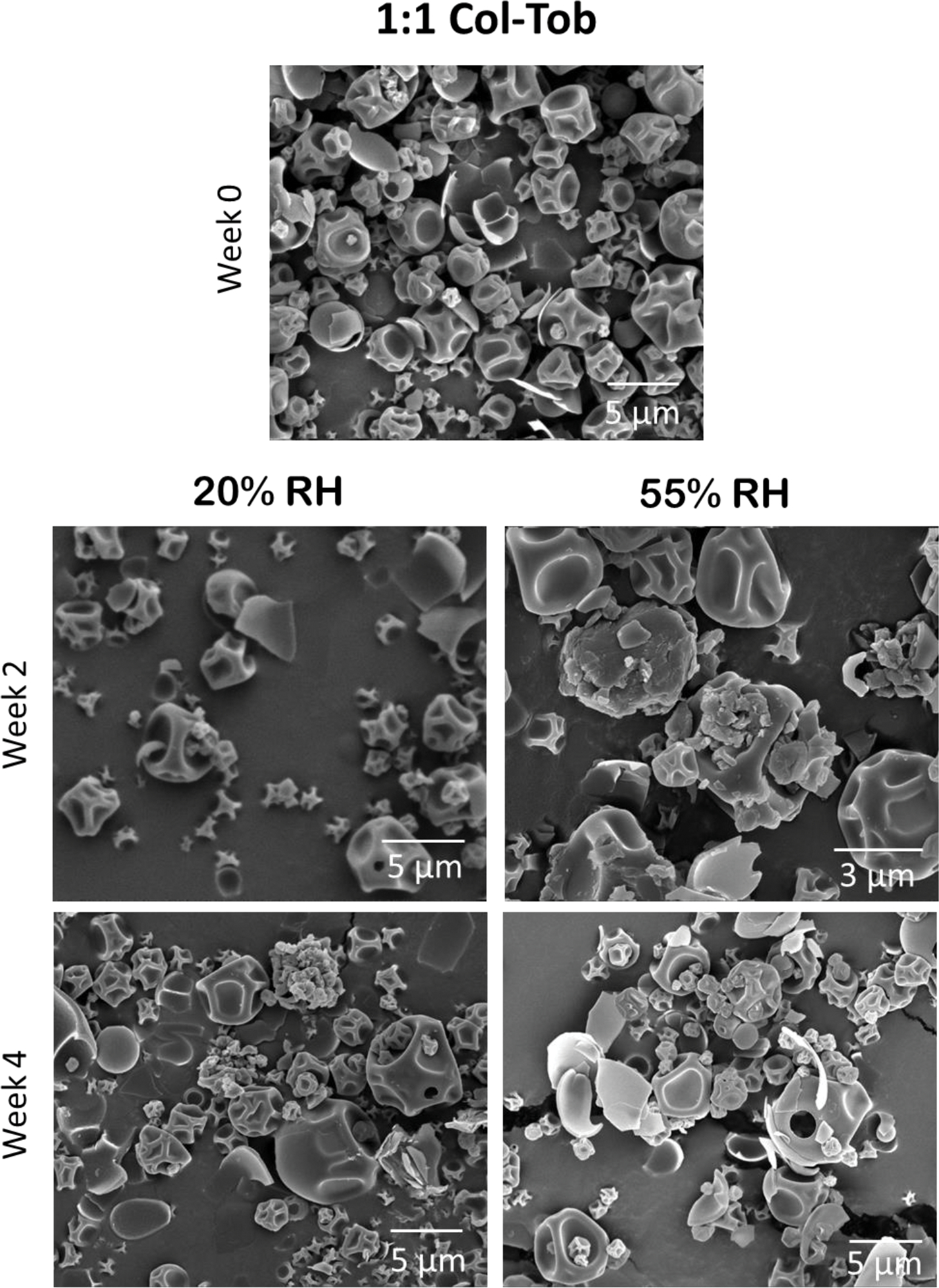
Representative SEM images of SD 1:1 combination formulation at the start and after 2 and 4 weeks of storage at 20 and 55% RH
ACKNOWLEDGEMENTS
This publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institute of Health under Award Numbers R01AI132681 and R01AI146160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.L. is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow (APP1157909).
Footnotes
CONFLICT OF INTEREST
MUA, MAKA, JL and QTZ are inventors of PCT/US2021/030393.
REFERENCES
- 1.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: The prato polymyxin consensus. Lancet Infect Dis. 2015;15(2):225–34. 10.1016/s1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 2.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55(7):3284–94. 10.1128/Aac.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurjar M. Colistin for lung infection: An update. J Intensive Care. 2015;3(1):1–12. 10.1186/s40560-015-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob Agents Chemother. 2014;58(5):2570–9. 10.1128/aac.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nang SC, Azad MAK, Velkov T, Zhou Q, Li J. Rescuing the last-line polymyxins: Achievements and challenges. Pharmacol Rev. 2021;73(2):679. 10.1124/pharmrev.120.000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghardt JM, Kloft C, Sharma A. Inhaled therapy in respiratory disease: The complex interplay of pulmonary kinetic processes. Can Respir J. 2018;2018:2732017. 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velkov T, Rahim NA, Zhou QT, Chan H-K, Li J. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Adv Drug Deliv Rev. 2015;85:65–82. 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzler E, Fraidenburg DR, Scardina T, Danziger LH. Inhaled antibiotics for gram-negative respiratory infections. Clin Microbiol Rev. 2016;29(3):581–632. 10.1128/cmr.00101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou QT, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan H-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99. 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MU, Velkov T, Lin Y-W, Yun B, Nowell CJ, Zhou F, et al. Potential toxicity of polymyxins in human lung epithelial cells. Antimicrob Agents Chemother. 2017;61(6):e02690–16. 10.1128/AAC.02690-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Ahmed MU, Li J, Azad MAK. Inhalation formulations of antimicrobial compounds. PCT/US2021/030393 ed. United States of America: Purdue Research Foundation; 2022. [Google Scholar]
- 12.Herrmann G, Yang L, Wu H, Song Z, Wang H, Hoiby N, et al. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J Infect Dis. 2010;202(10):1585–92. 10.1086/656788. [DOI] [PubMed] [Google Scholar]
- 13.Kashyap S, Kaur S, Sharma P, Capalash N. Combination of colistin and tobramycin inhibits persistence of Acinetobacter baumannii by membrane hyperpolarization and down-regulation of efflux pumps. Microb Infect. 2021;23(4–5):104795. 10.1016/j.micinf.2021.104795. [DOI] [PubMed] [Google Scholar]
- 14.Tappenden P, Harnan S, Uttley L, Mildred M, Carroll C, Cantrell A. Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: Systematic review and economic model. Health Technol Assess. 2013;17(56):v–xvii, 1–181. 10.3310/hta17560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riethmuller J, Herrmann G, Graepler-Mainka U, Hellwig D, Heuer HE, Heyder S, et al. Sequential inhalational tobramycin-colistin-combination in CF-patients with chronic P. aeruginosa colonization - an observational study. Cell Physiol Biochem. 2016;39(3):1141–51. 10.1159/000447821. [DOI] [PubMed] [Google Scholar]
- 16.Antoniu SA, Cojocaru I. Inhaled colistin for lower respiratory tract infections. Expert Opin Drug Deliv. 2012;9(3):333–42. 10.1517/17425247.2012.660480. [DOI] [PubMed] [Google Scholar]
- 17.Zhou QT, Gengenbach T, Denman JA, Yu HH, Li J, Chan HK. Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. AAPS J. 2014;16(1):37–47. 10.1208/s12248-013-9537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunaugh AD, Smyth HDC. Formulation techniques for high dose dry powders. Int J Pharm. 2018;547(1–2):489–98. 10.1016/j.ijpharm.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50(9):1209–27. https://rc.rcjournal.com/content/50/9/1209.short. [PubMed] [Google Scholar]
- 20.Traini D, Young PM. Delivery of antibiotics to the respiratory tract: An update. Expert Opin Drug Deliv. 2009;6(9):897–905. 10.1517/17425240903110710. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT. Powder production and particle engineering for dry powder inhaler formulations. Current Pharm Des. 2015;21(27):3902–16. 10.2174/1381612821666150820111134. [DOI] [PubMed] [Google Scholar]
- 22.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vehring R, Foss WR, Lechuga-Ballesteros D. Particle formation in spray drying. J Aerosol Sci. 2007;38(7):728–46. 10.1016/j.jaerosci.2007.04.005. [DOI] [Google Scholar]
- 24.Zhou QT, Morton DAV, Heidi HY, Jacob J, Wang J, Li J, et al. Colistin powders with high aerosolisation efficiency for respiratory infection: Preparation and in vitro evaluation. J Pharm Sci. 2013;102(10):3736–47. 10.1002/jps.23685. [DOI] [PubMed] [Google Scholar]
- 25.Jong T, Li J, Morton DAV, Zhou QT, Larson I. Investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J Pharm Sci. 2016;105(3):1156–63. 10.1016/S0022-3549(15)00189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan A, Farkas D, Longest W, Hindle M. Characterization of excipient enhanced growth (EEG) tobramycin dry powder aerosol formulations. Int J Pharm. 2020;591:120027. 10.1016/j.ijpharm.2020.120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JGY, Chan H-K, Prestidge CA, Denman JA, Young PM, Traini D. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur J Pharm Biopharm. 2013;83(2):285–92. 10.1016/j.ejpb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Mangal S, Park H, Zeng L, Heidi HY, Lin Y-w, Velkov T, et al. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. Int J Pharm. 2018;548(1):443–53. 10.1016/j.ijpharm.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangal S, Xu R, Park H, Zemlyanov D, Shetty N, Lin Y-W, et al. Understanding the impacts of surface compositions on the in-vitro dissolution and aerosolization of co-spray-dried composite powder formulations for inhalation. Pharm Res. 2018;36(1):6. 10.1007/s11095-018-2527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shetty N, Ahn P, Park H, Bhujbal S, Zemlyanov D, Cavallaro A, et al. Improved physical stability and aerosolization of inhalable amorphous ciprofloxacin powder formulations by incorporating synergistic colistin. Mol Pharm. 2018;15(9):4004–20. 10.1021/acs.molpharmaceut.8b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoppentocht M, Akkerman OW, Hagedoorn P, Frijlink HW, de Boer AH. The cyclops for pulmonary delivery of aminoglycosides; a new member of the Twincer family. Eur J Pharm Biopharm. 2015;90:8–15. 10.1016/j.ejpb.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Hoppentocht M, Akkerman OW, Hagedoorn P, Alffenaar J-WC, van der Werf TS, Kerstjens HAM, et al. Tolerability and pharmacokinetic evaluation of inhaled dry powder tobramycin free base in non-cystic fibrosis bronchiectasis patients. PLOS ONE. 2016;11(3):e0149768. 10.1371/journal.pone.0149768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newhouse MT, Hirst PH, Duddu SP, Walter YH, Tarara TE, Clark AR, et al. Inhalation of a dry powder tobramycin pulmosphere formulation in healthy volunteers. Chest. 2003;124(1):360–6. 10.1378/chest.124.1.360. [DOI] [PubMed] [Google Scholar]
- 34.Shetty N, Park H, Zemlyanov D, Mangal S, Bhujbal S, Zhou QT. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int J Pharm. 2018;544(1):222–34. 10.1016/j.ijpharm.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty N, Zhang Y, Park H, Zemlyanov D, Shah D, He A, et al. Surface composition and aerosolization stability of an inhalable combinational powder formulation spray dried using a three-fluid nozzle. Pharm Res. 2020;37(11):1–12. 10.1007/s11095-020-02937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanchaert B, Huang S, Wach K, Adams E, Van Schepdael A. Assay development for aminoglycosides by HPLC with direct UV detection. J Chromatographic Sci. 2017;55(3):197–204. 10.1093/chromsci/bmw169. [DOI] [PubMed] [Google Scholar]
- 37.Pharmacopeia US. USP<905> Uniformity of dosage units. Rockville (MD): The United States Pharmacopeial Convention, Inc.; 2011. [Google Scholar]
- 38.Wilson S, Wilson L, Fitzpatrick LE, Brundle CR, Wilson G, Evans CA. Encyclopedia of materials characterization: Surfaces, interfaces, thin films. United Kingdom: Butterworth-Heinemann; 1992. [Google Scholar]
- 39.Woodruff DP. Modern techniques of surface science. 3rd ed. Cambridge university press; 2016. [Google Scholar]
- 40.Bhujbal SV, Zemlyanov DY, Cavallaro A, Mangal S, Taylor LS, Zhou QT. Qualitative and quantitative characterization of composition heterogeneity on the surface of spray dried amorphous solid dispersion particles by an advanced surface analysis platform with high surface sensitivity and superior spatial resolution. Mol Pharm. 2018;15(5):2045–53. 10.1021/acs.molpharmaceut.8b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavielle L, Schultz J. Surface properties of carbon fibers determined by inverse gas chromatography: Role of pretreatment. Langmuir. 1991;7(5):978–81. 10.1021/la00053a027. [DOI] [Google Scholar]
- 42.Schultz J, Lavielle L, Martin C. The role of the interface in carbon fibre-epoxy composites. J Adhesion. 1987;23(1):45–60. 10.1080/00218468708080469. [DOI] [Google Scholar]
- 43.Van Oss CJ. Acid—base interfacial interactions in aqueous media. Colloids Surf A. 1993;78:1–49. 10.1016/0927-7757(93)80308-2. [DOI] [Google Scholar]
- 44.Van Oss CJ, Good RJ, Chaudhury MK. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir. 1988;4(4):884–91. 10.1021/la00082a018. [DOI] [Google Scholar]
- 45.Shetty N, Zeng L, Mangal S, Nie H, Rowles MR, Guo R, et al. Effects of moisture-induced crystallization on the aerosol performance of spray dried amorphous ciprofloxacin powder formulations. Pharm Res. 2018;35(1):1–13. 10.1007/s11095-017-2281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng XM, Martin GP, Marriott C. Particulate interactions in dry powder formulation for inhalation. 1st ed. London: CRC Press; 2001. [Google Scholar]
- 47.Zhou QT, Armstrong B, Larson I, Stewart PJ, Morton DAV. Understanding the influence of powder flowability, fluidization and de-agglomeration characteristics on the aerosolization of pharmaceutical model powders. Eur J Pharm Sci. 2010;40(5):412–21. 10.1016/j.ejps.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Instructions for use: TOBI Podhaler. Novartis Pharmaceutical Corporation. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/201688s008lbl.pdf. 2016. [Google Scholar]
- 49.Colobreathe. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/colobreathe. 2022. Accessed 10 July 2022. [Google Scholar]
- 50.Das S, Larson I, Young P, Stewart P. Surface energy changes and their relationship with the dispersibility of salmeterol xinafoate powders for inhalation after storage at high RH. Eur J Pharm Sci. 2009;38(4):347–54. 10.1016/j.ejps.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Kwok PCL, Tunsirikongkon A, Glover W, Chan H-K. Formation of protein nano-matrix particles with controlled surface architecture for respiratory drug delivery. Pharm Res. 2011;28(4):788–96. 10.1007/s11095-010-0332-2. [DOI] [PubMed] [Google Scholar]
- 52.Adi H, Traini D, Chan HK, Young PM. The influence of drug morphology on aerosolisation efficiency of dry powder inhaler formulations. J Pharm Sci. 2008;97(7):2780–8. 10.1002/jps.21195. [DOI] [PubMed] [Google Scholar]
- 53.Wallace SJ, Li J, Nation RL, Prankerd RJ, Velkov T, Boyd BJ. Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: Implications for solution stability and solubilization. J Phys Chem B. 2010;114(14):4836–40. 10.1021/jp100458x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mestres C, Alsina MA, Busquets MA, Murányi I, Reig F. Interaction of colistin with lipids in liposomes and monolayers. Int J Pharm. 1998;160(1):99–107. 10.1016/s0378-5173(97)00301-3. [DOI] [Google Scholar]
- 55.Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, et al. L-leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm. 2016;102:132–41. 10.1016/j.ejpb.2016.02.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


