Summary
The actions of the immune system are finely tuned, involving complex communication and coordination between diverse immune and non-immune cells across the tissues of the body. A healthy immune system requires a precise balance between immunity and tolerance. Regulatory T cells (Tregs) have long been appreciated as one of the master regulators of this balance; their importance is underscored by the autoimmunity that develops in mice and humans when Tregs are missing or dysfunctional. In addition to the immunoregulatory roles of Tregs in suppressing autoimmunity and inflammation via control of adaptive and innate immune responses, several non-immune modulatory functions of Tregs have been identified in recent years. In this review, we have highlighted the growing literature on the action of Tregs in metabolism, stem cell maintenance, tissue repair, and angiogenesis. Alongside Tregs’ immune suppressive role, these non-suppressive activities comprise a key function of Tregs in regulating health and disease. As Tregs receive increasing attention as therapeutic targets, understanding their non-canonical functions may become an important feature of Treg-directed interventions.
Keywords: regulatory T cell, autoimmunity, tissue regulation
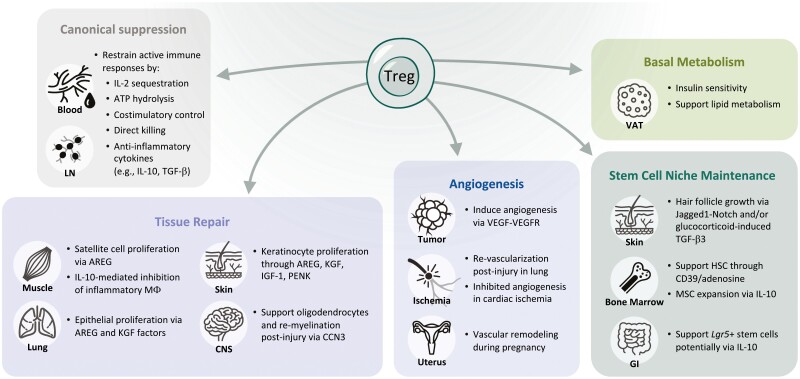
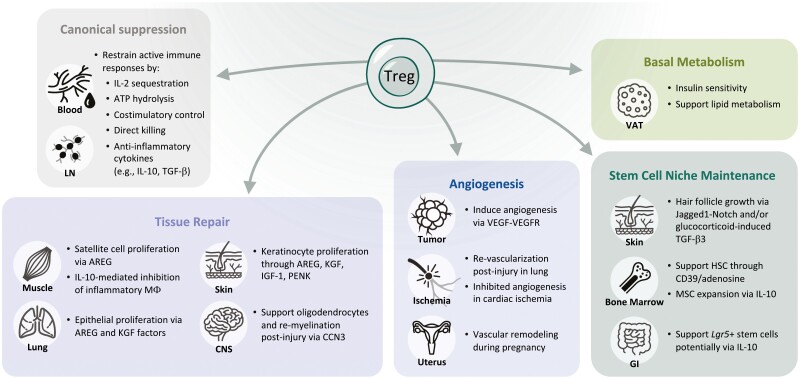
Diverse functions of Tregs beyond immune suppression. As detailed in the canonical suppression box, Tregs use several mechanisms to dampen proliferation and function of effector T cells and other immune cells. Tregs also have myriad effects on tissue repair, angiogenesis, basal metabolism, and maintenance of the stem cell niche.
Graphical Abstract
Graphical Abstract.
Introduction
To specialize in detecting dangerous pathogens, cells of the immune system continually patrol the blood and tissues of the body. While lymphoid organs such as the bone marrow, spleen, and lymph nodes are critical for the development, organization, and efficient expansion of immune cells, barrier tissues (including the skin, lung, and gut) are the predominant sites of first contact with exogenous antigens in our environment. Immune cells in these tissues have evolved mechanisms to reside there and continually sample antigens to decide if, and when, to trigger an immune response.
While recognition and removal of pathogens is often considered the primary function of the immune system, it is equally important that the immune system effectively decide when not to trigger an immune response. To accomplish this, the immune system must efficiently identify ‘self’ or non-pathogenic ‘non-self’ and ensure that the powerful defenses used against pathogens do not cause irreversible tissue damage to the host. Indeed, one of the most debilitating immune deficiencies can be seen in people suffering from Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, wherein patients suffer from a multitude of autoimmune disorders, with most succumbing within the first few months of life [1–3]. Most IPEX patients have a mutation in the gene encoding forkhead box P3 (FOXP3), a transcription factor that was found to be critical for CD4+ regulatory T-cell (Treg) fate in mice [4, 5]. While FOXP3 is the most frequent driver of IPEX, mutations in other genes can cause IPEX-like disease. Interestingly, while several of these genes (CTLA4, IL2RA, STAT5B) are also critical for Treg function, some of them, such as DOCK8 and STAT1, have wider roles in the immune system and may allow other cells to override Treg suppression [6–8]. Overall, the devastating effects seen when vast immune dysregulation prevents the appropriate maintenance of tolerance underscores the important role Tregs play as the preeminent regulators of the immune response.
There are multiple classes of Tregs, including conventional (CD4+Foxp3+) [9], Type 1 regulatory T cells (CD4+Foxp3-IL10+) [10], Th3 (CD4+Foxp3-TGFb+, variable IL4/IL10) [11] and, more recently, CD8+ regulatory T cells (multiple populations defined, including CD8+CD28low/-; CD8+CD122+Ly49+) [12–15]. High-resolution gene expression studies revealed that each of these broad classes are heterogeneous and contain multiple subsets of distinct Treg subpopulations. Conventional Tregs, broadly defined as CD4+Foxp3+CD25+ T cells in mouse and human, are found in most tissues of the body and are the predominant regulatory cell of the immune system. While it is unclear whether Tregs become truly resident in organs throughout the body, many do undergo phenotypic and transcriptional changes while localized in a particular tissue, which have been discussed elsewhere recently [16–18].
Our knowledge of the roles immune cells play in tissues beyond their canonical functions has expanded tremendously in the last several years. It has been observed that immune cells can affect non-immune cell function to maintain homeostasis under steady state and expedite a return to it after the resolution of injury or infection. While these tissue-supportive roles have been appreciated for some time in specific contexts, such as the microglia in the brain [19] or the intraepithelial lymphocytes in the gut [20], the importance of Tregs in tissue regulation has only recently been appreciated. As discussed previously, loss of Tregs causes early onset lethal autoimmunity in mice and humans. Due to this phenotype, it was initially difficult to directly study the effect of Treg depletion or loss of function in adult animals or in specific tissues without inducing severe autoimmunity. However, the engineering of inducible genetic Foxp3 deletion models along with transient antibody depletion have enabled investigation of short-term tissue-specific effects. More recently, the description of tissue-specific Treg transcription factors, such as PPAR-γ in visceral adipose tissue (VAT) Tregs, has allowed for even more targeted deletion of a subset of Tregs in specific tissues [21].
With these techniques, there is a growing body of research indicating that Treg function extends to roles in homeostasis in various tissues. Here, we will focus on the growing literature describing Treg functions in tissue homeostasis, from basal metabolism and maintenance of stem cell niches, to re-establishing homeostasis after tissue damage (Fig. 1).
Figure 1:
Diverse functions of Tregs beyond immune suppression. As detailed in the canonical suppression box, Tregs use several mechanisms to dampen proliferation and function of effector T cells and other immune cells. Tregs also have myriad effects on tissue repair, angiogenesis, basal metabolism, and maintenance of the stem cell niche. Roles in tissue repair and mostly been documented in the muscle, lung, skin, and central nervous system (CNS). Treg-secreted AREG, and KGF are critical for inducing proliferation of epithelial cells in the lung and skin and satellite cells in the muscle. In the CNS, Tregs can support oligodendrocytes ability to re-myelinate neurons following injury. Tregs can be pro- or anti-angiogenic depending on the tissue and model. Tregs have been the most well studied and have a clear role in supporting angiogenesis in tumors to enable growth. Their role in ischemia is less clear: Tregs are required for re-vascularization in the lung following injury but appear to inhibit angiogenesis following cardiac ischemia. Tregs also support vascular remodeling that occurs in the uterus during pregnancy. Fat Tregs, specifically those in the visceral adipose tissue (VAT), are the best described tissue Tregs and have clear roles in regulating insulin sensitivity and supporting lipid metabolism. Finally, Tregs support the unique stem cell niches in the skin, bone marrow, and the gut. Through diverse mechanisms, they can directly maintain stem cell quiescence and indirectly affect these cells by restricting expansion of supportive mesenchymal stromal cells. Overall, while some of these findings have been validated in humans, it is important to note that much of the data comes from studies in mice.
Treg suppression
The canonical and well-characterized role of Tregs is in the suppression of immune responses. For the purpose of this review, we define suppression as those Treg functions that directly control adaptive and innate immune responses through regulation of immune cell activation or direct killing of pathologically activated immune cells. The primary mechanisms of immune suppression by Tregs are as follows: sequestration of interleukin (IL)-2 via expression of the high-affinity receptor alpha chain CD25 (IL2RA); secretion of anti-inflammatory cytokines such as TGF-β, IL-10, and IL-35; hydrolysis of ATP to the immunosuppressive molecule adenosine via CD39 (ENTPD1) and CD73 (NT5E); blocking costimulatory CD80 and CD86 signals via robust expression of CTLA-4; and through direct killing of inflammatory and autoreactive cells using cytotoxic molecules including perforin and granzyme [22] (Fig. 1). Together, these processes mediate Tregs’ core function of suppressing pathological immune responses and controlling autoimmunity and inflammation. While these immunosuppressive processes can play an important role in tissue homeostasis and repair, we will be discussing roles that extend beyond these canonical Treg functions.
Non-canonical Treg functions
Modulation of basal metabolism
The population of Tregs in VAT surrounding vital organs was the first tissue-specific population to be deeply characterized and is the most well understood at present. The Benoist–Mathis lab pioneered much of the work on VAT Tregs [21, 23]. In addition to describing this population, they demonstrated the functional importance of these cells utilizing Foxp3 promoter-driven DTR-mediated depletion of Tregs. This model showed enhanced depletion of VAT Tregs compared with splenic Tregs and resulted in an increase in insulin sensitivity at early time points [23]. Longer time points, which would presumably allow for more profound metabolic alterations, were not possible due to the development of global autoimmunity in these mice. Subsequent work showed that mice fed a high-fat diet (HFD) had a drastic reduction in VAT Tregs that was correlated with dysregulation of insulin resistance and glucose levels [21].
More recently, PPAR-γ was found to be the major transcription factor controlling VAT Tregs [21, 24, 25]; and this discovery ushered in a new wave of targeted studies. Treatment of HFD mice with a PPAR-γ agonist, pioglitazone (Pio), increased VAT Treg numbers and normalized some of these metabolic phenotypes in control mice, while mice with PPAR-γ deletion in Foxp3-expressing cells were not rescued [21]. Gain-of-function studies where VAT Tregs (and lymphoid-tissue Tregs) were expanded with IL-2/anti-IL-2 complexes also improved insulin resistance and glucose levels in HFD mice, providing further evidence that Tregs are involved in metabolic control [23]. Together, these results show a strong correlation between Treg numbers and diet-induced metabolic dysfunction and demonstrate Tregs are one of the key mediators of PPAR-γ-driven rescue from metabolic dysfunction.
Further investigation into the mechanisms involved in VAT Treg function found that PPAR-γ+ VAT Tregs express ST2 and rely on IL-33 to populate the VAT [26, 27]. Treatment of obese mice, which have fewer fat Tregs, with IL-33 expands Tregs to numbers comparable to healthy mice and restores insulin sensitivity [26]. While IL-33 signaling also affects ILCs, macrophages, and other cells, the importance of ST2 signaling in the maintenance of VAT Tregs was demonstrated with a Treg-specific KO of the receptor [24]. Signaling through the alarmin IL-33 has emerged as an important pathway for Treg function, promoting their expansion [28] as well as contributing to various canonical and non-canonical Treg functions, some of which will be described later in this review, particularly as it relates to the expression of Amphiregulin (AREG).
Support of the stem cell niche
Stem cell niches must support steady-state cell turnover, while also responding to damage and enabling a return to homeostasis. Proliferation and differentiation of cells within these niches must be tightly regulated. Interestingly, several instances of direct Treg interactions and regulation of stem cells in the bone marrow (BM), skin, and intestine have been documented and will be detailed below.
Tregs are present at a much higher frequency in the BM compared with most other tissues (upward of 40% of CD4 T cells [29]). High-resolution images of the BM indicate that Tregs reside in the otherwise immune-privileged hematopoietic stem cell (HSC) niche and that the majority of HSCs in the BM were localized near Tregs [30]. Taking advantage of the need for CXCR4 expression for Treg homing to the BM [29], a Foxp3creCxcr4flox/flox mouse model was used to selectively reduce Tregs in the BM. This led to increased expansion and colony-forming ability of HSCs, indicating HSC were more prone to proliferate and differentiate to form multi-lineage colonies in the absence of Tregs [31]. Importantly, BM, but not lymph node, Tregs inhibited HSC colony formation in vitro, indicating that these tissue Tregs can directly affect the activation state of HSC. The Treg-mediated inhibition of HSC expansion was reversed upon antioxidant treatment, suggesting that Tregs maintained HSC quiescence by protecting HSCs from oxidative stress [31]. It was further demonstrated that this antioxidant activity was mediated by Treg-generated adenosine, as Treg-specific deletion of Entpd1 (CD39), which catalyzes the hydrolysis of ATP to generate immunosuppressive adenosine, or inhibition of the adenosine receptor A2AR, produced the same effect [31]. Overall, these data indicate that Tregs serve to protect the quiescence of BM stem cells and inhibit them from aberrantly differentiating.
Studies examining HSC engraftment following transplantation have also revealed roles for Tregs in the BM HSC niche. Transfer of HSC niche Tregs (defined as CD150hi) into an allogenic-HSC model led to improved HSC quiescence and survival and promoted significantly better allogenic-HSC engraftment than transfer of CD150lo Tregs [31]. This effect was also dependent on adenosine, as CD39 deletion in the transferred Tregs reduced HSC engraftment [31]. A second recent study found that Treg depletion prior to HSC transplant resulted in significantly less engraftment [32]. However, instead of the Tregs directly affecting HSCs, Treg-produced IL-10 led to increased expansion of mesenchymal stromal cells (MSCs) and HSCs in vivo, and a lack of MSC-mediated HSC support in vitro [32]. Interestingly, the depletion of BM Tregs in this study did not cause overt inflammation or expansion of other T cells in the BM, implying that Tregs and the IL-10 are not acting broadly to suppress immunity in the BM. Together, these studies provide support for a model in which Tregs produce adenosine and directly protect HSCs from oxidative stress to maintain their quiescence, and this promotes their long-term maintenance. Further studies are needed to dissect the direct and indirect effects of Tregs on HSCs themselves and other immune cells in the niche.
Treg regulation of stem cell niches is not limited to the hematopoietic system. In the skin, hair follicles contain a stem cell niche that is required for the cyclical renewal of hair [33] and hair follicle stem cell (HFSC) function has been correlated with Treg function in humans [34]. Tregs have been observed localized around the HFSC niche, near the bulge of the hair follicle [35–37].
Two-photon imaging on Foxp3-GFP mice further found that Tregs in close proximity to HFSC were less spherical than Tregs located farther away suggesting direct cell–cell contact [38]. During the telogen phase of hair growth, the HFSC are quiescent. Active hair growth occurs during the anagen phase, which can be triggered by senescence of the hair or depilation. Treg numbers fluctuate with these phases and are most abundant in the late telogen phase [38]. Mice that lack all T cells (Rag2−/−) have a delay in anagen induction in response to depilation-induced hair regeneration, as well as during the hair follicle cycle [38]. Specific depletion of Tregs after DTR treatment in Foxp3-DTR mice results in the inability to grow back hair after depilation, with the HFSC losing Ki67 expression in the telogen-to-anagen transition. This interaction was found to be dependent on Notch signaling, as Treg-specific depletion of the Notch ligand Jag-1 in Foxp3Cre/CreJag1fl/fl mice phenocopied the effects of systemic Treg depletion on hair growth [38].
Recently, it was discovered that glucocorticoid receptor (GR) signaling in skin Tregs was critical for HFSC proliferation and hair regrowth in both the depilation model and the natural hair growth cycle [39]. Using a combination of genetic mouse models and in vitro systems, the authors demonstrated that TGF-β3 production was induced in Tregs downstream of GR signaling, which was then required to overcome inhibitory BMP signals and activation HFSC proliferation. Importantly, no changes in immune cell numbers or cytokine production were observed in the skin, supporting the idea that Tregs control HFSCs separate from any immunosuppressive functions.
The intestinal stem cell (ISC) niche may be one of the best described stem cell microenvironments; however, there is still much unknown about the roles that lymphocytes, particularly Tregs, play in this niche. A recent paper by Biton et al. queried sc-RNAseq of WT and Lgr5−/− intestinal epithelial cells (IECs) to identify ISC-immune interactions and characterized the interactions between MHCII+ intestinal stem cells and T helper subsets, including Tregs [40]. In an in vitro organoid system, induced Tregs or IL-10 caused more self-renewal of the stem cells compared with conventional T cells and their associated Th cytokines [40]. Although IL-10 is considered a major Treg effector cytokine, the in vitro Tregs did not make substantial IL-10; therefore, further studies are needed to uncover the exact mechanisms by which Tregs are supporting SC proliferation. Interestingly, these interactions were dependent on MHCII expression in the stem cells, indicating that they directly present antigen and interact with T cells. Furthermore, depletion of Tregs using the Foxp3-DTR genetic mouse model led to increased proliferation of intestinal stem cells and aberrant differentiation into mature cell types [40]. Follow-up studies that conditionally delete IL-10 or other Treg-derived factors will be critical to dissect how Tregs are mediating these changes.
Based on the substantive data detailing the key role, Tregs play in regulating and maintaining the stem cell niche in bone marrow, skin, and gut, there is active research into Treg function in stem cell function in other tissues. Interestingly, Rag-deficient mice do not show overt defects with self-renewal of any of these tissues [41, 42]. This could be due to several factors including the lack of other immune cells or the presence of redundant non-immune mechanisms to maintain the stem cell niche.
Facilitation of tissue repair
Tregs have long been known to play a role in resolution following an immune challenge or barrier breach, but the major role described has been the direct suppression of immune cell function, infiltration, and inflammation. More recently, identification of the molecular mechanisms involved have revealed that Tregs can also have direct effects on non-immune cells to promote healing of an injured tissue. Tregs can produce various growth factors in a tissue-specific matter to promote repair in different tissues such as keratin growth factor (KGF) in the lung, basic fibroblast growth factor 2 in the gut, neurotrophin factor in the spinal cord, neuroregulin-1 in heart, and insulin growth factor 1 in the retina [43–45]. In addition to these, AREG is used by Tregs to facilitate repair across multiple tissues. AREG is an epidermal growth factor (EGF) family protein that is produced by numerous cell types [46]. Early mouse knockout studies indicated that AREG had minimal effects under homeostatic conditions [47], but more recent studies found that AREG expression was important for some immune functions [48, 49]. Still, the specific role of Treg-produced AREG has not been appreciated until recently.
There have been several studies exploring the role that Tregs have in muscle repair following injury. Burzyn et al. showed for the first time that Tregs accumulated at sites of muscle injury [50], and IL-33 was later identified as a key factor driving their recruitment [51]. Depletion of Tregs, whether by genetic mouse models or a Treg-specific deletion of IL-33 receptor, significantly decreases satellite cell proliferation and muscle repair following cardiotoxin-induced injury. Muscle Tregs exhibited robust expression of Areg, and administration of AREG itself or delivery of IL-33 to increase Treg accumulation rescued repair defects in old mice. At least some of the effects of Tregs on muscle repair also involved their immunosuppressive functions. Specifically, Tregs act to restrain IFN-γ production by NK and effector T cells, macrophage activation, and inflammation in injured muscle [52, 53].
Still, there is clear evidence that Tregs can directly affect muscle cells, separate from their ability to broadly dampen inflammation. The main cellular target of AREG was satellite cells, which proliferate, differentiate, and eventually form new myofibers in response to muscle injury. In vivo, the muscle Tregs were closely associated with the satellite cells [50]. In vitro studies have demonstrated that Tregs can directly affect the proliferation and colony forming capacity of satellite cells [50, 54]. Additionally, colony formation could be enhanced ex vivo by treatment with AREG. Together, these results underscore the key role that Tregs and AREG play in muscle repair.
Tregs have been shown to play a central role in tissue repair in the lung as well. Transfer of Tregs into lymphocyte-deficient Rag−/− mice in a model of acute lung injury showed enhanced resolution of the injury, while depletion of Tregs in WT mice aggravated the tissue damage [55].
At least some of these effects were mediated by CD103+ Tregs migrating to the lung and producing KGF to support epithelial cell proliferation following acute respiratory distress syndrome [43, 56]. Similar to muscle, Treg-derived AREG has been demonstrated to play a role in lung injury repair. Specifically, Arpaia et al. utilized T cell- and Treg-specific Areg KO mice to demonstrate that Treg-derived AREG was critical for lung repair and control of tissue damage following viral challenge [57]. AREG production was induced by Treg detection of the alarmins IL-18 and IL-33 and did not require TCR activation. A lack of AREG production in T cells or Tregs did not affect anti-viral immune responses or suppression. Sequencing of lung Tregs following infection indicated that IL-10+IL-18R- Tregs expressed genes encoding suppressive effector molecules (i.e., CD25, CD39), while the IL-10-IL-18R+ Tregs expressed genes involved in ECM generation and tissue repair [57]. Harb et al. recently expanded upon this model by demonstrating that in COVID patients and mouse viral infection models, upregulation of Notch4 in Tregs was critical for IL-18R expression and subsequent AREG production [58]. Altogether, these studies reveal critical roles for Tregs in protecting lung tissue from damage following infection. Further studies are needed to investigate the which specific Treg-secreted proteins are mediating this protection beyond AREG.
In the skin, the predominant cell type mediating repair is the keratinocyte, which migrate to the wound site, proliferate, and differentiate to restore the tissue [59]. AREG has been shown to directly drive keratinocyte proliferation [46], and skin Tregs express both AREG [60] and its receptor EGFR [61]. EGFR deletion in Tregs resulted in reduced infiltration of Tregs at the wound site and delayed wound closure [38]. Dissecting the exact relationships at play is complicated in this context, as several phases of the wound response overlap and resolution includes suppression of inflammation; however, several lines of study suggest Tregs can directly affect keratinocytes and promote re-epithelialization. For example, Tregs have been shown to make KGF and IGF-1, which promote epidermal regeneration [43, 45, 62]. While Tregs are not the exclusive producers of these factors, in vitro studies have demonstrated that Tregs can directly support epithelial cell proliferation through producing KGF [33]. Additionally, Tregs isolated from UVB-irradiated skin were able to induce keratinocyte outgrowth in an AREG and proenkephalin (PENK)-dependent manner [63]. Still, more targeted genetic and in vitro studies are required to determine whether Treg deletion or modulation are truly critical for keratinocyte responses during wound healing.
As in muscle, lung, and skin, Tregs found in the brain also express high levels of the IL-33 receptor ST2 and AREG [27]. A direct role for this pathway has not yet been described, but there is evidence that Tregs themselves also play an important role in central nervous system (CNS) tissue repair. Though the CNS is often considered to be an immune-privileged environment due to the blood–brain barrier, peripheral immune cells do coordinate with CNS-resident immune cells to aid in recovery following acute CNS trauma [64]. Tregs are an important component of this immune response, despite making up a relatively small proportion of these infiltrating immune cells [65]. For example, Tregs can promote myelin regeneration after acute CNS injury by promoting oligodendrocyte differentiation [65]. Though Tregs are not strictly required for the remyelination process per se, Foxp3-DTR transgenic mice depleted of Tregs exhibited significantly fewer differentiated oligodendrocytes post-injury, resulting in significantly fewer remyelinated neurons [65]. This pro-regenerative property of Tregs seems to be driven in part by soluble factors, as media collected from Treg cultures was able to drive regeneration. Importantly, increasing the Treg residence in the CNS by ectopic expression of IL-2 led to reduced neuroinflammation and prevented neurological damage during recovery in a model of traumatic brain injury [66]. It remains to be seen whether AREG drives Treg-driven CNS repair or if a novel pathway is utilized by neural Tregs.
Overall, while the precise mechanisms remain unknown, the studies reviewed here illustrate the diverse roles Tregs play in repair and the control of tissue damage in various context. As our understanding of the exact pathways involved grows, we can begin to exploit these pathways to develop new therapies to promote tissue healing following injury or infection.
Regulation of angiogenesis
Angiogenesis is the process of forming new blood vessels from existing vasculature. The production of these new blood vessels can play an important role in tissue repair, fetal development, the female reproductive system, and cancer. The literature surrounding the influence of Tregs in angiogenesis is still growing, but there is ample evidence that Tregs can be important regulators of this process [67].
One of the best characterized examples of Tregs modulating angiogenesis comes from tumor model systems, where there is mounting evidence that Tregs are robustly pro-angiogenic in the tumor microenvironment. Initial data pointed only to correlations between increased Treg numbers, vascular endothelial growth factor (VEGF) expression, and angiogenesis in tumors [68], but follow-up work has identified Tregs as a direct producer of VEGF and modulator of angiogenesis [69]. Facciabene et al. showed that tumor cells under hypoxic conditions secreted CCL28, which led to the recruitment of CCR10-expressing Tregs and increased angiogenesis. Additionally, Tregs were found to produce substantially higher amounts of VEGF than conventional T cells, and VEGF was further induced under hypoxic conditions. Finally, depletion of Tregs using an anti-CD25 antibody led to reduced VEGF-A and microvascular density, and reduced tumor volumes [69]. Additional studies have shown that circulating Treg proportions are a biomarker for VEGFR inhibitor responsiveness [70], and that Treg proliferation can be directly regulated by VEGF-VEGFR blockade [71]. The latter observation is particularly interesting in that it shows that Tregs can act not only as a source of VEGF but can also respond to this angiogenic growth factor. Together, these studies provide strong evidence for a non-immunosuppressive role of Tregs in supporting tumor growth via induction of angiogenesis.
While available evidence indicates a clear pro-angiogenic role for Tregs in the tumor microenvironment, Tregs have been shown to both induce and inhibit angiogenesis in other tissues and disease settings. The role of Tregs in ischemia has been studied in multiple organ systems. As described below, the results indicate that Tregs play a primarily pro-angiogenic role, but activity varies by tissue site. In the lung, genetic Treg deficiency led to reduced angiogenesis in a left pulmonary artery ligation model of ischemia [72]. This effect was reversed by transplant of CD4+CD25hi Tregs and was proposed to be dependent on the macrophage chemoattractant lipopolysaccharide-induced CXC chemokine (Cxcl5; LIX) [72]. Conversely, in cardiac ischemia injury models, Tregs have been shown to be anti-angiogenic due to their ability to induce endothelial cell apoptosis via DLL4/Notch signaling [73]. Furthermore, in chronic cardiac ischemia models, Tregs expanded but became pathogenic resulting in reduced immunosuppression and increased anti-angiogenic properties [74]. Tregs were able to support tube formation in vitro as well, providing evidence that they directly affect the endothelial cells of the heart. In this study, TNFR1 expression on the Tregs, which was induced following chronic ischemia, was required for the inhibition of angiogenesis [74].
Tregs also play an important role in regulating angiogenesis in the female reproductive system, particularly with respect to embryo implantation and pregnancy. Using a spontaneous abortion-prone mouse strain, Woidacki et al. found that transfer of Tregs improved vascular remodeling, placental development, and resulted in reduced fetal death [75]. This effect was IL-10 dependent and was associated with increased recruitment of uterine mast cells.
Together, this growing body of literature suggests that Tregs can positively or negatively regulate angiogenesis depending on tissue and disease context. The mechanisms used to modulate angiogenesis include classical pathways such as regulation of VEGF signaling, and novel indirect mechanisms such as modulation of endothelial cells by DLL4/Notch or TNFR1 signaling. As angiogenesis is an important facet of normal homeostatic processes and a key driver of disease, this additional role of Tregs must be considered alongside their immunosuppressive functions.
Closing statement
We are only beginning to understand the full versatility of Tregs in regulatory functions beyond suppression. These non-suppressive activities largely occur in non-lymphoid tissues, and to date, several key molecules have been identified as critical for these activities, most notably AREG to mediate repair, IL-33 to attract Tregs, and VEGF/VEGFR to modulate angiogenesis. However, the field is just scratching the surface of the complex molecular mechanisms that likely underly Tregs’ interactions within tissues. Furthermore, while there is some evidence that Tregs can directly affect stem cells and epithelial cells, there is still much unknown about which cells Tregs physically interact with and which cells are affected more broadly by secreted factors. Highly multiplexed imaging and spatial transcriptomic studies are needed to better understand these questions and to describe which cellular neighborhoods Tregs reside in. Together with more sophisticated in vitro co-culture systems, these studies will enable the field to gain a better understanding of how Tregs affect the myriad of other immune and non-immune tissue cells.
The centrality of Tregs in coordinating the immune response and tissue regulation have led to increasing interest in Tregs as a therapeutic target for pharmacological modulation and cell therapy [76, 77]. In particular, understanding how these myriad Treg activities are altered in autoimmune and inflammatory pathologies is an area of growing interest. With the rapidly increasing number of functions attributable to Tregs, it is critical to consider the effects of any therapeutic manipulation of these cells on both the immune system and the tissue cells. A better understanding of these functions and the exact signaling pathways controlling them will enable us to better predict net outcomes in each disease and tissue. Similarly, an increasingly granular understanding of Treg cell heterogeneity and functions within specific tissues will inform therapies that allow us to control Treg activities in a modular fashion to best reverse pathologies. Given the auto-reactive nature of Tregs and their plasticity to be polarized towards T effector phenotypes, analysis of genes and pathways that control these processes in Tregs is critical to gaining a better understanding of how we may control these functions by modulating novel targets.
Together with mouse genetic and especially human studies on the non-immune function of Tregs, these approaches are likely to help us to understand which Treg subsets or pathways should be modulated to optimally enhance immune-regulation, while balancing effects on tissue homeostasis and repair.
Glossary
Abbreviations:
- AREG
amphiregulin
- BM
bone marrow
- CNS
central nervous system
- EGF
epidermal growth factor
- FOXP3
forhead box P3
- GR
glucocorticoid receptor
- HFD
high-fat diet
- HFSC
hair follicle stem cell
- HSC
hematopoietic stem cell
- IEC
intestinal epithelial cell
- IL
interleukin
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X-linked
- ISC
intestinal stem cell
- KGF
keratin growth factor
- LIX
lipopolysaccharide-induced CXC chemokine
- MSC
mesenchymal stromal cells
- PENK
proenkephalin
- Pio
pioglitazone
- Tregs
regulatory T cells
- VAT
visceral adipose tissue
- VEGF
vascular endothelial growth factor.
Contributor Information
Jillian L Astarita, TRex Biosciences, South San Francisco, CA, USA.
Claudia X Dominguez, TRex Biosciences, South San Francisco, CA, USA.
Corey Tan, TRex Biosciences, South San Francisco, CA, USA.
Jovanny Guillen, TRex Biosciences, South San Francisco, CA, USA.
Mariela L Pauli, TRex Biosciences, South San Francisco, CA, USA.
Rosario Labastida, TRex Biosciences, South San Francisco, CA, USA.
Jose Valle, TRex Biosciences, South San Francisco, CA, USA.
Melanie Kleinschek, TRex Biosciences, South San Francisco, CA, USA.
Jesse Lyons, TRex Biosciences, South San Francisco, CA, USA.
Ali A Zarrin, TRex Biosciences, South San Francisco, CA, USA.
Conflict of interests
All authors are employed by Trex Biosciences.
Funding
No funding was used to generate this manuscript.
Data availability
Not applicable.
Author contributions
J.L.A., C.X.D., J.L., and A.A.Z. defined the scope and conceptualized the manuscript. J.L.A., C.X.D. and J.L. reviewed the literature, and wrote the manuscript with help from C.T, J.G., M.L.P., R.L., J.V., M.K., and A.A.Z. with major contributions from J.L.A. and C.X.D. Finalized revisions were completed by J.L.A., C.X.D., J.L., M.K. and A.A.Z. A.A.Z. edited the manuscript and supervised.
Permission to reproduce (for relevant content)
Not applicable.
Clinical trial registration
Not applicable.
The animal research adheres to the ARRIVE guidelines
Not applicable.
References
- 1. Ben-Skowronek I. IPEX syndrome: genetics and treatment options. Genes (Basel) 2021, 12,323–35. doi: 10.3390/genes12030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001, 27, 20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova J-L, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001, 27, 18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 4. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY.. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005, 22, 329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 5. Hori S, Nomura T, Sakaguchi S.. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6. Gambineri E, Ciullini Mannurita S, Hagin D, et al. Clinical, immunological, and molecular heterogeneity of 173 patients with the phenotype of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Front Immunol 2018, 9, 2411. doi: 10.3389/fimmu.2018.02411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyazaki H, Hoshi N, Kohashi M, Tokunaga E, Ku Y, Takenaka H, et al. A case of autoimmune enteropathy with CTLA4 haploinsufficiency. Intest Res 2022, 20, 144–9. doi: 10.5217/ir.2020.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alroqi FJ, Charbonnier LM, Keles S, Ghandour F, Mouawad P, Sabouneh R, et al. DOCK8 deficiency presenting as an IPEX-like disorder. J Clin Immunol 2017, 37, 811–9. doi: 10.1007/s10875-017-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M.. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995, 155, 1151–64. [PubMed] [Google Scholar]
- 10. Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK.. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006, 212, 28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 11. Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev 2001, 182, 207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 12. Vuddamalay Y, Attia M, Vicente R, Pomié C, Enault G, Leobon B, et al. Mouse and human CD8 + CD28 low regulatory T lymphocytes differentiate in the thymus. Immunology 2016, 148, 187–96. doi: 10.1111/imm.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koh DR, Fung-Leung WP, Ho A, Gray D, Acha-Orbea H, Mak TW.. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8-/- mice. Science 1992, 256, 1210–3. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 14. Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H.. Inhibition of follicular T-helper cells by CD8 + regulatory T cells is essential for self tolerance. Nature 2010, 467, 328–32. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang H, Zhang SL, Pernis B.. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science 1992, 256, 1213–5. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 16. Panduro M, Benoist C, Mathis D.. Tissue Tregs. Annu Rev Immunol 2016, 34, 609–33. doi: 10.1146/annurev-immunol-032712-095948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lui PPW, Cho I, Ali N.. Tissue Regulatory T cells. 2020, 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 2019, 50, 493–504.e7. doi: 10.1016/j.immuni.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q, Barres BA.. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 2018, 18, 225–42. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- 20. Cheroutre H, Lambolez F, Mucida D.. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 2011, 11, 445–56. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–53. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Josefowicz SZ, Lu LF, Rudensky AY.. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012, 30, 531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009, 15, 930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, et al. TCR transgenic mice reveal stepwise, multi-site acquisition of the distinctive Fat-Treg phenotype. Cell 2018, 174, 285–299.e12. doi: 10.1016/j.cell.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li C, Muñoz-Rojas AR, Wang G, Mann AO, Benoist C, Mathis D.. PPARγ marks splenic precursors of multiple nonlymphoid-tissue Treg compartments. Proc Natl Acad Sci USA 2021, 118, 1–8. doi: 10.1073/pnas.2025197118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, Levings MK.. IL-33 reverses an obesity-induced deficit in visceral adipose tissue ST2+ T regulatory cells and ameliorates adipose tissue inflammation and insulin resistance. J Immunol 2015, 194, 4777–83. doi: 10.4049/jimmunol.1500020. [DOI] [PubMed] [Google Scholar]
- 27. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol 2015, 16, 276–85. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 28. Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–8. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zou L, Barnett B, Safah H, LaRussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res 2004, 64, 8451–5. doi: 10.1158/0008-5472.can-04-1987. [DOI] [PubMed] [Google Scholar]
- 30. Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 2011, 474, 216–9. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirata Y, Furuhashi K, Ishii H, Li HW, Pinho S, Ding L, et al. CD150(high) bone marrow tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell 2018, 22, 445–453.e5. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camacho V, Matkins VR, Patel SB, et al. Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight 2020, 5, 1–8. doi: 10.1172/jci.insight.135681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blanpain C, Fuchs E.. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 2009, 10, 207–17. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–7. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chow Z, Mueller SN, Deane JA, Hickey MJ.. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J Immunol 2013, 191, 3049–56. doi: 10.4049/jimmunol.1203205. [DOI] [PubMed] [Google Scholar]
- 36. Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, et al. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol 2013, 190, 4483–7. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. J Clin Invest 2014, 124, 1027–36. doi: 10.1172/jci72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ali N, Zirak B, Rodriguez RS, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 2017, 169, 1119–1129 e11. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Hu X, Liang Y, Yu J, Li H, Shokhirev MN, et al. Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Nat Immunol 2022, 23, 1086–97. doi: 10.1038/s41590-022-01244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell 2018, 175, 1307–1320.e22. doi: 10.1016/j.cell.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE.. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992, 68, 869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 42. Shinkai Y, Rathbun G, Lam KP, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992, 68, 855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 43. Dial CF, Tune MK, Doerschuk CM, Mock JR.. Foxp3(+) regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am J Respir Cell Mol Biol 2017, 57, 162–73. doi: 10.1165/rcmb.2017-0019oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song X, Dai D, He X, Zhu S, Yao Y, Gao H, et al. Growth factor FGF2 cooperates with Interleukin-17 to repair intestinal epithelial damage. Immunity 2015, 43, 488–501. doi: 10.1016/j.immuni.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 45. Hui SP, Sheng DZ, Sugimoto K, Gonzalez-Rajal A, Nakagawa S, Hesselson D, et al. Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev Cell 2017, 43, 659–672.e5. doi: 10.1016/j.devcel.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 46. Zaiss DMW, Gause WC, Osborne LC, Artis D.. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015, 42, 216–26. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999, 126, 2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 48. Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR.. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science 2006, 314, 1746–1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- 49. Meulenbroeks C, Van Weelden H, Schwartz C, Voehringer D, Redegeld FA, Rutten VP, et al. Basophil-derived amphiregulin is essential for UVB irradiation-induced immune suppression. J Investig Dermatol 2015, 135, 222–8. doi: 10.1038/jid.2014.329. [DOI] [PubMed] [Google Scholar]
- 50. Burzyn D, Kuswanto W, Kolodin D, Shadrach J L, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 2016, 44, 355–67. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Panduro M, Benoist C, Mathis D.. Treg cells limit IFN-γ production to control macrophage accrual and phenotype during skeletal muscle regeneration. Proc Natl Acad Sci USA 2018, 115, E2585–93. doi: 10.1073/pnas.1800618115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Villalta SA, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med 2014, 258, 142–64. doi: 10.1126/scitranslmed.3009925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Castiglioni A, Corna G, Rigamonti E, Basso V, Vezzoli M, Monno A, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One 2015, 10, e0128094. doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 2009, 119, 2898–913. doi: 10.1172/jci36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol 2014, 7, 1440–51. doi: 10.1038/mi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory T cells in tissue protection. Cell 2015, 162, 1078–89. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harb H, Benamar M, Lai PS, Contini P, Griffith JW, Crestani E, et al. Notch4 signaling limits regulatory T-cell-mediated tissue repair and promotes severe lung inflammation in viral infections. Immunity 2021, 54, 1186–1199.e7. doi: 10.1016/j.immuni.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Piipponen M, Li D, Landen NX.. The immune functions of keratinocytes in skin wound healing. Int J Mol Sci 2020, 21, 8790–816. doi: 10.3390/ijms21228790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Delacher M, Imbusch CD, Weichenhan D, Breiling A, Hotz-Wagenblatt A, Träger U, et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat Immunol 2017, 18, 1160–72. doi: 10.1038/ni.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nosbaum A, Prevel N, Truong HA, Mehta P, Ettinger M, Scharschmidt TC, et al. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol 2016, 196, 2010–4. doi: 10.4049/jimmunol.1502139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nielsen MM, Witherden DA, Havran WL.. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 2017, 17, 733–45. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shime H, Odanaka M, Tsuiji M, et al. Proenkephalin+ regulatory T cells expanded by ultraviolet B exposure maintain skin homeostasis with a healing function. Proc Natl Acad Sci USA 2020, 117, 20696–705. doi: 10.1073/pnas.2000372117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dokalis N, Prinz M.. Resolution of neuroinflammation: mechanisms and potential therapeutic option. Semin Immunopathol 2019, 41, 699–709. doi: 10.1007/s00281-019-00764-1. [DOI] [PubMed] [Google Scholar]
- 65. Dombrowski Y, O’Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci 2017, 20, 674–80. doi: 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yshii L, Pasciuto E, Bielefeld P, Mascali L, Lemaitre P, Marino M, et al. Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat Immunol 2022, 23, 878–91. doi: 10.1038/s41590-022-01208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Luznik Z, Anchouche S, Dana R, Yin J.. Regulatory T cells in angiogenesis. J Immunol 2020, 205, 2557–65. doi: 10.4049/jimmunol.2000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gupta S, Joshi K, Wig JD, Arora SK.. Intratumoral FOXP3 expression in infiltrating breast carcinoma: Its association with clinicopathologic parameters and angiogenesis. Acta Oncol 2007, 46, 792–7. doi: 10.1080/02841860701233443. [DOI] [PubMed] [Google Scholar]
- 69. Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011, 475, 226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 70. Bencsikova B, Budinska E, Selingerova I, et al. Circulating T cell subsets are associated with clinical outcome of anti-VEGF-based 1st-line treatment of metastatic colorectal cancer patients: a prospective study with focus on primary tumor sidedness. BMC Cancer 2019, 19, 687. doi: 10.1186/s12885-019-5909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013, 73, 539–49. doi: 10.1158/0008-5472.can-12-2325. [DOI] [PubMed] [Google Scholar]
- 72. D’Alessio FR, Zhong Q, Jenkins J, Moldobaeva A, Wagner EM.. Lung angiogenesis requires CD4(+) forkhead homeobox protein-3(+) regulatory T cells. Am J Respir Cell Mol Biol 2015, 52, 603–10. doi: 10.1165/rcmb.2014-0278oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huang MT, Dai YS, Chou YB, Juan YH, Wang CC, Chiang BL.. Regulatory T cells negatively regulate neovasculature of airway remodeling via DLL4-Notch signaling. J Immunol 2009, 183, 4745–54. doi: 10.4049/jimmunol.0804371. [DOI] [PubMed] [Google Scholar]
- 74. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 2019, 139, 206–21. doi: 10.1161/circulationaha.118.036065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Woidacki K, Meyer N, Schumacher A, Goldschmidt A, Maurer M, Zenclussen AC.. Transfer of regulatory T cells into abortion-prone mice promotes the expansion of uterine mast cells and normalizes early pregnancy angiogenesis. Sci Rep 2015, 5, 13938. doi: 10.1038/srep13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Raffin C, Vo LT, Bluestone JA.. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020, 20, 158–72. doi: 10.1038/s41577-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pilat N, Sprent J.. Treg therapies revisited: tolerance beyond deletion. Front Immunol 2020, 11, 622810. doi: 10.3389/fimmu.2020.622810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.