Summary
While inflammation may not be the cause of disease, it is well known that it contributes to disease pathogenesis across a multitude of peripheral and central nervous system disorders. Chronic and overactive inflammation due to an effector T-cell-mediated aberrant immune response ultimately leads to tissue damage and neuronal cell death. To counteract peripheral and neuroinflammatory responses, research is being focused on regulatory T cell enhancement as a therapeutic target. Regulatory T cells are an immunosuppressive subpopulation of CD4+ T helper cells essential for maintaining immune homeostasis. The cells play pivotal roles in suppressing immune responses to maintain immune tolerance. In so doing, they control T cell proliferation and pro-inflammatory cytokine production curtailing autoimmunity and inflammation. For nervous system pathologies, Treg are known to affect the onset and tempo of neural injuries. To this end, we review recent findings supporting Treg’s role in disease, as well as serving as a therapeutic agent in multiple sclerosis, myasthenia gravis, Guillain–Barre syndrome, Parkinson’s and Alzheimer’s diseases, and amyotrophic lateral sclerosis. An ever-broader role for Treg in the control of neurologic disease has been shown for traumatic brain injury, stroke, neurotrophic pain, epilepsy, and psychiatric disorders. To such ends, this review serves to examine the role played by Tregs in nervous system diseases with a focus on harnessing their functional therapeutic role(s).
Keywords: regulatory T cells, autoimmunity, neurodegenerative disease, neuroimmunology, inflammation
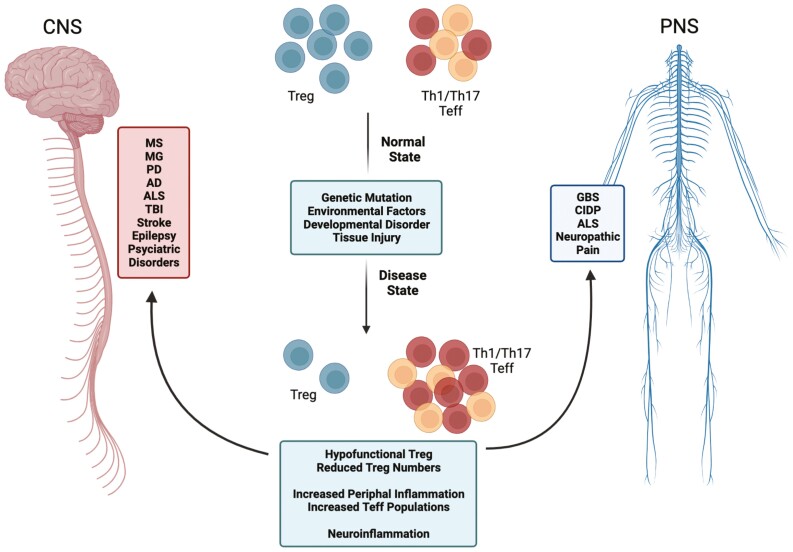
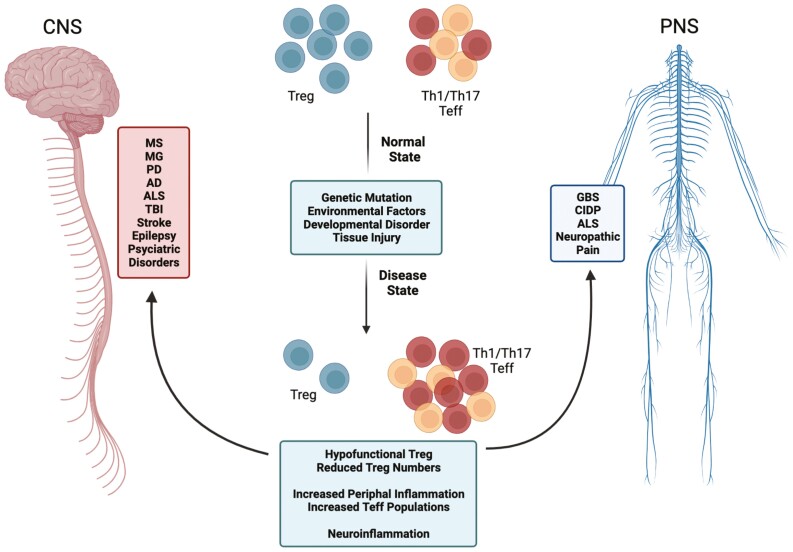
Regulatory T cell:T effector cell imbalances resulting from genetic mutation, neurotoxic environmental factors, developmental disorders, and/or tissue injury lead to an aberrant proinflammatory immune response in a multitude of nervous system pathologies. To counteract the peripheral and neuroinflammatory responses, research is being focused on regulatory T cell enhancement as a therapeutic target in both peripheral and central nervous system disease.
Graphical Abstract
Graphical Abstract.
Introduction
While inflammation itself may not cause nervous system disease, it likely contributes to disease pathogenesis once the disease has been initiated. Emerging evidence suggests aberrant innate and adaptive immune responses in many peripheral nervous systems (PNS) and central nervous system (CNS) pathologies [1]. Previously, the CNS was considered an immune privileged site, but recent studies have indicated that the CNS regularly undergoes immune maintenance and surveillance. It is also understood that an overactive immune response within the brain can lead to autoimmunity, tissue and cellular injury, and degeneration of the nervous system at lesion sites. To overcome this overactive immune response, researchers have turned their focus to modulating the immune response into a protective phenotype by altering the adaptive immune response. A large portion of the cell-mediated adaptive immune response to inflammation and disease is carried out by CD4+ T helper (Th) cells that are generated in the thymus following positive and negative selection against self-antigen. After migrating into the periphery, they are further polarized into Th subsets through various cytokines and transcriptional regulation to become either Th1, Th2, Th17, or regulatory T cells (Treg) (Fig. 1). Th1 cells preferentially produce interferon-gamma (IFN-γ) as a response to viral infection and tumors, whereas Th17 cells produce IL-17 and IL-22 to defend against extracellular pathogens at the mucosal and epithelial levels. However, both cell types have been linked to aberrant activation leading to the pathogenesis of the autoimmune and neurodegenerative disease [2]. Th2 cells produce IL-4, IL-5, and IL-13 to assist with humoral responses.
Figure 1:
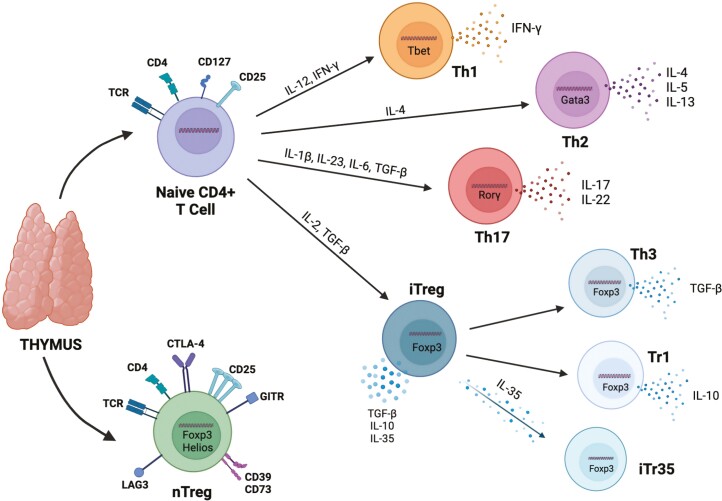
CD4+ T helper cell differentiation. Naïve CD4+ T helper (Th) cells and naïve Regulatory T cells (nTreg) are generated in the thymus after undergoing positive and negative selection against self-antigen. nTreg are naturally derived and express high levels of FoxP3 and Helios transcription factors, along with the T cell receptor (TCR or CD3), CD4, low levels of CD127, and high levels of CD25 (IL-2Rα), CTLA-4, GITR, CD39, CD73, and LAG3 to maintain their natural suppressive function. Other naïve CD4+ T helper cells leave the thymus where they differentiate into Th1, Th2, Th17, or induced Treg (iTreg) based on polarizing cytokines and transcription factor expression. Th1 cells are polarized in the presence of IL-12, IFN-γ, and Tbet. They primarily produce IFN-γ and are considered to be pro-inflammatory effector populations. Th2 cells are polarized by IL-4 and generally express Gata3 and produce IL-4, IL-5, and IL-12 to mediate allergy and humoral responses. Proinflammatory Th17 cells are generated in the presence of IL-1β, IL-23, IL-6, and TGF-β. They are defined by RORγt expression and production of IL-17 and IL-22. In addition, immunosuppressive Tregs can be induced in the periphery from Foxp3-Th cells (iTreg) when IL-2 and TGF-β are present. These cells will begin to express the same immunosuppressive markers as nTreg, as well as stable expression of the FoxP3 transcription factor. They produce anti-inflammatory cytokines, TGF-β, IL-10, and IL-35. iTreg can be further broken down into TGF-β-producing Th3, IL-10-producing Tr1, and IL-35-producing iTr35 depending on their predominant cytokine production.
Treg are an anti-inflammatory subset of CD4+ T lymphocytes responsible for suppressing pro-inflammatory immune responses of other T helper subsets to maintain immune homeostasis. In both rodents and humans, their cellular phenotype is defined by high expression of IL-2 receptor alpha (CD25) and the transcription factor Forkhead box protein P3 (FOXP3), with low expression of the IL-7 receptor (CD127) [3]. Stable FOXP3 expression is crucial for Treg development, function, and persistence. Mutation in the transcription factor results in Immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome characterized by aberrant Treg function and the inability to adequately control the immune response [4]. Treg stability and immunosuppressive function are defined by the methylation status of the Treg-specific demethylated region (TSDR), a non-coding region within the FOXP3 locus [5]. Supplementary markers of Treg phenotype, suppressive capacity, migratory capacity, and function vary among subsets but may include CTLA-4, CD39, CD73, GITR, LAG3, Helios, Stat5, and various surface integrins [6]. Variation in these markers likely depends on the microenvironment, activation state, cell:cell interactions, and level of inflammation. Along with the aforementioned phenotypic markers, Tregs can be divided into two major subsets, including natural Tregs (nTregs) and induced Tregs and/or peripherally-derived Treg (iTregs). nTregs are generated in the thymus, arise from CD4+ T lymphocytes in the presence of transforming growth factor beta (TGF-β) and IL-2, and display the classical phenotype of CD3+CD4+CD25+CD127lowFOXP3+ with the presence of the transcription factor Helios [7]. iTregs are induced in the periphery due to their plastic nature and can be categorized into multiple subsets based on modulations in cell surface marker expression, intracellular markers, and cytokine production. These subsets include IL-10-producing Tregs (Tr1), TGF-β-producing Tregs (Th3), and IL-35–producing Treg (iTr35) (Fig. 1). Therefore, their phenotypic plasticity makes them an appealing target for the therapeutic modulation of proinflammatory and overactive adaptive immune responses. The varying cellular phenotypes can be sorted utilizing extracellular markers and phenotyped by their expression of intracellular markers including the presence of various cytokines and transcription factors. Following isolation, cells can be cultured and evaluated for suppressive function utilizing cell proliferation assays. In addition, there are reports of brain-resident Treg that have an activated memory phenotype [8]. These cells likely arise from Treg within the blood that enters through the choroid plexus, through the glia limitans, or through extravasation across the blood-brain barrier due to increased expression of adhesion molecules and chemokine receptors during activation. These Tregs can stay in the brain for an extended period resulting in an established resident Treg population. During this time, brain-resident Tregs are engaged with self-antigens and are primed for the expression of anti-inflammatory mediators such as amphiregulin and IL10. However, the exact nature and identification of the self-antigens by brain-resident Tregs still remain unresolved.
To carry out their role, Tregs maintain immunosuppression via both direct and indirect mechanisms [3, 6, 7]. Direct mechanisms include inhibition of antigen presentation via cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and lymphocyte activation protein 3 (LAG3) and cytolysis of effector cells through the secretion of perforins and granzymes. Indirect mechanisms include secretion of anti-inflammatory cytokines, IL-10, IL-35, and TGF-β, presence of CD39, an ATPase, located on the cell surface along with the co-receptor CD73, and the ability to sequester IL-2 required for effector cell proliferation in the surrounding environment. These immunosuppressive mechanisms allow the immune system to function appropriately by mounting an immune response to foreign antigens without leading to tissue damage or cellular death. Therefore, due to their vital immunosuppressive function, dysfunction in Treg populations may lead to autoimmune disease and the progression of other inflammatory or neuroinflammatory conditions (Fig. 2). Thus, they are becoming increasingly more popular as potential disease targets, especially in nervous system pathologies in which low Treg numbers and/or decreased function are linked to disease pathogenesis or severity. Here, we discuss recent findings investigating the contribution of Treg in nervous system pathologies involving autoimmune diseases including multiple sclerosis (MS), myasthenia gravis (MG), Guillain–Barre Syndrome (GBS), and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). We also assess Treg-associated effects in neurodegenerative diseases including Parkinson’s disease (PD), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS), traumatic brain injury (TBI) and stroke, neuropathic pain, epilepsy, and mental health disorders including post-traumatic stress disorder (PTSD), psychosis, and anxiety/depression.
Figure 2:
Adaptive immune system imbalance in nervous system pathologies. In normal, healthy environments, Treg and Th1/Th17 Teff exist in a homeostatic state. Following genetic mutation, environmental factors, developmental disorders, and/or tissue injury resulting in disease, a Treg:Teff imbalance occurs in response to a chronic and overactive pro-inflammatory immune response both the in the brain and the periphery. The immune imbalance is likely due to hypofunctional Treg that are unable to effectively migrate to and suppress the overactive immune response, a reduction in functional circulating Treg, increased levels of peripheral and neuroinflammation, and ultimately, increased proinflammatory Teff populations leading to accelerated disease progression. Nervous system pathologies affected by this imbalance include those in the central nervous system (CNS) such as multiple sclerosis (MS), myasthenia gravis (MG), Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), tramautic brain injury (TBI), stroke, epilepsy, post-tramautic stress disorder (PTSD), depression, anxiety, and psychosis. Peripheral nervous system (PNS) diseases include Guillian–Barre syndrome (GBS), chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), ALS, and neuropathic pain.
Treg dysfunction and nervous system pathologies
Autoimmune diseases of the central and peripheral nervous system
Multiple sclerosis (MS)
MS is a chronic inflammatory demyelinating disease with an unknown etiology. It is characterized by cognitive impairments, vision abnormalities, muscle weakness, pain, fatigue, muscle spasms, coordination and balance issues, changes in sensation, and paralysis in extreme cases, all likely the result of demyelination [9]. The exact pathogenesis of the disease is unknown, but it is widely accepted to be immune-mediated and attributed to myelin-specific effector T cells that target the myelin sheath leading to an autoimmune response within the CNS. The most common animal model for studying the disease process is experimental autoimmune encephalomyelitis (EAE) [10]. EAE shares some disease characteristics such as demyelination, neuroinflammation, neuronal death/damage, and autoreactive T cell infiltration, which can be induced through immunization with self-antigens such as myelin oligodendrocyte glycoprotein (MOG), myelin basic protein (MBP), or proteolipoprotein (PLP). Due to the engagement of the adaptive immune system in the disease course and autoimmune dysfunction, the EAE model has been largely utilized to investigate the role of autoreactive T effector cells (Teff) and Treg in disease progression [11]. However, both human and animal models of MS have shown discrepancies in the contribution of Treg to disease pathology. Evaluations into low Treg numbers and disease progression and susceptibility are being carried out to address these discrepancies. It has been shown that TGF-β signaling is dysfunctional in MS, which is likely linked to Treg dysfunction [12]. Additionally, studies reveal that MS subjects have reduced IL-10-secreting Treg in the periphery and an increase in IL-21-secreting cells, resulting in a proinflammatory immune imbalance in diseased states [13]. Studies utilizing Treg-deficient MS mice indicate that adoptive transfer of Treg promotes regeneration and oligodendrocyte progenitor cell proliferation, and an additional EAE study utilizing Treg ablation displayed that Tregs are crucial for remyelination and stunting the neuroinflammatory response in chronic stages of the disease [14, 15]. However, other studies have reported a detrimental role of immunosuppressive Treg in disease progression. Evaluation of immunosuppressive surface marker expressions such as CD73 and CD103 correlates with disease severity, and MS patients have been found with increased peripheral activated Treg and decreased resting Treg when compared to healthy controls [16, 17].
In addition, a phase I clinical assessment reported that adoptive transfer of Treg into patients with relapsing-remitting MS revealed no adverse events and was safe and tolerable [18]. Additonally, ex vivo expanded Treg from MS patients restored their dysfunction, decreased their methylation status, and enhanced their immunosuppressive capacity [19]. In newly identified MS cases, peripheral Treg levels were significantly lower than in treated subjects or healthy controls [20]. However, following Interferon beta-1 alpha (INFβ1α) therapy, a drug shown to significantly slow disease progression, Treg populations were increased without modulating other immune markers. Likewise, in secondary progressive MS, there is a decrease in CD4+ and CD8+ T cells with enrichment in Th2, Treg, and Teff populations following Siponimod therapy [21]. The humoral response was also altered with this therapy, shifting the response into a regulatory B cell signature. Studies utilizing silymarin have reported similar results and Treg restoration [22]. Exploration of lymphoid aggregates in the brains of MS subjects also reports a lack of Treg in the CNS, indicating that they may not negatively participate in disease progression and their decreased presence may in fact contribute to it [23].
Myasthenia gravis (MG)
MG primarily involves degeneration of the neuromuscular junction by autoantibodies to the acetylcholine receptor or muscle-specific tyrosine kinase resulting in muscle weakness and fatigue [24]. It is well known that activated T cells and plasma cells are involved in the production of pathogenic autoantibodies and induction of the inflammatory cascade at this junction. Many studies have focused on the role of the adaptive immune response in disease progression, and the disease is considered to be largely T-cell mediated [25, 26]. Studies indicate increased numbers of Th1 and Th17 cells along with their cell-associated cytokines such as IL-1β, IL-6, IL-17, IFN-γ, and tumor necrosis factor-alpha (TNF-α), with an aberration in Treg subsets that is linked to the pathogenesis of the disease [27, 28]. It has also been shown that individuals with MG have decreased expression of CTLA-4, FOXP3, and IL-10, indicating a possible dysfunction in Treg subsets [29]. Additionally, evaluations of Treg function and Th17 phenotypes in diseased states reveal significant defects in suppressive capacity, as well as a Treg:Th17 imbalance that may further disease progression [30, 31]. Findings suggest that mitochondrial dysfunction within the Treg population may be an additional culprit [32]. Therefore, recent studies in humans and rat models of experimental autoimmune MG (EAMG) are focusing on the use of Treg-inducing agents such as melatonin or adoptive transfer of autologous Treg to fix the immune imbalance observed in disease [33, 34].
Guillain–Barre syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP)
GBS is an immune-mediated acute inflammatory disorder within the peripheral nervous system that is characterized by infiltration of autoreactive inflammatory cells that cause degeneration of myelin and axonal damage [35]. Disease pathogenesis is thought to be mediated by Th1 effector cells and a disturbance of the Th1/Th2 and Th17/Treg balances within the system [36, 37]. Experimental autoimmune neuritis (EAN) is generally utilized to model the disease in animals. CIDP is an immune-mediated peripheral nervous system disease with similar pathology to GBS [38]. The etiology of CIDP remains largely unknown, but like GBS, T-cell activation and the presence of myelin protein antibodies are believed to play an important role in disease pathogenesis. In addition, both diseases have been linked to peripheral Treg dysfunction. Subjects in acute stage GBS exhibit significantly reduced peripheral Treg numbers when compared to healthy controls, but when suppressive function and FOXP3 levels were compared, there were no differences, suggesting that a short-term reduction of circulating functional Tregs is enough to speed pathogenesis [39]. Treatment with immune modulators such as immunoglobulins, Bifidobacterium, or decitabine is linked to restoration in Treg numbers, reduction in Th2 and Th17 phenotypes, and enhancement of IL-10 and TGF-β1 secretion [37, 39–42]. In addition, a rat model of EAN suggests that Treg has a therapeutic effect by significantly reducing infiltration of inflammatory cells in the sciatic nerve and rescuing myelin and axonal damage [43]. Studies in CIDP show similar Treg phenotypes such as the decreased number and defective suppressive function when compared to healthy controls [44, 45]. Flow cytometric analysis revealed minimal changes in other immune subsets, and peak Treg dysfunction appeared during the progressive or relapsing phases of the disease, indicating their potential protective role [45].
Neurodegenerative diseases
Parkinson’s Disease (PD)
Parkinson’s Disease is the most common neurodegenerative movement disorder. It is characterized by the loss of dopaminergic neurons along the nigrostriatal tract and the presence of intraneuronal inclusions of modified or misfolded alpha-synuclein (α-syn) called Lewy Bodies [46]. In addition, both peripheral immune alterations and neuroinflammation have been implicated in disease progression and neurodegeneration [47]. PD brain analyses reveal increased microgliosis and immune cell infiltration, along with the increased presence of proinflammatory cytokines such as IL-6, TNF-α, IL1-β, and IFN-γ in both the CNS and periphery [48, 49]. Due to these inflammatory associations, peripheral immune cell dysfunction has been studied in multiple models of the disease, as well as in clinical studies. Peripheral blood analysis has demonstrated alterations in CD4+ T helper cells and CD8+ cytotoxic T lymphocytes [50–52]. In addition, PD patients display increased proinflammatory subsets with a coordinated decrease in anti-inflammatory cells. Multiple studies also suggest a T-cell-mediated response to aggregated forms of alpha-synuclein, which has been supported by their presence in all disease stages [53]. Some clinical evaluations confirm that patients with PD have fewer Tregs with decreased cell function when compared to healthy volunteers [51, 54–56] These findings are also supported in both acute and chronic animal models of PD [57–59]. PD animals exhibit fewer Treg and more Th1 and Th17 cells during the disease course.
An additional clinical evaluation suggests an even greater regulatory impairment in disease that corresponds to decreased levels of multiple suppressive cell subsets, including suppressor Tregs, active Tregs, Type 1 Treg (Tr1) cells, IL-10-producing CD4 and CD8 cells, and tolerogenic dendritic cells, suggesting a global defect in the ability to suppress an overactive immune response [52]. Therefore, researchers are focusing on mechanisms to fix the regulatory impairment observed in the disease. Augmentation, induction, and/or adoptive transfer of Treg results in neuronal survival and attenuation of neuroinflammation in neurotoxin and α-syn models of disease [55, 60–63]. In addition, both in vivo and ex vivo expansion of dysfunctional Treg isolated from PD subjects restores their suppressive function [54, 55, 64]. However, some clinical studies report opposite findings and suggest an increase in Treg as the disease progresses [65]. Additionally, a study evaluating disease progression and peripheral blood T and B cell populations in a transgenic A53T mouse model of PD suggests an increase in CD3+ and CD4+ T cell populations, with a decrease in CD19+ B cells in early stages, followed by movement impairments and increased T helper cell subsets, including Tregs by 10 months of age [66].
Alzheimer’s Disease (AD)
Alzheimer’s Disease is the most prevalent neurodegenerative disorder. It is characterized by memory loss, deficits in cognition, language impairment, and behavior disturbances [67]. Clinical hallmarks include the presence of proteinaceous inclusions containing amyloid beta (Aβ) and neurofibrillary tangles (NFT) containing tau. Both are thought to contribute to neuronal damage and loss and likely play a role in the neuroinflammatory cascade connected to the disease. Additionally, like PD, T cells have been shown to be dysfunctional in AD and are involved in disease pathogenesis by secreting proinflammatory mediators resulting in infiltration into the brain and interaction with resident microglia [68, 69]. Studies report a Th17/Th1/Treg imbalance with increased IL-17 and decreased IL-10 levels in serum and cerebral spinal fluid (CSF), as well as higher proportions of effector memory T cells and fewer Tregs and naïve T cells when compared to controls [68–71]. Also, early depletion of Tregs correlates with the acceleration of cognitive impairment and restoration of Tregs restores cognition [72]. Adoptive transfer of Tregs in a 3xTg-AD mouse model also improved cognitive function, reduced deposition of Aβ plaques, and ultimately ameliorated disease progression [73]. However, even with these findings, some studies report the beneficial role of breaking Treg-mediated immune tolerance to maintain the activation of microglia for Aβ plaque removal to enhance cognitive impairment [74, 75]. It is argued that suppressing this beneficial microglial immune response will lead to increased Aβ burden and enhance cognitive decline. These findings suggest a controversial role of the protective effects of Treg in AD. Unfortunately, protective inhibition of Treg for the treatment of AD and clearance of Aβ has not been replicated despite efforts by multiple research groups.
Amyotrophic lateral sclerosis (ALS)
ALS, also known as Lou Gehrig’s and motor neuron disease (MND), is a fatal neurodegenerative disorder characterized by rapid neuronal degeneration and inflammation in upper and lower motor neurons [76]. The cause of the disease is unknown, but several factors have been linked to its pathogenesis including the presence of misfolded proteins, oxidative stress, glial activation, mitochondrial dysfunction, and neuronal inflammation. Like most other neurodegenerative diseases, the immune system has been implicated in disease pathogenesis, and altering the neuroinflammatory response would likely be beneficial for slowing disease progression. Clinical evidence demonstrates that ALS patients have a dramatic reduction in Treg numbers that are not effective in suppressing immune responses [77, 78]. Epigenetic evaluations also show higher methylation of the TSDR in ALS patient Tregs [77]. In addition, decreased Treg levels are correlated with increased rates of disease progression and death [79, 80] Rapidly progressing patients display reduced mRNA levels of FOXP3, TGF-β, IL4, and GATA3 indicating decreased Th2 populations as well [81]. For this reason, Tregs and Th2 cells are now being considered as promising targets for neuroprotection in ALS. In support of this, evaluations in the SOD1G93A mouse model indicate that Treg expansion preserves motor neuron soma size and suppresses microglial and astrocytic immunoreactivity in the spinal cord along with increased neurotrophic factor production within the spinal cord and peripheral nerves [80]. Although, clinical evaluation using dimethyl fumarate to positively affect Treg numbers has indicated that there was a lack of efficacy in primary and secondary endpoints for slowing disease progression [82]. However, direct infusions of autologous Tregs have been safe and well-tolerated in ALS patients [83]. Treg number and immunosuppressive function were increased following infusion and were correlated with slowing disease progression.
Traumatic injury within the CNS
Traumatic brain injury (TBI) and stroke
TBI is a complex two-step brain injury in which there is a primary lesion along with secondary brain injury [84]. Initially, there is mechanical stress followed by several cellular and biochemical events such as oxidative stress, mitochondrial dysfunction, inflammation, and ultimately, cell death. In addition, it has been shown that there is massive infiltration of circulating immune cells into the CNS in both TBI patients and animal models of cortical injury [85]. This inflammatory response has dual beneficial and detrimental roles in the brain depending on time, nature of the response, and phenotype of cells entering the site of inflammation. Studies evaluating the role of Treg in the inflammatory cascade and tissue clean-up phase following TBI are limited, but some results show that the depletion of Tregs results in proinflammatory effector T cell infiltration into the brain, increased reactive microgliosis, and elevated IFN-γ expression, leading to increased motor deficits and tissue damage [86]. Evaluation of peripheral Treg phenotypes and frequency in TBI patients compared to normal controls revealed no difference among groups; however, the time-course of peripheral Treg frequencies changes post injury [87]. Circulating Treg number positively corresponded to survival and post-injury recovery, with their peak levels at day 14, indicating that Tregs likely play an active role in tissue recovery following damage. Their efficacy has been linked to maintaining the Treg/Th17 balance via increased TGF-β and decreased nuclear factor-κB (NFκB) signaling to reduce neurological impairment normally associated with the neuroinflammatory cascade following injury [88].
Stroke occurs when a blood clot blocks and/or narrows an artery leading to the brain. This blockage results in hypoxia and neuroinflammation [89]. Following stroke initiation, an inflammatory process is activated resulting in neuronal death, microglial activation, disruption of the blood-brain barrier, lymphocyte infiltration, and proinflammatory cytokines and reactive oxygen species release into the surrounding environment. Therefore, the immune system plays a pivotal role in the pathophysiology of stroke. Like TBI, Treg evaluation in stroke is limited, but it is suggested that Tregs play a role in immune regulation and self-tolerance following ischemic stroke, ultimately contributing the survival outcome [90]. It is hypothesized that Tregs decrease over activation of the immune response and may have a beneficial role. However, there are still some controversies about how Tregs contribute to neuroprotection following stroke. The discrepancy likely arises from varying Treg number and function depending on the site of action and the surrounding inflammatory microenvironment [91]. Animal models of ischemic stroke show a large infiltration of Tregs into the brain in the chronic phase of the disease due to increased IL-2, IL-33, serotonin, CCL1, and CCL20 [92, 93]. These infiltrating Treg suppressed astrogliosis through the induction of amphiregulin and are also shown to decrease MMP9 and CCL2 levels [94]. It is also suggested that the infiltrating Treg promote a pro-regenerative environment during all stages of recovery [95]. Immediately following ischemic stroke, subjects have significantly elevated circulating Treg that peaks two days post-injury [96]. Correlation analyses indicate that subjects with lower numbers of circulating Treg have a higher risk of early neurological deterioration and infection than those with higher numbers of circulating Treg. Taken together, these findings suggest that Treg may participate in the recovery of both TBI and ischemic stroke patients, making them in a potential therapeutic target for both injuries.
Additional nervous system pathologies
Neuropathic pain, epilepsy, and mental health disorders
Neuropathic pain is a chronic disease that is generally caused by progressive nerve damage that can occur as the result of comorbid disease, injury, or infection [97]. It is a lesion of the somatosensory system including damage to peripheral fibers and central neurons in which there are imbalances between the excitatory and inhibitory signaling systems. In addition, there is increasing evidence of the role of inflammation in neuropathic pain. Neuropathic pain is thought to be mediated by IFN-γ-producing Th1 cells [98]. When FOXP3+ Treg are depleted in animals with a chronic-constriction injury of the sciatic nerve, inhibition of pain is eliminated, and there is a dramatic Th1 cell infiltration into the spinal cord [99]. In a partial sciatic nerve ligation mouse model, it is shown that Tregs also infiltrate and proliferate at the site of injury [100]. These cells suppressed the development of pain through inhibition of the Th1 inflammatory response, and they reduced neuronal damage and neuroinflammation in the sensory ganglia through IL-10 signaling. In a model of traumatic painful neuroma following a neurotomy, Tregs reduced the ratio of M1/M2 macrophages, ultimately reducing inflammation-induced pain [101]. A recent study in patients with neuropathic pain displayed a Th17/Treg imbalance in which circulating Tregs were increased and Th17 were decreased [102]. This was confirmed through evaluation of mRNA levels of FOXP3, TGF-β, and RORγt. The increased levels of Treg along with the presence of neuropathic pain are likely due to ongoing stress and an attempt to alleviate pain. However, it remains to be elucidated whether these alterations contribute to pathogenesis in any detrimental way.
Epilepsy is a neurological disease characterized by the presence of recurrent seizures that is associated with lesions in the CNS [103]. Impairments in the activation state and resolution of inflammation following lesion formation have been associated with the development of epilepsy. Inflammatory events are noted within the neuronal tissue, at the BBB, and in the periphery. Proinflammatory IL-17 and granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing T cells are concentrated in epileptic sites, where increased presence of effector T cells correlates directly with disease severity, and Treg depletion with elevated seizure severity [104]. In childhood epilepsy patients, the proportion of Th17 cells and expression of IL17A and RORγt is significantly higher than healthy controls [105]. Subjects also have significantly lower levels of circulating Treg and expression of FOXP3, GITR, and CTLA-4. Childhood epilepsy T cell signature shows a shift toward proinflammatory IL17 production, altered natural killer (NK) cell subsets, and unchanged CD4+ and CD8+ levels [106]. Evaluation of intercellular signaling revealed the loss of inhibitor/regulatory networks leading to pathogenic responses in the neuroinflammatory immune cell cascade. However, in studies of temporal lobe epilepsy, subjects had higher levels of IL-10-producing Treg when compared to age-matched controls [107]. Subjects also display elevations in many additional immune markers such as HLA-DR, CD69, CTLA-4, IL-23R, IFN-γ, TNF-α, and IL-17. Correlation analyses revealed that the frequency of Treg correlated with the age of seizure onset. However, whether Treg increases are linked to seizure activity or due to the perturbed inflammatory response that is present is yet to be revealed.
Apart from diseases attributable to detectable lesion formation and nerve cell damage in the CNS and PNS, the immune system and inflammation have also been linked to many mental health disorders including psychosis, PTSD, anxiety, and depression. Many of these psychiatric disorders and symptoms have been linked to autoimmunity and are generally associated with stressed dysregulation of glutamatergic and monoaminergic systems leading to neurotransmitter release and uptake abnormalities [108]. The exact mechanisms remain elusive, but neuroinflammation appears to be linked to this dysfunction. Additional clinical and animal model evaluations propose that Tregs are hypofunctional in these disease states and may contribute to their presence and/or worsening of disease [109–111]. Studies show that impaired Treg leads to astrocytic and microglial overactivation in schizophrenia, along with decreased levels of HLA-DR+ memory Treg and dendritic cells [109, 112]. These alterations were linked to more severe cognitive deficits and negative symptoms associated with disease [112]. Although, in another study evaluating Th17/Treg balance and NK shifts in relation to psychosis and social stress, it was observed that there were no significant differences in Th1, Th2, Treg, or NK numbers between groups [113]. However, high psychosis liability was linked to increased Treg, decreased NK, and increased Th17 number, potentially due to the high levels of stress associated with disease. Likewise, altered Teff and Treg ratios are observed in bipolar disorder; however, there is some indication that although considered detrimental in autoimmunity, Th17 cells may play a role in functional and structural integrity of the brain with Tregs suppressing this potentially protective response [114, 115].
Studies in PTSD, depression, and anxiety have also reported similar findings to psychosis. Evaluation of PTSD and non-PTSD individuals reveal a substantial reduction in both number and function of naïve T cells and Treg following traumatic stress [110, 116, 117]. This altered peripheral immune response may explain why subjects have increased susceptibility to infection, autoimmunity, and inflammation. Patients with major depressive disorder also show a reduced percentage of Treg compared to controls [118]. Additionally, it is reported that after anti-depressant therapy, Treg populations are restored to normal levels, making them an appealing therapeutic target [119]. Given the findings in animal models and some clinical studies, boosting Treg cell function and/or activity may be a potential interventional approach for reducing neuropathic pain development, altering epilepsy, and assisting with psychiatric disorder treatments.
Regulatory T-cell-enhancing therapies
Currently, there are multiple approaches to generate and/or expand Tregs both in vitro and in vivo. These methods are targeted at enhancing native Treg stability, durability, and/or trafficking capabilities, engineering antigen-specific Treg, and inducing Treg number and function through the use of immunomodulators or adoptive transfer (Fig. 3). Each of these strategies has its own benefits for generating an anti-inflammatory response and potential for disease specificity.
Figure 3:
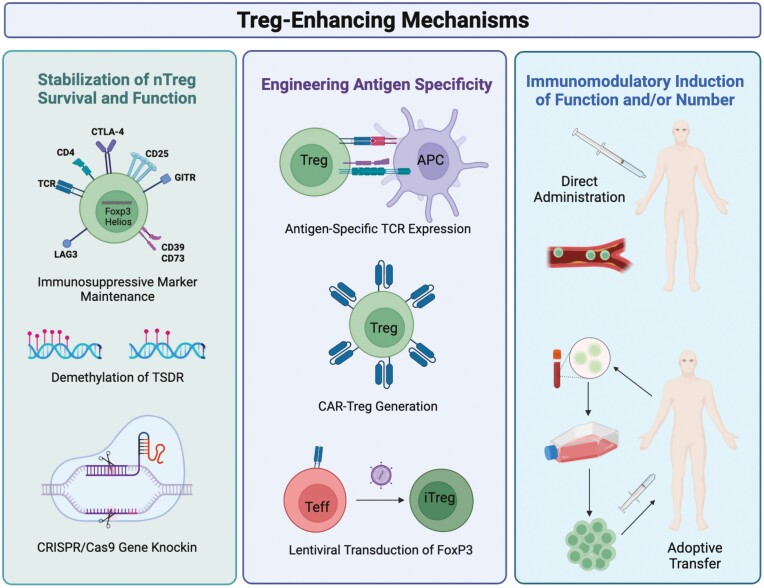
Mechanisms of enhancing natural Treg (nTreg) and induced Treg (iTreg) for the treatment of nervous system pathologies. Currently, there are three main areas of research dedicated to Treg-enhancing therapies: Stabilization of nTreg survival and function, engineering antigen-specific Treg responses, and utilization of immune-modulatory agents to induce peripheral populations. To stabilize nTreg populations, immunosuppressive markers and transcription factors can be maintained through polarizing cytokines and cell activation, demethylation of the Treg-specific demethylated region (TSDR) using methyltransferases, and CRISPR/Cas9 gene editing to generate stable expression of FoxP3. To engineer antigen specificity, researchers are focusing on antigen-specific T cell receptor (TCR) expression, chimeric antigen receptor (CAR) expression, and transformation of antigen-specific Teff into Treg through lentiviral transduction of FoxP3. To generate iTreg populations, direct administration of Treg-inducing agents such as low-dose IL-2, GM-CSF, bee venom, or CD3 mAb are being utilized. Additionally, ex vivo expansion of dysfunctional Treg using these immune agents followed by autologous adoptive transfer is being explored.
First, Tregs display phenotypic plasticity through cytokine signaling and input from the surrounding microenvironment. For instance, Tregs have the ability to express different master regulatory transcription factors to generate functionally distinct subsets with increased trafficking to sites of inflammation, enhanced suppressive function, and/or tissue repair processes. Several animal studies have shown that a small subset of Treg cells can lose FOXP3 expression when there is an IL-2 deficiency or an abundance of proinflammatory cytokines, shifting them into an effector phenotype [120]. On the other hand, increased expression of intracellular markers and transcriptional factors such as Helios and Ikaros zinc finger (IkZF) leads to a stable and highly immunosuppressive phenotype [121]. In addition, the ability to modulate methylation status of the TSDR on the FOXP3 intron will shift stability and suppressive function as well. In this case, epigenetic modifiers, such as DNA methyltransferase, histone demethylase, and/or methyltransferase, can help to stabilize Treg [122]. Lastly, with the invention and feasibility of clustered regularly interspaced short palindromic repeats (CRISPR) gene editing systems, some research has been focused on manipulating Treg stability and trafficking capacity through selective gene knockout or knockin [123–126]. For instance, in a mouse study, the CRISPR/Cas9 system was utilized to stabilize FOXP3 expression by introducing either dCas9-TET1CD of methylcytosine dioxygenase or dCas9-p300CD of histone aceltytransferase with guide RNAs targeted to the foxp3 gene locus. Addition of dCas9-p300CD promoted expression of Treg phenotypic genes and enhanced suppression activity [125]. However, only a few studies have done so in primary immune cells, specifically Treg, and little is known about the relationship between artificial genome editing and its effect on epigenetic regulation endogenously.
Next, there are several approaches to generate antigen-specific Treg to enhance their specificity over polyclonal Treg. However, their expansion is difficult due to their low frequency in the body. Therefore, researchers are focusing on modifying polyclonal Tregs by introducing synthetic receptors in the form of a chimeric antigen receptor (CAR) or an engineered T cell receptor (TCR) to directly recognize antigen itself or in an MHC-antigen configuration. Preclinical models of ulcerative colitis, rheumatoid arthritis (RA), Type-1 Diabetes (T1D), MS, graft-versus-host disease (GVHD), and transplantation have displayed that engineering antigen-specific Treg has increased immunosuppressive efficiency when compared to polyclonal populations due to their ability to migrate and accumulate in sites of injury or inflammation [127–129]. Their antigen specificity also allows both targeted suppression and non-specific suppression due to their natural suppressive abilities. For example, Treg expressing a transgenic TCR specific for MBP or PLP suppressed both antigen-specific T cells and polyclonal Teff in close proximity [129]. However, in human studies, TCR specificity would require MHC compatibility, which varies among subjects, ultimately limiting their clinical utility. Therefore, researchers have begun focusing on CAR Treg to overcome this potential problem.
CARs are engineered receptors that provide the T cell with an ability to target a specific protein as well as activate the cell simultaneously. They are comprised of an antigen-binding domain, a transmembrane domain, and an intracellular domain that leads to cell function and activation signaling cascades [130]. Previously, CAR T cells have shown efficacy in blood cancer, models of colitis, and transplantation [131–133]. Their advantage lies in their ability to recognize aberrant proteins in target tissues and their lack of MHC restriction, making them applicable to a larger number of subjects. Work performed using models for colitis, GVHD, and skin transplantations have shown that the use of CAR-engineered Treg enhances cell migration to sites of inflammation or injury, better suppression of Teff responses and proliferation, and reduces tissue and cellular injury [129]. However, some studies report that CAR Treg may have cytotoxic effects leading to perforin and granzyme-mediated cytolysis resulting in potential tissue damage and cellular death. Additionally, CAR T cells in cancer studies have also been linked to development of a cytokine “storm” and potential neuronal cytotoxicity; however, it is unknown if CAR Treg may have this unwanted affect as well. A final approach is to convert antigen-specific Teff into Treg by overexpression of FOXP3. Conversion of antigen-specific Teff using lentiviral transduction of FOXP3 successfully shifts cells into a stable and activated Treg phenotype in cellular, preclinical, and clinical settings [134]. However, there is evidence suggesting that these induced cells differ in function and persistence when compared to naïve Treg, likely due to their lack of endogenous Treg suppressive markers and mechanisms such as surface expression of CD39/CD73, CTLA-4, and programmed cell death protein 1 (PD1). Therefore, modulating antigen-specific Teff into Treg may not result in a suitably suppressive cell type when utilized in in vivo inflammatory models of disease.
Lastly, the use of immune modulators, such as cytokines or peptides, or adoptive transfer of autologous Treg to increase cell number or function in diseased states is under active investigation in multiple nervous system pathologies [58, 59, 135]. It is well known that the growth factor, IL-2, is essential for Treg generation, induction, and stabilization [136]. Selective Treg induction can be achieved through low-dose IL-2 therapy due to their increased affinity to the ligand. Therefore, signaling via the IL-2R using low doses of IL-2 promotes Treg cell persistence and survival while limiting the effect on other T cell subsets. Therapeutic efficacy has been shown in animal models of disease and in clinical trials for GVHD, T1D, ulcerative colitis, EAE/MS, and ALS [137–141]. Likewise, a study utilizing astrocyte-targeted gene delivery of IL-2 increased brain-resident Treg and resulted in a neuroprotective and anti-inflammatory profile in models of TBI, ischemic stroke, and MS without altering peripheral immune responses [83]. This suggests that brain-specific IL-2 administration is a promising delivery platform with therapeutic potential for many neuroinflammatory pathologies. To increase sensitivity and Treg selectivity, modified forms of IL-2 such as monoclonal antibodies (mAbs) and PEGylated versions are also being evaluated for their efficacy [142, 143]. While individuals are not currently utilizing these modifications in nervous system pathologies, it is a likely future avenue, given the increased focus on Treg in these diseases.
Additional agents under investigation include CD3 mAb, bee venom, GM-CSF, rapamycin, and other immune-modulatory drugs or peptides [58, 59, 135, 144–146]. Work from our own laboratory has supported the immune transformative effects of GM-CSF in both murine models and clinical assessments of PD [54, 55, 62, 63, 147]. Treatment results in a dose-dependent increase in Treg populations, increased immunosuppressive markers, alterations in anti-inflammatory CD4+ T cell gene expression, decreased proinflammatory cytokine levels, decreased neuroinflammation, and enhanced neuronal survival in both acute and chronic models of PD and AD [54, 55, 59, 62, 63, 147]. In PD patients, GM-CSF treatment slowed disease progression and resulted in significant improvement in motor output that correlated with increased Treg number and function [54, 55]. Finally, some studies suggest that increasing Treg number and function using ex vivo stimulation followed by adoptive transfer of autologous Treg would be beneficial [64, 148]. Studies show that re-introduction of functional Treg in patients with remitting-relapsing MS or ALS may result in positive disease outcomes by slowing disease progression [18, 19, 149].
Conclusion
Evaluation of the innate and adaptive immune responses in nervous system pathologies has revealed that the immune system plays a critical role in disease pathogenesis or protection depending on the type of response generated. The neuroinflammatory cascade and microenvironment present in the disease states discussed here are generally Teff-mediated, and Treg have the capacity to positively influence the inflammatory response in most cases. However, their limited number and function in many nervous system pathologies lead to disease progression and increased disease severity. Tregs have the ability to maintain self-tolerance, inhibit detrimental and neurotoxic immune responses, suppress Teff-mediated neurodegeneration, and suppress peripheral inflammation associated with disease outcomes as well. Therefore, efforts to enhance or induce Tregs is under active investigation. Because Treg cells are highly specific and immunosuppressive, they should be considered as potent therapeutic agents for the treatment of nervous system pathologies that are linked to neuroinflammation. Enhancing their number or function can be achieved in many ways, such as enhancing stability, durability, and trafficking, artificially engineering their antigen specificity, or using immunomodulators to induce peripheral populations. However, due to the relatively new investigations into this cell population for these indications, clinical translation of Treg-based therapies may still require additional investigation into quantity or dose of Treg required, Treg-mediated mechanisms of suppression, timing of manipulation and/or adoptive transfer, and antigen specificity in each disorder.
Acknowledgements
The authors would like to thank the series editor, Dr. David Tough, for allowing us to contribute to this review series. We would also like to thank BioRender for use of their graphics program and the INBRE grant from NIH (2P20GM103427) for supporting a site license to EndNote software.
Abbreviations
- α-syn
alpha-synuclein
- β
amyloid beta
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- CAR
chimeric antigen receptor
- CCL
chemokine (C-C motif) ligand
- CD
cluster of differentiation
- CIDP
chronic inflammatory demyelinating polyradiculoneuropathy
- CNS
central nervous system
- CRISPR
clustered regularly interspaced short palindromic repeats
- CSF
cerebral spinal fluid
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- DNA
deoxyribonucleic acid
- EAE
experimental autoimmune encephalomyelitis
- EAMG
experimental autoimmune myasthenia gravis
- EAN
experimental autoimmune neuritis
- FOXP3
forkhead box protein P3
- GBS
Guillain-Barre Syndrome
- GITR
Glucocorticoid-induced TNFR-related protein
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GVHD
graft-versus-host disease
- HLA-DR
Human Leukocyte Antigen – DR
- IFN-γ
interferon gamma
- IFNβ1α
Interferon beta-1 alpha
- Ikzf
Ikaros zinc finger
- IL
interleukin
- IPEX
Immunodysregulation polyendocrinopathy enteropathy X-linked
- iTreg
induced regulatory T cell
- LAG3
Lymphocyte-activation protein 3
- mAb
monoclonal antibody
- MBP
myelin basic protein
- MG
myasthenia gravis
- MHC
major histocompatibility complex
- MND
motor neuron disease
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NFκB
Nuclear factor-κB
- NFT
neurofibrillary tangles
- NK
natural killer
- nTreg
natural regulatory T cell
- PD
Parkinson’s disease
- PD1
programmed cell death protein 1
- PEG
polyethylene glycol
- PLP
proteolipoprotein
- PNS
peripheral nervous system
- PTSD
post-traumatic stress disorder.
Contributor Information
Katherine E Olson, Department of Pharmacology and Experimental Neuroscience, Center for Neurodegenerative Disorders, University of Nebraska Medical Center, Omaha, Nebraska 68198, USA.
R L Mosley, Department of Pharmacology and Experimental Neuroscience, Center for Neurodegenerative Disorders, University of Nebraska Medical Center, Omaha, Nebraska 68198, USA.
Howard E Gendelman, Department of Pharmacology and Experimental Neuroscience, Center for Neurodegenerative Disorders, University of Nebraska Medical Center, Omaha, Nebraska 68198, USA.
Conflict of Interest
We declare no conflict of interest.
Funding
This work is supported by the University of Nebraska Foundation, which includes community donations from the Carol Swarts, M.D. Emerging Neuroscience Research Laboratory, the Margaret R. Larson Professorship, the Eisenberg Parkinson’s Research Fund, and the Frances and Louie Blumkin and Harriet Singer Research Foundations and National Institutes of Health grants P01 DA028555, R01 NS36126, P01 NS31492, P01 MH64570, P01 NS43985, P30 MH062261, and R01 AG043540 (HEG), and 2R01 NS034239 (HEG and RLM)
References
- 1. Skaper SD, Facci L, Zusso M, Giusti P.. An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci 2018, 12, 72. doi: 10.3389/fncel.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gonzalez H, Pacheco R.. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation 2014, 11, 201. doi: 10.1186/s12974-014-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grover P, Goel PN, Greene MI.. Regulatory T cells: regulation of identity and function. Front Immunol 2021, 12, 750542. doi: 10.3389/fimmu.2021.750542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bacchetta R, Barzaghi F, Roncarolo MG.. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci 2018, 1417, 5–22. doi: 10.1111/nyas.13011. [DOI] [PubMed] [Google Scholar]
- 5. Alvarez Salazar EK, et al. Methylation of FOXP3 TSDR underlies the impaired suppressive function of tregs from long-term belatacept-treated kidney transplant patients. Front Immunol 2017, 8, 219. doi: 10.3389/fimmu.2017.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savage PA, Klawon DEJ, Miller CH.. Regulatory T cell development. Annu Rev Immunol 2020, 38, 421–53. doi: 10.1146/annurev-immunol-100219-020937. [DOI] [PubMed] [Google Scholar]
- 7. Schmitt EG, Williams CB.. Generation and function of induced regulatory T cells. Front Immunol 2013, 4, 152. doi: 10.3389/fimmu.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liston A, Dooley J, Yshii L.. Brain-resident regulatory T cells and their role in health and disease. Immunol Lett 2022, 248, 26–30. doi: 10.1016/j.imlet.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 9. Yamout BI, Alroughani R.. Multiple sclerosis. Semin Neurol 2018, 38, 212–25. doi: 10.1055/s-0038-1649502. [DOI] [PubMed] [Google Scholar]
- 10. Constantinescu CS, Farooqi N, O’Brien K, Gran B.. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 2011, 164, 1079–106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson AP, Harp CT, Noronha A, Miller SD.. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol 2014, 122, 173–89. doi: 10.1016/B978-0-444-52001-2.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee PW, Severin ME, Lovett-Racke AE.. TGF-beta regulation of encephalitogenic and regulatory T cells in multiple sclerosis. Eur J Immunol 2017, 47, 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haque R, Kim Y, Park K, Jang H, Kim SY, Lee H, et al. Altered distributions in circulating follicular helper and follicular regulatory T cells accountable for imbalanced cytokine production in multiple sclerosis. Clin Exp Immunol 2021, 205, 75–88. doi: 10.1111/cei.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dombrowski Y, O’Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci 2017, 20, 674–80. doi: 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McIntyre LL, Greilach SA, Othy S, Sears-Kraxberger I, Wi B, Ayala-Angulo J, et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol Dis 2020, 140, 104868. doi: 10.1016/j.nbd.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tapia-Maltos MA, Treviño-Frenk I, García-González HB, Rosetti M, Barriga-Maldonado V, Morales-Ramírez F, et al. Identification of regulatory T cell molecules associated with severity of multiple sclerosis. Mult Scler 2021, 27, 1695–705. doi: 10.1177/1352458520977045. [DOI] [PubMed] [Google Scholar]
- 17. Verma ND, Lam AD, Chiu C, Tran GT, Hall BM, Hodgkinson SJ.. Multiple sclerosis patients have reduced resting and increased activated CD4(+)CD25(+)FOXP3(+)T regulatory cells. Sci Rep 2021, 11, 10476. doi: 10.1038/s41598-021-88448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duffy SS, Keating BA, Moalem-Taylor G.. Adoptive Transfer of Regulatory T Cells as a Promising Immunotherapy for the Treatment of Multiple Sclerosis. Front Neurosci 2019, 13, 1107. doi: 10.3389/fnins.2019.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lifshitz GV, Zhdanov DD, Lokhonina AV, Eliseeva DD, Lyssuck EY, Zavalishin IA, et al. Ex vivo expanded regulatory T cells CD4(+)CD25(+)FoxP3(+)CD127(Low) develop strong immunosuppressive activity in patients with remitting-relapsing multiple sclerosis. Autoimmunity 2016, 49, 388–96. doi: 10.1080/08916934.2016.1199020. [DOI] [PubMed] [Google Scholar]
- 20. Ebrahimimonfared M, Ganji A, Zahedi S, Nourbakhsh P, Ghasami K, Mosayebi G.. Characterization of regulatory T-cells in multiple sclerosis patients treated with interferon Beta-1a. CNS Neurol Disord Drug Targets 2018, 17, 113–8. doi: 10.2174/1871527317666180327122435. [DOI] [PubMed] [Google Scholar]
- 21. Wu Q, Mills EA, Wang Q, Dowling CA, Fisher C, Kirch B, et al. Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis. JCI Insight 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shariati M, Shaygannejad V, Abbasirad F, Hosseininasab F, Kazemi M, Mirmosayyeb O, et al. Silymarin restores regulatory T cells (Tregs) function in Multiple Sclerosis (MS) patients in vitro. Inflammation 2019, 42, 1203–14. doi: 10.1007/s10753-019-00980-9. [DOI] [PubMed] [Google Scholar]
- 23. Bell L, Lenhart A, Rosenwald A, Monoranu CM, Berberich-Siebelt F.. Lymphoid aggregates in the CNS of progressive multiple sclerosis patients lack regulatory T cells. Front Immunol 2019, 10, 3090. doi: 10.3389/fimmu.2019.03090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ.. Treatment of myasthenia gravis. Neurol Clin 2018, 36, 311–37. doi: 10.1016/j.ncl.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milani M, Ostlie N, Wang W, Conti-Fine, BM.. T cells and cytokines in the pathogenesis of acquired myasthenia gravis. Ann N Y Acad Sci 2003, 998, 284–307. doi: 10.1196/annals.1254.032. [DOI] [PubMed] [Google Scholar]
- 26. Cao Y, Amezquita RA, Kleinstein SH, Stathopoulos P, Nowak RJ, O’Connor KC.. Autoreactive T cells from patients with myasthenia gravis are characterized by elevated IL-17, IFN-gamma, and GM-CSF and diminished IL-10 production. J Immunol 2016, 196, 2075–84. doi: 10.4049/jimmunol.1501339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Danikowski KM, Jayaraman S, Prabhakar BS.. Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 2017, 14, 117. doi: 10.1186/s12974-017-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uzawa A, Kuwabara S, Suzuki S, Imai T, Murai H, Ozawa Y, et al. Roles of cytokines and T cells in the pathogenesis of myasthenia gravis. Clin Exp Immunol 2021, 203, 366–74. doi: 10.1111/cei.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu W, Ren M, Ghosh S, Qian K, Luo Z, Zhang A, et al. Defects of CTLA-4 are associated with regulatory T cells in myasthenia gravis implicated by intravenous immunoglobulin therapy. Mediators Inflamm 2020, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thiruppathi M, Rowin J, Li Jiang Q, Sheng JR, Prabhakar BS, Meriggioli MN.. Functional defect in regulatory T cells in myasthenia gravis. Ann N Y Acad Sci 2012, 1274, 68–76. doi: 10.1111/j.1749-6632.2012.06840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villegas JA, Van Wassenhove J, Le Panse R, Berrih-Aknin S, Dragin N.. An imbalance between regulatory T cells and T helper 17 cells in acetylcholine receptor-positive myasthenia gravis patients. Ann N Y Acad Sci 2018, 1413, 154–62. doi: 10.1111/nyas.13591. [DOI] [PubMed] [Google Scholar]
- 32. Wang N, Yuan J, Karim MR, Zhong P, Sun YP, Zhang HY, et al. Effects of mitophagy on regulatory T cell function in patients with myasthenia gravis. Front Neurol 2020, 11, 238. doi: 10.3389/fneur.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang T, Niu C, Sun C, Ma Y, Guo R, Ruan Z, et al. Melatonin exerts immunoregulatory effects by balancing peripheral effector and regulatory T helper cells in myasthenia gravis. Aging (Albany NY) 2020, 12, 21147–60. doi: 10.18632/aging.103785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aricha R, Reuveni D, Fuchs S, Souroujon MC.. Suppression of experimental autoimmune myasthenia gravis by autologous T regulatory cells. J Autoimmun 2016, 67, 57–64. doi: 10.1016/j.jaut.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 35. Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat Rev Neurol 2019, 15, 671–83. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi P, Qu H, Nian D, Chen Y, Liu X, Li Q, et al. Treatment of Guillain–Barre syndrome with Bifidobacterium infantis through regulation of T helper cells subsets. Int Immunopharmacol 2018, 61, 290–6. doi: 10.1016/j.intimp.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 37. Maddur MS, Rabin M, Hegde P, Bolgert F, Guy M, Vallat JM, et al. Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain–Barre syndrome patients. Immunol Res 2014, 60, 320–9. doi: 10.1007/s12026-014-8580-6. [DOI] [PubMed] [Google Scholar]
- 38. Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH.. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev 2017, 1, CD010369. doi: 10.1002/14651858.CD010369.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chi LJ, Wang HB, Zhang Y, Wang WZ.. Abnormality of circulating CD4(+)CD25(+) regulatory T cell in patients with Guillain–Barre syndrome. J Neuroimmunol 2007, 192, 206–14. doi: 10.1016/j.jneuroim.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 40. Zhang G, Wang Q, Song Y, Cheng P, Xu R, Feng X, et al. Intravenous immunoglobulin promotes the proliferation of CD4(+)CD25(+) Foxp3(+) regulatory T cells and the cytokines secretion in patients with Guillain–Barre syndrome in vitro. J Neuroimmunol 2019, 336, 577042. doi: 10.1016/j.jneuroim.2019.577042. [DOI] [PubMed] [Google Scholar]
- 41. Wang H, Li L, Zhang Y, Pan SC, Chen AQ, Qian WD.. Expression and significance of CD4(+)CD25(+)CD127(−) regulatory T cells in peripheral blood of patients with different phenotypes of Guillain–Barre syndrome. Int J Clin Exp Med 2015, 8, 19126–31. [PMC free article] [PubMed] [Google Scholar]
- 42. Fagone P, Mazzon E, Chikovani T, Saraceno A, Mammana S, Colletti G, et al. Decitabine induces regulatory T cells, inhibits the production of IFN-gamma and IL-17 and exerts preventive and therapeutic efficacy in rodent experimental autoimmune neuritis. J Neuroimmunol 2018, 321, 41–8. doi: 10.1016/j.jneuroim.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 43. Wang FJ, Cui D, Qian WD.. Therapeutic effect of CD4+CD25+ regulatory T cells amplified in vitro on experimental autoimmune neuritis in rats. Cell Physiol Biochem 2018, 47, 390–402. doi: 10.1159/000489919. [DOI] [PubMed] [Google Scholar]
- 44. Sanvito L, Makowska A, Gregson N, Nemni R, Hughes RA.. Circulating subsets and CD4(+)CD25(+) regulatory T cell function in chronic inflammatory demyelinating polyradiculoneuropathy. Autoimmunity 2009, 42, 667–77. doi: 10.3109/08916930903140907. [DOI] [PubMed] [Google Scholar]
- 45. Chi LJ, Wang HB, Wang WZ.. Impairment of circulating CD4+CD25+ regulatory T cells in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst 2008, 13, 54–63. doi: 10.1111/j.1529-8027.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 46. Kalia LV, Lang AE.. Parkinson’s disease. Lancet 2015, 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 47. Rocha EM, De Miranda B, Sanders LH.. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 2018, 109, 249–57. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 48. MacMahon Copas AN, McComish SF, Fletcher JM, Caldwell MA.. The pathogenesis of Parkinson’s disease: a complex interplay between astrocytes, microglia, and T lymphocytes? Front Neurol 2021, 12, 666737. doi: 10.3389/fneur.2021.666737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seo J, Park J, Kim K, Won J, Yeo HG, Jin YB, et al. Chronic infiltration of T lymphocytes into the brain in a non-human primate model of Parkinson’s disease. Neuroscience 2020, 431, 73–85. doi: 10.1016/j.neuroscience.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 50. Magistrelli L, Storelli E, Rasini E, Contaldi E, Comi C, Cosentino M, et al. Relationship between circulating CD4+ T lymphocytes and cognitive impairment in patients with Parkinson’s disease. Brain Behav Immun 2020, 89, 668–74. doi: 10.1016/j.bbi.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 51. Zhao X, Jin T, Zheng C, Ma D, Zhang Y.. Imbalance of circulating Tfh/Tfr cells in patients with Parkinson’s disease. Front Neurol 2020, 11, 572205. doi: 10.3389/fneur.2020.572205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alvarez-Luquin DD, Arce-Sillas A, Leyva-Hernández J, Sevilla-Reyes E, Boll MC, Montes-Moratilla E, et al. Regulatory impairment in untreated Parkinson’s disease is not restricted to Tregs: other regulatory populations are also involved. J Neuroinflammation 2019, 16, 212. doi: 10.1186/s12974-019-1606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kustrimovic N, Comi C, Magistrelli L, Rasini E, Legnaro M, Bombelli R, et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation 2018, 15, 205. doi: 10.1186/s12974-018-1248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olson KE, Namminga KL, Lu Y, Schwab AD, Thurston MJ, Abdelmoaty MM, et al. Safety, tolerability, and immune-biomarker profiling for year-long sargramostim treatment of Parkinson’s disease. EBioMedicine 2021, 67, 103380. doi: 10.1016/j.ebiom.2021.103380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gendelman HE, Zhang Y, Santamaria P, Olson KE, Schutt CR, Bhatti D, et al. Evaluation of the safety and immunomodulatory effects of sargramostim in a randomized, double-blind phase 1 clinical Parkinson’s disease trial. NPJ Parkinsons Dis 2017, 3, 10. doi: 10.1038/s41531-017-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, et al. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol 2012, 7, 927–38. doi: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karaaslan Z, Kahraman ÖT, Şanlı E, Ergen HA, Ulusoy C, Bilgiç B, et al. Inflammation and regulatory T cell genes are differentially expressed in peripheral blood mononuclear cells of Parkinson’s disease patients. Sci Rep 2021, 11, 2316. doi: 10.1038/s41598-021-81961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwab AD, Thurston MJ, Machhi J, Olson KE, Namminga KL, Gendelman HE, et al. Immunotherapy for Parkinson’s disease. Neurobiol Dis 2020, 137, 104760. doi: 10.1016/j.nbd.2020.104760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Machhi J, Kevadiya BD, Muhammad IK, Herskovitz J, Olson KE, Mosley RL, et al. Harnessing regulatory T cell neuroprotective activities for treatment of neurodegenerative disorders. Mol Neurodegener 2020, 15, 32. doi: 10.1186/s13024-020-00375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang Y, Liu Z, Cao BB, Qiu YH, Peng YP.. Treg cells attenuate neuroinflammation and protect neurons in a mouse model of Parkinson’s disease. J Neuroimmune Pharmacol 2020, 15, 224–37. doi: 10.1007/s11481-019-09888-5. [DOI] [PubMed] [Google Scholar]
- 61. Kim KH, Kim M, Lee J, Jeon HN, Kim SH, Bae H.. Comparison of the protective effects of bee venom extracts with varying PLA2 compositions in a mouse model of Parkinson’s Disease. Toxins (Basel) 2019, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Olson KE, Kosloski-Bilek LM, Anderson KM, Diggs BJ, Clark BE, Gledhill JM Jr, et al. Selective VIP receptor agonists facilitate immune transformation for dopaminergic neuroprotection in MPTP-intoxicated mice. J Neurosci 2015, 35, 16463–78. doi: 10.1523/JNEUROSCI.2131-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Olson KE, Namminga KL, Lu Y, Thurston MJ, Schwab AD, de Picciotto S, et al. Granulocyte-macrophage colony-stimulating factor mRNA and Neuroprotective Immunity in Parkinson’s disease. Biomaterials 2021, 272, 120786. doi: 10.1016/j.biomaterials.2021.120786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thome AD, Atassi F, Wang J, Faridar A, Zhao W, Thonhoff JR, et al. Ex vivo expansion of dysfunctional regulatory T lymphocytes restores suppressive function in Parkinson’s disease. NPJ Parkinsons Dis 2021, 7, 41. doi: 10.1038/s41531-021-00188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garfias S, Tamaya Domínguez B, Toledo Rojas A, Arroyo M, Rodríguez U, Boll C, et al. Peripheral blood lymphocyte phenotypes in Alzheimer and Parkinson’s diseases. Neurologia (Engl Ed) 2022, 37, 110–21. doi: 10.1016/j.nrleng.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 66. Idova GV, Al’perina EL, Gevorgyan MM, Tikhonova MA, Zhanaeva SY.. Content of peripheral blood T- and B-cell subpopulations in transgenic A53T mice of different age (a model of Parkinson’s disease). Bull Exp Biol Med 2021, 170, 401–4. doi: 10.1007/s10517-021-05075-w. [DOI] [PubMed] [Google Scholar]
- 67. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet 2021, 397, 1577–90. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oberstein TJ, Taha L, Spitzer P, Hellstern J, Herrmann M, Kornhuber J, et al. Imbalance of circulating Th17 and regulatory T cells in Alzheimer’s disease: a case control study. Front Immunol 2018, 9, 1213. doi: 10.3389/fimmu.2018.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun L, Ju T, Wang T, Zhang L, Ding F, Zhang Y, et al. Decreased netrin-1 and correlated Th17/Tregs balance disorder in abeta1-42 induced Alzheimer’s disease model rats. Front Aging Neurosci 2019, 11, 124. doi: 10.3389/fnagi.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. D’Angelo C, Goldeck D, Pawelec G, Gaspari L, Di Iorio A, Paganelli R.. Exploratory study on immune phenotypes in Alzheimer’s disease and vascular dementia. Eur J Neurol 2020, 27, 1887–94. doi: 10.1111/ene.14360. [DOI] [PubMed] [Google Scholar]
- 71. Ciccocioppo F, Lanuti P, Pierdomenico L, Simeone P, Bologna G, Ercolino E, et al. The characterization of regulatory T-cell profiles in Alzheimer’s disease and multiple sclerosis. Sci Rep 2019, 9, 8788. doi: 10.1038/s41598-019-45433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 2016, 139, 1237–51. doi: 10.1093/brain/awv408. [DOI] [PubMed] [Google Scholar]
- 73. Baek H, Ye M, Kang GH, Lee C, Lee G, Choi DB, et al. Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget 2016, 7, 69347–57. doi: 10.18632/oncotarget.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang Y, He Z, Xing Z, Zuo Z, Yuan L, Wu Y, et al. Influenza vaccination in early Alzheimer’s disease rescues amyloidosis and ameliorates cognitive deficits in APP/PS1 mice by inhibiting regulatory T cells. J Neuroinflammation 2020, 17, 65. doi: 10.1186/s12974-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, et al. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun 2015, 6, 7967. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers 2017, 3, 17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 77. Beers DR, Zhao W, Wang J, Zhang X, Wen S, Neal D, et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight 2017, 2, e89530. doi: 10.1172/jci.insight.89530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rashid Chehreh Bargh S, Tafakhori A, Masoumi F, Rahmani F, Ahmadi M, Namdar A, et al. Evaluation of regulatory T lymphocytes and IL2Ra and FOXP3 gene expression in peripheral mononuclear cells from patients with amyotrophic lateral sclerosis. Ir J Med Sci 2018, 187, 1065–71. doi: 10.1007/s11845-018-1793-2. [DOI] [PubMed] [Google Scholar]
- 79. Giovannelli I, Heath P, Shaw PJ, Kirby J.. The involvement of regulatory T cells in amyotrophic lateral sclerosis and their therapeutic potential. Amyotroph Lateral Scler Frontotemporal Degener 2020, 21, 435–44. doi: 10.1080/21678421.2020.1752246. [DOI] [PubMed] [Google Scholar]
- 80. Sheean RK, McKay FC, Cretney E, Bye CR, Perera ND, Tomas D, et al. Association of regulatory T-cell expansion with progression of amyotrophic lateral sclerosis: a study of humans and a transgenic mouse model. JAMA Neurol 2018, 75, 681–9. doi: 10.1001/jamaneurol.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med 2013, 5, 64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vucic S, Henderson RD, Mathers S, Needham M, Schultz D, Kiernan MC, et al. Safety and efficacy of dimethyl fumarate in ALS: randomised controlled study. Ann Clin Transl Neurol 2021, 8, 1991–9. doi: 10.1002/acn3.51446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thonhoff JR, Beers DR, Zhao W, Pleitez M, Simpson EP, Berry JD, et al. Expanded autologous regulatory T-lymphocyte infusions in ALS: a phase I, first-in-human study. Neurol Neuroimmunol Neuroinflamm 2018, 5, e465. doi: 10.1212/NXI.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR.. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant 2017, 26, 1118–30. doi: 10.1177/0963689717714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alam A, Thelin EP, Tajsic T, Khan DZ, Khellaf A, Patani R, et al. Cellular infiltration in traumatic brain injury. J Neuroinflammation 2020, 17, 328. doi: 10.1186/s12974-020-02005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kramer TJ, Hack N, Brühl TJ, Menzel L, Hummel R, Griemert EV, et al. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-gamma gene expression in acute experimental traumatic brain injury. J Neuroinflammation 2019, 16, 163. doi: 10.1186/s12974-019-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li M, Lin YP, Chen JL, Li H, Jiang RC, Zhang JN.. Role of regulatory T cell in clinical outcome of traumatic brain injury. Chin Med J (Engl) 2015, 128, 1072–8. doi: 10.4103/0366-6999.155094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen C, Hu N, Wang J, Xu L, Jia XL, Fan X, et al. Umbilical cord mesenchymal stem cells promote neurological repair after traumatic brain injury through regulating Treg/Th17 balance. Brain Res 2022, 1775, 147711. [DOI] [PubMed] [Google Scholar]
- 89. Feske SK. Ischemic Stroke. Am J Med 2021, 134, 1457–64. doi: 10.1016/j.amjmed.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 90. Wang HY, Ye JR, Cui LY, Chu SF, Chen NH.. Regulatory T cells in ischemic stroke. Acta Pharmacol Sin 2022, 43, 1–9. doi: 10.1038/s41401-021-00641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liesz A, Kleinschnitz C.. Regulatory T cells in post-stroke immune homeostasis. Transl Stroke Res 2016, 7, 313–21. doi: 10.1007/s12975-016-0465-7. [DOI] [PubMed] [Google Scholar]
- 92. Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–50. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 93. Guo S, Luo Y.. Brain Foxp3(+) regulatory T cells can be expanded by Interleukin-33 in mouse ischemic stroke. Int Immunopharmacol 2020, 81, 106027. doi: 10.1016/j.intimp.2019.106027. [DOI] [PubMed] [Google Scholar]
- 94. Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain 2017, 140, 1914–31. doi: 10.1093/brain/awx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang Y, Liesz A, Li P.. Coming to the rescue: regulatory t cells for promoting recovery after ischemic stroke. Stroke 2021, 52, e837–41. doi: 10.1161/STROKEAHA.121.036072. [DOI] [PubMed] [Google Scholar]
- 96. Santamaria-Cadavid M, Rodríguez-Castro E, Rodríguez-Yáñez M, Arias-Rivas S, López-Dequidt I, Pérez-Mato M, et al. Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol 2020, 20, 68. doi: 10.1186/s12883-020-01648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Baron R, Binder A, Wasner G.. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010, 9, 807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 98. Galvin DA, C M.. The role of T-lymphocytes in neuropathic pain initiation, development of chronicity and treatment. Brain Behav Immun Health 2021, 18, 100371. doi: 10.1016/j.bbih.2021.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ding W, You Z, Chen Q, Yang L, Doheny J, Zhou X, et al. Gut microbiota influences neuropathic pain through modulating proinflammatory and anti-inflammatory T cells. Anesth Analg 2021, 132, 1146–55. doi: 10.1213/ANE.0000000000005155. [DOI] [PubMed] [Google Scholar]
- 100. Davoli-Ferreira M, de Lima KA, Fonseca MM, Guimarães RM, Gomes FI, Cavallini MC, et al. Regulatory T cells counteract neuropathic pain through inhibition of the Th1 response at the site of peripheral nerve injury. Pain 2020, 161, 1730–43. doi: 10.1097/j.pain.0000000000001879. [DOI] [PubMed] [Google Scholar]
- 101. Chen H, Jiang L, Zhang D, Chen J, Luo X, Xie Y, et al. Exploring the correlation between the regulation of macrophages by regulatory T cells and peripheral neuropathic pain. Front Neurosci 2022, 16, 813751. doi: 10.3389/fnins.2022.813751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Luchting B, Rachinger-Adam B, Heyn J, Hinske LC, Kreth S, Azad SC.. Anti-inflammatory T-cell shift in neuropathic pain. J Neuroinflammation 2015, 12, 12. doi: 10.1186/s12974-014-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Beghi E, Giussani G, Sander JW.. The natural history and prognosis of epilepsy. Epileptic Disord 2015, 17, 243–53. doi: 10.1684/epd.2015.0751. [DOI] [PubMed] [Google Scholar]
- 104. Xu D, Robinson AP, Ishii T, Duncan DS, Alden TD, Goings GE, et al. Peripherally derived T regulatory and gammadelta T cells have opposing roles in the pathogenesis of intractable pediatric epilepsy. J Exp Med 2018, 215, 1169–86. doi: 10.1084/jem.20171285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ni FF, Li CR, Liao JX, Wang GB, Lin SF, Xia Y, et al. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy. Seizure 2016, 38, 17–22. doi: 10.1016/j.seizure.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 106. Kumar P, Shih DCW, Lim A, Paleja B, Ling S, Li Yun L, et al. Pro-inflammatory, IL-17 pathways dominate the architecture of the immunome in pediatric refractory epilepsy. JCI Insight 2019, 4, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vieira ELM, de Oliveira GNM, Lessa JMK, Gonçalves AP, Oliveira ACP, Bauer ME, et al. Peripheral leukocyte profile in people with temporal lobe epilepsy reflects the associated proinflammatory state. Brain Behav Immun 2016, 53, 123–30. doi: 10.1016/j.bbi.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 108. Severance EG, Tveiten D, Lindstrom LH, Yolken RH, Reichelt KL.. The gut microbiota and the emergence of autoimmunity: relevance to major psychiatric disorders. Curr Pharm Des 2016, 22, 6076–86. doi: 10.2174/1381612822666160914183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Corsi-Zuelli F, Deakin B, de Lima MHF, Qureshi O, Barnes NM, Upthegrove R, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health 2021, 17, 100330. doi: 10.1016/j.bbih.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, et al. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun 2009, 23, 1117–24. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 111. Westfall S, Caracci F, Zhao D, Wu QL, Frolinger T, Simon J, et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun 2021, 91, 350–68. doi: 10.1016/j.bbi.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fernandez-Egea E, Vértes PE, Flint SM, Turner L, Mustafa S, Hatton A, et al. Peripheral immune cell populations associated with cognitive deficits and negative symptoms of treatment-resistant schizophrenia. PLoS One 2016, 11, e0155631. doi: 10.1371/journal.pone.0155631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Counotte J, Drexhage HA, Wijkhuijs JM, Pot-Kolder R, Bergink V, Hoek HW, et al. Th17/T regulator cell balance and NK cell numbers in relation to psychosis liability and social stress reactivity. Brain Behav Immun 2018, 69, 408–17. doi: 10.1016/j.bbi.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 114. Poletti S, de Wit H, Mazza E, Wijkhuijs AJM, Locatelli C, Aggio V, et al. Th17 cells correlate positively to the structural and functional integrity of the brain in bipolar depression and healthy controls. Brain Behav Immun 2017, 61, 317–25. doi: 10.1016/j.bbi.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 115. Corsi-Zuelli F, Deakin B.. Impaired regulatory T cell control of astroglial overdrive and microglial pruning in schizophrenia. Neurosci Biobehav Rev 2021, 125, 637–53. doi: 10.1016/j.neubiorev.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 116. Morath J, Gola H, Sommershof A, Hamuni G, Kolassa S, Catani C, et al. The effect of trauma-focused therapy on the altered T cell distribution in individuals with PTSD: evidence from a randomized controlled trial. J Psychiatr Res 2014, 54, 1–10. doi: 10.1016/j.jpsychires.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 117. Jergovic M, Bendelja K, Vidović A, Savić A, Vojvoda V, Aberle N, et al. Patients with posttraumatic stress disorder exhibit an altered phenotype of regulatory T cells. Allergy Asthma Clin Immunol 2014, 10, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Grosse L, Carvalho LA, Birkenhager TK, Hoogendijk WJ, Kushner SA, Drexhage HA, et al. Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology (Berl) 2016, 233, 1679–88. doi: 10.1007/s00213-015-3943-9. [DOI] [PubMed] [Google Scholar]
- 119. Mohd Ashari NS, Mohamed Sanusi SNF, Mohd Yasin MA, Che Hussin CM, Wong KK, Shafei MN.. Major depressive disorder patients on antidepressant treatments display higher number of regulatory T cells. Malays J Pathol 2019, 41, 169–76. [PubMed] [Google Scholar]
- 120. Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol 2016, 17, 1322–33. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Seng A, Yankee TM.. The role of the ikaros family of transcription factors in regulatory T cell development and function. J Clin Cell Immunol 2017, 8, 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Iizuka-Koga M, Nakatsukasa H, Ito M, Akanuma T, Lu Q, Yoshimura A.. Induction and maintenance of regulatory T cells by transcription factors and epigenetic modifications. J Autoimmun 2017, 83, 113–21. doi: 10.1016/j.jaut.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 123. Lam AJ, Lin DTS, Gillies JK, Uday P, Pesenacker AM, Kobor MS, et al. Optimized CRISPR-mediated gene knockin reveals FOXP3-independent maintenance of human Treg identity. Cell Rep 2021, 36, 109494. doi: 10.1016/j.celrep.2021.109494. [DOI] [PubMed] [Google Scholar]
- 124. Van Zeebroeck L, Arroyo Hornero R, Côrte-Real BF, Hamad I, Meissner TB, Kleinewietfeld M.. Fast and efficient genome editing of human FOXP3(+) regulatory T cells. Front Immunol 2021, 12, 655122. doi: 10.3389/fimmu.2021.655122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Okada M, Kanamori M, Someya K, Nakatsukasa H, Yoshimura A.. Stabilization of Foxp3 expression by CRISPR-dCas9-based epigenome editing in mouse primary T cells. Epigenetics Chromatin 2017, 10, 24. doi: 10.1186/s13072-017-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cortez JT, Montauti E, Shifrut E, Gatchalian J, Zhang Y, Shaked O, et al. CRISPR screen in regulatory T cells reveals modulators of Foxp3. Nature 2020, 582, 416–20. doi: 10.1038/s41586-020-2246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Skuljec J, Chmielewski M, Happle C, Habener A, Busse M, Abken H, et al. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front Immunol 2017, 8, 1125. doi: 10.3389/fimmu.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Koristka S, Kegler A, Bergmann R, Arndt C, Feldmann A, Albert S, et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J Autoimmun 2018, 90, 116–31. doi: 10.1016/j.jaut.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 129. Zhang Q, Lu W, Liang CL, Chen Y, Liu H, Qiu F, et al. Chimeric antigen receptor (CAR) treg: a promising approach to inducing immunological tolerance. Front Immunol 2018, 9, 2359. doi: 10.3389/fimmu.2018.02359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Feins S, Kong W, Williams EF, Milone MC, Fraietta JA.. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol 2019, 94, S3–9. doi: 10.1002/ajh.25418. [DOI] [PubMed] [Google Scholar]
- 131. Hong M, Clubb JD, Chen YY.. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020, 38, 473–88. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 132. Chmielewski M, Hahn O, Rappl G, Nowak M, Schmidt-Wolf IH, Hombach AA, et al. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology 2012, 143, 1095–107.e2. doi: 10.1053/j.gastro.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 133. Singh AK, McGuirk JP.. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol 2020, 21, e168–78. doi: 10.1016/S1470-2045(19)30823-X. [DOI] [PubMed] [Google Scholar]
- 134. Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 2008, 16, 194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 135. Olson KE, Gendelman HE.. Immunomodulation as a neuroprotective and therapeutic strategy for Parkinson’s disease. Curr Opin Pharmacol 2016, 26, 87–95. doi: 10.1016/j.coph.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Fan MY, Low JS, Tanimine N, Finn KK, Priyadharshini B, Germana SK, et al. Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep 2018, 25, 1204–1213.e4. doi: 10.1016/j.celrep.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hirakawa M, Matos TR, Liu H, Koreth J, Kim HT, Paul NE, et al. Low-dose IL-2 selectively activates subsets of CD4(+) Tregs and NK cells. JCI Insight 2016, 1, e89278. doi: 10.1172/jci.insight.89278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dong S, Hiam-Galvez KJ, Mowery CT, Herold KC, Gitelman SE, Esensten JH, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 2021, 6, 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Moorman CD, Sohn SJ, Phee H.. Emerging therapeutics for immune tolerance: tolerogenic vaccines, T cell therapy, and IL-2 therapy. Front Immunol 2021, 12, 657768. doi: 10.3389/fimmu.2021.657768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li J, Zhang Z, Du S, Guo Q.. Interleukin 2 ameliorates autoimmune neuroinflammation by modulating the balance of T helper 17 cells and regulatory T cells in mouse. Ann Clin Lab Sci 2021, 51, 529–34. [PubMed] [Google Scholar]
- 141. Giovannelli I, Bayatti N, Brown A, Wang D, Mickunas M, Camu W, et al. Amyotrophic lateral sclerosis transcriptomics reveals immunological effects of low-dose interleukin-2. Brain Commun 2021, 3, fcab141. doi: 10.1093/braincomms/fcab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ptacin JL, Caffaro CE, Ma L, San Jose Gall KM, Aerni HR, Acuff NV, et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat Commun 2021, 12, 4785. doi: 10.1038/s41467-021-24987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mizui M. Natural and modified IL-2 for the treatment of cancer and autoimmune diseases. Clin Immunol 2019, 206, 63–70. doi: 10.1016/j.clim.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 144. Kasahara K, Sasaki N, Yamashita T, Kita T, Yodoi K, Sasaki Y, et al. CD3 antibody and IL-2 complex combination therapy inhibits atherosclerosis by augmenting a regulatory immune response. J Am Heart Assoc 2014, 3, e000719. doi: 10.1161/JAHA.113.000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Alves S, Churlaud G, Audrain M, Michaelsen-Preusse K, Fol R, Souchet B, et al. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain 2017, 140, 826–42. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- 146. Ye M, Chung HS, Lee C, Yoon MS, Yu AR, Kim JS, et al. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J Neuroinflammation 2016, 13, 10. doi: 10.1186/s12974-016-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Olson KE, Namminga KL, Schwab AD, Thurston MJ, Lu Y, Woods A, et al. Neuroprotective activities of long-acting granulocyte-macrophage colony-stimulating factor (mPDM608) in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Intoxicated Mice. Neurotherapeutics 2020, 17, 1861–77. doi: 10.1007/s13311-020-00877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Faridar A, Thome AD, Zhao W, Thonhoff JR, Beers DR, Pascual B, et al. Restoring regulatory T-cell dysfunction in Alzheimer’s disease through ex vivo expansion. Brain Commun 2020, 2, fcaa112. doi: 10.1093/braincomms/fcaa112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Thonhoff JR, Simpson EP, Appel SH.. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr Opin Neurol 2018, 31, 635–9. doi: 10.1097/WCO.0000000000000599. [DOI] [PubMed] [Google Scholar]