Since the discovery of regulatory T cells (Tregs) as crucial regulators of immune tolerance against self-antigens, these cells have become a promising tool for the induction of donor-specific tolerance in transplantation medicine. The therapeutic potential of increasing in vivoTreg numbers for a favorable Treg to Teff cell ratio has already been demonstrated in several sophisticated pre-clinical models and clinical pilot trials. In addition to improving cell quantity, enhancing Treg function utilizing engineering techniques led to encouraging results in models of autoimmunity and transplantation. Here we aim to discuss the most promising approaches for Treg-enhancing therapies, starting with adoptive transfer approaches and ex vivoexpansion cultures (polyclonal vs. antigen specific), followed by selective in vivostimulation methods. Furthermore, we address next generation concepts for Treg function enhancement (CARs, TRUCKs, BARs) as well as the advantages and caveats inherit to each approach. Finally, this review will discuss the clinical experience with Treg therapy in ongoing and already published clinical trials; however, data on long-term results and efficacy are still very limited and many questions that might complicate clinical translation remain open. Here, we discuss the hurdles for clinical translation and elaborate on current Treg-based therapeutic options as well as their potencies for improving long-term graft survival in transplantation.
Keywords: Treg, transplantation, tolerance
Graphical Abstract
Graphical Abstract.
Introduction
Recent innovations in surgical techniques and therapeutics led to remarkable improvements considering short-term graft survival in solid organ transplantation (SOT) patients. Long-term graft survival, however, has not improved much in the last decades [1]. Despite continuous immunosuppression patients experience late graft loss due to chronic rejection that cannot be properly controlled. Moreover, they suffer from increased morbidity and mortality associated with the unspecific suppression of the immune system and the toxicity of immunosuppressive drugs [2, 3]. Therefore, the induction of immunological tolerance, namely the acceptance of an allograft in the absence of chronic immunosuppression, is the ultimate goal in transplantation. In rare cases of liver and kidney transplantation a state of ‘operational tolerance’ with stable graft function without chronic immunosuppression was achieved; however, occurrence was unpredictable, spontaneous, and late after transplantation [4–6].
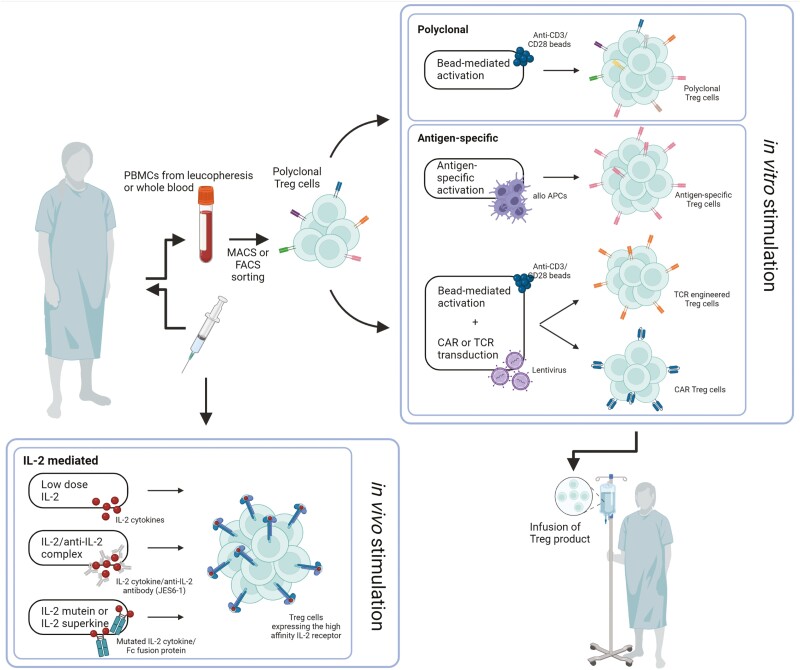
Regulatory immune cells have been recognized as key players in immune homeostasis and have been suggested as perfect candidates for deliberate induction of donor-specific tolerance in SOT. As a result, several approaches favoring tolerance induction through regulatory T-cell (Treg) enhancement have been described in preclinical models and clinical trials (Fig. 1).
Figure 1:
approaches of Treg-based therapy in transplantation medicine. (APC, antigen-presenting cells; PBMCs, peripheral blood mononuclear cells; created with BioRender.com)
Heterogeneity of regulatory T cells
The first evidence of so-called suppressor T lymphocytes goes back to 1969 [7]; however, their existence was under debate for the next 25 years. Since the ‘official’ discovery of Tregs in 1995 as a small CD4 + T-cell subpopulation with high levels of IL-2Rα (CD25) expression and the capacity of protecting thymectomized mice from autoimmunity [8], we learned a lot about the crucial role of Tregs in immune responses [9, 10]. A major break-through came in 2003 when the X-linked gene FOXP3 was identified as main transcription factor responsible for Treg phenotype and function. Disruption of FOXP3 leads to early onset of multi-organ inflammation and fatal autoimmune disease in mice and men [11–13]. For example, mutations in FOXP3 result in the development of immunodysregulation polyendocrinopaty enteropathy X-linked (IPEX) syndrome in male humans [14]. Utilizing a mouse model homologue of FOXP3 deficiency (scurfy mice), the first rescue experiments using adoptive Treg transfer were successfully performed, paving the way for these cells to be applied in therapy of IPEX patients and other autoimmune diseases with dysfunctional FOXP3 expression [13, 15, 16].
Tregs comprise about 5-10% of circulating CD4 + T cells and are characterized by the constitutive and high expression of CD25 and FOXP3 [17]. While FOXP3 expression in mice is specific for Tregs, in humans it has been shown that CD4 + CD25-effector T cells (Teff) are capable of transiently expressing the transcription factor [18]. Moreover, it has been demonstrated that FOXP3 expression in human Tregs, contrary to murine Tregs, is not homogenous with several splicing variants that have been identified in the last years. There are two main isoforms that are expressed at comparable levels by human Tregs and are distinguished by the full-length expression of FOXP3 or lack of exon 2. The deficiency of exon 2 leads to the inability of FOXP3 interaction and inhibition of RORα and RORγt contrasting the development of Th17 cells [19]. A third isoform lacking the exon 2 as well as exon 7 has been described as Th17 differentiation facilitating [20]. In addition, it has been shown that loss of the full length FOXP3 is associated with impaired lineage stability [20]. Inflammatory conditions, like in autoimmune diseases may result in conversion of FOXP3 + Tregs into Th17 cells, contributing to disease progression and impairing immune homeostasis, therefore stable full length expression of FOXP3 is a prerequisite for Treg cell therapy [21]. Analysis of human splicing variants during Treg isolation and later adoptive cell transfer is hindered not only by the fact that FOXP3 is an intracellular marker, but also by the limited options for anti-FOXP3 antibodies recognizing exon 2 or Δ2 a as well as no available antibody against exon 7 or Δ7 [22]. However, in humans an inverse correlation of FOXP3 expression and expression of the IL-7 receptor (CD127) α chain was identified, establishing CD4 + CD25 + CD127low as surface markers for human Treg identification [23, 24]. In 2009, Miyara et al. classified human Tregs based on their expression levels of FOXP3 and CD45RA in naïve/resting (FOXP3low CD45RA+), effector-type (FOXP3high CD45RA-) and cytokine-producing (FOXP3low CD45RA-) Tregs [25]. In fact, there are studies demonstrating superior proliferation capacity of human naïve CD45RA + Tregs with higher stability and suppressive function when compared to effector or memory phenotype Tregs [26]. However, although overall Treg numbers increase, the amount of naïve Tregs decreases with age, complicating the use of CD45RA + Tregs for adoptive cell transfer in adults and older patients [27].
In addition, circulating Tregs can be divided according to their origin of differentiation since they develop in the thymus (tTreg), the periphery (pTreg) as well as in vitro (iTregs) in presence TGF-β and IL-2 [9]. While thymus derived Tregs mainly recognize self-antigens, pTregs which develop from CD4 + conventional T cells (Tconv) have been shown to recognize ‘non-self’, showing a similar T-cell receptor (TCR) repertoire as conventional T cells. While in mice the differentiation between tTregs and pTregs is possible by neuropilin 1 (NRP1) expression, in human no marker has been identified yet [28]. Recent literature suggests the expression of Helios to discriminate between tTregs and pTregs in human, although its validity remains contentious [29–31]. Besides, different papers state that the analysis of epigenetic DNA methylation levels on the non-coding conserved region of the FOXP3 gene, namely the Treg-specific demethylation region (TSDR), is still the only reliable way to distinguish between these subsets in humans [32, 33].
Suppressive capacity of regulatory T cells
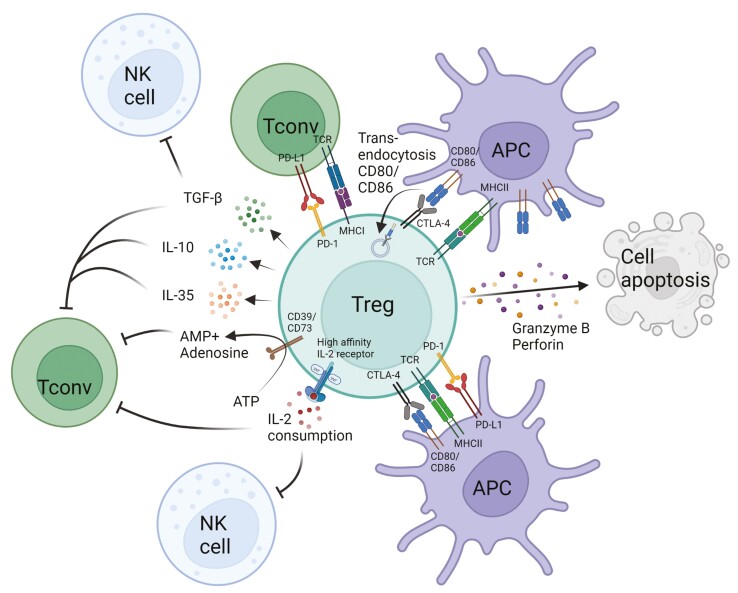
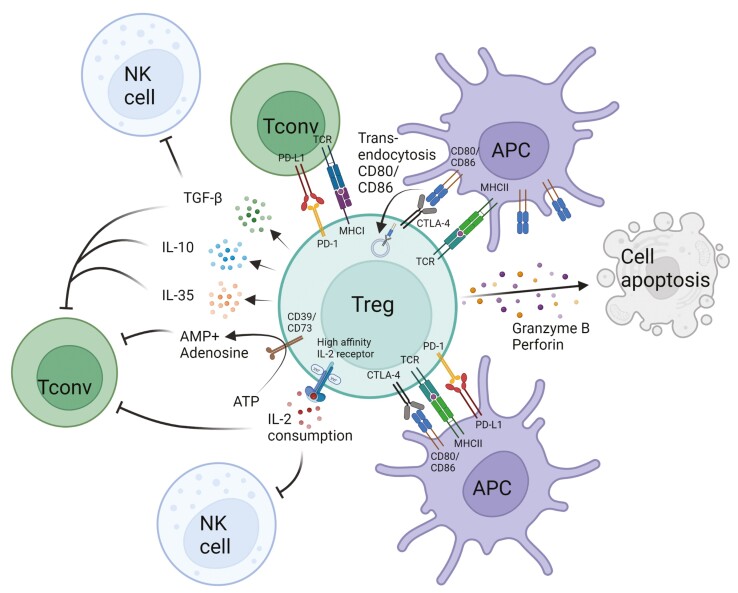
Tregs suppress immune responses by cell-contact dependent and independent mechanisms (Figure 2). These processes include the secretion of anti-inflammatory cytokines such as TGF-β, IL-10, and IL-35 to suppress Tconv and natural killer (NK) cells [34–36]. In addition, Tregs are capable of excess IL-2 consumption by the expression of the IL-2 high-affinity receptor complex IL-2αβγ, limiting the availability of IL-2 for other IL-2 responsive subsets (NK, CD8, etc.) [8, 37, 38]. The expression of cell surface receptor like cytotoxic T lymphocyte antigen 4 (CTLA-4) on Tregs negatively regulates antigen-presenting cell (APC) function by binding to CD80/CD86 and subsequent blockade of CD28 ligation by Tconv [39, 40]. Tregs have been shown to deplete CD80/86 by trogocytosis [41, 42], therefore actively reducing costimulatory molecules on APCs. Furthermore, the release of lytic proteins such as perforin and granzyme B are able to directly kill target antigen expressing APCs [43]. The surface expression of CD39 and CD73 on Tregs mediates conversion of ATP to AMP causing further reduction of Tconv proliferation [44].
Figure 2:
mechanisms of Treg-mediated suppression. (APC, antigen-presenting cell; Tconv, conventional T cell; NK cell, natural killer cell; created with BioRender.com)
Notably, a variety of these Treg-mediated suppressive mechanisms occur in an antigen unspecific manner, resulting in suppression of Teff cells with diverse specificities [45]. Tregs are capable of shaping a microenvironment promoting the attraction of other immunosuppressive cell populations. This effect was first described by Gershon and Kondo in 1971 as ‘infectious tolerance’ and suggests that adoptively transferred Tregs may not need to persist indefinitely, but long enough to transfer the suppressive capacities to other immune cells [46]. In 1993 the Waldmann group studied this effect in a murine skin transplantation setting. Thymectomized mice (CBA/Ca) received a tolerizing protocol utilizing an induction therapy combining CD4 as well as CD8 non-depleting antibodies and a allogeneic skin graft (B10.BR). Upon challenge with naïve recipient-type lymphocytes, infectious tolerance was conferred to freshy infused cells and sustained tolerance was shown by indefinite survival of another donor-type skin graft. If CD4+, but not CD8 + T cells were depleted before naïve lymphocyte infusion, however, skin grafts were rejected, suggesting again a profound role of CD4 + T cells in tolerance induction. Furthermore, they showed that coexistence of tolerized host and freshly transferred naïve cells from the same mouse strain for 2 weeks in vivo led to tolerance against a second same-donor skin graft (B10.BR) even after selective depletion of tolerized host cells, impressively demonstrating the effects of ‘infectious tolerance’ in transplantation [47]. Notably, infectious tolerance was also seen in humans were Tregs conveyed suppressor activity to conventional CD4 + T helper cells [48], again proving the importance of Tregs for cell-based therapies.
Regulatory T cells: therapeutic approaches
Adoptive cell therapy
There are several open questions regarding the best in vitro expansion approach for adoptive cell therapy, regarding Treg source, isolation method, culture conditions and specificity. Tregs can be isolated from the donor, recipient, or even third-parties (‘off-the-shelf product’) and isolation sites include peripheral blood mononuclear cells (PBMCs), umbilical cord-blood (UCB), and (pediatric) thymi. Isolation techniques and markers used for sorting of the starting cell populations as well as conditions for expansion cultures also vary between published studies. Moreover, the right cell dose in clinical (or pre-clinical in vivo) studies has yet to be determined and cannot simply be extrapolated from in vitro suppressor assays.
Treg source and isolation
Whereas most clinical studies use PBMC-derived cells, it has been reported that comparable frequencies of Tregs can be found within the UCB. Moreover cells isolated from UCB are largely naïve, since the UBC is almost devoid of CD25 + antigen-experienced memory and effector T cells, making it a highly suitable source for Treg isolation in the clinical setting [49, 50]. One notable drawback of using UBC is the very low yield of Tregs per unit. A challenge that may be overcome by pooling multiple donors [51]. Pediatric thymi routinely obtained during heart surgery are in addition a valuable source for Tregs [52]. More recently, Lombardi et al. were able to generate a good manufacturing practice (GMP)-compatible protocol for the expansion of pediatric thymus-derived CD3 + CD4 + CD25 + CD127- (Tregs) as well as CD3 + CD4 + CD25 + CD127- CD45RA + (RA + Tregs) cells, paving the way for future clinical application [53]. Indeed, a phase I/II clinical trial for prevention of heart transplant rejection in children using autologous Tregs isolated from thymic tissue is currently ongoing (NCT04924491). Besides the UCB and the thymus, peripheral blood (PB) remains the main source used for Treg cellular products in clinical trials [54]. In theory, Tregs for adoptive cell therapy can be obtained from the recipient, the donor or even unrelated third-parties. In a pre-clinical study our group could directly show the superiority of recipient-based Tregs for tolerance induction via hematopoietic chimerism in transplantation [55]. Moreover, autologous (recipient-derived) Treg therapy has been demonstrated to be feasible and safe in numerous clinical studies [56, 57]. Interestingly, allogeneic (donor-derived) Tregs have shown superior effectiveness in preventing graft versus host disease (GvHD) after allogeneic stem cell transplantation [58]. Hoffman et al. developed the first GMP-compliant isolation method for magnetic sorting of CD4 + CD25 + T cells from standard leukapheresis products using the CliniMACS system (Miltenyi Biotec, Bisley, UK) [59]. This system utilizes a two-step magnetic bead isolation involving a CD8+/CD19 + depletion step followed by the enrichment of CD25 + cells via positive selection. However, multiple parameters for stricter Treg selection are not applicable in this system, representing an important limitation of this approach. In addition, the purity of bead-isolated Tregs regarding their FOXP3 expression is reportedly limited to about 80% raising the concern of contamination with activated Teff cells that potentially cause graft damage upon injection into the patient [60]. Therefore fluorescence activated cell sorting (FACS) of Tregs based on selected cell markers by flow cytometry gained importance as a method allowing high purity Treg (>99%) isolation [61]. In recent times effort was made in developing a GMP-compliant closed FACS-system for clinical application, allowing the use of multiple parameters and quality of expression during Treg isolation, e.g. CD25high, CD62Lhigh, CD127low, CD45RA, which is not possible using the European Medicines Agency (EMA) approved CliniMACS system. In the human setting it was demonstrated that only Treg cultures originated from CD4+, CD25high, CD45RA + sorted cells maintained FOXP3 stability as well as high suppressive capacity following in vitro cell culture [26, 62]. Based on these results, FACS sorting for Treg isolation is already part of clinical kidney transplantation trials (NCT02088931; NCT03867617) [63, 64].
In vitro expansion
Besides the challenge of manufacturing highly pure Treg cell products, the amount of cells needed for adoptive transfer is another hurdle to overcome for efficient Treg cell therapy. Based on mouse models for tolerance induction, it is suggested that in vivo Treg numbers need to be increased by 33% in order to reach a Treg:Teff ratio shown to elicit effective Treg-based suppression [65, 66]. What has to be taken in account is the amount of Teff that is expected within the recipient. Without lympho-depleting pre-treatment, it is stated that 49-79 × 109 Tregs are needed to reach clinically efficacious numbers. If immunosuppressive drugs such as anti-thymocyte globulin (ATG) are administered however, it is suggested that 3-5 × 109 Tregs are sufficient [66]. First routine ex vivo expansion cultures for polyclonal Treg products used beads coated with anti-CD3 and anti-CD28 in the presence of high-dose IL-2. Subsequent, cell culture condition optimization protocols adding rapamycin and TGF-β suggested further improvements in purity of in vitro expanded Treg cultures. With the supplementation of rapamycin, a mammalian target of rapamycin (mTOR) inhibitor, potential contamination of the Treg cell product with Teff is mitigated due to insensitivity of Tregs to mTOR blockade [67]. In expansion culture protocols containing rapamycin and TGF-β, Tregs showed higher suppressive capacity and stable FOXP3 expression due to increased TSDR demethylation [68]. These polyclonally expanded Tregs exhibit a wide range of TCR specificities with their main effects relying on bystander immunosuppression trough antigen-independent mechanisms [69].
Besides, there is growing evidence suggesting that antigen-specific Tregs are more efficient in regulating immunological responses compared to polyclonal expanded Tregs [70]. These statements are based on the superior homing capacity of antigen-specific Tregs, allowing localized and more potent regulation of inflammatory processes [71]. A variety of approaches for antigen-specific Treg expansion have been developed over the last years. After discovering that murine naïve CD4 + T cells have potential to develop into iTregs if stimulated with IL-2 and TGF-β in vitro, antigen-specific Teff as source for antigen-specific iTregs came into the center of attention [72]. Yet, these iTregs do not show a stable suppressive phenotype if re-introduced into inflammatory environment and they are likely to re-gain their pro-inflammatory characteristics in vivo [73]. Transgenic (over)expression of FOXP3 via lentivirus-based transduction in antigen-specific Tconv, however, lead to high levels of stable FOXP3 expression accompanied by suppressive capacities in vitro [74, 75] as well as in vivo [76, 77]. In addition, these iTregs maintained stability in inflammatory in vitro as well as in vivo conditions [77]. This approach requires retroviral transduction techniques which are associated with not only safety concerns, but also high production costs and vector capacity constraints [78]. However, one safety risk, the random insertion of FOXP3, was successfully addressed recently by utilizing advanced genetic tools such as TALEN or CRISPR/Cas9 to induce high expression of FOXP3 in Teff cells via homology directed repair (HDR) genome editing [79, 80].
Besides, different approaches of allospecific in vitro priming were developed. Putnam et al., for example, made use of allogeneic DCs whereas Sagoo et al. utilized donor-specific B cells to generate human Tregs with direct allospecificity in vitro. In both studies donor-specific Tregs succeeded over polyclonal Tregs in protecting human skin xenografts from alloimmune response-mediated injury [71, 81]. In addition, Jiang et al. made use of human leucocyte antigen A2 (HLA A2) peptide (138-170aa) pulsed immature DCs to generate human Tregs with defined antigen specificity via indirect recognition and demonstrated cell-contact-dependent effective suppression of Tconv cells [82]. Studies performed in murine skin allograft or GvHD models obtained similar results, strengthening the hypothesis of better efficacy of donor-specific Tregs [65, 83, 84]. Moreover, it was demonstrated that due to antigen-specificity, lower Treg cell numbers are needed to achieve sufficient suppression compared with polyclonal Tregs [71, 81].
Regardless of the fact that antigen-specific Tregs are more potent than polyclonally expanded Tregs, the main limitation of generating these cells is the low precursor frequency as well as challenging cell culture requirements. Another approach to generate targeted immunosuppressive Tregs is to introduce antigen-specific transgenic TCR or synthetic chimeric antigen receptors (CAR) into polyclonal Tregs. Utilizing genetically modified α and β TCR chains to obtain Tregs with a certain antigen-specificity already achieved promising results in autoimmune diseases [85, 86], GvHD [84], and transplantation [87]. With regard to the latter, it was demonstrated that engineered Tregs were even more efficient at tolerance induction when not only transduced with a TCR specific for direct but also indirect allorecognition in a murine heart allograft model (BALB/c →C57BL/6) [88]. These results again highlight the clinical potential of genetically engineered Tregs. However, there are some limitations to this approach such as the transduction of antigen-specific TCRs isolated from Teff into Treg.
In vivo stimulation
Since in vitro expansion and adoptive Treg transfer require advanced GMP-compliant cell culture conditions, are accompanied by high costs and risk of contamination, different approaches for in vivo stimulation of Tregs are part of ongoing investigations.
Low dose IL-2
Treg survival as well as stability and function are dependent on (exogenous) IL-2 [89] and IL-2 deficiency results in Treg apoptosis [90]. Therefore, IL-2 became an interesting therapeutic option for enhancing Treg efficacy in vivo. In fact, low dose IL-2 therapy resulted in preferential expansion and activation of Tregs, whereas high dose treatment led to expansion of NK cells as well as cytotoxic T lymphocytes (CTL) and caused serious side effects [91, 92]. These observations are based on the expression of IL-2 receptors with different IL-2 binding affinities. Whereas CTLs only express β and γ receptor subunits resulting in low IL-2 binding affinity, Tregs express the IL-2 high-affinity receptor complex consisting of the α, β, and γ chains enabling activation and expansion even with low IL-2 availability [93]. Utilizing low-dose IL-2 administration, promising results have been demonstrated in clinical trials of patients with hepatitis C virus-induced vasculitis [94], GvHD [95], as well as Type 1 diabetes (T1D) [96]. In addition, Tahvildari et al. showed that low-dose IL-2 treatment led to increased levels of Tregs with only mild expansion of Teffs in a murine model of corneal transplantation and therefore improved allograft survival [97]. Clinical use of IL-2 therapy is however limited by the short serum half-life and dose-dependent toxicities [98, 99].
IL-2 complexes
Almost 30 years ago, Finkelman et al. could show that the complexation of cytokines and respective neutralizing anti-cytokine antibodies would increase the half-life and biological effect of cytokines in vivo [100]. A decade later, Boyman and Sprent discovered that IL-2 complexed to a specific anti-IL-2 monoclonal antibody, namely JES6-1, increased not only the duration and magnitude of the cytokine response but was able to selectively stimulate target cells. This approach predominantly led to the expansion of Tregs with only minor increase of (IL-2 responsive) CTLs and NK cells. This effect was suggested to be based on the binding of the JES6-1 antibody to a site of IL-2 that is essential for the interaction with the β (CD122) but not α (CD25) IL-2 receptor subunit, favoring the cytokine binding towards the IL-2 high affinity receptor, preventing potential side-effects seen in IL-2 high dose therapy [93]. The potential of IL-2/JES6-1 complexes to increase Treg levels and therefore decrease inflammation was already demonstrated in a murine model of fully mismatched islet cell transplantation. In fact, after 3 consecutive days of injecting IL-2/JES6-1 complexes, Treg levels within CD4 + T cells of the spleen were increased 5-fold and resulted in indefinite acceptance of the majority of grafts [101]. More importantly, it was demonstrated that administration of IL-2/JES6-1 complexes synergize with rapamycin and a short-term treatment of anti-IL-6 to significantly prolong survival of fully mismatched skin grafts (BALB/c →C57BL/6) [102]. Recently, researchers have been able to develop specific human anti-IL-2 receptor antibodies, demonstrating selective in vivo Treg expansion and suppressive potency in a mouse model of T1D, experimental autoimmune encephalomyelitis and xenogeneic GvHD [103]. These promising findings might pave the way for future IL-2 complex-based clinical trials [104].
Polyclonal vs. antigen-specific Tregs
Whether polyclonal or antigen-specific Tregs are the future of Treg-based treatment remains another controversial topic regarding adoptive Treg transfer approaches. In fact, pre-clinical studies demonstrated that antigen-specific Tregs are more potent at inhibiting Teff in vitro as well as in vivo if compared to polyclonal Tregs [70]. However, studies on non-human primates suggesting lower efficacy of ex vivo induced antigen-specific Tregs with evidence of loss of regulatory mechanisms upon in vivo introduction [105]. In addition, antigen-specific Treg development requires challenging cell culture conditions as seen in the ARTEMIS trial where generation of the Treg product failed in 5 out of 10 patients enrolled in this study (NCT02474199). These results ignite the discussion whether polyclonal Tregs are more feasible for clinical translation of this therapeutic approach. Although it is stated that the bystander suppressive capacity of polyclonal expanded Tregs may cause increased susceptibility to opportunistic infections or tumor development due to unspecific suppression of immune responses and therefore limit the effect in transplantation settings [69, 106], human trials did not detect signs of over-immunosuppression after polyclonal Treg transfer. In addition, no events of Tregs converting into donor-specific Teff occurred in kidney transplant recipients, supporting the feasibility of polyclonal over antigen-specific Tregs [107, 108].
Next generation approaches
Engineered Tregs: CARs and TRUCKs
Another approach to confer antigen specificity is the expression of specific chimeric antigen receptors (CAR)s on immune cells. CARs are chimeric fusion proteins containing an extracellular antigen binding site of a monoclonal antibody fused to T-cell stimulatory and costimulatory intracellular domains [109]. One major advantage of CAR T cells compared with TCR transgenic T cells is the ability to recognize specific antigens without the requirement of antigen processing and presentation, independent of MHC classes I and II. Utilizing CAR-engineered Teff cells led to promising results in blood cancer settings [110, 111] which also gave rise to the development of advanced CAR constructs. The introduction of an intracellular costimulatory domain in addition to the single CD3ξ intracellular signaling domain, for example, resulted in better T-cell activation and proliferation [112]. More importantly, it has been demonstrated that Tregs engineered with this ‘second-generation’ CARs targeting HLA-A2 successfully prevented xenogeneic GvHD in a humanized mouse transplantation model in vivo [113]. In addition, precise antigen-specific action of HLA-A2 targeting CAR Tregs was verified in a side-to-side skin transplantation model demonstrating prolonged survival of HLA-A2 expressing grafts while no effect was seen for HLA-A2 non-expressing transplants [114]. In addition, migration of Tregs towards the desired site of impact has been shown to be crucial for therapeutic Treg-mediated suppression in transplantation [115]. It was demonstrated that donor-specific CAR Tregs are able to migrate into the targeted tissue where they are capable of not only delaying skin graft rejection but also decrease B cell responses and donor specific antibody production, whereas allospecific memory and graft rejection in sensitized mice was not attenuated [116]. Besides, Tregs are capable of exerting bystander suppression in inflammatory sites without direct targeting of cell surface antigens. This was demonstrated using CAR Tregs specific for citrullinated vimentin (CV), a protein abundantly and almost exclusively found in the inflamed joint extracellular matrix in rheumatoid arthritis (RA) patients. In detail, CV-specific CAR Tregs were able to proliferate when co-cultured with synovial fluid from the joints of RA patients, suggesting that CV within the extracellular matrix was sufficient to activate CAR Tregs specific for CV [117]. This approach offers the opportunity of using the bystander suppressive capacity of Tregs in inflammatory settings where direct targeting of antigen expressing cells might be disadvantageous based on reported cytotoxic activity of CAR Tregs in specific cases [43]. In addition, there are ongoing investigations regarding third- and fourth-generation (known as TRUCK) CAR Tregs that include further costimulatory domains or co-express cytokines and transcription factors in order to maximize the potential of CAR Tregs for individual disease treatment [118]. At present CAR Treg production is dependent mainly on γ-retroviral or lentiviral vectors known to be accompanied by not only safety concerns but also high manufacturing costs [78]. Nevertheless, first human clinical trials are now evaluating the safety and feasibility of HLA-A*02 recognizing CAR Tregs in HLA-A2 mismatched liver (NCT05234190) and kidney (NCT04817774) transplant recipients.
Reducing off-target effects for in vivo Treg expansion: the orthogonal IL-2R/IL-2 system and the SynNotch receptor system
To further minimize the risk of side effects caused by immunosuppressive therapy or IL-2 administration for in vivo Treg expansion, Tregs expressing engineered IL-2 receptors that only engage with specific engineered IL-2 cytokines enable precise treatment approaches. It was already demonstrated that T cells transduced with an orthogonal IL-2Rβ subunit selectively bind a mutant IL-2 showing only minor interaction with wild-type IL-2Rβ in vivo. Furthermore, adoptive cell transfer (ACT) of orthogonal IL-2Rβ transduced effector CD4 + and CD8 + T cells resulted in elevated anti-tumor immune responses upon orthogonal IL-2 injection in a pre-clinical cancer model [119]. Recently, efficacy of orthogonal IL-2 receptor engineered Tregs was tested in a murine mixed chimerism model. It was demonstrated that administration of orthogonal IL-2 significantly increased orthogonal Treg numbers in vivo without promoting the expansion of other T-cell subsets. More importantly, this approach led to promising results involving donor hematopoietic cell engraftment with heart allograft acceptance at a later time point [120].
Another option to further improve Treg-based therapy could be the recently developed synthetic Notch (SynNotch) receptor system containing the Notch receptor regulatory core domain fused to custom-made extracellular recognition and intracellular transcriptional domains [121]. Upon synNotch receptor activation via target antigen binding the specific intracellular transcription factor is released and enters the nucleus in order to initiate expression of a certain gene. This system is completely independent of T-cell-based signaling pathways allowing customized antigen-specific T-cell response programs including the production of certain cytokines, therapeutic mediators, regulating transcription factors, or other immune response modulating adjuvants [122]. Promising results have already been obtained in studies on murine cancer models utilizing synNotch Teff cells [122] pathing the way for application in other immune cell types such as Tregs. Here, the synNotch system could be used in combination with the orthogonal IL-2R engineered Tregs to produce orthogonal IL-2 in an antigen-dependent positive feedback loop [117].
Cytokine engineering: IL-2 muteins/IL-2 superkines
Engineering IL-2 to not only extend its half-life, but also improve its effectiveness in vivo led to the development of IL-2 ‘superkines’ or IL-2 muteins. The combination of screening IL-2 mutants for superior interaction with the β- and γ-subunit of the IL-2 receptor and developments of chimeric proteins by fusion of the cytokine to proteins like albumin or IgG for half-life extension, already led to promising results in cancer studies [104, 123, 124]. Recent development of IL-2 muteins with preferred binding to the α (CD25) IL-2 receptor subunit for favored interaction with the high-affinity IL-2 receptor represents another in vivo approach for selective Treg expansion. These new IL-2 muteins resemble the biological function of IL-2/JES6-1 mab complexes with promising results in treatment of type I diabetes in a pre-clinical murine model [125]. Moreover, there are ongoing clinical trials evaluating the safety and efficacy of Efavaleukin Alfa, a human IL-2 mutein Fc fusion protein selectively expanding human Tregs in vivo, being evaluated in GvHD (NCT03422627) and systemic lupus erythematosus (NCT03451422).
Tackling the humoral response: BAR T cells
Recently, new chimeric immune receptor (CIR) T cells with specificities against antibody-producing B cells were developed. These B-cell antibody receptor (BAR) T cells consist of an antigen or antigen fragment which can be recognized by certain B cell receptors on the cell surface fused to an intracellular costimulatory or T-cell signaling domain [117]. Like CAR Tregs, BAR T cells recognize antigens independent of MHC, resulting in the suppression of antigen-specific B cells. This was already demonstrated in pre-clinical mouse models of allergy [118] as well as hemophilia A [119]. In transplantation settings, humoral alloimmune responses still account for the majority of late graft loss due to DSA development [120] and chronic humoral rejection. Thus, HLA-BAR T cells might be an attractive treatment approach as these cells could be utilized for the prevention of de novo DSA development through suppression of allospecific B cells [121]. In addition, effective recipient desensitization protocols for preformed DSA clearance remain wanted. Therefore, it was suggested that CD8 + T cells transduced with HLA-specific BAR in combination with drugs accounting for global plasma cell depletion could be a promising application for desensitization of patients with preformed DSA by targeting donor HLA-specific memory B cells [121]. What remains an uncertainty concerning the therapeutic use of BAR T cells is the fact that the alloantigen expressed on its cell surface might be recognized by recipient leucocyte T-cell receptors. This would result in destruction of BAR T cells and could potential trigger a ‘cytokine storm’ due to systemic inflammatory processes leading to excessive T-cell activation [121, 123]. In order to translate this approach into a clinical setting these limitations have to be addressed first.
‘Off the shelf’ Tregs
High costs accompanied to GMP-compliant in vitro Treg expansion together with low baseline autologous cell numbers still are one of the limiting factors for large scale application of adoptive Treg therapy. This problem is even more pronounced in transplant patients receiving immunosuppressive treatment for underlying autoimmune diseases or chronical illness as well as patients on the waiting list for re-transplantation. As a result, the development of ‘off-the shelf’ T-cell products remains in the center of scientific attention. Different approaches have been investigated over the years including third party Tregs obtained from UCB or donor bone marrow-derived endogenous Tregs [126] with the goal of creating a biobank for Tregs for maximum MHC matching. Having a Treg biobank with ready-to-use Tregs at any time would expand the availability of Treg therapies from living-donation settings to recipients of DBD and DCD donors. After transfer however, extrinsic Tregs might still face the problem of host-mediated elimination due to the expression of foreign MHC molecules. The approach of using the CRISPR-Cas9 system to achieve HLA deficient Tregs led to NK cell-mediated elimination of these cells due to lack of canonical HLA molecules on the cell surface. The expression of non-canonical HLA-E or HLA-G, which are NK inhibitory receptor ligands, could be one possibility of solving this issue [127]. Besides, the expression of CD47, commonly known as the ‘do-not-eat-me-signal’ for macrophages, was suggested to be of additional benefit in this approach [128].
‘Off the shelf’ CAR Tregs could represent another opportunity to circumventing the need for high quantity autologous Tregs and challenging cell culture conditions to generate antigen-specific Tregs. CAR Tregs can be generated fast and in large numbers from naive or polyclonal expanded Tregs and are, as mentioned above, able to recognize antigens presented within the MHC and in a non-MHC restricted manner. After in vivo transfer, however, off-target effects due to missing the intended destination might be an issue. Thus, including suicide genes acting as ‘safety switch’ in case of pan-immunosuppression could be a solution. RQR8 or huEGFRt have been successfully tested as suicide genes in murine models where cells expressing these surface proteins were targeted and sufficiently deleted by the respective monoclonal antibody rituximab/cetuximab [129, 130] and are now part of a CAR-Treg human liver transplantation trial (NCT05234190). For prevalent clinical application of CAR Tregs, however, some other safety concerns remain. Even though to date no issues regarding viral-based transfer of the CAR to Tregs were reported, there is still the need for alternative gene-editing tools such as CRISPR-Cas9 or TALENs since testing and production of viral vectors is not only time consuming but also expensive. Moreover, random genome insertion of the transgene is a negative property of viral vectors that can cause oncogenic genetic changes. However, utilizing CRISPR-Cas9 or TALENs enables the replacement of endogenous TCR with a specific CAR if targeting the endogenous T-cell receptor-α constant (TRAC). These modifications would minimize the risk of side-effects such as alloreactivity resulting in GvHD, facilitating the development of ‘off the shelf’ CAR T-cell products [131, 132]. Another approach to reach this aim is the use of modular or universal CAR. Pierini et al. for example developed a murine CAR consisting of an anti-FITC scFv portion fused to a CD28 and CD3 co-stimulatory domain, termed mAbCAR. This synthetic receptor is suggested to bind any FITC conjugated mAb enabling fast generation of CAR Tregs directed against a variety of antigens. The efficacy of this system was demonstrated in a murine model by significantly prolonging the survival of fully mismatched islets and skin grafts if H-2Dd-mAbCAR Tregs were injected [133]. These promising results display a first step towards another valuable tool for antigen-specific ‘off the shelf’ Treg therapy.
Clinical experience
Many clinical trials have already introduced Treg cellular therapy into clinical trials to treat autoimmune diseases, hematopoietic stem cell transplantation and SOT recipients [134, 135]. The clinical experience with Treg therapy in SOT in ongoing and already published clinical trials is summarized in Table 1.
Table 1:
ongoing clinical trials adopting regulatory T-cell therapy in solid organ transplantation (search date September 15, 2022)
| Study ID | Phase | Age | Title | Product | Dose | Status | Location |
|---|---|---|---|---|---|---|---|
| Renal transplantation—endogenous Treg expansion | |||||||
| NCT02417870 | I/II | 18-75 (adult, older adult) | Ultra-low Dose Subcutaneous IL-2 in Renal Transplantation | Low-dose recombinant IL-2 (proleukin) | — | Terminated (June 2021) |
Brigham and Women`s Hospital, Boston, US |
| Renal transplantation—adoptive Treg therapy | |||||||
| NCT02088931 | I | 18-50 (adult) |
Treg Adoptive Therapy for Subclinical Inflammation in Kidney Transplantation (TASK) | CD4 + CD127lo/-CD25 + polyclonally expanded Tregs | 3.2 × 108 | Completed (July 2022) |
University of California, San Francisco, US |
| NCT02091232 | I | >18 (adult, older adult) | Infusion of T-Regulatory Cells in Kidney Transplant Recipients (The ONE Study) | Tregs (recipient) stimulated with donor PBMCs and belatacept | 4-9 × 108 | Completed (Nov 2021) |
Massachusetts General Hospital, Boston, US |
| NCT04817774 | I/II | 18-70 (adult, older adult) | Safety and Tolerability Study of Chimeric Antigen Receptor T-Reg Cell Therapy in Living Donor Renal Transplant Recipients | CD4 + CD45RA + CD25 + CD127low/- HLA-A*02 specific CAR Tregs | — | Recruiting (Dec 2021) |
University Hospitals Leuven Leuven, Belgium (and 3 other centers) |
| NCT03943238 | I | 18-65 (adult, older adult) | TLI, TBI, ATG & Hematopoietic Stem Cell Transplantation and Recipient T Regs Therapy in Living Donor Kidney Transplantation | Autologous polyclonally expanded Tregs | starting at 25 × 106/kg | Recruiting (May 2022) |
Stanford University Palo Alto, Northwestern University Chicago, US |
| NCT03284242 | n/a | 18-65 (adult, older adult) | A Pilot Study Using Autologous Regulatory T Cell Infusion Zortress (Everolimus) in Renal Transplant Recipients | Autologous polyclonally expanded Tregs | n/a | Recruiting (May 2022) |
University of Kentucky Medical Center Lexington, Kentucky, US |
| NCT02711826 | I/II | >18 (adult, older adult) | Treg Therapy in Subclinical Inflammation in Kidney Transplantation | Autologous polyclonally expanded Tregs | 5.5 ± 4.5 × 108 | Recruiting (March 2022) |
University of California at San Francisco, US (and 5 other centers) |
| NCT02145325 | I | 18-65 (adult, older adult) | Trial of Adoptive Immunotherapy With TRACT to Prevent Rejection in Living Donor Kidney Transplant Recipients | Autologous polyclonal expanded CD4 + CD25 + nTregs | 0.5-5 × 106 | Completed (Oct 2019) |
Northwestern University Comprehensive Transplant Center Chicago, Illinois, US |
| NCT03867617 | I/II | >18 (adult, older adult) | Cell Therapy for Immunomodulation in Kidney Transplantation | Autologous polyclonally expanded CD4 + CD127lo/- CD25 + CD45RA Tregs | 0.3-1.5 × 107 | Recruiting (Sep 2019) |
Medical University of Vienna, Vienna, Austria |
| NCT01446484 | I/II | 1-18 (child) | Treatment of Children With Kidney Transplants by Injection of CD4 + CD25 + FoxP3 + T Cells to Prevent Organ Rejection | Autologous CD4 + CD25 + CD127low FoxP3 + Tregs | 2 × 108 | Unknown (Nov 2011) |
Russian state Medical University, Moscow, Russian Federation |
| NCT02371434 | I/II | 18-65 (adult, older adult) | The ONE Study nTreg Trial (ONEnTreg13) | Autologous polyclonally expanded CD4 + CD25 + FoxP3 + nTregs | 0.5-3 × 106 | Completed (Feb 2020) |
Charité University Medicine, Berlin, Germany |
| NCT02244801 | I | 18-70 (adult, older adult) | Donor-Alloantigen-Reactive Regulatory T Cell (darTreg) Therapy in Renal Transplantation (The ONE Study) | Donor alloantigen reactive Tregs (darTregs) | 3 × 108; 9 × 108 | Completed (Oct 2018) |
University of California San Francisco, US |
| NCT02129881 | I/II | >18 (adult, older adult) | The ONE Study UK Treg Trial | Autologous polyclonally expanded Tregs | 1-10 × 106/kg | Completed (Jan 2019) |
Guy’s Hospital London, UK |
| ISRCTN 11038572 |
II | >18 (adult, older adult) | TWO study: cell therapy trial in renal transplantation | Autologous polyclonally expanded Tregs | 5-10 × 106/kg | Recruiting (June 2022) |
Oxford Transplant Centre, Churchill Hospital, Oxford, UK |
| Liver transplantation—endogenous Treg expansion | |||||||
| NCT02739412 | II | 18-65 (adult, older adult) | Efficacy of Low Dose, SubQ Interleukin-2 (IL-2) to Expand Endogenous Regulatory T-Cells in Liver Transplant Recipients | Low-dose recombinant IL-2 (proleukin) | 0.30 MIU per meter squared body surface area; for 4 weeks | Active, not recruiting (May 2021) |
Beth Israel Deaconess Medical Center Boston, Massachusetts, US |
| NCT02949492 | IV | 18-50 (adult) |
Low-dose IL-2 for Treg Expansion and Tolerance (LITE) | Low-dose recombinant IL-2 (proleukin) | — | Terminated (Aug 2019) |
Kings Collage Hospital London, UK |
| Liver transplantation—adoptive cell therapy | |||||||
| NCT01624077 | I | 10-60 (child, adult) | Safety Study of Using Regulatory T Cells Induce Liver Transplantation Tolerance | Autologous polyclonally TGF-β induced CD4 + CD25 + CD127- Tregs | 1 × 106/kg | Unknown (Feb 2015) |
Nanjing Medical University Nanjing, Jiangsu, China |
| NCT03654040 | I/II | 18-70 (adult, older adult) |
Liver Transplantation With Tregs at UCSF | Autologous expanded donor alloantigen reactive Tregs (arTregs) | 30-90 × 106 total Treg cells | Recruiting (Aug 2021) |
University of California, San Francisco San Francisco, California, US |
| NCT03577431 | I/II | 18-70 (adult, older adult) |
Liver Transplantation With Tregs at MGH | Autologous expanded donor alloantigen reactive CD4 + CD25 + CD127- Treg cells (arTregs) | 2.5-125 × 106 | Recruiting (Nov 2021) |
Massachusetts General Hospital: Transplantation Boston, Massachusetts, United States |
| NCT02474199 | I/II | 18-70 (adult, older adult) |
Donor Alloantigen Reactive Tregs (darTregs) for Calcineurin Inhibitor (CNI) Reduction (ARTEMIS) | Autologous donor alloantigen reactive Tregs (darTregs) | 3-5 × 108 | Completed (Feb 2021) | University of California at San Francisco San Francisco, US Northwestern University Comprehensive Transplant Ctr Chicago, US Mayo Clinic in Rochester Rochester, US |
| NCT02188719 | I | 21-70 (adult, older adult) | Donor-Alloantigen-Reactive Regulatory T Cell (darTregs) in Liver Transplantation (deLTa) | Autologous donor alloantigen reactive Tregs (darTregs) | 2.5-96 × 107 | Terminated (Sep 2020) - has results |
University of California at San Francisco San Francisco, US Northwestern University Comprehensive Transplant Ctr Chicago, US Mayo Clinic in Rochester Rochester, US |
| NCT02166177 | I/II | 18-70 (adult, older adult) | Safety and Efficacy Study of Regulatory T Cell Therapy in Liver Transplant Patients (ThRIL) | Autologous polyclonally expanded Tregs | 0.5-1; 3-4.5 × 106/kg | Completed (Jan 2019) |
Kings College Hospital London, UK |
| UMIN-000015789 | I/II | 18-65 (adult, older adult) | Tolerance induction by a regulartory T cell-based cell therapy in living donor liver transplantation | Donor-reactive Treg-enriched cell product | 0.23-6.37 × 106 Tregs/kg | Recruiting (until July 2012) Data published 2016 [136] |
Hokkaidou University Graduate School of Medicine, Japan |
| NCT05234190 | I/II | 18-70 (adult, older adult) | Safety and Clinical Activity of QEL-001 in A2-mismatch Liver Transplant Patients (LIBERATE) | Autologous CAR Tregs targeting HLA-A2 (HLA-A2 CAR-Treg) | — | Recruiting (Feb 2022) |
Cambridge University Hospitals NHS Foundation Trust Cambridge, UK Royal Free London NHS Foundation Trust London, UK King’s College Hospital NHS Foundation Trust London, UK |
Translational hurdles
Although the importance of Tregs for long-term survival of allografts has been demonstrated in several pre-clinical as well as clinical trials, there is still no single (pre-clinical) study showing that Tregs alone are sufficient to induce tolerance in immunocompetent hosts or even significantly prolong SOT survival. There are multiple clinical trials demonstrating the safety of adoptively transferred Tregs in combination with immunosuppressants in kidney as well as liver transplantation (Table 1). However, Treg therapy for the induction of a pro-tolerogenic state with intention to wean the patients off immunosuppressive drugs was successfully tested in only one clinical liver transplantation trial by Todo et al [136, 137]. This approach is pursued in further ongoing liver transplantation studies (NCT03654040; NCT03577431). The use of Treg therapy based weaning protocols in heart and lung transplantation, however, remains challenging due to the lack of reliable biomarkers or functional assays identifying tolerant patients and no applicable rescue options in case of treatment-based rejection [138]. Although several studies correlate increased Treg numbers with improved lung transplant function and protective effect against chronic lung allograft dysfunction in animals and humans, Treg therapy still requires in-advance preparation time [139, 140]. Since planned living organ donation is only feasible for kidney and liver transplantation, these treatment options remain challenging for heart and lung transplantation. Recently, it was shown in a rat model of lung transplantation that in vitro expanded recipient-derived Tregs administered to the transplant during ex vivo perfusion resulted in successful inhibition of early alloimmune responses post-transplantation, claiming to provide an approach suitable for clinical translation to lung transplantation from deceased donors [141]. However, whether Treg-mediated tolerance induction is sufficiently stable to endure integrity during other opportunistic infections throughout the patient’s life is pending. In addition, several studies suggest differences in success of graft acceptance depending on the type of organ transplanted, implying the need for organ-specific approaches. Whereas liver transplant patients (especially pediatric patients) are the most common to develop ‘operational tolerance’, late after transplantation, this is a really rare event in kidney transplantation. Skin and intestines are considered to be the most immunogenic organs [136, 142]; however, the detailed mechanisms responsible for this phenomenon remain controversial [143].
Immunosuppressive drugs
As mentioned above, using autologous ex vivo expanded Tregs is time-consuming and does not allow fast application. Thus, it only can be implemented in planned living-donor transplant settings making this approach suitable for living-donor kidney and liver transplantation only. In addition, there are several studies demonstrating negative effects of transplantation-related immunosuppressive drugs on Treg cell numbers or Treg-related suppressive capacities, directly interfering with the feasibility of autologous Treg therapy. Calcineurin inhibitors, such as Tacrolimus or cyclosporine A, lead to decreased Treg numbers in liver and kidney transplant patients since this medication impedes IL-2 transcription which is crucial for survival and maintenance of Tregs. In a murine model of skin transplantation, it was shown that this treatment further results in less efficient Treg-mediated suppression of Teff [144]. Besides, there is evidence that co-stimulation blockade with CTLA4-Ig leads to decreased skin graft acceptance in a pre-clinical mouse model due to inhibition of IL-2 complex-mediated in vivo Treg expansion. Moreover a loss of suppressive function is suggested, based on limitation of crucial CD28-dependent signals [145]. On the other hand, in vitro studies on murine polyclonal-induced iTregs revealed no negative impact but rather improvement of Treg generation and suppressive function in the presence of the co-stimulation blocker CTLA4Ig [146]. Besides, there are studies stating that moderate CD80/CD86 blockade using CTLA4-Ig may be beneficial for pTreg development in both, rodents and humans [147, 148]. These suggestions are based on observations claiming that activation of Teff cells requires higher CD80/CD86 expression than Treg activation since partial blockade prevented Teff cells emergence, but maintained Treg homeostasis [147, 148]. Despite these controversial results, FDA approved costimulation blockers are used in patients with autoimmune disease and after renal transplantation. Further in-depth studies on the combination of Treg-based therapy with CTLA4-Ig are clearly needed.
Other immunomodulatory drugs commonly used in transplanted patients are suggested to favor in vivo Treg action. Methylprednisolone, for example, is a corticosteroid widely used to treat transplant patients facing acute rejection. The administration of this substance during kidney rejection also leads to an altered T-cell composition that is beneficial for a highly-suppressive DRhigh CD45RA− Treg population [149]. Furthermore, as anti-thymocyte globulin (ATG) gained interest as induction therapy in transplantation setting in the last years, studies involving Tregs revealed that under low-dose ATG regimen, circulating in vivo Treg numbers in renal transplant recipients are reduced, but to a lesser extent than Teff, shifting the Treg:Teff ratio to a pro-tolerogenic setting. In addition, it was stated that recovery of Tregs was faster after ATG administration if compared to Teff [150]. Moreover, the FDA approved mTOR inhibitor rapamycin (sirolimus and everolimus) leads to inhibition of Teff cells while sparing Tregs in vivo as well as in vitro. In fact, a 4-fold higher amount of peripheral Treg has been detected in kidney transplant patients receiving sirolimus if compared to patients receiving cyclosporine [151]. However, it is noteworthy that this increase in Tregs is rather due to higher treatment sensitivity of Tconv and does not promote Treg expansion [152]. Nevertheless, combining rapamycin with Treg expansion therapy is a promising treatment approach.
Concluding remarks
Pre-clinical studies reaching from in vivo and ex vivo expanded polyclonal to in vitro induced antigen-specific and genetically modified Tregs provided promising results for the improvement of long-term graft survival in context with immunosuppression minimization. Multiple approaches of Treg-based therapy that are now being evaluated for efficacy and safety in human trials. Whereas the ONE study consortium and others demonstrated feasibility and safety of multiple regulatory cell products [56, 153], to date there is only one published study to prove efficacy of Treg therapy in clinical transplantation [136].
The enhancement of Treg function by introducing CARs with tissue specific chimeric receptors, a FOXP3 phenotype lock module as well as a ‘safety switch’ is now part of a human phase I/II liver transplantation clinical trial (NCT05234190).
However, there are still plenty of open questions regarding Treg stability, Treg source, specificity, mode of action and migration toward the intended site of action. Approaches aiming for ‘off the shelf’ universal Tregs or ‘delayed tolerance’ approaches would furthermore expand their therapeutic potential for organ donation after circulatory death (DCD) and brain death (DBD) settings. Recent developments and new strategies such as genetic engineering tools will further enhance the potential of Treg cells as immunotherapy to minimize/avoid conventional immunosuppression and increase quality of life of transplant patients.
Acknowledgements
We would like to thank Anna M. Weijler for helpful discussions.
Abbreviations
- ACT
adoptive cell transfer
- APC
antigen-presenting cell
- ATG
anti-thymocyte globulin
- BAR
B-cell antibody receptor
- CAR
chimeric antigen receptor
- CIR
chimeric immune receptor
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T lymphocyte antigen 4
- DBD
donation after brainstem death
- DCD
Organ donation after circulatory death
- DSA
donor-specific antibody
- FACS
fluorescence activated cell sorting
- GMP-compatible
good manufacturing practice compatible
- GvHD
graft versus host disease
- HDR
homology directed repair
- HLA
human leucocyte antigen
- IPEX
immunodysregulation polyendocrinopaty enteropathy X-linked syndrome
- MHC
major histocompatibility complex
- mTOR
mammalian target of rapamycin
- NK
natural killer cell
- NRP1
neuropilin 1
- PBMCs
peripheral blood mononuclear cells
- SOT
solid organ transplantation
- TCR
T-cell receptor
- Teff
effector T cells
- TRAC
T-cell receptor-α constant
- Treg
regulatory T cell
- TSDR
Treg-specific demethylation region
- UCB
umbilical cord blood
Contributor Information
Romy Steiner, Department of General Surgery, Medical University of Vienna, Vienna, Austria; Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria; Center for Biomedical Research, Medical University of Vienna, Vienna, Austria.
Nina Pilat, Department of General Surgery, Medical University of Vienna, Vienna, Austria; Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria; Center for Biomedical Research, Medical University of Vienna, Vienna, Austria.
Funding
This work was supported by the Austrian Science Fund (P31186-B28 to NP).
Conflict of Interests
The authors declare that there are no competing interests.
Author Contributions
R.S. wrote and critically revised the manuscript. N.P. wrote and critically revised the manuscript. Both authors approved the final version of the manuscript.
Ethical Approval
The animal research adheres to the ARRIVE guidelines: not applicable.
Data Availability
Not applicable.
Permission to Reproduce
Manuscript does not use any third party copyrighted material.
Clinical Trial Registration
Not applicable.
References
- 1. Wekerle T, Segev D, Lechler R, Oberbauer R.. Strategies for long-term preservation of kidney graft function. Lancet 2017, 389, 2152–62. [DOI] [PubMed] [Google Scholar]
- 2. Katabathina V, Menias CO, Pickhardt P, Lubner M, Prasad SR.. Complications of immunosuppressive therapy in solid organ transplantation. Radiol Clin North Am 2016, 54, 303–19. doi: 10.1016/j.rcl.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3. Bamoulid J, Staeck O, Halleck F, Khadzhynov D, Brakemeier S, Dürr M, et al. The need for minimization strategies: current problems of immunosuppression. Transpl Int 2015, 28, 891–900. doi: 10.1111/tri.12553. [DOI] [PubMed] [Google Scholar]
- 4. Sánchez-Fueyo A. Hot-topic debate on tolerance: immunosuppression withdrawal. Liver Transpl 2011, 17(Suppl 3):S69–73. doi: 10.1002/lt.22421. [DOI] [PubMed] [Google Scholar]
- 5. Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 2010, 120, 1848–61. doi: 10.1172/jci39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu XQ, Hu ZQ, Pei YF, Tao R.. Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects. Hepatobiliary Pancreat Dis Int 2013, 12, 12–33. [DOI] [PubMed] [Google Scholar]
- 7. Nishizuka Y, Sakakura T.. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 1969, 166, 753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 8. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M.. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995, 155, 1151–64. [PubMed] [Google Scholar]
- 9. Sakaguchi S, Yamaguchi T, Nomura T, Ono M.. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 10. Vandenbark AA, Offner H.. Critical evaluation of regulatory T cells in autoimmunity: are the most potent regulatory specificities being ignored?. Immunology 2008, 125, 1–13. doi: 10.1111/j.1365-2567.2008.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khattri R, Cox T, Yasayko SA, Ramsdell F.. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003, 4, 337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12. Hori S, Nomura T, Sakaguchi S.. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 13. Fontenot JD, Gavin MA, Rudensky AY.. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003, 4, 330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001, 27, 20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 15. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 2001, 27, 68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 16. Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM.. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol 2008, 38, 1814–21. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH.. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 2001, 193, 1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur J Immunol 2007, 37, 21–3. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- 19. Sambucci M, Gargano F, De Rosa V, De Bardi M, Picozza M, Placido R, et al. FoxP3 isoforms and PD-1 expression by T regulatory cells in multiple sclerosis. Sci Rep 2018, 8, 3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mailer RK, Joly AL, Liu S, Elias S, Tegner J, Andersson J.. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci Rep 2015, 5, 14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014, 20, 62–8. [DOI] [PubMed] [Google Scholar]
- 22. Mailer RKW. Alternative splicing of FOXP3-virtue and vice. Front Immunol 2018, 9, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006, 203, 1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood K.. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation 2010, 90, 1321–7. doi: 10.1097/tp.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, et al. Only the CD45RA+ subpopulation of CD4+CD25 high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 2006, 108, 4260–7. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 27. Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol 2010, 184, 4317–26. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 28. Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209(10):1723–1742, s1. doi: 10.1084/jem.20120914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, et al. A role for the transcription factor Helios in human CD4(+)CD25(+) regulatory T cells. Mol Immunol 2010, 47, 1595–600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shevach EM, Thornton AM.. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 2014, 259, 88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P, et al. Differences in expression level of helios and neuropilin-1 do not distinguish thymus-derived from extrathymically-induced CD4+Foxp3+ regulatory T cells. PLoS One 2015, 10, e0141161e0141161. doi: 10.1371/journal.pone.0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol 2008, 38, 1654–63. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 33. Huehn J, Polansky JK, Hamann A.. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage?. Nat Rev Immunol 2009, 9, 83–9. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura K, Kitani A, Strober W.. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 2001, 194, 629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 36. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 37. D’Cruz LM, Klein L.. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 2005, 6, 1152–9. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 38. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ.. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007, 8, 1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 39. Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F.. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology 2006, 118, 240–9. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S.. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA 2008, 105, 10113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol 2019, 20, 218–31. doi: 10.1038/s41590-018-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ.. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004, 21, 589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 44. Antonioli L, Pacher P, Vizi ES, Haskó G.. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013, 19, 355–67. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity 2015, 43, 896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gershon RK, Kondo K.. Infectious immunological tolerance. Immunology 1971, 21, 903–14. [PMC free article] [PubMed] [Google Scholar]
- 47. Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993, 259, 974–7. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 48. Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH.. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med 2002, 196, 255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riley JL, June CH, Blazar BR.. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 2009, 30, 656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011, 117, 1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Milward K, Issa F, Hester J, Figueroa-Tentori D, Madrigal A, Wood KJ.. Multiple unit pooled umbilical cord blood is a viable source of therapeutic regulatory T cells. Transplantation 2013, 95, 85–93. doi: 10.1097/tp.0b013e31827722ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dijke IE, Hoeppli RE, Ellis T, Pearcey J, Huang Q, McMurchy AN, et al. Discarded human thymus is a novel source of stable and long-lived therapeutic regulatory T cells. Am J Transplant 2016, 16, 58–71. [DOI] [PubMed] [Google Scholar]
- 53. Romano M, Sen M, Scottà C, Alhabbab RY, Rico-Armada A, Lechler RI, et al. Isolation and expansion of thymus-derived regulatory T cells for use in pediatric heart transplant patients. Eur J Immunol 2021, 51, 2086–92. [DOI] [PubMed] [Google Scholar]
- 54. Safinia N, Vaikunthanathan T, Fraser H, Thirkell S, Lowe K, Blackmore L, et al. Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget 2016, 7, 7563–77. doi: 10.18632/oncotarget.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pilat N, Klaus C, Hock K, Baranyi U, Unger L, Mahr B, et al. Polyclonal recipient nTregs are superior to donor or third-party Tregs in the induction of transplantation tolerance. J Immunol Res 2015, 2015, 562935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pilat N, Sprent J.. Treg therapies revisited: tolerance beyond deletion. Front Immunol 2020, 11, 622810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Landwehr-Kenzel S, Muller-Jensen L, Kuehl JS, Abou-El-Enein M, Hoffmann H, Muench S, et al. Adoptive transfer of ex vivo expanded regulatory T cells improves immune cell engraftment and therapy-refractory chronic GvHD. Mol Ther 2022, 30, 2298–314. doi: 10.1016/j.ymthe.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoffmann P, Boeld TJ, Eder R, Albrecht J, Doser K, Piseshka B, et al. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol Blood Marrow Transplant 2006, 12, 267–74. [DOI] [PubMed] [Google Scholar]
- 60. Di Ianni M, Del Papa B, Zei T, Iacucci Ostini R, Cecchini D, Cantelmi MG, et al. T regulatory cell separation for clinical application. Transfus Apher Sci 2012, 47, 213–6. [DOI] [PubMed] [Google Scholar]
- 61. Scheffold A. How can the latest technologies advance cell therapy manufacturing?. Curr Opin Organ Transplant 2014, 19, 621–6. [DOI] [PubMed] [Google Scholar]
- 62. Canavan JB, Scottà C, Vossenkämper A, Goldberg R, Elder MJ, Shoval I, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 2016, 65, 584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am J Transplant 2017, 17, 2945–54. doi: 10.1111/ajt.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Oberbauer R, Edinger M, Berlakovich G, Kalhs P, Worel N, Heinze G, et al. A prospective controlled trial to evaluate safety and efficacy of in vitro expanded recipient regulatory T cell therapy and tocilizumab together with donor bone marrow infusion in HLA-mismatched living donor kidney transplant recipients (Trex001). Front Med (Lausanne) 2020, 7, 634260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI.. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood 2007, 109, 827–35. [DOI] [PubMed] [Google Scholar]
- 66. Tang Q, Lee K.. Regulatory T-cell therapy for transplantation: how many cells do we need?. Curr Opin Organ Transplant 2012, 17, 349–54. [DOI] [PubMed] [Google Scholar]
- 67. Fraser H, Safinia N, Grageda N, Thirkell S, Lowe K, Fry LJ, et al. A rapamycin-based GMP-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol Ther Methods Clin Dev 2018, 8, 198–209. doi: 10.1016/j.omtm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mathew JM, J HV, LeFever A, Konieczna I, Stratton C, He J, et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci Rep 2018, 8, 7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thornton AM, Shevach EM.. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 2000, 164, 183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 70. Selck C, Dominguez-Villar M.. Antigen-specific regulatory T cell therapy in autoimmune diseases and transplantation. Front Immunol 2021, 12, 661875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G.. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med 2011, 3, 83ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Freudenberg K, Lindner N, Dohnke S, Garbe AI, Schallenberg S, Kretschmer K.. Critical role of TGF-β and IL-2 receptor signaling in Foxp3 induction by an inhibitor of DNA methylation. Front Immunol 2018, 9, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bhela S, Varanasi SK, Jaggi U, Sloan SS, Rajasagi NK, Rouse BT.. The plasticity and stability of regulatory T cells during viral-induced inflammatory lesions. J Immunol 2017, 199, 1342–52. doi: 10.4049/jimmunol.1700520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aarts-Riemens T, Emmelot ME, Verdonck LF, Mutis T.. Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(-) cells. Eur J Immunol 2008, 38, 1381–90. doi: 10.1002/eji.200737590. [DOI] [PubMed] [Google Scholar]
- 75. Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 2008, 16, 194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 76. Andersen KG, Butcher T, Betz AG.. Specific immunosuppression with inducible Foxp3-transduced polyclonal T cells. PLoS Biol 2008, 6, e276e276. doi: 10.1371/journal.pbio.0060276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Passerini L, Rossi Mel E, Sartirana C, Fousteri G, Bondanza A, Naldini L, et al. CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci Transl Med 2013, 5, 215ra174. [DOI] [PubMed] [Google Scholar]
- 78. Raes L, De Smedt SC, Raemdonck K, Braeckmans K.. Non-viral transfection technologies for next-generation therapeutic T cell engineering. Biotechnol Adv 2021, 49, 107760. doi: 10.1016/j.biotechadv.2021.107760. [DOI] [PubMed] [Google Scholar]
- 79. Honaker Y, Hubbard N, Xiang Y, Fisher L, Hagin D, Sommer K, et al. Gene editing to induce FOXP3 expression in human CD4(+) T cells leads to a stable regulatory phenotype and function. Sci Transl Med 2020, 12, eaay6422. doi: 10.1126/scitranslmed.aay6422. [DOI] [PubMed] [Google Scholar]
- 80. Goodwin M, Lee E, Lakshmanan U, Shipp S, Froessl L, Barzaghi F, et al. CRISPR-based gene editing enables FOXP3 gene repair in IPEX patient cells. Sci Adv 2020, 6, eaaz0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant 2013, 13, 3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jiang S, Camara N, Lombardi G, Lechler RI.. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood 2003, 102, 2180–6. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 83. Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 2008, 14, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Trenado A, Sudres M, Tang Q, Maury S, Charlotte F, Grégoire S, et al. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol 2006, 176, 1266–73. doi: 10.4049/jimmunol.176.2.1266. [DOI] [PubMed] [Google Scholar]
- 85. Fujio K, Okamoto A, Araki Y, Shoda H, Tahara H, Tsuno NH, et al. Gene therapy of arthritis with TCR isolated from the inflamed paw. J Immunol 2006, 177, 8140–7. doi: 10.4049/jimmunol.177.11.8140. [DOI] [PubMed] [Google Scholar]
- 86. Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA 2009, 106, 19078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jethwa H, Adami AA, Maher J.. Use of gene-modified regulatory T-cells to control autoimmune and alloimmune pathology: is now the right time?. Clin Immunol 2014, 150, 51–63. [DOI] [PubMed] [Google Scholar]
- 88. Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest 2008, 118, 3619–28. doi: 10.1172/jci33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abbas AK, Trotta E, D RS, Marson A, Bluestone JA.. Revisiting IL-2: biology and therapeutic prospects. Sci Immunol 2018, 3, eaat1482. doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- 90. Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O’Gorman WE, et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 2010, 185, 6426–30. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McDermott DF, Atkins MB.. Application of IL-2 and other cytokines in renal cancer. Expert Opin Biol Ther 2004, 4, 455–68. doi: 10.1517/14712598.4.4.455. [DOI] [PubMed] [Google Scholar]
- 92. Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M.. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. J Immunol 2009, 183, 4904–12. doi: 10.4049/jimmunol.0900284. [DOI] [PubMed] [Google Scholar]
- 93. Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J.. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006, 311, 1924–7. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 94. Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011, 365, 2067–77. doi: 10.1056/nejmoa1105143. [DOI] [PubMed] [Google Scholar]
- 95. Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011, 365, 2055–66. doi: 10.1056/nejmoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2013, 1, 295–305. doi: 10.1016/s2213-8587(13)70113-x. [DOI] [PubMed] [Google Scholar]
- 97. Tahvildari M, Omoto M, Chen Y, Emami-Naeini P, Inomata T, Dohlman TH, et al. In vivo expansion of regulatory T cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. Transplantation 2016, 100, 525–32. doi: 10.1097/tp.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Boyman O, Sprent J.. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012, 12, 180–90. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 99. Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA.. In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol 1985, 134, 157–66. [PubMed] [Google Scholar]
- 100. Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol 1993, 151, 1235–44. [PubMed] [Google Scholar]
- 101. Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 2009, 206, 751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pilat N, Wiletel M, Weijler AM, Steiner R, Mahr B, Warren J, et al. Treg-mediated prolonged survival of skin allografts without immunosuppression. Proc Natl Acad Sci USA 2019, 116, 13508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Trotta E, Bessette PH, Silveria SL, Ely LK, Jude KM, Le DT, et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med 2018, 24, 1005–14. doi: 10.1038/s41591-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Vazquez-Lombardi R, Loetsch C, Zinkl D, Jackson J, Schofield P, Deenick EK, et al. Potent antitumour activity of interleukin-2-Fc fusion proteins requires Fc-mediated depletion of regulatory T-cells. Nat Commun 2017, 8, 15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ezzelarab MB, Zhang H, Sasaki K, Lu L, Zahorchak AF, van der Windt DJ, et al. Ex vivo expanded donor alloreactive regulatory T cells lose immunoregulatory, proliferation, and antiapoptotic markers after infusion into ATG-lymphodepleted, nonhuman primate heart allograft recipients. Transplantation 2021, 105, 1965–79. doi: 10.1097/tp.0000000000003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen KL, McKenna DH, et al. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol Blood Marrow Transplant 2013, 19, 1271–3. doi: 10.1016/j.bbmt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Roemhild A, Otto NM, Moll G, Abou-El-Enein M, Kaiser D, Bold G, et al. Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial. BMJ 2020, 371, m3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Harden PN, Game DS, Sawitzki B, Van der Net JB, Hester J, Bushell A, et al. Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am J Transplant 2021, 21, 1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sadelain M, Rivière I, Riddell S.. Therapeutic T cell engineering. Nature 2017, 545, 423–31. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013, 5, 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Porter DL, Levine BL, Kalos M, Bagg A, June CH.. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011, 365, 725–33. doi: 10.1056/nejmoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mohseni YR, Tung SL, Dudreuilh C, Lechler RI, Fruhwirth GO, Lombardi G.. The future of regulatory T cell therapy: promises and challenges of implementing CAR technology. Front Immunol 2020, 11, 1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest 2016, 126, 1413–24. doi: 10.1172/jci82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dawson NA, Lamarche C, Hoeppli RE, Bergqvist P, Fung VC, McIver E, et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 2019, 4, e123672. doi: 10.1172/jci.insight.123672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009, 30, 458–69. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sicard A, Lamarche C, Speck M, Wong M, Rosado-Sánchez I, Blois M, et al. Donor-specific chimeric antigen receptor Tregs limit rejection in naive but not sensitized allograft recipients. Am J Transplant 2020, 20, 1562–73. [DOI] [PubMed] [Google Scholar]
- 117. Raffin C, Zhou Y, Piccoli L, Lanzavecchia A, Sadelain M, Bluestone JA.. Development of citrullinated-vimentin-specific CAR for targeting Tregs to treat autoimmune rheumatoid arthritis. The Journal of Immunology. 2016, 196210(1 Supplement):19–19. [Google Scholar]
- 118. Rosado-Sánchez I, Levings MK.. Building a CAR-Treg: going from the basic to the luxury model. Cell Immunol 2020, 358, 104220. [DOI] [PubMed] [Google Scholar]
- 119. Sockolosky JT, Trotta E, Parisi G, Picton L, Su LL, Le AC, et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 2018, 359, 1037–42. doi: 10.1126/science.aar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hirai T, Ramos TL, Lin PY, Simonetta F, Su LL, Picton LK, et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J Clin Invest 2021, 131, e139991. doi: 10.1172/JCI139991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, et al. Engineering customized cell sensing and response behaviors using synthetic Notch receptors. Cell 2016, 164, 780–91. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T cells with customized therapeutic response programs using synthetic Notch receptors. Cell 2016, 167, 419–432.e16. doi: 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012, 484, 529–33. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Merchant R, Galligan C, Munegowda MA, Pearce LB, Lloyd P, Smith P, et al. Fine-tuned long-acting interleukin-2 superkine potentiates durable immune responses in mice and non-human primate. J ImmunoTher Cancer 2022, 10, e003155. doi: 10.1136/jitc-2021-003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Khoryati L, Pham MN, Sherve M, Kumari S, Cook K, Pearson J, et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci Immunol 2020, 5, eaba5264. doi: 10.1126/sciimmunol.aba5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Parmar S, Liu X, Tung SS, Robinson SN, Rodriguez G, Cooper LJ, et al. Third-party umbilical cord blood-derived regulatory T cells prevent xenogenic graft-versus-host disease. Cytotherapy 2014, 16, 90–100. doi: 10.1016/j.jcyt.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol 2017, 35, 765–72. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 2019, 37, 252–8. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Philip B, Kokalaki E, Mekkaoui L, Thomas S, Straathof K, Flutte B, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 2014, 124, 1277–87. doi: 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- 130. Paszkiewicz PJ, Fräßle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest 2016, 126, 4262–72. doi: 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, et al. Multiplex genome-edited T-cell manufacturing platform for "Off-the-Shelf" adoptive T-cell immunotherapies. Cancer Res 2015, 75, 3853–64. doi: 10.1158/0008-5472.CAN-14-3321 [DOI] [PubMed] [Google Scholar]
- 132. Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJC, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–17. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pierini A, Iliopoulou BP, Peiris H, Pérez-Cruz M, Baker J, Hsu K, et al. T cells expressing chimeric antigen receptor promote immune tolerance. JCI Insight 2017, 2, e92865. doi: 10.1172/jci.insight.92865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Raffin C, Vo LT, Bluestone JA.. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020, 20, 158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hippen KL, Hefazi M, Larson JH, Blazar BR.. Emerging translational strategies and challenges for enhancing regulatory T cell therapy for graft-versus-host disease. Front Immunol 2022, 13, 926550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016, 64, 632–43. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 137. Mathew JM, Leventhal JR.. Cell therapy can enable minimization of immunosuppression. Nat Rev Nephrol 2020, 16, 486–7. doi: 10.1038/s41581-020-0330-5. [DOI] [PubMed] [Google Scholar]
- 138. Mastoridis S, Martínez-Llordella M, Sanchez-Fueyo A.. Biomarkers and immunopathology of tolerance. Curr Opin Organ Transplant 2016, 21, 81–7. [DOI] [PubMed] [Google Scholar]
- 139. Ius F, Salman J, Knoefel AK, Sommer W, Nakagiri T, Verboom M, et al. Increased frequency of CD4(+) CD25(high) CD127(low) T cells early after lung transplant is associated with improved graft survival - a retrospective study. Transpl Int 2020, 33, 503–16. doi: 10.1111/tri.13568. [DOI] [PubMed] [Google Scholar]
- 140. Gauthier JM, Harrison MS, Krupnick AS, Gelman AE, Kreisel D.. The emerging role of regulatory T cells following lung transplantation. Immunol Rev 2019, 292, 194–208. doi: 10.1111/imr.12801. [DOI] [PubMed] [Google Scholar]
- 141. Miyamoto E, Takahagi A, Ohsumi A, Martinu T, Hwang D, Boonstra KM, et al. Ex vivo delivery of regulatory T-cells for control of alloimmune priming in the donor lung. Eur Respir J 2022, 59, 2100798. doi: 10.1183/13993003.00798-2021. [DOI] [PubMed] [Google Scholar]
- 142. Starzl TE, Zinkernagel RM.. Transplantation tolerance from a historical perspective. Nat Rev Immunol 2001, 1, 233–9. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jones ND, Turvey SE, Van Maurik A, Hara M, Kingsley CI, Smith CH, et al. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J Immunol 2001, 166, 2824–30. doi: 10.4049/jimmunol.166.4.2824. [DOI] [PubMed] [Google Scholar]
- 144. Whitehouse G, Gray E, Mastoridis S, Merritt E, Kodela E, Yang JHM, et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci USA 2017, 114, 7083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Charbonnier LM, Vokaer B, Lemaître PH, Field KA, Leo O, Le Moine A.. CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplant 2012, 12, 2313–21. doi: 10.1111/j.1600-6143.2012.04184.x. [DOI] [PubMed] [Google Scholar]
- 146. Pilat N, Mahr B, Gattringer M, Baranyi U, Wekerle T.. CTLA4Ig improves murine iTreg induction via TGFβ and suppressor function in vitro. J Immunol Res 2018, 2018, 2484825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant 2008, 8, 2086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA.. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol 2004, 34, 2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 149. Seissler N, Schmitt E, Hug F, Sommerer C, Zeier M, Schaier M, et al. Methylprednisolone treatment increases the proportion of the highly suppressive HLA-DR(+)-Treg-cells in transplanted patients. Transpl Immunol 2012, 27, 157–61. doi: 10.1016/j.trim.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 150. Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant 2010, 10, 2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ruggenenti P, Perico N, Gotti E, Cravedi P, D’Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007, 84, 956–64. doi: 10.1097/01.tp.0000284808.28353.2c. [DOI] [PubMed] [Google Scholar]
- 152. Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 2011, 3, 83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sánchez-Fueyo A, Whitehouse G, Grageda N, Cramp ME, Lim TY, Romano M, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant 2020, 20, 1125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.