Summary
Foxp3+CD4+ regulatory T cells (Tregs) are famous for their role in maintaining immunological tolerance. With their distinct transcriptomes, growth-factor dependencies and T-cell receptor (TCR) repertoires, Tregs in nonlymphoid tissues, termed “tissue-Tregs,” also perform a variety of functions to help assure tissue homeostasis. For example, they are important for tissue repair and regeneration after various types of injury, both acute and chronic. They exert this influence by controlling both the inflammatory tenor and the dynamics of the parenchymal progenitor-cell pool in injured tissues, thereby promoting efficient repair and limiting fibrosis. Thus, tissue-Tregs are seemingly attractive targets for immunotherapy in the context of tissue regeneration, offering several advantages over existing therapies. Using skeletal muscle as a model system, we discuss the existing literature on Tregs’ role in tissue regeneration in acute and chronic injuries, and various approaches for their therapeutic modulation in such contexts, including exercise as a natural Treg modulator.
Keywords: Tregs, tissue regeneration, tissue repair, skeletal muscle, muscular dystrophy, exercise
Regulatory T cells (Tregs) play an important role in tissue repair and regeneration in response to various types of injury, rendering them attractive potential therapeutic targets. Tregs’ role in skeletal muscle regeneration after acute and chronic injury and their future therapeutic application in related pathologies is discussed.
Graphical Abstract
Graphical Abstract.
Introduction
The lineage-defining transcription factor of Foxp3+CD4+ regulatory T cells (or Tregs) was identified almost two decades ago [1–3]. This landmark co-discovery propelled Tregs from the shadowy realm of “suppressor cells” to the limelight of immunological research. It eventually became clear that Tregs control most types of immune reaction—including autoimmunity, allergy, inflammation, anti-tumor responses and anti-microbe responses—by regulating the activities of most innate and adaptive immunocyte types [4]. This superpower made Tregs and their products attractive candidates as immunotherapeutic agents [5, 6].
The therapeutic potential of Tregs was even further enhanced by the discovery of so-called “tissue-Tregs” [7]. During the initial decade after their discovery, essentially all studies on Foxp3+CD4+ T cells examined those circulating through the blood and lymphoid organs. The functional focus evolved from Treg impacts on other T cells, to effects on all adaptive immunocytes to influences on all immunocytes, whether adaptive or innate. In 2009, a unique population of Foxp3+CD4+ T cells was found in the visceral adipose tissue (VAT) of lean, “middle-aged” mice [8]. VAT Tregs have a transcriptome, T-cell-receptor (TCR) repertoire, and growth/survival factor dependencies that are distinct from those of their lymphoid-organ counterparts. Importantly, they control local and systemic inflammation and metabolism, at least in part by regulating the activities of local parenchymal [8] and stromal [9] cells. Subsequently, analogous populations of tissue-distinct Tregs were found at a multiplicity of sites, including skeletal muscle [10], skin [11], the colonic lamina propria [12], cardiac muscle [13], lungs [14], the liver [15], and the central nervous system [16]. These studies have revealed that the functional purview of tissue-Tregs extends well beyond protection from microbe and tumor challenges to tissue homeostasis at several junctures, e.g. regulation of metabolism, orchestration of tissue repair/regeneration, and regulation of stem/progenitor cell activities. Thus, the heightened promise of tissue-Tregs as immunotherapeutic agents rests on three points: (i) that recognition of local antigen(s) by their TCRs will encourage their accumulation at the site(s) where they are most needed; (ii) that specific accumulation at these sites will avoid the generalized immunosuppression or immunostimulation that systemic impoverishment or enrichment of Tregs can induce; and (iii) that their homeostatic activities will lend an additional therapeutic boost.
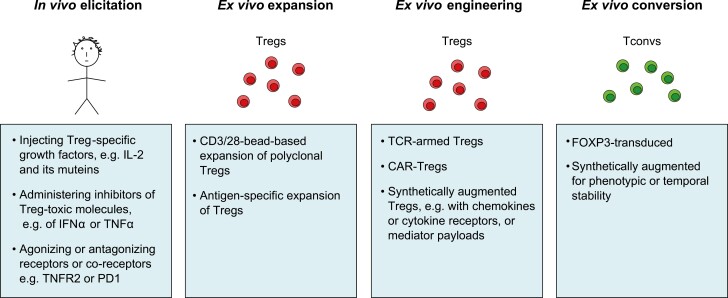
The options for performing Treg-based immunotherapy are manifold. This topic has been expertly reviewed quite recently [5, 6]; so we will just outline the major approaches here. As illustrated in Fig. 1, they can be grouped into four major classes. First, there are methods to preferentially elicit Foxp3+CD4+ cells in vivo: via injecting specific Treg growth factors [e.g. low-dose interleukin (IL)-2 or IL-2 variants], by administering inhibitors of molecules toxic to Tregs [e.g. dampening interferon (IFN)α or tumor necrosis factor (TNF)α], or through agonism or antagonism of receptors or co-receptors (e.g. agonism of TNFR2). The advantage of such strategies is that they avoid cell isolations and transfers, but their optimum application awaits the development of specific targeting methods in order to avoid systemic complications. Second are methods to expand isolated Tregs ex vivo and transfer them. The simplest strategy for expansion is CD3/28-bead-based expansion of polyclonal Tregs, but the low frequency of any particular Treg specificity in the blood renders this option relatively ineffective in most contexts. Antigen-specific Treg expansion ameliorates this problem although it introduces additional complexities–for example, which antigen? Third, methods for engineering more performant Tregs are becoming increasingly popular, sophisticated, and ingenious. These options may entail targeting a designated tissue by introducing a TCR that recognizes an antigen specifically expressed at that site or, alternatively, a chimeric antigen receptor (CAR) composed of an external antibody domain that binds to some molecule preferentially located there and an internal signaling domain that integrates into the Treg signaling network. And/or these options may involve synthetic augmentation of Treg performance, such as bestowing new chemokine or cytokine receptors or adding soluble mediator “payloads.” Fourth are methods to ex vivo convert Foxp3-CD4+ conventional T cells (Tconvs) to Tregs. Transduction of FOXP3 is the most common variant of this strategy, often coupled with some sort of synthetic augmentation, as mentioned above. These so-called induced Tregs or iTregs have been the subject of some skepticism because they are thought to be less stable and less authentic than thymus-generated Tregs [17].
Figure 1:
Treg-based therapeutic strategies. The increasing armamentarium of Treg-based treatments. See Introduction for details. Treg, Foxp3+CD4+ T cell; Tconv, Foxp3−CD4+ conventional T cell; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; R, receptor; TCR, T cell receptor; CAR, chimeric antigen receptor.
A few general concerns related to Treg-based therapies should be mentioned. One is the notion that Tregs might convert to pathogenic effector cells in the inflammatory lesion they are meant to control [18]. However, it is worth noting that evidence for this phenomenon, mostly derived from murine Treg-transfer models or from in vitro culture systems, has been down-played because the Treg preparations typically employed for such experiments are too-often contaminated with low levels of effector T cells or with cells not fully committed to the Treg lineage, a caveat that was, in general, not fully ruled out [19, 20]. Thus, employing highly purified, fully committed, thymus-generated Tregs or synthetic equivalents is prescribed for clinical applications. In addition, of the many ongoing and completed Treg-therapy trials, this issue has not proven problematic to our knowledge. A second point is the notion that Tregs depend on IL-2, which may be low or declining in the target lesion as therapy proceeds, and thus might have to be co-administered. This issue is likely to be most relevant to polyclonal Treg therapies and to survival of circulating Tregs as tissue-Tregs are known to be maintained, even expanded, by other growth factors, e.g. IL-33, IL-18 [7]. In addition, a recent clinical trial pointed out the dangers of co-administering IL-2 and Tregs, i.e. expansion of host T and NK effector cells [21].
As its title foretells, the focus of this review is the potential application of Treg-enhancing therapies to tissue regeneration. We have chosen skeletal muscle as a model system and will speculate on how the armamentarium of Treg-based treatments might be harnessed to ameliorate acute or chronic muscle pathologies. Current clinical trials in this area are limited and, thus, there is vast potential for this treatment strategy. Lastly, we will propose exercise as a natural Treg modulator.
Treg therapies in acute muscle injury
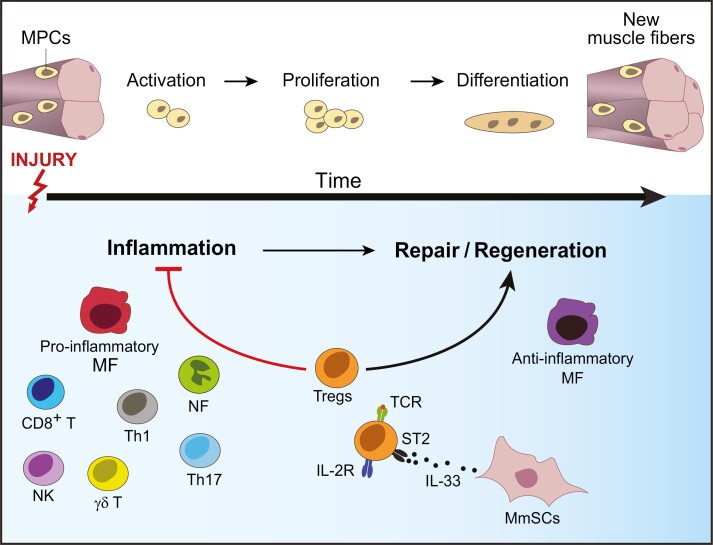
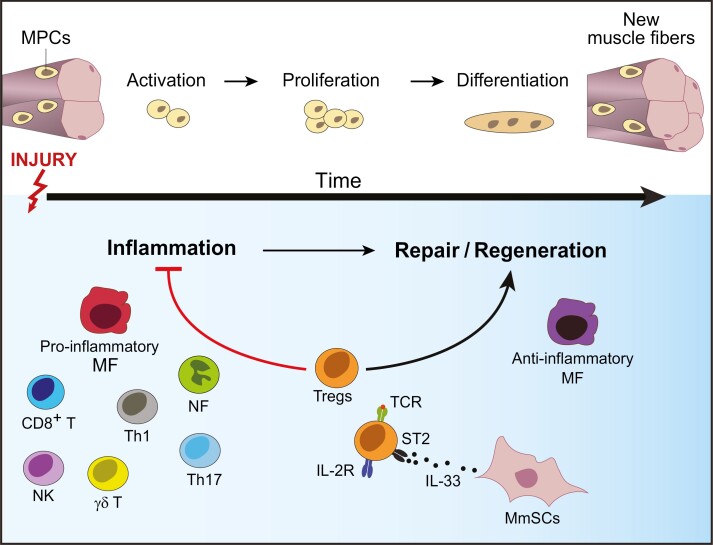
Tissue regeneration is an orchestrated, multi-cellular process that follows a strict temporal order. Its dynamics are well characterized in the case of skeletal muscle. As the largest organ in the body, skeletal muscle is commonly injured due to a variety of reasons, including mechanical trauma, thermal stress, myo- or neuro-toxic agents, and ischemia [22]. Injury results in degradation and necrosis of myofibers. Replacement of damaged myofibers is initiated by a pool of muscle-progenitor cells (MPCs) located in close apposition to muscle fibers [23]. In response to acute injury, quiescent MPCs become activated, proliferate, differentiate, migrate and fuse to form new myofibers (Fig. 2) [24, 25]. In addition, early after injury, muscle mesenchymal stromal cells (MmSCs) get activated, proliferate, and form temporary extracellular matrix (ECM) that acts as a scaffold for the regenerating myofibers [26–29]. Remodeling and degrading ECM at later stages of regeneration is essential for efficient repair since excessive deposition results in fibrosis, impairing the restoration of normal tissue function and increasing susceptibility to re-injury [27, 30].
Figure 2:
Tregs in skeletal muscle injury. In response to skeletal muscle injury, muscle-progenitor cells (MPCs) get activated, proliferate, and differentiate to form new muscle fibers (upper panel). Various immunocytes dynamically accumulate at the site of injury (lower panel), starting with neutrophils (NFs), followed by pro-inflammatory macrophages (MFs), natural killer (NK), γδT cells, CD8+ T cells and T helper 1 (TH1) and TH17 cells. The subsequent reparative stage is dominated by anti-inflammatory MFs and Tregs that are essential for effective regeneration. Accrual of muscle Tregs in injured muscle is dependent on T-cell receptor (TCR) stimulation by local antigens, in addition to trophic cytokines, such as IL-33 produced by muscle mesenchymal stromal cells (MmSCs).
The inflammatory processes accompanying tissue injury also strongly influence the outcome of repair [31]. Early after injury, there is a rapid, transient influx of neutrophils (NFs) followed by pro-inflammatory macrophages (MFs), which are required for clearance of dead cells and cellular debris. In addition, injury invokes the rapid accumulation of various types of innate and adaptive lymphocytes, such as natural killer (NK), effector αβT, and γδT cells (Fig. 2) [32, 33]. The pro-inflammatory mediators produced by these accruing immunocytes—such as TNFα, IFNγ and IL-17A—are crucial for activating MPCs and trigging their proliferation [33–35]. Yet, the duration and magnitude of this initial inflammatory phase need to be tightly regulated since chronic exposure of MPCs to the same inflammatory mediators paradoxically blocks their differentiation into myocytes, resulting in impaired tissue repair [32, 36–38]. Additionally, uncontrolled inflammation results in fibrosis and scar formation [30]. Thus, within a few days of the insult, the muscle micro-environment transitions to an anti-inflammatory, pro-regenerative state. This shift is most pronounced for MFs, which switch to anti-inflammatory Ly6Clow phenotypes with various pro-regenerative functions, such as matrix remodeling and promotion of angiogenesis [39–42]. This dynamically and temporally regulated inflammatory response is a defining hallmark of efficient tissue repair that has been observed in a diversity of other tissues.
Over the last decade, Tregs became increasingly appreciated as tissular “rheostats” of muscle repair [10, 32, 43–45]. At steady-state, skeletal muscle harbors a small Treg population [10]. In response to acute injury, this population rapidly expands, reaching its numerical peak 3–4 days post-injury, a timepoint that marks a transition of the muscle milieu from a pro- to an anti-inflammatory state, before declining thereafter (Fig. 2) [10, 43, 44]. Treg accrual in skeletal muscle results from a combination of local proliferation, most pronounced 1 day after injury, and sphingosine-1-phosphate receptor (S1pr)-mediated emigration from lymphoid tissues [45]. Notably, decreased Treg recruitment to skeletal muscle after injury contributes to their diminished accumulation in aging animals [45]. The muscle microenvironment strongly influences muscle Tregs, as evidenced by their distinct transcriptional profiles compared with those of lymphoid-tissue Tregs [10, 46]. Muscle Tregs express a core set of nonlymphoid-tissue-Treg genes, including elevated levels of chemokine receptors, such as CCR2, CCR4, and CCR8 [10, 46]. The corresponding ligands for these receptors are rapidly upregulated by muscle stromal cells and infiltrating immunocytes after injury [47, 48], and therefore may aid Treg homing to the site of damage. Muscle Tregs also have an activated, effector-like phenotype, with preferential expression of key immunosuppressive molecules such as IL-10 and CTLA-4, which may arm them to control the strong inflammation induced upon injury [10, 43]. Compared with other tissue-Treg compartments, such as VAT or colon, muscle Tregs are distinguished by their continuous exchange with the circulating Treg pool [10, 46]. In addition, muscle Tregs have high proliferation rates at the early stages of injury, which is reflected in their upregulation of cell cycle and growth genes [10, 46].
The functional relevance of muscle Tregs in acutely injured muscle was demonstrated using genetic models allowing punctual Treg ablation, which exhibited compromised regeneration and tissue fibrosis, a consequence of inefficient repair [10]. Tregs employ at least two mechanisms to promote muscle regeneration. First, they control the inflammatory tenor of regenerating muscle by reining in immunocytes and promoting the pro- to anti-inflammatory shift in infiltrating myeloid cells [10]. In Treg-less mice, resolution of inflammation is impaired, with a persistent accumulation of NFs and inflammatory MFs. For example, Treg control of IFNγ production by NK and effector T cells is essential for controlling MF phenotype and function in regenerating muscle [32, 43]. Independently, Tregs exert their pro-regenerative power in a non-immunological fashion by directly interacting with MPCs and promoting their accrual. This effect is mediated at least in part by muscle Treg production of amphiregulin (Areg), a member of the epidermal growth factor family [10]. Thus, upon Treg ablation, MPCs exhibit reduced clonal efficiency, a deficiency that can be reversed by Areg administration [10]. Whether the anti-inflammatory and pro-reparative functions of Tregs are exerted by the same cells is currently under study. Single-cell RNA sequencing (scRNA-seq) analysis identified a considerable degree of transcriptional heterogeneity in muscle Tregs [46]. Intriguingly, muscle Treg subsets after injury are dynamic and evolve across the course of regeneration, raising the possibility that different Treg functions, such as dampening of inflammation and promotion of repair might be the roles of dynamically different Treg subtypes.
Treg accrual in muscle results from a combination of antigen- and cytokine-driven stimulation (Fig. 2). A characteristic of Tregs in acutely injured muscle is their restricted, clonally expanded TCR repertoire [10]. Intriguingly, one particular TCR clone was identified repeatedly in muscle Tregs isolated from multiple, independent animals, a strikingly rare incidence in the highly diverse TCR repertoires of different individuals [10]. This observation strongly suggests that muscle Tregs are responding to local antigens. Indeed, a transgenic mouse (tg) line harboring the rearranged transgenes encoding the TCRα and TCRβ chains of this particular clone (mTreg24 TCR-tg mice) shows enhanced Treg accumulation in injured muscle and improved muscle repair [49]. In addition to TCR-driven proliferation, the increase in muscle Tregs after acute injury, unlike that of their lymphoid-tissue counterparts, is strongly dependent on the alarmin cytokine, IL-33. Muscle insult induces rapid production of IL-33, primarily from MmSCs [45]. Treg-specific ablation of the IL-33 receptor (ST2) impairs their accumulation and hampers the regeneration process [45]. The IL-33/Treg axis is of high relevance in the context of muscle aging since diminished IL-33-dependent accumulation of muscle Tregs contributes, at least in part, to the regeneration deficiency in aging animals [45]. Disrupted IL-33-mediated Treg accrual in aging muscle is primarily due to decreased IL-33 production by MmSCs, and IL-33 supplementation boosts muscle Treg accumulation after injury and improves muscle repair [45].
Currently, treatment of acute muscle injury is limited to rest, ice, compression, and elevation (RICE), nonsteroidal anti-inflammatory drugs (NSAIDs), and physical therapy [22]. The objective of RICE is to minimize the size of the initial injury, inflammation, and subsequently the resulting scar. Yet, the impact of RICE has not been confirmed in randomized clinical trials [22]. While NSAIDs can offer analgesia and dampen inflammation, interfering with the early inflammatory process can hamper regeneration since NSAIDs can inhibit MPC proliferation [50, 51]. Chronic use of NSAIDs is also not recommended as it can cause serious gastrointestinal and renal side-effects, hypertension, and other systemic complications. The proposed ability of physical activity to promote efficient repair after acute injury is attributed, at least in part, to its immunomodulatory activity. We will elaborate on the role of exercise and immunocyte regulation via Tregs in an upcoming section.
Considering the limited therapeutic palette for acute muscle injuries, Treg-based therapies present a potentially more precise and effective alternative to traditional approaches. As highlighted in Fig. 1, enhancing Treg activity in regenerating muscle could potentially be achieved via the administration of Treg-trophic factors. Considering its preferential activity on tissue- (in particular, muscle) Tregs, IL-33-based therapies are likely to exhibit superior therapeutic specificity than IL-2 equivalents, which can cause systemic expansion of Tregs. IL-33 potently enhances muscle Treg accumulation [45], and Tregs expressing ST2 exhibit enhanced suppressive activity compared with that of their ST2- counterparts [52]. Yet, the effects of systemic IL-33 administration need to be thoroughly evaluated because of the multiple cellular targets of IL-33, including MFs, eosinophils, and type-2 lymphocytes [53]. An alternative approach, with better specificity towards tissue-Tregs, would be engineered fusion proteins of IL-2 and IL-33, which show therapeutic activity better than that of IL-2 and IL-33, alone or in combination, in models of nonlymphoid tissue inflammation [54, 55]. In light of the restricted TCR repertoire of muscle Tregs, administration of engineered antigen-specific Tregs targeting muscle antigens is a promising approach. Antigen-specific Tregs exert more potent suppressive activity in mouse models than polyclonal Tregs [56, 57]. Moreover, specific antigen-TCR interactions can promote Treg accumulation at the site of injury, as evidenced by the preferential accrual of Tregs from mTreg24 TCR-tg mice in injured skeletal muscle in comparison with other lymphoid and nonlymphoid tissues [49]. In addition to suppressing Tconvs targeting the same antigens, antigen-specific Tregs can exert bystander suppression of other T and non-T immunocytes at the site of injury [6]. The remarkable recent advances in gene-editing technologies offer the opportunity to arm muscle-specific Tregs with additional functional molecules with the potential to enhance tissue repairs, such as IL-10 or Areg. In addition, modulating these Tregs to stably express particular transcription factors that foster their acquisition of the tissue-Treg program, such as BATF [58–60], can potentially enhance their homing and function in injured tissues. One pre-requisite for the success of Tregs engineered in this manner is identifying peptide antigens that can selectively activate and expand the muscle Treg compartment. So far, only a limited number of antigens recognized by Tregs at any location have been identified. Various approaches for scanning T-cell antigens have been successfully employed for the design of immunotherapies [61]. However, most of them have so far been employed to uncover ligands for CD8+ T cells, in particular those recognizing limited viral proteomes. Recently, a peptide screen of VAT Treg antigens identified surrogate agonists that can specifically expand this population [62]. Employing similar approaches is likely to inform Treg cell therapies for acute muscle injuries.
Treg therapies in muscular dystrophies
Muscular dystrophies are a group of genetic diseases characterized by progressive skeletal-muscle weakness and degeneration. Within this group are Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD), both X-linked diseases caused by mutations in the gene encoding dystrophin (also known as dystrophinopathies) [63–65]. DMD is associated with the most severe clinical symptoms while BMD has a later onset and milder clinical presentation. The nature of these conditions depends on the amount of residual dystrophin in the muscle [66]. Disease severity is also related to the type of dystrophin mutation, with frameshift mutations associated with more severe disease (DMD) and mutations that preserve the reading frame with the less severe disorder (BMD), although some exceptions do exist [66–68].
Dystrophin is part of the dystrophin-associated protein complex (DAPC), a group of interacting muscle-fiber proteins that span the cytoskeleton, cell membrane, and extracellular matrix [69]. Dystrophin is located in the cytoplasm and links the intracellular actin network to the transmembrane element of the DAPC. A deficiency in dystrophin leads to the breakdown of the DAPC, which dramatically affects the structural integrity and contractile activity of skeletal muscle [70]. Over time, the muscle progressively degenerates and is replaced by fibrosis and fat, resulting in a devastating clinical course. DMD patients usually require a wheelchair by ages 10–12, need assisted ventilation around age 20, and eventually succumb to cardiac and/or respiratory failure between ages 20 and 40 [71].
In contrast to the acute toxin-induced injury discussed earlier, the pathogenesis of dystrophinopathies is multi-factorial. Known contributors include the weakening of the sarcolemma, which is an important muscle-fiber structure that helps control mechanical stress during muscle contraction [70]. Another contributor is free-radical damage, as reactive oxygen, and nitrogen species are elevated in DMD [70]. Despite these numerous mechanisms, one major consequence and, subsequently, the contributor is the inflammation provoked by muscle damage [70, 72]. In the setting of acute skeletal-muscle injury, as was described in detail earlier, a response by the immune system is a typical and necessary correlate of muscle regeneration [73]. There is a highly dynamic and orchestrated reaction by numerous immunocyte types, which facilitates the regeneration process immediately after injury through normal tissue restoration [73]. However, in the context of DMD, there is a repetitive muscle injury, which results in chronic inflammation that exacerbates muscle damage [72]. The importance of inflammation in the pathogenesis of DMD has been highlighted by both human and mouse data. For DMD patients, systemic corticosteroids, which are strong immunosuppressants, have been the mainstay therapy for many years. Long-term corticosteroid treatment improves muscle strength and function, prolonging ambulation, and delaying pulmonary and cardiac dysfunction [74–76]. It also results in a reduction in the risk of secondary deficits such as scoliosis [74–76]. Muscle biopsies taken pre- and post-treatment in DMD patients given corticosteroids for 6 months showed a significant reduction in immunocyte numbers in the muscle tissue [77]. In mice, the mdx mutant strain, which harbors an alteration in the gene encoding dystrophin, is used as a genetic model of DMD. Depletion of specific myeloid and lymphoid populations substantially reduces muscle damage in this model [78–81]. Taken together, these mouse and human data underscore inflammation as an important driver in the pathogenesis of dystrophinopathies.
Tregs are a critical immunocyte subset due to their potent immunosuppressive activities. While typically a small population in skeletal muscle at homeostasis, Treg numbers are significantly elevated in mdx mouse and human DMD/BMD muscle [10, 43, 82]. Administration of an anti-CD25 mAb to mdx mice, used to deplete Tregs in this context because both dystrophin and Foxp3 are located on the X-chromosome, led to an increased inflammatory infiltrate in skeletal muscle according to both histology and an elevation in serum creatine kinase (CK) levels, an indicator of muscle damage [10, 43]. At the whole-tissue level, there was also an upregulation of transcripts encoding factors that promote fibrosis, a critical component of muscular dystrophy pathology [10]. A genetic mouse model permitting specific ablation of Tregs in mdx mice also revealed Tregs to be critical restraints on interferon IFNγ production by Tconvs [83]. IFNγ is pathogenic in mdx mice as it promotes a more inflammatory tenor in the muscle MF compartment and its genetic deletion resulted in reduced disease severity [83].
Given that inflammation has a major pathogenic role in muscular dystrophy and that Tregs exert numerous beneficial immunosuppressive influences in mdx mice, Tregs have the potential for the treatment of dystrophinopathies in humans. In comparison with corticosteroid administration, which results in global dampening of the immune system and severe adverse side-effects, Treg-based therapies can provide immunosuppressive activity with a predilection for sites of inflammation. As outlined in the Introduction, systemic low-dose IL-2 is an effective method for expanding Tregs in vivo [84]. In mdx mice, administration of IL-2/anti-IL-2 complexes (which extend the half-life of IL-2) leads to an increase in Tregs in the muscle but not in the spleen, which results in a dramatic decrease in skeletal muscle inflammation and lowers serum CK [10, 43]. The expression of IL-10, a key anti-inflammatory molecule, is increased in the muscle of so-treated mdx mice, although the functional importance of this increase was not established [43]. The fact that systemic Treg augmentation leads to selective enrichment of Tregs in the muscle and an associated increase in the level of muscle IL-10 argues for greater specificity of this therapeutic approach.
Interestingly, as was observed in acute muscle injury, CD4+ T cells are clonally expanded in muscle tissue of both mdx mice and DMD patients, indicating a response to one or more muscle antigens [10, 85, 86]. Indeed, when the mdx mutation was crossed into the mTreg24 mouse line, wherein T cells highly preferentially express α and β TCR chains from a Treg clone expanded in acute muscle upon injury, there was enhanced accumulation of Tregs in the skeletal muscle and improved muscle regeneration [49]. These data suggest a potential overlap between Treg clones and antigens found in chronic and acute models of injury in mice. With the identification of Treg clones expanded in human DMD muscle or of the antigens they are responding to, engineered Tregs with specific TCRs or CARs might prove to be an attractive therapeutic approach. Instead of simply boosting systemic numbers of Tregs, this approach would allow for the highly specific accumulation of Tregs within injured muscle and presumably more effective local immunosuppression and less systemic suppression. Although not yet studied in muscular dystrophy, Tregs from acutely injured muscle can also directly improve muscle regeneration through the production of Areg, which enhances myogenic differentiation, as noted earlier [10]. In the context of muscular dystrophy, one could postulate that transferred Tregs, in addition to suppressing the chronic inflammation, may have other undiscovered functions that directly enhance muscle regeneration, depending on the extent to which they can take on the mantle of true muscle Tregs after transfer.
Another aspect of employing Treg-based therapies for muscular dystrophy is their use in combination with approaches attempting to restore functional dystrophin in diseased muscle tissues. Currently, there are numerous dystrophin-restoring therapies in development: exon-skipping using antisense oligonucleotides to restore the reading frame in patients with out-of-frame dystrophin mutations; CRISPR/Cas9 editing to restore the reading frame of the dystrophin gene through directed DNA breaks; and adeno-associated viruses (AAVs) to deliver essential pieces of the dystrophin gene to the muscle [70]. While these are promising approaches, many delivery vectors can provoke immune responses [87]. Additionally, there have been numerous reports of dystrophin-specific autoreactive T cell responses in patients with DMD [88, 89]. These individuals do not produce full-length dystrophin protein and, as a result, do not sufficiently purge cognate self-reactive T cells during their thymic maturation and subsequent peripheral residence. Therefore, coupling dystrophin-restoring approaches with Treg-based therapies could allow for improvements in muscle function while limiting major side-effects associated with vector immunogenicity and dystrophin autoimmunity.
While DMD and BMD are major types of muscular dystrophy, there are numerous other diseases that similarly result in repetitive muscle damage and chronic inflammation. Limb-girdle muscular dystrophies are a diverse group of diseases caused by mutations in any one of more than 20 different genes, resulting in weakening and degeneration of the pelvic and shoulder girdle muscles [72]. While the exact nature of the inflammatory infiltrate has yet to be studied in-depth, patients have improved with corticosteroids [90]. Beyond muscular dystrophy, there are non-inherited inflammatory myopathies, a heterogenous group of diseases that share the common feature of immunocyte-mediated muscle injury. The most common diseases of this group are dermatomyositis (DM), polymyositis (PM), immune-mediated necrotizing myopathy (IMNM), and inclusion-body myositis (IBM). Our knowledge of the exact pathogeneses of these inflammatory myopathies is incomplete, but corticosteroids are often used and result in improved muscle strength.
Treg-based therapies aim to enrich Tregs at sites of inflammation, Tregs engineered with particular TCRs or CARs being the most specific, thereby resulting in localized effects. Although Tregs have numerous roles in skeletal muscle, given that an important function is immunosuppression, we speculate that Treg-based therapies could help control chronic inflammation with fewer side-effects and, thus, be applicable across a wide swath of muscle diseases.
Exercise as a natural Treg modulator
In the previous sections of this review, we highlighted Treg-enhancing therapies for the treatment of acute muscle injury and muscular dystrophies. Here, we will summarize what is known about the relationship between exercise and Treg activities and will propose mechanisms by which exercise may act as a natural Treg modulator. Finally, we will integrate these concepts with the prior sections to provide specific insight into how exercise may favorably impact the pathology of acute and chronic muscle injuries.
Exercise has been prescribed as an intervention to enhance health and stave off disease for millennia [91]. The concept of exercise as medicine is supported by a preponderance of evidence for an inverse relationship between physical activity level and all-cause mortality risk [92, 93]. It has been suggested that this relationship reflects the anti-inflammatory effects of exercise [94], which work to counteract the chronic inflammation associated with modern afflictions such as cardiovascular disease and type 2 diabetes. Indeed, such diseases arise from metabolic derangements in response to low physical activity and excessive nutrient availability, which is now known to impact the configuration and function of the immune system [95].
In addition to its direct effects on metabolic homeostasis via enhancing sensing and oxidation of nutrients by muscle and adipose tissues [96, 97], exercise has profound immunomodulatory potential. Schulz first documented this potential at the turn of the 20th century in a paper describing exercise-induced leukocytosis [98]. This phenomenon has been attributed to increased blood flow and elevated concentrations of catecholamines and cortisol [99]. Indeed, β-adrenergic blockade achieved via propranolol administration attenuates exercise-induced leukocytosis [100]. In contrast, lymphocytopenia occurs in the period of recovery after exercise cessation and persists for 24–48 h [99, 101]. Although early reports attributed this effect to increased apoptosis, it is more likely to reflect increased lymphocyte extravasation into peripheral tissues. Recent studies measuring apoptosis and the expression of adhesion molecules such as CD18, CD53, and CD54 on circulating immunocytes after exercise support this interpretation [102–104]. Furthermore, high-intensity exercise, especially modalities involving loading during the lengthening (eccentric) phase of muscular contraction, results in myofibrillar disruptions [105, 106] and myocellular release of damage-associated molecular patterns (DAMPs) such as mitochondrial DNA, ATP, Tenascin C, and HMGB-1 [107]. These factors may activate muscle-resident stroma and immunocytes, leading to the formation of chemokine gradients that would attract cells mobilized to the blood during exercise [108]. Notably, the magnitude and duration of lymphocytosis and lymphocytopenia in response to exercise are dependent on exercise intensity. The age, sex and training history of the organism as well as the mode, duration, and frequency of exercise are additional variables affecting the reported immunomodulatory effects. For a summary of innate and adaptive blood immunocyte responses to exercise, the reader is referred to two excellent reviews on exercise immunology [109, 110].
Changes in Treg frequency, number, and function in peripheral blood in response to various human and rodent exercise regimens have also been the subject of recent reviews [111, 112]. Although these reviews highlighted many acute and chronic exercise interventions that augment the representation of Tregs in the circulation, there are also many studies documenting no change or diminished Treg counts. These discrepancies are almost certainly consequences of heterogeneity in the cohorts tested, exercise regimens used, and the times at which blood samples were collected in relation to exercise. Given the immediate increase and subsequent reduction in Treg representation in peripheral blood after exercise [101], the time of analysis is of critical importance. Yet many studies have taken only one pre- and one post-exercise sample, and some chose a very early post-exercise timepoint (<24 h), while others looked only late into recovery (>3 d). Given the role of local Tregs in responses to acute and chronic muscle injury discussed in the previous sections of this review, it is possible that studies documenting reduced Treg presence in the blood late during exercise recovery are merely looking where Tregs sojourned on their way to sites of need. Taking this perspective, one would expect lower Treg counts in the blood after prolonged, high-intensity exercise capable of inflicting significant tissue damage, such as a marathon race, and this result is what has been documented [101, 113]. Further support for this perspective comes from analyses demonstrating increased proportion and function of Tregs in non-muscle peripheral tissues of exercised vis-à-vis sedentary mice after experimental injury [114–116]. Aside from histological observations of inflammation in intensely exercised rat and human muscles [105, 117, 118], there is a dearth of information on immunocyte activities in skeletal muscles after exercise. Fluorocytometric analysis of paired blood and muscle samples after exercise of various durations and intensities will be critical for finding a definitive answer to whether exercise modulates muscle-Treg numbers and functions. Despite the paucity of studies looking at skeletal-muscle immunocytes after exercise, there are a few well-documented exercise-induced adaptations that may potentially support Treg accumulation and function in muscle. We propose that increased local lactate concentration, increased production of the tryptophan metabolite kynurenic acid (Kyna), and changes in the gut microbiota are three mechanisms by which exercise might modulate muscle Treg numbers and/or functions.
During intense exercise, carbohydrate metabolism is the dominant metabolic pathway used to fuel ATP production to sustain muscular work [119]. As exercise intensity increases, the production of lactate exceeds the rate of pyruvate oxidation in the tricarboxylic acid (TCA) cycle, which leads to an accumulation of lactate in myofibers and in the muscle extracellular space. This high-lactate environment, although painful for the athlete, may suit Tregs well: studies comparing the metabolic phenotypes of Tconvs and Tregs have shown that the latter is more dependent on oxidative phosphorylation [120, 121] and can use lactate in low-glucose environments as a source of pyruvate to fuel TCA cycle and electron transport chain activities to support suppressive function [122, 123].
Exercise also enhances the production of the tryptophan metabolite Kyna via PGC-1α-dependent upregulation of kynurenine aminotransferases (KATs) [124, 125]. Kyna is a ligand for the aryl hydrocarbon receptor (AhR) and G-protein-coupled receptor 35 (GPR35) [126]. Interestingly, muscle injury increases the expression of GPR35 and AhR on Tregs several fold [10], and AhR signaling promotes the generation of Tregs in other contexts [127, 128]. Kyna-mediated modulation has been proposed for circulating Tregs [111, 112]; it will be interesting to explore this mechanism in muscle-localized Treg populations.
Finally, exercise training produces significant changes in the gut microbiota [129–132]. Remarkably, 6 weeks of endurance exercise increases Faecalibacterium and Lachnospira communities while decreasing Bacteroides in the guts of lean human participants [129], and chronic endurance exercise promotes an elevation in short-chain fatty acid production in the gut [129, 132]. Changes in the microbiota influence Treg generation and phenotype in the gut via microbe-dependent metabolites [133–135], and Tregs traffic between the gut and extra-gut tissues [136]. Therefore, changes in the gut microbiota could be a potential mechanism of Treg modulation by long-term exercise.
As alluded to above, muscle injuries are commonly treated according to the RICE principle, which was first introduced in 1978 [137]. However, the American College of Sports Medicine now suggests a gentle movement of an afflicted area within the first 24 h after injury, followed by progressively challenging physical activity after 48–72h. Furthermore, controlled muscular contractions elicited in a previously injured area by transcutaneous electrical nerve stimulation is a common practice in physical therapy for treating acute and chronic muscular injuries. Thus, although there are no randomized clinical trials comparing post-injury immobilization to exercise, the widespread implementation of movement-based therapies after muscle injury by practitioners of sports medicine and physical therapy raises the question of whether exercise might be both the “poison” and the cure for sports-related muscular injuries. We propose that, through the mechanisms described above, exercise might mobilize Tregs to then home to injured sites to support the transition from pro-inflammatory to pro-repair processes. Furthermore, exercise might be prophylactic against excessive inflammatory responses subsequent to acute injury by increasing the number of Tregs in muscle.
Exercise as a therapy for muscular dystrophies was once avoided due to the “work overload” theory, which predicted deleterious effects on muscle function. However, mdx mice allowed to exercise voluntarily for several weeks do not display worsened hindlimb muscle, diaphragm, or cardiac muscle pathology [138, 139]. Instead, exercise-trained mdx mice, have improved hindlimb muscle function compared with sedentary mdx mice, despite running significantly less per day than age-matched healthy mice [140, 141]. Cardiac function in dystrophic mice is also improved by voluntary exercise [139]. Impressively, a meta-analysis of studies investigating the effect of exercise on muscular dystrophy patients found a significant association between exercise and improvement in endurance during walking [142]. It will be useful to know whether these benefits of exercise coincide with Treg modulation. Indeed, if exercise augments Treg representation and function in healthy and mdx muscles, then the mechanisms mediating exercise-induced Treg modulation may be elucidated and used in active or sedentary individuals to facilitate muscle repair. Thus, exercise or exercise-based treatments tailored to modulate Treg activities may be a novel therapeutic approach to treating acute and chronic muscle injuries.
Acknowledgements
None.
Abbreviations
- AAVs
adeno-associated viruses
- AhR
aryl hydrocarbon receptor
- BMD
Becker muscular dystrophy
- CAR
chimeric antigen receptor
- CK
creatine kinase
- DAMPs
damage-associated molecular patterns
- DAPC
dystrophin-associated protein complex
- DM
dermatomyositis
- DMD
Duchenne muscular dystrophy
- GPR35
G-protein-coupled receptor 35
- IBM
inclusion-body myositis
- IFNα
interferon-α
- IL-2
interleukin-2
- IMNM
immune-mediated necrotizing myopathy
- KATs
kynurenine aminotransferases
- NK
natural killer
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PM
polymyositis
- RICE
rest, ice, compression, and elevation
- S1pr
sphingosine-1-phosphate receptor
- scRNA-seq
single-cell RNA sequencing
- TCA
tricarboxylic acid
- Tconvs
conventional T cells
- TCR
T-cell receptor
- TNFα
tumor necrosis factor-α
- Tregs
regulatory T cells
- VAT
visceral adipose tissue
Contributor Information
Bola S Hanna, Department of Immunology, Harvard Medical School and Evergrande Center for Immunologic Diseases, Harvard Medical School and Brigham and Women’s Hospital; Boston, USA.
Omar K Yaghi, Department of Immunology, Harvard Medical School and Evergrande Center for Immunologic Diseases, Harvard Medical School and Brigham and Women’s Hospital; Boston, USA.
P Kent Langston, Department of Immunology, Harvard Medical School and Evergrande Center for Immunologic Diseases, Harvard Medical School and Brigham and Women’s Hospital; Boston, USA.
Diane Mathis, Department of Immunology, Harvard Medical School and Evergrande Center for Immunologic Diseases, Harvard Medical School and Brigham and Women’s Hospital; Boston, USA.
Conflict of Interest
DM is a cofounder of TRexBio and is a cofounder, member of the Scientific Advisory Board, and member of the Abata Advisory Board of Abata Therapeutics.
Funding
The lab’s work in this area is supported by grants from the National Institutes of Health (NIH) (R01 AR070334) and the JPB Foundation to DM; BSH and PKL received fellowships from the Deutsche Forschungsgemeinschaft (HA 8510/1) and the NIH (F32 AG072874), respectively; and OKY was supported by an NIH training grant (T32GM007753).
Data Availability
Not applicable. This paper did not generate new data.
Author Contributions
All authors contributed to the literature review for and to the writing and revision of this article.
Permission to reproduce material from other sources
Not applicable.
References
- 1. Fontenot JD, Gavin MA, Rudensky AY.. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003, 4, 330–6. [DOI] [PubMed] [Google Scholar]
- 2. Hori S, Nomura T, Sakaguchi S.. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 3. Khattri R, Cox T, Yasayko SA, Ramsdell F.. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003, 4, 337–42. [DOI] [PubMed] [Google Scholar]
- 4. Plitas G, Rudensky AY.. Regulatory T cells: differentiation and function. Cancer Immunol Res 2016, 4, 721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira LMR, Muller YD, Bluestone JA, Tang Q.. Next-generation regulatory T cell therapy. Nat Rev Drug Discov 2019, 18, 749–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raffin C, Vo LT, Bluestone JA.. Treg cell-based therapies: challenges and perspectives. Nat Rev Immunol 2020, 20, 158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muñoz-Rojas AR, Mathis D.. Tissue regulatory T cells: regulatory chameleons. Nat Rev Immunol 2021, 21, 597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009, 15, 930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol 2019, 4, eaaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell 2013, 155, 1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK.. Response to self antigen imprints regulatory memory in tissues. Nature 2011, 480, 538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014, 513, 564–8. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol 2014, 307, H1233–42. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al. A distinct function of regulatory T cells in tissue protection. Cell 2015, 162, 1078–89. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delacher M, Imbusch CD, Weichenhan D, Breiling A, Hotz-Wagenblatt A, Trager U, et al. Genome-wide DNA-methylation landscape defines specialization of regulatory T cells in tissues. Nat Immunol 2017, 18, 1160–72. doi: 10.1038/ni.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–50. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 17. Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 2007, 27, 786–800. [DOI] [PubMed] [Google Scholar]
- 18. Bailey-Bucktrout SL, Bluestone JA.. Regulatory T cells: stability revisited. Trends Immunol 2011, 32, 301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science 2010, 329, 1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, et al. Plasticity of foxp3(+) T cells reflects promiscuous foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 2012, 36, 262–75. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 21. Dong S, Hiam-Galvez KJ, Mowery CT, Herold KC, Gitelman SE, Esensten JH, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 2021, 6, e147474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baoge L, Van Den Steen E, Rimbaut S, Philips N, Witvrouw E, Almqvist KF, et al. Treatment of skeletal muscle injury: a review. ISRN Orthop 2012, 2012, 689012. doi: 10.5402/2012/689012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charge SB, Rudnicki MA.. Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004, 84, 209–38. [DOI] [PubMed] [Google Scholar]
- 24. Sambasivan R, Yao R, Kissenpfennig A, Van WL, Paldi A, Gayraud-Morel B, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 2011, 138, 3647–56. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 25. Lepper C, Partridge TA, Fan CM.. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011, 138, 3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G.. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011, 138, 3625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 2015, 21, 786–94. [DOI] [PubMed] [Google Scholar]
- 28. Munoz-Canoves P, Serrano AL.. Macrophages decide between regeneration and fibrosis in muscle. Trends Endocrinol Metab 2015, 26, 449–50. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Zhao W, Ransohoff RM, Zhou L.. Identification and function of fibrocytes in skeletal muscle injury repair and muscular dystrophy. J Immunol 2016, 197, 4750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahdy MAA. Skeletal muscle fibrosis: an overview. Cell Tissue Res 2019, 375, 575–88. [DOI] [PubMed] [Google Scholar]
- 31. Arnold L, Henry A, Poron F, Baba-Amer Y, van RN, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007, 204, 1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panduro M, Benoist C, Mathis D.. Treg cells limit IFN-γ production to control macrophage accrual and phenotype during skeletal muscle regeneration. Proc Natl Acad Sci USA 2018, 115, E2585–93. doi: 10.1073/pnas.1800618115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mann AO, Hanna BS, Munoz-Rojas AR, Sandrock I, Prinz I, Benoist C, et al. IL-17A-producing γδT cells promote muscle regeneration in a microbiota-dependent manner. J Exp Med 2022, 219, e20211504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng M, Nguyen MH, Fantuzzi G, Koh TJ.. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am J Physiol Cell Physiol 2008, 294, C1183–91. [DOI] [PubMed] [Google Scholar]
- 35. Chen SE, Jin B, Li YP.. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol 2007, 292, C1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Villalta SA, Deng B, Rinaldi C, Wehling-Henricks M, Tidball JG.. IFN-g promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J Immunol 2011, 187, 5419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Londhe P, Davie JK.. Gamma interferon modulates myogenesis through the major histocompatibility complex class II transactivator, CIITA. Mol Cell Biol 2011, 31, 2854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Londhe P, Davie JK.. Interferon-gamma resets muscle cell fate by stimulating the sequential recruitment of JARID2 and PRC2 to promoters to repress myogenesis. Sci Signal 2013, 6, ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Segawa M, Fukada S, Yamamoto Y, Yahagi H, Kanematsu M, Sato M, et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res 2008, 314, 3232–44. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40. Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, et al. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther 2015, 23, 1189–200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Varga T, Mounier R, Patsalos A, Gogolak P, Peloquin M, Horvath A, et al. Macrophage PPARγ, a lipid activated transcription factor controls the growth factor GDF3 and skeletal muscle regeneration. Immunity 2016, 45, 1038–51. doi: 10.1016/j.immuni.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG.. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 2012, 189, 3669–80. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med 2014, 6, 258ra–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castiglioni A, Corna G, Rigamonti E, Basso V, Vezzoli M, Monno A, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS One 2015, 10, e0128094. doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 2016, 44, 355–67. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dispirito JR, Zemmour D, Ramanan D, Cho J, Zilionis R, Klein AM, et al. Molecular diversification of regulatory T cells in nonlymphoid tissues. Sci Immunol 2018, 3, eaat5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oprescu SN, Yue F, Qiu J, Brito LF, Kuang S.. Temporal dynamics and heterogeneity of cell populations during skeletal muscle regeneration. iScience 2020, 23, 100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varga T, Mounier R, Horvath A, Cuvellier S, Dumont F, Poliska S, et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol 2016, 196, 4771–82. doi: 10.4049/jimmunol.1502490. [DOI] [PubMed] [Google Scholar]
- 49. Cho J, Kuswanto W, Benoist C, Mathis D.. T cell receptor specificity drives accumulation of a reparative population of regulatory T cells within acutely injured skeletal muscle. Proc Natl Acad Sci USA 2019, 116, 26727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bondesen BA, Mills ST, Kegley KM, Pavlath GK.. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 2004, 287, C475–83. [DOI] [PubMed] [Google Scholar]
- 51. Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjaer M, et al. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol (1985) 2009, 107, 1600–11. doi: 10.1152/japplphysiol.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, et al. IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol 2014, 193, 4010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Molofsky AB, Savage AK, Locksley RM.. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015, 42, 1005–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stremska ME, Dai C, Venkatadri R, Wang H, Sabapathy V, Kumar G, et al. IL233, an IL-2-IL-33 hybrid cytokine induces prolonged remission of mouse lupus nephritis by targeting Treg cells as a single therapeutic agent. J Autoimmun 2019, 102, 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stremska ME, Jose S, Sabapathy V, Huang L, Bajwa A, Kinsey GR, et al. IL233, a novel IL-2 and IL-33 hybrid cytokine, ameliorates renal injury. J Am Soc Nephrol 2017, 28, 2681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM.. CD25+ CD4+ T Cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004, 199, 1467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jaeckel E, von Boehmer H, Manns MP.. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes 2005, 54, 306–10. [DOI] [PubMed] [Google Scholar]
- 58. Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol 2015, 16, 276–85. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 59. Hayatsu N, Miyao T, Tachibana M, Murakami R, Kimura A, Kato T, et al. Analyses of a mutant Foxp3 allele reveal BATF as a critical transcription factor in the differentiation and accumulation of tissue regulatory T cells. Immunity 2017, 47, 268–283.e9. doi: 10.1016/j.immuni.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 60. Delacher M, Imbusch CD, Hotz-Wagenblatt A, Mallm JP, Bauer K, Simon M, et al. Precursors for nonlymphoid-tissue Treg cells reside in secondary lymphoid organs and are programmed by the transcription factor BATF. Immunity 2020, 52, 295–312.e11. doi: 10.1016/j.immuni.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Joglekar AV, Li G.. T cell antigen discovery. Nat Methods 2021, 18, 873–80. [DOI] [PubMed] [Google Scholar]
- 62. Fernandes RA, Li C, Wang G, Yang X, Savvides CS, Glassman CR, et al. Discovery of surrogate agonists for visceral fat Treg cells that modulate metabolic indices in vivo. eLife 2020, 9, e58463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kunkel LM, Hejtmancik JF, Caskey CT, Speer A, Monaco AP, Middlesworth W, et al. Analysis of deletions in DNA from patients with Becker and Duchenne muscular dystrophy. Nature 1986, 322, 73–7. doi: 10.1038/322073a0. [DOI] [PubMed] [Google Scholar]
- 64. Hoffman EP, Brown RH Jr, Kunkel LM.. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 65. Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP.. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990, 345, 315–9. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 66. Darras BT, Urion DK, Ghosh PS.. Dystrophinopathies. In: Margaret PA, David BE, Ghayda MM, Roberta AP, Stephanie EW, Lora JHB, Karen WG, Anne A (eds.), GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 67. Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM.. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 1988, 2, 90–5. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 68. Aartsma-Rus A, Van Deutekom JC, Fokkema IF, van Ommen GJ, Den Dunnen JT.. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 2006, 34, 135–44. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- 69. Gao QQ, McNally EM.. The dystrophin complex: structure, function, and implications for therapy. Compr Physiol 2015, 5, 1223–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A.. Duchenne muscular dystrophy. Nat Rev Dis Primers 2021, 7, 13. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mercuri E, Bonnemann CG, Muntoni F.. Muscular dystrophies. Lancet 2019, 394, 2025–38. [DOI] [PubMed] [Google Scholar]
- 72. Tidball JG, Welc SS, Wehling-Henricks M.. Immunobiology of inherited muscular dystrophies. Compr Physiol 2018, 8, 1313–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 2017, 17, 165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gloss D, Moxley RT III, Ashwal S, Oskoui M.. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY.. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev 2016, 5, CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018, 17, 251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hussein MR, et al. The effects of glucocorticoid therapy on the inflammatory and dendritic cells in muscular dystrophies. Int J Exp Pathol 2006, 87, 451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wehling M, Spencer MJ, Tidball JG.. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol 2001, 155, 123–31. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hodgetts S, Radley H, Davies M, Grounds MD.. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord 2006, 16, 591–602. [DOI] [PubMed] [Google Scholar]
- 80. Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG.. Helper (CD4+) and cytotoxic (CD8+) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 2001, 98, 235–43. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- 81. Wehling-Henricks M, Sokolow S, Lee JJ, Myung KH, Villalta SA, Tidball JG.. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet 2008, 17, 2280–92. doi: 10.1093/hmg/ddn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Eghtesad S, Jhunjhunwala S, Little SR, Clemens PR.. Rapamycin ameliorates dystrophic phenotype in mdx mouse skeletal muscle. Mol Med 2011, 17, 917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Villalta SA, Deng B, Rinaldi C, Wehling-Henricks M, Tidball JG.. IFN-γ promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J Immunol 2011, 187, 5419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J.. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006, 311, 1924–7. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 85. Gussoni E, Pavlath GK, Miller RG, Panzara MA, Powell M, Blau HM, et al. Specific T cell receptor gene rearrangements at the site of muscle degeneration in Duchenne muscular dystrophy. J Immunol 1994, 153, 4798–805. [PubMed] [Google Scholar]
- 86. Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, et al. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest 2009, 119, 1583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chamberlain JS. Gene therapy of muscular dystrophy. Hum Mol Genet 2002, 11, 2355–62. [DOI] [PubMed] [Google Scholar]
- 88. Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med 2010, 363, 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Flanigan KM, Campbell K, Viollet L, Wang W, Gomez AM, Walker CM, et al. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum Gene Ther 2013, 24, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Komaki H, Hayashi YK, Tsuburaya R, Sugie K, Kato M, Nagai T, et al. Inflammatory changes in infantile-onset LMNA-associated myopathy. Neuromuscul Disord 2011, 21, 563–8. doi: 10.1016/j.nmd.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 91. Tipton CM. The history of “Exercise Is Medicine” in ancient civilizations. Adv Physiol Educ 2014, 38, 109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bouchard C, Blair SN, Katzmarzyk PT.. Less sitting, more physical activity, or higher fitness?. Mayo Clin Proc 2015, 90, 1533–40. [DOI] [PubMed] [Google Scholar]
- 93. Cabanas-Sanchez V, Guallar-Castillon P, Higueras-Fresnillo S, Garcia-Esquinas E, Rodriguez-Artalejo F, Martinez-Gomez D.. Physical activity, sitting time, and mortality from inflammatory diseases in older adults. Front Physiol 2018, 9, 898. doi: 10.3389/fphys.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA.. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011, 11, 607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 95. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–85. [DOI] [PubMed] [Google Scholar]
- 96. Egan B, Zierath JR.. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 2013, 17, 162–84. [DOI] [PubMed] [Google Scholar]
- 97. Wallberg-Henriksson H, Zierath JR.. Exercise remodels subcutaneous fat tissue and improves metabolism. Nat Rev Endocrinol 2015, 11, 198–200. [DOI] [PubMed] [Google Scholar]
- 98. Schulz, G. In: von Ziemssen H, Verlag von F.C.W. Vogel (eds.), Deutshes Archiv fur Klinische Medicin. Leipzig, 1893, 234–281. [Google Scholar]
- 99. Pedersen BK, Toft AD.. Effects of exercise on lymphocytes and cytokines. Br J Sports Med 2000, 34, 246–51. doi: 10.1136/bjsm.34.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ahlborg B, Ahlborg G.. Exercise leukocytosis with and without beta-adrenergic blockade. Acta Med Scand 1970, 187, 241–6. doi: 10.1111/j.0954-6820.1970.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 101. Clifford T, et al. T-regulatory cells exhibit a biphasic response to prolonged endurance exercise in humans. Eur J Appl Physiol 2017, 117, 1727–37. doi: 10.1007/s00421-017-3667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rooney BV, Bigley AB, LaVoy EC, Laughlin M, Pedlar C, Simpson RJ.. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: a detailed temporal analysis of leukocyte extravasation. Physiol Behav 2018, 194, 260–7. [DOI] [PubMed] [Google Scholar]
- 103. Simpson RJ, Florida-James GD, Whyte GP, Guy K.. The effects of intensive, moderate and downhill treadmill running on human blood lymphocytes expressing the adhesion/activation molecules CD54 (ICAM-1), CD18 (β2 integrin) and CD53. Eur J Appl Physiol 2006, 97, 109–21. [DOI] [PubMed] [Google Scholar]
- 104. Simpson RJ, Florida-James GD, Whyte GP, Black JR, Ross JA, Guy K.. Apoptosis does not contribute to the blood lymphocytopenia observed after intensive and downhill treadmill running in humans. Res Sports Med 2007, 15, 157–74. [DOI] [PubMed] [Google Scholar]
- 105. Armstrong RB, Ogilvie RW, Schwane JA.. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 1983, 54, 80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 106. Fridén J, Sjostrom M, Ekblom B.. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 1983, 4, 170–6. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- 107. Jones B, Hoyne GH.. The role of the innate and adaptive immunity in exercise induced muscle damage and repair. J Clin Cell Immunol 2017, 8, 482. [Google Scholar]
- 108. Gong T, Liu L, Jiang W, Zhou R.. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 2020, 20, 95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 109. Pedersen BK, Hoffman-Goetz L.. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 2000, 80, 1055–81. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 110. Gleeson M. Immune function and exercise. Eur J Sport Sci 2004, 4, 52–66. doi: 10.1080/17461390400074304. [DOI] [Google Scholar]
- 111. Dorneles GP, Dos Passos AAZ, Romao PRT, Peres A.. New insights about regulatory T cells distribution and function with exercise: the role of immunometabolism. Curr Pharm Des 2020, 26, 979–90. [DOI] [PubMed] [Google Scholar]
- 112. Proschinger S, Winker M, Joisten N, Bloch W, Palmowski J, Zimmer P, et al. The effect of exercise on regulatory T cells: A systematic review of human and animal studies with future perspectives and methodological recommendations. Exerc Immunol Rev 2021, 27, 142–66. [PubMed] [Google Scholar]
- 113. Perry C, et al. Endurance exercise diverts the balance between Th17 cells and regulatory T cells. PLoS One 2013, 8, e74722. doi: 10.1371/journal.pone.0074722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fernandes P, de Mendonca OL, Bruggemann TR, Sato MN, Olivo CR, Arantes-Costa FM, et al. Physical exercise induces immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front Immunol 2019, 10, 854. doi: 10.3389/fimmu.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lowder T, Dugger K, Deshane J, Estell K, Schwiebert LM, et al. Repeated bouts of aerobic exercise enhance regulatory T cell responses in a murine asthma model. Brain Behav Immun 2010, 24, 153–9. doi: 10.1016/j.bbi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xie Y, Li Z, Wang Y, Xue X, Ma W, Zhang Y, et al. Effects of moderate- versus high- intensity swimming training on inflammatory and CD4(+) T cell subset profiles in experimental autoimmune encephalomyelitis mice. J Neuroimmunol 2019, 328, 60–7. doi: 10.1016/j.jneuroim.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 117. Malm C, Sjodin TL, Sjoberg B, Lenkei R, Renstrom P, Lundberg IE, et al. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol 2004, 556, 983–1000. doi: 10.1113/jphysiol.2003.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Marklund P, Mattsson CM, Wahlin-Larsson B, Ponsot E, Lindvall B, Lindvall L, et al. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol 2013, 114, 66–72. [DOI] [PubMed] [Google Scholar]
- 119. Hargreaves M, Spriet LL.. Skeletal muscle energy metabolism during exercise. Nat Metab 2020, 2, 817–28. [DOI] [PubMed] [Google Scholar]
- 120. Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015, 125, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 2011, 186, 3299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Angelin A, Gil-de-Gomez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab 2017, 25, 1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 2021, 591, 645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [DOI] [PubMed] [Google Scholar]
- 125. Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol 2016, 310, C836–40. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 126. Cervenka I, Agudelo LZ, Ruas JL.. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [DOI] [PubMed] [Google Scholar]
- 127. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA.. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 2010, 185, 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, et al. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep 2017, 21, 2277–90. doi: 10.1016/j.celrep.2017.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018, 50, 747–57. [DOI] [PubMed] [Google Scholar]
- 130. Clarke SF, Murphy EF, O’Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–20. [DOI] [PubMed] [Google Scholar]
- 131. Petersen LM, Bautista EJ, Nguyen H, Hanson BM, Chen L, Lek SH, et al. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017, 5, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 2019, 25, 1104–9. doi: 10.1038/s41591-019-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Arpaia N, Campbell C, Fan X, Dikiy S, van DV, Deroos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–9. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–5. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Magnuson AM, Thurber GM, Kohler RH, Weissleder R, Mathis D, Benoist C.. Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc Natl Acad Sci USA 2015, 112, 1511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mirkin G, Hoffman M.. The Sports Medicine Book. Boston: Little, Brown, 1978. [Google Scholar]
- 138. Dupont-Versteegden EE, McCarter RJ, Katz MS.. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. J Appl Physiol (1985) 1994, 77, 1736–41. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- 139. Kogelman B, Putker K, Hulsker M, Tanganyika-de WC, van der Weerd L, Aartsma-Rus A, et al. Voluntary exercise improves muscle function and does not exacerbate muscle and heart pathology in aged Duchenne muscular dystrophy mice. J Mol Cell Cardiol 2018, 125, 29–38. doi: 10.1016/j.yjmcc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 140. Hayes A, Williams DA.. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol (1985) 1996, 80, 670–9. doi: 10.1152/jappl.1996.80.2.670. [DOI] [PubMed] [Google Scholar]
- 141. Call JA, McKeehen JN, Novotny SA, Lowe DA.. Progressive resistance voluntary wheel running in the mdx mouse. Muscle Nerve 2010, 42, 871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Gianola S, Castellini G, Pecoraro V, Monticone M, Banfi G, Moja L.. Effect of muscular exercise on patients with muscular dystrophy: a systematic review and meta-analysis of the literature. Front Neurol 2020, 11, 958. doi: 10.3389/fneur.2020.00958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. This paper did not generate new data.