ABSTRACT
We report the first identification of a fluconazole-resistant Candida parapsilosis (FR-Cp) strain in our hospital, which subsequently caused an outbreak involving 17 patients (12 deaths) within a 26-bed French intensive care unit. Microsatellite genotyping confirmed that all FR-Cp isolates belonged to the same clone. Given recent reports of rapid dissemination of these emerging clones, routine testing of azole susceptibility for all Candida parapsilosis isolates should be encouraged, at least in ICU patients.
KEYWORDS: outbreak, Candida parapsilosis, fluconazole resistance, infection prevention and control, intensive care unit
INTRODUCTION
Fluconazole-resistant C. parapsilosis (FR-Cp) clones have been recently involved in long-lasting nosocomial outbreaks in clinical wards, with high morbidity and mortality rates (1, 2). FR-Cp is characterized by its ability to form tenacious biofilms on medical devices, lower susceptibility to some chlorine-based disinfectants, and high potential for cross-transmission, which complicates prevention and control (1 to 3). Moreover, there seems to be a link between the ability of the fungus to spread clonally and the presence of the Y132F alteration conferring resistance to fluconazole (2).
In the 26-bed ICU of the 950-bed teaching Bichat hospital (Paris, France), only patients at risk for invasive candidiasis (i.e., with prolonged mechanical ventilation, vascular devices, recent surgical procedures, immune suppression, and/or treated by broad-spectrum antibiotics) are routinely screened for Candida spp. colonization on swabs from mouth, axilla, groin, and anus. Isolates are identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry. MICs of fluconazole, voriconazole, and amphotericin B are measured only for Candida strains causing invasive infections, by the gradient concentration strip method (Etest; bioMérieux). Isolates with MIC ≥8 mg/L for fluconazole, and ≥1 mg/L for voriconazole are considered resistant (4). Since there is no breakpoint for interpretation of MIC of amphotericin B with the Etest technique, we use the epidemiological cutoff endpoint (ECV), i.e., 2 mg/L (5).
On 21 May 2021, a FR-Cp strain was detected, for the first time in our hospital, on swabs collected from mouth, axilla, groin, and anus of a first ICU patient. On May 24, a FR-Cp strain was isolated from blood culture of a second patient on mechanical ventilation and extracorporeal membrane oxygenation (ECMO) for COVID-19 acute respiratory distress syndrome. Following disclosure of the two cases, we changed our screening policy and initiated systematic FR-Cp screening of all patients admitted in the ICU, and collected 9 environmental samples in the room of a FR-Cp patient in June 2021 (tap, sink, washbasin, syringe pump, ECMO module, trolley, 2 bedside tables, and power outlets). All patients with a FR-Cp strain detected on either blood, urine, central venous catheter tip, or a superficial swab sample (axilla, groin, anus, mouth) were considered cases.
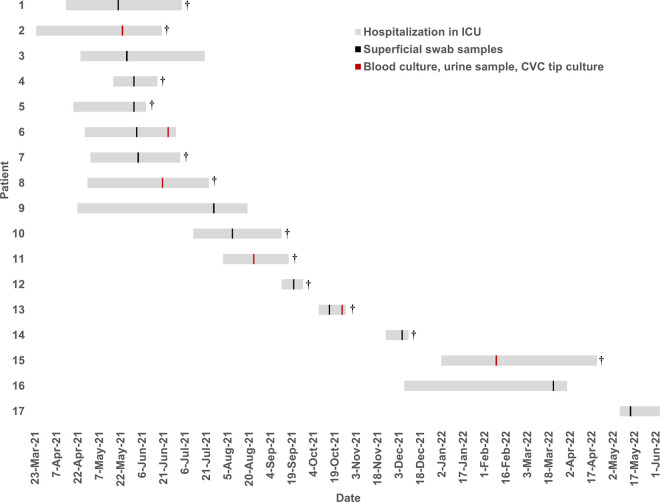
As displayed in Fig. 1 and the supplemental table, between May 2021 and May 2022, 18 FR-Cp isolates were detected in 17 patients and in 1 environment sample (washbasin). Based on definitions of the French expert consensus-based clinical practice guidelines (6), 6 patients had confirmed invasive infections: 3 non-catheter-related candidemia, 2 catheter-related sepsis (local and systemic signs of infection, associated with positive catheter tip culture and partial resolution of the clinical symptoms after catheter removal), and 1 orchi-epididymitis with prostatic abscess. Overall, 12 patients died in the ICU.
FIG 1.
Chronogram of cases of fluconazole-resistant C. parapsilosis (FR-Cp) infections or colonizations over the study period. Gray bars represent the dates of stay in the ICU. Vertical lines figure the date of detection of the FR-Cp: black, superficial swab samples (axilla, groin, anus, mouth); red, blood, urine, central venous catheter tip. †, patients who died during ICU stay.
Specific infection control measures were implemented in May 2021 (7): cases were cohorted and placed on contact isolation and had daily chlorhexidine gluconate bathing. Environment daily disinfection with paracetic acid-based solutions was initiated, and a terminal cleaning of the ICU was performed in August and September 2021. In May 2022, 9 additional environmental samples were collected, and all were negative.
All isolates were also resistant to voriconazole while keeping susceptibility to echinocandins and amphotericin B. The A395T mutation that confers the Y132F amino acid substitution was present in all FR-Cp strains. Microsatellite genotyping (8, 9) confirmed that all FR-Cp isolates belonged to the same clone.
Candida sp. is known to spread within ICUs from health care workers’ hands or contaminated patient care equipment (10). Persistent dormant Y132F environmental reservoir was also suspected to fuel clonal nosocomial outbreaks (11).
In our report, there was a larger gap between the first 13 cases detected in May–October 2021, and the 4 cases detected between November 2021 and May 2022. During the first phase of the outbreak, cross-transmission likely occurred between patients, exacerbated by concomitant circulation of SARS-CoV-2 over the period. Breaks in infection prevention and control measures related to workload (1, 2) and change in the case mix of patients (immunomodulatory drugs, increase of length of stay, invasive procedures, i.e., ECMO, wider use of azoles for suspicion of Aspergillus infections) may have contributed to the spread. However, the last four cases were detected several weeks after, suggesting persistence of an unknown environmental reservoir even if only one environmental sample was positive. Thus, the role of an environmental reservoir in dissemination remains unclear.
In 2020, 95 Candida screenings were performed in the ICU, and 19 resulted positive for Candida parapsilosis. At that time, according to national guidelines, only isolate Candida parapsilosis strains causing invasive infections were tested for susceptibility to azoles (and all were susceptible to fluconazole). Therefore, we cannot definitely rule out if the FR-Cp strain was already circulating in the hospital before disclosure of the first invasive infection in May 2021.
In February 2022, isolates harboring the Y132F ERG11 gene substitution were reported for the first time in patients admitted to a Spanish hospital, without prior azole exposure (12). This observation suggests that the strain may become endemic in hospital settings. Whether the strain also circulates in the community, which may constitute a reservoir for introduction in health care settings, is still unknown. Our report illustrates that the spread of FR-Cp isolates is possibly underestimated since antifungal susceptibility testing was not routinely performed on isolates from colonized patients.
Besides Candida auris, FR-Cp is an emerging invasive multiresistant pathogen of great concern in the ICU, because of high potential of dissemination of currently circulating clones, and high mortality rate. Detection is challenging, and may contribute to its spread, particularly in facilities with important workload like in the context of the COVID-19 pandemic. To monitor trends of resistance and quickly identify clustered cases, routine testing of azole susceptibility for all Candida parapsilosis isolates should be encouraged.
Ethics approval.
The investigation was a part of infection control response and was not considered research subject to institutional review board approval; therefore, written informed consent by participants was not required.
No personal identifiable data for case-patients are included in this report.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Thomaz DY, de Almeida JN, Sejas ONE, Del Negro GMB, Carvalho GOMH, Gimenes VMF, de Souza MEB, Arastehfar A, Camargo CH, Motta AL, Rossi F, Perlin DS, Freire MP, Abdala E, Benard G. 2021. Environmental clonal spread of azole-resistant Candida parapsilosis with Erg11-Y132F mutation causing a large candidemia outbreak in a Brazilian cancer referral center. JoF 7:259. 10.3390/jof7040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fekkar A, Blaize M, Bouglé A, Normand A-C, Raoelina A, Kornblum D, Kamus L, Piarroux R, Imbert S. 2021. Hospital outbreak of fluconazole-resistant Candida parapsilosis: arguments for clonal transmission and long-term persistence. Antimicrob Agents Chemother 65:e02036-20. 10.1128/AAC.02036-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta AK, Ahmad I, Summerbell RC. 2002. Fungicidal activities of commonly used disinfectants and antifungal pharmaceutical spray preparations against clinical strains of Aspergillus and Candida species. Med Mycol 40:201–208. 10.1080/mmy.40.2.201.208. [DOI] [PubMed] [Google Scholar]
- 4.Wayne P. 2020. M59—Epidemiological cutoff values for antifungal susceptibility testing, 3rd ed. Clinical and Laboratory Standards Institute, Malvern, PA. [Google Scholar]
- 5.Espinel-Ingroff A, Sasso M, Turnidge J, Arendrup M, Botterel F, Bourgeois N, Bouteille B, Canton E, Cassaing S, Dannaoui E, Dehais M, Delhaes L, Dupont D, Fekkar A, Fuller J, Garcia-Effron G, Garcia J, Gonzalez GM, Govender NP, Guegan H, Guinea J, Houzé S, Lass-Flörl C, Pelaez T, Forastiero A, Lackner M, Magobo R. 2021. Etest ECVs/ECOFFs for detection of resistance in prevalent and three nonprevalent Candida spp. to triazoles and amphotericin B and Aspergillus spp. to caspofungin: further assessment of modal variability. Antimicrob Agents Chemother 65:e0109321. 10.1128/AAC.01093-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timsit J-F, Baleine J, Bernard L, Calvino-Gunther S, Darmon M, Dellamonica J, Desruennes E, Leone M, Lepape A, Leroy O, Lucet J-C, Merchaoui Z, Mimoz O, Misset B, Parienti J-J, Quenot J-P, Roch A, Schmidt M, Slama M, Souweine B, Zahar J-R, Zingg W, Bodet-Contentin L, Maxime V. 2020. Expert consensus-based clinical practice guidelines management of intravascular catheters in the intensive care unit. Ann Intensive Care 10:118. 10.1186/s13613-020-00713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. 2022. Infection prevention and control for Candida auris. https://www.cdc.gov/fungal/candida-auris/c-auris-infection-control.html. Retrieved 11 January 2023.
- 8.Diab-Elschahawi M, Forstner C, Hagen F, Meis JF, Lassnig AM, Presterl E, Klaassen CHW. 2012. Microsatellite genotyping clarified conspicuous accumulation of Candida parapsilosis at a cardiothoracic surgery intensive care unit. J Clin Microbiol 50:3422–3426. 10.1128/JCM.01179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DendroUPGMA. 2022. DendroUPGMA: Dendrogram construction using the UPGMA algorithm. http://genomes.urv.es/UPGMA/. Retrieved 16 August 2022.
- 10.Hernández-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, Moncada-Barrón D, Alvarez-Verona E, Hernández-Delgado L, Torres-Narváez P, Lavalle-Villalobos A. 2010. Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr 169:783–787. 10.1007/s00431-009-1109-7. [DOI] [PubMed] [Google Scholar]
- 11.Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, Litvintseva AP. 2017. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 55:2996–3005. 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcoceba E, Gómez A, Lara-Esbrí P, Oliver A, Beltrán AF, Ayestarán I, Muñoz P, Escribano P, Guinea J. 2022. Fluconazole-resistant Candida parapsilosis clonally related genotypes: first report proving the presence of endemic isolates harbouring the Y132F ERG11 gene substitution in Spain. Clin Microbiol Infect 28:1113–1119. 10.1016/j.cmi.2022.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01130-22-s0001.docx, DOCX file, 0.6 MB (567.2KB, docx)
Supplemental material. Download aac.01130-22-s0002.tif, TIF file, 0.7 MB (703.9KB, tif)