Abstract
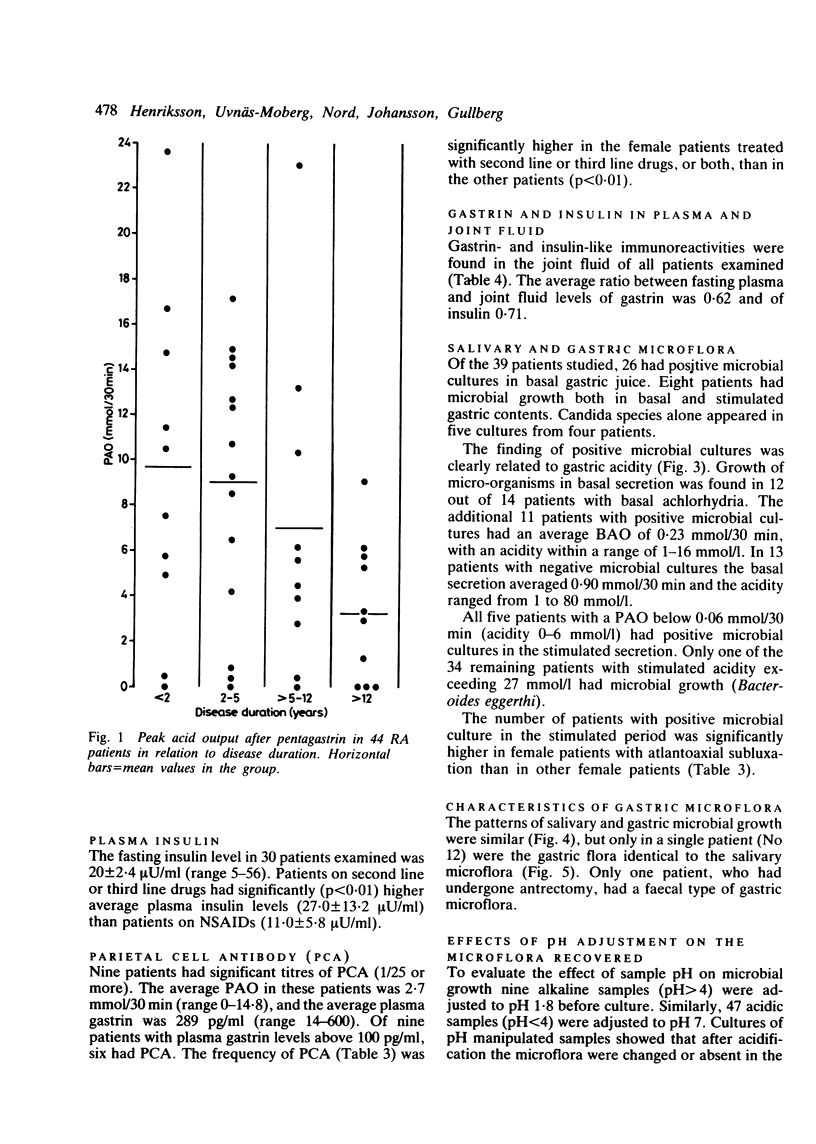
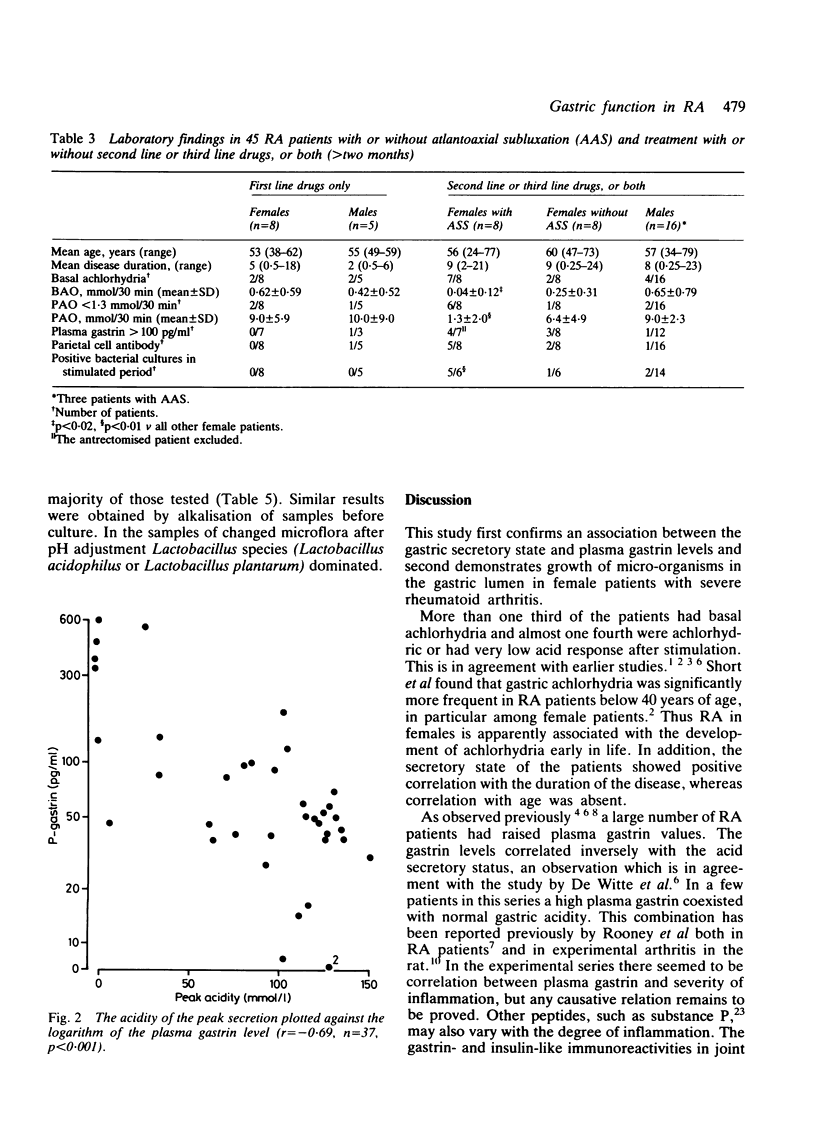
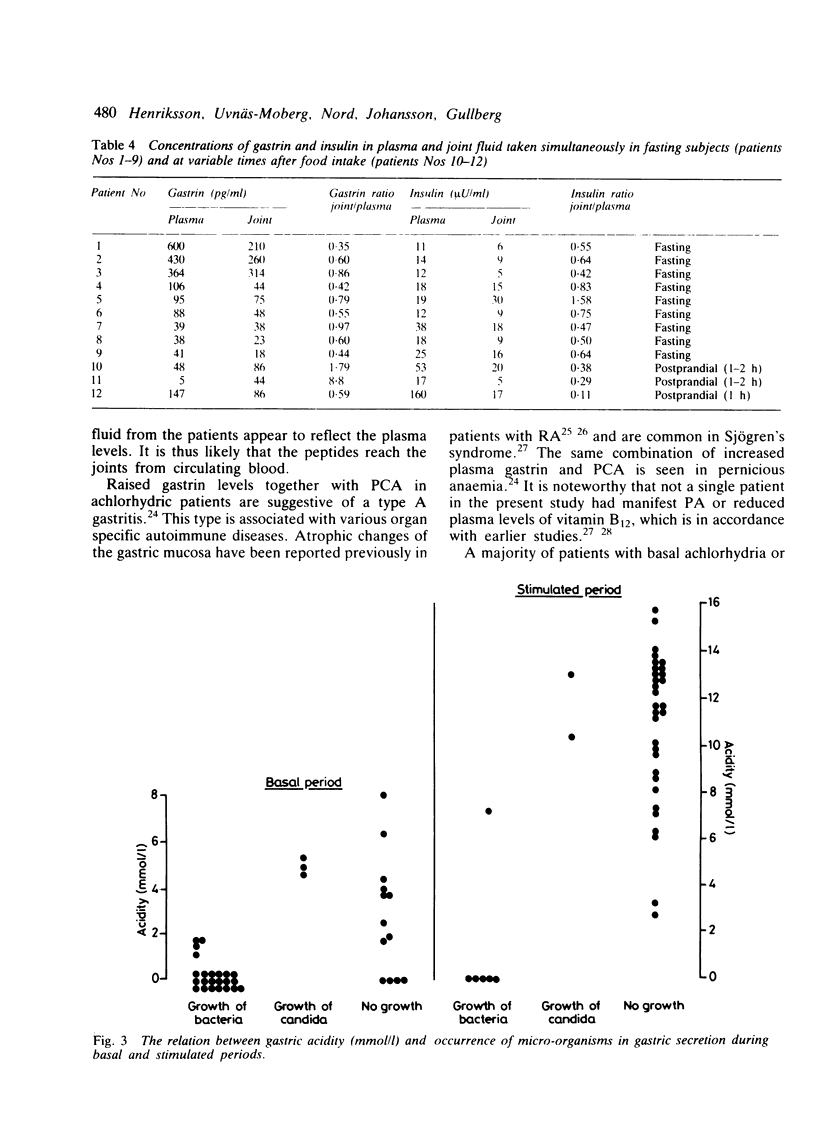
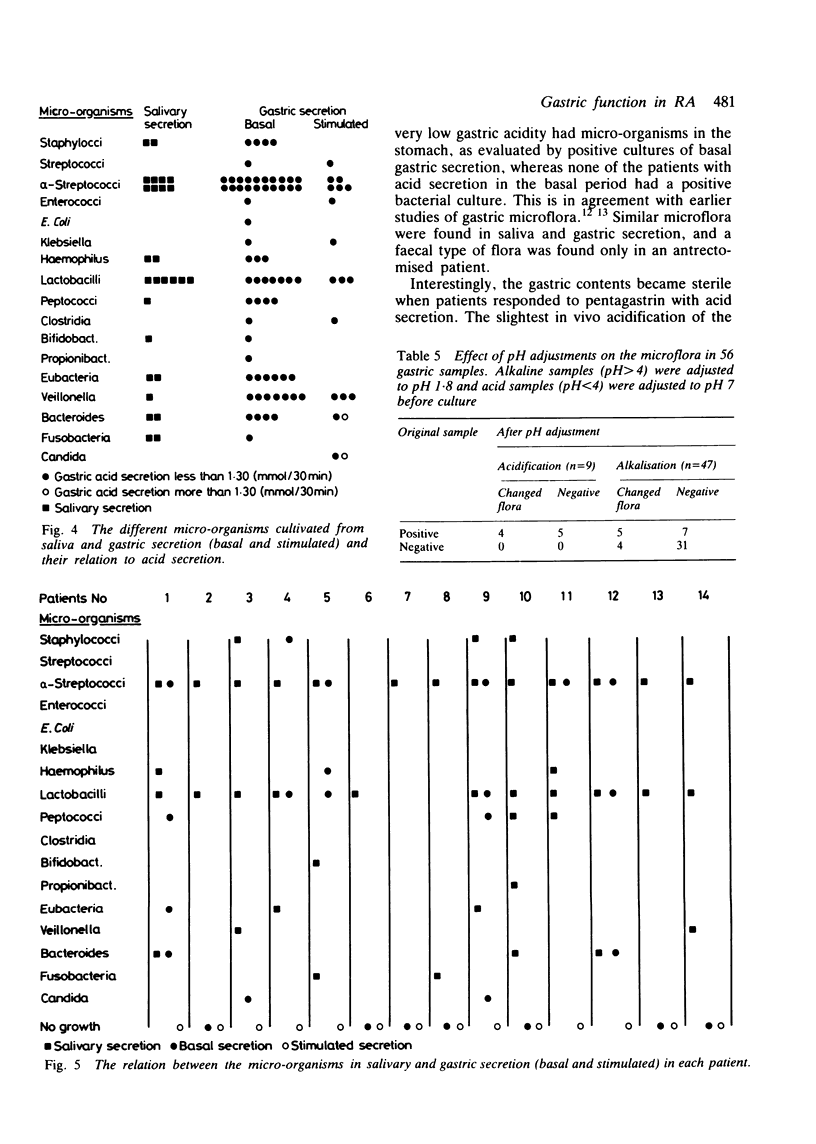
The relation between the basal and stimulated gastric acid secretion, plasma gastrin, and the gastric microflora was examined in 45 patients with rheumatoid arthritis. Sixteen patients (36%) had basal achlorhydria, and of these, 10 (22%) had achlorhydria or hypochlorhydria after stimulation with pentagastrin. The peak acid output and acidity showed inverse correlation with the disease duration but were not associated with age or with the degree of physical disability. Hypergastrinaemia was found in nine patients (20%), of whom 6 (13%) had significant titres of parietal cell antibody. The acidity of the peak acid output showed negative correlation with plasma gastrin. It was confirmed that the gastric secretory state is a determinant of plasma gastrin levels and in addition influences the growth of micro-organisms in the gastric lumen. The type of microflora in the non-acid stomach was similar to that found in the saliva. A subgroup of eight females was identified who showed low gastric acid secretion rates, positive bacterial cultures, and atlantoaxial subluxation. Gastrin- and insulin-like immunoreactivities were found in joint fluid. The concentrations reflected their plasma levels, suggesting that the peptides are not released at the inflammatory site, but rather that they reach synovial fluid from circulating blood.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Heimdahl A., Nord C. E. Effect of phenoxymethylpenicillin and clindamycin on the oral, throat and faecal microflora of man. Scand J Infect Dis. 1979;11(3):233–242. doi: 10.3109/inf.1979.11.issue-3.11. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Louyot P., Tamisier J. N., Jeanpierre J., Pourel J., Zannetti A., Bertrand P. Le conportement gastrique au cours des rhumatismes inflammatoires sous incidence médicamenteuse. Rev Rhum Mal Osteoartic. 1974 Dec;41(12):725–732. [PubMed] [Google Scholar]
- Marcolongo R., Bayeli P. F., Montagnani M. Gastrointestinal involvement in rheumatoid arthritis: a biopsy study. J Rheumatol. 1979 Mar-Apr;6(2):163–173. [PubMed] [Google Scholar]
- Maury C. P., Törnroth T., Teppo A. M. Atrophic gastritis in Sjögren's syndrome. Morphologic, biochemical, and immunologic findings. Arthritis Rheum. 1985 Apr;28(4):388–394. doi: 10.1002/art.1780280406. [DOI] [PubMed] [Google Scholar]
- Pitcher C. S., Lindsay D. J., Hill A. G. Absorption of vitamin B12 in rheumatoid arthritis. Ann Rheum Dis. 1970 Sep;29(5):533–536. doi: 10.1136/ard.29.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney P. J., Dick W. C., Imrie R. C., Buchanan K. D. Animal model to study the relationship between immunoreactive gastrin and inflammatory arthritis. Nature. 1973 Dec 21;246(5434):497–498. doi: 10.1038/246497a0. [DOI] [PubMed] [Google Scholar]
- Rooney P. J., Dick W. C., Imrie R. C., Turner D., Buchanan K. D., Ardill J. On the relationship between gastrin, gastric secretion, and adjuvant arthritis in rats. Ann Rheum Dis. 1978 Oct;37(5):432–435. doi: 10.1136/ard.37.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney P. J., Grennan D. M., Sturrock R. D., Brooks P. M., Dick W. C. Serum immunoreactive gastrin: specificity for rheumatoid arthritis, bimodality of distribution, and failure of effect of anti-inflammatory drugs. Ann Rheum Dis. 1976 Feb;35(1):40–45. doi: 10.1136/ard.35.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowden D. R., Taylor I. L., Richter J. A., Pinals R. S., Levine R. A. Is hypergastrinaemia associated with rheumatoid arthritis? Gut. 1978 Nov;19(11):1064–1067. doi: 10.1136/gut.19.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Wallensten K., Uvnäs B. Release of gastrin on stimulation of the sciatic and brachial nerves of the cat. Acta Physiol Scand. 1978 Jul;103(3):349–351. doi: 10.1111/j.1748-1716.1978.tb06226.x. [DOI] [PubMed] [Google Scholar]
- Winfield J., Cooke D., Brook A. S., Corbett M. A prospective study of the radiological changes in the cervical spine in early rheumatoid disease. Ann Rheum Dis. 1981 Apr;40(2):109–114. doi: 10.1136/ard.40.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


