Background:
This study aimed to evaluate the clinical efficacy of minimally invasive puncture and drainage (MIPD) versus trepanation and drainage in the treatment of chronic subdural hematoma (CSDH).
Methods:
PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and Wanfang database were searched for studies on the treatment of CSDH by MIPD and trepanation and drainage. By reading the title, abstract and full text, and screening according to the inclusion and exclusion criteria, the qualified articles were confirmed. Subsequently, the literature quality was evaluated based on the Cochrane Handbook for Systematic Reviews of Interventions, and the data of the research subjects and the primary outcome measures were extracted for meta-analysis with RevMan 5.1 software.
Results:
Ten articles were included, with a total of 1000 patients. According to the meta-analysis, the 2 groups showed no statistical difference in CSDH recurrence rate (P > .05). The operation time, intraoperative blood loss, and incidence of postoperative adverse reactions were lower and the cure rate was higher in the MIPD group compared with trepanation and drainage group (all P < .05). By drawing the funnel plot of the outcome measures with heterogeneity, it can be seen that the distribution on both sides of the funnel was basically symmetrical, suggesting a low deviation possibility of the analysis results and reliable reference significance of our findings.
Conclusion:
Compared with trepanation and drainage, MIPD has better clinical effects and higher safety in treating CSDH and can effectively reduce surgery-induced damage, which is worth popularizing in clinical practice.
Keywords: chronic subdural hematoma, clinical efficacy, meta-analysis, minimally invasive puncture and drainage, trepanation and drainage
1. Introduction
Chronic subdural hematoma (CSDH) refers to intracranial hemorrhage occurring in the subdural space more than 3 weeks after injury. Due to the enlargement of the hematoma, significant space-occupying effects will be produced, leading to the compression of the ventricles and brainstem and resulting in clinical manifestations such as vomiting, disturbance of consciousness, headache, increased intracranial pressure, and even acute cerebral infarction in severe cases.[1,2] Accounting for about one-tenth of cases with intracranial hematoma, CSDH often occurs on the convex surface of the frontotemporal hemisphere, with the accumulated blood volume as much as 100 to 300 mL.[3] Therefore, for CSDH, computed tomography (CT) diagnosis and surgical intervention should be carried out in time to ensure the prognosis and recovery of patients.[4] According to statistics, the current incidence of CSDH is about 13.5/100,000, but it can reach 50 to 70 per 100,000 among the elderly over 65 years old.[5] At present, the pathogenesis of CSDH is not thoroughly elucidated, and it is clinically believed to be associated with brain atrophy, increased venous tone, and hypercoagulability.[5]
Trepanation and drainage are the most commonly used surgical method for CSDH. Despite relatively ideal surgical effects, this procedure is limited by complicated surgical procedures and large trauma.[6] In addition, this operation comes with a high incidence of postoperative complications, which may not only affect patients’ postoperative rehabilitation but also cause secondary injuries.[7] The rapid development of minimally invasive technology in neurosurgery in recent years has driven the emergence of minimally invasive puncture and drainage (MIPD) for the treatment of CSDH.[8] This procedure is mainly to select a reasonable puncture point based on the results of skull CT examination and perform surgical drainage on the hematoma site after quickly penetrating the skull and dura mater.[9] Although there have been many studies comparing the effect of trepanation and drainage versus MIPD, the experimental results are still controversial. For example, some studies believe that MIPD greatly simplifies the surgical process compared with trepanation and drainage, which has a very high application value in the treatment of CSDH.[10] But evidence has also revealed unsatisfactory efficacy of MIPD for some complicated hematomas, with the possibility of prognostic recurrence.[11]
Faced with this situation, this paper will use the principles and methods of evidence-based medicine to conduct a meta-analysis of the effects of trepanation and drainage versus MIPD in the treatment of CSDH, aiming to provide a reliable reference for future clinical trials when choosing CSDH treatment procedures, thus providing a more reliable guarantee for patients’ prognosis and rehabilitation.
2. Materials and methods
2.1. Literature search
We searched relevant literature in the PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and Wanfang database, with the keywords set as “Chronic subdural hematoma,” “Minimally Invasive,” “minimally invasive puncture and drainage” and “Trepanation and drainage,” and the search period set from the construction of the database to November 11, 2022.
2.2. Literature selection criteria
Inclusion criteria: studies with CSDH patients as the research participants; comparative analysis of the efficacy of MIPD versus trepanation and drainage in the treatment of CSDHs. Exclusion criteria: unpublished literature; literature that cannot view the full text; literature with obvious logical errors or design defects; literature with a lack of measurement criteria for objective evaluation indicators; literature in which the author declares that there is a conflict of interest.
2.3. Literature quality evaluation
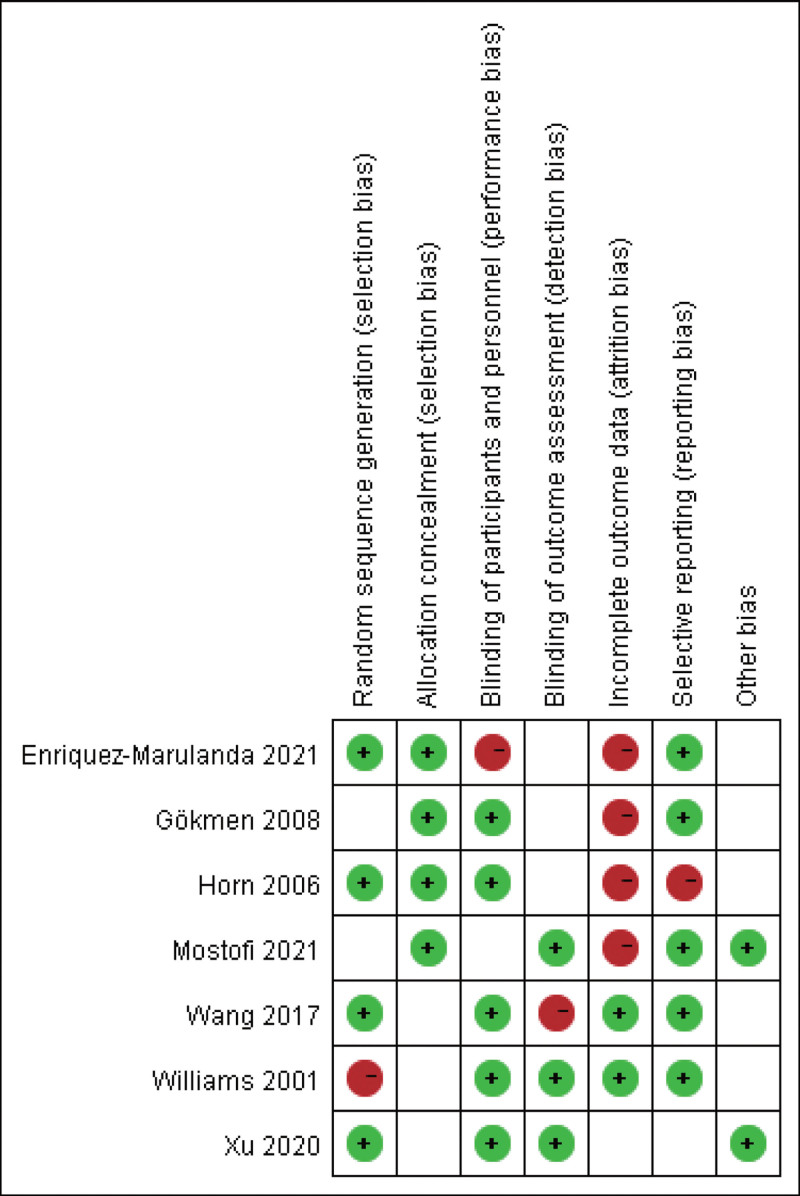
The included articles were evaluated by 2 reviewers independently by referring to the bias risk evaluation standard of the Cochrane Handbook for Systematic Reviews of Interventions, with the specific evaluation contents as follows: generation of randomization; concealment of allocation; blinding; no selective outcome reporting; other sources of biases. The literature quality was rated as Grade A (all the above indicators are clearly indicated), B (some of the above indicators are clearly indicated), or C (none of the above indicators are clearly stated). Any disagreements or discrepancies were addressed by consulting a third reviewer.
2.4. Data extraction
After reading the full text, 2 evaluators independently completed the data extraction, which mainly included the selection criteria, sample size, grouping method and process, basic data of research subjects, research conditions, intervention measures, outcome measures, follow-up time, and statistical methods. When multi-outcome research was involved, the measures related to this paper were extracted, and the indicators with different measurement units were converted before statistical analysis. When there were differences in the extraction results between the 2 reviewers, the opinion of the third reviewer was sought.
2.5. Outcome indicators
Operation time (the time from entering the operating room to leaving the operating room after completing the operation). Intraoperative blood loss (the amount of bleeding during surgery). Cure rate: according to the CSDH rehabilitation standard,[12] a cure referred to basically disappeared symptoms such as obnubilation, memory loss, and unresponsiveness, with postoperative CT results indicating completely removed hematoma without residue; the cure rate = the number of cured people/total number of people × 100%. Safety: adverse reactions (or complications) from operation to discharge were recorded. Recurrence rate: CSDH recurrence during postoperative follow-up was recorded.
2.6. Statistics analysis
Meta-analysis was carried out with RevMan 5.1 software. Heterogeneity analysis was carried out, with I2 ≤ 50% and I2 > 50% indicating the absence and presence of heterogeneity, respectively, in which case, the fixed-effects model and the random-effects model were adopted for analysis, respectively. Measurement data were analyzed by mean difference and enumeration data by odds ratio. A funnel plot was drawn to evaluate the publication bias, and the significance level was set as P < .05.
2.7. Ethics statement
Ethical approval was not required for this study, because this study is a meta-analysis.
3. Results
3.1. Study characteristics
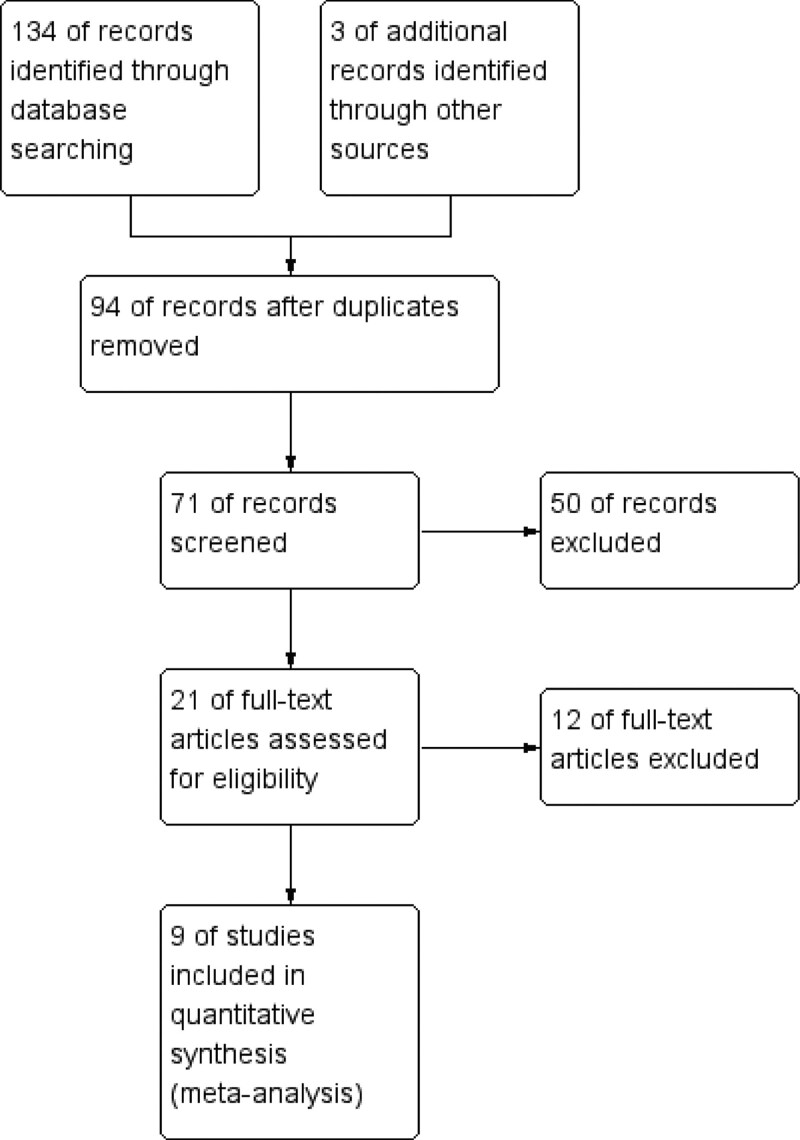
A total of 134 related articles were retrieved in the initial search. After reading the title, abstract, and full text and screening according to the inclusion and exclusion criteria, 7 articles[13–19] meeting the criteria were finally included, as shown in the literature screening flowchart Figure 1. The basic characteristics and quality grades of the literature are shown in Table 1 and Figure 2. A total of 1922 cases were included in these studies, of which 1171 cases treated by trepanation and drainage were regarded as the control group (CG), and another 751 cases treated by MIPD were regarded as the observation group (OG).
Figure 1.
Literature retrieval and screening process.
Table 1.
Basic characteristics of the included literature.
| Author | Year | Control group | Observation group | Outcome | ||
|---|---|---|---|---|---|---|
| n | Age | n | Age | |||
| Horn[13] | 2006 | 55 | 57.6 ± 5.9 | 24 | 59.1 ± 6.8 | ‡,§, |
| Mostofi[14] | 2021 | 555 | 62 ± 5 | 524 | 61 ± 7 | ‡,§ |
| Enriquez-Marulanda[15] | 2021 | 357 | Not specified | 45 | Not specified | ‡,§, |
| Gökmen[16] | 2008 | 38 | 50–70 | 32 | 53–69 | *,†,‡, |
| Wang[17] | 2017 | 38 | 59.14 ± 8.63 | 45 | 60.42 ± 6.12 | *,†,‡,§ |
| Xu[18] | 2020 | 91 | Not specified | 67 | Not specified | *,†, |
| Williams[19] | 2001 | 37 | 62–71 | 14 | 59–73 | *,†,‡, |
*Operation time. †Intraoperative blood loss; ‡Cure rate. §Safety. Recurrence rate.
Figure 2.
Literature quality rating.
3.2. Comparison of operation time
Five studies compared and analyzed operation time, with obvious heterogeneity (I2 > 50%) among the articles, so the random-effects model was used for analysis. As indicated by Figure 3, OG had a shorter operation time than CG (P < .05).
Figure 3.
Forest diagram of comparison of operation time.
3.3. Comparison of intraoperative blood loss
The intraoperative blood loss was compared and analyzed in 4 studies with heterogeneity among these articles (I2 > 50%). The results of the random-effects model analysis are presented in Figure 4, which shows less intraoperative blood loss in OG as compared to CG (P < .05).
Figure 4.
Forest diagram of comparison of intraoperative blood loss.
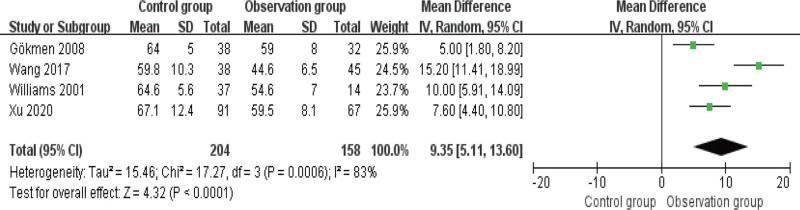
3.4. Comparison of cure rate
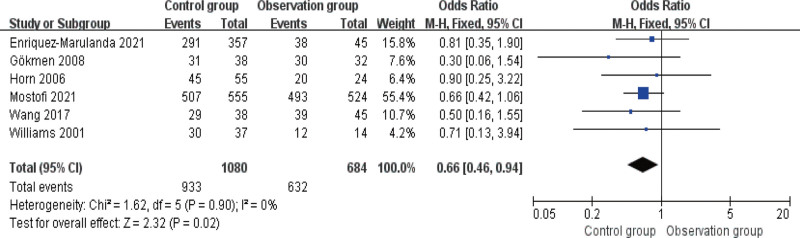
The cure rate was compared in 6 studies, and the fixed-effects model was adopted for analysis as there was no heterogeneity among these articles (I2 ≤ 50%). As indicated by Figure 5, the cure rate is higher in OG versus CG (P < .05).
Figure 5.
Forest diagram of comparison of cure rate.
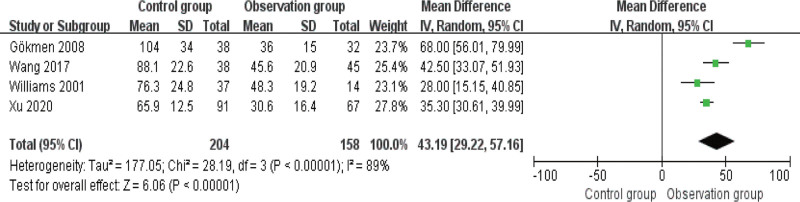
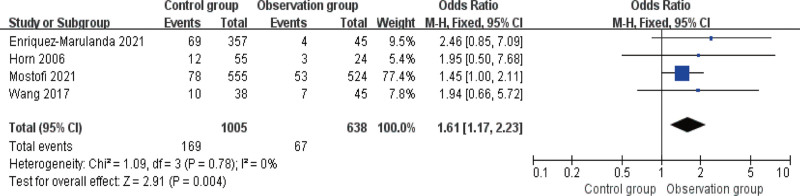
3.5. Comparison of safety
There was no heterogeneity among the 4 articles that reported the incidence of post-treatment adverse reactions, I2 ≤ 50%. As shown in Figure 6, there was a statistically significant difference in the incidence of adverse reactions (or complications) between the 2 groups, with a lower incidence in OG (P < .05).
Figure 6.
Forest diagram of comparison of safety.
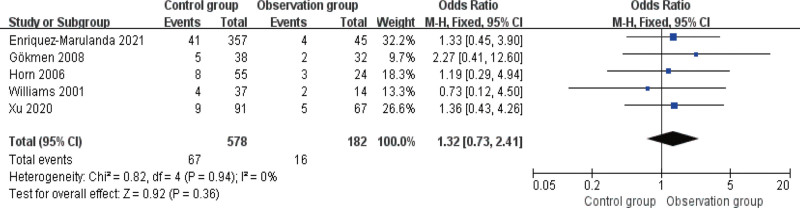
3.6. Comparison of recurrence rate
Five studies reported the post-treatment recurrence rate, with no heterogeneity among them (I2 > 50%). The results of the fixed-effects model analysis (Fig. 7) revealed no statistical inter-group difference in the recurrence rate (P > .05).
Figure 7.
Forest diagram of comparison of recurrence rate.
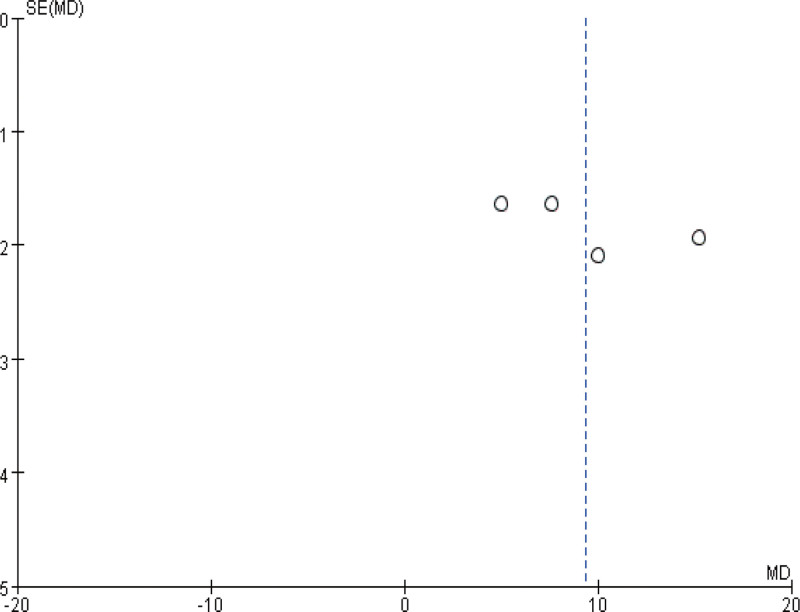
3.7. Publication bias
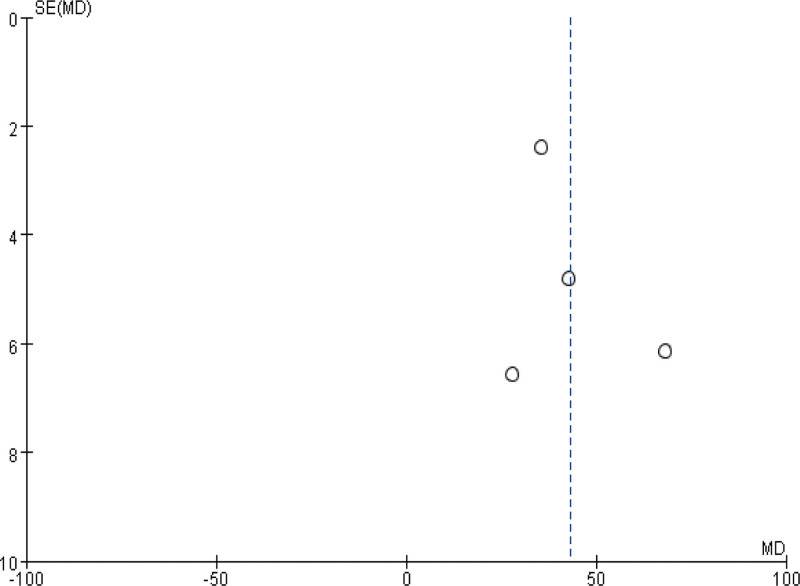
Finally, a funnel plot was plotted for heterogeneous outcomes (operation time and intraoperative blood loss) in the above observations to observe the publication bias. As shown in Figures 8 and 9, the distribution on both sides of the funnel plot is basically symmetrical, suggesting a small possibility of outcome bias and reliable reference significance of the findings.
Figure 8.
The funnel diagram of operation time.
Figure 9.
The funnel diagram of intraoperative blood loss.
4. Discussion
At present, the incidence of CSDH keeps in parallel with the aggravation of global aging.[20] Meanwhile, CSDH is one of the major neurosurgical diseases recognized to cause the death and disability of elderly patients, accounting for about 10% of intracranial hemorrhage, with over 20% of CSDH patients seriously disabled due to poor neurological function recovery.[21] Therefore, timely and accurate surgical treatment is the key to ensuring patients’ prognosis and recovery.[22] However, the efficacy of trepanation and drainage and MIPD, as 2 major surgical methods for CSDH, have been controversial. Therefore, this study conducts a meta-analysis of the therapeutic effects of the 2 surgical methods, aiming to provide a more reliable reference for clinical practice.
This meta-analysis compared the effects of MIPD and trepanation and drainage in the treatment of CSDH. The results showed that the operation time and intraoperative blood loss of CSDH patients under the treatment of MIPD were markedly reduced compared with those receiving trepanation and drainage, while the cure rate and safety were improved, indicating that MIPD is better in the treatment of CSDH, with less trauma to patients. This is also consistent with the results of most studies,[23–25] which can support our analysis results. It is pointed out that thorough irrigation and drainage is the key to CSDH surgery.[26] MIPD can carry out continuous and closed convection irrigation during and after operation, and completely remove old hematoceles and blood clots in a short time, achieving an effective cure rate.[27] Besides, the rigid puncture needle used in the MIPD allows for close contact of it with the scalp, bone hole, and dura hiatus, which can effectively prevent air from entering, avoid contact between gas and intracranial tissues and prevent complications such as tension pneumocranium and intracranial infection[28] Moreover, the skull defect caused by a hard puncture is only a few millimeters and usually heals completely on its own. The incision-free operation also avoids the risk of cerebrospinal fluid leakage and infection.[29] This is also one of the main reasons for the lower incidence of adverse reactions (complications) after MIPD. On the other hand, since CSDH surgery is usually performed under local anesthesia, patients can retain consciousness during the operation, and the shortening of operation time will also effectively reduce the psychological burden of patients and the postoperative rehabilitation cycle.[30] Furthermore, although MIPD has higher requirements for surgical operation, it has lower medical costs, which is more conducive to its clinical promotion and popularization.[31] And in the comparison of postoperative recurrence rate, we found no marked difference in the recurrence rate between the 2 procedures, suggesting that both trepanation and drainage and MIPD have a relatively stable effect on the treatment of CSDH. Of course, it may also be caused by the inconsistent follow-up time after operation. For example, the recurrence rate calculated by Xu et al[18] was the recurrence within 1 year after operation, while Horn et al[13] only carried out a follow-up investigation for 3 months. In view of this situation, we will carry out clinical trials as soon as possible for analysis and confirmation.
Of course, this research needs further improvement due to the limited conditions. For example, the allocation methods of most studies are not explained in detail, and there is a lack of multicenter, double-blind clinical randomized controlled trials. In addition, although this study is a comparative analysis of the 2 surgical procedures, the basic treatment is also an extremely important link in CSDH. However, the basic treatment plan of patients is not mentioned in some papers, which may also contribute to some degree of bias in results. Finally, surgical experience is also an important factor affecting the effectiveness of surgery. But due to the different medical levels, the included literature also has certain differences in surgical operation, which will inevitably affect the therapeutic effect. In this regard, we believe that a large-scale multicenter double-blind clinical randomized controlled study is warranted to compare the effects of MIPD versus trepanation and drainage in the treatment of CSDH, and to observe the short- and long-term prognosis of patients, so as to provide a better reference for clinical selection of CSDH treatment procedures.
5. Conclusion
Compared with trepanation and drainage, MIPD has better clinical effects and higher safety in treating CSDH and can effectively reduce surgery-induced damage, which is worth popularizing in clinical practice. In the future, we still need to carry out more clinical research on MIPD and trepanation and drainage in the treatment of CSDH, so as to provide a better reference for the clinical selection of treatment modalities.
Author contributions
Conceptualization: Guangfeng Li, Lele Du, Fuhua Yu.
Data curation: Guangfeng Li, Lele Du, Fuhua Yu.
Funding acquisition: Guangfeng Li, Fuhua Yu.
Investigation: Guangfeng Li, Lele Du.
Methodology: Lele Du, Fuhua Yu.
Resources: Guangfeng Li, Lele Du, Fuhua Yu.
Software: Guangfeng Li.
Validation: Guangfeng Li, Lele Du, Fuhua Yu.
Visualization: Lele Du.
Writing – original draft: Guangfeng Li.
Writing – review & editing: Fuhua Yu.
Abbreviations:
- CG
- control group
- CSDH
- chronic subdural hematoma
- CT
- computed tomography
- MIPD
- minimally invasive puncture and drainage
- OG
- observation group
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Li G, Du L, Yu F. Clinical efficacy of minimally invasive puncture and drainage versus trepanation and drainage for chronic subdural hematoma: Systematic review and meta-analysis. Medicine 2023;102:11(e32860).
Contributor Information
Guangfeng Li, Email: 36547817@qq.com.
Lele Du, Email: 14578914@qq.com.
References
- [1].Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020;141:339–45. [DOI] [PubMed] [Google Scholar]
- [2].Stubbs DJ, Vivian ME, Davies BM, et al. Incidence of chronic subdural haematoma: a single-centre exploration of the effects of an ageing population with a review of the literature. Acta Neurochir (Wien). 2021;163:2629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dinsmore J, Wiles MD. Surgical management of chronic subdural haematoma: looking beyond anaesthetic technique. Anaesthesia. 2022;77:519–22. [DOI] [PubMed] [Google Scholar]
- [4].Sherrod BA, Baker C, Gamboa N, et al. Preoperative MRI characteristics predict chronic subdural haematoma postoperative recurrence: a meta-analysis. Br J Neurosurg. 2021;35:527–31. [DOI] [PubMed] [Google Scholar]
- [5].Monserrate A, De Jesus O. Huge chronic subdural haematoma formation in an encephalomalacia stroke cavity. BMJ Case Rep. 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Link TW, Schwarz JT, Paine SM, et al. Middle meningeal artery embolization for recurrent chronic subdural hematoma: a case series. World Neurosurg. 2018;118:e570–4. [DOI] [PubMed] [Google Scholar]
- [7].Krieg SM, Aldinger F, Stoffel M, et al. Minimally invasive decompression of chronic subdural haematomas using hollow screws: efficacy and safety in a consecutive series of 320 cases. Acta Neurochir (Wien). 2012;154:699–705; discussion 705. [DOI] [PubMed] [Google Scholar]
- [8].Wan Y, Fei X, Jiang D, et al. Clinical observation of treatment of chronic subdural hematoma with novel double needle minimally invasive aspiration technology. J Craniofac Surg. 2017;28:646–9. [DOI] [PubMed] [Google Scholar]
- [9].Latini MF, Fiore CA, Romano LM, et al. [Minimally invasive treatment of chronic subdural hematoma in adults. results in 116 patients]. Neurologia. 2012;27:22–7. [DOI] [PubMed] [Google Scholar]
- [10].Umana GE, Salvati M, Fricia M, et al. A review of remote intracerebral hemorrhage after chronic subdural hematoma evacuation. J Neurol Surg A Cent Eur Neurosurg. 2022;83:368–76. [DOI] [PubMed] [Google Scholar]
- [11].Majovsky M, Masopust V, Netuka D, et al. Flexible endoscope-assisted evacuation of chronic subdural hematomas. Acta Neurochir (Wien). 2016;158:1987–92. [DOI] [PubMed] [Google Scholar]
- [12].Bounajem MT, Campbell RA, Denorme F, et al. Paradigms in chronic subdural hematoma pathophysiology: current treatments and new directions. J Trauma Acute Care Surg. 2021;91:e134–41. [DOI] [PubMed] [Google Scholar]
- [13].Horn EM, Feiz-Erfan I, Bristol RE, et al. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. 2006;65:150–3; discussion 153. [DOI] [PubMed] [Google Scholar]
- [14].Mostofi K, Peyravi M, Moghaddam BG. Minimally invasive surgical approach for treatment of chronic subdural hematoma, outcome in 1079 patients. Turk Neurosurg. 2021;31:18–23. [DOI] [PubMed] [Google Scholar]
- [15].Enriquez-Marulanda A, Gomez-Paz S, Salem MM, et al. Middle meningeal artery embolization versus conventional treatment of chronic subdural hematomas. Neurosurgery. 2021;89:486–95. [DOI] [PubMed] [Google Scholar]
- [16].Min X, Bo Y, Cunzu W, et al. Minimally invasive approach in bilateral chronic subdural hematomas: surgical selection and outcome in 74 cases. Turk Neurosurg. 2017;27:380–5. [DOI] [PubMed] [Google Scholar]
- [17].Wang K, Chen D, Cao X, et al. A prospective comparative study of twist drill craniostomy versus burr hole craniostomy in patients with chronic subdural hematoma. Turk Neurosurg. 2017;27:60–5. [DOI] [PubMed] [Google Scholar]
- [18].Xu M, Wang WH, Zhu SQ, et al. Effects of minimally invasive approaches on chronic subdural hematoma by novel YL-1 puncture needle and burr-hole methods. Acta Neurol Belg. 2020;120:37–42. [DOI] [PubMed] [Google Scholar]
- [19].Williams GR, Baskaya MK, Menendez J, et al. Burr-hole versus twist-drill drainage for the evacuation of chronic subdural haematoma: a comparison of clinical results. J Clin Neurosci. 2001;8:551–4. [DOI] [PubMed] [Google Scholar]
- [20].Rauhala M, Helen P, Huhtala H, et al. Chronic subdural hematoma-incidence, complications, and financial impact. Acta Neurochir (Wien). 2020;162:2033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stubbs DJ, Davies B, Hutchinson P, et al. Challenges and opportunities in the care of chronic subdural haematoma: perspectives from a multi-disciplinary working group on the need for change. Br J Neurosurg. 2022;36:600–8. [DOI] [PubMed] [Google Scholar]
- [22].Tamura R, Sato M, Yoshida K, et al. History and current progress of chronic subdural hematoma. J Neurol Sci. 2021;429:118066. [DOI] [PubMed] [Google Scholar]
- [23].Chari A, Kolias AG, Santarius T, et al. Twist-drill craniostomy with hollow screws for evacuation of chronic subdural hematoma. J Neurosurg. 2014;121:176–83. [DOI] [PubMed] [Google Scholar]
- [24].Waqas M, Vakhari K, Weimer PV, et al. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg. 2019;126:228–36. [DOI] [PubMed] [Google Scholar]
- [25].Haldrup M, Ketharanathan B, Debrabant B, et al. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir (Wien). 2020;162:777–84. [DOI] [PubMed] [Google Scholar]
- [26].Hess RM, OConnor TE, Khan A, et al. Minimally invasive approach to subdural hematoma treatment using IRRAflow catheter and middle meningeal artery embolization. Cureus. 2021;13:e13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang J, Liu X, Fan X, et al. The use of endoscopic-assisted burr-hole craniostomy for septated chronic subdural haematoma: a retrospective cohort comparison study. Brain Res. 2018;1678:245–53. [DOI] [PubMed] [Google Scholar]
- [28].Omura T, Fukushima Y, Yoshikawa G, et al. Cerebral hyperperfusion syndrome after a burr hole drainage surgery for chronic subdural hematoma. World Neurosurg. 2019. [DOI] [PubMed] [Google Scholar]
- [29].Eun J, Ahn JG. Selection of an appropriate surgical method for the management of chronic subdural hematoma in a patient with poor physical status. Asian J Neurosurg. 2021;16:164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kolias AG, Sinha R, Park H, et al. Surgical management of chronic subdural hematomas: in need of better evidence. Acta Neurochir (Wien). 2013;155:183–4. [DOI] [PubMed] [Google Scholar]
- [31].Opsenak R, Fejercak T, Hanko M, et al. Is there an impact of subdural drainage duration and the number of burr holes on the recurrence rate of unilateral chronic subdural haematoma? Rozhl Chir. 2020;99:29–33. [DOI] [PubMed] [Google Scholar]