Abstract
1,1,2,2-tetrafluoro-2-[1,1,1,2,3,3-hexafluoro-3-(1,1,2,2-tetrafluoroethoxy)propan-2-yl]oxyethane-1-sulfonic acid (PFESA-BP2) was first detected in 2012 in the Cape Fear River downstream of an industrial manufacturing facility. It was later detected in the finished drinking water of municipalities using the Cape Fear River for their water supply. No toxicology data exists for this contaminant despite known human exposure. To address this data gap, mice were dosed with PFESA-BP2 at 0, 0.04, 0.4, 3, and 6 mg/kg-day for 7 days by oral gavage. PFESA-BP2 was detected in the sera and liver of all treated mice. Treatment with PFESA-BP2 significantly increased the size of the liver for all mice at 3 and 6 mg/kg-day. At the 6 mg/kg-day dose, the liver more than doubled in size compared to the control group. Male mice treated with 3 and 6 mg/kg-day and females treated with 6 mg/kg-day demonstrated significantly elevated serum markers of liver injury. The percent of PFESA-BP2 in serum relative to the amount administered was similar in male and female mice, ranged from 9 to 13%, and did not demonstrate a dose dependence. The percent accumulation in the liver of the mice varied by sex, ranged from 30 to 65%, and correlated positively with increasing dose level.
1. Introduction
Perfluoroalkyl substances (PFASs) have been detected in the global environment, including points far from sites of production and/or use.1 The unique stability of the carbon-fluorine bond results in PFASs having exceedingly long environmental half-lives.2 Concerns about PFASs have resulted in establishment of national and state regulations for some PFASs and voluntary advisory levels for others.3 Public concerns and regulatory guidelines have focused on a small number of PFASs. Although there are currently thousands of compounds categorized as PFASs 4, there have been only approximately 1223 PFAS historically registered in commerce in the US, with 602 actively in commerce today. 5
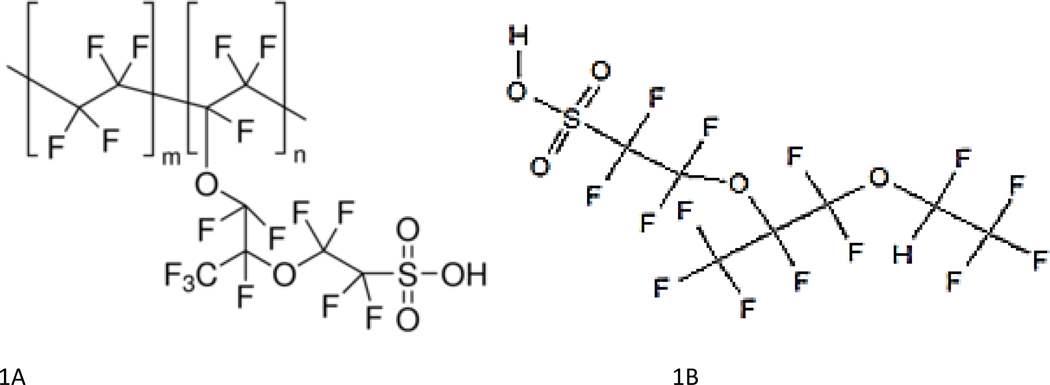
1,1,2,2-tetrafluoro-2-[1,1,1,2,3,3-hexafluoro-3-(1,1,2,2-tetrafluoroethoxy)propan-2-yl]oxyethane-1-sulfonic acid (PFESA-BP2 CAS #749836–20-2) is known to exist as a by-product of manufacturing Nafion polymer (Figure 1). PFESA-BP2 has not been the subject of a pre-manufacture notice and review under the US Toxic Substance Control Act, which is required only for chemicals intended for a commercial purpose. PFESA-BP2 is a 7-carbon sulfonate with an isotopic mass of 463.93 amu and with two internal ether oxygens, giving it a mass and general structure (length) that is similar to perfluorooctanoic sulfonic acid (PFOS - 498.93 amu). These similarities could be used to infer a longer half-life and possibly similar toxicity. Because the compound is a by-product of Nafion, a Dupont sulfonated tetrafluoroethylene-based polymer, it also has been referred to as Nafion by-product 2.
Figure 1.
Structure of the Nafion Polymer (A) and PFESA-BP2 (B)
Traditional wastewater treatment processes, including those used by the North Carolina fluorochemical manufacturer, Chemours, are not effective in removing PFAS. 6, 7 The lack of sufficient treatment has resulted in the discharge of many different PFASs into water sources, including the discharge PFAS by-products into the Cape Fear River. 8
In 2012, two PFESA byproducts (i.e. PFESA-BP2 and perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid (PFESA-BP1 CAS #29311–67-9)) were detected in North Carolina’s Cape Fear River, downstream of an industrial manufacturing facility. 8 In a September 2017 publicly available report to the North Carolina Department of Environmental Quality, the United States Environmental Protection Agency (USEPA) used a non-targeted analytical method to estimate PFESA-BP2 concentrations in Chemours discharge effluent and the Cape Fear River downstream of manufacturing as 45,200 ng/L and 2,075 ng/L, respectively. 9 These reported PFESA-BP2 concentrations were provided as gross estimates because a PFESA-BP2 standard was unavailable at that time. As such, these concentrations assume that the mass spectrometer responded to the non-targeted analyte as if it were GenX [2,3,3,3-Tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)-propanoic acid, PFPrOPrA, CAS #13252-13-6], for which a standard was available. The report suggests such estimates are accurate to within 10-fold of the estimated value.
In July 2017, North Carolina’s Brunswick County drinking water provider (H2Go) began bi-weekly sampling for PFESA-BP2, with concentration estimates ranging from non-detectable (ND) to 134 ng/L in their finished drinking water. 10 North Carolina’s Department of Environmental Quality (NCDEQ) reported PFESA-BP2 in private wells near the industrial manufacturing facility with concentrations up to 125 ng/L. 11 With the availability of an authentic standard provided by the manufacturer, subsequent studies corroborated PFESA-BP2 contamination in finished drinking water 12, but also in 99% of serum samples from public volunteers from this same region.13 The study demonstrated the presence of PFESA-BP2 is likely isolated to the area downstream of the NC industrial manufacturing facility because serum samples from residents of Raleigh, NC, Chapel Hill, NC, Durham, NC and Dayton, Ohio did not demonstrate the presence of this compound. These studies demonstrate the presence of PFESA-BP2 contamination in water sources within the Cape Fear River Basin, as well as the widespread presence of this compound in human serum samples from this same region.
Despite the known presence of PFESA-BP2 in the environment and in human blood, there are no known toxicology studies utilizing PFESA-BP2. Previous studies on perfluorooctanoic acid (PFOA) and PFOS have demonstrated that these compounds bioaccumulate in the liver and serum of affected animals (rat, mouse, rabbit, monkey), and induce liver toxicity. 14 15
Given the potential health effects associated with PFAS compounds and the presence of PFESA-BP2 in human serum, this study examined the hepatotoxic effects and bioaccumulation of PFESA-BP2 in adult mice exposed by oral gavage for seven days (0.04 – 6 mg/kg-day).
2. Methods
2.1. Animals
Balb-c mice, 10–12-week-old males and females, were obtained from Charles River Laboratories (Raleigh, NC, USA). The animals arrived at the National Health and Environmental Effects Research Laboratory (NHEERL) animal facility post-weaning and allowed to acclimate for at least 5 days prior to initiation of the experiments. Animals were randomly selected and housed by treatment group in polycarbonate cages on heat-treated pine shaving bedding in animal rooms with a controlled temperature range (22–26°C) and a 12:12-h light–dark cycle. Animals were fed commercial rodent chow (Purina Prolab) and water ad libitum. All studies were conducted after approval by the Institutional Animal Care and Use Committee (IACUC) using recommendations of the 2011 National Research Council (NRC) “Guide for the Care and Use of Laboratory Animals” and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals.16
2.2. Experimental Design
Animals were dosed with PFESA-BP2 for seven consecutive days by gavage using 20-gauge stainless steel feeding needles. Doses of 0, 0.04, 0.4, 3, and 6 mg/kg-day were administered once daily in the afternoon. The number of animals ranged from 10 to 24 per dose group, divided equally between males and females (Table 1). PFESA-BP2 was obtained from Chemours (78.8% purity - 14% potassium fluoride (KF) – 6.6% (1,1,2,2-Tetrafluoro-2-(1,2,2,2-tetrafluoroethoxy)ethanesulfonic acid (NVHOS CAS #801209–99-4)). A stock dosing solution was prepared by dissolving PFESA-BP2 in ethanol (EtOH) followed by dilution with deionized (DI) water for a final concentration of 1 g/L in 90:10 DI H2O:EtOH. The stock solution was diluted with DI water to establish dosing solution concentrations for each treatment at a dosing volume of 0.2 mL per day. The final PFESA-BP2 concentration in the dosing solutions ranged from 0.002 to 0.8 g/L (data not shown). The control group received the carrier of Picopure water with an ethanol concentration equal to the dosing solution with the highest ethanol concentration which was always the high dose males (not to exceed 7.15% ethanol).
Table 1.
Effects of PFESA-BP2 on average body weights, liver weights, clinical serum chemistry, and serum/liver PFESA-BP2 concentrations in Balb-c mice after 7 days of treatment. The sample sizes for the data presented here, which varied by dose group and variable, are demonstrated in Table S1.
| Males | |||||
|---|---|---|---|---|---|
| 0 | 0.04 mg/kg-day | 0.4 mg/kg-day | 3 mg/kg-day | 6 mg/kg-day | |
| Number of Mice Dosed | 12 | 10 | 15 | 10 | 5 |
| Body Weight (g) | 22.9±0.37 | 23.2±0.55 | 23.6±0.42 | 23.2±0.47 | 23.9±0.69 |
| Liver Weight (g) | 1.29±0.03 | 1.33±0.05 | 1.37±0.04 | 2.02±0.04 *** | 2.79±0.06 *** |
| Liver/Body Weight (%) | 5.62±0.08 | 5.75±0.12 | 5.83±0.09 | 8.70±0.10 *** | 11.7±0.15 *** |
| Number of Mice with Visual Liver Reticulation | 0 | 1 | 2 | 10 | 5 |
| ALT (log 10 U/L) | 1.81±0.06 | 1.79±0.09 | 1.72±0.07 | 2.04±0.08 | 2.34±0.11 *** |
| AST (log 10 U/L) | 2.08±0.07 | 2.07±0.10 | 1.94±0.08 | 2.15±0.09 | 2.24±0.13 |
| GLDH | 1.15±0.04 | 1.19±0.06 | 1.05±0.05 | 1.35±0.05 * | 1.71±0.08 *** |
| BUN (mg/dl) | 9.07±0.35 | 9.11±0.48 | 9.08±0.44 | 9.09±0.41 | 9.26±0.62 |
| Albumin (g/dl) | 3.30±0.08 | 3.32±0.11 | 3.44±0.09 | 3.56±0.10 | 3.59±0.14 |
| Globulin (g/dl) | 2.07±0.05 | 2.09±0.07 | 2.16±0.06 | 2.25±0.06 | 2.35±0.09 * |
| Total Protein (g/dl) | 5.37±0.11 | 5.42±0.17 | 5.59±0.13 | 5.81±0.14 | 5.93±0.21 |
| Glucose (mg/dl) | 201±9.52 | 194±13.9 | 192±10.8 | 200±12.0 | 159±17.57 |
| Tbil (mg/dl) | 0.41±0.07 | 0.31±0.10 | 0.30±0.08 | 0.35±0.09 | 0.30±0.12 |
| Triglycerides (mg/dl) | 319±32.6 | 325±33.0 | 328±29.5 | 341±23.8 | 374±31.9 |
| Cholesterol (mg/dl) | 128±9.05 | 122±9.75 | 129±8.70 | 140±7.00 | 122±9.43 |
|
| |||||
| Females | |||||
| 0 | 0.04 mg/kg-day | 0.4 mg/kg-day | 3 mg/kg-day | 6 mg/kg-day | |
|
| |||||
| Number of Mice Dosed | 12 | 10 | 15 | 10 | 6 |
| Body Weight (g) | 18.9±0.23 | 18.7±0.34 | 19.0±0.27 | 19.5±0.29 | 20.0±0.43 * |
| Liver Weight (g) | 0.98±0.03 | 0.94±0.04 | 0.96±0.03 | 1.67±0.03 *** | 2.38±0.05 *** |
| Liver/Body Weight (%) | 5.20±0.13 | 5.08±0.19 | 5.09±0.15 | 8.29±0.16 *** | 11.5±0.24 *** |
| Number of Mice with Visual Liver Reticulation | 0 | 3 | 1 | 7 | 5 |
| ALT (log 10 U/L) | 1.92±0.08 | 1.85±0.12 | 1.88±0.09 | 2.01±0.10 | 2.49±0.15 ** |
| AST (log 10 U/L) | 2.26±0.07 | 2.22±0.10 | 2.28±0.08 | 2.13±0.08 | 2.54±0.12 |
| GLDH | 1.40±0.07 | 1.31±0.10 | 1.23±0.08 | 1.35±0.08 | 1.75±0.12 * |
| BUN (mg/dl) | 8.31±0.42 | 8.11±0.53 | 8.30±0.42 | 9.07±0.46 | 8.92±0.69 |
| Albumin (g/dl) | 3.33±0.09 | 3.26±0.14 | 3.35±0.11 | 3.53±0.12 | 3.43±0.17 |
| Globulin (g/dl) | 1.79±0.06 | 1.73±0.09 | 1.84±0.07 | 2.09±0.08 * | 2.08±0.12 |
| Total Protein (g/dl) | 5.12±0.15 | 4.98±0.22 | 5.17±0.18 | 5.62±0.19 | 5.52±0.28 |
| Glucose (mg/dl) | 224±12.1 | 227±17.7 | 213±14.1 | 212±15.2 | 235±22.4 |
| Tbil (mg/dl) | 0.24±0.06 | 0.32±0.09 | 0.45±0.07 | 0.31±0.08 | 0.35±0.11 |
| Triglycerides (mg/dl) | 191±39.5 | 185±33.8 | 200±25.5 | 324±25.0 * | 280±36.1 |
| Cholesterol (mg/dl) | 113±23.8 | 114±23.5 | 134±18.2 | 99.6±17.8 | 112±25.7 |
The statistics for this table are based use F-test p-value from ANOVA; Averages demonstrated for each group with standard error
p ≤ 0.05
p ≤ 0.001
p ≤0.0001 relative to control
Approximately 24 hours after the seven-day dosing was completed, all animals were anesthetized by CO2 inhalation, weighed, euthanized by exsanguination, and necropsied. Animals were weighed during the exposure period and at the time of necropsy. Blood was obtained transdermally from the heart with a 25-gauge 5/8 in needle attached to a 1 mL syringe. Whole blood was collected in 0.5 mL serum separator tubes, allowed to clot at room temperature, centrifuged at 13000 rpm for 1.5 min, and serum isolated. Serum samples were stored at −20°C in 2.5 mL high density polyethylene (HDPE) tubes until analysis. The liver was removed from each animal, weighed, and divided into samples. One portion of the liver was stored in foil at −20°C for PFESA-BP2 analysis, another portion was fixed in 10% neutral buffered formalin for 48 hrs before being transferred to 70% ethanol for histopathology, and a third portion was placed in RNAlater and stored at −20°C for PCR analysis at a later time.
2.3. Histopathology
Samples of liver from one male and one female mouse from the control, 0.4 mg/kg-day and 3 mg/kg-day treatment group were viewed microscopically to study the appearance of the cells by Pathogenesis LLC. Livers from the 0.04 mg/kg-day and 6 mg/kg-day group were not analyzed with histopathology. Each block was sectioned at 5 microns and stained with hematoxylin and eosin according to a previously published methodology.17 Tissue sections were evaluated microscopically without the evaluator having prior knowledge of the treatment group. Histologic features were scored using a semi-quantitative scoring scheme with 0 = no change to 4 = severe change.17 Numbers of individual apoptotic hepatocytes (consistent with apoptosis) and mitotic figures were counted in each of ten 400X fields centered on a central vein.
For computer-aided image analysis of Zone 3 (centrilobular) hepatocytes, multiple photomicrographs at 1000X magnification were collected from at least 5 randomly selected hepatic lobules per mouse. The area of 30 individual hepatocytes from Rappaport Zone 3 of each liver was calculated using the lasso tool in Photoshop (lasso to outline individual hepatocytes > Image > Analysis> Record Measurement), Adobe Photoshop CC 2017.
2.4. Clinical Chemistry
All serum clinical chemistry analyses were carried out using the Randox Daytona Plus instrument (Belfast, Northern Ireland). Hepatic cell and bile duct injury was assessed by determining the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamate dehydrogenase (GLDH), and bilirubin. Markers for potential renal injury included serum concentrations of blood urea nitrogen (BUN) and creatinine. Serum glucose, total protein, and albumin were measured as markers of general toxicity. All assays were performed using reagents obtained from the instrument manufacturer.
2.5. Extraction and Analysis of PFESA-BP2 from Tissue and Serum
PFESA-BP2 was extracted from serum and tissue samples using methods presented in.18 In brief, liver samples were weighed in 15 mL HDPE centrifuge tubes and homogenized at approximately a 3:1 DI:sample wet weight ratio using an Omni-Prep Multi Sample Homogenizer. Liver homogenate and serum samples from mice treated with PFESA-BP2 were diluted at variable ratios with DI water to bring the concentrations within the values of the external calibration curve. Serum and liver homogenates from control mice were analyzed directly. The diluted samples (50 μL) were pipetted into a fresh 15 mL HDPE centrifuge tube, followed by 100 μL of 0.1 M formic acid. After vortex-mixing, 0.5 mL of cold acetonitrile (ACN) was added to each tube. Samples were vortex-mixed again and then centrifuged at 1000 rpm for 3 minutes. The supernatant (100 μL) was combined with 300 μL of 2.5mM ammonium acetate in HDPE vials. Approximately 10% of the samples were extracted in duplicate.
Samples were analyzed using an Agilent 1100 series HPLC equipped with an Eclipse Plus C8 column (2.1 × 50 mm, 3.5 μm; Agilent) interfaced to an Agilent 6210 series Accurate-Mass MS-TOF system with negative electrospray ionization (ESI). The mobile phase system consisted of 0.4 mM ammonium formate in 95:5 deionized water:methanol (A) and 95:5 methanol:deionized water (B). Quantification of PFESA-BP2 was based on comparison of a single ion peak area in negative mode 462.9326 [M-H]- to the response of an external standard curve created by spiking variable levels of standard into control liver homogenate or serum. The standard used for quantification was provided by the manufacturer as an 1% aqueous solution. Analytical blanks (i.e. ACN and Pico-pure water) were analyzed with every run. When appropriate, isotopically labeled (C13) PFOA purchased from Wellington Laboratories Inc. was used as the internal standard for quantification of the liver and serum concentrations.
2.6. Statistical Evaluation
All variables were analyzed separately by sex with two-way main effects ANOVAs, testing for changes due to PFESA-BP2 treatment, after allowing a separate intercept for each block. Each treatment group was compared to vehicle controls with pairwise t-tests, using Dunnett’s adjustment for multiple comparisons. The ANOVA assumptions of normality and homogeneity of variance were examined using the Shapiro-Wilk and Levene’s tests. ALT, AST, GLDH were analyzed on the log10 scale.
Results and Discussion
2.7. Toxicity
No changes in the animals’ appearance or overt behavior were observed during the dosing period. There were no changes in body weights during dosing except for in the 6 mg/kg-day female group where the body weight increased significantly relative to the initial weight and relative to the weight change in the controls (Table 1). The relative and absolute liver weights increased significantly in the 0.4 mg/kg-day and higher dose groups for the males and in the 3 mg/kg-day and higher dose groups for the females (Table 1). At the 6 mg/kg-day dose level, the livers doubled in size compared to the control group for both males and females (Table 1). At necropsy, livers of 3 and 6 mg/kg-day mice were enlarged and pale, and the surfaces were reticulated (i.e. pattern of individual liver lobules made visible due to color change of hepatocytes). The control group contained no animals with reticulated livers (Table 1).
Samples from the control, 0.4 mg/kg-day and 3 mg/kg-day treatment group were viewed microscopically to study the appearance of the cells. Livers from the 0.04 mg/kg-day and 6mg/kg-day dose group were not analyzed with histopathology. Histopathology revealed hepatocyte hypertrophy predominantly in the centrilobular portion of the liver lobule (Rappaport Zone 3) for the 3 mg/kg-day dose group (Figure 2). The hypertrophy extended to a lesser degree into Zone 2. Mitotic figures were another change observed in the 3 mg/kg-day livers and may indicate a response by the liver not seen in the control and 0.4 mg/kg-day mice. Intracytoplasmic vacuoles were present in all treatment groups and were recorded as fine to moderately large, sharp-edged, clear vacuoles consistent with lipid accumulation or as vacuoles with less distinct borders consistent with glycogen accumulation. The vacuoles consistent with glycogen accumulation did not vary between zones of the liver lobule or treatment group, whereas the vacuoles consistent with lipid accumulation were observed in Zones 2 and 3 and had slightly increased numbers in the 3 mg/kg-day livers compared to the control group. When hypertrophy is present, it is common to develop initially around the central vein and spread outward as seen in the 3 mg/kg-day mice. Larger group numbers would need to be evaluated to determine if cell death and intracytoplasmic vacuoles are significant in the higher dose.
Figure 2.
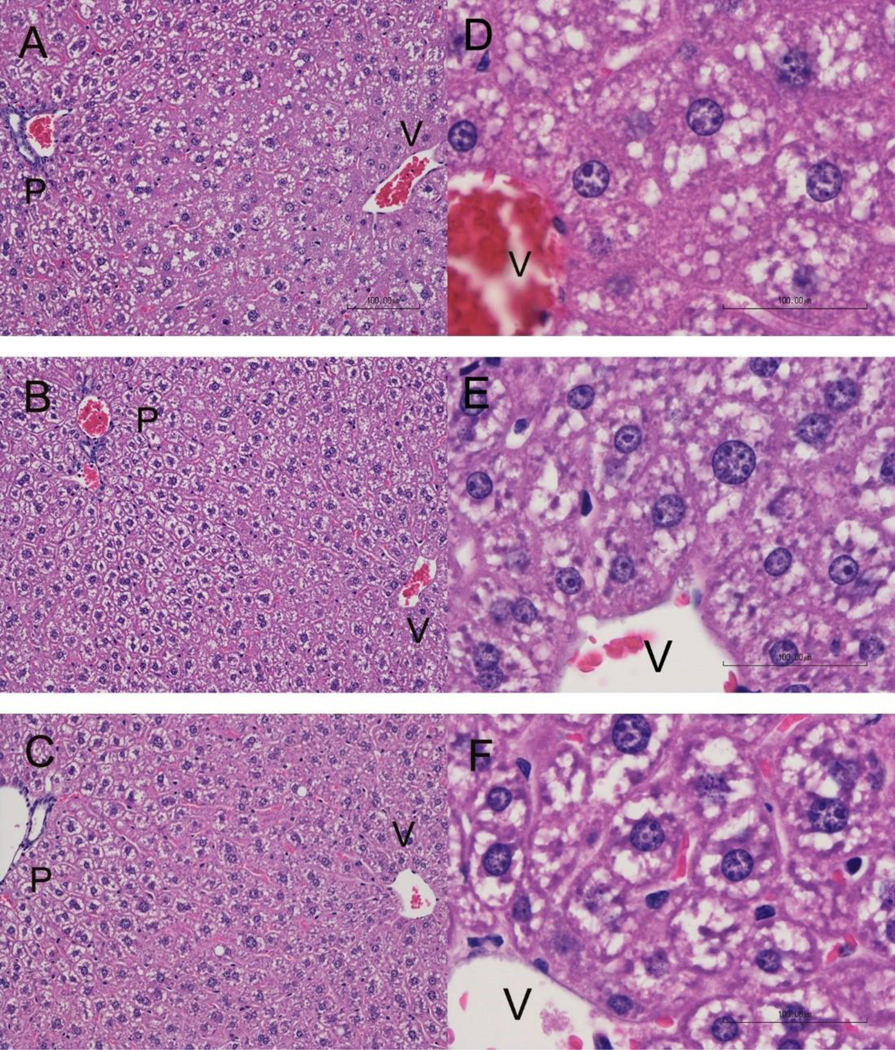
Liver histopathology for Balb-c mice receiving PFESA-BP2 at 3 mg/kg-day (A, D), 0.4 mg/kg-day (B, E), or vehicle (C, F). Livers from the 3 mg/kg-day dose group demonstrated increased cytoplasmic volume and density of cytoplasmic contents of centrilobular hepatocytes surrounding the central vein (V) compared with hepatocytes closer to the portal region (P), a change which was not observed in liver from the lower concentration of PFESA-BP2 or vehicle mice. Slides A, B, and C are at 100x magnification; slides D, E, and F are at 400x magnification.
Serum liver function markers indicative of hepatotoxicity were detected in both sexes within the 3 and 6 mg/kg-day treatment groups. Elevated ALT concentrations occurred in the 6 mg/kg/day treatment for both sexes and in the 3 mg/kg/day male dose group (Table 1). Increased GLDH was seen in both 3 and 6 mg/kg-day males and the 6 mg/kg-day females. Elevated serum protein levels occurred in males with significant increases in both globulin and total proteins at the 3 and 6 dose levels. For females, only the globulin levels were increased for both the 3 and 6 mg/kg-day dose levels.
2.8. PFESA-BP2 Bioaccumulation
All analytical blanks were negative for PFESA-BP2. The coefficient of determination (R2) was greater than 0.98 for all standard curves. Average PFESA-BP2 serum concentrations ranged from 0.47 μg/mL in the 0.04 mg/kg-day dose group to 88 μg/mL in the 6 mg/kg-day dose group (Table 2). The average serum concentration at the lowest dose level was between 100 and 200-fold higher than the average PFESA-BP2 concentrations reported in serum from the residents of Wilmington, NC 13. It is notable that bioaccumulation did occur with the presence of two internal ether oxygens, suggesting molecular length (and mass) increase retention in biological systems. The average PFESA-BP2 liver concentrations ranged from 1.4 μg/g in the 0.04 mg/kg-day female mice to 240 μg/g in the 6 mg/kg-day male mice (Table 2). The concentrations of PFESA-BP2 in the serum and liver are in the range of previously reported mouse serum PFOA/PFOS concentrations14, 19–21 (Lau et al. 2006, Thibodeaux et al., 2003, Wolf et al., 2008, Guo et al. 2019). For example, samples collected from WT mice dosed with PFOA at 3 mg/kg-day for seven days demonstrated average serum concentrations of ~33.3 μg/mL. 20 This value is slightly lower than the 3 mg/kg-day serum concentrations reported here (~48 μg/mL), but it is unclear if the lower values are attributed to compound differences or the strain of mouse treated for the experiment.
Table 2.
Bioaccumulation rates for PFESA-BP2 in serum and liver relative to total dose administered.
| Male | ||||
|---|---|---|---|---|
| 0.04 mg/kg-day | 0.4 mg/kg-day | 3 mg/kg-day | 6 mg/kg-day | |
| Dosing Solution Concentration (g/L) | 4.0 | 36.8 | 344 | 716 |
| Total Administered (μg) | 5.6 | 51.52 | 481.6 | 1002.4 |
| Serum Concentration (μg/mL) | 0.51±0.07b | 3.99±0.28 | 47.0±3.45 | 83.9±17.1 |
| Liver Concentration (μg/g) | 2.41±0.38 | 20.1±3.23 | 143±31.2 | 235±30.9 |
| Serum Accumulation (μg) a | 0.69 | 5.51 | 63.8 | 117 |
| Liver Accumulation (μg) | 3.21 | 27.5 | 289 | 656 |
| % Serum Accumulation | 12% | 11% | 13% | 12% |
| % Liver Accumulation | 57% | 53% | 60% | 65% |
|
| ||||
| Female | ||||
| 0.04 mg/kg-day | 0.4 mg/kg-day | 3 mg/kg-day | 6 mg/kg-day | |
|
| ||||
| Dosing Solution Concentration (g/L) | 3.15 | 30.1 | 313 | 624 |
| Total Administered (μg) | 4.41 | 42.14 | 438.2 | 873.6 |
| Serum Concentration (μg/mL) | 0.44±0.17 | 3.55±0.98 | 48.0±14.4 | 92.9±83.9 |
| Liver Concentration (μg/g) | 1.43±0.12 | 17.5±4.37 | 129±41.4 | 208±24.2 |
| Serum Accumulation (μg) | 0.48 | 3.95 | 54.8 | 109 |
| Liver Accumulation (μg) | 1.34 | 16.8 | 215 | 495 |
| % Serum Accumulation | 11% | 9% | 12% | 12% |
| % Liver Accumulation | 30% | 40% | 49% | 57% |
Serum volumes estimated assuming serum accounts for 5.85% of the total body weight
Averages demonstrated for each group with one standard deviation
The percent of PFESA-BP2 in serum relative to the amount administered, ranging from 9 to 13%, was similar in male and female mice and did not demonstrate a direct relationship with dose (Table 2). The percent accumulation in the liver of the mice, ranging from 30 to 65%, varied by sex and correlated positively with increasing dose level (Table 2). Higher accumulations in the liver compared to serum could have implications for the human population in cases where PFESA-BP2 was identified in serum.13
PFESA-BP2 was detected at low levels (<0.3 μg/g) in two of the control livers analyzed. The contamination is assumed to be due to reuse of necropsy instruments across animals because it was present in only two of the livers and was not present in the serum for these animals, as well as dosing protocol would not allow occurrence of cross-contamination with dosing instruments. Since the serum levels are an order of magnitude lower than that in the livers of mice treated with PFESA-BP2, this contamination is not expected to affect the toxicity and bioaccumulation results.
2.9. Summary
The results presented here demonstrate that short term (7 day) exposures to PFESA-BP2 significantly increased liver weights in treated mice following doses as low as 0.4 mg/kg-day in male mice and more than doubled the size of the liver in both male and female mice at 6 mg/kg-day. Elevated serum liver function tests indicate that injury occurs at PFESA-BP2 doses of 3 mg/kg-day and higher in both sexes with males apparently more sensitive than the females. There were no adverse effects detected at the 0.04 and 0.4 mg/kg-day doses compared to the control group. At the lowest dose (0.04 mg/kg-day - ~500 ppb), serum levels were 100- to 200-fold higher than median serum concentration from humans exposed to PFESA-BP2 through drinking water (~3 ppb).13 To our knowledge this is the first toxicology study of PFESA-BP2. Given that this chemical induces hepatotoxic effects comparable to those associated with other PFASs, additional toxicology studies are warranted. Genomic analysis and more histopathological evaluations can be explored with tissues collected in this study. Future work should include extended in vivo treatments to simulate a chronic environmental exposure covering different developmental lifestages.
Supplementary Material
3 Acknowledgements
Histopathology samples were analyzed by Dr. Elizabeth Whitley, Pathogenesis, LLC.
References
- 1.Giesy JP; Kannan K, Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 2001, 35 (7), 1339–42. [DOI] [PubMed] [Google Scholar]
- 2.Banks RE; Smart BE; Tatlow JC, Organofluorine Chemistry: Principles and Commercial Applications. Springer US: 2013. [Google Scholar]
- 3.Interstate Technology and Resource Council (ITRC)), PFAS Facts Sheets PFAS-1 2018. www.itrcweb.org. [Google Scholar]
- 4.Wang Z; DeWitt JC; Higgins CP; Cousins IT, A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environmental science & technology 2017, 51 (5), 2508–2518. [DOI] [PubMed] [Google Scholar]
- 5.Agency USEP, EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan. U.S. Environmental Protection Agency: Washington, D.C., 2019. [Google Scholar]
- 6.Gallen C; Eaglesham G; Drage D; Nguyen TH; Mueller JF, A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 2018, 208, 975–983. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W; Zhang Y; Taniyasu S; Yeung LW; Lam PK; Wang J; Li X; Yamashita N; Dai J, Distribution and fate of perfluoroalkyl substances in municipal wastewater treatment plants in economically developed areas of China. Environmental pollution 2013, 176, 10–7. [DOI] [PubMed] [Google Scholar]
- 8.Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C, Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ Sci Technol 2015, 49 (19), 11622–30. [DOI] [PubMed] [Google Scholar]
- 9.Buckley T. Laboratory PFAS Report No. 6 for NC DEQ: Chemours Process Samples; US EPA: October 25, 2017, 2017. [Google Scholar]
- 10.H2GO PFC Sampling. https://www.h2goonline.com/PFCSampling. [Google Scholar]
- 11.NCDEQ Expanded PFAS Analysis on DEQ-Collected Private Wells Associated with Chemours-Fayetteville; 2018. [Google Scholar]
- 12.Hopkins ZR; Sun M; DeWitt JC; Knappe DRU, Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. Journal - AWWA 2018, 110 (7), 13–28. [Google Scholar]
- 13.Katlorz N, PFAS Blood Sample Results. 2018. https://genxstudy.ncsu.edu/files/2019/08/Wilmington-Nov-2018-blood-results.pdf. [Google Scholar]
- 14.Lau C; Thibodeaux JR; Hanson RG; Narotsky MG; Rogers JM; Lindstrom AB; Strynar MJ, Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci 2006, 90 (2), 510–8. [DOI] [PubMed] [Google Scholar]
- 15.Yang B; Zou W; Hu Z; Liu F; Zhou L; Yang S; Kuang H; Wu L; Wei J; Wang J; Zou T; Zhang D, Involvement of oxidative stress and inflammation in liver injury caused by perfluorooctanoic acid exposure in mice. BioMed research international 2014, 2014, 409837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guide for the Care and Use of Laboratory Animals. In National Academies Press (US), Animals C. f. t. U. o. t. G. f. t. c. a. U. o. L., Ed. 2011. [PubMed] [Google Scholar]
- 17.Chernoff N; Hill DJ; Chorus I; Diggs DL; Huang H; King D; Lang JR; Le TT; Schmid JE; Travlos GS; Whitley EM; Wilson RE; Wood CR, Cylindrospermopsin toxicity in mice following a 90-d oral exposure. Journal of toxicology and environmental health. Part A 2018, 81 (13), 549–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiner JL; Nakayama SF; Delinsky AD; Stanko JP; Fenton SE; Lindstrom AB; Strynar MJ, Analysis of PFOA in dosed CD1 mice. Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reproductive toxicology 2009, 27 (3–4), 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thibodeaux JR; Hanson RG; Rogers JM; Grey BE; Barbee BD; Richards JH; Butenhoff JL; Stevenson LA; Lau C, Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci 2003, 74 (2), 369–81. [DOI] [PubMed] [Google Scholar]
- 20.Wolf DC; Moore T; Abbott BD; Rosen MB; Das KP; Zehr RD; Lindstrom AB; Strynar MJ; Lau C, Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-alpha knockout and wild-type mice. Toxicol Pathol 2008, 36 (4), 632–9. [DOI] [PubMed] [Google Scholar]
- 21.Guo H; Wang J; Yao J; Sun S; Sheng N; Zhang X; Guo X; Guo Y; Sun Y; Dai J, Comparative Hepatotoxicity of Novel PFOA Alternatives (Perfluoropolyether Carboxylic Acids) on Male Mice. Environmental science & technology 2019, 53 (7), 3929–3937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.