Abstract
Background and Aims
The introduction of Coronavirus disease 2019 (COVID-19) vaccines urged all Thais to seek prevention of serious illness and death from COVID-19. However, immunocompromised individuals might not be able to achieve an efficient immune response from these vaccines. This study aimed to evaluate the cost-effectiveness and budget impact of introducing Evusheld (tixagevimab plus cilgavimab) for three patient groups—organ transplant, autoimmune disease, and dialysis patients, from the Thai government perspective.
Methods
A Markov decision model was developed to compare the use of Evusheld plus COVID-19 vaccines versus COVID-19 vaccines alone. The methodology followed the National HTA Guidelines of Thailand. Model input parameters were collected locally from retrospective data and from a literature review.
Results
Evusheld helped prevent COVID-19 infection, severe infection, and death in all three patient groups. Using the Thai threshold of 160,000 Thai Baht (THB) per quality-adjusted life year (QALY) gained, the only scenario found to be cost-effective was that of dialysis patients with inadequate immune response, with an incremental cost-effectiveness ratio (ICER) of 54,700 THB per QALY gained. To make a policy of Evusheld provision cost-effective in other groups, the price of Evusheld had to be lower (a reduction of 44–88% of its current price). The results of one-way sensitivity analysis indicated that the cost-effectiveness of Evusheld was sensitive to changes in the rate of infection, cost and efficacy of Evusheld, proportion of inadequate immune responses, and the probability of moving from a ‘recovered’ to ‘susceptible’ status.
Conclusion
Among three COVID-19-vaccinated immunocompromised patient populations, this study concluded that Evusheld was cost-effective for dialysis patients with inadequate immune response to the COVID-19 vaccine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40258-023-00796-7.
Key Points for Decision Makers
| Evusheld shows benefit in helping prevent illness and death among immunocompromised individuals who are unable to achieve an efficient response to COVID-19 vaccines. |
| Similar to other low- and middle-income countries, Thailand needs evidence on cost-effectiveness and budget impact to inform decision making with regard to the introduction of Evusheld and other expensive medicines to ensure the sustainability of universal health coverage (UHC). |
| As shown in the study results, a policy of screening the level of immunity before giving Evusheld can make Evusheld more cost effective. |
Introduction
Coronavirus disease 2019 (COVID-19) vaccines have been proven to be effective in protecting people from COVID-19 [1]. Nevertheless, some people with weakened immune systems may have a poor response to the vaccines or may not be eligible for them at all. Their existing health conditions put them at a higher risk of severe illness and death from COVID-19 compared to the general population.
Recently, the PROVENT Phase III clinical trial of Evusheld, which is the combination of two monoclonal antibodies called tixagevimab and cilgavimab, found that Evusheld could provide protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections among 3441 participants [2]. These findings resulted in authorisation in the USA and other countries, including Thailand, for the use of Evusheld to reduce the risk of COVID-19 among those who have an inadequate immune response to COVID-19 vaccines or those who cannot receive a vaccine due to severe allergic reactions [3, 4].
Despite the known benefits, Evusheld is not a substitute for COVID-19 vaccination [5]. Primary protection from COVID-19 is still through vaccination. For those with medical conditions that may lead to an inadequate immune response from the COVID-19 vaccine, Evusheld can be given after vaccination.
In response to the COVID-19 pandemic, which remains a concern in Thailand, the Ministry of Public Health (MOPH) is considering using Evusheld for preexposure prevention in high-risk groups. However, the inclusion of this expensive medicine requires evidence on value for money and the budgetary implications of its introduction.
Therefore, this study was commissioned by the Thai government to assess the cost-effectiveness and budget impact of the use of Evusheld for preexposure prophylaxis for COVID-19 in a group at high-risk of severe COVID-19 infection and death.
Methods
This was a cost-utility analysis to estimate the expected costs and health gains associated with the use of Evusheld plus COVID-19 vaccines versus COVID-19 vaccines alone for the preexposure prophylaxis for COVID-19 among those who are at an increased risk of severe illness or death due to COVID-19. The study population included people who had received an organ transplant, autoimmune disease patients (i.e., systemic lupus erythematosus, immune thrombocytopenic purpura, Sjögren’s syndrome, mixed connective tissue disease, immune encephalitis and myositis), and patients undergoing renal dialysis. The total number of patients in each group and the incidence of COVID-19 infection, hospitalisation and mortality data were collected from COVID-19 national registries: COLAB and COWARD, which are managed by the Thai MOPH. COLAB is a national registry for all health service facilities in Thailand that offer the COVID-19 reverse-transcription polymerase chain reaction (RT‒PCR) test. All test results must be reported to this database. COWARD is another national database that registers all COVID-19 patients who require hospitalisation and the treatment outcomes. The average starting age of the cohort was 54 years [2].
The analysis compared 3 scenarios: (i) COVID-19 vaccines as the current standard of prevention of COVID-19 infection in which 77% of the Thai population has received at least two doses of COVID-19 vaccines (data from COLAB and COWARD); (ii) Evusheld Policy 1, in which all eligible people were given Evusheld two to four weeks after receiving the second or booster dose(s) of COVID-19 vaccine; and (iii) Evusheld Policy 2, in which only people with low protective immunity through vaccination were provided with Evusheld. The last scenario aimed to assess a new strategy of providing Evusheld exclusively to individuals with low levels of anti-spike IgG (immunoglobulin formed after infection or immunisation) antibodies (e.g., below 264 BAU/mL), which correlated with neutralising activity [6] and hence requires a screening test. Given the present COVID-19 vaccination programme in Thailand, the vaccines used among eligible patients were a mixture of inactivated, viral vector and mRNA vaccines.
A model-based economic evaluation was constructed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), following the National Health Technology Assessment Guidelines of Thailand [7].
The analysis was conducted using the perspective of public health care payers in Thailand. According to the trial data, the Evusheld efficacy was assumed to last 6 months [2, 5]; consequently, the 6-month time horizon was used with a cycle length of one week. Hence, no discounting was applied for the outcomes over the study period (see Sect. 2.3). Finally, the data presented here were collected and analysed during mid-April through to early June 2022.
Model Structure
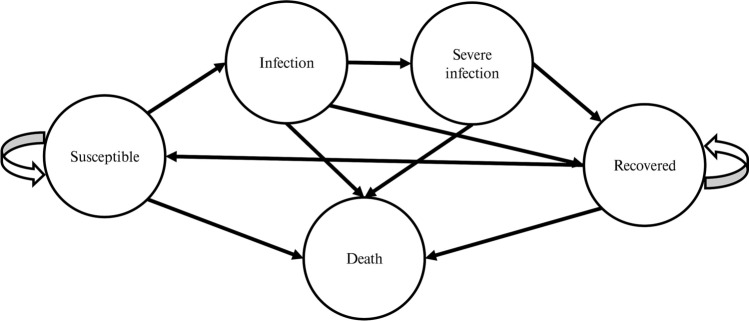
Figure 1 displays a Markov model developed for this study. In the model, individuals are classified into five mutually exclusive health stages, namely, ‘susceptible’, ‘infection’, ‘severe infection’, ‘recovered’ and ‘death’.
Fig. 1.
Markov model representing events that could occur during the spread of COVID-19
A cohort of each group of patients entered the Markov model: 8325 organ transplant patients, 331,378 autoimmune disorder patients, and 17,652 dialysis patients. These numbers represent each group of patients registered in the Thai MOPH. Given that all individuals received at least two doses of COVID-19 vaccines, only people with low levels of neutralising antibodies were assumed to be susceptible to COVID-19. The model started when an individual entered the ‘susceptible’ stage, i.e., low levels of neutralising antibodies. Following this, individuals could become ‘infected’, and then progress to ‘severe infection’. The ‘infection’ and ‘severe infection’ states were transient, i.e., in the next cycle, the individual would move to either the ‘recovered’ or ‘death’ stage. Once ‘recovered’, an individual was no longer infected but could become ‘susceptible’ to the disease again in the next cycles. Each state was associated with the mortality rate of COVID-19-related illness or other causes.
Model Inputs
Model inputs (Table 1) comprised treatment efficacy, transitional probabilities, direct medical costs and utilities.
Table 1.
Input parameters used in the cost-effectiveness model
| Parameters | Value (SE) | References |
|---|---|---|
| Transitional probabilities | ||
| Probability of COVID-19 infection | ||
| Organ transplant patients | 0.003 (0.003)a | COLAB and COWARD |
| Autoimmune disorder patients | 0.002 (0.001) | |
| Dialysis patients | 0.005 (0.005) | |
| Probability of severe COVID-19 infection | ||
| Organ transplant patients | 0.049 (0.009) | COLAB and COWARD |
| Autoimmune disorder patients | 0.012 (0.001) | |
| Dialysis patients | 0.076 (0.006) | |
| Probability of death from severe COVID-19 infection | ||
| Organ transplant patients | 0.332 (0.091) | COLAB and COWARD |
| Autoimmune disorder patients | 0.344 (0.037) | |
| Dialysis patients | 0.608 (0.043) | |
| Probability of moving from ‘recovered’ to ‘susceptible’ | 0.026 (0.007) | Assumption |
| Direct medical costs (THB) | ||
| The treatment of mild to moderate COVID-19 | ||
| Organ transplant patients | 100,620 (21,050) | UCS hospitalisation database |
| Autoimmune disorder patients | 105,930 (10,370) | |
| Dialysis patients | 123,500 (3260) | |
| The treatment of severe COVID-19 | ||
| Organ transplant patients | 300,600 (87,420) | UCS hospitalization database |
| Autoimmune disorder patients | 280,140 (33,600) | |
| Dialysis patients | 251,500 (8360) | |
| Cost of quarantine at designated facilities | 15,200 (3790) | |
| Utility | ||
| ‘Susceptible’ | 0.818 (0.02) | [14] |
| ‘Infection’ | 0.693 (0.01) | [14] |
| ‘Severe infection’ | 0.515 (0.13) | [14] |
| ‘Recovered’ | 0.818 (0.02) | [14] |
SE standard error
aThe SE is assumed to be equal to the mean in the sensitivity analysis
The efficacy of Evusheld (relative risk reduction) was based on the Phase III PROVENT trial [2]. Data from the trial at a median of 6 months showed a greater reduction in symptomatic COVID-19 cases in the Evusheld group than in the placebo group, with a reduction in relative risk of 82.8% (95% CI: 65.8, 91.4). In this study, we assume a similar efficacy of Evusheld in preventing mild to severe COVID-19 infection.
Transitional probabilities between health states were obtained from COLAB and COWARD, which are national registries for COVID-19 diagnosis and treatment, respectively. In addition, the probability of moving from the ‘recovered’ to ‘susceptible’ state was estimated from the half-life of Evusheld, which lasts 6 months [2, 5], and the number of participants in the Evusheld arm from the PROVENT trial (N = 3441) was used for a standard error estimation. This means that most patients protected by Evusheld are unlikely to develop COVID-19 in the next 6 months. This assumption was also applied to the case of COVID-19 recovery, meaning that most recovered patients are unlikely to have another COVID-19 infection within 6 months, although it was evident that some patients may be infected by 2 weeks at the earliest [8–10]. The age-adjusted mortality rate for the general population was identified from Thai life tables [11]. Patients infected with non-severe COVID-19 were assumed to die from general causes. The proportion of patients with inadequate anti-S titres after screening was assumed to be equal to 39% [12].
The estimated price of Evusheld obtained from the Department of Disease Control, MOPH was 29,000 THB. The screening cost for the anti-spike IgG (anti-S) titre was estimated at 450 THB per patient from the Department of Medical Science, MOPH. Total direct medical costs were derived from a hospitalization database under Thailand’s universal coverage scheme (UCS) of the National Health Security Office during January 2020 and October 2021. The cost of treatment of COVID-19 patients was obtained from the general ward setting, while treatment costs for severe cases were taken from the intensive care unit or ICU setting. All costs were converted to 2022 values using the Thai consumer price index [13] and presented in Thai Baht (THB) (approximately THB 36 = USD 1 in 2022).
Utility values of patients with COVID-19 were gathered from a study conducted in another country in Asia, i.e., Iran [14]. Given that our study population had underlying health conditions, the utility of patients’ underlying diseases was applied to the utility of the ‘susceptible’ and ‘recovered’ stages. We adjusted utilities of ‘infection’ (multiplying the utility value of the ‘susceptible’ stage by the utility value of patients requiring hospitalisation) and ‘severe infection’ (multiplying the utility value of the ‘susceptible’ stage by the utility of patients requiring intensive care) for patients’ underlying diseases, which were reported in the same study.
Outcomes
The primary outcomes of interest were the number of infections, severe infections, and deaths from COVID-19, as well as total costs and quality-adjusted life years (QALYs). These were estimated for each cohort (total population starting in the model) representing a period of 6 months, with the exception of incremental QALYs from COVID-19 death averted, which were estimated over a lifetime horizon. Estimated QALYs lost from premature death were considered in the outcome analysis. Future benefits in terms of QALYs gained from death averted were discounted at 3%, as per the guideline recommendation [7].
The incremental cost-effectiveness ratio (ICER) in Thai Baht (THB) per QALY gained of each policy choice was presented to assess the cost-effectiveness of the technology. To be considered good value for money (or cost-effective) in Thailand, Evusheld had to offer an additional unit of health gain at or below a willingness-to-pay (WTP) threshold of 160,000 THB per QALY [15].
Sensitivity Analysis and Threshold Analysis
We performed a one-way sensitivity analysis and a probabilistic sensitivity analysis (PSA) to account for the effect of the assumptions used and parameter uncertainty in the model, respectively.
For the one-way sensitivity analysis, parameters were varied within their 95% confidence intervals (CIs), with the exception of parameters for which the variance information was not available, such as the price of Evusheld and screening cost of anti-spike IgG. In this case, the standard error was assumed to be 30% of the mean as agreed upon by experts during the consultation meeting on June 2, 2022 [16]. The most influential variables are reported in a tornado diagram.
Parameter distributions for the PSA were assigned based on Briggs et al [17]. Beta distribution was used for efficacy, transition probabilities, and utility, while gamma distribution was assigned to cost parameters. Parameter values were drawn at random from the assigned distributions using Monte Carlo simulation with 1000 iterations. The results were summarised using cost-effectiveness acceptability curves.
Additionally, an expected value of perfect information (EVPI) analysis was conducted to compare the expected net benefit of the optimal strategy obtained using perfect information and the expected net benefit given the current information. This can help to further quantify the extent of uncertainty and the value of potential future research to inform a decision. We calculated the population EVPI per year for the total number of cohorts in the model at different WTP thresholds.
An expected value of partially perfect information (EVPPI) analysis was also conducted to identify parameters with the highest impact on uncertainty and further aid in making decisions regarding the need for future research. In this EVPPI analysis, we created a model simulation using the WTP threshold of 160,000 THB per QALY. The EVPPI was calculated for the following parameters: relative risk reduction of COVID-19 infection and severe infection due to Evusheld, probability of moving from the ‘susceptible’ to ‘infection’ stage, probability of moving from the ‘infection’ to ‘severe infection’ stage, probability of moving from ‘severe infection’ to ‘death’, probability of moving from ‘recovered’ to ‘susceptible’, treatment cost of mild COVID-19 cases, treatment cost of severe COVID-19 cases, and cost of quarantine.
Finally, a threshold analysis of the Evusheld price was performed to uncover the maximum price at which the drug would still be considered cost effective.
Budget Impact Analysis
Budget impact analysis was also conducted using the health care payer perspective to assess the affordability of offering Evusheld to the target population. The budget impact was estimated over 6 months, focusing on a single cohort with an initial dose of Evusheld. Uptake was assumed to be 100% to ensure that this prediction was not underestimated, as the government will use this information for financial planning.
Model Validation
Face and predictive validity were examined. The model’s structure, parameter values, and assumptions were presented first to the research partners and then to a broader range of stakeholders, including relevant departments under the MOPH (i.e., the Department of Disease Control, FDA, the Department of Medical Science, and the Health Technical Office), the Royal College of Physicians of Thailand, health professionals, academics and the Pharmaceutical Research and Manufacturers Association [16]. In addition, the model predicted that the number of COVID-19 infections and deaths in the model cohorts were similar to those in the original data obtained from the COLAB and COWARD registries (see Supplementary Information Table S1 and Fig. SF1).
Results
Base‑Case Analysis
Table 2 reports the main outcomes. Over a 6-month period, Evusheld reduced COVID-19 hospitalisations and deaths compared to the COVID-19 vaccine alone. Regardless of whether immunity was screened before the provision of Evusheld, there was no difference in health outcomes between screening and no screening for vaccination-induced immunity before administering Evusheld, only a cost difference. The new policy options with Evusheld had higher costs when compared to the current approach. The total costs of Evusheld Policy 1 (i.e., offering Evusheld to all target groups) were double the total costs of Evusheld Policy 2 (i.e., offering Evusheld to the low immunity population after the screening test) across all three patient groups.
Table 2.
Outcomes and costs in the economic evaluation of Evushelda
| Organ transplant patients (N = 8325) | Autoimmune disease patients (N = 331,378) | Dialysis patients (N = 17,652) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| COVID-19 vaccine | Evusheld Policy 1b | Evusheld Policy 2c | COVID-19 vaccine | Evusheld Policy 1b | Evusheld Policy 2c | COVID-19 vaccine | Evusheld Policy 1b | Evusheld Policy 2c | |
| Outcomes (6 months, undiscounted) | |||||||||
| Number of COVID-19 infections | 377 | 67 | 67 | 10,494 | 1839 | 1839 | 1267 | 228 | 228 |
| Number of severe infections | 18 | 1 | 1 | 115 | 3 | 3 | 91 | 3 | 3 |
| Number of deaths from COVID-19 | 6 | 0.2 | 0.2 | 38 | 1 | 1 | 53 | 2 | 2 |
| QALYs | 3388 | 3389 | 3389 | 134,889 | 134,917 | 134,917 | 7174 | 7186 | 7186 |
| Cost (6 months, undiscounted) | |||||||||
| Total costs (THB) | 48,957,300 | 249,292,900 | 105,769,900 | 1,303,276,900 | 9,833,612,700 | 4,120,656,000 | 198,701,900 | 544,177,000 | 239,856,500 |
| Cost-effectiveness (vs COVID-19 vaccine as a reference) | |||||||||
| Incremental cost (THB) | Reference | 200,335,600 | 56,812,600 | Reference | 8,530,335,800 | 2,817,379,100 | Reference | 345,475,100 | 41,154,600 |
| Incremental QALYs (lifetime, discounted) | 80 | 80 | 558 | 558 | 753 | 753 | |||
| ICER (THB/QALY) | 2,489,600 | 706,000 | 15,295,000 | 5,051,500 | 458,900 | 54,700 | |||
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year, THB Thai Baht
aThe numbers presented in the table were rounded to the nearest whole number
bEvusheld Policy 1 refers to the provision of Evusheld to all patients at least 2–4 weeks after the COVID-19 vaccine
cEvusheld Policy 2 refers to the provision of Evusheld to individuals who had low levels of protective immunity at least 2-4 weeks after the COVID-19 vaccine
A base-case analysis that considered all scenarios, found that no scenarios were cost effective (except for Evusheld Policy 2 among dialysis patients) using the threshold of 160,000 THB per QALY. Compared to COVID-19 vaccination alone, the policy of providing Evusheld for dialysis patients who had a low immune response to vaccination (Evusheld Policy 2) was cost effective with an ICER of 54,700 THB per QALY.
Sensitivity and Threshold Analyses
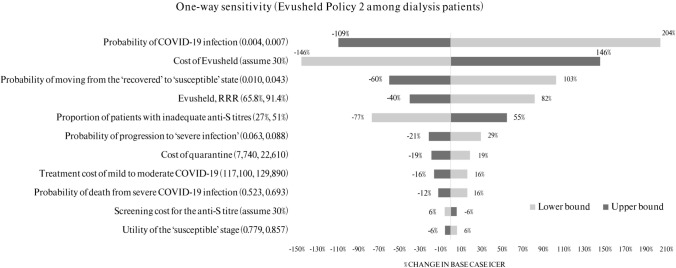
The results of one-way sensitivity analysis indicated that the favourable cost-effectiveness result of Evusheld Policy 2 among dialysis patients was most sensitive to changes in the rate of breakthrough COVID-19 infections, cost and efficacy of Evusheld, probability of moving from ‘recovered’ to ‘susceptible’, and the proportion of patients with inadequate anti-S titres. Other variables that had a significant impact on the base case ICER are shown in Fig. 2. The remaining parameters are not presented in the tornado diagram, as they were unlikely to have a substantial impact on the ICER (less than 5%).
Fig. 2.
Results of one-way sensitivity analysis of Evusheld Policy 2 among dialysis patients
Overall, given the changes in one-way sensitivity analysis, Evusheld Policy 2 among dialysis patients remained cost effective. Similar results were found in other patient groups (see Supplementary Information Fig. SF2–6).
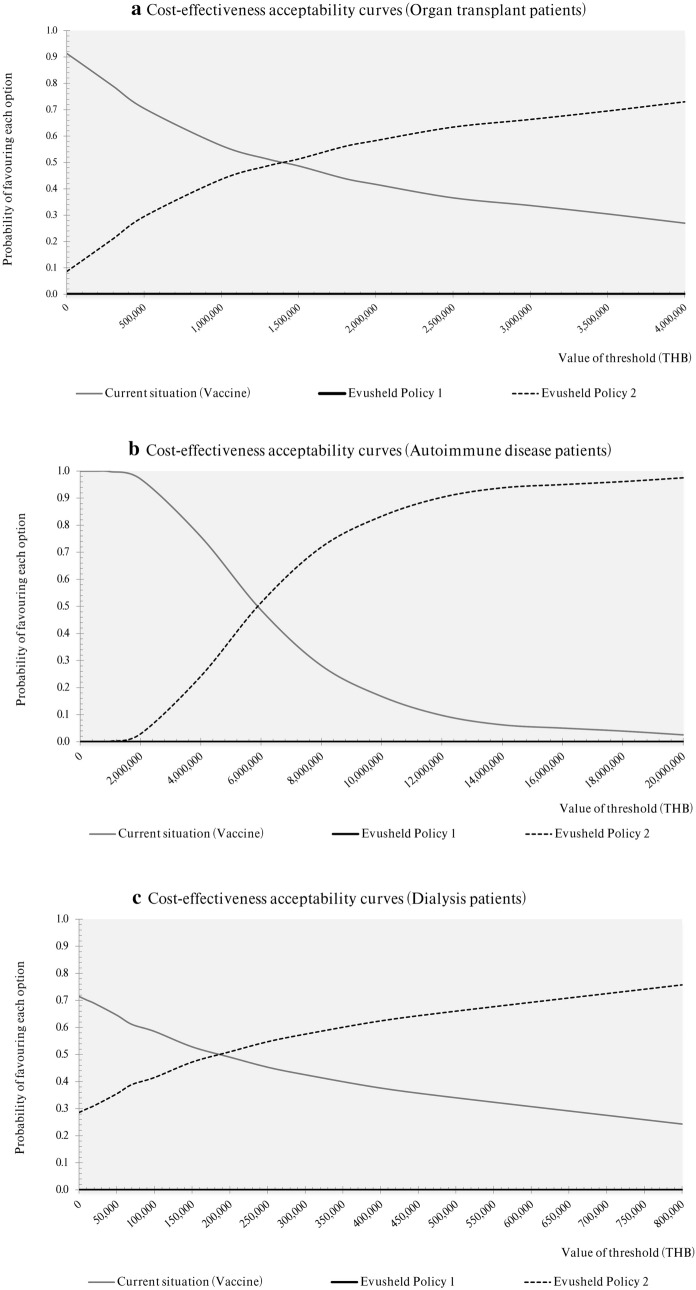
The PSA results (Fig. 3a–c) revealed that at the current Thai WTP threshold, the standard of care policy of offering COVID-19 vaccines was the most cost-effective choice with a 100% probability of being cost effective for autoimmune disease patients, 85% for organ transplant patients and 52% for dialysis patients. Of the three subgroups considered under Evusheld Policy 2, dialysis patients had the highest probability of the drug being cost effective, i.e., 51% certainty, at a WTP threshold of 200,000 THB per QALY. To compare with organ transplant and autoimmune disease patients, a similar level of certainty (51%) could only be achieved at WTP thresholds of 1,500,000 and 6,000,000 THB per QALY, respectively. Evusheld Policy 1 was dominated by the other two policy options at any value of the WTP threshold.
Fig. 3.
a–c Acceptability curves of cost-effectiveness at the different ceiling thresholds of the three policy options to prevent COVID-19 among a organ transplant patients, b autoimmune disease patients, and c dialysis patients
Threshold analysis identified that in order for Evusheld Policy 1 to be cost effective in all patient groups, the price of Evusheld would have to be reduced by approximately 44–88%—6500, 3500, and 16,300 THB for organ transplant, autoimmune disease, and dialysis patients, respectively. Moreover, if the price of Evusheld was to be reduced to 15,000 or 7800 THB, Evusheld Policy 2 would become cost effective for organ transplant and autoimmune disease patients, respectively.
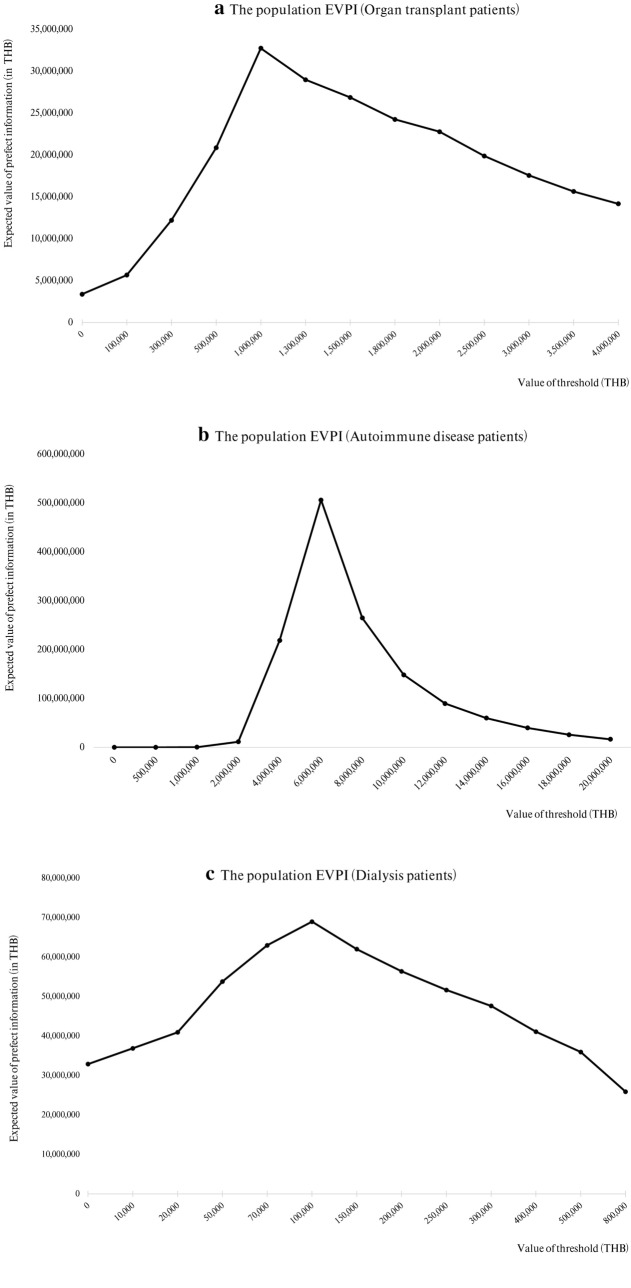
Population EVPI analysis showed a maximum of 33 million THB at a WTP of 1 million THB per QALY for organ transplant patients, 506 million THB at a WTP of 6 million THB per QALY for autoimmune disease patients, and 69 million THB at a WTP of 100,000 THB per QALY for dialysis patients (Fig. 4a–c).
Fig. 4.
a–c Population expected value of perfect information (EVPI) at the different thresholds for a organ transplant patients, b autoimmune disease patients, and c dialysis patients
The uncertainty of each parameter is shown in the EVPPI analysis results. Given a WTP of 160,000 THB per QALY, the EVPPI of all parameters among autoimmune disease patients was equal to zero, which means that there is no expected value in conducting future research on parameters in this group of patients. The EVPPI results in the organ transplant group showed that the probability of moving from ‘susceptible’ to ‘infection’ had the largest influence on uncertainty (1.6 million THB), while all other parameters were equal to zero. The results of EVPPI among dialysis patients revealed five parameters that had the biggest effect on uncertainty: the probability of moving from ‘susceptible’ to ‘infection’ (60 million THB), relative risk reduction of COVID-19 infection (210,000 THB), relative-risk reduction of severe COVID-19 infection (160,000 THB), probability of moving from ‘recovered’ to ‘susceptible’ (90,000 THB), and direct medical cost of severe COVID-19 cases (88,000 THB).
Budget Impact Analysis
Over the time horizon of 6 months, Evusheld policies increased government spending compared to the current policy (Table 3). Although it would save treatment and quarantine costs, a policy of implementing Evusheld would lead to additional budget spending on drug and screening tests for anti-S titre.
Table 3.
A comparison of budget impact (million Thai Baht [THB]) under different scenarios of preventing COVID-19 in high-risk groupsa
| COVID-19 vaccine | Evusheld Policy 1b | Evusheld Policy 2c | |
|---|---|---|---|
| Organ transplant patients (N = 8325) | |||
| Screening for anti-S titre | – | – | 4 |
| Evusheld | – | 241 | 94 |
| Treatment and quarantine | 49 | 8 | 8 |
| Total budget (million THB) | 49 | 249 | 106 |
| Additional budget | Reference | 200 | 57 |
| Autoimmune disease patients (N = 331,378) | |||
| Screening for anti-S titre | – | – | 149 |
| Evusheld | – | 9610 | 3748 |
| Treatment and quarantine | 1303 | 224 | 224 |
| Total budget (million THB) | 1303 | 9834 | 4121 |
| Additional budget | Reference | 8530 | 2818 |
| Dialysis patients (N = 17,652) | |||
| Screening for anti-S titre | – | – | 8 |
| Evusheld | – | 512 | 200 |
| Treatment and quarantine | 199 | 32 | 32 |
| Total budget (million THB) | 199 | 544 | 240 |
| Additional budget | Reference | 345 | 41 |
aThe numbers presented in the table were rounded to the nearest whole number in a million THB
bEvusheld Policy 1 refers to the provision of Evusheld to all patients at least 2–4 weeks after the COVID-19 vaccine
c Evusheld Policy 2 refers to the provision of Evusheld to individuals who had low levels of protective immunity at least 2–4 weeks after the COVID-19 vaccine
It is interesting to note that Evusheld Policy 2, with a screening test for anti-S titre before providing Evusheld, represented less than half of the total cost of Evusheld Policy 1.
Discussion
According to the scenarios evaluated in this study, providing Evusheld after COVID-19 vaccines was found to be preferable in terms of health outcomes when compared with a COVID-19 vaccine alone. Although the Evusheld policy yielded more health benefits, it was more costly than the current policy pursued by the Thai government. At the current price of Evusheld, only Evusheld Policy 2 among dialysis patients represented good value for money in the Thai context. This study can help guide priority setting for immunocompromised populations to receive Evusheld in the country. It should be noted that differences in the value for money of Evusheld across population groups stemmed from differences in COVID-19 infection risk in each population in Thailand; hence, our results may not be transferrable to other settings.
As the cost of Evusheld is high and it is very likely that booster shots will need to be administered every 6 months, implementing the Evusheld policy can result in a considerable financial burden on the Thai government in the long term. A price negotiation would lessen this burden and allow an opportunity to provide Evusheld to a wider group of patients. We believe that equity considerations will need to be incorporated when this decision is made.
To the best of our knowledge, this is the first study to assess the cost-effectiveness and budget impact of preexposure prevention of COVID-19 with Evusheld. Our study applied real-world (local) epidemiologic and cost data in the Thai setting, which could be considered a strength of our study. Furthermore, the results of this study were used to inform the decision-making of the Thai government; this study could be seen as an example of how economic evidence can be applied to address policy questions in a timely manner during a pandemic. In late June 2022, the Thai cabinet chaired by the Prime Minister reviewed the study results, and the Thai MOPH subsequently procured Evusheld for chronic kidney disease and organ transplant patients as of July 2022 [18]. The policy remains active even though Thailand downgraded COVID-19 from a “dangerous communicable disease” to one that needs “monitoring” starting from 1 October 2022.
Notably, there could be a change since this study. The efficacy of Evusheld used in this study came from the PROVENT trial, in which immunocompromised persons accounted for 73%. It should be noted that no participants had previously received a COVID-19 vaccine, which might not be the same as the current situation in Thailand, where the majority of these immunocompromised patients are fully vaccinated. Moreover, the trial was conducted before the emergence of the Omicron variant. Although a recent study demonstrated that Evusheld retained activity against Omicron [19], new variants of the coronavirus could be identified in the future against which Evusheld may be less effective. Recent evidence from the in vitro studies [20, 21] also indicated that Evusheld is losing its neutralising effect against the current variants. This may decrease cost-effectiveness of Evusheld, which warrants further investigation. Given this, one of the policy recommendations is that the MOPH should continue to monitor the neutralising activity of Evusheld against emerging SARS-CoV-2 variants.
The proportion of patients with inadequate anti-S titres was derived from real-world data in France, which should incorporate testing performance [12]. Nevertheless, different countries may have different tests used for measuring anti-S titres, and this may overestimate or underestimate the value for money of Evusheld Policy 2 if the test performance in a new setting is lower or higher than the test used in France, respectively. Moreover, given that the parameter ‘the proportion of patients with inadequate anti-S titres’ was used, we cannot be certain that the screening test has 100% accuracy.
Probabilistic sensitivity analysis revealed that there was substantial decision uncertainty. Data from the COLAB and COWARD registries represent a real-world setting; hence, it is possible that the number of patients, COVID-19 infections, and deaths are underreported. This may lead to an underestimation of the health benefits and budget required for purchasing Evusheld. We encourage additional data collection and reporting due to the paucity of evidence currently available.
We did not include costs or outcomes from a long COVID disease duration beyond 6 months, which may result in underestimating the impact and value for money of Evusheld. However, the analysis assessing the impact of long COVID prevention on QALYs showed that the conclusion of the cost-effectiveness results (ICER values) remains the same (see Supplementary Information Table S2). Also, we also did not consider the quality of life lost beyond one week in the case of hospitalisation in these groups of immunocompromised patients as they may require a longer time before full recovery. However, data on the recovery period of these patients are not available in COWARD. This may result in underestimating the benefit of Evusheld.
Finally, lifetime QALY gains resulting from COVID-19 deaths averted in a 6-month period could be overestimated in this analysis and produce more favourable ICERs since a repeat dose of Evusheld every 6 months may be needed, as some patients will not be completely protected against COVID-19 throughout their lifetime. If we exclude QALYs gained from lives saved from prophylaxis longer than 6 months, no Evusheld policies would be cost-effective (see Supplementary Information Table S2). Nevertheless, the assumption was verified by Thai experts in the stakeholder consultation meeting held at the end of this study on 2 June 2022 [16]. The stakeholders agreed on lifetime prophylaxis because the assumption using a 6-month time horizon to avert COVID-19 deaths would be drastically underestimated.
Conclusion
This study suggests that providing Evusheld following COVID-19 vaccination to dialysis patients who have had an inadequate immune response is a cost-effective policy option in Thailand.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Health Technical Office, Ministry of Public Health, Thailand for funding support. The findings, interpretations and conclusions expressed in this article do not necessarily reflect the views of the aforementioned funding agency. The authors thank Yaroslava Zemlyanska for proofreading support. The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript.
Declarations
Funding
This study was funded by the Health Technical Office, Ministry of Public Health, Thailand (65-006).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and have given their approval for this version to be published.
Ethics approval
This study did not involve any human participants. Therefore, no ethics approval was needed.
Consent to participate
Not applicable
Consent for publication (from patients/participants)
Not applicable
Availability of data and material
All relevant data are within the paper.
Code availability
Data used in the economic evaluation model were obtained from a third party and are not publicly available due to privacy restrictions. To ask for permission, please submit a written document to Health Intervention and Technology Assessment Program (HITAP), Ministry of Public Health, Thailand at hitap@hitap.net.
References
- 1.Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise J. Covid-19: Evusheld is approved in UK for prophylaxis in immunocompromised people. BMJ. 2022;376:o722. doi: 10.1136/bmj.o722. [DOI] [PubMed] [Google Scholar]
- 4.Kmietowicz Z. Covid-19: monoclonal antibodies authorised in US as alternative to vaccines for certain groups. BMJ. 2021;375:n3064. doi: 10.1136/bmj.n306. [DOI] [PubMed] [Google Scholar]
- 5.Administration USF&D. FDA authorizes revisions to Evusheld dosing. Updated June 29, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing. Accessed 11 July 11 2022.
- 6.Harvala H, Robb ML, Watkins N, et al. Convalescent plasma therapy for the treatment of patients with COVID-19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus Med. 2021;31(3):167–175. doi: 10.1111/tme.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaikledkaew U, Teerawattananon Y. Guidelines for health technology assessment in Thailand (Second Edition). J Med Assoc Thai. 2014;97(5). [PubMed]
- 8.Adrielle Dos Santos L, Filho PGG, Silva AMF, et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J Infect. 2021;82(3):399–406. doi: 10.1016/j.jinf.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correia AM, Borges V, Isidro J, et al. Potential recurrence of COVID-19 in a healthcare professional: SARS-CoV-2 genome sequencing confirms contagiousness after re-positivity. Int J Infect Dis. 2021;112:318–320. doi: 10.1016/j.ijid.2021.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zayet S, Royer PY, Toko L, Pierron A, Gendrin V, Klopfenstein T. Recurrence of COVID-19 after recovery ? A case series in health care workers, France. Microbes Infect. 2021;23(4–5):104803. doi: 10.1016/j.micinf.2021.104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Thai Working Group on Burden of Disease and Injuries. Burden of disease and injury of the Thai population. Thailand: Report, International Health Policy Program, Ministry of Public Health. 2014.
- 12.Goulenok T, Delaval L, Delory N, et al. Pre-exposure anti-SARS-cov-2 monoclonal antibodies in severely immunocompromised patients with immune-mediated inflammatory diseases. Lancet Rheumatol. 2022 doi: 10.1016/s2665-9913(22)00099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bureau of Trade & Economics Indices Ministry of Commerce. Consumer price index of Thailand year 2022 base year 2019, 2022. http://www.price.moc.go.th/price/cpi/index_new_all.asp. Accessed 22 Apr 2022.
- 14.Alinia C, Yaghmaei S, Abdullah FZ, et al. The health-related quality of life in Iranian patients with Covid-19. BMC Infect Dis. 2021 doi: 10.1186/s12879-021-06170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Health Economic Working Group. Meeting of the health economic working group on 22 May 2013.2013. Thailand: Report for Ministry of Public Health.
- 16.Health Intervention and Technology Assessment Program. The consultation meeting on the research project “Economic evaluation of Evusheld for pre-exposure prevention of COVID-19 in high-risk populations”. 2022. https://www.hitap.net/documents/183935.
- 17.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: OUP; 2006. [Google Scholar]
- 18.Signing Ceremony “Evusheld” between Minister of Public Health, Thailand and AstraZeneca. Bureau of Information Office of the Permanent Secretary of Ministry of Public Health. 6 July 2022. https://pr.moph.go.th/?url=pr/detail/2/04/176033/. Accessed 8 Aug 2022.
- 19.Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422.e13–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuver R, Shah GL, Korde NS, et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell. 2022;40(6):590–591. doi: 10.1016/j.ccell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.