The Centers for Disease Control and Prevention has recommended that moderately/severely immunosuppressed individuals receive an additional dose (AddDose) of COVID-19 vaccine at least 28 days after an initial mRNA vaccine series, or at least 2 months after a single adenovirus vector vaccine [1]. Rheumatoid arthritis (RA) is frequently treated with immunosuppressive disease-modifying anti-rheumatic drugs (DMARDs), meaning that most patients with RA are eligible for an AddDose. The American College of Rheumatology suggests patients interrupt use of (“hold”) certain DMARDs around the time of COVID-19 vaccination in attempt to boost immunogenicity [2]. A small number of reports have noted no significant change in RA disease activity pre- vs. post-vaccination against COVID-19, but they assessed RA disease activity infrequently and did not compare results for patients who held vs. continued DMARDs. Furthermore, data are lacking on the assessment of immune cellular populations possibly correlated with RA disease activity around the time of COVID-19 vaccination.
We conducted a prospective observational study of patients with RA treated at Brigham and Women’s Hospital who were previously vaccinated against COVID-19 (2 doses of mRNA vaccine or 1 dose of adenovirus vector vaccine). Subjects enrolled between July and November 2021, prior to receiving an AddDose. RA disease activity was assessed weekly using the validated patient-reported RA Disease Activity Index-5 (RADAI-5) from enrollment through 4 weeks post-AddDose via online data entry [3]. Subjects completed an additional online survey 2 days after the AddDose regarding vaccine reactogenicity and whether they held or continued DMARDs. We compared mean RADAI-5 in the 4 weeks pre-AddDose vs. the 4 weeks post-AddDose using the generalized estimating equation in SAS (version 9.4) to account for correlated data among individual subjects. We aimed to enroll 60 subjects to achieve 91% power to detect a 15% non-inferiority margin in RADAI-5 post- vs. pre-AddDose.
Among 71 subjects, mean age was 62 (SD 12) years and 85% were female. Methotrexate (42%) and TNF inhibitors (38%) were the most common DMARDs; 49% held at least one DMARD (see Table 1 for additional characteristics). Symptoms after the AddDose most often included injection site pain/swelling (72%) and fatigue (52%) (see Supplement 1). RADAI-5 was normally distributed pre- and post-AddDose. The mean RADAI-5 was 3.20 (SD 0.23) pre-AddDose compared to 3.25 (SD 0.23) after (difference of 1.6%, p=0.51) (see Supplement 2). Figure 1 displays mean RADAI-5 in 35 (49%) subjects that held DMARDs and 36 (51%) subjects that continued all DMARDs. Mean RADAI-5 did not significantly differ pre- vs. post-AddDose among subjects that held DMARDs, nor in subjects that continued all DMARDs. Mean change in RADAI-5 between pre- vs. post-AddDose did not significantly differ based on whether subjects held vs. continued DMARDs (p for interaction = 0.16).
Table 1.
Characteristics of study subjects
| All subjects (N=71) | Subset of subjects analyzed by flow cytometry (N=27)++ | |
|---|---|---|
| Age, mean (SD) | 62.2 (11.8) | 62.8 (13.3) |
| Female, n (%) | 60 (84.5) | 20 (74.1) |
| White, n (%) | 67 (95.7)+ | 25 (92.6) |
| Seropositive rheumatoid arthritis, n (%) | 62 (87.3) | 27 (100.0) |
| Brand of initial vaccine series, n (%) | ||
| Pfizer | 36 (50.7) | 11 (40.7) |
| Moderna | 30 (42.3) | 12 (44.4) |
| Johnson & Johnson | 5 (7.0) | 4 (14.8) |
| Self-reported RA flare after initial COVID-19 vaccine series, n (%) | 13 (18.3) | 7 (25.9) |
| COVID-19 infection prior to additional dose, n (%) | 1 (1.4) | 0 (0) |
| Mean (SD) days from completion of initial vaccine series to additional dose | 177.8 (48.1) | 184.6 (50.5) |
| DMARDs at the time of additional dose, n (%)* | ||
| Methotrexate | 30 (42.3) | 16 (59.3) |
| TNFi | 27 (38.0) | 13 (48.1) |
| Prednisone | 15 (21.1) | 3 (11.1) |
| JAKi | 11 (15.5) | 4 (14.8) |
| Abatacept | 5 (7.0) | 0 (0) |
| Tocilizumab | 5 (7.0) | 1 (3.7) |
| Rituximab | 3 (4.2) | 2 (7.4) |
| Other | 13 (18.3) | 2 (7.4) |
| None | 3 (4.2) | 0 (0) |
| Held DMARD(s) around the time of additional dose, n (%)** | 35 (49.3) | 16 (59.3) |
Abbreviations: RA, rheumatoid arthritis. DMARDs, disease-modifying anti-rheumatic drugs. TNFi, tumor necrosis factor inhibitor. JAKi, janus kinase inhibitor.
1 subject provided pre- and post-AddDose blood samples but did complete post-AddDose rheumatoid arthritis disease activity assessments. This subject only contributed flow cytometry data was not included among the 71 subjects in the primary analysis.
Among 70 subjects with data on race
DMARD groups are not mutually exclusive
Among 35 subjects that held DMARDs, 21 held all DMARDs and 14 held at least one but not all DMARDs
Figure 1. RA disease activity and lymphocyte subsets among subjects that held at least 1 DMARD (top panels) and those that continued all DMARDs (bottom panels) around the time of the additional dose of COVID-19 vaccine.
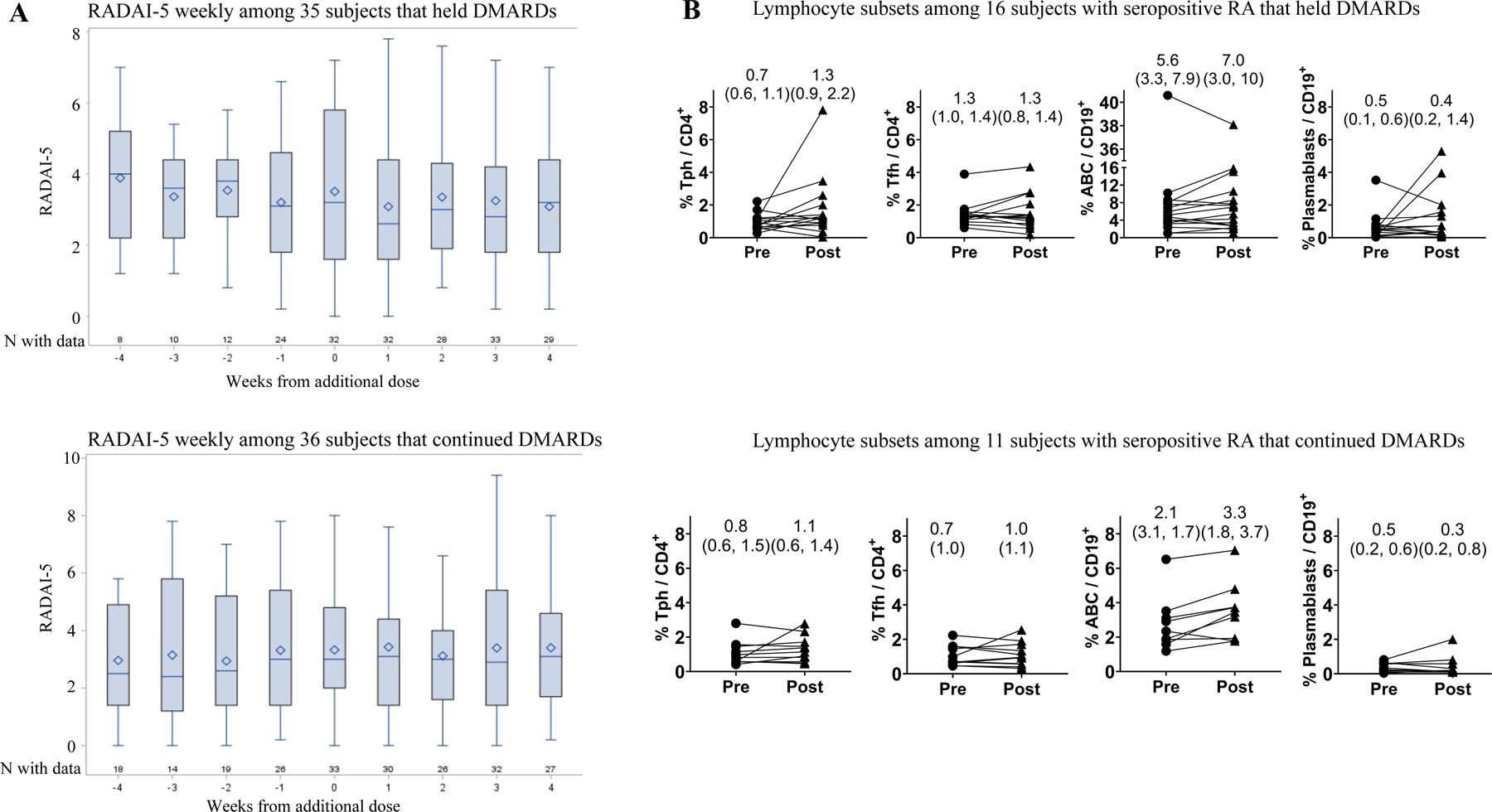
A) Box-and-whisker plots for RADAI-5 (Rheumatoid Arthritis Disease Activity Index-5) indicate the median (line), mean (diamond), 25th percentile (bottom of box), 75th percentile (top of box), and minimum and maximum values (whiskers). B) Lymphocyte populations were gated as follows: Tph (CD4+ PD-1hi CXCR5−), Tfh (CD4+ PD-1hi CXCR5−), ABCs (CD19+ CD21− CD11C+), plasmablasts (CD19+ CD27hi CD38hi). Patients treated with rituximab (n=3) were included in T cell analyses but excluded from B cell analyses. Summary data on each panel include median (IQR) % lymphocyte population pre- and post-additional dose. Significance testing of flow cytometry data used Wilcoxon paired tests with Bonferroni correction for multiple testing. All comparisons of pre- vs. post- were non-significant (p>0.0125).
For a subset of 27 patients with seropositive RA, flow cytometry quantified percentages of 4 lymphocyte populations associated with immune activation in RA -- T peripheral helper (Tph) cells, T follicular helper (Tfh) cells, age-associated B cells (ABC), and plasmablasts -- in blood samples obtained pre-AddDose and week 4 post-AddDose [4–6]. Subject characteristics are shown in Table 1. Frequencies of these lymphocyte populations did not significantly differ between the pre- and post-AddDose timepoints in either subjects that held at least one DMARD (n=16) or subjects that continued all DMARD (n=11) (Figure 1).
Limitations of this study include a relatively small number of participants, though the study was adequately powered to detect a difference of 15% in RA disease activity pre- vs. post-AddDose. The number of participants providing blood for flow cytometry analyses was low, particularly in subgroups by holding vs. continuing DMARDs, and results should be interpreted cautiously.
These results suggest that RA disease activity, measured weekly with a validated patient-reported outcome, is stable around the time of an AddDose of COVID-19 vaccine. Lymphocyte subsets of interest in RA were also similar before and after the AddDose, supporting the observation of stable patient-reported RA disease activity. Holding DMARDs was not associated with greater RA disease activity following the additional dose.
Supplementary Material
Funding information:
ModernaTx provided support to Brigham and Women’s Hospital for an Investigator Sponsored Study. Additional support for the investigators’ time came from the National Institutes of Health (K23 AR075070, L30 AR070514, K23 AR076453, K08 AR072791, P30 AR072577, P30 AR070253). Dr. Rao receives support from the Clinical Scientist Development Award from the Doris Duke Charitable Foundation.
Competing Interests
ModernaTx provided support for this investigator-sponsored study (PI: Solomon) with payments made directly to Brigham and Women’s Hospital.
SKT: research support to Brigham and Women’s Hospital from NIH; consulting fees from NGM Biopharmaceuticals
KY: research support to Brigham and Women’s Hospital from NIH; consulting fees from OM1, Inc.
AHJ: research support to Brigham and Women’s Hospital from Amgen
DR: research support to Brigham and Women’s Hospital from Doris Duke Charitable Foundation, Jansen, Merck, NIH; scientific advisory board member for Bristol Myers Squibb; patent submitted on Tph cells as a biomarker in autoimmune disease; consulting fees from Jansen
DHS: research support to Brigham and Women’s Hospital from ModernaTx, Amgen, Abbvie, CorEvitas, and NIH; royalties from UpToDate for a chapter related to NSAIDs
JS, JE, MGW, KH, LC, IA, KEM: none
Funding, Grant/award information
ModernaTX, Inc
Doris Duke Charitable Foundation
- U.S. Department of Health and Human Services, National Institutes of Health
- K08 AR072791
- K23 AR075070
- K23 AR076453
- L30 AR070514
- P30 AR070253
- P30 AR072577
Footnotes
Ethical Approval Information
This study involves human participants and was approved by an Ethics Committee(s) or Institutional Board(s).
Name of Review Board: Mass General Brigham Institutional Review Board
Protocool # 2021P00763
This study does not involve animal subjects.
Patient and Public Involvement
Patients or the public were not included in the design, conduct, reporting, or dissemination plans of our research.
Data Sharing Statement
Requests for access to unpublished de-identified data will be reviewed by the authors and subject to a data use agreement with Brigham and Women’s Hospital.
References
- [1].Centers for Disease Control and Prevention. Covid-19 vaccines for moderately or severely immunocompromised people. 2021. Dec 10. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
- [2].American College of Rheumatology. Covid-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases. 2021. Dec 10. https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf.
- [3].Leeb BF, Haindl PM, Maktari A, et al. Patient-centered rheumatoid arthritis disease activity assessment by a modified radai. J Rheumatol 2008;35:1294–9. [PubMed] [Google Scholar]
- [4].Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral t helper cell subset drives b cells in rheumatoid arthritis. Nature 2017;542(7639):110–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rubtsov AV, Rubtsova K, Fischer A, et al. Toll-like receptor 7 (tlr7)-driven accumulation of a novel cd11c+ b-cell population is important for the development of autoimmunity. Blood 2011;118(5):1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kerkman PF, Rombouts Y, van der Voort EI, et al. Circulating plasmablasts/plasmacells as a source of anticitrullinated protein antibodies in patients with rheumatoid arthritis. Ann Rheum Dis 2013;72(7):1259–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to unpublished de-identified data will be reviewed by the authors and subject to a data use agreement with Brigham and Women’s Hospital.


