Abstract
Purpose
To describe the phenotype of CLN-associated retinal dystrophy in a subset of patients at the Columbia University Medical Center, United States, and the Hospital das Clínicas de Pernambuco, Brazil, in comparison to the published literature.
Methods
Eleven patients with confirmed biallelic variants in the CLN genes were evaluated via dilated fundus examination, clinical imaging, and full-field electroretinogram. A thorough literature search was conducted to determine previously published variants and associated phenotypes.
Results
Genetic testing confirmed the presence of variants in CLN3, CLN7/MFSD8, CLN8, and GRN/CLN11. Five novel variants were identified, and four novel phenotypes of previously published alleles were described. The phenotype differed among patients with variants in the same gene and sometimes among patients with the same allele.
Conclusions
Substantial phenotypic variability among variants in the CLN genes makes identification of genotype–phenotype or allele–phenotype correlations challenging. Further study is required to establish an extensive database for adequate patient counseling.
Keywords: CLN3, MFSD8, CLN8, GRN, ffERG, nonsyndromic, rod-cone dystrophy, maculopathy
The neuronal ceroid lipofuscinoses (NCLs) are a group of genetically and phenotypically diverse inherited lysosomal storage disorders that commonly result in severe vision loss and progressive neurodegeneration in the form of cognitive deterioration, movement disorders, seizures, and shortened life expectancy.1 To date, 13 unique genes have been identified to cause NCL: PPT1, TPP1, DNAJC5, CLN3, CLN5, CLN6, MFSD8, CLN8, CTSD, GRN, ATP13A2, CTSF, and KCDT7.2
The NCLs vary in age of onset, symptoms at onset, and disease progression. The classic infantile form has an onset between the ages of 6 and 24 months, the late-infantile form at the ages of 2 and 5 years, the juvenile form at the age of 4 and 8 years, and the adult form at the age of 13 to 25.2 Protracted disease courses have also been reported in certain genes, such as CLN3, with age of onset in the third to fifth decade of life.3–5 CLN3-associated NCL, also known as Batten disease, presents as classic juvenile neuronal ceroid lipofuscinosis (JNCL) where ocular symptoms precede neurologic symptoms. JNCL commonly presents between the age of 4 and 8 years with a decrease in central vision and rapidly progresses to severe vision loss in the first decade following disease onset.6,7 The visual acuities of patients with advanced disease may be as low as light perception.1 The onset of neurologic symptoms, such as speech disturbances, seizures, and motor decline, typically occurs between the ages of 10 and 20.6,8 The average life span of these patients is approximately 20 years.6,8
In contrast to CLN3, the other genes of interest in this study do not always present with ocular symptoms first. GRN-associated NCL, also known as CLN11, results in disease onset in early adulthood, between the ages of 20 and 25.2 Of the few reported cases in the literature, several patients have presented first with visual loss followed by generalized seizures 3 to 5 years later,9,10 while others have demonstrated seizures and other neurologic symptoms prior to visual impairment.10,11 On the other hand, CLN8-associated disease causes variant late-infantile onset NCL (vLINCL) and begins with neurologic involvement. Symptoms of CLN8-associated NCL begin between the ages of 2 and 7 years with the onset of seizures and deterioration of motor skills.1 Disease progression is rapid with onset of myoclonus, cognitive impairment, and visual loss within 2 years from the time of diagnosis. Similarly, MFSD8/CLN7 also leads to a form of vLINCL, with symptoms of ataxia and developmental regression beginning between the ages of 3 and 6 years.12–15 Disease progression occurs rapidly with onset of epileptic seizures, psychomotor deterioration, myoclonus, loss of vision, and premature death within the second decade.12–15
In addition to the classic systemic diseases associated with these genes, cases of isolated retinal disease have been reported in the literature.16–21 Herein, we report 11 patients with both isolated retinal and systemic disease positive for biallelic variants in CLN3, MFSD8, CLN8, and GRN. Detailed phenotypic and genetic analysis of the patients’ variants expands the known body of literature, which is especially important for a set of conditions with no known or previously identified phenotype–genotype or phenotype–allele correlation. Further, with phase 1/2 gene therapy clinical trials under way for neurologic disease caused by variants in CLN3 (ClinicalTrials.gov: NCT03770572), a clear understanding of the NCLs is essential.
Methods
Patient Selection
A retrospective review of 11 patients from eight unrelated families with confirmed genetic diagnosis of biallelic variants in the CLN genes was conducted. Clinical evaluations were performed at the Edward S. Harkness Eye Institute at Columbia University Irving Medical Center (New York, NY, USA) and the Department of Ophthalmology of the Hospital das Clínicas de Pernambuco at the Federal University of Pernambuco (Recife, Pernambuco, Brazil). Patients were evaluated by ophthalmic examination, multimodal imaging, and full-field electroretinography (ffERG). Informed consent was waived due to the retrospective nature of the study design and the minimal risk conferred to patients as described in Columbia University Institutional Review Board–approved protocol AAAR8743. All procedures were reviewed and in accordance with the tenets of the Declaration of Helsinki.
Clinical Evaluation
Patients were labeled according to their symptoms at presentation into rod-cone dystrophy, interchangeably referred to as retinitis pigmentosa (RP) for the purpose of this article; cone-rod dystrophy (CRD); and the corresponding systemic disease—patients V and XI. All patients underwent complete ocular examination, including best-corrected visual acuity (BCVA) testing, slit-lamp examination, and dilated fundus examination. Patients’ pupils were maximally dilated with 1% tropicamide and 2.5% phenylephrine before examination. Snellen visual acuities (in feet) were converted into logMAR as per Holladay,22 where values of 2.6, 2.7, and 2.8 were assigned for patients who could only count fingers, perceive hand motion, or had only light perception, respectively.
Imaging studies including spectral domain optical coherence tomography (SD-OCT; Spectralis HRA2; Heidelberg Engineering, Heidelberg, Germany), short wavelength autofluorescence (SW-AF) (Spectralis HRA2, Heidelberg Engineering [patients I–IV, VI–VIII]; Canon, CR2 Plus-AF, Tokyo, Japan [patients V, IX–XI]). Color fundus photography was obtained using 55° Clarus (Carl Zeiss Meditec USA, Inc., Dublin, CA, USA [patients VI–VII]), wide field (PLC, Dunfermline, UK [patients I–IV]), or Canon, CR2 Plus-AF (patients V, IX–XI). ffERG was obtained for 8 of 11 patients using Dawson, Trick, and Litzkow electrodes and Ganzfeld stimulation using a Diagnosys Espion Electrophysiology System (Diagnosys LLC, Lowell, MA, USA [patients I, III–IV, VI–VIII]) or Roland (Ronald Consult, Brandenburg an der Havel, Germany [patients V, XI]) according to the International Society for Clinical Electrophysiology of Vision standards.23
Genetic Analysis
Genomic DNA was isolated from peripheral blood. Samples were analyzed via Spark Therapeutics Invitae Inherited Retinal Disorders Panel, Gene Dx Retinal Dystrophy Xpanded Panel, Massachusetts Eye and Ear Panel for Retinal Genes, Columbia University research whole-exome sequencing, New York Genome Center whole-genome sequencing, and Mendelics Next-Generation Sequencing Epilepsy Panel. All identified variants were evaluated for pathogenicity against published literature and the Leiden Open Variation Database and ClinVar databases.
Results and Discussion
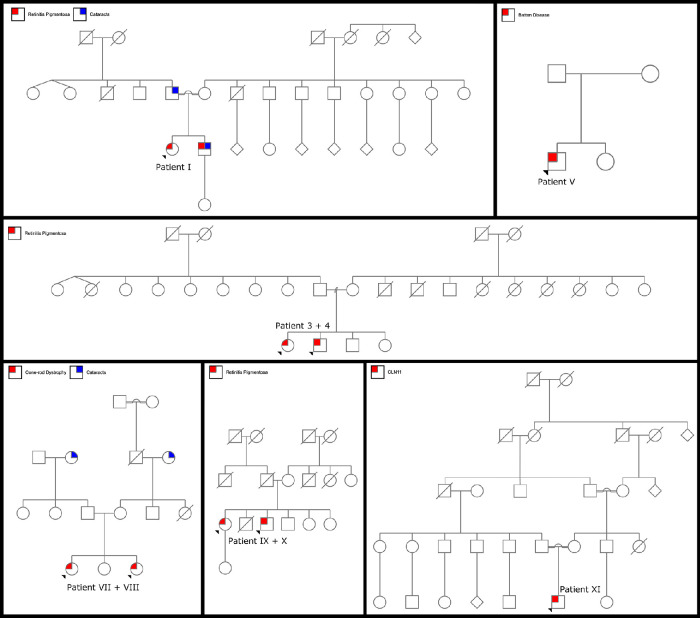
The clinical, genetic, and demographic information of these patients is summarized in Table 1. The age at onset of symptoms ranged from 5 to 27 years, with a mean of 22 years. BCVA ranged from 0 to 2.8 with a mean logMAR score of 0.85. Eight patients had more than one visit. With a range of 1 to 12 visits, the mean follow-up time for these patients with more than one visit was 2.6 years. Pedigrees for each patient are included (Fig. 1). All patients except for patients V, VI, and XI had a positive family history of retinal degeneration. Color fundus photography was available for all patients except patient VIII. Autofluorescence fundus photography and OCT imaging was available for all patients. ffERG testing was obtained in 8 of 11 patients.
Table 1.
Demographic, Genetic, and Clinical Summary of 11 Patients With CLN-Associated Retinal Disease
| Patient | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | I | II | III | IV | V | VI | VII | VIII | IX | X | XI |
| Ethnicity | Haitian | Haitian | Sicilian, Irish | Sicilian, Irish | North Brazilian | Italian | Polish | Polish | Mixed European and African ancestry | Mixed European and African ancestry | North Brazilian |
| Sex | F | F | F | M | M | F | F | F | F | M | M |
| Age at onset, y | 16 | 32 | 19 | 17 | 5 | 18 | 16 | 15 | 37 | 32 | 21 |
| Age at last visit, y | 33 | 38 | 21 | 22 | 7 | 63 | 24 | 17 | 41 | 34 | 22 |
| Ocular symptoms | |||||||||||
| Decreased acuity | – | + | – | – | + | – | + | + | – | – | – |
| Photophobia | – | + | + | – | – | – | + | – | – | – | + |
| Nyctalopia | + | – | + | + | – | + | – | – | + | + | + |
| BCVA at presentation, logMAR | 0.9, 1.1 | 0.4, 0.3 | 0.5, 0.3 | 0.2, 0 | 1.6, 1.6 | 1.8, 1.8 | 0.8, 0.8 | 0.1, 0.1 | 0, 0 | 0, 0 | 0.8, 1 |
| BCVA at last visit, logMAR | 0.7, 1 | NA | 0.3, 0.4 | NA | 2.8, 2.8 | 2.8, 2.8 | 1.8, 1.8 | 0.7, 0.6 | 0, 0 | 0, 0 | NA |
| Neurologic symptoms | |||||||||||
| Seizures | – | – | – | – | + | – | – | – | – | – | + |
| Follow-up, y | 2 | 0 | 2 | 0 | 1 | 11 | 7 | 4 | 1 | 1 | 0 |
| Number of visits | 5 | 1 | 5 | 1 | 3 | 12 | 7 | 4 | 2 | 2 | 1 |
| Diagnosis | arRP | arCRD | arRP | arRP | Batten disease | arRP | arCRD | arCRD | arRP | arRP | CLN11 |
| Electroretinography findings | Extinguished scotopic responses; 30-Hz flicker severely diminished and delayed | No ERG | Scotopic rod response severely diminished, low B/A ratio on scotopic maximum; 30-Hz flicker moderately diminished and delayed | Scotopic rod response severely diminished, low B/A ratio on scotopic maximum; 30-Hz flicker moderately diminished and delayed | Extinguished scotopic and photopic responses | Extinguished scotopic responses; 30-Hz flicker severely diminished and delayed | Extinguished scotopic responses; 30-Hz flicker moderately diminished and delayed | Scotopic rod WNL, low B/A ratio on scotopic maximum; 30-Hz flicker borderline diminished without delay | No ERG | No ERG | Scotopic rod response severely diminished, low B/A ratio on scotopic maximum; 30-Hz flicker borderline diminished without delay |
| Consanguinity | Yes | Yes | No | No | No | No | Yes | Yes | No | No | Yes |
| Gene | CLN3 | CLN3 | CLN3 | CLN3 | CLN3 | MFSD8 | MFSD8 | MFSD8 | CLN8 | CLN8 | GRN |
| Variant 1 | c.884A>G p.Glu295Gly | c.175G>A p.Ala59Thr | Deletion of exons 8–9 | Deletion of exons 8–9 | Deletion of exons 8–9 | c.1361T>C p.Met454Thr | c.1361T>C p.Met454Thr | c.1361T>C p.Met454Thr | c.509C>G p.Thr170Arg | c.509C>G p.Thr170Arg | c.767_768insCC p.Ile256ThrfsTer28 |
| Variant 2 | Homozygous | Homozygous | c.1213C>T p.Arg405Trp | c.1213C>T p.Arg405Trp | Homozygous | c.863+2dup | c.754+2T>A | c.754+2T>A | c.779C>T p.Pro260Leu | c.779C>T p.Pro260Leu | Homozygous |
arCRD, autosomal recessive cone rod dystrophy; arRP, autosomal recessive retinitis pigmentosa; NA, non-applicable.
Figure 1.
Pedigrees for nine patients with confirmed biallelic variants in the CLN genes. Patients I, III, IV, and V have two confirmed variants in the CLN3 gene. Patients VII and VIII have two confirmed variants in the MFSD8/CLN gene. Patients IX and X have two confirmed variants in the CLN8 gene. Patient X has two confirmed variants in the GRN/CLN11 gene.
CLN3
Clinical Summary
Patient I experienced trouble with nighttime driving at the age of 16. At the age of 22, the patient was diagnosed incidentally on routine eye examination and referred to a retinal specialist. Patient II noticed poor central vision and photophobia at the age of 33. She presented for clinical correlation following genetic testing. Patient III presented at the age of 19 for flashing lights but notes that she has had nyctalopia since childhood. Her brother, patient IV, presented after genetic testing revealed he was positive for the same genetic variants as his sister, noting he had also experienced nyctalopia since childhood. At the age of 5, patient V began to experience a rapid decrease in central and peripheral vision with color blindness. He presented for a precise diagnosis of his disease.
Consanguinity was reported in patients I and II, both of whom were Haitian in origin; the parents of the probands were first and second cousins, respectively. Patient I had an affected brother with the same genetic variant. Patient II had a father diagnosed with cone-rod dystrophy and an older brother diagnosed with retinitis pigmentosa; no genetic results were available for either family member. Patients III, IV, and V did not report consanguinity. While siblings III and IV were both affected, no other family members were affected. Patients I to IV had no history or any signs or symptoms of neurologic disease. Patient V, now age 7, had undergone extensive neurologic evaluation, which revealed no abnormalities. Magnetic resonance imaging of the brain was also within normal limits.
Imaging and Electrophysiology
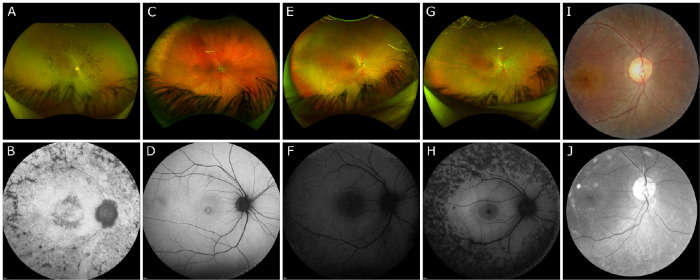
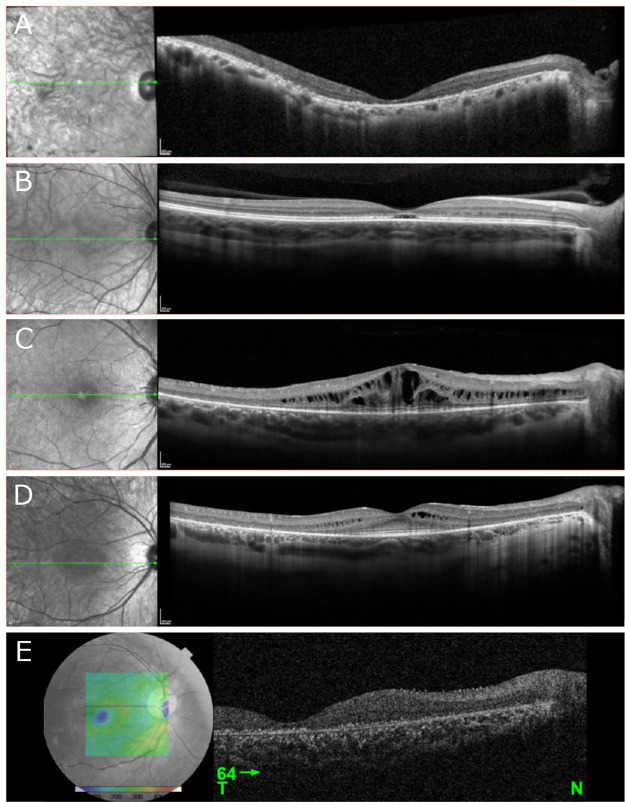
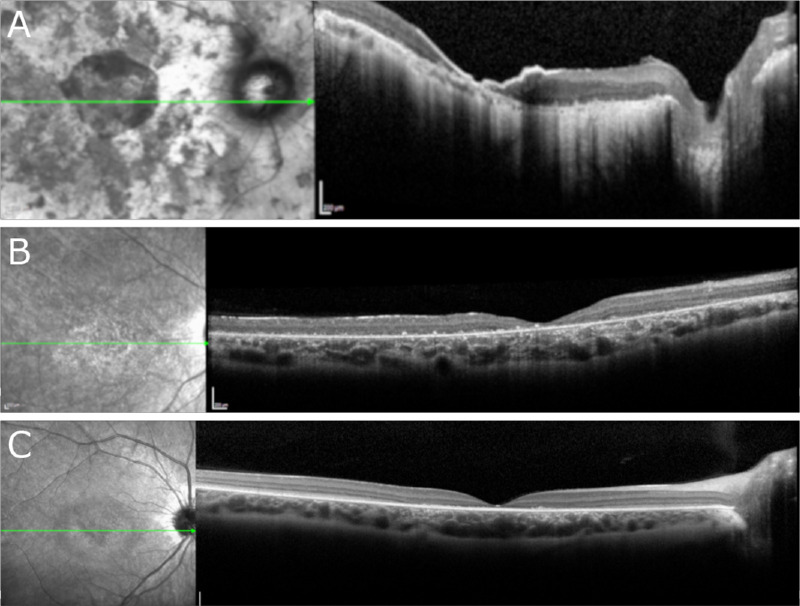
Wide-field Optos fundus photography of patient I demonstrated a mid-peripheral ring sparing the arcades (Fig. 2A). Color fundus photography in patients II to V was largely unremarkable (Figs. 2C, E, G, I). SW-AF of patient I demonstrated a ring of bone spicules outside the arcades with a smaller ring of hypoautofluorescence surrounding the fovea (Fig. 2B). Patient II demonstrated bull's-eye atrophy in the macula on SW-AF (Fig. 2D). SW-AF for patient III revealed bone spicules outside the arcades sparing the superior retina and cystoid macula edema (CME) (Fig. 2F). Patient IV showed bone spicules adjacent to the arcades and a central hypoautofluorescent ring on SW-AF (Fig. 2H). Red-free fundus photography for patient V revealed no abnormal findings (Fig. 2J). OCT imaging of patients I and V revealed diffuse retinal thinning, loss of outer retinal structure, and loss of the ellipsoid zone (Figs. 3A, E). Patient II demonstrated normal retinal architecture with foveal optical gap on OCT (Fig. 3B). OCT of patients III and IV revealed severe and mild CME, respectively, with preservation of the ellipsoid zone within the fovea and peripheral retinal thinning and loss of outer retinal layers (Figs. 3C, D).
Figure 2.
Color and autofluorescence fundus photography in five patients with confirmed biallelic variants in CLN3. (A, B) On wide-field Optos imaging, patient I demonstrated a mid-peripheral ring of pigmentation consistent with bone spicules, sparing the arcades. SW-AF revealed hypoautofluorescence consistent with a ring of bone spicules outside the arcades with a smaller ring of hypoautofluorescence surrounding the fovea. (C, D) Patient II had no abnormal findings on wide-field Optos imaging but demonstrated a hypoautofluorescent pattern of bull's-eye atrophy in the macula on SW-AF. (E, F) Wide-field Optos imaging was within normal limits for patient III. SW-AF revealed bone spicules outside the arcades sparing the superior retina and cystoid macula edema. (G, H) Patient IV had no abnormal findings on wide-field Optos imaging. SW-AF demonstrated bone spicules adjacent to the arcades and a relatively preserved ring of autofluorescence surrounding foveal hypoautofluorescence. (I, J) Both Zeiss fundus and red-free fundus photography revealed no abnormalities for patient V.
Figure 3.
OCT performed in five patients with confirmed biallelic variants in CLN3. (A, E) OCT imaging revealed diffuse retinal thinning, loss of outer retinal structure, and loss of the ellipsoid zone in patients I and V, respectively. (B) Imaging of patient II demonstrated normal extrafoveal architecture with a foveal optical gap. (C, D) Patients III and IV demonstrated severe and mild cystoid macular edema, respectively, with preservation of the ellipsoid zone within the fovea and peripheral retinal thinning and loss of outer retinal layers.
ffERG testing of patient I revealed extinguished scotopic responses bilaterally with severely diminished and delayed 30-Hz flicker responses (Table 1). No ffERG testing was conducted for patient II. Patients III and IV both showed severely diminished scotopic rod response with low B/A ratios on scotopic maximum testing. These patients also demonstrated moderately diminished and delayed 30-Hz flicker testing. Both scotopic and photopic responses were completely extinguished on ffERG testing of patient V.
Analysis by Genotype
The most common variant in this cohort of patients was a large 1.02-kb deletion affecting exons 8 and 9 [NM_001042432.1], previously known as exons 7 and 8 [NM_000086.2], found among three patients: patients III and IV, who were heterozygous for the variant, and patient V, who was homozygous. This variant has been well characterized and is known to cause Batten disease when homozygous,1,24,25 as seen in patient V. Further, patients who are heterozygous for the 1.02-kb deletion have presented with both protracted disease courses and isolated retinal disease.3–5,16–19,21,26,27
Patients III and IV were heterozygous for the 1.02-kb deletion in association with the c.1213C>T;p.(Arg405Trp) variant, which has been previously reported in both homozygous and compound heterozygous states (Table 2).18,26,27 Among the eight published cases of isolated retinal disease, the ocular phenotype is consistent with a diagnosis of RP, including symptoms of nyctalopia beginning between age 10 and 30 years. Seven of eight patients, now aged 36 to 60 years, have no evidence of neurologic disease at most recent follow-up. Only one patient, homozygous for this variant, now age 30, has developed neurologic symptoms of cogwheel rigidity and word-finding difficulties.27 Patients III and IV have a phenotype consistent with the published literature of RP but they may be too young, at the ages of 21 and 22 years, to determine if they will have an isolated or protracted disease course. It is important to note, however, that a previously published patient with the same combination of variants as patients III and IV, who was last examined at age 49 years, has not developed neurologic symptoms (Table 2).18 Future study is required to understand the interactions between these variant combinations, as well as the presence of possible modifying gene or genes, as the literature has reported patients homozygous for the c.1213C>T;p.(Arg405Trp) who have developed neurologic symptoms, whereas patients reported to be compound heterozygous, even in combination with the 1.02-kb deletion known to cause Batten disease, have not developed neurologic problems.
Table 2.
Previously Unpublished Genotypes and Phenotypes of Study Patients
| Characteristic | Patient(s) Affected | Phenotype | Novel? | Published Patient 1 | Published Patient 2 | Published Patient 3 |
|---|---|---|---|---|---|---|
| CLN3 | ||||||
| c.175G>A p.Ala59Thr | II (homozygous) |
Visual symptoms onset at age 32 as decreased central vision, diagnosed with CRD; now age 38 without neurologic involvement | No | Patient c.175G>A;p.(Ala59Thr) heterozygous in combination with 1.02-kb deletion; visual symptoms onset at age 36 as nyctalopia and tunnel vision, diagnosed with RP; age 50 diagnosed with AIR with decreased central vision; now age 56 without neurologic involvement (Chen et al.16) | NA | NA |
| c.1213C>T p.Arg405Trp | III–IV (heterozygous) | Siblings heterozygous in combination with 1.02-kb deletion; visual symptoms onset in teens as nyctalopia, diagnosed with RP; ERG low B/A ratio; now age 21 and 22 without neurologic involvement | No | Two homozygous patients, visual symptoms onset in 30s as nyctalopia, ERG low B/A ratio; now age 36 and 51 without neurologic involvement (Ku et al.18) | Patient c.1213C>T;p.(Arg405Trp) heterozygous in combination with 1.02-kb deletion; visual symptoms onset before age 10 as tunnel vision; now age 49 without neurologic involvement (Ku et al.18) | Patient homozygous, visual symptoms onset at age 12 as nyctalopia and tunnel vision; experienced cogwheel rigidity at age 25; now age 30 with word-finding difficulty (Kuper et al.27) |
| Deletion of exons 8–9 | III–IV (heterozygous) and V (homozygous) | Heterozygous—see above; homozygous—visual symptoms onset at age 5, diagnosed with Batten disease; now age 7 LP without neurologic involvement | No | Many reports of Batten disease versus isolated retinal disease, most common variant, very well studied | ||
| MFSD8 | ||||||
| c.1361T>C p.(Met454Thr) | VI–VIII (heterozygous) | See below | No | 3 siblings all heterozygous in combination with c.1235C>T;p.(Pro412Leu); visual symptoms onset at ages 15–20 as decreased central vision; nyctalopia starting at the ages of 38–40 years; now aged 40–50 without neurologic involvement (Smirnov et al.20) | 2 siblings, both homozygous; visual symptoms onset at ages 15–20 as decreased central vision; nyctalopia starting at the ages of 38–40 years old; now aged 40–50 without neurologic involvement (Smirnov et al.20) | 6 patients from 6 unrelated families, all homozygous; decreased central vision onset between ages 19 and 30, nyctalopia later; longest follow-up to age 78, all patients without neurologic involvement (Khan et al.17) |
| c.754+2T>A | VII–VIII (heterozygous) | Siblings heterozygous in combination with c.1361T>C;p.(Met454Thr); visual symptoms onset in teens with decreased central vision, diagnosed with CRD; now 24 and 17 without neurologic involvement | No | Patient homozygous; diagnosed with typical vLINCL, motor symptoms onset before visual symptoms; optic atrophy diagnosed at age 5 (Siintola et al.15) | Patient heterozygous in combination with c.1103- 2delA; diagnosed with typical vLINCL, motor symptoms before visual symptoms; optic atrophy diagnosed at age 5 (Kousi et al.12) | NA |
When available, similar genotypes with the corresponding phenotype are presented for comparison. AIR, autoimmune retinopathy; LP, light perception.
Patient II presented homozygous for a previously reported variant, c.175G>A;p.(Ala59Thr), in association with a novel phenotype. One patient has been published in the literature with this variant: a compound heterozygote in combination with the 1.02-kb deletion.16 The published patient presented at the age of 36 years for nyctalopia with progressive visual field loss and was diagnosed with atypical RP. Patient II first experienced decreased central acuity and photophobia and was diagnosed with CRD. The difference in these ocular phenotypes is unusual and may potentially be explained by the homozygosity of the variant identified in patient II as compared to the compound heterozygosity of the variant seen in the patient in the literature, similar to how patients who are homozygous for the 1.02-kb deletion develop Batten disease while those who are compound heterozygous may present with isolated retinal disease. Further study is needed to determine the expression of CLN3 variants between rod and cone photoreceptors and potentially understand the cause of this phenotypic variability of rod-cone versus cone-rod dystrophy between genotypes.
The final unique variant in our cohort was found in patient I, who was homozygous for a novel variant: c.884A>G;p.(Glu295Gly). Patient I presented with nyctalopia at the age of 16 and was diagnosed with RP. Now at the age of 33 years, the patient has no neurologic symptoms, suggesting a likely case of isolated retinal disease. However, a previously published variant affecting the same amino acid, c.883G>A;p.(Glu295Lys), has been known to cause protracted JNCL3–5 with onset of neurologic symptoms as late as 45 years and death secondary to epileptic complications as late as age 5.4 As both variants affect the same amino acid residue, it is worth discussing the amino acid changes. Glutamate contains an acidic, polar side chain while glycine has a neutral, nonpolar side chain, and lysine contains a basic, polar side chain.28 While the change from glutamate to glycine replaces a large, hydrophilic side chain with a small, neutral side change, replacing glutamate with lysine introduces a basic side chain in place of an acidic side chain.28 Further study is required to establish the severity of these two variants on protein structure and function. Even with the differences between the amino acid change, these genotypes may produce a similar disease course. As such, patient I should undergo careful neurologic evaluation to monitor for the development of subtle neurologic symptoms.
With a phase 1/2 gene therapy clinical trial currently under way for neurologic disease associated with Batten disease (ClinicalTrials.gov: NCT03770572), it is incredibly important to characterize variants responsible for protracted JNCL versus isolated retinal disease. Patients with a seemingly isolated retinal disease course may develop neurologic symptoms later in life, such as in the case of c.1213C>T;p.(Arg405Trp)27 or c.883G>A;p.(Glu295Lys)4 disease, and as such may benefit from future treatments available for neurologic symptoms of CLN3-associated neurologic disease.
MFSD8/CLN7
Clinical Summary
Patient VI was diagnosed at the age of 18 years due to the onset of nyctalopia. She presented for care following retirement of her previous provider. Patient VII experienced a sudden decrease in vision over a period of 4 months and an increase in photophobia at the age of 16 years. The patient was referred for specialized care. Her sister, patient VIII, presented following familial genetic testing, which showed she was positive of the same genetic variant as her sister. At presentation, she was experiencing decreased central acuity. Patient VI did not report consanguinity. Patients VII and VIII noted their great grandparents were first cousins. While siblings VII and VIII were both affected, no other family members were affected. Patients VI to VIII had no history or any signs or symptoms of neurologic disease.
Imaging and Electrophysiology
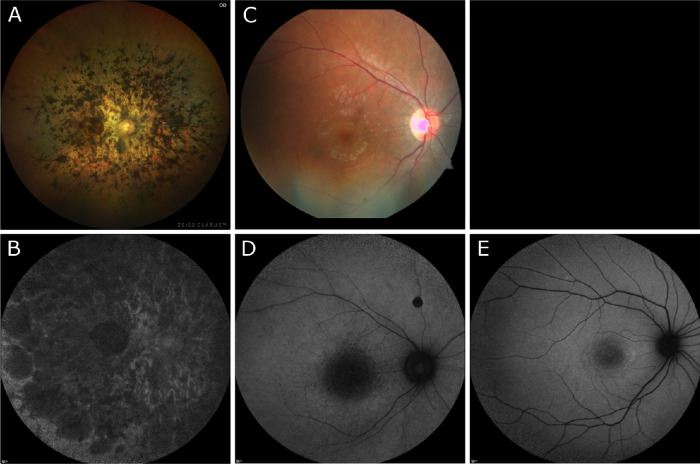
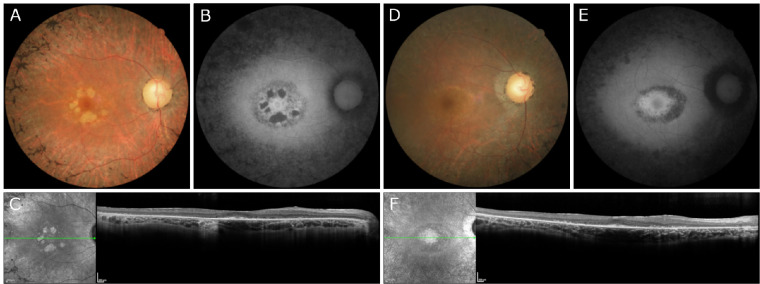
Zeiss Clarus imaging of patient VI demonstrated dense pigment migration progressing inward from the mid-periphery and encroaching on the macula (Fig. 4A). Zeiss color imaging of patient VII showed bull's-eye macular atrophy (Fig. 4C). No color images were available for patient VIII. SW-AF of patient VI demonstrated diffuse patchy atrophy throughout the macula and beyond the arcades (Fig. 4B). SW-AF in patient VII revealed hypoautofluorescence in the macula indicative of substantial atrophy (Fig. 4D). Patient VIII demonstrated bull's-eye atrophy in the macula on SW-AF (Fig. 4E). OCT imaging of patients VI and VII revealed diffuse retinal thinning, loss of outer retinal structure, and loss of the ellipsoid zone in patients VI to VII (Figs. 5A, B). Patient VIII demonstrated foveal loss of retinal layers with preservation of retinal architecture peripherally on OCT (Fig. 5C).
Figure 4.
Color and autofluorescence fundus photography in three patients with confirmed biallelic variants in MFSD8/CLN7. (A, B) Zeiss Clarus fundus photos of patient VI demonstrated relatively dense pigment migration extending across the mid-periphery and macula. SW-AF showed bone spicules adjacent to the arcades and a relatively preserved ring of autofluorescence surrounding foveal hypoautofluorescence. (C, D) Patient VII demonstrated bull's-eye macular atrophy on Zeiss fundus photography, and SW-AF revealed hypoautofluorescence in the macula indicative of substantial atrophy. (E) No color imaging was obtained for patient VIII. SW-AF demonstrated a hypoautofluorescent pattern of bull's-eye atrophy in the macula.
Figure 5.
OCT performed in three patients with confirmed biallelic variants in MFSD8/CLN7. (A, B) OCT imaging revealed diffuse retinal thinning, loss of outer retinal structure, and loss of the ellipsoid zone in patients VI and VII, respectively. (C) OCT of patient VIII showed an isolated loss of retinal layers in the fovea.
ffERG testing of patients VI and VII demonstrated extinguished scotopic responses bilaterally and severely diminished and delayed responses on 30-Hz flicker testing (Table 1). ffERG of patient VIII demonstrated diminished scotopic rod and maximum responses with a low B/A ratio and moderately diminished amplitudes, borderline normal, with no implicit time delay on 30-Hz flicker testing (Table 1). Patients VII and VIII were diagnosed with cone-rod dystrophy clinically. While the clinical presentation of patient VIII was consistent with cone-rod dystrophy, the ffERG testing can be described as macular dystrophy.
Analysis by Genotype
Patients VI to VIII presented with biallelic variants in CLN7/MFSD8 and were all found to have novel phenotypes. All three patients were heterozygous for the c.1361T>C;p.(Met454Thr) variant. This variant has been associated previously, independent of homozygosity or compound heterozygosity, with systemic vLINCL21,29 and isolated CRD (Table 2).17,21 Of the previously described patients in the literature, 11 have not developed neurologic symptoms, the oldest of whom was 78 years old at latest evaluation. Patient VI, who was compound heterozygous for the c.1361T>C;p.(Met454Thr) variant in combination with a novel variant c.863+2, presented with nyctalopia at age 19 years and was diagnosed with RP, as opposed to CRD. Further study of this novel variant, specifically its effect on gene splicing and its position within the 12 predicted transmembrane domains of the MFSD8 gene,15 may elucidate the cause of the novel phenotype described in patient VI.
While patients VII and VIII have a phenotype of CRD consistent with the published literature of isolated retinal disease in the c.1361T>C;p.(Met454Thr) variant, the second variant, c.754+2T>A, has been previously associated only with syndromic vLINCL disease in both homozygous and compound heterozygous states (Table 2).12,15 This novel presentation of isolated retinal disease associated with the c.754+2T>A variant can be attributed to its unique combination with the c.1361T>C;p.(Met454Thr) variant. The c.754+2T>A variant is located in intron 8 of the MFSD8 gene and leads to altered splicing,15 while the c.1361T>C variant is located within the 11th transmembrane domain and affects protein folding.29 It is possible that the combination of these variants produces a milder protein defect than each variant individually, explaining the novel phenotype of isolated retinal disease associated with the c.7542T>A variant.
Overall, the phenotypic variability in the MFSD8 gene is extreme. Several authors have proposed a spectrum of disease from isolated macular involvement to generalized rod-cone dystrophy to systemic vLINCL.17 Specifically, Priluck and Breazzano30 describe a case of nonsyndromic macular dystrophy in a patient with compound heterozygous variants c.154G>A;p.(Gly52Arg) and c.1006G>C;p.(Gluc336Gln), corresponding to exons 3 and 11, presenting with foveal optical gap and no peripheral disease. Further study is required to understand the interactions between different alleles and their combined effects on protein structure and function that could explain this phenotypic variability.
CLN8
Clinical Summary
Patients IX and X began experiencing nyctalopia at ages 37 and 32 years, respectively. They presented for specialized care. While siblings IX and X were both affected, no other family members were affected. No consanguinity was reported. Patient IX had no history or any signs or symptoms of neurologic disease. Patient X had a history of mild childlike behavior; the remainder of the neurologic examination was unremarkable.
Imaging and Electrophysiology
Color fundus photography in patients IX and X revealed perifoveal atrophy with the presence of bone spicules along the vascular arcades (Figs. 6A, D). On SW-AF, patients IX and X demonstrated bone spicules around the periphery with a relatively preserved paramacular ring of autofluorescence and perifoveal atrophy (Figs. 6B, E). Further, patient IX showed patchy retinal pigment epithelium (RPE) atrophy within the macula (Fig. 6E). OCT imaging of patients IX and X showed preservation of ellipsoid zone within the fovea and peripheral retinal thinning and loss of outer retinal layers (Figs. 6C, F). No ffERG testing was conducted for either patient.
Figure 6.
Fundus photography and OCT performed in two patients with confirmed biallelic variants in CLN8. (A, D) Zeiss Clarus fundus photography demonstrated perifoveal atrophy with the presence of bone spicules along the vascular arcades in patients IX and X, respectively. (B, E) SW-AF of patients IX and X, respectively, revealed bone spicules surrounding the arcades 360 degrees with central hyperautofluorescent focus. (C, F) OCT imaging of patients IX and X, respectively, showed preservation of ellipsoid zone within the fovea and loss of outer retinal layers in the periphery.
Analysis by Genotype
Patients IX and X were heterozygous for two novel variants in CLN8: c.509C>G;p.(Thr170Arg) and c.779C>T;p.(Pro260Leu). Both patients began experiencing nyctalopia at ages 37 and 32 years, respectively. Neither patient, now 41 and 34 years, experienced neurologic symptoms besides a mild intellectual disability described as childish behavior in patient XI. From this history, it can be assumed that patients X and XI have a course of isolated retinal disease. To the best of our knowledge, this is the first report of nonsystemic CLN8 disease. In addition to the classical form of CLN8 vLINCL, which typically presents with seizure onset during childhood followed by rapid disease progression to myoclonus, cognitive impairment, and visual loss within 2 years from the time of diagnosis,1 reports of a milder disease course have also been described.31 Sanchez et al.31 describe two siblings who presented with seizures and retinitis pigmentosa. Sibling 1 experienced seizures at age 7 years followed by the onset of ocular symptom at age 13 years, while sibling 2 experienced ocular symptoms at age 5 years, prior to seizure onset at age 16 years. Both patients, described at ages 13 and 22 years, have not experienced progression of neurologic symptoms. These cases, in combination with the phenotype identified in patients IX and X, suggest three distinct phenotypes of CLN8 disease: isolated retinal disease, mild systemic disease, and vLNCL.
GRN/CLN11
Clinical Summary
Patient XI presented at the age of 21 years for evaluation of photophobia and progressive nyctalopia after a causative variant was identified on genetic testing conducted for refractive seizures. Patient XI reported consanguinity in the family. Patient XI had a history of generalized tonic-clonic seizures beginning at the age of 16 years. No other neurologic symptoms were present at the latest follow-up.
Imaging and Electrophysiology
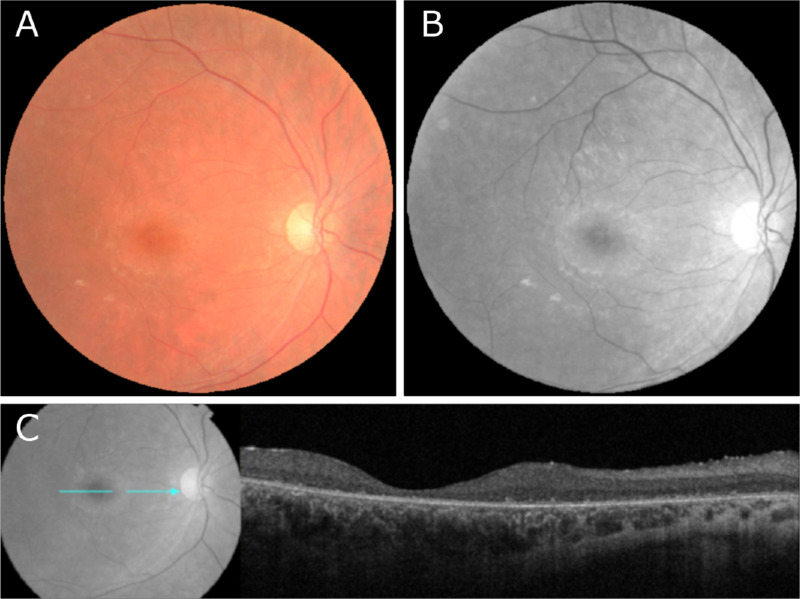
Color and red-free fundus photography in patient XI was largely unremarkable (Figs. 7A, B). OCT imaging of patient XI revealed diffuse retinal thinning, loss of outer retinal structure, and loss of the ellipsoid zone (Fig. 7C). ffERG testing revealed severely diminished scotopic rod response, a low B/A ratio on scotopic maximum response, and 30-Hz flicker testing with borderline diminished responses without delay (Table 1).
Figure 7.
Fundus photography and OCT performed in a patient with confirmed biallelic variants in GRN/CLN11. (A, B) Zeiss color and red-free autofluorescence imaging in patient XI revealed no abnormalities. (C) OCT imaging of patient XI revealed diffuse retinal thinning, loss of outer retinal structure, and loss of the ellipsoid zone.
Analysis by Genotype
Patient XI was homozygous for the GRN novel variant c.767_768insCC;p.(Ile256ThrfsTer28) and demonstrated a typical course of systemic CLN11 disease. A similar genotype has been reported in two patients previously, c.768_769dup;p.(Gln257frameshift), once in association with systemic CLN11 disease and once in association with spastic ataxia without ocular symptoms (Table 3).11,32 Although previously assumed that homozygous GRN variants lead to CLN11, of the 11 with homozygous variants in GRN, 4 have presented with atypical disease course, with 2 developing ocular symptoms prior to neurologic disease and 2 never developing ocular symptoms at all.32 Huin et al.32 have proposed that this finding could be due to hypomorphic variants as studies of progranulin levels, the protein product of the GRN gene, showed residual progranulin levels in homozygous patients similar to heterozygous patients. We agree that the differences in protein synthesis caused by different variants may explain the phenotypic variability in GRN-associated disease and suggest similar studies of protein product should be conducted for the other CLN genes. Further, we highlight the importance of proper patient follow-up and educated counseling for patients with variants in GRN asNA three patients with seemingly isolated RP have developed neurologic symptoms consistent with systemic CLN11 disease 17 to 41 years after the onset of visual symptoms.32
Table 3.
Previously Published Genotypes and Phenotypes of Study Patients in Comparison to the Genotype and Phenotypes Found in the Literature
| Characteristic | Patient(s) Affected | Phenotype | Novel? | Similar Genotype? | Published Patient 1 | Published Patient 2 | Published Patient 3 |
|---|---|---|---|---|---|---|---|
| CLN3 | |||||||
| c.884A>G p.Glu295Gly | I (homozygous) | Visual symptoms onset age 16 as nyctalopia, diagnosed with RP, BCVA 0.7 and 1.0; now age 33 without neurologic involvement | Yes | Yes | Patient c.883G>A;p.(Glu295Lys) heterozygous in combination with 1.02-kb deletion; protracted systemic disease; visual symptoms age 6, blind age 14, neurologic symptoms age 20 (panic attacks), seizures age 37, died age 39 (Aberg et al.3) | Siblings c.883G>A;p.(Glu295Lys) heterozygous in combination with 1.02-kb deletion; protracted systemic disease; visual symptoms onset at age 5 and 5, blind age 12 and 13; sibling 1, neurologic symptoms age 45, seizures age 50, died age 51; sibling 2, age 39, no neurologic symptoms (Wisniewski et al.4) | Patient c.883G>A;p.(Glu295Lys) heterozygous in combination with 1.02-kb deletion; protracted systemic disease; visual symptoms age 6, blind age 13; neurologic symptoms age 19 (panic attacks), now age 30 with polyneuropathy (Lauronen et al.5) |
| MFSD8 | |||||||
| c.863+2dup | VI (heterozygous) | Patient heterozygous in combination with c.1361T>C;p.(Met454Thr); visual symptoms onset age 19 as nyctalopia and tunnel vision, diagnosed with RP; now age 63 without neurologic involvement | Yes | Yes | Patient homozygous for c.863+1G>C splice cite variant; diagnosed with vLINCL; motor symptoms onset age 2 years, seizures age 3 (Kousi et al.12) | Patient heterozygous c.863+3_4insT splice cite variant in combination with c.154G>A;p.(Gly52Arg); diagnosed with vLINCL; motor symptoms onset age 3 years, seizures age 4 (Aiello et al.34) | Patient heterozygous c.863+3_4insT splice cite variant in combination with c.2T>C;p.(Met1Thr); diagnosed with vLINCL; motor symptoms onset age 5 years, seizures age 7 (Aiello et al.34) |
| CLN8 | |||||||
| c.509C>G p.Thr170Arg | IX–X (heterozygous) | Siblings heterozygous in combination with c.779C>T;p.(Pro260Leu); visual symptoms onset age 37 and 32 as nyctalopia; now age 41 and 34 without neurologic involvement | Yes | Yes | Siblings homozygous for c.509C>T;p.(Thr170Met); symptoms onset age 4 and age 8 with isolated neurologic symptoms without vision problems (Mitchell et al.33) | NA | NA |
| c.779C>T p.Pro260Leu | IX–X (heterozygous) | Siblings heterozygous in combination with c.509C>G;p.(Thr170Arg); visual symptoms onset age 37 and 32 as nyctalopia; now age 41 and 34 without neurologic involvement | Yes | No | NA | NA | NA |
| GRN | |||||||
| c.767_768insCC p.Ile256ThrfsTer28 | XI (homozygous) | Neurologic symptoms onset age 16 as seizures; visual symptoms onset age 19 as nyctalopia and tunnel vision; age 22 difficulty walking; diagnosed with CLN11 | Yes | Yes | Patient homozygous for c.768_769dup;p.(Gln257frameshift); neurologic symptoms onset age 21, seizures at age 25; eye exam unable due to poor cooperation (Faber et al.11) | Patient homozygous for c.768_769dup;p.(Gln257frameshift); neurologic symptoms onset age 16 as seizures; age 19 diagnosed with RP for significant visual loss; myoclonus age 26, died age 27 (Huin et al.32) |
Conclusion
Herein we report nine patients with isolated retinal disease and two patients with systemic NCL disease, all with confirmed variants in four genes previously associated with systemic NCL. Five patients (I–V) with biallelic variants in CLN3 were included, and only patient V had a previous diagnosis of Batten disease. Five of the variants identified in these patients were unique and one was novel. A total of three patients (VI–VIII) with two confirmed variants in MFSD8/CLN7 were evaluated, none of whom had neurologic symptoms at most recent presentation. Of the three unique variants identified in these patients, one was novel. Two siblings (IX–X) with biallelic novel variants in CLN8 were included, and one patient (XI) with a novel homozygous variant in GRN/CLN11 with systemic NCL was also included in this study. With this report, we hope to expand the known phenotypes for each variant, presenting previously unpublished genotypes and phenotypes.
The wide phenotypic variability of the CLN genes makes a phenotype–genotype correlation difficult to establish. Further, no apparent allele–phenotype correlation can be drawn as patients homozygous for an allele can present with isolated retinal disease, in the form of RP and CRD, or systemic disease. We hypothesize that this phenotypic variability is a result of the presence of a modifying gene or genes, interactions between individual alleles leading to additive effects, hypomorphic effects of different variants, and/or the effects of changes to different domains of the CLN genes. Further study is required to elucidate the cause of this phenotypic variability. Until such a time when a genotype–phenotype correlation can be established, clinicians must take extra care when counseling patients as seemingly isolated retinal courses of disease can potentially be devastating protracted disease courses; studies have reported the onset of neurologic complications as late as 45 years of age. Extensive study is required to establish a correlation, if it exists, to help counsel patients on their expected prognosis and determine if patients with presumed isolated retinal disease should be considered for treatment of neurologic symptoms such as those currently under development for CLN3 systemic disease.
Acknowledgments
The Jonas Children's Vision Center is supported by the NIH R01EY09076, 5P30CA013696, U01 EY030580, U54OD020351, R24EY028758, R24EY027285, 5P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437, the Schneeweiss Stem Cell Fund, New York State [SDHDOH01-C32590GG-3450000], the Foundation Fighting Blindness New York Regional Research Center Grant [TA-NMT-0116-0692-COLU], Nancy & Kobi Karp, the Crowley Family Funds, The Rosenbaum Family Foundation, Alcon Research Institute, the Gebroe Family Foundation, the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA.
Disclosure: M. Kolesnikova, None; J.R. Lima de Carvalho Jr., None; J.K. Oh, None; M. Soucy, None; A. Demirkol, None; A.H. Kim, None; S.H. Tsang, Receives financial support from Abeona Therapeutics, Inc. and Emendo. He is also the founder of Rejuvitas and is on the scientific and clinical advisory board for Nanoscope Therapeutics and Medical Excellence Capital; M.P. Breazzano, None
References
- 1. Mole SE. The genetic spectrum of human neuronal ceroid-lipofuscinoses. Brain Pathol. 2004; 14: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mole SE, Anderson G, Band HA, et al.. Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis. Lancet Neurol. 2019; 18: 107–116. [DOI] [PubMed] [Google Scholar]
- 3. Aberg L, Lauronen L, Hamalainen J, Mole SE, Autti T.. A 30-year follow-up of a neuronal ceroid lipofuscinosis patient with mutations in CLN3 and protracted disease course. Pediatr Neurol. 2009; 40: 134–137. [DOI] [PubMed] [Google Scholar]
- 4. Wisniewski KE, Zhong N, Kaczmarski W, et al.. Compound heterozygous genotype is associated with protracted juvenile neuronal ceroid lipofuscinosis. Ann Neurol. 1998; 43: 106–110. [DOI] [PubMed] [Google Scholar]
- 5. Lauronen L, Munroe PB, Jarvela I, et al.. Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology. 1999; 52: 360–365. [DOI] [PubMed] [Google Scholar]
- 6. Wisniewski KE, Zhong N, Philippart M.. Pheno/genotypic correlations of neuronal ceroid lipofuscinoses. Neurology. 2001; 57: 576–581. [DOI] [PubMed] [Google Scholar]
- 7. Bohra LI, Weizer JS, Lee AG, Lewis RA.. Vision loss as the presenting sign in juvenile neuronal ceroid lipofuscinosis. J Neuroophthalmol. 2000; 20: 111–115. [DOI] [PubMed] [Google Scholar]
- 8. Williams RE, Aberg L, Autti T, Goebel HH, Kohlschutter A, Lonnqvist T.. Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochim Biophys Acta. 2006; 1762: 865–872. [DOI] [PubMed] [Google Scholar]
- 9. Smith KR, Damiano J, Franceschetti S, et al.. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012; 90: 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canafoglia L, Morbin M, Scaioli V, et al.. Recurrent generalized seizures, visual loss, and palinopsia as phenotypic features of neuronal ceroid lipofuscinosis due to progranulin gene mutation. Epilepsia. 2014; 55: e56–e59. [DOI] [PubMed] [Google Scholar]
- 11. Faber I, Prota JR, Martinez AR, Lopes-Cendes I, Franca MCJ.. A new phenotype associated with homozygous GRN mutations: complicated spastic paraplegia. Eur J Neurol. 2017; 24: e3–e4. [DOI] [PubMed] [Google Scholar]
- 12. Kousi M, Siintola E, Dvorakova L, et al.. Mutations in CLN7/MFSD8 are a common cause of variant late-infantile neuronal ceroid lipofuscinosis. Brain. 2009; 132: 810–819. [DOI] [PubMed] [Google Scholar]
- 13. Topcu M, Tan H, Yalnizoglu D, et al.. Evaluation of 36 patients from Turkey with neuronal ceroid lipofuscinosis: clinical, neurophysiological, neuroradiological and histopathologic studies. Turk J Pediatr. 2004; 46: 1–10. [PubMed] [Google Scholar]
- 14. Wheeler RB, Sharp JD, Mitchell WA, et al.. A new locus for variant late infantile neuronal ceroid lipofuscinosis-CLN7. Mol Genet Metab. 1999; 66: 337–338. [DOI] [PubMed] [Google Scholar]
- 15. Siintola E, Topcu M, Aula N, et al.. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Hum Genet. 2007; 81: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen FK, Zhang X, Eintracht J, et al.. Clinical and molecular characterization of non-syndromic retinal dystrophy due to c.175G>A mutation in ceroid lipofuscinosis neuronal 3 (CLN3). Doc Ophthalmol. 2019; 138: 55–70. [DOI] [PubMed] [Google Scholar]
- 17. Khan KN, El-Asrag ME, Ku CA, et al.. Specific alleles of CLN7/MFSD8, a protein that localizes to photoreceptor synaptic terminals, cause a spectrum of nonsyndromic retinal dystrophy. Invest Ophthalmol Vis Sci. 2017; 58: 2906–2914. [DOI] [PubMed] [Google Scholar]
- 18. Ku CA, Hull S, Arno G, et al.. Detailed clinical phenotype and molecular genetic findings in CLN3-associated isolated retinal degeneration. JAMA Ophthalmol. 2017; 135: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roosing S, van den Born LI, Sangermano R, et al.. Mutations in MFSD8, encoding a lysosomal membrane protein, are associated with nonsyndromic autosomal recessive macular dystrophy. Ophthalmology. 2015; 122: 170–179. [DOI] [PubMed] [Google Scholar]
- 20. Smirnov VM, Nassisi M, Solis Hernandez C, et al.. Retinal phenotype of patients with isolated retinal degeneration due to CLN3 pathogenic variants in a French retinitis pigmentosa cohort. JAMA Ophthalmol. 2021; 139: 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zare-Abdollahi D, Bushehri A, Alavi A, et al.. MFSD8 gene mutations; evidence for phenotypic heterogeneity. Ophthalmic Genet 2019; 40: 141–145. [DOI] [PubMed] [Google Scholar]
- 22. Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004; 30: 287–290. [DOI] [PubMed] [Google Scholar]
- 23. McCulloch DL, Marmor MF, Brigell MG, et al.. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015; 130: 1–12. [DOI] [PubMed] [Google Scholar]
- 24. Siintola E, Lehesjoki AE, Mole SE.. Molecular genetics of the NCLs—status and perspectives. Biochim Biophys Acta. 2006; 1762: 857–864. [DOI] [PubMed] [Google Scholar]
- 25. Mole SE, Williams RE, Goebel HH.. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005; 6: 107–126. [DOI] [PubMed] [Google Scholar]
- 26. Wang F, Wang H, Tuan HF, et al.. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014; 133: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuper WFE, van Alfen C, van Eck L, et al.. A case of unexpected adult-onset neurologic decline in CLN3-associated retinal degeneration. JAMA Ophthalmol. 2017; 135: 1451–1453. [DOI] [PubMed] [Google Scholar]
- 28. Pommie C, Levadoux S, Sabatier R, Lefranc G, Lefranc MP.. IMGT standardized criteria for statistical analysis of immunoglobulin V-REGION amino acid properties. J Mol Recognit. 2004; 17: 17–32. [DOI] [PubMed] [Google Scholar]
- 29. Patino LC, Battu R, Ortega-Recalde O, et al.. Exome sequencing is an efficient tool for variant late-infantile neuronal ceroid lipofuscinosis molecular diagnosis. PLoS One. 2014; 9: e109576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Priluck AZ, Breazzano MP.. Novel MFSD8 mutation causing non-syndromic asymmetric adult-onset macular dystrophy [published online July 8, 2022]. Ophthalmic Genet. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez RL, Yan J, Richards S, et al.. Atypical presentation of neuronal ceroid lipofuscinosis type 8 in a sibling pair and review of the eye findings and neurological features. Am J Ophthalmol Case Rep. 2016; 4: 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huin V, Barbier M, Bottani A, et al.. Homozygous GRN mutations: new phenotypes and new insights into pathological and molecular mechanisms. Brain. 2020; 143: 303–319. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell WA, Wheeler RB, Sharp JD, et al. Turkish variant late infantile neuronal ceroid lipofuscinosis (CLN7) may be allelic to CLN8. Eur J Paediatr Neurol. 2001:5(Suppl A): 21–27, 10.1053/ejpn.2000.0429. [DOI] [PubMed] [Google Scholar]
- 34. Aiello C, Terracciano A, Simonati A, et al. Mutations in MFSD8/CLN7 are a frequent cause of variant-late infantile neuronal ceroid lipofuscinosis. Hum Mutat. 2009; 30(3): E530–E540, 10.1002/humu.20975. [DOI] [PubMed] [Google Scholar]