Abstract
In 1987, the government passed legislation to protect brand-name pharmaceutical firms against competition from generic drug brands in exchange for economic investment in Canadian pharmaceutical research and development (R&D). Since 2002, brand-name pharmaceutical companies' R&D investments have fallen short of their commitment, while Canadians now pay the fourth highest drug prices of all the Organisation for Economic Co-operation and Development member countries. In this article, we examine the degree to which brand-name pharmaceutical companies have fallen short of their promises, discuss whether a patent policy is the best strategy to secure Canadian pharmaceutical R&D funding and propose practical alternatives to this arrangement.
Abstract
En 1987, le gouvernement adoptait une loi pour protéger les entreprises pharmaceutiques de médicaments de marque contre la concurrence des médicaments génériques, en échange d'investissements économiques dans la recherche et le développement (R et D) pharmaceutiques au Canada. Depuis 2002, les investissements en R et D effectués par les sociétés pharmaceutiques de médicaments de marque ont été inférieurs à leurs engagements, alors que le Canada figure en quatrième position des prix les plus élevés pour les médicaments parmi les pays membres de l'Organisation de coopération et de développement économiques. Dans cet article, nous examinons dans quelle mesure les sociétés pharmaceutiques de médicaments de marque ont brisé leurs promesses. Nous nous demandons aussi si une politique sur les brevets constitue la meilleure stratégie pour garantir le financement canadien dans la R et D pharmaceutique et nous proposons des alternatives pratiques à cet arrangement.
Introduction
The affordability of medicines represents a natural conflict between profit for pharmaceutical companies and cost to consumers. To balance these conflicts, the Canadian government has implemented policies to ensure that pharmaceutical companies are successful and drug prices are affordable.
In 1987, the government passed legislation (Bill C-22) to protect brand-name pharmaceutical firms against competition from generic drug brands (Lexchin 1993). In exchange, the Pharmaceutical Manufacturers Association of Canada (PMAC), now Innovative Medicines Canada (IMC) – which represents all the major brand-name pharmaceutical companies in Canada – entered into an agreed-upon but legally unenforceable promise of economic investment in Canadian pharmaceutical research and development (R&D) (Lexchin 1993), as a trade-off against higher medicine costs.
The IMC pledged to grow their R&D investments to 10% of the total revenue in return for increased patent protection of their brand-name drugs. However, since 2002, IMC companies' R&D investments have declined steadily and fallen significantly short of their commitments. Meanwhile, Canadians now pay the fourth highest drug prices of all countries in the Organisation for Economic Co-operation and Development (OECD).
The IMC publicly defends its position by claiming that the original definition of R&D (based on the 1987 Scientific Research and Experimental Development [SR&ED] tax credit eligibility) is outdated (IMC 2022). Instead, they choose to use the Frascati Manual definition of R&D and data collected by Statistics Canada (OECD 2015). In this article, we examine the validity of the IMC's claims and discuss options available to the Canadian government to ensure that the pharmaceutical industry upholds its end of the Bill C-22 bargain.
Did Government Policy Changes Favour IMC Companies?
In the 1960s, three government reports concluded that Canadian drug prices were among the highest in the world and that patent protection was the primary cause for this (Canada, Restrictive Trade Practices Commission 1963; Hall 1964; Harley 1967). To combat this, the Canadian government introduced a bill in 1969 to extend the use of compulsory licences (CLs) (Lexchin 1993). CLs essentially negate patents, allowing generic companies to procure licences upon payment of appropriate royalties, to manufacture versions of the brand-name drugs with imported active pharmaceutical ingredients (APIs).
Under CLs, the generic drug industry in Canada grew rapidly, and many more generic drugs were produced at more affordable prices (Lexchin 1993). From 1975 to 1982, Canada had some of the lowest drug prices, deflated by national gross domestic product, among all the member nations of the OECD (Jacobzone 2000). Canadian consumers saved approximately $211 million on prescription drugs in 1983 alone (Eastman 1985), whereas multinational pharmaceutical companies lost only 3.1% of the Canadian market to generic drugs that year (Eastman 1985).
PMAC lobbied the government against compulsory licensing throughout the 1970s, despite CLs posing no significant threat to their economic position (Eastman 1985). In 1985, the Canadian government committed to negotiating a free-trade agreement with the US, which led to pressure from the US government to eliminate compulsory licensing. In 1987, the Canadian government passed Bill C-22, which eliminated CLs for the first seven years of a new drug being in the market, or 10 years if components of the drug were imported, which was the case for most generic drugs (Lexchin 1993).
The extended patent protection provided by Bill C-22 resulted in a delay in the introduction of generic drugs into the market, leading to increased drug costs. In 1993, Bill C-91 was passed following the signing of the North American Free Trade Agreement (NAFTA) and the Trade Related Aspects of Intellectual Property Rights agreement, eliminating all forms of compulsory licensing (Lexchin 1997). The outcome of this step was a further increase in drug costs for Canadians (Lexchin 1993). From 1985 to 2000, average drug prices increased by 296%, more than double the percentage increases in total healthcare expenditures of 144% (CIHI 2003).
To comply with NAFTA, Canada also extended patents from 17 years from the time patents were granted to 20 years after patent filing. The 2014 Comprehensive Economic and Trade Agreement (CETA) between Canada and the European Union further extended patent life by up to two years (Lexchin and Gagnon 2014), and has been estimated to eventually cost Canadians over $500 million annually (Lexchin and Gagnon 2014).
Promises Made by the PMAC in Exchange for Patent Protection Policies
In exchange for extended patent protection, PMAC (now IMC) pledged to increase their member companies' investments in Canadian pharmaceutical R&D from less than 5% of the total revenues to 10%, thereby growing the industry through job creation and innovation. In a 1993 letter sent to the minister of Industry, Science & Technology, Michael Wilson, the president of PMAC, Judith Erola, pledged that member companies would invest 10% of revenues into R&D by 1996 (Statistics Canada, Personal Communication, March 21, 2022) and that the commitment would stand as long as the provisions of Bill C-22 remained in effect. According to the letter, IMC member companies were projected to spend $2 billion on Canadian pharmaceutical R&D between 1992 and 1996 and $3 billion between 1997 and 2002. In addition, in hearings before the House of Commons Legislative Committee on Bill C-22, John Zabriskie, the then-president of Merck Frosst Canada, testified that the industry would create 2,000 new R&D jobs between 1988 and 1995 (Library of Parliament 1987). Although the legislation would cause drug prices to increase, this cost was predicted to be offset by economic benefits from R&D growth. R&D investments would be reported annually by the Patented Medicine Prices Review Board (PMPRB), an independent body established under the Patent Act as part of Bill C-22 (Lexchin 1997).
Canada Kept Its Part of the Agreement, Has the IMC?
Bill C-22 is still in effect, but according to the PMPRB, pharmaceutical R&D expenditures peaked in 1997 at 12.9% of revenue and remained above 10% until 2002, when they began declining (PMPRB 2020). By 2019, the PMPRB reported that IMC member companies spent only 3.9% of their revenues funding Canadian pharmaceutical R&D. By examining sales revenues and R&D spending of IMC member companies between 2010 and 2020 (PMPRB 2020), we estimated that the shortfall in R&D investment over this time period exceeds $7 billion. Consequently, the expected benefits to Canadians from increased R&D investments, including increased employment and research advancement, have not materialized.
Differing Claims from PMPRB and IMC about R&D Expenditures
The PMPRB reports annually on IMC members companies' R&D spending by drug patentees. The IMC disputes these reports, claiming their R&D investments are underestimated owing to the PMPRB using what IMC terms an “outdated” definition of R&D. However, the PMPRB's definition is the one that the IMC originally committed to in 1993: R&D activities that are eligible for SR&ED tax credits under the 1987 tax guidelines. The IMC's commitment should therefore be upheld to this original definition. The PMPRB reported that the R&D spending in 2019 for all pharmaceutical patentees was $893.2 million and $652.6 million for the 32 IMC companies that held patents that year (PMPRB 2019).
In contrast, the IMC claimed that in 2019, $2.2 billion was spent by pharmaceutical companies on R&D, quoting data published by Statistics Canada (IMC 2022), which uses the Frascati Manual definition of R&D that includes activities in the social sciences and humanities, including donations and grants given to organizations that support community programs, education and environmental activism, as well as research in the social sciences, such as bioethics. The Frascati definition also includes research performed outside Canada, usually clinical trials, and spending on routine regulatory affairs and administration expenses for phases I to III of clinical trials, as well as pharmacovigilance studies. These are all activities that do not qualify for SR&ED tax credits under the 1987 guidelines. Though supportive of research in other ways, these activities do not fulfill the original objectives of the agreement with IMC to create research jobs, advance science and grow the Canadian pharmaceutical R&D industry.
Statistics Canada estimated R&D expenditures by the entire Canadian pharmaceutical industry (including IMC members, non-IMC brand-name companies and generic firms) in 2019 as being within a range of $1.6–$2.2 billion (Figure 1) (Statistics Canada 2022). The IMC used the upper bound of the Statistics Canada estimate and did not disclose the fact that this amount included R&D expenditures by non-IMC companies. In fact, Statistics Canada separately reported that the R&D expenditures of only IMC companies was between $0.9 and $1.4 billion in 2019 (Figure 1), nearly a billion dollars less than what the IMC claims (IMC 2022; Statistics Canada 2022).
Figure 1.
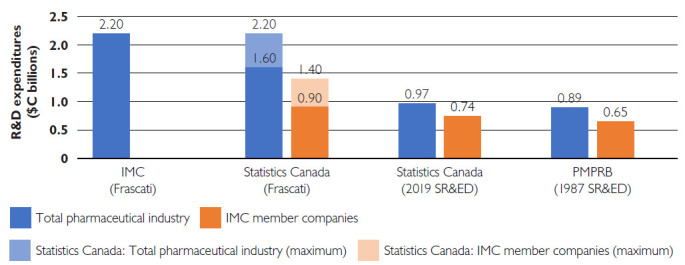
R&D expenditure reports for the pharmaceutical industry and IMC member companies in 2019
Figures are reported by the IMC, Statistics Canada and the PMPRB, using definitions as stated in parentheses. Statistics Canada reports a range of values, depicted by the lighter shaded areas of the bars (IMC 2022; PMPRB 2019; Statistics Canada 2022).
IMC = Innovative Medicines Canada; PMPRB = Patented Medicine Prices Review Board; R&D = research and development; SR&ED = Scientific Research and Experimental Development.
In addition to publishing R&D expenditures using the Frascati Manual method, Statistics Canada also reports expenditures eligible for SR&ED tax credits (using the updated 2014 definition). Statistics Canada estimates that SR&ED expenditures by IMC companies were $738 million, including both in-house and out-sourced R&D (Figure 1) (Statistics Canada 2022). The PMPRB reports that R&D expenditures by IMC companies that qualified for the 1987 SR&ED tax credits were $652.6 million in 2019 (Figure 1) (PMPRB 2019). Both the PMPRB and Statistics Canada estimates are well below the $2.2 billion that the IMC quoted, and well short of their 1987 commitment (IMC 2022).
Is Patent Policy the Best Strategy to Secure Pharmaceutical Investment in R&D?
The Canadian government's agreement with PMAC was based on the premise that strong patent protection leads to higher revenues and greater local R&D investment by pharmaceutical companies. This reasoning was based on industry claims that the ability of PMAC to invest in pharmaceutical R&D was limited due to the revenue lost to the generic drug industry and that more robust patent protection would enable them to increase their investments.
However, data from multiple OECD countries show that patent protections and the resulting higher drug prices are not correlated with R&D investments (see Appendix 1, available at www.longwoods.com/content/27038), both in 1987 (Figure A1), when Bill C-22 was passed, and more recently in 2017 (Figure A2) (OECD 2009, 2022, 2023a, 2023b). There is no support for the industry's claim that patent protection leads to greater R&D investments. Canada has the fourth highest drug prices among OECD member countries, yet it has one of the lowest contributions toward R&D investments from pharmaceutical companies. The patent policy should, therefore, not be used to secure local pharmaceutical investment. Instead, policies directed toward this goal should target public investment in R&D, tax incentives and innovation infrastructure such as hospitals and university research facilities.
What Should Be Done about the IMC's Unfulfilled Commitment?
The IMC made a commitment on behalf of its member companies to invest 10% of their revenues into Canadian pharmaceutical R&D in response to Bill C-22. The bill has remained in effect since its passing in 1987 and resulted in significantly increased drug spending by Canadians. Yet, the IMC R&D funding commitments were met only until 2002. Clearly, this approach of naively accepting non-enforceable commitments from industry does not work, and future policy must be enforced by legislation.
To redress the broken IMC promises, we propose two non-mutually exclusive policy options. First, companies can be required to contribute a percentage of revenues to an independent biomedical research trust fund, akin to the UK-based Wellcome Trust (https://wellcome.org/), for future Canadian R&D. The Wellcome Trust is a global charitable foundation with a portfolio that stands at £38.2 billion and that funds vaccine and antibiotic development, mental health research and climate change strategies through support for both basic research and clinical trials (Wellcome n.d.). This approach will establish a sustainable and growing research fund that can truly spur innovative research, create jobs, develop the pharmaceutical industry and strengthen the economy in Canada.
A second alternative is to impose a surtax on promotional spending by companies that would be directed into a research fund similar to that launched by the Agenzia Italiana del Farmaco (AIFA) in Italy (Italian Medicines Agency [AIFA] Research & Development Working Group 2010). AIFA is a regulatory institution operating within the Italian Ministry of Health to promote and fund independent research on drugs. Financing for this program comes through a unique policy that requires all international and national pharmaceutical companies operating in Italy to contribute 5% of their yearly promotional expenditures to a national fund for independent research. The program generated €45 million in the first three years.
Conclusion
In conclusion, pharmaceutical companies have benefited from high drug prices resulting from increased patent protection, but they have not fulfilled their commitments to increase pharmaceutical R&D investment in Canada. Legislation should be passed to enforce their commitments, and the Canadian government should consider alternative strategies to grow the pharmaceutical R&D industry, such as those successfully implemented in other countries.
Note
All currencies are in Canadian dollars unless noted otherwise.
Contributor Information
Shoo K. Lee, Professor Emeritus, University of Toronto, Honorary Staff Physician, Mount Sinai Hospital, Toronto, ON.
Sukhy K. Mahl, Assistant Director, MiCare Research Centre, Mount Sinai Hospital, Toronto, ON.
Jessica J. Green, Writer, MiCare Research Centre, Mount Sinai Hospital, Toronto, ON.
Joel Lexchin, Professor Emeritus, School of Health Policy and Management, York University, Toronto, ON.
References
- Canada, Restrictive Trade Practices Commission. 1963. Report Concerning the Manufacture, Distribution and Sale of Drugs. Department of Justice. Retrieved April 15, 2021. <https://catalog.hathitrust.org/Record/000964039/Home>.
- Canadian Institute of Health Information (CIHI). 2003. Drug Expenditure in Canada, 1985–2003. Retrieved February 6, 2023. <https://publications.gc.ca/collections/Collection/H115-27-2003E.pdf>.
- Eastman H.C. 1985. Report of the Commission of Inquiry on the Pharmaceutical Industry (HD9670/.C2/C2). Minister of Supply and Services Canada. Retrieved April 15, 2021. <https://publications.gc.ca/collections/collection_2016/bcp-pco/CP32-46-1985-1-eng.pdf>. [Google Scholar]
- Hall E.M. 1964. Royal Commission on Health Services: Volume I. Retrieved April 15, 2021. <https://publications.gc.ca/collections/collection_2016/bcp-pco/Z1-1961-3-1-1-eng.pdf>.
- Harley H.C. 1967. Second (Final) Report of the Special Committee of the House of Commons on Drug Costs and Prices. House of Commons Committees, 27th Parliament, 1st Session: Special Committee on Drug Costs and Prices, Report. Retrieved April 15, 2021. <https://parl.canadiana.ca/view/oop.com_HOC_2701_3_5/3?r=0&s=1>.
- Innovative Medicines Canada (IMC). 2022, March 31. PMPRB Continues to Ignore the Value of Innovative Medicines for Canadians. Retrieved April 20, 2022. <http://innovativemedicines.ca/pmprb-continues-ignore-value-innovative-medicines-canadians/>.
- Italian Medicines Agency (AIFA) Research & Development Working Group. 2010. Feasibility and Challenges of Independent Research on Drugs: The Italian Medicines Agency (AIFA) Experience. European Journal of Clinical Investigation 40(1): 69–86. doi:10.1111/j.1365-2362.2009.02226.x. [DOI] [PubMed] [Google Scholar]
- Jacobzone S. 2000. Pharmaceutical Policies in OECD Countries: Reconciling Social and Industrial Goals. OECD Labour Market and Social Policy Occasional Papers No. 40. OECD Publishing. doi:10.1787/323807375536. [Google Scholar]
- Lexchin J. 1993. Pharmaceuticals, Patents, and Politics: Canada and Bill C-22. International Journal of Social Determinants of Health and Health Services 23(1): 147–60. doi:10.2190/UCWG-YBR3-X3L0-NWYT. [DOI] [PubMed] [Google Scholar]
- Lexchin J. 1997. After Compulsory Licensing: Coming Issues in Canadian Pharmaceutical Policy and Politics. Health Policy 40 (1): 69–80. Lexchin, J. 1997. doi:10.1016/S0168-8510(96)00886-X. [DOI] [PubMed] [Google Scholar]
- Lexchin J., Gagnon M.-A. 2014. CETA and Pharmaceuticals: Impact of the Trade Agreement between Europe and Canada on the Costs of Prescription Drugs. Globalization and Health 10: 30. doi:10.1186/1744-8603-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Library of Parliament. 1987. Canadian Parliamentary Historical Resources. House of Commons Committees, 34th Parliament, 3rd Session: Legislative Committee on Bill C-91. Retrieved January 31, 2023. <https://parl.canadiana.ca/view/oop.com_HOC_3403_80_1/498>. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). 2009. ANBERD: Business Enterprise R&D by Industry (ISIC Rev. 2) [Archived]. Retrieved August 24, 2022. <https://stats.oecd.org/Index.aspx?DataSetCode=ANBERD_REV2#>.
- Organisation for Economic Co-operation and Development (OECD). 2015. Frascati Manual 2015: Guidelines for Collecting and Reporting Data on Research and Experimental Development. The Measurement of Scientific, Technological and Innovation Activities. OECD Publishing. doi:10.1787/9789264239012-en. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). 2022. ANBERD (Analytical Business Enterprise R&D) database (ISIC Rev. 4). Retrieved August 24, 2022. <https://stats.oecd.org/Index.aspx?DataSetCode=ANBERD_REV4>.
- Organisation for Economic Co-operation and Development (OECD). 2023a. Pharmaceutical Spending (Indicator). Retrieved August 23, 2022. <https://data.oecd.org/healthres/pharmaceutical-spending.htm?wpmobileexternal=true&wpmobileexternal=true>.
- Organisation for Economic Co-operation and Development (OECD). 2023b. Population (Indicator). Retrieved August 23, 2022. <https://data.oecd.org/pop/population.htm>.
- Patented Medicine Prices Review Board (PMPRB). 2019. Annual Report 2019. Government of Canada. Retrieved June 17, 2021. <https://www.canada.ca/content/dam/pmprb-cepmb/documents/reports-and-studies/annual-report/2019/pmprb-ar-2019-en.pdf>.
- Patented Medicine Prices Review Board (PMPRB). 2020. Annual Report 2020. Retrieved June 17, 2021. <https://www.canada.ca/content/dam/pmprb-cepmb/documents/reports-and-studies/annual-report/2020/pmprb-ar-2020-en.pdf>.
- Statistics Canada. 2022. The Canadian Research and Development Pharmaceutical Sector, 2019. Catalogue No. 11-621-M. Retrieved July 1, 2021. <https://www150.statcan.gc.ca/n1/en/pub/11-621-m/11-621-m2022002-eng.pdf?st=mzM2Z7ge>.
- Wellcome. n.d. Who We Are. Retrieved January 31, 2023. <https://wellcome.org/who-we-are>.


