Abstract
The larval stages of the cestode parasites belonging to the genus Echinococcus grow within internal organs of humans and a range of animal species. The resulting diseases, collectively termed echinococcoses, include major neglected tropical diseases of humans and livestock. Echinococcus larvae are outwardly protected by the laminated layer (LL), an acellular structure that is unique to this genus. The LL is based on a fibrillar meshwork made up of mucins, which are decorated by galactose-rich O-glycans. In addition, in the species cluster termed E. granulosus sensu lato, the LL features nano-deposits of the calcium salt of myo-inositol hexakisphosphate (Insp6). The main purpose of our article is to update the immunobiology of the LL. Major recent advances in this area are (i) the demonstration of LL “debris” at the infection site and draining lymph nodes, (ii) the characterization of the decoy activity of calcium Insp6 with respect to complement, (iii) the evidence that the LL mucin carbohydrates interact specifically with a lectin receptor expressed in Kupffer cells (Clec4F), and (iv) the characterization of what appear to be receptor-independent effects of LL particles on dendritic cells and macrophages. Much information is missing on the immunology of this intriguing structure: we discuss gaps in knowledge and propose possible avenues for research.
Keywords: mucin, complement, Echinococcus, macrophage, dendritic cell, Clec4F
Introduction
The laminated layer (LL) is the crucial structure at the host-parasite interface in the parasitic diseases collectively known as echinococcoses. We reviewed the biology of the LL including structural aspects in depth in 2011 and 2015 (Díaz et al., 2011a, 2011b, 2015). For most of the present article, we therefore focus on the immunological side, on which there is significant recent information. Our discussion makes emphasis on the interactions of LL with host complement, antibodies, dendritic cells, and macrophages, but it does touch on additional aspects of immunology.
A primer on Echinococcus and the echinococcoses
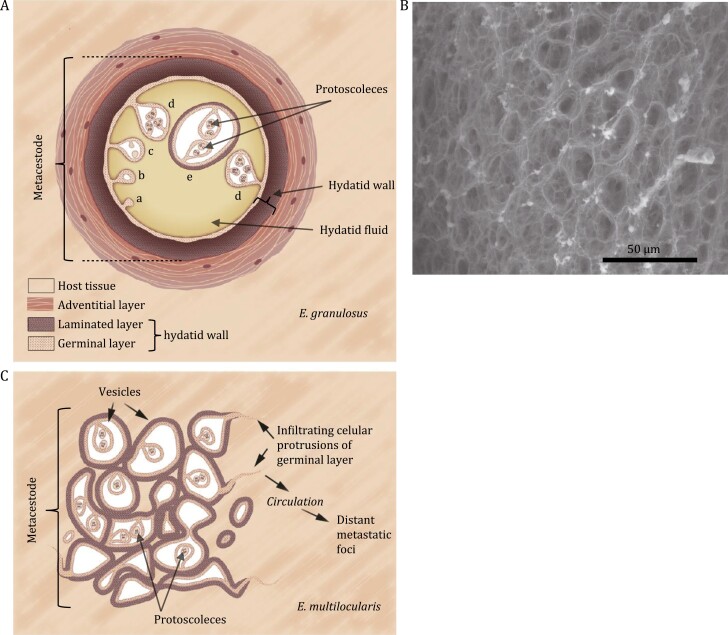
Echinococcus is a genus of taeniid cestode platyhelminths (tapeworms). The larval stages of the species within this genus lodge in internal organs of mammals including humans, causing the diseases collectively known as echinococcoses (Brunetti and White, 2012; Thompson and Jenkins, 2014; Thompson, 2017; Wen et al., 2019; Lightowlers et al., 2021). Within this disease complex, cystic echinococcosis (CE; also known as hydatid disease) has the broadest geographical distribution, and it is considered a neglected tropical disease (Agudelo Higuita et al., 2016). CE is caused by a cluster of species that can be discriminated only by molecular means, and that have mainly ungulates as the natural hosts of their larval stages. This cluster, termed E. granulosus sensu lato, encompasses E. granulosus sensu stricto and four other species (Lymbery, 2017). Because of lack of full species information in many cases, throughout this article, “E. granulosus” refers to E. granulosus sensu lato unless otherwise specified. The larval E. granulosus parasite (metacestode) is a fluid-filled, bladder like structure known as hydatid, which can attain tens of cm in diameter in the mammalian host (Thompson, 2017) (Fig. 1A). CE can affect virtually any internal organ, but liver, and lungs are the most commonly affected. The other important echinococcosis is alveolar echinococcosis (AE), caused by E. multilocularis. In this case, the metacestode forms a series of interconnected tubules and chambers that invades the host’s liver (Fig. 1B). Natural hosts are wild rodents, and human infection is life-threatening if untreated (Brunetti and White, 2012; Thompson, 2017; Casulli et al., 2019; Lightowlers et al., 2021).
Figure 1.
Structure of Echinococcus metacestodes and the LL. General structure of the E. granulosus (A) and E. mutilocularis metacestodes (B). In (A), a–d represent stages in the development of protoscoleces. Note that the “adventitial layer” often surrounding E. granulosus metacestodes is the fibrous host reaction, and that metacestode plus adventitial layer form the so called “hydatid cyst”. Reproduced and modified with permission from reference (Thompson, 2017) (published by Elsevier). (C) Appearance of the E. granulosus LL under the scanning electron microscope, using critical point drying. The image corresponds to material from a natural infection in cattle, and was obtained by Prof. Aline Miller and Ziqing Zhou (Department of Chemical Engineering, Manchester Institute of Biotechnology, University of Manchester, UK).
Echinococcosis normally results from the ingestion of Echinococcus eggs, passed out with the feces of a canid host that harbors the adult tapeworm. After their ingestion, eggs release oncospheres that traverse the gut wall and are carried via blood or lymph to solid organs where metacestodes develop (Thompson, 2017). In addition to the described (primary) form of infection, echinococcosis can also develop after infection by protoscoleces, called “secondary infection”. Protoscoleces (Fig. 1A and 1B) are parasite forms normally destined to infect the primary host but are capable of reverse development into metacestodes when released into mammalian tissues (Thompson, 2017). In addition to being often employed experimentally for infecting mice, this secondary infection route causes natural infections when protoscoleces escape from the metacestode, as it can happen during surgery.
Irrespective of species differences, Echinococcus larvae develop as fluid-filled chambers bounded by a thin layer of live parasite tissue, called the germinal layer (Fig. 1A and 1B). The acellular LL is synthesized directionally by germinal layer towards the host; germinal layer plus LL together form what is known as the hydatid wall (especially in CE). While the E. multilocularis LL usually has tens of µm thickness, in E. granulosus it reaches hundreds of µm to over a mm in thickness (Reinehr et al., 2020). The LL is deployed 14–20 days post infection after primary infections, and in approximately twice this time after secondary infections (Díaz et al., 2011a). The LL precludes direct contact between the germinal layer and host immune cells, thus affording parasite cells partial protection against immune effectors (Gottstein et al., 2002; Díaz et al., 2011b).
The systemic immune responses to larval Echinococcus are, as can be expected of responses to helminths, biased towards Th2, although Th1, as well as Th17, components are also detected (Zhang et al., 2012; Díaz, 2017; Gottstein et al., 2017; Díaz et al., 2018; Wang et al., 2018; Yasen et al., 2021). As should also be expected of parasites capable of long-lived infections, Echinococcus larvae elicit strong regulatory responses in the host, in which the immune-suppressive cytokine TGF-β and Foxp3+ regulatory T cells appear to be central (Mejri et al., 2011; Pang et al., 2014a, 2014b, 2018; Gottstein et al., 2017; Díaz et al., 2018; Wang et al., 2018; Fratini et al., 2020). Accordingly, the local response to the metacestode is usually remarkably subdued. However, mismatch between host and parasite species (e.g. cattle or pig infection by E. granulosus sensu stricto) results in a granulomatous-type local inflammatory response, which is damaging to the parasite (Díaz et al., 2000, 2018; Sakamoto and Cabrera, 2003; Basika et al., 2012; Riesle et al., 2014; Thompson, 2017).
A summary of the LL structure
The fabric of the LL is a meshwork of mucins (Casaravilla and Díaz, 2010; Hulsmeier et al., 2010; Díaz et al., 2011a), i.e. non-globular polypeptide backbones (apomucins) decorated by multiple (mucin-type) O-glycans. Animal mucins usually form loose, highly hydrated aggregates, like in mucus (Thornton et al., 2018). The LL mucins also form a highly hydrated gel-like structure (Irigoín et al., 2004; Díaz et al., 2011a). However, different from mucus, the LL mucin meshwork forms a physically coherent elastic structure, which in the case of E. granulosus allows the hydatid to be turgid. Under the transmission electron microscope the mucin fabric appears as a disorganized meshwork of fine fibrils (Morseth, 1967; Sakamoto and Sugimura, 1969; Richards et al., 1983). By scanning electron microscopy using critical point drying, the E. granulosus appears sponge-like (Fig. 1C).
The sequences of the LL apomucins have been deduced from genomic and transcriptomic data, but without any proteomic confirmation to date (Díaz et al., 2011a, 2015; Parkinson et al., 2012; Tsai et al., 2013; Zheng et al., 2013). These sequences code for (mostly short) secreted apomucins, each comprising a short non-glycosylated N-terminal extension, a mucin domain with a high predicted density of O-glycosylation, and a C-terminal signal for the incorporation of a glycosylphosphatidylinositol anchor (Parkinson et al., 2012; Tsai et al., 2013; Zheng et al., 2013); more details are given in Díaz et al., (2015).
The glycans decorating the LL mucins have been elucidated for E. granulosus and E. multilocularis (Hülsmeier et al., 2002; Díaz et al., 2009, 2015; Hulsmeier et al., 2010; Lin et al., 2013; del Puerto et al., 2016). They are based on the conventional mucin O-glycan cores 1 and 2, and are elongated and capped mostly with Gal residues. Therefore, most of the di- or tri-saccharide motifs exposed for interaction with host lectins (carbohydrate-binding proteins) or antibodies feature either α- or β-Gal as terminal monosaccharide residues. The E. multilocularis glycans are less elongated than their E. granulosus counterparts, generating subtle differences in the Gal-rich motifs present (Díaz et al., 2015; del Puerto et al., 2016). E. multilocularis metacestodes in culture lose all viability in the presence of α- or β-galactosidases, suggesting that the intact LL glycans are not needed solely for protection from the host (Wang et al., 2021).
The E. granulosus LL is formed, in addition to the mucin meshwork, by the calcium salt of myo-inositol hexakisphosphate (Insp6). Insp6 is a ubiquitous nuclear and cytosolic metabolite in eukaryotes (Raboy, 2003); in addition, in vacuolar compartments in plant seeds, it forms solid deposits with various metal ions (Madsen and Brinch-Pedersen, 2020). Insp6 is insoluble in the presence of several divalent and trivalent metal ions, including Ca2+ at the concentrations found in extracellular and vesicular system compartments in animals (Veiga et al., 2006). In E. granulosus, Insp6 reaches a vesicular system compartment, precipitates as calcium salt, and is exocytosed from the GL onto the LL in the form of deposits measuring approximately 40 nm in diameter (Morseth, 1967; Richards et al., 1983; Irigoín et al., 2004). The deposits are easily detected in the LL by transmission electron microscopy, appearing as naturally electron-dense “granules” interspersed among the mucin fibrils (Morseth, 1967; Richards et al., 1983; Irigoín et al., 2004). The adaptation is not common to the genus Echinococcus, as it is absent, by chemical, and/or ultrastructural evidence, in E. multilocularis and E. vogeli (Ingold et al., 2001; Irigoín et al., 2002).
The LL owes its name to its concentric “laminations” observable by light microscopy or scanning electron microscopy at low magnification, in E. granulosus specially (Ingold et al., 2001; Díaz et al., 2011a; Reinehr et al., 2020). These “laminations” appear to result from different degrees of compaction of the mucin fibrils and calcium Insp6 deposits (Morseth, 1967).
The E. granulosus LL is freely permeable to macromolecules up to the size of immunoglobulin G (150 kDa) at least (Coltorti and Varela-Díaz, 1974). Indirect evidence suggests some limitation to the permeation of larger molecules (750 kDa and above) (Breijo et al., 2008; Barrios et al., 2019). The calcium Insp6 deposits represent a truly enormous surface available for adsorption of soluble host proteins (Casaravilla et al., 2006). In agreement, the bulk of the abundant host proteins found in the LL (other than immunoglobulins and terminal complement components) can be solubilized by calcium chelators (Díaz et al., 2000a, 2000b; Basika et al., 2012; Barrios et al., 2019) (our unpublished observations).
In sum, we have a basic understanding of LL structure, but major knowledge gaps remain. Among several aspects, proteomic data on the mucins would be very valuable, confirming the inferred apomucin sequences and allowing to explore their possible post-translational modifications (not restricted to glycosylation).
Molecular immunological aspects: the LL and host complement
The E. granulosus sensu lato LL activates host complement only poorly (Irigoín et al., 1996; Ferreira et al., 2001). This surely reflects an adaptation to minimize complement-mediated inflammation, known to be detrimental to the parasite (Ferreira et al., 2000; Breijo et al., 2008). The most powerful pro-inflammatory peptide of complement arises from the cleavage of C5, i.e. in the first step of the terminal complement pathway (Merle et al., 2015a). Possibly in agreement, the control of complement activation on LL-derived materials is more evident at the terminal complement pathway level than at the level of activation of C3, the central component (Irigoín et al., 1996). C3 activation is a self-amplified process, and this is central to the alternative complement pathway. The major physiological regulator curtailing C3b amplification is factor H (Merle et al., 2015b). Factor H both displaces factor B from activated C3 (C3b) and acts as a cofactor for the proteolytic inactivation of C3b by factor I (Sánchez-Corral et al., 2018). C3b inactivation on the LL in vitro is verified to proceed rapidly on the mucins and even more rapidly on calcium Insp6 (Irigoín et al., 2008; Barrios et al., 2019). The whole of the factor I-cofactor activity for C3b inactivation detected in hydatid wall extracts corresponds to factor H derived from the host, rather than any parasite-derived regulators (Díaz et al., 1997). Together, these data indicate that factor H has functionally relevant affinities for calcium Insp6 and for certain (unidentified) sites on the mucins. Thus, amplification of C3 activation/deposition on the LL is avoided by offering binding sites for host factor H (Irigoín et al., 2008; Barrios et al., 2019).
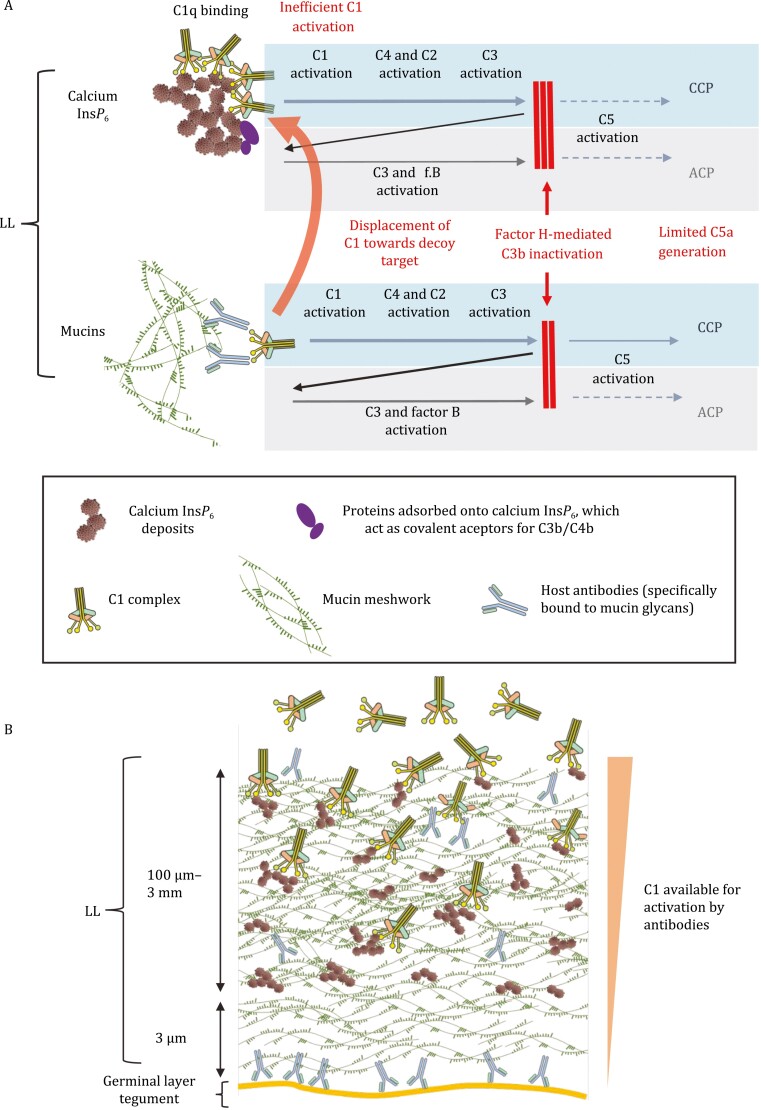
Complement control based on the prevention of C3b amplification could be overridden by strong activation of the lectin and/or classical pathways (Merle et al., 2015b). The LL does not activate the human lectin pathway in vitro (Barrios et al., 2019). However, as detailed in the following section, the LL mucins are targets of host antibodies, and part of these belong to classes or sub-classes that activate the classical pathway (Barrios et al., 2019). Antibodies bound to antigens initiate the classical pathway by binding (through their Fc portions) to C1q, the recognition subunit of the C1 complex (Merle et al., 2015b). Interestingly, most of the C1q-binding capacity of the LL is not due to antibodies but rather due to calcium Insp6 (Barrios et al., 2019). However, C1 activation on calcium Insp6 is inefficient, similar to what is observed on other polyanionic molecules that bind C1q (Garlatti et al., 2010; Barrios et al., 2019). Also, C3b deposited on calcium Insp6 is inactivated particularly fast. In sum, the net effect of C1q binding to Insp6 is probably to limit local availability of C1 for activation on antibodies, without making a contribution to the activation of C5 (Barrios et al., 2019). We envisage that this mechanism may be particularly important for the avoidance of classical pathway activation by antibodies bound to the surface of the germinal layer (Riesle et al., 2014), where such activation may damage the parasite through the lytic mechanism of complement. In this hypothesis, the absence of calcium Insp6 in the 3 μm of LL closest to the germinal layer (Richards et al., 1983) may keep complement activation (however inefficient) away from the parasite cells. Our in vitro data also suggested that C1 complex permeation through the LL is restricted (Barrios et al., 2019). Thus, the E. granulosus LL probably minimizes C1 complex access to antibodies bound to the germinal layer through a combination of a sieve effect and the abundance of a decoy target for C1q (Fig. 2).
Figure 2.
Proposed model summarizing complement activation and control on the LL and the hydatid wall. (A) Although the lectin pathway is not activated by the LL, the structure interacts both with the classical (CCP) and the alternative complement pathways (ACP). Calcium Insp6 in the LL binds C1 complex through its C1q component. Although inefficiently, this causes C1 activation and initiates the CCP. This results in deposition of the activated forms of the CCP component C4 (C4b) and of the central component C3 (C3b) on calcium Insp6 itself. As Insp6 has no covalent acceptors for C4/C3, this deposition is bridged by adsorbed serum proteins. C3b deposited on calcium Insp6 is very rapidly inactivated in factor H-dependent fashion, which also means that ACP activation is curtailed. C1 binding to antibodies bound to the mucins is reduced as a consequence of the abundant calcium Insp6 acting as a decoy target for C1. Any remaining CCP activation on the mucins, as well as ACP activation, is curtailed as a consequence of factor H having affinity for (unknown sites in) the mucins. Overall, calcium Insp6 re-directs CCP activation from the mucin-bound antibodies onto itself, without either enhancing or reducing C5 activation on the LL. (B) The high binding capacity of calcium Insp6 deposits for C1q, combined with a restriction to the access of C1 imposed by the LL mucin gel would prevent the binding of functional C1 to the germinal layer tegument. This would avoid CCP activation triggered by antibodies bound to the germinal layer. In other words, the re-directioning of CCP activation onto calcium Insp6, although “neutral” when considering C5 activation by the LL only, would have a parasite-protective role when the germinal layer is also considered. The previously reported absence of calcium Insp6 deposits in the innermost 3 µm of the LL would prevent bystander damage to the germinal layer tegument by activation CCP initiated on calcium Insp6. Part (B) has been reproduced from reference (Barrios et al., 2019) (published by Elsevier).
In sum, the E. granulosus LL appears to control antibody-dependent classical pathway activation by using calcium Insp6 as a decoy, and alternative pathway activation/amplification by accumulating host factor H. In terms of perspectives, it would be of particular interest to analyze complement activation and control on the E. multilocularis LL, in view of absence of calcium Insp6 in this species.
A subjective, chronological, account of the complex path that led us to find the calcium Insp6 deposits and understand their interactions with complement is given in Box 1.
Box 1. A convoluted research story: complement and calcium inositol hexakiphosphate deposits.
This Box gives a subjective account of the discovery of calcium Insp6 deposits in the E. granulosus LL, in the context of research initially aimed at understanding the regulation of host complement activation on the LL. Part of the motivation for this Box is to pay tribute to the memory of Robert B. (“Bob”) Sim, who passed away last year (Díaz, 2021). Bob was a world authority in complement biochemistry, who developed most of his career at the University of Oxford. In 1993, Bob enthusiastically embraced a collaborative project to analyze complement evasion by E. granulosus, with Ana María Ferreira (Universidad de la República, Uruguay). In this context, Bob supervised the corresponding author’s doctoral Thesis, which was specifically aimed to find molecular mechanisms explaining poor host complement activation on the hydatid wall (Irigoín et al., 1996; Ferreira et al., 2001).
In the context of the Thesis mentioned, we detected an activity in hydatid wall extracts that inhibited the formation, and hence the activity, of the alternative complement pathway C3 convertase in assays using purified components (Díaz et al., 1999). This activity was independent of host factor H, which we had previously observed to be abundant in the same extracts (Díaz et al., 1997). Under the assay conditions, the novel activity prevented the proteolytic activation of factor B, an indispensable step in alternative pathway C3 convertase formation. It did not do so by inhibiting factor D, the proteinase that cleaves factor B. Hence the “inhibitor” had to block factor B’s binding to C3b (or, in our assay, amidated C3, which is a C3b analog), a step that is indispensable for factor B activation. The “inhibitor” was heat-stable, and behaved in gel permeation and dialysis as if it was a small macromolecule (Díaz et al., 1999).
The rest of the advances described in this Box were made mostly in Uruguay, but with Bob Sim’s important participation as senior collaborator and complement expert. The “inhibitor” was identified as inositol hexakiphosphate (Insp6) (Irigoín et al., 2002). This compound has a high density of negative charge and therefore a very large hydrodynamic radius, explaining that it would behave like a macromolecule, though it is less than 1 kDa in molecular mass (Van der Kaay and Van Haastert, 1995). However, a difficult issue had surfaced: Insp6 interacts strongly and in complex ways with divalent cations, which are needed in for complement activation. In particular, the alternative pathway C3 convertase is a kinetically unstable, Mg2+-dependent complex, the half-life of which is enhanced if Ni2+ is used instead of Mg2+ (Fishelson and Müller-Eberhard, 1982) as it had been done in our initial assays. We therefore needed two major inputs before we could fully interpret the results of our extensive set of functional complement assays. One was the detailed quantitative description of Insp6-cation systems (Veiga et al., 2006; Torres et al., 2005). The second input was the verification that Insp6 is found in the LL as its insoluble calcium salt (Irigoín et al., 2002, 2004; Casaravilla et al., 2006). Our hydatid wall extracts, prepared in the presence of the divalent metal sequestering agent EDTA, contained soluble Insp6. In the presence of divalent cations, Insp6 re-precipitated non-adverted to us (and to different extents depending on the cation). The inhibitory activity in our assays was not due to depletion of free divalent cations. Rather, the colloidal Insp6 salts show specific interaction with factor B, in the presence of Ni2+ in particular, indeed preventing the factor B - C3b association (Irigoín et al., 2008). However, under the relevant combination of conditions, i.e. calcium Insp6 and Mg2+ as the metal forming the convertase, only weak inhibition of complement activation is detected using purified components, and none in whole serum. In agreement, when human serum was put through a calcium Insp6 column, factor B was not specifically retained. Instead, the SDS-PAGE profile of the eluate included the characteristic band pattern of C1q (Irigoín et al., 2008). The specific elimination of Insp6 from the LL weakened the covalent deposition of C3b, suggesting that the calcium Insp6 deposits acted as initiation sites for the classical complement pathway (Irigoín et al., 2008).
More recently, we determined that indeed, the native calcium Insp6 deposits present in the LL bind C1q (and consequently C1 complex) avidly, and cause classical pathway initiation (Barrios et al., 2019). However, activation of C1 bound to Insp6 is inefficient compared to activation on antibodies. Most of the C3b deposited downstream of this activation binds to calcium Insp6 itself (Barrios et al., 2019), a phenomenon that was not detectable under the conditions of our previous assays (Irigoín et al., 2008). C3b deposited on calcium Insp6 is extremely rapidly inactivated (Barrios et al., 2019), and this inactivation must depend on host factor H according to our early results (Díaz et al., 1997). Thus, we concluded that similar to other polyanions, calcium Insp6 has, in addition to its strong affinity for C1q, some affinity for factor H (Barrios et al., 2019). Calcium Insp6 probably contributes most sites for factor H sequestration on the LL [although the LL mucins likely also have sites as C3b inactivation on them is also rather fast (Irigoín et al., 2008)].
In sum, we began by studying factor H in the LL. We then found Insp6 by following an activity on complement that turned out not to be biologically relevant. In the way, we observed that calcium Insp6, the insoluble salt actually found in the LL, binds C1q and initiates classical pathway activation. However, this initiation does not make a net contribution to activation of the terminal complement pathway, partly because C3b on calcium Insp6 is very efficiently inactivated. This in turn means that the calcium Insp6 surface must has affinity for factor H, somehow taking us back to the beginning of the story. As explained in the main text, we now think that calcium Insp6 acts a decoy target for the classical pathway that minimizes its antibody-dependent activation on the germinal layer (Barrios et al., 2019).
Molecular immunological aspects: antibodies targeting the LL
The E. granulosus LL has abundant, strongly-bound host antibodies (Varela-Díaz and Coltorti, 1973; Coltorti and Varela-Díaz, 1974; Taherkhani et al., 2007; Riesle et al., 2014, Barrios et al., 2019). In human infection, these antibodies belong to IgG1, IgG2, IgG4, IgA, and IgE classes/sub-classes (Barrios et al., 2019). Most host antibodies remain bound after eliminating calcium Insp6 with EDTA (Basika et al., 2012), suggesting that they are bound to the LL mucins. Although antibodies directed to the short non-glycosylated stretches of the LL predicted apomucins cannot be ruled out, in all likelihood an overwhelming majority of antibodies associating with the LL in vivo are bound to the mucin glycans. In agreement, Echinococcus infections prominently feature anti-carbohydrate and/or LL-reactive antibodies (Russi et al., 1974; Gottstein et al., 1994; Dai et al., 2001; Walker et al., 2004; Díaz et al., 2011b, 2016).
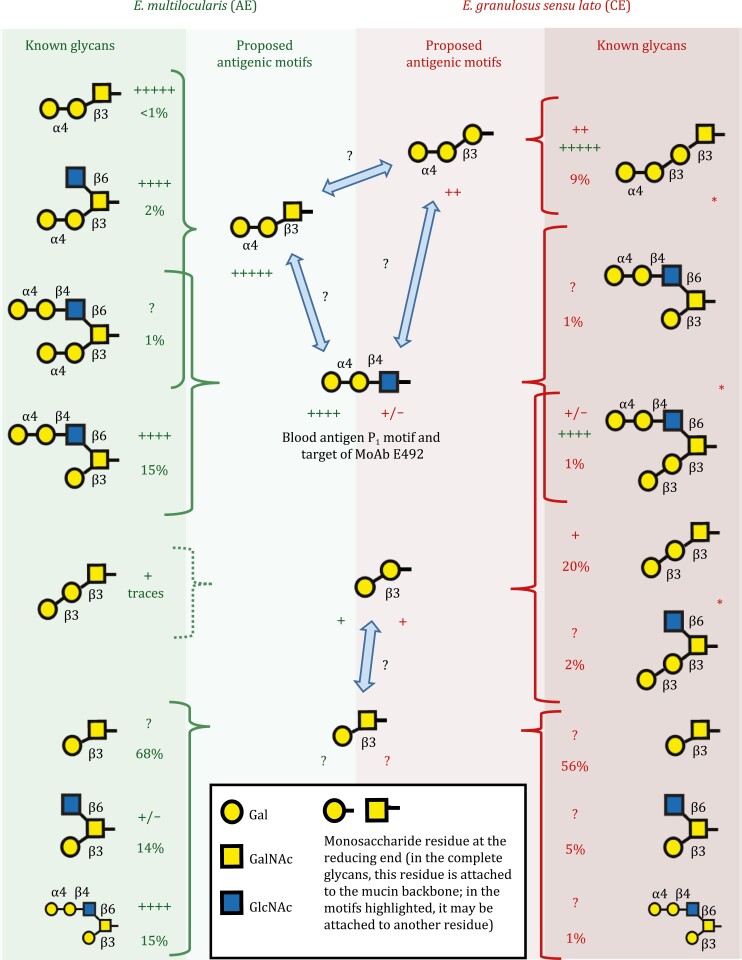
The fine specificity of serum antibodies targeting the LL glycans has been mapped with the help of synthetic versions of those glycans (Koizumi et al., 2009). Human antibody responses to these synthetic glycans are generally stronger in AE than in CE (Hada et al., 2021). The immunodominant tri-saccharide motif in AE is Galα1-4Galβ1-3GalNAc (Koizumi et al., 2011; Yamano et al., 2012) (Fig. 3). The motif is present in its unmodified form in a glycan that is not abundant in the E. multilocularis LL (Hulsmeier et al., 2010; Díaz et al., 2015; del Puerto et al., 2016). However, glycans that carry variations of the same motif in which the GalNAc residue bears a GlcNAcβ1-6 branch are more abundant, antigenic, and probably cross-reactive with the unmodified motif (Hulsmeier et al., 2010; del Puerto et al., 2016; Hada et al., 2021). The Galα1-4Galβ1-4GlcNAc motif is also strongly antigenic in AE (Koizumi et al., 2011; Hada et al., 2021). In human CE, antibody responses against LL glycans appear to be dominated by motifs terminated in Galα, including Galα1-4Galβ1-3Gal (Díaz et al., 2011b; Yamano et al., 2012; Hada et al., 2021). However, the Galα1-4Galβ1-4GlcNAc motif appears not to be strongly antigenic in CE (Russi et al., 1974; Díaz et al., 2011b; Hada et al., 2021), in contrast to the situation in AE. In addition, antibody responses occur in CE against Galβ1-3Galβ1-3GalNAc (Hada et al., 2021). Although these responses are weak when measured against the synthetic glycan, they may be biologically significant as the glycan is very abundant in the E. granulosus LL (Díaz et al., 2009). The antigeniticy of the most abundant glycan in E. granulosus (Galβ1-3GalNAc) has not been measured. E. multilocularis infection antibodies are observed to react with glycans that are found only in the E. granulosus LL (Hada et al., 2021). This reactivity may be explained by cross-reactions, as also summarized in Fig. 3.
Figure 3.
Major carbohydrate antibody epitopes in the E. multilocularis and E. granulosus LL. Information on E. multilocularis and E. granulosus is given in green and red font, respectively (and/or on green or red background). The main LL mucin glycans in each species (Díaz et al., 2009; Hulsmeier et al., 2010; Lin et al., 2013; del Puerto et al., 2016) are shown at either side of the figure. Antigenicity of the synthetic glycan against human infection sera (Koizumi et al., 2011; Yamano et al., 2012; Hada et al., 2021) (from +/‐ to +++++), and percent molar abundance in the LL (Díaz et al., 2009; del Puerto et al., 2016) are indicated for each structure. Glycans are grouped by the tri-saccharide or di-saccharide non-reducing terminal motifs that they contain, and some glycans contain more than one motif (a glycan that had to be represented twice for each species is shown in a smaller size in the second instances). For simplicity, branching at position 6 of GalNAc is taken not to abrogate the antigenicity of the motifs containing this sugar. Asterisks denote that, for E. granulosus, additional less abundant glycans exist that carry each of the motifs. The deduced antigenic motifs as such are shown in the center of the figure, with an indication of their inferred antigenicity in human infection. For some E. granulosus glycans that have not been detected in E. multilocularis (del Puerto et al., 2016), antigenicity towards E. multilocularis infection sera (Hada et al., 2021) is indicated in green. Such antigenicity may be explained by shared motifs or, in the absence of shared motifs, by cross-reaction with related motifs. The proposed cross-reactions between related motifs are indicated by light blue double arrows. Note that although no direct evidence exists for the motif Galβ1-3GalNAc being antigenic, the motif is highlighted because: (i) it is very abundant in the LL, and (ii) its possible antigenicity in E. multilocularis infection would explain why the related motif Galβ1-3Gal is antigenic in this species (Hada et al., 2021) in spite of being present only at trace levels (del Puerto et al., 2016). Also note that the glycan Galα1-4Galβ1-3Galβ1-3Galβ1-3GalNAc (not depicted) was not found to be antigenic against either E. multilocularis or E. granulosus infection sera (Hada et al., 2021), which does not fit the overall picture; we do not have an explanation for this observation.
Three published mouse monoclonal antibodies are known to react with the Echinococcus LL. The E492 antibody (IgG) (Baz et al., 1999) reacts with the Galα1-4Galβ1-4GlcNAc motif (Lin et al., 2013) (Fig. 3). This motif, previously mentioned as a target of AE infection antibodies, is also responsible for the reactivity of human anti-P1 blood group antibodies with the LL (Russi et al., 1974). The E492 antibody reacts with the hydatid wall and with other structures both in E. granulosus and E. multilocularis (Baz et al., 1999; Walker et al., 2004), consistent with the wide occurrence of the target motif in the LL O-glycans, as well as in N-glycans and glycolipids found in other Echinococcus structures (Khoo et al., 1997; Lin et al., 2013; del Puerto et al., 2016; Díaz et al., 2016). The EmG3 antibody (IgM) also reacts strongly with the E. granulosus and E. multilocularis LL as well as with other Echinococcus structures (Grimm et al., 2020b; Reinehr et al., 2020). Its broad pattern of reactivity suggests that it may also recognize a carbohydrate epitope with broad distribution in Echinococcus, but its specificity is unknown. In contrast with the previous situations, the Em2G11 antibody (IgG) reacts with the E. multilocularis LL but not with the E. granulosus LL or with other E. multilocularis stuctures (Dai et al., 2001; Barth et al., 2012; Reinehr et al., 2020). The antibody binds carbohydrates (Dai et al., 2001), but it does not react with any of a number of synthetic glycans representative of the E. multilocularis LL glycans (Koizumi et al., 2011; del Puerto et al., 2016), and its specificity remains unknown.
Shed LL materials
It is biological commonsense that the LL must turn over in order to allow the larval parasite to grow in size. In other words, the outermost strata of the LL, synthesized when the parasite was smaller, must be shed, and new strata incorporated from the inside. Given the massive size of the LL, this process has the potential to release considerable amounts of a parasite-derived “debris” within the host’s tissues. The accumulation and spread of such “debris” was first documented in E. multilocularis (Barth et al., 2012; Reinehr et al., 2020), and more recently in E. granulosus (Grimm et al., 2020a) infections in humans, and was achieved with the help of monoclonal antibodies Em2G11 and EmG3. The debris takes the form of particles given the names “SPEMs” and “SPEGs” (for “small particles of E. multilocularis/granulosus”), which measure 2–20 μm (in the parasite’s vicinity), or less than 1 μm (in the draining lymph nodes) (Barth et al., 2012; Grimm et al., 2020a, 2020b; Reinehr et al., 2020) (Thomas Barth, University of Ulm, personal communication). In the vicinity of the parasite, SPEMs/SPEGs are found in the histologically altered area close to the parasite and also in the surrounding vital host tissue (Grimm et al., 2020a, 2020b). The particles also reach the local blood vessels, and in liver they are found predominantly in the sinusoids, i.e. the vascular space. In E. granulosus infections in particular, particles were additionally found to be very abundant in a sample of pleural effusion (liquid escaping from the metacestode into the pleural space), as free particles and within macrophages. SPEMs/SPEGs also reach most of the regional lymph nodes in human infections (Grimm et al., 2020a). SPEMs/SPEGs have not yet been studied in natural or experimental animal infections.
The abundance of particulate LL material suggests the additional possibility of soluble LL-derived materials circulating in host fluids. Although this possibility has not yet been studied in vivo, E. multilocularis metacestodes in culture release soluble molecules recognized by the E492 antibody (Walker et al., 2004).
In sum, it can be expected that local host immune cells encounter the surface of the LL as such but much more commonly encounter LL-derived particles, both locally, and in draining lymph nodes. It will be important to characterize further the dissemination of LL-derived materials in infected hosts, including the soluble mucins, which might be detected with the help of the LL-reactive monoclonal antibodies available.
Innate cell recognition of the LL: mechanisms dependent on Clec4F
The very abundant mucin glycans of the LL can be expected to engage host innate immunity via carbohydrate receptors (lectins), helping to shape host responses to larval Echinococcus. When the LL mucins were probed in vitro with a panel of recombinant lectin receptors expressed by mammalian innate immune cells, the only panel member to bind clearly was Clec4F (Hsu et al., 2013). Clec4F is a cell-surface receptor that has been studied almost exclusively in rodents, in which it is only expressed in Kupffer cells (Fadden et al., 2003; Yang et al., 2013; Taylor et al., 2019), the sessile liver macrophages exposed to the vascular space. Rodent Clec4F has specificity for glycans terminated in GalNAc or Gal (Fadden et al., 2003; Coombs et al., 2006; Yang et al., 2013; Taylor et al., 2019). This monosaccharide specificity is consistent with the LL mucin glycans being predominantly terminated in Gal (Díaz et al., 2009; Hulsmeier et al., 2010; Lin et al., 2013; del Puerto et al., 2016), and in fact several defined E. granulosus and/or E. multilocularis LL mucin glycans are bound by recombinant Clec4F in vitro (Hsu et al., 2013). We have recently obtained definitive evidence that mouse Kupffer cells take up E. granulosus LL mucins in Clec4F-dependent fashion in vivo (unpublished results) but the downstream consequences of this engagement/uptake are not known.
Rodent Clec4F participates in the clearance by Kupffer cells of platelets that have lost the terminal sialic acid residues from their surface glycans, thereby exposing sub-terminal Gal residues (Li et al., 2017; Jiang et al., 2021). Such a function is not expected to be associated to the generation of pro-inflammatory signals. Hence Clec4F matches the profile of a receptor that would be “chosen” through evolution by an immunity-evading parasite for binding its shed molecules and presumably helping in their clearance (Díaz and Allen, 2007). The liver-specific expression of Clec4F in rodents would also match the fact that the liver is the primary infection site of larval E. multilocularis in its rodent hosts. The liver was also probably the primary anatomical site for the larval stages of the ancestors of the genus Echinococcus, thought to infect rodents (Hoberg et al., 2000). In the natural hosts of larval E. granulosus, the situation is more complex: non-liver (particularly lung) infections are common (Thompson, 2017; Lightowlers et al., 2021), and Clec4F is probably expressed extra-hepatically in addition to in the liver (Taylor et al., 2019). In particular in sheep, which is the main host for E. granulosus sensu stricto, Clec4F is expressed (at the mRNA level at least) in liver, spleen, and lymph nodes (Taylor et al., 2019) (our unpublished observations). Importantly, Clec4F expressed in Kupffer cells may be relevant even if the parasite is not located in the liver, as shed LL materials can reach draining lymph nodes (Grimm et al., 2020a), and conceivably more distant anatomical sites including the liver.
The question of the relevance of Clec4F in human echinococcosis leads to a more complex discussion. Clec4F expression in liver (i.e. in Kupffer cells) has been lost in primates (Taylor et al., 2019). Further, in humans in particular, the Clec4F gene does not encode a functional lectin (Taylor et al., 2019). Importantly, mouse Clec4F appears not to function alone in the clearance of de-sialylated platelets by Kupffer cells. Rather, Clec4F seems to function as part of a system encompassing the asialoglycoprotein receptor (ASGR) and the macrophage Gal-type lectins (MGLs), also expressed in mouse Kupffer cells (Deppermann et al., 2020; Jiang et al., 2021), and having GalNac/Gal-type specificities (Coombs et al., 2006). In our reasoning, this opens the possibility that Kupffer cells in different mammalian species use different combinations of GalNac/Gal-type lectins to clear de-sialylated platelets. This in turn would mean that human Kupffer cells may express lectins that bind LL mucins even if they do not express functional Clec4F. In this respect, we did not detect clear-cut binding of recombinant (soluble, dimeric) human ASGR or MGL to LL mucins in vitro (Hsu et al., 2013), but this could not reflect the situation with the native (cell-surface, trimeric) receptors. If the ASGR does bind LL mucins, hepatocytes may contribute to the clearance of LL mucins (especially soluble), possibly in all host species as hepatocyte ASGR expression is conserved in mammals (Grewal, 2010). It should be borne in mind that humans are accidental hosts of larval Echinococcus parasites, and play no role in infection transmission. Hence, if lectin receptors are important for the handling of shed LL materials by the host, it is conceivable that such a mechanism is defective in humans.
In sum, Clec4F and possibly other lectin receptors of similar specificity probably contribute to the clearance of shed LL mucins by Kupffer cells and possibly further phagocytic cells. In terms of future perspectives, it will be important to characterize how the accumulation and circulation of LL materials is influenced by cellular capture mediated by Clec4F and possibly other lectins. A related point is the kinetics of digestion of LL-derived materials in host phagocytic cells including Kupffer cells. An additional point is whether the engagement of the host lectin receptors by LL materials generates intracellular signals beyond those needed for internalization. It will also be important to determine the identity and functionality of Clec4F-expressing cells present in lymphoid tissues of ungulate natural hosts of E. granulosus.
Innate cell recognition of the LL: mechanisms independent of Clec4F
The pattern of Clec4F expression across cell types in the ruminant natural hosts of larval E. granulosus is not known. However, it is very likely at least some immune cells types that encounter LL particles in vivo will not express Clec4F. Focusing on dendritic cells (DC) and macrophages in particular, the most commonly used mouse cell models do not express Clec4F and are therefore suitable to study Clec4F-independent interactions with the LL. Such cell models include mouse bone marrow derived DC differentiated in the presence of GMCSF (GMCSF-BMDC) and bone marrow derived macrophages (BMDM). Both of these cell types do respond to E. granulosus LL particles, with up-regulation of CD86 (without concomitant up-regulation of CD40 or cytokine production) (Casaravilla et al., 2014). In the additional presence of TLR agonists (intended to mimic endogenous TLR agonists released in vivo as a consequence of tissue damage), the particles blunt CD40 up-regulation and IL-12 production while potentiating IL-10 production (Casaravilla et al., 2014). These responses to LL particles are not due to bound host antibodies (Casaravilla et al., 2014). They are also not significantly altered by removal of calcium Insp6, by oxidation of the LL mucin glycans with periodate, or by catalytic hydrolysis of conventional proteins in the material (Casaravilla et al., 2014). However, reduction of disulfides in the material strongly diminishes the responses (Casaravilla et al., 2014; Pittini et al., 2019; Casaravilla et al., 2020). The single cysteine residue in the predicted LL apomucins is present in a non-glycosylated N-terminal sequence (Tsai et al., 2013): the corresponding synthetic peptide, dimerized via disulfide, does not imitate or competitively inhibit the responses elicited by LL particles (Pittini et al., 2019). As disulfide reduction weakens the LL mucin meshwork (Casaravilla and Díaz, 2010), an alternative possibility is that disulfides are needed for LL particles to maintain certain physical characteristics required for the cellular responses observed. This possibility is consistent with the observations that neither soluble nor solubilized and plate-bound LL mucins elicit responses similar to those triggered by the particles (Casaravilla et al., 2014; Pittini et al., 2019). In broad agreement, a soluble mucin fraction from the E. multilocularis LL does not elicit changes in surface markers in GMCSF-BMDC (Margos et al., 2011). So together the evidence suggests that the responses to LL particles depend on supra-molecular properties of the mucin gel, and not on conventional interactions between molecular-level agonistic motifs and cellular receptors.
Myeloid cells are thought to respond to particles by a mechanism independent of conventional cellular receptors, as studied for materials with adjuvant properties like alum, sodium urate, and polystyrene beads (Ng et al., 2008; Flach et al., 2011; Shi, 2012; Mu et al., 2018). This mechanism, termed “membrane affinity triggered signaling” (MATS) requires that the cells adhere to the particles strongly. Responses triggered via this mechanism are abrogated in the presence of inhibitors of actin dynamics or inhibitors of phosphatidylinositol 3-kinase (PI3K) class I (which acts in concert with actin filaments (Lindmo and Stenmark, 2006; Flach et al., 2011; Shi, 2012). Myeloid cell responses to LL particles were completely abrogated by inhibitors of actin dynamics and of PI3K class I, in agreement with a MATS-like mechanism (Casaravilla et al., 2014; Pittini et al., 2019; Casaravilla et al., 2020). In further agreement, responses to particulate adjuvants thought to act by MATS can take place in the absence of particle internalization (Ng et al., 2008; Flach et al., 2011) and LL particles of sizes far too large for cellular internalization elicit responses similarly to smaller particles (Pittini et al., 2019; Casaravilla et al., 2020). Particulate adjuvants thought to act via MATS activate the NLRP3 inflammasome in DC primed with TLR adjuvants in vitro, or without need of such priming in vivo (Kool et al., 2008; Eisenbarth et al., 2008; Khameneh et al., 2017). The same activity is shown by LL particles on GMCSF-BMDC, again dependent on actin dynamics and PI3K class I but not on particle internalization (Casaravilla et al., 2020). NLRP3-dependent IL-1β, indicative of NLRP3 inflammasome activation is also detected in vivo after particle injection into the peritoneal cavity (Casaravilla et al., 2020). Thus, we see a MATS-like mechanism as a reasonable explanation for the capacity of LL particles to elicit responses in myeloid cells in a manner not dependent on any particular molecular-level motifs but instead dependent on their physical presentation. It is noteworthy that particles previously proposed to act via MATS are rigid in nature (crystals, polystyrene beads), whereas LL mucin particles are based on an aqueous gel and hence soft and flexible.
Exposure to LL particles does not cause activation of the central signaling module NF-κB or MAP kinases, or interfere with their activation in response to LPS, in GMCSF-BMDC. However, it does blunt the activation of Akt, an effector of PI3K class I, induced by LPS (Pittini et al., 2019). Similarly muted Akt activation is observed in response to disparate signals capable of activating PI3K class I, in GMCSF-BMDC and inflammatory macrophages exposed to LL particles (Seoane et al., 2016). This may seem hard to reconcile with PI3K class I being absolutely required for cells to respond to the particles. However, it should be first noted that Akt activation is blunted but not fully inhibited in the presence of the particles, and presumably the same happens with PI3K upstream of Akt. Moreover, an attractive explanatory hypothesis can be put forward based on details of the MATS mechanism. The interaction between the DC plasma membrane and polystyrene beads causes local accumulation of the lipid phosphatidylinositol 4,5-bisphosphate (PIP2) in the inner leaflet of the plasma membrane (Mu et al., 2018). This brings about recruitment of the cytosolic protein moesin and exposure of an atypical ITAM sequence that initiates MATS signaling. PIP2 is the substrate of PI3K class I (Hawkins and Stephens, 2015), and PIP2 availability can regulate the enzyme’s activity in some contexts (Saito et al., 2003; Schwartzberg, 2003). We envisage that PIP2 may be strongly accumulated in the plasma membrane area in contact with LL particles, and that this may result in diminished availability of PIP2 for agonist-induced PI3K class I activity, and hence in blunted Akt activation. In our hypothesis, PIP2 sequestration would take place to a larger extent during interaction with LL particles than it does during interaction with alum in particular, since alum does not blunt Akt activation in GMCSF-BMDC (Casaravilla et al., 2020).
Blunted Akt activation in response to LL particles results in diminished phosphorylation of Akt’s direct target GSK3, and these changes explain that GMCSF-BMDC exposed to the particles do not up-regulate CD40 normally in response to TLR agonists (Casaravilla et al., 2014; Pittini et al., 2019) (Fig. 4). Slightly blunted CD40 up-regulation in the presence of LL particles is also seen in peritoneal cavity DC in vivo (Casaravilla et al., 2014). The interaction between CD40 and its ligand CD154 is a central activating signal in the immune system, and is required for induction of most CD4+ T-cell responses by DC (Díaz et al., 2021).
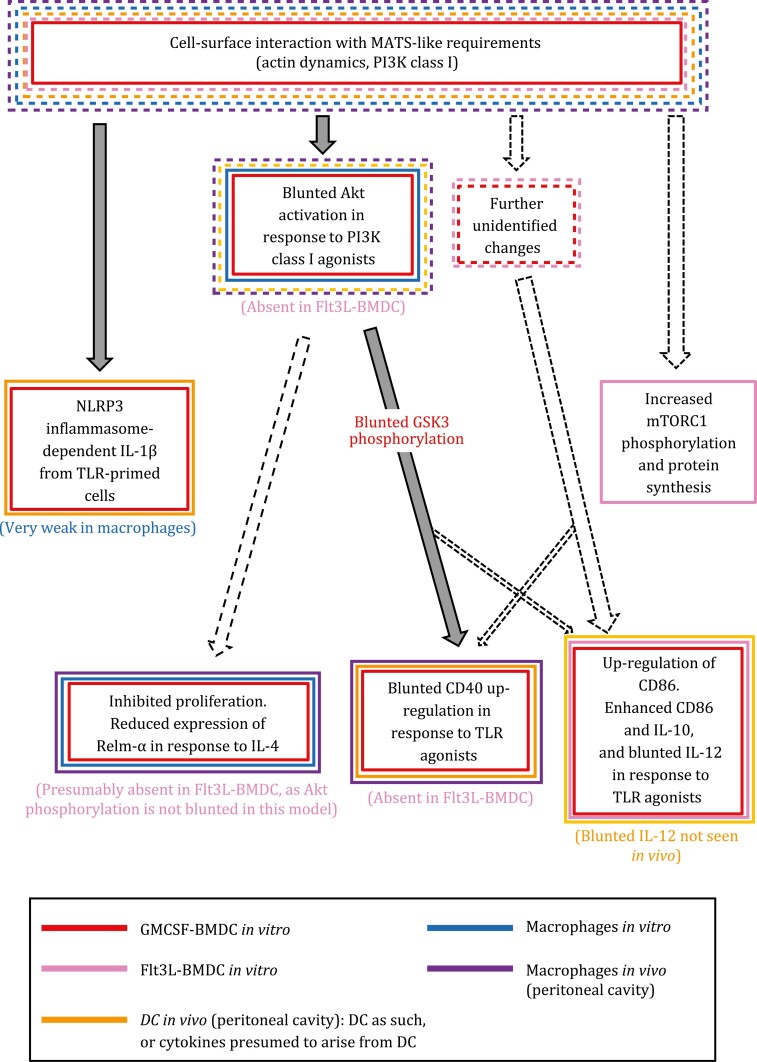
Figure 4.
Summary of proposed responses to LL particles observed in DC and macrophages, excluding any lectin receptor-dependent responses. The diagram attempts to summarize responses tentatively attributed to a MATS-like mechanism. The solid color-coded rectangles indicate the cell models in which there is supporting evidence for the corresponding event. The dotted rectangles, which have the same color code, indicate that the event is deduced from context to take place in the particular cell model. Grey arrows indicate mechanistic evidence, whereas empty dotted arrows indicate deduced mechanistic links, narrow arrows indicating weak possible links. The diagram is based on references (Casaravilla et al., 2014; Seoane et al., 2016; Pittini et al., 2019; Casaravilla et al., 2020; Rodrigues et al., 2021). The in vitro macrophage data correspond to BMDM or thioglycollate medium-elicited peritoneal cavity macrophages. The alterations in CD40, CD86, IL-10, and IL-12 in GMCSF-BMDC are known to be independent of NLRP3 and of PI3K class III (needed for NLRP3 inflammasome activation by LL particles) (Casaravilla et al., 2020). Although not shown in the figure, the mTORC1 complex is (indirectly) activated by Akt (in addition to other pathways). Thus, enhanced mTORC1 activation after exposure to LL particles, observed in Flt3L-BMDC (Rodrigues et al., 2021), is unlikely to occur in GMCSF-BMDC and macrophages, which respond to the particles with decreased capacity to activate Akt in response to PI3K class I agonists (Seoane et al., 2016; Pittini et al., 2019).
As mentioned, LL particles diminish Akt activation and blunt CD40 up-regulation in GMCSF-BMDC. However, a different picture is seen in experiments using BMDC differentiated in the presence of Flt3L (Flt3L-BMDC). These are a model for splenic (and likely other resident) DC, whereas GMCSF-BMDC are a model for inflammatory monocyte-derived DC (Naik, 2008). Flt3L-BMDC do not show reduced Akt or GSK3 phosphorylation in the presence of LL particles (Pittini et al., 2019). They also do not show muted CD40 up-regulation, and in fact they show the opposite trend (Pittini et al., 2019). One of the major downstream targets indirectly activated by Akt is the mTORC1 complex (Manning and Toker, 2017). Flt3L-BMDC are reported to respond to LL particles with enhanced mTOR phosphorylation and overall protein synthesis rate, suggestive of increased activation of the mTORC1 complex (Rodrigues et al., 2021). These observations strongly suggest that different DC sub-types can respond differently to LL particles (Fig. 4). Somewhat similarly, Flt3L-BMDC, but not GMCSF-BMDC, up-regulate CD40 in response to Schistosoma mansoni soluble egg antigen (Webb et al., 2017). Interestingly, CD40 up-regulation in this context depends on a type I interferon response by the DC themselves, which is necessary for induction of CD4+ T-cell responses to the parasite preparation.
LL-derived materials, particulate, or soluble, may also influence myeloid cells through non-intrinsic proteins that become adsorbed onto the structural mucins and in particular onto calcium Insp6 (Basika et al., 2012). We speculate that adsorbed protein explains the induction of arginase (a marker of macrophage of M(IL-4) phenotype (Rückerl and Allen, 2014)) and inhibition of nitric oxide production in macrophages by soluble LL extracts previously observed (Amri and Touil-Boukoffa, 2015). We wash the materials extensively with high ionic strength to remove adsorbed proteins and do not observe LL-induced arginase or M(IL-4) markers in macrophages in vitro (Díaz et al., 2016; Seoane et al., 2016). Arginase induction in the cited paper (Amri and Touil-Boukoffa, 2015) was abrogated by mannan, suggesting participation of the mannose receptor, known to induce arginase expression in macrophages in some contexts (Garrido et al., 2011; Nzoumbou et al., 2017). Potential mannose receptor ligands extracts are high mannose N-glycans (Khoo et al., 1997; Ezekowitz et al., 1988) probably derived from adsorbed glycoproteins, as N-glycans altogether are not intrinsic constituents of the LL (Díaz et al., 2009).
In sum myeloid cell types not expressing Clec4F respond to LL particles by mechanisms that appear to be receptor-independent and depend on physical properties of the particles. These mechanisms are akin to those explaining responses to particulate adjuvants, but certain differences with responses to adjuvants are apparent.
In terms of future perspectives, further research would be worthwhile on Flt3L-BMDC responses to LL particles, including assessing if there is a type I interferon response similar to that observed for S. mansoni soluble egg antigen (Webb et al., 2017). Flt3L-BMDC include both conventional and plasmacytoid DC (Naik et al., 2005): the analysis of plasmacytoid DC responses to LL materials holds particular interest, as this cell type has been recently found to be enriched near the parasite in human cystic echinococcosis (Yasen et al., 2021). In addition, the study of myeloid cell responses to LL particles should incorporate a systematic analysis of the influence of host proteins adsorbed in vivo, using homologous (e.g. mouse-mouse) systems. Experiments with myeloid cells should also address further whether LL particles induce the expression of central regulatory molecules such as TGF-β, PD-L1, and PD-L2.
Impact of laminated layer materials on immune biology of the echinococcoses: interactions with response induction
Adaptive immune responses, including T-cell responses, are readily detectable in Echinococcus infections (Wang and Gottstein, 2016; Díaz, 2017; Gottstein et al., 2017). The phenotype of DC in the parasite’s vicinity has been analyzed in experimental intraperitoneal E. multilocularis infection. The cells (defined only in terms of CD11c+ expression) were found to express lower protein levels of CD40, CD80, CD86, and MHCII than cells from naïve mice (Mejri et al., 2011). They were also found to have augmented levels of mRNA for TGF-β, and to suppress mitogen-induced T-cell proliferation ex-vivo. This is broadly consistent with the previously mentioned importance of regulatory T-cells in responses to larval Echinococcus.
The histological data on SPEMs/SPEGs discussed above indicate that extracellular LL particles must contact DCs both at the tissue site and in draining lymph nodes. The available data do not allow a safe inference on the impact of such conditioning on the polarization of the subsequent adaptive response. Some characteristics of GMCSF-BMDC conditioned by E. granulosus LL particles (low CD40, high IL-10, and low IL-12 in the additional presence of TLR agonists) suggest a tolerogenic profile. However, other characteristics (higher than basal CD86, IL-1β secretion in the presence of TLR agonists) suggest the opposite (Casaravilla et al., 2014; Casaravilla et al., 2020). Also, as mentioned, Flt3L-BMDC conditioned by LL particles up-regulate CD40 normally, unlike GMCSF-BMDC (Pittini et al., 2019). Injection of LL particles triggers antigen-specific IL-13 and IL-10 responses, without detectable IL-5 or interferon-γ, suggesting a regulatory/Th2 bias (our unpublished results). Our group is currently analyzing the potential of LL particles to expand regulatory T cells in vivo, with promising results.
For LL particles to be a source of antigens for T-cell-dependent responses, DC must phagocytose the particles, at the tissue site and/or in lymph nodes. Although we have not observed phagocytosis in vitro (Pittini et al., 2019), opsonization with antibodies and/or complement, which likely takes place in vivo, has not been assessed. Such opsonization could involve natural anti-carbohydrate antibodies (Milland and Sandrin, 2006) in addition to antibodies elicited by the infection. The overall capacity to internalize antigens is not inhibited in DC exposed to LL particles, as Flt3L-BMDC conditioned with the particles endocytose ovalbumin in vitro (Rodrigues et al., 2021), and similarly conditioned GMCSF-BMDC phagocytose plastic beads (our unpublished data). GMCSF-BMDC conditioned with LL particles are functional in terms of antigen presentation, as (once pulsed with ovalbumin peptide) they efficiently induce the proliferation of ovalbumin-specific CD4+ T cells in vitro (Seoane et al., 2016). As mentioned, injection of LL particles triggers detectable antigen-specific responses, which implies that the particles are taken up and processed by antigen-presenting cells in vivo (our unpublished results). In addition to particles, DC could incorporate soluble LL mucins: a soluble E. multilocularis LL fraction was shown to be taken up by resident mouse peritoneal cells in vitro (Dai et al., 2001).
Within lymph nodes in human infections, SPEGs are found both in the sinuses and in the germinal centers, i.e. the sites in which B cells are stimulated with the collaboration of T cells. Within germinal centers, the particles co-localize with follicular dendritic cells (FDC). FDC are cells specialized in the long-term retention of antigens opsonized by antibody and/or complement for the stimulation of B cells differentiating in the germinal center (Rezk et al., 2013). Co-localization with FDC suggests that LL particles effectively become opsonized in the infection context. Consistently, the mechanisms of complement activation and control outlined above imply that the LL becomes opsonized with iC3b and its downstream product C3dg, for which FDC express the receptor CR2 (Carroll and Isenman, 2012). Lymph nodes that contain SPEMs/SPEGs are statistically larger than patients’ lymph nodes that do not contain these particles, as well as larger than lymph nodes from control individuals (Grimm et al., 2020a), suggesting ongoing immune responses. Although these data do not imply causal relationships, they do suggest that SPEMs/SPEGs are a probable source of antigens for T-cell dependent B-cell responses, which again implies that DC internalize the particles in vivo. Consistent with our proposal that the LL mucins are poor in T-cell epitopes (Díaz et al., 2011b), neither of two different purified mucin fractions from the E. multilocularis LL elicits proliferation of spleen cells from infected mice in vitro, in contrast to preparations that contain conventional parasite antigens (Dai et al., 2001; Walker et al., 2004; Margos et al., 2011). According to our unpublished proteomic studies, it is unlikely that the (E. granulosus) LL structure includes conventional (non-mucin) proteins. We reason that an alternative source of T-cell antigens present in SPEMs/SPEGs in vivo are conventional parasite proteins (potentially including the major diagnostic antigens (Díaz et al., 2016) adsorbed onto the structural LL components. As mentioned, in E. granulosus, calcium Insp6 can adsorb conventional proteins (Casaravilla et al., 2006; Basika et al., 2012). In experiments in which LL-derived from natural infections is injected into mice (as in our own experiments mentioned in the previous paragraphs), strongly-bound proteins from the natural host may also act as T cell antigens.
SPEMs/SPEGs may also collaborate towards T-cell independent B-cell responses, as previously suggested (Díaz et al., 2011b). Antibodies induced in experimental E. multilocularis infection reactive with a LL mucin fraction are mostly T cell-independent (Dai et al., 2001). The induction of T-independent, LL-reactive antibodies may require priming by cross-reactive, non-LL, components exposed by the infecting parasite stages: in experimental E. multilocularis infection, LL-reactive antibodies are observed when mice are infected with protoscoleces i.p. but not when they are infected with eggs orally (Walker et al., 2004). Protoscoleces (from E. granulosus) are known to contain glycoconjugates that react with the antibody E492 (also reactive with the hydatid wall, as mentioned) that elicit T-cell independent antibodies after their injection in mice (Mourglia-Ettlin et al., 2011).
Capture of LL materials by the liver, and by Kupffer cells in particular, could be a major player influencing adaptive immune response induction in Echinococcus infections. Such capture could limit the amount of LL materials that reaches the spleen. In addition, such capture might generate parasite-specific regulatory responses that temper effector adaptive responses against the parasite. The liver (under non-inflammatory conditions) carries out extra-lymphoid tolerogenic antigen presentation, which can impact at a systemic level (Lüth et al., 2008; Thomson and Knolle, 2010; Heymann and Tacke, 2016). Kupffer cells in particular play a major role in tolerogenic antigen presentation to CD4+ T cells (You et al., 2008; Heymann et al., 2015). Although nothing is known about the cells present in lymphoid tissues of non-rodent non-primate mammals that express Clec4F (Taylor et al., 2019), it is plausible that these cells may also contribute to induction of tolerance to parasite antigens.
In broader terms, the presence of molecules with potential to induce regulatory responses in the LL would also be suggested by the effects of a soluble extract on whole spleen cells stimulated in vitro with LPS: in cells treated with the extract, transcription of Th1-inflammatory molecules (IFN-γ, IL-1β, TNF-α) was decreased whereas transcription of regulatory markers (TGF-β and Foxp3) was increased (Benazzouz et al., 2021). It should however be cautioned that the mentioned extract is likely to contain host (human) proteins as major components. Also, a mucin fraction from the E. multilocularis LL strongly suppresses spleen cell proliferation induced by conventional parasite antigens or mitogen (Walker et al., 2004). On the other hand, injection of LL particles in the absence of another challenge is reported to elicit inflammatory cytokines, suggesting that they have an inflammatory potential (Shakibapour et al., 2020; Benazzouz et al., 2021).
In sum, we lack direct information on what is the impact of DC conditioning by LL materials on subsequent adaptive responses. It is likely that LL particles have an inflammatory potential that is associated with their physical presentation: disparate particulate materials have inflammatory and adjuvant properties (Harris et al., 2010; Maughan et al., 2015). However, in the case of the LL this inflammatory potential has probably been curtailed to a minimum through evolution. The limitation by LL particles of Akt activation on inflammatory DC models and its downstream effects (Seoane et al., 2016; Pittini et al., 2019) may reflect this adaptation. DC and other conventional antigen-presenting cells must be able to take up LL particles in vivo, presumably with the help of opsonization by antibody and/or complement. In terms of the LL as a source of antigens, it is unclear if the LL mucins can provide T cell epitopes, and whether these mucins can elicit, or only amplify, T-independent B-cell responses. However, conventional antigens are probably taken up together with the mucin particles. The lectin-mediated uptake of LL materials by Kupffer cells may be an important player, by limiting the availability of these materials for interaction with conventional antigen-presenting cells and lymphocytes and/or by inducing regulatory responses to parasite antigens.
Impact of laminated layer materials on the immune biology of the echinococcoses: interactions with effector mechanisms
The LL operates as a barrier against direct contact between parasite cells and host immune cells. However, this does not offer complete protection to the parasite. In vitro, nitric oxide can diffuse through the LL and kill E. granulosus larvae (Steers et al., 2001). In vivo in natural infections, local granulomatous inflammation strongly correlates with low parasite viability (Díaz et al., 2018). The previous discussion on complement suggests that the E. granulosus LL has been perfected through evolution for minimal generation of complement-derived pro-inflammatory factors. In agreement, early in secondary E. granulosus experimental infections, surviving parasites show little inflammation and complement deposition on the newly formed LL; in contrast, those parasites that are killed before deploying a LL show complement deposition on their surface and are surrounded by inflammatory cells (Breijo et al., 2008).
In natural infections by larval Echinococcus, local inflammation when present can be eosinophil-rich, but it most often features macrophages and macrophage-derived cells types as central effector cells (Díaz et al., 2018). In experimental intra-hepatic E. multilocularis infection, depletion of macrophages during the first 6 weeks facilitates parasite establishment and perhaps also parasite development after LL deployment (Wang et al., 2020). We will therefore focus on macrophages in the following discussion.
Macrophage accumulation at infection sites can obviously be brought about by recruitment of their monocyte precursors. However, macrophage accumulation can also be caused by macrophage proliferation at the site, and this process is marked in type 2 contexts such as helminth infections (Rückerl and Allen, 2014; Jenkins and Allen, 2021). Such supra-homeostatic macrophage proliferation can be induced by the trophic factor that maintains macrophages numbers under basal conditions (M-CSF or CSF1) or by type 2 cytokines including IL-4 (Rückerl and Allen, 2014; Jenkins and Allen, 2021). The contributions of macrophage recruitment and proliferation to macrophage accumulation in chronic Echinococcus infections have not been assessed.
LL particles at low doses inhibit macrophage proliferation induced by exogenous M-CSF or IL-4, apparently as a consequence of their capacity to inhibit PI3K/Akt signaling induced by these agonists (Seoane et al., 2016). This activity has the potential to curtail macrophage accumulation in the vicinity of the parasite (given that IL-4 is present among host responses in the echinococcoses (Ma et al., 2014; Pang et al., 2014a, 2014b; Wang et al., 2014; Petrone et al., 2015), thus in principle helping parasite survival. However, higher doses of LL particles also cause recruitment of monocytes that differentiate into macrophages (Seoane et al., 2016) (our unpublished results).
Macrophages are highly plastic cells, and as such capable of being anti-parasite effectors as well as of suppressing effector responses (Ruckerl et al. 2014). The major recognized macrophage polarization program in type 2 contexts is the M(IL-4) phenotype, so termed because the major responsible cytokine is IL-4 (Ruckerl et al. 2014). Strong macrophage M(IL-4) differentiation takes place in experimental intra-hepatic E. multilocularis infection, both before, and after LL deployment (Wang et al., 2020). Also, depletion of macrophages in this system decreases local fibrosis, in agreement with M(IL-4) macrophages being associated with fibrosis in other systems (Ruckerl et al. 2014). Although as mentioned depletion of macrophages favors parasite establishment/development in this system, an-anti-parasitic role for M(IL-4) macrophages should not be inferred, as there is also an early wave of “classically activated” macrophages that may contribute to the result (Wang et al., 2020). The enzyme arginase, an M(IL-4) marker (Rückerl and Allen, 2014), is upregulated in macrophages and other local cells in experimental intraperitoneal E. granulosus sensu stricto infection (Cao et al., 2020). This apparently results in a reduction in circulating levels of arginine and consequent decrease in CD3ζ expression in T cells (Cao et al., 2020), as reported in other systems (Rodriguez et al., 2003).
The effects of LL materials on M(IL-4) phenotypes appear to be complex. LL particles in vitro and at low doses in vivo inhibit the induction (by exogenous IL-4) of the M(IL-4) marker Relm-α but not that of ChiL3 (also known as Ym1). This is also probably explained by the blunting of PI3K class I/Akt activation, as induction of Relm-α depends heavily on this pathway whereas induction of ChiL3 does not (Heller et al., 2008; Weisser et al., 2011; Rückerl et al., 2012; Byles et al., 2013; Covarrubias et al., 2016). However, at high doses in vivo, LL particles cause up-regulation of Relm-α, ChiL3, and arginase ((Seoane et al., 2016); our unpublished results).
Helminth immune evasion probably rests mostly on the regulatory, rather than on type 2, arm of responses (Díaz and Allen, 2007). As mentioned, evidence is growing for strong regulatory responses in the echinococcoses (Mejri et al., 2011; Pang et al., 2014a, 2014b; Gottstein et al., 2017; Díaz et al., 2018; Pang et al., 2018; Wang et al., 2018; Fratini et al., 2020). In macrophages in particular, M(IL-4) markers can co-exist with different levels of expression of immune-stimulatory (e.g. CD40, CD80, CD86) and immune-inhibitory molecules (e.g. PD-L1, PD-L2), and these can differ across macrophage sub-types at a given infection site (Campbell et al., 2018). In experimental intraperitoneal E. multilocularis infection, local macrophages express levels of CD80/CD86 similar to those in naïve mice and levels of CD40 below those of naïve mice (Mejri et al., 2006). Macrophages from the infected mice are poorer than those from naïve mice at stimulating antigen-specific T-cell proliferation, and they suppress T-cell proliferation induced by a mitogen. In the mitogen system, an agonistic CD40 antibody potentiates the T-cell stimulatory capacity of macrophages from naïve mice but not of those from infected mice, suggesting defective capacity to respond via CD40 (Mejri and Gottstein, 2006). Decreased CD40 up-regulation (in the presence of TLR agonists in vitro) is observed in BMDM exposed to E. granulosus LL particles (Casaravilla et al., 2014). Speculatively, contact of macrophages with SPEGs/SPEMs in vivo may decrease their CD40 expression and capacity to interact with CD4+ T cells, thus curtailing macrophage effector responses that require T cell help. Also, negative impacts of E. granulosus and E. multilocularis LL materials on macrophage effector functions is suggested by in vitro experiments analyzing nitric oxide production (Steers et al., 2001; Andrade et al., 2004).
More broadly, a potential of LL materials to blunt effector responses is suggested by major amelioration of dextran sulfate-induced colitis and reduction in plasma TNF-α and IFN-γ in mice injected i.p. with LL particles (Soufli et al., 2015).
In sum, the LL as such acts a barrier against cell-mediated effector mechanisms and it is evolutionarily optimized to minimize complement-dependent cell recruitment. LL particles can inhibit macrophage accumulation by proliferation. Whereas it is clear that LL materials can antagonize classical macrophage effector functions and suppress some responses via arginase, data are still missing on the capacity of LL materials to induce expression of (non-Th2) immune-inhibitory molecules, in macrophages, and other cell types.
Conclusions
The LL primarily fulfills a physical defensive function for larval Echinococcus parasites. The growth of these larvae requires the LL to be turned over, generating debris in the host tissues and in draining lymph nodes. There must have been strong evolutionary pressure for this debris to avoid triggering inflammatory responses that, considering its sheer abundance, would endanger the host’s life. This pressure is reflected in the subtle interactions with complement, which we now understand to a reasonable degree for E. granulosus. Since attaining an overall lack of pro-inflammatory signals often requires active downregulation, Echinococcus may have developed the capacity to deliver anti-inflammatory signals to host immune cells via LL materials. Despite some interesting hints, the search for such signals has proven hard. A major difficulty is that particulate materials have immunological activities by virtue of being particulate, making it impossible to choose an “inert” particulate material that acts as a point of comparison. The LL mucin glycans engage host Clec4F, expressed in most mammalian Kupffer cells, which surely provides a means for handling of LL materials by the host. Finally, it is obvious that host responses to LL materials must be shaped by parasite-derived immune regulators unrelated to the LL. It is crucial to characterize such immune regulators, and the excretion-secretion products of metacestodes are the main starting point (Nono et al., 2012; Nono et al., 2020).
Acknowledgements
The authors are grateful to Gustavo Salinas (Universidad de la República and Institut Pasteur Montevideo, Uruguay) for valuable help with PCR assays to analyze expression of sheep Clec4F. We apologize to those authors whose contributions could not be included because of lack of space. We apologize to those authors whose contributions could not be included because of lack of space.
Abbreviations
- ACP
alternative complement pathway
- AE
alveolar echinococcosis
- ASGR
asialoglycoprotein receptor
- BMDC
bone marrow derived DC
- BMDM
bone marrow derived macrophages
- CCP
classical complement pathway
- CE
cystic echinococcosis
- DC
dendritic cell(s)
- FDC
follicular dendritic cells
- Flt3L-BMDC
BMDC differentiated in the presence of Flt3L
- Gal
galactose
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- GMCSF-BMDC
BMDC differentiated in the presence of GMCSF
- Insp6
myo-inositol hexakisphosphate
- LL
laminated layer
- MATS
membrane affinity triggered signaling
- MGL
macrophage galactose-type lectin
- SPEGs
small particles of Echinococcus granulosus
- SPEMs
small particles of Echinococcus multilocularis
Contributor Information
Álvaro Díaz, Área Inmunología, Departamento de Biociencias (Facultad de Química) and Cátedra de Inmunología, Instituto de Química Biológica (Facultad de Ciencias), Universidad de la República, Montevideo, Uruguay.
Anabella A Barrios, Área Inmunología, Departamento de Biociencias (Facultad de Química) and Cátedra de Inmunología, Instituto de Química Biológica (Facultad de Ciencias), Universidad de la República, Montevideo, Uruguay.
Leticia Grezzi, Área Inmunología, Departamento de Biociencias (Facultad de Química) and Cátedra de Inmunología, Instituto de Química Biológica (Facultad de Ciencias), Universidad de la República, Montevideo, Uruguay.
Camila Mouhape, Área Inmunología, Departamento de Biociencias (Facultad de Química) and Cátedra de Inmunología, Instituto de Química Biológica (Facultad de Ciencias), Universidad de la República, Montevideo, Uruguay.
Stephen J Jenkins, Centre for Inflammation Research, Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, EH8 9JU, UK.
Judith E Allen, Lydia Becker Institute of Immunology and Inflammation, School of Biological Sciences, University of Manchester, Manchester Academic Health Sciences Centre, Manchester, M13 9NQ, UK.
Cecilia Casaravilla, Área Inmunología, Departamento de Biociencias (Facultad de Química) and Cátedra de Inmunología, Instituto de Química Biológica (Facultad de Ciencias), Universidad de la República, Montevideo, Uruguay.
Declarations
The authors declare no conflict of interest.
This work was supported by Agencia Nacional de Investigación e Innovación, Government of Uruguay (ANII) (grant FCE_1_2019_1_156234; to author CC) and a by Comisión Sectorial de Investigación Científica, Universidad de la República, Uruguay (“CSIC Iniciación” grant id 575 year 2019; to author LG).
In memory of Bob Sim.
This article is dedicated to the memory of Robert B. (“Bob”) Sim, who passed away in 2021. Bob was a world authority in complement biochemistry and an outstanding scientific mentor. Part of the advances summarized in this article were made possible by Bob’s direct contribution, his inspirational supervision of the article’s corresponding author, and his generous advice and help with reagents.
References
- Agudelo Higuita NI, Brunetti E, McCloskey C.. Cystic echinococcosis. J Clin Microbiol 2016;54:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri M, Touil-Boukoffa C.. A protective effect of the laminated layer on Echinococcus granulosus survival dependent on upregulation of host arginase. Acta Trop 2015;149:186–194. [DOI] [PubMed] [Google Scholar]
- Andrade MA, Siles-Lucas M, Espinoza Eet al. Echinococcus multilocularis laminated-layer components and the E14t 14-3-3 recombinant protein decrease NO production by activated rat macrophages in vitro. Nitric Oxide 2004;10:150–155. [DOI] [PubMed] [Google Scholar]
- Barrios AA, Grezzi L, Miles Set al. Inefficient and abortive classical complement pathway activation by the calcium inositol hexakisphosphate component of the Echinococcus granulosus laminated layer. Immunobiology 2019;224:710–719. [DOI] [PubMed] [Google Scholar]
- Barth TFE, Herrmann TS, Tappe Det al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis 2012;6:e1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basika T, Muñoz N, Casaravilla Cet al. Phagocyte-specific S100 proteins in the local response to the Echinococcus granulosus larva. Parasitology 2012;139:271–283. [DOI] [PubMed] [Google Scholar]
- Baz A, Richieri A, Puglia Aet al. Antibody response in CD4-depleted mice after immunization or during early infection with Echinococcus granulosus. Parasite Immunol 1999;21:141–150. [DOI] [PubMed] [Google Scholar]
- Benazzouz S, Amri M, Wang Jet al. In vitro immunoregulatory activity and anti-inflammatory effect of Echinococcus granulosus laminated layer. Acta Trop 2021;218:105886. [DOI] [PubMed] [Google Scholar]
- Breijo M, Anesetti G, Martínez Let al. Echinococcus granulosus: the establishment of the metacestode is associated with control of complement-mediated early inflammation. Exp Parasitol 2008;118:188–196. [DOI] [PubMed] [Google Scholar]
- Brunetti E, White AC.. Cestode infestations: hydatid disease and cysticercosis. Infect Dis Clin N Am 2012;26:421–435. [DOI] [PubMed] [Google Scholar]
- Byles V, Covarrubias AJ, Ben-Sahra Iet al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun 2013;4:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SM, Knipper JA, Ruckerl Det al. Myeloid cell recruitment versus local proliferation differentiates susceptibility from resistance to filarial infection. Elife 2018;7:e30947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Gong W, Zhang Xet al. Arginase promotes immune evasion of Echinococcus granulosus in mice. Parasit Vectors 2020;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC, Isenman DE.. Regulation of humoral immunity by complement. Immunity 2012;37:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaravilla C, Brearley C, Soule Set al. Characterization of myo-inositol hexakisphosphate deposits from larval Echinococcus granulosus. FEBS J 2006;273:3192–3203. [DOI] [PubMed] [Google Scholar]
- Casaravilla C, Pittini Á, Rückerl Det al. Unconventional maturation of dendritic cells induced by particles from the laminated layer of larval Echinococcus granulosus. Infect Immun 2014;82:3164–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaravilla C, Díaz A.. Studies on the structural mucins of the Echinococcus granulosus laminated layer. Mol Biochem Parasitol 2010;174:132–136. [DOI] [PubMed] [Google Scholar]
- Casaravilla C, Pittini A, Rückerl Det al. Activation of the NLRP3 inflammasome by particles from the Echinococcus granulosus laminated layer. Infect Immun 2020;88:e00190-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casulli A, Barth TFE, Tamarozzi F.. Echinococcus multilocularis. Trends Parasitol 2019;35:738–739. [DOI] [PubMed] [Google Scholar]
- Coltorti EA, Varela-Díaz VM.. Echinococcus granulosus: penetration of macromolecules and their localization on the parasite membranes of cysts. Exp Parasitol 1974;35:225–231. [DOI] [PubMed] [Google Scholar]
- Coombs PJ, Taylor ME, Drickamer K.. Two categories of mammalian galactose-binding receptors distinguished by glycan array profiling. Glycobiology 2006;16:1C–7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias AJ, Aksoylar HI, Yu Jet al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. Elife 2016;5:e11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai WJ, Hemphill A, Waldvogel Aet al. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of alphabeta+ CD4+ T cells. Infect Immun 2001;69:6074–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppermann C, Kratofil RM, Peiseler Met al. Macrophage galactose lectin is critical for Kupffer cells to clear aged platelets. J Exp Med 2020;217:e20190723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz A. Immunology of cystic echinococcosis (hydatid disease). Br Med Bull 2017;124:121–133. [DOI] [PubMed] [Google Scholar]
- Díaz A. Robert Braidwood (Bob) Sim. 1951–2021: a disciple’s perspective. Viruses 2021;13:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz A, Fernández C, Pittini Áet al. The laminated layer: recent advances and insights into Echinococcus biology and evolution. Exp Parasitol 2015;158:23–30. [DOI] [PubMed] [Google Scholar]
- Díaz A, Fontana EC, Todeschini ARet al. The major surface carbohydrates of the Echinococcus granulosus cyst: mucin-type O-glycans decorated by novel galactose-based structures. Biochemistry 2009;48:11678–11691. [DOI] [PubMed] [Google Scholar]
- Díaz A, Casaravilla C, Irigoín Fet al. Understanding the laminated layer of larval Echinococcus I: structure. Trends Parasitol 2011a;27:204–213. [DOI] [PubMed] [Google Scholar]
- Díaz A, Allen JE.. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur J Immunol 2007;37:3319–3326. [DOI] [PubMed] [Google Scholar]
- Díaz A, Casaravilla C, Allen JEet al. Understanding the laminated layer of larval Echinococcus II: immunology. Trends Parasitol 2011b;27:264–273. [DOI] [PubMed] [Google Scholar]
- Díaz A, Casaravilla C, Barrios AAet al. Parasite molecules and host responses in cystic echinococcosis. Parasite Immunol 2016;38:193–205. [DOI] [PubMed] [Google Scholar]
- Díaz A, Ferreira A, Sim RB.. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J Immunol 1997;158:3779–3786. [PubMed] [Google Scholar]
- Díaz A, González-Alayón I, Pérez-Torrado Vet al. CD40-CD154: a perspective from type 2 immunity. Semin Immunol 2021;53:101528. [DOI] [PubMed] [Google Scholar]
- Díaz A, Ibarguren S, Breijo Met al. Host-derived annexin II at the host-parasite interface of the Echinococcus granulosus hydatid cyst. Mol Biochem Parasitol 2000b;110:171–176. [DOI] [PubMed] [Google Scholar]
- Díaz A, Irigoín F, Ferreira Fet al. Control of host complement activation by the Echinococcus granulosus hydatid cyst. Immunopharmacology 1999;42:91–98. [DOI] [PubMed] [Google Scholar]
- Díaz A, Sagasti C, Casaravilla C.. Granulomatous responses in larval taeniid infections. Parasite Immunol 2018;40:e12523. [DOI] [PubMed] [Google Scholar]
- Díaz A, Willis AC, Sim RB.. Expression of the proteinase specialized in bone resorption, cathepsin K, in granulomatous inflammation. Mol Med 2000a;6:648–659. [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O’Connor Wet al. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008;453:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz RA, Stahl PD.. The structure and function of vertebrate mannose lectin-like proteins. J Cell Sci Suppl 1988;9:121–133. [DOI] [PubMed] [Google Scholar]
- Fadden AJ, Holt OJ, Drickamer K.. Molecular characterization of the rat Kupffer cell glycoprotein receptor. Glycobiology 2003;13:529–537. [DOI] [PubMed] [Google Scholar]
- Ferreira AM, Breijo M, Sim RBet al. Contribution of C5-mediated mechanisms to host defence against Echinococcus granulosus hydatid infection. Parasite Immunol 2000;22:445–453. [DOI] [PubMed] [Google Scholar]
- Ferreira AM, Díaz A, Fernández Cet al. Assessment of in vivo complement activation on the Echinococcus granulosus hydatid cyst wall. Parasite Immunol 2001;23:655–658. [DOI] [PubMed] [Google Scholar]
- Fishelson Z, Müller-Eberhard HJ.. C3 convertase of human complement: enhanced formation and stability of the enzyme generated with nickel instead of magnesium. J Immunol 1982;129:2603–2607. [PubMed] [Google Scholar]
- Flach TL, Ng G, Hari Aet al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med 2011;17:479–487. [DOI] [PubMed] [Google Scholar]
- Fratini F, Tamarozzi F, Macchia Get al. Proteomic analysis of plasma exosomes from Cystic Echinococcosis patients provides in vivo support for distinct immune response profiles in active vs inactive infection and suggests potential biomarkers. PLoS Negl Trop Dis 2020;14:e0008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlatti V, Chouquet A, Lunardi Tet al. Cutting edge: C1q binds deoxyribose and heparan sulfate through neighboring sites of its recognition domain. J Immunol 2010;185:808–812. [DOI] [PubMed] [Google Scholar]
- Garrido VV, Dulgerian LR, Stempin CCet al. The increase in mannose receptor recycling favors arginase induction and Trypanosoma cruzi survival in macrophages. Int J Biol Sci 2011;7:1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B, Dai W, Walker Met al. An intact laminated layer is important for the establishment of secondary Echinococcus multilocularis infection. Parasitol Res 2002;88:822–828. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Wunderlin E, Tanner I.. Echinococcus multilocularis: parasite-specific humoral and cellular immune response subsets in mouse strains susceptible (AKR, C57B1/6J) or ‘resistant’ (C57B1/10) to secondary alveolar echinococcosis. Clin Exp Immunol 1994;96:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B, Soboslay P, Ortona Eet al. Immunology of alveolar and cystic echinococcosis (AE and CE). In Advances in Parasitology. Amsterdam, Netherlands: Elsevier, 2017, 96, 1–54. [DOI] [PubMed] [Google Scholar]
- Grewal, P. K. The ashwell–morell receptor. In Methods in Enzymology. Amsterdam, Netherlands: Elsevier, 2010, 479, 223–241. [DOI] [PubMed] [Google Scholar]
- Grimm J, Beck A, Nell Jet al. Combining computed tomography and histology leads to an evolutionary concept of hepatic alveolar echinococcosis. Pathogens 2020b;9:E634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm J, Nell J, Hillenbrand Aet al. Immunohistological detection of small particles of Echinococcus multilocularis and Echinococcus granulosus in lymph nodes is associated with enlarged lymph nodes in alveolar and cystic echinococcosis. PLoS Negl Trop Dis 2020a;14:e0008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada N, Morita T, Ueda Tet al. Synthesis of the carbohydrate moiety of glycoproteins from the parasite Echinococcus granulosus and their antigenicity against human sera. Molecules 2021;26:5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Sharp FA, Lavelle EC.. The role of inflammasomes in the immunostimulatory effects of particulate vaccine adjuvants. Eur J Immunol 2010;40:634–638. [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Stephens LR.. PI3K signalling in inflammation. Biochim Biophys Acta 2015;1851:882–897. [DOI] [PubMed] [Google Scholar]
- Heller NM, Qi X, Junttila ISet al. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal 2008;1:ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann F, Peusquens J, Ludwig-Portugall Iet al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015;62:279–291. [DOI] [PubMed] [Google Scholar]
- Heymann F, Tacke F.. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13:88–110. [DOI] [PubMed] [Google Scholar]
- Hoberg EP, Jones A, Rausch RLet al. A phylogenetic hypothesis for species of the genus Taenia (Eucestoda: Taeniidae). J Parasitol 2000;86:89–98. [DOI] [PubMed] [Google Scholar]
- Hsu T-L, Lin G, Koizumi Aet al. The surface carbohydrates of the Echinococcus granulosus larva interact selectively with the rodent Kupffer cell receptor. Mol Biochem Parasitol 2013;192:55–59. [DOI] [PubMed] [Google Scholar]
- Hülsmeier AJ, Gehrig PM, Geyer Ret al. A major Echinococcus multilocularis antigen is a mucin-type glycoprotein. J Biol Chem 2002;277:5742–5748. [DOI] [PubMed] [Google Scholar]
- Hulsmeier AJ, Deplazes P, Naem Set al. An Echinococcus multilocularis coproantigen is a surface glycoprotein with unique O-gycosylation. Glycobiology 2010;20:127–135. [DOI] [PubMed] [Google Scholar]
- Ingold K, Dai W, Rausch RLet al. Characterization of the laminated layer of in vitro cultivated Echinococcus vogeli metacestodes. J Parasitol 2001;87:55–64. [DOI] [PubMed] [Google Scholar]
- Irigoín F, Laich A, Ferreira AMet al. Resistance of the Echinococcus granulosus cyst wall to complement activation: analysis of the role of Insp6 deposits. Parasite Immunol 2008;30:354–364. [DOI] [PubMed] [Google Scholar]
- Irigoín F, Casaravilla C, Iborra Fet al. Unique precipitation and exocytosis of a calcium salt of myo -inositol hexakisphosphate in larval Echinococcus granulosus. J Cell Biochem 2004;93:1272–1281. [DOI] [PubMed] [Google Scholar]
- Irigoín F, Ferreira F, Fernández Cet al. myo-Inositol hexakisphosphate is a major component of an extracellular structure in the parasitic cestode. Echinococcus granulosus. 2002;362:27–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoín F, Würzner R, Sim RBet al. Comparison of complement activation in vitro by different Echinococcus granulosus extracts. Parasite Immunol 1996;18:371–375. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, Allen JE.. The expanding world of tissue-resident macrophages. Eur J Immunol 2021;51:1882–1896. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Tang Y, Hoover Cet al. Kupffer cell receptor CLEC4F is important for the destruction of desialylated platelets in mice. Cell Death Differ 2021;28:3009–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khameneh HJ, Ho AW, Spreafico Ret al. The Syk-NFAT-IL-2 pathway in dendritic cells is required for optimal sterile immunity elicited by alum adjuvants. J Immunol 2017;198:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo K-H, Nieto A, Morris HRet al. Structural characterization of the N-glycans from Echinococcus granulosus hydatid cyst membrane and protoscoleces. Mol Biochem Parasitol 1997;86:237–248. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Yamano K, Schweizer Fet al. Synthesis of the carbohydrate moiety from the parasite Echinococcus multilocularis and their antigenicity against human sera. Eur J Med Chem 2011;46:1768–1778. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Hada N, Kaburaki Aet al. Synthetic studies on the carbohydrate moiety of the antigen from the parasite Echinococcus multilocularis. Carbohydr Res 2009;344:856–868. [DOI] [PubMed] [Google Scholar]
- Kool M, Pétrilli V, De Smedt Tet al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 2008;181:3755–3759. [DOI] [PubMed] [Google Scholar]
- Li Y, Fu J, Ling Yet al. Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells. Proc Natl Acad Sci USA 2017;114:8360–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowlers MW, Gasser RB, Hemphill Aet al. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int J Parasitol 2021;51:1167–1192. [DOI] [PubMed] [Google Scholar]
- Lin G, Todeschini AR, Koizumi Aet al. Further structural characterization of the Echinococcus granulosus laminated layer carbohydrates: the blood-antigen P1-motif gives rise to branches at different points of the O-glycan chains. Glycobiology 2013;23:438–452. [DOI] [PubMed] [Google Scholar]
- Lindmo K, Stenmark H.. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 2006;119:605–614. [DOI] [PubMed] [Google Scholar]
- Lüth S, Huber S, Schramm Cet al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest 2008;118:3403–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymbery AJ. Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus. Adv Parasitol 2017;95:111–145. [DOI] [PubMed] [Google Scholar]
- Ma X, Wang L, Zhao Het al. Th17 cells are associated with the Th1/Th2-cell balance during Echinococcus multilocularis infection. Mol Med Rep 2014;10:236–240. [DOI] [PubMed] [Google Scholar]
- Madsen CK, Brinch-Pedersen H.. Globoids and phytase: the mineral storage and release system in seeds. IJMS 2020;21:7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Toker A.. AKT/PKB signaling: navigating the network. Cell 2017;169:381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos MC, Grandgirard D, Leib Set al. In vitro induction of lymph node cell proliferation by mouse bone marrow dendritic cells following stimulation with different Echinococcus multilocularis antigens. J Helminthol 2011;85:128–137. [DOI] [PubMed] [Google Scholar]
- Maughan CN, Preston SG, Williams GR.. Particulate inorganic adjuvants: recent developments and future outlook. J Pharm Pharmacol 2015;67:426–449. [DOI] [PubMed] [Google Scholar]
- Mejri N, Gottstein B.. Intraperitoneal Echinococcus multilocularis infection in C57BL/6 mice affects CD40 and B7 costimulator expression on peritoneal macrophages and impairs peritoneal T cell activation. Parasite Immunol 2006;28:373–385. [DOI] [PubMed] [Google Scholar]
- Mejri N, Müller J, Gottstein B.. Intraperitoneal murine Echinococcus multilocularis infection induces differentiation of TGF-β-expressing DCs that remain immature: immature dendritic cells in chronic alveolar echinococcosis. Parasite Immunol 2011;33:471–482. [DOI] [PubMed] [Google Scholar]
- Merle NS, Church SE, Fremeaux-Bacchi Vet al. Complement system part I—molecular mechanisms of activation and regulation. Front Immunol 2015b;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle NS, Noe R, Halbwachs-Mecarelli Let al. Complement system part II: role in immunity. Front Immunol 2015a;6:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milland J, Sandrin MS.. ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens 2006;68:459–466. [DOI] [PubMed] [Google Scholar]
- Morseth DJ. Fine structure of the hydatid cyst and protoscolex of Echinococcus granulosus. J Parasitol 1967;53:312–325. [PubMed] [Google Scholar]
- Mourglia-Ettlin G, Amezcua-Vesely MC, Fraga Ret al. Echinococcus granulosus glycoconjugates induce peritoneal B cell differentiation into antibody-secreting cells and cytokine production. Parasite Immunol 2011;33:621–631. [DOI] [PubMed] [Google Scholar]
- Mu L, Tu Z, Miao Let al. A phosphatidylinositol 4,5-bisphosphate redistribution-based sensing mechanism initiates a phagocytosis programing. Nat Commun 2018;9:4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NSet al. Cutting edge: generation of splenic CD8+ and CD8‐ dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol 2005;174:6592–6597. [DOI] [PubMed] [Google Scholar]
- Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol 2008;86:439–452. [DOI] [PubMed] [Google Scholar]
- Ng G, Sharma K, Ward SMet al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 2008;29:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nono JK, Lutz MB, Brehm K.. Expansion of host regulatory T cells by secreted products of the tapeworm Echinococcus multilocularis. Front Immunol 2020;11:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nono JK, Pletinckx K, Lutz MBet al. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl Trop Dis 2012;6:e1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzoumbou-Boko R, De Muylder G, Semballa Set al. Trypanosoma musculi infection in mice critically relies on mannose receptor-mediated arginase induction by a TbKHC1 kinesin H chain homolog. J Immunol 2017;199:1762–1771. [DOI] [PubMed] [Google Scholar]
- Pang N, Zhang F, Li Set al. TGF-β/Smad signaling pathway positively up-regulates the differentiation of Interleukin-9-producing CD4+ T cells in human Echinococcus granulosus infection. J Infect 2018;76:406–416. [DOI] [PubMed] [Google Scholar]
- Pang N, Zhang F, Ma Xet al. TGF-β/Smad signaling pathway regulates Th17/Treg balance during Echinococcus multilocularis infection. Int Immunopharmacol 2014a;20:248–257. [DOI] [PubMed] [Google Scholar]
- Pang N, Zhang F, Ma Xet al. Th9/IL-9 profile in human echinococcosis: their involvement in immune response during infection by Echinococcus granulosus. Mediat Inflamm 2014b;2014:781649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J, Wasmuth JD, Salinas Get al. A transcriptomic analysis of Echinococcus granulosus larval stages: implications for parasite biology and host adaptation. PLoS Negl Trop Dis 2012;6:e1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone L, Vanini V, Petruccioli Eet al. IL-4 specific-response in whole blood associates with human Cystic Echinococcosis and cyst activity. J Infect 2015;70:299–306. [DOI] [PubMed] [Google Scholar]
- Pittini A, Martínez-Acosta YE, Casaravilla Cet al. Particles from the Echinococcus granulosus laminated layer inhibit CD40 upregulation in dendritic cells by interfering with Akt activation. Infect Immun 2019;87:e00641–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Puerto L, Rovetta R, Navatta Met al. Negligible elongation of mucin glycans with Gal β1-3 units distinguishes the laminated layer of Echinococcus multilocularis from that of Echinococcus granulosus. Int J Parasitol 2016;46:311–321. [DOI] [PubMed] [Google Scholar]
- Raboy V. myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003;64:1033–1043. [DOI] [PubMed] [Google Scholar]
- Reinehr M, Micheloud C, Grimm Fet al. Pathology of echinococcosis: a morphologic and immunohistochemical study on 138 specimens with focus on the differential diagnosis between cystic and alveolar echinococcosis. Am J Surg Pathol 2020;44:43–54. [DOI] [PubMed] [Google Scholar]
- Rezk SA, Nathwani BN, Zhao Xet al. Follicular dendritic cells: origin, function, and different disease-associated patterns. Hum Pathol 2013;44:937–950. [DOI] [PubMed] [Google Scholar]
- Richards KS, Arme C, Bridges JF.. Echinococcus granulosus equinus: an ultrastructural study of the laminated layer, including changes on incubating cysts in various media. Parasitology 1983;86:399–405. [DOI] [PubMed] [Google Scholar]
- Riesle S, García MP, Hidalgo Cet al. Bovine IgG subclasses and fertility of Echinococcus granulosus hydatid cysts. Vet Parasitol 2014;205:125–133. [DOI] [PubMed] [Google Scholar]
- Rodríguez PC, Zea AH, DeSalvo Jet al. l-Arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol 2003;171:1232–1239. [DOI] [PubMed] [Google Scholar]
- Rodrígues CR, Nicolao MC, Chop Met al. Modulation of the mTOR pathway plays a central role in dendritic cell functions after Echinococcus granulosus antigen recognition. Sci Rep 2021;11:17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückerl D, Jenkins SJ, Laqtom NNet al. Induction of IL-4Rα-dependent microRNAs identifies PI3K/Akt signaling as essential for IL-4-driven murine macrophage proliferation in vivo. Blood 2012;120:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückerl D, Allen JE.. Macrophage proliferation, provenance, and plasticity in macroparasite infection. Immunol Rev 2014;262:113–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russi S, Siracusano A, Vicari G.. Isolation and characterization of a blood P1 active carbohydrate antigen of Echinococcus granulosus cyst membrane. J Immunol 1974;112:1061–1069. [PubMed] [Google Scholar]
- Saito K, Tolias KF, Saci Aet al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity 2003;19:669–678. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Cabrera PA.. Immunohistochemical observations on cellular response in unilocular hydatid lesions and lymph nodes of cattle. Acta Trop 2003;85:271–279. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sugimura M.. Studies on echinococcosis. XXI. Electron microscopical observations on general structure of larval tissue of multilocular Echinococcus. Jpn J Vet Res 1969;17:67–80. [PubMed] [Google Scholar]
- Sánchez-Corral P, Pouw RB, López-Trascasa Met al. Self-damage caused by dysregulation of the complement alternative pathway: relevance of the factor H protein family. Front Immunol 2018;9:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg PL. Amplifying Btk’s signal. Immunity 2003;19:634–636. [DOI] [PubMed] [Google Scholar]
- Seoane PI, Rückerl D, Casaravilla Cet al. Particles from the Echinococcus granulosus laminated layer inhibit IL-4 and growth factor-driven Akt phosphorylation and proliferative responses in macrophages. Sci Rep 2016;6:39204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibapour M, Shojaie B, Yousofi Darani H.. Immunization with hydatid cyst wall antigens can inhibit breast cancer through changes in serum levels of Th1/Th2 cytokines. Int J Prev Med 2020;11:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. To forge a solid immune recognition. Protein Cell 2012;3:564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufli I, Toumi R, Rafa Het al. Crude extract of hydatid laminated layer from Echinococcus granulosus cyst attenuates mucosal intestinal damage and inflammatory responses in Dextran Sulfate Sodium induced colitis in mice. J Inflamm 2015;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers NJ, Rogan MT, Heath S.. In-vitro susceptibility of hydatid cysts of Echinococcus granulosus to nitric oxide and the effect of the laminated layer on nitric oxide production. Parasite Immunol 2001;23:411–417. [DOI] [PubMed] [Google Scholar]
- Taherkhani H, Zeyhle E, Rogan MT.. Antibody responses in human cystic hydatid disease to the laminated layer of Echinococcus granulosus. Parasitol Res 2007;101:647–652. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Snelling T, Smith DFet al. Absence of a human ortholog of rodent Kupffer cell galactose-binding receptor encoded by the CLEC4f gene. Glycobiology 2019;29:332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RCA. Biology and systematics of Echinococcus. Adv Parasitol 2017;95:65–109. [DOI] [PubMed] [Google Scholar]
- Thompson RCA, Jenkins DJ.. Echinococcus as a model system: biology and epidemiology. Int J Parasitol 2014;44:865–877. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Knolle PA.. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 2010;10:753–766. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sharpe C, Ridley C.. Intracellular processing of human secreted polymeric airway mucins. Ann ATS 2018;15:S154–S158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Domínguez S, Cerdá MFet al. Solution behaviour of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J Inorg Biochem 2005;99:828–840. [DOI] [PubMed] [Google Scholar]
- Tsai IJ, Zarowiecki M, Holroyd Net al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 2013;496:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kaay J, Van Haastert PJ.. Desalting inositolpolyphosphates by dialysis. Anal Biochem 1995;225:183–185. [DOI] [PubMed] [Google Scholar]
- Varela-Díaz VM, Coltorti EA.. The presence of host immunoglobulins in hydatid cyst membranes. J Parasitol 1973;59:484–488. [PubMed] [Google Scholar]
- Veiga N, Torres J, Domínguez Set al. The behaviour of myo-inositol hexakisphosphate in the presence of magnesium(II) and calcium(II): protein-free soluble Insp6 is limited to 49 μM under cytosolic/nuclear conditions. J Inorg Biochem 2006;100:1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Baz A, Dematteis Set al. Isolation and characterization of a secretory component of Echinococcus multilocularis metacestodes potentially involved in modulating the host-parasite interface. Infect Immun 2004;72:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang CS, Fang BBet al. Dual role of hepatic macrophages in the establishment of the Echinococcus multilocularis metacestode in mice. Front Immunol 2020;11:600635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, von Gunten S, Beldi Get al. Digest the sugar, kill the parasite: a new experimental concept in treating alveolar echinococcosis. Pharmacology 2021;106:3–8. [DOI] [PubMed] [Google Scholar]
- Wang J, Cardoso R, Marreros Net al. Foxp3+ T regulatory cells as a potential target for immunotherapy against primary infection with Echinococcus multilocularis eggs. Infect Immun 2018;86:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lin R, Zhang Wet al. Transcriptional profiles of cytokine/chemokine factors of immune cell-homing to the parasitic lesions: a comprehensive one-year course study in the liver of E. multilocularis-infected mice. PLoS One 2014;9:e91638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gottstein B.. Immunoregulation in larval Echinococcus multilocularis infection. Parasite Immunol 2016;38:182–192. [DOI] [PubMed] [Google Scholar]
- Webb LM, Lundie RJ, Borger JGet al. Type I interferon is required for T helper (Th) 2 induction by dendritic cells. EMBO J 2017;36:2404–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser SB, McLarren KW, Voglmaier Net al. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol 2011;41:1742–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Vuitton L, Tuxun Tet al. Echinococcosis: advances in the 21st century. Clin Microbiol Rev 2019;32:e00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K, Koizumi A, Takeda Tet al. Galα1-4Galβ1-3GalNAc is the dominant epitope of Em2 antigen, the mucin-type glycoprotein from Echinococcus multilocularis. Parasitol Res 2012;111:795–805. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Chen JB, Tsai TFet al. CLEC4F is an inducible C-Type Lectin in F4/80-positive cells and is involved in alpha-galactosylceramide presentation in liver. PLoS One 2013;8:e65070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen A, Sun W, Aini Aet al. Single-Cell RNA sequencing reveals the heterogeneity of infiltrating immune cell profiles in the hepatic cystic echinococcosis microenvironment. Infect Immun 2021;89:e0029721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Q, Cheng L, Kedl RMet al. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 2008;48:978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wen H, Li Jet al. Immunology and immunodiagnosis of cystic echinococcosis: an update. Clin Dev Immunol 2012;2012:101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhang W, Zhang Let al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat Genet 2013;45:1168–1175. [DOI] [PubMed] [Google Scholar]