Abstract
Global climate change is leading to higher ambient temperatures, and more frequent heatwaves. To date, impacts of ambient extreme heat on childhood morbidity have been understudied, although - given children’s physiologic susceptibility, with smaller body surface-to-mass ratios, and many years of increasing temperatures ahead - there is an urgent need for better information to inform public health policies and clinical approaches. In this review, we aim to: (1) identify pediatric morbidity outcomes previously associated with extreme heat, (2) to identify predisposing co-morbidities which may make children more susceptible to heat-related outcomes, and (3) to map the current body of available literature.
A scoping review of the current full-text literature was conducted using the Arksey and O’Malley framework (2015). Search terms for (1) pediatric population, (2) heat exposures, (3) ambient conditions, and (4) adverse outcomes were combined into a comprehensive PubMed and Medline literature search. Of the 1753 publications identified, total of 20 relevant studies were ultimately selected based on selection criteria of relevance to U.S. urban populations.
Most identified studies supported positive associations between high extreme temperature exposures and Heat-related Illness, Dehydration/ Electrolyte Imbalance, General Symptoms, Diarrhea and Digestion Disorders, Infectious Diseases/ Infections, Asthma/ Wheeze, and Injury. Most studies found no association with Renal Disease, Cardiovascular Diseases, or Diabetes Mellitus. Results were mixed for Other Respiratory Diseases, and Mental Health/ Psychological Disorders. Very few of the identified studies examined susceptibility by pre-existing conditions; Cystic Fibrosis was the only co-morbidity for which we found significant evidence.
Further research is needed to understand the nuances of associations between extreme heat and specific outcomes – particularly how associations may vary by child age, sex, race/ ethnicity, community characteristics, and other pre-existing conditions.
Keywords: Pediatric morbidity, heat wave, extreme heat, climate change, childhood morbidity, hot temperatures, high ambient temperatures, hot weather, warm season, children, child, vulnerability, chronic conditions
1. Introduction
Global climate change is a leading public health concern in our world today. It is regarded as one of the largest impending social, environmental, and health threats we are facing this century. Climate change will result in an increase in ambient temperatures, more frequent and extreme heat waves, and more frequent extreme weather events around the world (Meehl and Tebaldi 2004).
To date, the bulk of the heat-health research has focused on risks to older adults (Bunker et al 2016). In contrast, a strikingly small number of studies have identified impacts of heat on morbidity, and especially childhood morbidity. The pediatric population is vastly understudied yet has specific physiologic and developmental susceptibilities to heat (Blum et al. 1998; Bunyavanich et al. 2003) – including children’s physiologic susceptibility with smaller body surface-to-mass ratios, rapidly developing bodies, and long future life with many years of high temperatures ahead. Because childhood health sets the trajectory for future adult health and productivity, there is an urgent need for better information to inform public health policies and clinical approaches.
Children differ greatly from adults in their response to heat due to their rapid growth and development, smaller body size, greater skin surface area relative to body mass, and lesser ability to regulate core body temperature (Calkins et al 2016). Some studies investigate age-stratified mortality rates, but very few investigate specific childhood morbidities, with even fewer studies stratifying by age group or identifying susceptibility factors among children. It has been particularly noted that more research is needed within age groups of the pediatric population, because some impacts may be more pronounced in younger children (Sheffield et al 2018).
It is also possible that there are several unqiue predisposing conditions that make children more susceptible to poor health outcomes. Because children differ physioloigcally from adults, risks posed by well-established adult predisposing diseases, such as cardiovascular diseases, may differ as well. More research is needed to better understand what conditions put children at risk for poor health outcomes in high temperatures and heatwave situations.
There is a great need to understand children’s susceptibility to extreme heat, with an increasing number of individual studies on this population’s reaction to hot temperatures. Yet many studies have focused on mortality rather than morbidity, used broad indicators of morbidity [e.g., emergency department (ED) visits, rather than specific diagnoses], or not specifically focused on the pediatric populations (Xu et al 2014, Li et al 2015). To our knowledge, there are no existing reviews specific to pediatric morbidity assocaited with extreme heat. This scoping review aims to close this gap by detailing the scope of the published literature documenting assocaitions between extreme heat and pediatric morbidities.
1.1. Research Question
The primary goal of this study was to comprehensively review the literature to identify pediatric morbidity outcomes previously associated with ambient extreme heat, to help inform subsequent quantitative analyses. A secondary goal was to identify any evidence that specific pre-existing co-morbidities may be associated with greater susceptibility to heat, among children. Thus, our primary research questions were: For which child health outcomes is there substantial evidence of an association with extreme ambient heat? And, are there specific co-morbidities which have been shown to exacerbate impacts of extreme heat on child health?
2. Methods
This review followed a standard scoping review methodology (Arksey & O’Malley, 2015). Two key research questions were identified (stated above), a literature search was conducted using the defined search terms shown in Table 1, inclusion criteria were applied (Figure 1), and data from included sources was summarized and reported. This methodology allowed for the inclusion of multiple types of publications and study designs to produce a more thorough understanding of the range of pediatric morbidities associated with extreme heat. The inclusion criteria and methodology for this review were specified in a protocol in advance of the review.
Table 1.
Search Terms Used in PubMed.
| Search Component | Search Terms |
|---|---|
|
#1 Adverse Outcomes to focus on morbidity not mortality |
(((“emergency department”[Text Word] OR “casualty department”[Text Word] OR “child health”[Text Word] OR “hospital admission”[Text Word] OR “hospital admissions”[Text Word] OR “adverse effects”[Text Word] OR “health effects”[Text Word] OR risk[Text Word]))) |
|
#2 Heat Exposure Text and mesh terms |
((heat[Text Word] OR temperature[Text Word])))) OR ((“heat stroke”[Text Word] OR heatstroke[Text Word] OR “heat stress”[Text Word] OR hyperthermi*[Text Word]))) OR ((“Hot Temperature/adverse effects”[Mesh]) OR “Heat Stress Disorders”[Mesh]))) |
|
#3 Ambient Conditions to exclude acute heat |
((climat*[Text Word] OR weather[Text Word] OR ambient[Text Word] OR heatwave[Text Word]))) |
|
#4 Pediatric Population Test and mesh terms excluding preterm or premature births |
((((“Adolescent”[Mesh]) OR “Child”[Mesh]) OR “Infant”[Mesh:NoExp]) OR “Infant, Newborn”[Mesh:NoExp])) OR ((adolescen*[Text Word] OR child*[Text Word]))) OR ((((infan*[Text Word] OR neonat*[Text Word] OR newborn*[Text Word]))) NOT ((preterm[Text Word] OR prematur*[Text Word])) |
| Summary Search | #1 AND #2 AND #3 AND #4 |
Figure 1.
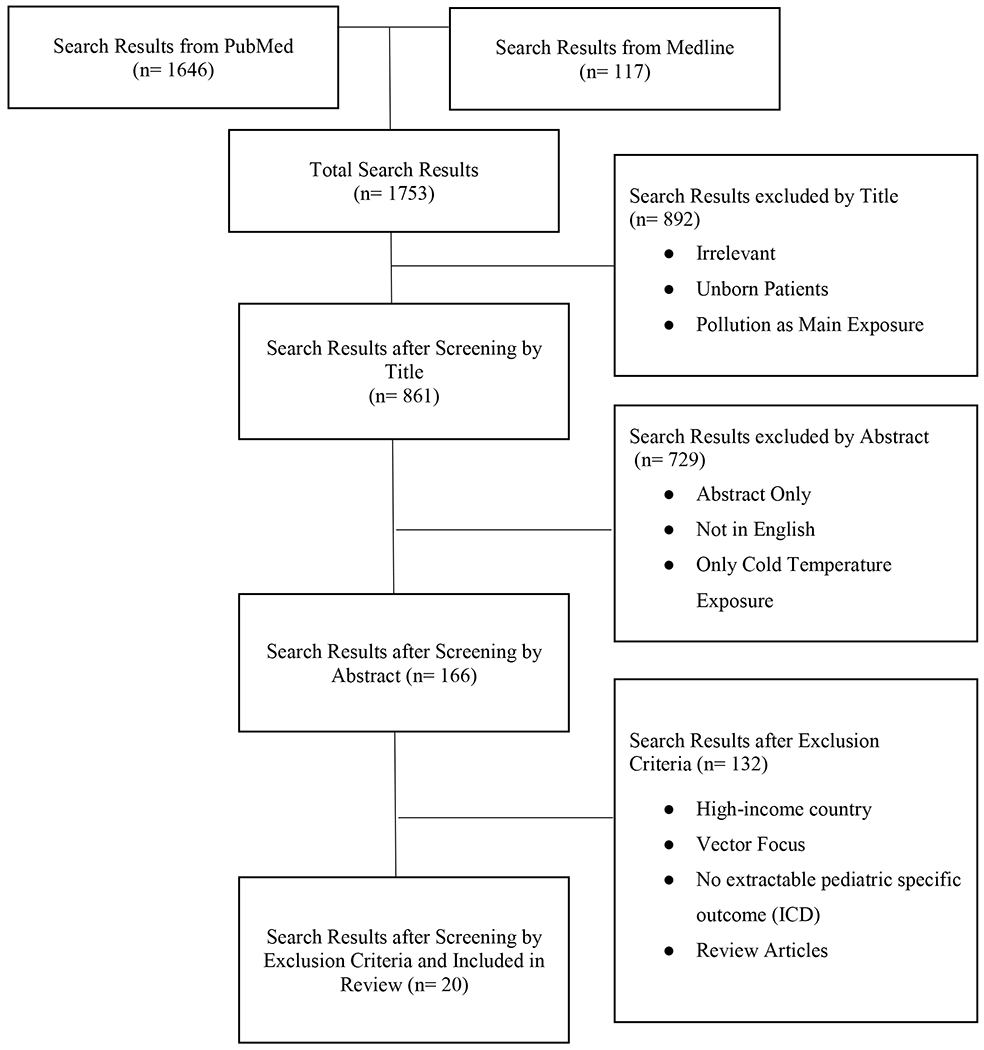
Flow Chart of the Literature Selection Process
Extractable data included specific ICD-9 or ICD-10 codes from pediatric populations exposed to extreme ambient heat, a range of temperature exposures examined, and effect estimates with confidence intervals. Each study was also reviewed for investigation of predisposing co-morbidities.
2.1. Database Search
Studies were identified by database searches of PubMed and Medline. No date restrictions were used in database searches. The search terms were developed with an experienced librarian with input from the research team. References from included literature were also searched for relevant studies not identified in the initial search results. The original electronic database search was conducted in December 2019, and updated in February 2020 and September 2021.
2.2. Search Terms
The search strategy included search terms for exposure to extreme heat, adverse effects, and the pediatric population. Both text and MeSH terms were included. Extreme Heat terms included hot temperature, heat stress, heat stroke, hyperthermia, and heat. Ambient temperature was specified using the search terms climate, weather, ambient, and heatwave. Adverse effects were investigated by including emergency department, casualty, hospital admission, adverse effects, risk, and health effects. The pediatric population was targeted by utilizing the search terms adolescent, child, and infant. The specific search terms are shown in Table 1.
2.3. Article Screening and Inclusion Criteria
Studies were included if they reported results specific to children aged 0 - 18 years of age, exposure to elevated ambient temperatures, and at least one morbidity outcome. Studies examining extreme heat and cold were included only if results were presented specifically for extreme heat, given the very different health outcomes, and very different biologic mechanisms, which may facilitate impacts of extreme cold and extreme heat on child health.
Studies were included from various settings relevant to the US urban population (i.e., high income) for relevance to future research in this population. All study designs were included. Studies containing information about previously-diagnosed conditions that might predispose children to adverse heat outcomes were also included.
Mortality studies were included in the initial review process but later excluded if they did not examine at least one morbidity outcome. Other excluded studies were those that were not published in English; used physiologic temperature therapy; focused solely on preterm children, premature birth outcomes, and unborn children; focused on pollution as the main exposure; or did not have extractable ICD-9 Codes (e.g., examining only total emergency department visits or ambulance call-outs). Also, while review articles were not included in data analysis, their references were reviewed for any studies that were relevant and not previously identified in search results; by this criteria, we identified several studies which included adult populations as well, but we include here only those reported results specific to children.
2.4. Assessment and Analysis
Electronic search results were downloaded into EndNote bibliographic software. In consultation with co-authors, the first author screened the titles and abstracts identified by the search and applied the selection criteria. Data extracted included morbidity outcomes, country(s) where outcome was experienced, and type of publication, as well as a short summary and any notes on the study. An overview of the collection process is shown in Figure 1. Most studies examined multiple outcomes, or examined associations within varying age strata, which we report here as separate results.
3. Results
3.1. Description of Included Studies
A total of 20 articles met the criteria for inclusion in this scoping review. Most (12) were studies of U.S. or Canadian populations, five based in Australia, and 3 from the U.K. and Europe. All studies examined data on outcome-specific emergency room visits or hospital admissions data.
3.2. Identified Pediatric Morbidity Outcomes
Relevant results were extracted for all outcomes reported in the original study. Extracted data included study design, age ranges of interest, exposure metric and interval of comparison, and effect estimates with confidence intervals.
Where temperature metrics are examined across multiple lag days prior to outcome, we report these as separate effect estimates or multi-day lags, as reported in the original study. In many cases, for brevity, we report only the significant lag day or case day results, but mark those studies where other lag were tested but not found significant.
All extracted results were initially sorted by morbidity (ICD-9/-10 code), and grouped where similar (i.e., ear infections were grouped under infectious disease/ infections).
As scoping reviews do not normally exclude studies based on design or analytic approach, comparison of the magnitude of effect size observed is not feasible. Instead, directions of association were compared to map the literature broadly. The final list of results is summarized in Table 2.
Table 2.
Identified Pediatric Morbidity Outcomes
| Author, date | Study location, period | Study Design | Sample characteristics* | Temperature metric | Outcomes | Main findings | Refinements/ Notes |
|---|---|---|---|---|---|---|---|
| Heat-Related Illness (ICD-9 992; ICD-10 T67 and X30) | |||||||
| Adeyeye et al., 2019 | New York State, USA; 2008-2012 | Case-crossover | 0-4 years (n = 124) 5-24 years (n= 2,344) |
Daily Tmax (per 1°C) | Hospital admissions, ED visits | RR (95% CI): 0-4 years: 1.32 (1.22, 1.43) ± 5- 24 years: 1.34 (1.31, 1.37) ± |
Warm season only (May -Sept) |
| Kingsley et al., 2016 | Rhode Island, USA; 2005-2012 | Poisson regression | 0-18 years (n = 4,716) | Daily Tmax (across 10°F increments) | ED admissions rate | Percent change (95% CI): 60 to 70°F: −2.2% (−10.7%, 7.0%) 65 to 75°F: −1.8% (−8.4%, 5.3%) 75 to 85°F = 16.6% (2.6%, 32.6%) 80 to 90°F = 31.1% (6.8%, 61.0%) |
Warm season only (Apr -Oct) |
| Knowlton et al., 2009 | California, USA; 2006 | Time series | 0-4 years | Heat wave vs. non-heat wave period | ED visits | RR (95% CI): 6.17 (2.58, 17.9) |
RR for ED visits only. |
| Lippmann et al., 2013 | North Carolina, USA; 2007-2008 | Time series | 0-9 years (n = 55) 10-18 years (n = 368) |
Daily Tmean (per 1°C above 15.6°C) | ED visits | IRR (95% CI): 0-9 years: 1.43 (1.33, 1.55) 10-18 years: 1.34 (1.30, 1.38) |
|
| Nitschke et al., 2011 | Adelaide, Australia; 1993-2009 | Case series | 0-4 years, 5-14 years | Heat wave vs. non-heat wave period | Hospital admissions, ED visits |
IRR (95% CI) for hospital admissions: 0-4 years: Heatwaves before 2008: 2.13 (1.27, 3.57) Ω 5-14 years: Heatwaves before 2008: 2.00 (0.95, 4.24) Ω IRR (95% CI) for ED visits: 0-4 years: Heatwaves before 2008: 1.58 (0.86, 2.91) 2008 heatwave: 0.92 (0.21, 4.27) 2009 heatwave: 3.36 (1.54, 7.30) 5-14 years: Heatwaves before 2008: 2.29 (1.21, 4.32) 2008 heatwave: 2.11 (0.62, 7.23) 2009 heatwave: 3.82 (1.40, 10.40) |
|
| Sheffield et al., 2018 | New York City, USA; 2005-2011 | Case-crossover | 0-4 years | Daily Tmax (per IQR: 13°F) | ED visits | Percent excess risk (95%CI): Lag 0: Age 1-3 years: 16.6% (3.0%, 31.9%)± |
Warm season only (May- Sept). |
| Toloo et al., 2014 | Brisbane, Australia; 2000-2012 | Time series analysis | 0-14 years | Daily Tmax >34°C, or >37°C, for 2+ days, vs. non-heat days | ED visits | RR (95% CI): >34°C: 3.00 (1.19 - 7.56) >37°C: 29.9 (7.27 - 112.6) |
Warm season only (Dec- Feb) |
| Van Loenhout et al., 2018 | Netherlands; 2002-2007 | Distributed lag non-linear Poisson regression | Age 0-14 years | 3 Tmax scenarios, vs 21 °C (ref) | ED admissions | RRs (95% CI): 1 day at 32°C: 1.15 (1.08, 1.22) 2 days at 28 °C: 1.14 (1.08, 1.20) 3 days at 26 °C: 1.12 (1.07, 1.17) |
Warm season only (May- Sept) |
| Winquist et al., 2016 | Atlanta, Georgia, USA; 1993-2012 | Time series | 5-18 years | Daily Tmax >=32°C vs 27°C | ED visits® | RR (95% CI) Lag 0: 4.59 (3.73, 5.65) ± Lag 1: 2.94 (2.41, 3.50) Lag 2: 1.75 (1.45, 2.11) Lag 3: 1.67 (1.39, 2.02) Lag 4: 1.22 (1.02, 1.46) |
Warm season only (May- Sept) |
| Dehydration and Electrolyte Imbalance (ICD-9 276; ICD-10 E86) | |||||||
| Basu et al., 2012 | California, USA; 2005 to 2008 | Case-crossover | 0-4 years, 5-18 years | Daily mean apparent temperature (per 10°F) | ER visits | Percent excess risk (95% CI): 0-4 years: Lag 0: 11.7% (5.6%, 18.1%) ± |
Warm season only (May-Sept) |
| Adeyeye et al., 2019 | New York State, USA; 2008-2012 | Case-crossover | 0-4 years (n = 7,078) 5-24 years (n= 8,856) |
Daily Tmax (per 1°C) | Hospital admissions, ED visits (dehydration) | RR (95% CI) 0-4 years: 1.00 (0.99, 1.01) ± 5- 24 years: 1.03 (1.02, 1.04) |
Warm season only (May-Sept) |
| Isaksen et al., 2016 | King County, WA, USA; 1990-2010 | Time series analysis | 0-4 years, 5-14 years | Humidex ¥ > 37.4 °C (99th %ile)] | Hospital admissions rate | Percent change (95% CI) per 1°C increase: 0-4 years: 17.7% (−4.2%, 44.5%) 5-14 years: 21.2% (−1.8%, 49.8%) |
Warm season only (May-Sept) |
| Knowlton et al., 2009 | California, USA; 2006 | Time series | 0-4 years | Heat wave vs. non-heat wave period | Hospital admissions, ED visits (electrolyte imbalance) | RR (95% CI): Hospital admissions: 1.06 (0.97, 1.16) ED visits: 1.19 (1.10, 1.30) |
|
| Nitschke et al., 2011 | Adelaide, Australia; 1993-2009 | Case series | 0-4 years, 5-14 years | Heat wave periods (daily Tmax >35°C for 3 consecutive days) vs non-heat wave periods | Hospital admission, ED visits (dehydration) |
IRR (95% CI) for hospital admissions: 0-4 years Heatwaves before 2008: 2.13 (1.27, 3.57) 5-14 years Heatwaves before 2008: 2.00 (0.95, 4.24) IRR (95% CI) for ED visits: 0-4 years Heatwaves before 2008: 1.58 (0.86, 2.91) 2008 heatwave: 0.92 (0.21, 4.27) 2009 heatwave: 3.36 (1.54, 7.30) 5-14 years Heatwaves before 2008: 2.29 (1.21, 4.32) 2008 heatwave: 2.11 (0.62, 7.23) 2009 heatwave: 3.82 (1.40, 10.40) |
Estimates are as reported above for HRI. Insufficient data to test 2008 and 2009 heatwaves separately. |
| Winquist et al., 2016 | Atlanta, GA, USA; 1993-2012 | Time series | 5-18 years | Daily Tmax >=32°C, vs 27°C | ED visits (dehydration) | RR (95% CI): 0-4 years Lag 0: 1.02 (0.95, 1.18) ± 5-18 years Lag 0: 1.12 (1.05, 1.20) ± Lag 1: 1.10 (1.03, 1.17) |
Warm season only (May- Sept) |
| General symptoms (ICD-9:780; ICD-10: R50-R69) | |||||||
| Sheffield et al., 2018 | New York City, USA; 2005-2011 | Time-stratified case-cross over | 0-4 years | Daily Tmax (per IQR: 13 °F) | ED visit | % excess risk (95%CI): Lag 0: Age 1-3 years: 10.1% (8.2%, 11.9%) ± |
Warm season only (May- Sept) |
| Renal Disease (ICD-9 580-589, ICD-10 N00, N399) | |||||||
| Isaken et al., 2016 | King County, WA, USA; 1990-2010 | Time series analysis | 0-4 years, 5-14 years | Humidex ¥ > 37.4 °C (99th %ile) | Hospital admissions (renal disorders) | Percentage change (95% CI) per 1°C increase: 0-4 years: 11.5% (−66.8, 275) 5-14 years: NE |
Warm season only (May-Sept) |
| Adeyeye et al., 2019 | New York State, USA; 2008 to 2012 | Case-crossover | 0-4 years (n= 44), 5-24 years (n= 600) |
Daily Tmax | Hospital admission/ED visits (acute kidney failure) | RR (95% CI) per 1°C increase: 0-4 years: 0.92 (0.84, 1.02) ± 5- 24 years: 1.02 (0.99, 1.05) ± |
Warm season only (May -Sept) |
| Knowlton et al., 2009 | California, USA; 2006 | Time series | 0-4 years | Heat wave vs. non-heat wave period | Hospital admissions, ED visits (acute kidney failure, nephritis) | RR (95% CI): Acute kidney failure: 0.61 (0.31, 1.15) Nephritis (hospitalization): 0.73 (0.49, 1.10) Nephritis (ED visit): 0.81 (0.44, 1.49) |
Insufficient data for ED visits for acute kidney failure. |
| Winquist et al., 2016 | Atlanta, GA, USA; 1993-2012 | Time series | 0-4 years | Daily Tmax >= 32°C, vs 27°C | ED visits (renal disorders) | RR (95% CI): Lag 0: 1.044 (0.923, 1.182) ± |
Warm season only (May- Sept) |
| Xiao et al., 2017 | Western Australia; 2006 to 2015 | Time series | 0–14 years | Severe or low-intensity heatwave [by Heatwave Severity Index (HWSI)], vs. non-heatwave | Hospital admission, ED visits (renal disorders) | RR (95% CI): Low intensity heatwave: 15.08 (14.47, 15.70) Severe/extreme heatwave: 16.50 (14.90, 18.10) |
Warm season only (Nov- April) |
| Infectious Disease/Infections (ICD-9 070-079 & 382, ICD-10 A00-A09 & H60) | |||||||
| Basu et al., 2012 | California, USA; 2005 to 2008 | Case-crossover | 0-4 years, 5-18 years | Daily mean apparent temperature | ED visits | % excess risk (95% CI) per 10°F increase: Lag 0: 0-4 years: 7.4% (2.2%, 13%) ± Lag 0: 5-18 years: 14.7% (16.8%, 29.4%) ± |
Warm season only (May-Sept) |
| Sheffield et al., 2018 | New York City, USA; 2005-2011 | Case-crossover | 0-4 years | Daily Tmax (per IQR: 13 °F) | ED visits | % excess risk (95%CI) per 13°F increase: Lag 0: Age 1-3 years: 4.9% (3.9%, 5.9%) ± |
Warm season only (May- Sept) |
| Wilk et al., 2020 | Southwestern Ontario, Canada; 2002-2019 | Distributed lag non-linear Poisson regression | 0-17 years, < 1 year, 1-12 years, 13-17 years | Daily Tmax >= 33.1°C (99th %ile) vs Tmax at minimum ED visit risk | ED visits (infectious diseases) | RR (95% CI) for ED visit: 0-17 years: 1.35 (1.07, 1.72) 1-12 years: 1.51 (1.13, 2.00) |
Warm season only (June-Aug) |
| Winquist et al., 2016 | Atlanta, GA, USA; 1993-2012 | Time series | 0-4 years, 5-18 years | Daily Tmax >= 32°C, vs 27°C | ED visits | RR (95% CI): 0-4 years Lag 0: 1.069 (1.010,1.131) ± 5-18 years Lag 0: 1.089 (1.012, 1.172) ± Lag 1: 1.120 (1.041, 1.205) Lag 6: 1.088 (1.015,1.167) |
Warm season only (May- Sept) |
| Diarrhea and Digestion Disorders (ICD-9: 564, 787; ICD-10: K00-K95, R19.7) | |||||||
| Xu et al., 2013 | Brisbane, Australia; 2003 to 2009 | Distributed lag non-linear Poisson regression | <5 years (n= 11,194) | Diurnal temperature range (DTR) vs 10 °C (ref) | ED admissions (diarrhea) | RRs (95% CI) per unit increase: Lag 1: RR= 1.03 (1.02,1.05) ± Lag 2: RR= 1.03 (1.01, 1.04) Lag 3: RR= 1.02 (1.01, 1.04) |
|
| Xu et al., 2014 | Brisbane, Australia; 2001 to 2010 | Distributed lag non-linear Poisson regression | 0- 14 years (n= 58,166) | Daily Tmean of 29.6 °C (99th %ile) vs 16.0 °C (ref) |
ED visits (diarrhea) | RRs (95% CI) of ED visits: 0-14 years Lag 0-1: RR= 1.08 (1.04, 1.13) ± 0-1 years Lag 0-1: RR= 1.06 (1.01, 1.13) ± 1-2 years Lag 0-1: RR= 1.17 (1.10, 1.25) ± 2-5 years Lag 0-1: RR= 1.07 (1.01, 1.13) ± 5-14 years Lag 0-1: RR= 1.03 (0.96, 1.11) ± |
|
| Sheffield et al., 2018 | New York City, USA; 2005-2011 | Time-stratified case-cross over | 0-4 years | Daily Tmax (per IQR: 13 °F) | ED visits (digestive disorders) | % excess risk (95%CI) per 13°F increase Lag O: Age 1-3 years: −0.3% (−3%, 2.4%) ± |
Warm season only (May- Sept) |
| Respiratory Disease (ICD-9 460-519, ICD-10 J00-J99) | |||||||
| Basu et al., 2012 | California, USA; 2005-2008 | Case-crossover | 0-4 years, 5-18 years | Daily mean apparent temperature | ER visits | % excess risk (95% CI) per 10°F increase: Lag 0: 0-4 years: −2.6% (−4.1%, −1.0%) ± |
Warm season only (May-Sept) |
| Kovats et al., 2004 | Greater London, UK; 1994-2000 | Time series | 0–4 years, 5–14 years | Daily Tmean vs 23°C (ref) | Hospital admissions | Percent change (95%CI) per °C: 0-4 years: 3.91% (−3.33%, 11.69%) 5-14 years: 5.20% (−3.04%, 14.15%) |
|
| Nitschke et al., 2011 | Adelaide, Australia; 1993-2009 | Case series | 0-4 years, 5-14 years | Heat wave periods (daily Tmax of 35°C or above for 3 consecutive days) vs non-heat wave periods | Hospital admissions, ED visits |
IRR (95% CI) for hospital admissions: 0-4 years Heatwaves before 2008: 0.88 (0.79, 0.98) 2008 heatwave: 0.83 (0.59, 1.2) 2009 heatwave: 1.03 (0.74, 1.42) 5-14 years Heatwaves before 2008: 1.00 (0.89, 1.13) 2008 heatwave: 1.02 (0.71, 1.47) 2009 heatwave: 0.65 (0.41, 1.04) IRR (95% CI) for ED visits: 0-4 years Heatwaves before 2008: 0.96 (0.89, 1.04) 2008 heatwave: 1.05 (0.89, 1.04) 2009 heatwave: 0.72 (0.62, 0.83) 5-14 years Heatwaves before 2008: 1.16 (0.91, 1.46) 2008 heatwave: 1.08 (0.70, 1.66) 2009 heatwave: 0.64 (0.36, 1.14) |
|
| O’Lenick et al., 2017 | Atlanta, GA, USA; 1993-2012 | Poisson generalized linear regression | 5–18 years (n = 161, 301) | Daily Tmax IQR (32 °C vs 27°C) | ED visits | RR (95% CI): Lag 0: 1.00 (0.98, 1.02) ± |
Warm season only (May-Sept) |
| Van Loenhout et al., 2018 | Netherlands; 2002-2007 | Distributed lag non-linear Poisson regression | 0-14 years | 3 Tmax scenarios, vs 21 °C (ref) | ED admissions | RRs (95% CI): 1 day of 32°C: 1.05 (1.02, 1.09) 2 days of 28 °C: 1.05 (1.02, 1.09) 3 days of 26 °C: 1.05 (1.02, 1.07) |
Warm season only (May- Sept) |
| Winquist et al., 2016 | Atlanta, GA, USA; 1993-2012 | Time series | 0-4 years, 5-18 years | Daily Tmax >=32°C, vs 27°C | ED visits | RR (95% CI): 0-4 years Lag 4: 0.98 (0.97, 0.99) ± Lag 5: 0.98 (0.97, 0.99) 5-18 years Lag 4: 0.96 (0.95, 0.98) ± Lag 5: 0.98 (0.97, 0.99) |
Warm season only (May- Sept) |
| Asthma/Wheeze (ICD-9 493, ICD-10 J45) | |||||||
| Figgs, 2018 | Douglas County NC, USA; 2011 & 2012 | Retrospective case-control | < 19 years | Model A: 2012 vs. 2011 admissions. Model B: Daily Tmean above vs. below median. |
ED admissions | OR (95% CI): Model A: 3.37 (2.72, 4.17) Model B: 3.35 (2.71, 4.15) |
Warm season only (28 May - 3 Aug) |
| O’Lenick et al., 2017 | Atlanta, Georgia, USA; 1993-2012 | Poisson generalized linear regression | 5–18 years (n = 51,360) | Daily Tmax IQR (32 vs 27°C) | ED visits | RR (95% CI): Lag 1: 1.04 (1.01, 1.07) ± Lag 2: 1.06 (1.03, 1.09) Lag 3: 1.06 (1.03, 1.09) Lag 4: 1.04 (1.01, 1.07) Lag 5: 1.04 (1.01, 1.07) 3-day average: 1.07 (1.04, 1.11) 5-day average: 1.12 (1.08, 1.17) 7-day average: 1.15 (1.10, 1.20) |
Warm season only (May-Sept) |
| Soneja et al., 2016 | Maryland, USA; 2000 to 2012 | Case-crossover | 0-4 years (n = 18, 043) 5-17 years (n = 16, 649) |
Daily Tmax > 95th %ile, vs other days | Hospital admissions | OR (95%CI): 0-4 years: Year-round: 0.94 (0.86, 1.02) Summer only: 1.08 (0.87, 1.34) 5-17 years: Year-round: 1.01 (0.92, 1.10) Summer only: 1.36 (1.05, 1.77) |
|
| Winquist et al., 2016 | Atlanta, GA, USA; 1993-2012 | Time series | 0-4 years, 5-18 years | Daily Tmax >= 32°C, vs 27°C | ED visits | RR (95% CI): 0-4 years Lag 0: 0.993 (0.967, 1.020) ± 5-18 years Lag 1: 1.042 (1.013, 1.072) ± Lag 2: 1.059 (1.030, 1.088) Lag 3: 1.058 (1.029, 1.087) Lag 4: 1.038 (1.010, 1.067) Lag 5: 1.037 (1.009, 1.065) |
Warm season only (May- Sept) |
| Cardiovascular Diseases (ICD-9: 390-459; ICD-10: I00-I99) | |||||||
| Adeyeye et al., 2019 | New York State, USA; 2008 to 2012 | Case-crossover | 0-4 years (n = 1,946) 5-24 years (n= 15,619) |
Daily Tmax | Hospitalization/ ED visits (cardiovascular disease) | RR (95% CI) per 1°C increase: 0-4 years: 1.01 (0.99, 1.03) ± 5- 24 years: 1.00 (0.99, 1.00) ± |
Warm season only (May -Sept) |
| Winquist et al., 2016 | Atlanta, Georgia, USA; 1993-2012 | Time series | 0-4 years, 5-18 years | Daily Tmax >=32°C, vs 27°C | ED visits (Dysrhythmia) | RR (95% CI): 0-4 years Lag 0: 1.15 (0.96, 1.37) ± 5-18 years Lag 0: 1.12 (0.96, 1.30) ± |
Warm season only (May-September) |
| Isaksen et al., 2016 | King County, Washington, USA; 1990 to 2010 | Time series analysis | 0-4 years, 5-14 years | Humidex ¥ > 37.4 °C (99th %ile) | ED visits (cardiovascular, cerebrovascular disease) | Percent change (95% CI) per unit increase: Cardiovascular disease: 0-4 years: −14.6% (−43.0%, 27.9%) ± 5-14 years: 2.2% (−26.8%, 42.9%) ± Cerebrovascular disease: 0-4 years: 0.6% (−2.3%, 3.6%) ± 5-14 years: −19.4% (−73.3%, 143%) ± |
Warm season only (May- Sept) |
| Van Loenhout et al., 2018 | Netherlands; 2002-2007 | Distributed lag non-linear Poisson regression | 0-14 years | 3 Tmax scenarios, vs 21 °C (ref) | ED visits (circulatory diseases) | RRs (95% CI): 1 day of 32°C: 0.99 (0.91, 1.09) 2 days of 28 °C: 1.00 (0.93, 1.09) 3 days of 26 °C: 1.01 (0.94, 1.08) |
Warm season only (May- Sept) |
| Diabetes Mellitus (ICD-9: 250; ICD-10: E08-E13) | |||||||
| Winquist et al., 2016 | Atlanta, Georgia, USA; 1993 to 2012 | Time series analysis | 5-18 years | Daily Tmax >= 32°C, vs 27°C | ED visits | RR (95% CI): Lag 0: RR= 1.044 (0.958, 1.136) ± |
Warm season only (May- Sept) |
| Isaksen et al., 2016 | King County, WA, USA; 1990-2010 | Time series analysis | 0-4 years 5-14 years |
Humidex ¥ | ED visits | Percent change (95% CI) per 1 ° increase: 0-4 years: −21.9% (−64.5%, 71.6%) 5-14 years: −11.9% (−30.3%, 11.5%) |
Warm season only (May- Sept) |
| Mental Health Disorders/Psychological Disorders (ICD-9 290-319, ICD-10 N00-N39) | |||||||
| Basu et al., 2017 | California, USA; 2005 to 2013 | Poisson regression | 6-18 years | Daily mean apparent temperature | ED visits | Percent excess risk (95%CI) per 10°F increase Mental health disorders: 7.3% (4.0%, 10.8%) Self-inflicted injury/ suicide: 6.7% (4.5%, 8.9%) |
Warm season (May- Oct): |
| Isaksen et al., 2016 | King County, WA, USA; 1990-2010 | Time series analysis | 0-4 years, 5-14 years | Humidex ¥ > 37.4 °C (99th %ile) | Hospital admissions | Percent change (95% CI) per 1 °C increase: 0-4 years: 17.8% (−31.4%, 102%) 5-14 years: −11% (−23.6%, 3.8%) |
Warm season only (May- Sept) |
| Injury (ICD-9 800-904, 910-929, 950-959, ICD-10 V00-Y99) | |||||||
| Parsons et al., 2010 | England & Wales, UK; 1996-2006 | Poisson regression | 0-15 years (n = 6, 063) | Daily Tmax × | ED visits (trauma) | IRR (95% CI): 1.019 (1.014, 1.025) |
|
| Sheffield et al., 2018 | New York City, USA; 2005-2011 | Case-crossover | 0-4 years | Daily Tmax (per IQR: 13 °F) | ED visits | % excess risk (95%CI) per 13°F increase: Lag 0: Age 1-3 years: 5.1% (3.8%, 6.4%) ± |
Warm season only (May- Sept). |
Many studies report only overall N, but test many outcomes. Outcome-specific sample sizes are not reported, expect where noted here.
Heat-related illness (HRI) defined differently in this study than others; includes ‘Disorders of electrolyte, fluid and acid-base balance, ‘Acute renal failure’ and ‘Effects of heat and light’.
Humidex is an apparent temperature index, combining temperature and relative humidity.
Insufficient data to test effects for hospital admissions.
Other lag days tested, non-significant results found.
Reference temperature not reported.
3.3. Morbidity Outcomes Identified
The most commonly-examined outcome was heat-related illness (HRI), though its definition varied somewhat across studies. All nine studies reported positive associations with heat metrics that included daily maximum temperature (Tmax), average temperature (Tmean), and heatwave vs. non-heatwave periods. In studies reporting associations across multiple lag days, the strongest associations were found on lag day 0 (case day), and generally comparable between older and younger children.
Six studies examined Dehydration/ Electrolyte Imbalance, with all but one reporting significant positive associations. Heat metrics varied across studies, though the only one that accounted for humidity (using a Humidex index) reported null results. Effects were stronger on the case day, where lag days were tested, and stronger and more significant, in several studies, among older vs. younger children.
One study reported positive significant associations between Tmax and general symptoms among children under age 4 (Sheffield et al., 2018). Two of the three studies examining diarrhea and digestion disorders found positive significant associations across multiple lag days among younger children (up to age 5).
All four studies of Infectious Diseases/ Infections reported positive significant associations across lag days up to 6 days prior to case days, and among children ranging from 0 to 18 years old. Likewise, all four studies of Asthma/ Wheeze reported significant positive associations, up to five days prior to case day, and particularly among older children (aged 5 - 18 years). Finally, both studies of injury outcomes reported significant positive associations with Tmax, particularly on the case day.
Most studies of Renal Disease found no significant association with various heat metrics with the exception of one Australian study that reported greater risk of ED visits or hospital admissions for renal disorders during either low- or high-intensity heatwaves, among all children aged 0 to 14 (Xiao et al., 2017). No studies reported significant associations between heat and Cardiovascular Diseases or Diabetes Mellitus in children.
Results were mixed for Other Respiratory Diseases (not asthma/ wheeze) as, among six studies, three were null, one reported consistent positive associations with Tmax across multiple days (Van Loenhout et al., 2018), and two reported protective associations, suggesting significantly lower risk of ED visit for respiratory disease with higher daily temperature; one found this protective association only among younger children (0 to 4 years old) (Basu et al., 2012), and the other found small but consistent protective associations for both older and younger children, four to five days prior to ED visit (Winquist et al., 2016).
Finally, there were mixed results for Mental Health/ Psychological Disorders, with one study reporting elevated risk of ED visits for mental health disorders and self-inflicted injury/ suicide with higher daily Tmean among children aged 6 to 18 years (Winquist et al., 2016). The other study (Isaksen et al., 2016) reported no association.
3.4. Age Reporting
Studies varied greatly in the child age range examined, but many studies reported results separately for younger (e.g., 0 to 4 years old) and older (e.g., 5 to 18 years old) children. Some outcomes (e.g., general symptoms) were exclusively examined among younger children (Sheffield et al., 2018), and some outcomes were examined only among older children (i.e., associations for asthma/ wheeze were stronger among older children, and mental health disorders and self-inflicted injury/ suicide were examined in Basu et al., 2017, only among 6 to 18 year-olds). In most cases, however, associations were fairly comparable across age ranges.
3.5. Predisposing Disease
Only two studies identified specific predisposing diseases in children, both identifying Cystic Fibrosis (CF) as a predisposing factor. One study found children with CF were more vulnerable to hypokalemia and metabolic alkalosis, and the other identified a vulnerability to Pseudomonas aeruginosa infections in high-heat situations. Results are shown in Table 3.
Table 3.
Identified Predisposing Factors for Adverse Heat Outcomes.
| Predisposing Disease | ICD-9 | ICD-10 | Source Name | Heat Effects | ICD-9 | ICD-10 |
|---|---|---|---|---|---|---|
| Cystic Fibrosis | 277 | E84 | Bates et al 1997 | hypokalemia | 276.8 | E87.6 |
| Cystic Fibrosis | 277 | E84 | Bates et al 1997 | metabolic alkalosis | 276.3 | E87.3 |
| Cystic Fibrosis | 277 | E84 | Psoter et al 2015 | Pseudomonas aeruginosa | 8.42 | B96.5 |
Discussion
This scoping review aimed to create a comprehensive list of pediatric morbidity outcomes identified in the literature as associated with extreme heat, and to identify predisposing illnesses that make children more vulnerable to morbid heat outcome. Using a scoping review approach, we were able to identify and combine many types of publications – each using different study designs, heat metrics, exposure intervals, outcomes, and populations. As such, our results do not provide a quantitative assessment of a specific question, but rather summarize the literature to date on heat and child morbidity, to shape research going forward.
We found predominantly positive associations between high extreme temperature exposures and Heat-related Illness, Dehydration/ Electrolyte Imbalance, General Symptoms, Diarrhea and Digestion Disorders, Infectious Diseases/ Infections, Asthma/ Wheeze, and Injury.
Those studies that considered Renal Disease, Cardiovascular Diseases, or Diabetes Mellitus generally did not find significant associations. Results were mixed for Other Respiratory Diseases, and Mental Health/ Psychological Disorders.
For those outcomes with mixed or close-to-null results, this finding could mean that the association is truly null, that the study was under-powered, or that the outcome categorization was too broad to identify a true association. Also, many studies were identified in the search process but later eliminated due to non-specific reporting of morbidity events, such as total emergency department visits, lacking specific ICD codes.
This review also aimed to identify areas needing more study within this body of literature. Notably, Cystic Fibrosis was the only pre-existing co-morbidity for which we found evidence of susceptibility. There is a great need to investigate other co-morbidities, and to examine other potential susceptibility factors (e.g., sex, race/ ethnicity, community characteristics, socioeconomic position), to better identify susceptible populations, and tailor heat-health interventions.
Xu et al (2013) noted that both hot and cold temperatures are associated with increased ED visits for acute exacerbations of childhood asthma, with male children aged 0-4 most vulnerable to heat effects (Xu et al 2013). Kenny et al states in a comprehensive review of diabetes literature that “diabetes tends to place individuals at greater risk for heat-related illness during heatwaves due to an impaired capacity to dissipate heat”, implying that diabetes is indeed a predisposing risk factor instead of an outcome (Kenny et al 2016). Finally, studies like Krämer et al note seasonal trends in pediatric eczema, showing exacerbations in both summer and winter temperatures, implying that ambient temperature can play a role in flare-ups in children (Krämer et al 2005).
Identifying specific outcomes associated with extreme heat is somewhat complicated by having an ICD code for “heat-related illness” (ICD-9 codes 992.0–992.9). This is a unique ICD-9 code in that, unlike most others, it presupposes the exposure - suggesting ample room for bias in diagnosis, as clinicians may be more inclined to use this diagnosis on hot days. Further, the code may entail many more specific outcomes (dehydration, exhaustion, etc.), and precludes more detailed analysis. More broadly, many of the studies identified reported results for very broad code ranges, rather than by specific diagnosis, complicating comparison between studies.
Strengths
The use of a scoping design allowed for the inclusion of many different types of study designs and thus also for the creation of a more comprehensive list of morbidity outcomes and better identification of areas needing additional study. This mapping will help guide future research in this understudied field of pediatric morbidity association with extreme heat.
Limitations
Included studies had inconsistent age groupings, with some including all children 18 years and under and others breaking groups of children into different, inconsistent age brackets. Because the study designs, heat metrics, and age ranges were so varied, any potential consistent age stratification or meta-analysis is virtually impossible.
The review was also limited to the data collection methods used in included studies when considering what should count as a predisposing disease risk factor. Future studies using more detailed health records should aim to distinguish more clearly between risk factors, including potential risk factors not reported here (e.g., sex, race/ ethnicity) and outcomes. As a scoping review does not exclude studies based on design or analytic approach, comparison of the magnitude of effect size observed is not feasible. Instead, we compared the direction of association to map the literature broadly.
Conclusion
This literature review created the most extensive list to date, to our knowledge, of pediatric morbidity outcomes associated with extreme heat in the literature as of November 2021. The prevalent health outcomes in the pediatric population positively associated with extreme heat were Heat-related Illness, Dehydration/ Electrolyte Imbalance, General Symptoms, Diarrhea and Digestion Disorders, Infectious Diseases/ Infections, Asthma/ Wheeze, and Injury.
Few to no significant associations were found for Renal Disease, Cardiovascular Diseases, or Diabetes Mellitus. Results were mixed for Other Respiratory Diseases, and Mental Health/ Psychological Disorders.
More research is needed as the pediatric population has been identified as vulnerable to extreme heat, yet it is clear that our understanding is not complete due to lack of specific results by age category and unclear health outcome coding systems.
This literature review can inform future surveillance and preventive measures during heat events, and serve as a basis for future researchers hoping to investigate pediatric morbidity due to extreme heat. With the looming threat of global climate change, studies like this are necessary to protect children and the public from preventable health burdens.
Acknowledgements
We would like to thank Kathleen Turner for her help and guidance.
Funding –
This study was funded by NIEHS (R01ES030717) (PES, JC); NIEHS (P30ES023515) (PES).
Footnotes
Conflict of Interest – The authors declare that they have no conflict of interest.
Ethics Approval – N/A
Consent to participate - N/A
Consent for publication - N/A
Code Availability – N/A
Availability of data and material –
N/A
References
- Adeyeye TE, Insaf TZ, Al-Hamdan MZ, Nayak SG, Stuart N, DiRienzo S, & Crosson WL (2019). Estimating policy-relevant health effects of ambient heat exposures using spatially contiguous reanalysis data. Environ Health, 18(1), 35. doi: 10.1186/s12940-019-0467-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arksey H, & O’Malley L (2015). Scoping studies: towards a methodological framework. International journal of social research methodology, 8(1), 19–32. [Google Scholar]
- Basu R, Gavin L, Pearson D, Ebisu K, & Malig B (2018). Examining the Association Between Apparent Temperature and Mental Health-Related Emergency Room Visits in California. Am J Epidemiol, 187(4), 726–735. doi: 10.1093/aje/kwx295 [DOI] [PubMed] [Google Scholar]
- Bates RD, Nahata MC, Jones JW, McCoy K, Young G, Cox S, Barson WJ. Pharmacokinetics and safety of tobramycin after once-daily administration in patients with cystic fibrosis. Chest. 1997. Nov 5;112(5):1208–13. doi: 10.1378/chest.112.5.1208. [DOI] [PubMed] [Google Scholar]
- Blum LN, Bresolin LB, Williams MA, From the AMA Council on Scientific Affairs (1998) Heat-related illness during extreme weather emergencies. JAMA 279(19):1514. doi: 10.1001/jama.279.19.1514 [DOI] [PubMed] [Google Scholar]
- Bunker A, Wildenhain J, Vandenbergh A, Henschke N, Rocklöv J, Hajat S, & Sauerborn R (2016). Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine, 6, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunyavanich S, Landrigan C, McMichael A, Epstein P (2003) The impact of climate change on child health. Ambul Pediatr 3(1):44–52 [DOI] [PubMed] [Google Scholar]
- Calkins MM, Isaksen TB, Stubbs BA, Yost MG, & Fenske RA (2016). Impacts of extreme heat on emergency medical service calls in King County, Washington, 2007-2012: relative risk and time series analyses of basic and advanced life support. Environ Health, 15, 13. doi: 10.1186/s12940-016-0109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figgs LW. Emergency department asthma diagnosis risk associated with the 2012 heat wave and drought in Douglas County NE, USA. Heart Lung. 2019. May-Jun;48(3):250–257. doi: 10.1016/j.hrtlng.2018.12.005. Epub 2019 Jan 24. [DOI] [PubMed] [Google Scholar]
- Green D, Bambrick H, Tait P, Goldie J, Schultz R, Webb L, … Pitman A (2015). Differential Effects of Temperature Extremes on Hospital Admission Rates for Respiratory Disease between Indigenous and Non-Indigenous Australians in the Northern Territory. Int J Environ Res Public Health, 12(12), 15352–15365. doi: 10.3390/ijerph121214988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harduar Morano L, Watkins S, & Kintziger K (2016). A Comprehensive Evaluation of the Burden of Heat-Related Illness and Death within the Florida Population. Int J Environ Res Public Health, 13(6). doi: 10.3390/ijerph13060551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksen TB, Yost MG, Hom EK, Ren Y, Lyons H, & Fenske RA (2015). Increased hospital admissions associated with extreme-heat exposure in King County, Washington, 1990-2010. Rev Environ Health, 30(1), 51–64. doi: 10.1515/reveh-2014-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny GP, Sigal RJ, & McGinn R (2016). Body temperature regulation in diabetes. Temperature, 3(1), 119–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley SL, Eliot MN, Gold J, Vanderslice RR, & Wellenius GA (2016). Current and Projected Heat-Related Morbidity and Mortality in Rhode Island. Environ Health Perspect, 124(4), 460–467. doi: 10.1289/ehp.1408826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton K, Rotkin-Ellman M, King G, Margolis HG, Smith D, Solomon G, … English P (2009). The 2006 California heat wave: impacts on hospitalizations and emergency department visits. Environ Health Perspect, 117(1), 61–67. doi: 10.1289/ehp.11594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats RS, Hajat S, & Wilkinson P (2004). Contrasting patterns of mortality and hospital admissions during hot weather and heat waves in Greater London, UK. Occup Environ Med, 61(11), 893–898. doi: 10.1136/oem.2003.012047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U, Weidinger S, Darsow U, Möhrenschlager M, Ring J, & Behrendt H (2005). Seasonality in symptom severity influenced by temperature or grass pollen: results of a panel study in children with eczema. Journal of investigative dermatology, 124(3), 514–523. [DOI] [PubMed] [Google Scholar]
- Li M, Gu S, Bi P, Yang J, & Liu Q (2015). Heat waves and morbidity: current knowledge and further direction-a comprehensive literature review. International journal of environmental research and public health, 12(5), 5256–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann SJ, Fuhrmann CM, Waller AE, & Richardson DB (2013). Ambient temperature and emergency department visits for heat-related illness in North Carolina, 2007–2008. Environmental research, 124, 35–42. [DOI] [PubMed] [Google Scholar]
- Macgregor DM (2003). Effect of weather on attendance with injury at a paediatric emergency department. Emerg Med J, 20(2), 204–205. doi: 10.1136/emj.20.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl GA, & Tebaldi C (2004). More intense, more frequent, and longer lasting heat waves in the 21st century. Science, 305(5686), 994–997. [DOI] [PubMed] [Google Scholar]
- Neut D, Fily A, Cuvellier JC, & Vallee L (2012). The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain, 13(1), 61–65. doi: 10.1007/s10194-011-0397-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke M, Tucker GR, & Bi P (2007). Morbidity and mortality during heatwaves in metropolitan Adelaide. Med J Aust, 187(11-12), 662–665. [DOI] [PubMed] [Google Scholar]
- Nitschke M, Tucker GR, Hansen AL, Williams S, Zhang Y, & Bi P (2011). Impact of two recent extreme heat episodes on morbidity and mortality in Adelaide, South Australia: a case-series analysis. Environ Health, 10, 42. doi: 10.1186/1476-069x-10-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Lenick CR, Winquist A, Chang HH, Kramer MR, Mulholland JA, Grundstein A, & Sarnat SE (2017). Evaluation of individual and area-level factors as modifiers of the association between warm-season temperature and pediatric asthma morbidity in Atlanta, GA. Environ Res, 156, 132–144. doi: 10.1016/j.envres.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons N, Odumenya M, Edwards A, Lecky F, Pattison G. Modelling the effects of the weather on admissions to UK trauma units: a cross-sectional study. Emerg Med J. 2011. Oct;28(10):851–5. doi: 10.1136/emj.2010.091058. Epub 2010 Nov 22. [DOI] [PubMed] [Google Scholar]
- Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc. 2015. Mar;12(3):385–91. doi: 10.1513/AnnalsATS.201408-400OC. [DOI] [PubMed] [Google Scholar]
- Sargen MR, Hoffstad O, & Margolis DJ (2014). Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol, 134(1), 51–57. doi: 10.1038/jid.2013.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield PE, Herrera MT, Kinnee EJ, & Clougherty JE (2018). Not so little differences: variation in hot weather risk to young children in New York City. Public Health, 161, 119–126. doi: 10.1016/j.puhe.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Braunstein M, & Lee-Wong M (2015). Association between climate factors, pollen counts, and childhood hay fever prevalence in the United States. J Allergy Clin Immunol, 135(2), 463–469. doi: 10.1016/j.jaci.2014.08.003 [DOI] [PubMed] [Google Scholar]
- Smith S, Elliot AJ, Hajat S, Bone A, Smith GE, & Kovats S (2016). Estimating the burden of heat illness in England during the 2013 summer heatwave using syndromic surveillance. J Epidemiol Community Health, 70(5), 459–465. doi: 10.1136/jech-2015-206079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneja S, Jiang C, Fisher J, Upperman CR, Mitchell C, Sapkota A. Exposure to extreme heat and precipitation events associated with increased risk of hospitalization for asthma in Maryland, U.S.A. Environ Health. 2016. Apr 27;15:57. doi: 10.1186/s12940-016-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toloo GS, Yu W, Aitken P, FitzGerald G, & Tong S (2014). The impact of heatwaves on emergency department visits in Brisbane, Australia: a time series study. Crit Care, 18(2), R69. doi: 10.1186/cc13826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenhout JAF, Delbiso TD, Kiriliouk A, Rodriguez-Llanes JM, Segers J, & Guha-Sapir D (2018). Heat and emergency room admissions in the Netherlands. BMC Public Health, 18(1), 108. doi: 10.1186/s12889-017-5021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk P, Gunz A, Maltby A, Ravichakaravarthy T, Clemens KK, Lavigne É, … & Vicedo-Cabrera AM (2021). Extreme heat and paediatric emergency department visits in Southwestern Ontario. Paediatrics & Child Health, 26(5), 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Grundstein A, Chang HH, Hess J, & Sarnat SE (2016). Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ Res, 147, 314–323. doi: 10.1016/j.envres.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Spicer T, Jian L, Yun GY, Shao C, Nairn J, … Weeramanthri TS (2017). Variation in Population Vulnerability to Heat Wave in Western Australia. Front Public Health, 5, 64. doi: 10.3389/fpubh.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Huang C, Turner LR, Su H, Qiao Z, & Tong S (2013). Is diurnal temperature range a risk factor for childhood diarrhea? PLoS One, 8(5), e64713. doi: 10.1371/journal.pone.0064713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Huang C, Hu W, Turner LR, Su H, & Tong S (2013). Extreme temperatures and emergency department admissions for childhood asthma in Brisbane, Australia. Occupational and environmental medicine, 70(10), 730–735. [DOI] [PubMed] [Google Scholar]
- Xu Z, Liu Y, Ma Z, Sam Toloo G, Hu W, & Tong S (2014). Assessment of the temperature effect on childhood diarrhea using satellite imagery. Sci Rep, 4, 5389. doi: 10.1038/srep05389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Sheffield PE, Su H, Wang X, Bi Y, & Tong S (2014). The impact of heat waves on children’s health: a systematic review. International journal of biometeorology, 58(2), 239–2471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A


