Abstract
Introduction
Bone mineral density (BMD) in adolescence is a crucial determinant in osteoporosis and fragility fractures in older age. Vitamin E is the most abundant lipid-soluble antioxidant present in the blood. However, the association of vitamin E status with BMD in children and adolescents remains unclear.
Methods
We first measured the association of vitamin E status (serum α- and γ tocopherol) with BMD in children and adolescents with the National Health and Nutrition Examination Survey (NHANES). Multiple linear regression models were performed to evaluate their relationship after adjusting for a large range of covariates. Stratified analyses and interaction tests were used to explore their effects on different genders, ages, and races/ethnicities.
Results
13,606 children and adolescents from NHANES (2005–2006, 2017–2018) were included in our analysis. Compared with the lowest α-tocopherol quartile, individuals in the highest α-tocopherol quartile are likelier to be Non-Hispanic White and have a higher value of poverty income ratio (PIR). They have a lower value of serum phosphorus and lumbar spine BMD. Every 1umol/L increase in serum α- and γ- tocopherol, the lumbar spine BMD decreased by -0.0016 and -0.0068 g/cm2. Compared with the lowest quartile serum α- and γ- tocopherol concentration, individuals in the highest quartile have a -0.0223 and -0.0329 g/cm2 lower mean BMD, respectively. Interaction effects suggest that the negative effect is more prominent among female youth, individuals aged 8–13 years, non-Hispanic whites, Mexican Americans, and non-Hispanic blacks.
Conclusions
Our study indicates serum α- and γ-tocopherol are negatively correlated with lumbar BMD. Age, gender, and race may have a modifying effect on this relationship. Our study has an important clinical implication. A higher vitamin E status for children and adolescents could not improve BMD, even decrease BMD. More prospective research with stronger evidence is needed to verify our findings and their underlying mechanisms.
Introduction
Osteoporosis is a condition characterized by decreased bone mass and impaired microarchitecture, causing bone pain and a higher risk of fragility fracture for older people [1]. Based on World Health Organization (WHO) standards, the global prevalence of osteoporosis among adults aged 50–59, 60–69, and 70–79 was 11.4%, 24.8, and 37.6% [2]. It is estimated that 22 million females and 5.5 million males have osteoporosis in European Union (EU), placing a significant health and economic burden on society [3]. The peak bone mass (PBM) in adolescence is a crucial determinant in the process of osteoporosis and fragility fractures in older age [4]. Evidence has shown that a 10% PBM increase was associated with a 50% decrease in fragility fracture risk in older age [5]. Boreham et al. reported that a 6.4% reduction in PBM during childhood and adolescence could double the risk of fragility fractures in adulthood [6]. Previous studies indicated that PBM formation could be influenced by genetics, diet and nutrition, physical activity, some diseases, and others [7–9].
As a lipid-soluble vitamin that cannot be synthesized in the body, vitamin E consists of two classes and eight isoforms, including tocotrienols (α, β, γ, δ) and four tocopherols (α, β, γ, δ). Alpha-tocopherol and gamma-tocopherol are two major forms in the body [10], which play a fundamental physiological role through their potent antioxidant properties [11, 12]. Vitamin E is associated with many diseases, including cancer, cardiovascular disease, renal disease, and dementia [13–16]. Basic and clinical research has also explored the association between a- and γ- tocopherol and bone metabolism but ended up with inconsistent results [17–19]. In a study conducted by Hermizi et al. [17], rats were given a 7 mg/kg dose of nicotine for the first two months and then 60 mg/kg vitamin E treatment in the following two months. The results showed that vitamin E reversed the bone loss caused by nicotine effects. Another study by Norazlina et al. revealed that vitamin E deficiency could reduce the spine bone mass in female rats [18]. Nevertheless, some animal studies also showed the negative effect of vitamin E on bone health. In a study by Fujita et al., they found that vitamin E could reduce bone mass by stimulating and enhancing the function of osteoclasts in the a-tocopherol transfer protein deficient mice [19]. Several epidemiological investigations on vitamin E and BMD have also reached different conclusions [20–22]. In a National Health and Nutrition Examination Survey (NHANES) study, Zhang et al. [20] found serum α-tocopherol was negatively associated with BMD in older Americans. In contrast, Yang et al.’s study [22] showed no relationship between dietary vitamin E intake and lumbar or femur BMD in older Scottish women. However, no study has investigated the relationship between vitamin E status and BMD in children and adolescents [23]. Therefore, our study evaluated the correlation between α- and γ-tocopherol and BMD in children and adolescents, aiming to provide more useful information for clinical and preventive purposes.
Methods
The use of data was approved by the ethics review board of the National Center for Health Statistics. Written consent was obtained from each participant.
Study population
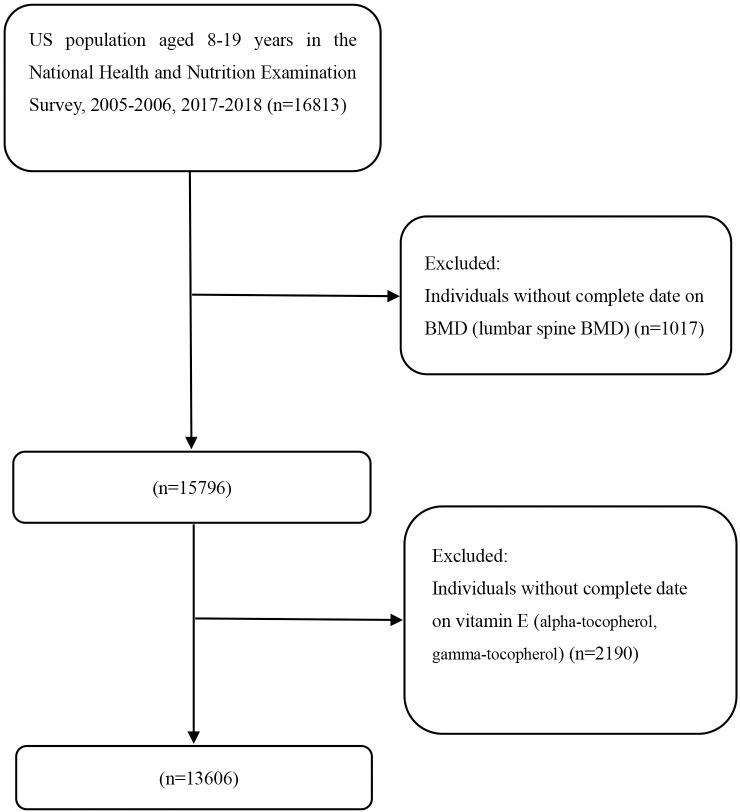
NHANES is a complex, multi-stage national nutrition survey that reflects the health status of the general U.S. population. NHANES currently collects and publicly releases data every two years. The National Center for Health Statistics (CNHS) approved all NHANES studies. Each participant signed an informed consent form in NHANES projects. We chose two cycles of NHANES (2005–2006, 2017–2018) since the data on serum vitamin E (alpha-tocopherol, gamma-tocopherol) exist only in the two cycles. Initially, 16,813 subjects aged 8–19 were found in NHANES 2005–2006 and 2017–2018. After excluding 3,207 subjects without serum vitamin E (alpha-tocopherol, gamma-tocopherol) and BMD (lumbar spine BMD), 13,606 eligible subjects are included in our analysis. Participant selection in this study is exhibited in Fig 1.
Fig 1. Flowchart identifying process of the NHANES participants inclusion and exclusion.
Variables
Alpha- and gamma-tocopherol
The serum vitamin E concentrations (alpha- and gamma-tocopherol) were measured by modified high-performance liquid chromatography using the photodiode array detection method. The researchers mixed 100 ml of serum with an ethanol solution containing two internal standards—nonapreno-beta-carotene and retinyl butyrate. Unknown analyte quantification was completed by comparing the peak height or peak area of a known quantity of the same analyte in the calibration solution. The alpha- and gamma-tocopherol concentration was compared with retinyl butyrate at 300 nm. Serum alpha- and gamma-tocopherol information was available for individuals older than 6 in selected NHANES cycles. Detailed information on alpha- and gamma-tocopherol could be obtained from LBXVIE and LBDGTCSI datasets on the NHANES website.
Bone mineral density
Since the femur BMD data were only available in individuals aged over 50 years, we selected lumbar spine BMD as the dependent variable in our analyses. The data on lumbar spine BMD was available for subjects older than eight years in selected cycles, which could be obtained from the DXXLSA dataset. The lumbar spine BMD was measured by Dual-energy X-ray absorptiometry (DXA) using Hologic densitometers (Hologic, Inc., Bedford, Massachusetts) with the software Apex 3.2. The lumbar spine BMD values were collected and standardized by professionals.
Other covariates
Other covariates were chosen based on the published studies [8, 24, 25]. The variance inflation factor (VIF) was used to detect co-linearity between multiple variables. Covariates were excluded if the VIF >5. Concomitant covariates in this study included race/ethnicity, gender, age, PIR (poverty income ratio), body mass index (BMI), serum phosphorus, and serum calcium. A higher PIR reflects better socioeconomic status and household income. Covariates of race/ethnicity, gender, age, and PIR were collected from structured questionnaires. Other covariates on collection and processing are detailed at https://www.cdc.gov/nchs/nhanes/.
Statistical analysis
All data and analyses were conducted with Package R 3.4 and EmpowerStats 4.0, adjusting for Mobile Examination Center (MEC) weights. The baseline characteristics of subjects were presented based on the quartile of alpha-tocopherol (Categories 1: 7.5–15.4 umol/L; Categories 2: 15.4–17.6 umol/L; Categories 3: 17.6–20.4 umol/L; Categories 4: >20.4 umol/L). We used weighted linear regression models and chi-square tests to compare continuous and categorical variables between groups. The relationship between serum α- and γ-tocopherol concentration and lumbar spine BMD was evaluated by weighted multivariate linear regression analyses. We built three models, including model 1 (unadjusted model), model 2 (adjusted for race/ethnicity, gender, and age), and model 3 (adjusted for race/ethnicity, gender, age, PIR, BMI, serum phosphorus, and serum calcium). The nonlinear association of α- and γ- tocopherol with lumbar BMD was also performed using smooth curve fits. After converting serum vitamin E concentrations to categorical variables (quartiles), another weighted multivariate linear regression model was performed. Then stratified analyses were performed by age (8–13; 14–19), gender (men, women), and race/ethnicity (Non-Hispanic White, Mexican American, Non-Hispanic Black, Other Hispanic, Other Race), and their interactions were also tested. P values< 0.05 were considered to be statistical significance.
Results
Overall, 13,606 participants were enrolled in this study, with an average age of 13.59 ± 3.37. Of these participants, 59.85% are non-Hispanic white, 14.16% are non-Hispanic black, 13.09% are Mexican American, 4.99% are other Hispanic, and 7.91% are other races. The mean serum α- and γ- tocopherol concentrations are 18.95 ± 4.99 and 4.56 ± 1.94 umol/L, respectively. The weighted baseline characteristics of participants are listed based on the alpha-tocopherol quartiles (Table 1). With the exception of total serum calcium (P = 0.071), no statistical differences are observed in participants’ characteristics. Compared with the lowest α-tocopherol quartile, individuals in the highest α-tocopherol quartile are likelier to be Non-Hispanic White and have a higher value of PIR. They have a lower value of serum phosphorus and lumbar spine BMD.
Table 1. Characteristics of the study population based on alpha-tocopherol quartiles.
| Alpha-tocopherol (umol/L) | |||||||
|---|---|---|---|---|---|---|---|
| total | Q1 (7.5–15.4) | Q2 (15.4–17.6) | Q3 (17.6–20.4) | Q4 (>20.4) | P value | ||
| Number of subjects (n) | 13606 | 3387 | 3396 | 3406 | 3417 | ||
| Age (years) | 13.59 ± 3.37 | 14.11 ± 2.95 | 13.65 ± 3.25 | 13.31 ± 3.53 | 13.40 ± 3.55 | <0.001 | |
| Gender (%) | <0.001 | ||||||
| Men | 52.11 | 59.92 | 49.09 | 49.39 | 51.23 | ||
| Women | 47.89 | 40.08 | 50.91 | 50.61 | 48.77 | ||
| Race/ethnicity (%) | <0.001 | ||||||
| Mexican American | 13.09 | 13.26 | 14.36 | 12.87 | 12.16 | ||
| Other Hispanic | 4.99 | 4.61 | 5.37 | 5.37 | 4.66 | ||
| Non-Hispanic White | 59.85 | 57.65 | 57.23 | 58.02 | 64.94 | ||
| Non-Hispanic Black | 14.16 | 18.65 | 16.28 | 13.74 | 9.73 | ||
| Other Race (Including Multi-Racial) | 7.91 | 5.83 | 6.76 | 10.00 | 8.51 | ||
| BMI | 22.14 ± 5.71 | 22.84 ± 5.84 | 22.12 ± 5.69 | 21.59 ± 5.28 | 22.13 ± 5.91 | <0.001 | |
| PIR | 2.64 ± 1.57 | 2.49 ± 1.56 | 2.58 ± 1.56 | 2.58 ± 1.51 | 2.85 ± 1.61 | <0.001 | |
| Serum total calcium (mmol/L) | 9.68 ± 0.25 | 9.66 ± 0.26 | 9.66 ± 0.25 | 9.68 ± 0.23 | 9.72 ± 0.25 | 0.071 | |
| Serum phosphorus (mmol/L) | 4.44 ± 0.57 | 4.45 ± 0.60 | 4.45 ± 0.56 | 4.45 ± 0.56 | 4.42 ± 0.56 | <0.001 | |
| Lumbar spine BMD (g/cm2) | 0.90 ± 0.19 | 0.92 ± 0.18 | 0.91 ± 0.19 | 0.88 ± 0.20 | 0.88 ± 0.19 | <0.001 | |
| Alpha-tocopherol (umol/L) | 18.95 ± 4.99 | - | - | - | - | - | |
| Gamma-tocopherol (umol/L) | 4.56 ± 1.94 | - | - | - | - | - | |
Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model. (%) for categorical variables. The P value was calculated by the weighted chi-square test. Abbreviation: BMI body mass index. BMD bone mineral density. PIR poverty income ratio.
Association between α-tocopherol and BMD
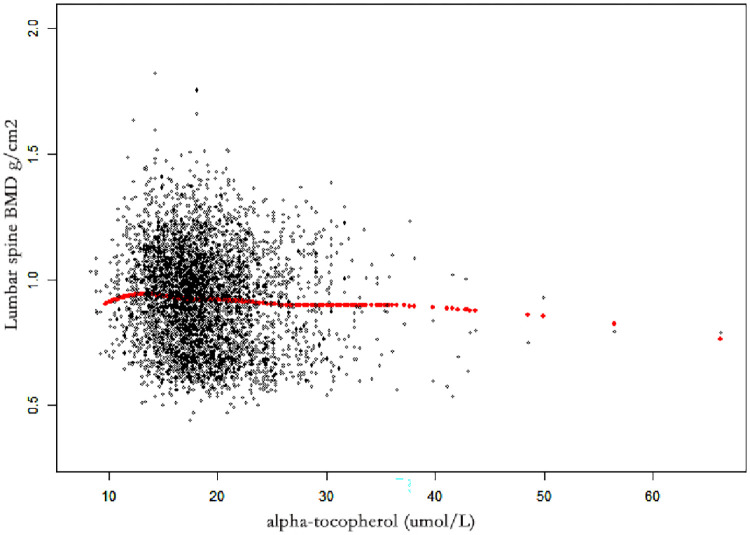
Table 2 shows the results of weighted multivariate regression analyses. Serum alpha-tocopherol was negatively correlated with BMD in all models. In model 3 (fully adjusted model), serum alpha-tocopherol was negatively linked with lumbar spine BMD (β = -0.0021 95% CI: -0.0025, -0.0017, P< 0.001) (Table 2 and Fig 2). Individuals in the highest quartile of serum α-tocopherol had a 0.0223 g/cm2 decreased lumbar BMD (β = -0.0223, 95% CI: -0.0279, -0.0166, P< 0.001) compared with the lowest quartile (Table 2).
Table 2. The association between serum alpha-tocopherol and lumbar spine BMD.
| Lumbar spine BMD (g/cm2) | |||
|---|---|---|---|
| Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | |
| Alpha-tocopherol (umol/L) | -0.0034 (-0.0041, -0.0028) <0.001 | -0.0017 (-0.0022, -0.0013) <0.001 | -0.0021 (-0.0025, -0.0017) <0.001 |
| Alpha-tocopherol categories | |||
| Q1 (7.5–15.4) | Reference | Reference | Reference |
| Q2 (15.4–17.6) | -0.0126 (-0.0222, -0.0030) 0.010 | 0.0024 (-0.0037, 0.0085) 0.440 | 0.0027 (-0.0033, 0.0086) 0.380 |
| Q3 (17.6–20.4) | -0.0434 (-0.0528, -0.0340) <0.001 | -0.0123 (-0.0183, -0.0064) <0.001 | -0.0119 (-0.0178, -0.0060) <0.001 |
| Q4 (>20.4) | -0.0480 (-0.0570, -0.0389) <0.001 | -0.0179 (-0.0237, -0.0122) <0.001 | -0.0223 (-0.0279, -0.0166) <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 |
| Subgroup analysis stratified by gender | |||
| Male | -0.0037 (-0.0046, -0.0028) <0.001 | -0.0003 (-0.0009, 0.0003) 0.358 | -0.0008 (-0.0014, -0.0002) 0.006 |
| Female | -0.0035 (-0.0044, -0.0027) <0.001 | -0.0030 (-0.0036, -0.0025) <0.001 | -0.0032 (-0.0037, -0.0026) <0.001 |
| P for interaction | 0.819 | <0.001 | <0.001 |
| Subgroup analysis stratified by age | |||
| 8–13 | -0.0038 (-0.0045, -0.0032) <0.001 | -0.0032 (-0.0038, -0.0026) <0.001 | -0.0027 (-0.0032, -0.0021) <0.001 |
| 14–19 | 0.0006 (-0.0000, 0.0012) 0.067 | 0.0008 (0.0001, 0.0014) 0.016 | 0.0002 (-0.0004, 0.0008) 0.546 |
| P for interaction | <0.001 | <0.001 | <0.001 |
| Subgroup analysis stratified by race/ethnicity | |||
| Mexican American | -0.0033 (-0.0041, -0.0025) <0.001 | -0.0027 (-0.0036, -0.0019) <0.001 | -0.0033 (-0.0041, -0.0025) <0.001 |
| Other Hispanic | 0.0030 (0.0001, 0.0060) 0.0449 | -0.0004 (-0.0021, 0.0014) 0.666 | -0.0010 (-0.0027, 0.0007) 0.260 |
| Non-Hispanic White | -0.0033 (-0.0045, -0.0022) <0.001 | -0.0018 (-0.0025, -0.0010) <0.001 | -0.0021 (-0.0028, -0.0013) <0.001 |
| Non-Hispanic Black | -0.0050 (-0.0065, -0.0035) <0.001 | -0.0020 (-0.0029, -0.0010) <0.001 | -0.0021 (-0.0030, -0.0011) <0.001 |
| Other races (Including multi-racial) | -0.0019 (-0.0046, 0.0007) 0.147 | -0.0007 (-0.0023, 0.0009) 0.368 | -0.0013 (-0.0030, 0.0003) 0.099 |
| P for interaction | <0.001 | 0.142 | 0.045 |
Model 1: no covariates were adjusted. Model 2: age, gender, and race/ethnicity were adjusted. Model 3: age, gender, race/ethnicity, BMI, PIR, serum calcium, and serum phosphorus. Abbreviation: BMI body mass index. BMD bone mineral density. PIR poverty income ratio.
Fig 2. The associations between α- tocopherol and lumbar spine BMD.
Each black point represents a sample. Age, gender, race/ethnicity, PIR, BMI, serum calcium, and serum phosphorus were adjusted. Abbreviations: PIR, poverty income ratio. BMI, body mass index. BMD, bone mineral density.
In stratified analysis, the negative relationship was pronounced in females (β = -0.0032, 95% CI: -0.0037, -0.0026, P< 0.001) than males (β = -0.0008, 95% CI: -0.0037, -0.0026, P<0.001) (P for interaction< 0.001) in model 3 (Table 2). The age-stratified analyses (8–13 and 14–19) showed that the negative association was more pronounced in individuals aged 8–13 (β = -0.0027, 95% CI: -0.0032, -0.0021, P<0.001) than those aged 14–19 (β = 0.0002, 95% CI: -0.0004, -0.0008, P = 0.546) (Table 2). For race/ethnicity, the negative association was pronounced in Non-Hispanic White (β = -0.0021, 95% CI: -0.0028, -0.0013, P<0.001), Non-Hispanic Black (β = -0.0021, 95% CI: -0.0030, -0.0011, P<0.001), and Mexican American (β = -0.0033, 95% CI: -0.0041, -0.0025, P< 0.001), but not in Other Hispanic (β = -0.0010, 95% CI: -0.0027, 0.0007, P = 0.260), and Other Races (β = -0.0013, 95% CI: -0.0030, 0.0003, P = 0.099) in the fully adjusted models (P for interaction = 0.045) (Table 2).
Association between γ-tocopherol and BMD
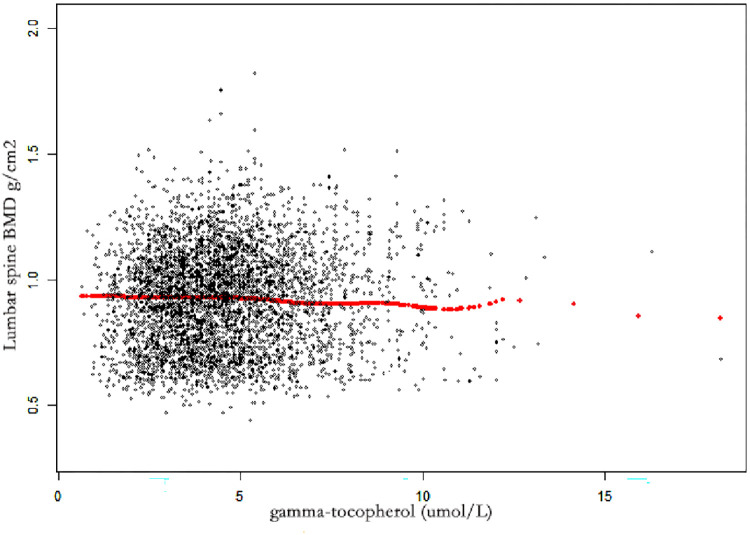
In three models, serum γ-tocopherol was negatively associated with lumbar BMD (Table 3). In model 3, serum alpha-tocopherol concentration was negatively associated with lumbar spine BMD (β = -0.0068 95% CI: -0.0079, -0.0057, P< 0.001) (Table 3 and Fig 3). Individuals in the highest quartile of serum γ-tocopherol had a 0.0329 g/cm2 decreased lumbar BMD (β = -0.0329, 95% CI: -0.0387, -0.0270, P<0.001) compared with the lowest quartile (Table 3).
Table 3. The association between serum gamma-tocopherol and lumbar spine BMD.
| Lumbar spine BMD g/cm2 | |||
|---|---|---|---|
| Model 1 β (95% CI) P value | Model 2 β (95% CI) P value | Model 3 β (95% CI) P value | |
| Gamma-tocopherol (umol/L) | -0.0038 (-0.0054, -0.0021) <0.001 | -0.0041 (-0.0051, -0.0030) <0.001 | -0.0068 (-0.0079, -0.0057) <0.001 |
| Gamma-tocopherol (umol/L) | |||
| Q1 (<1.09) | reference | Reference | reference |
| Q2 (1.09–2.18) | 0.0020 (-0.0067, 0.0107) 0.652 | 0.0040 (-0.0015, 0.0095) 0.153 | 0.0020 (-0.0034, 0.0073) 0.475 |
| Q3 (2.18–4.25) | 0.0098 (0.0009, 0.0188) 0.031 | -0.0035 (-0.0092, 0.0022) 0.227 | -0.0089 (-0.0145, -0.0032) 0.002 |
| Q4 (4.25–5.00) | -0.0200 (-0.0290, -0.0109) <0.001 | -0.0208 (-0.0266, -0.0151) <0.001 | -0.0329 (-0.0387, -0.0270) <0.001 |
| P for trend | 0.001 | <0.001 | <0.001 |
| Subgroup analysis stratified by gender | |||
| Male | -0.0055 (-0.0078, -0.0031) <0.001 | -0.0050 (-0.0065, -0.0035) <0.001 | -0.0077 (-0.0092, -0.0062) <0.001 |
| Female | -0.0042 (-0.0065, -0.0020) <0.001 | -0.0031 (-0.0046, -0.0017) <0.001 | -0.0062 (-0.0077, -0.0047) <0.001 |
| P for interaction | 0.461 | 0.034 | 0.061 |
| Subgroup analysis stratified by age | |||
| 8–13 | -0.0013 (-0.0029, 0.0002) 0.093 | -0.0028 (-0.0043, -0.0014) <0.001 | -0.0090 (-0.0105, -0.0076) <0.001 |
| 14–19 | 0.0013 (-0.0005, 0.0030) 0.157 | -0.0011 (-0.0029, 0.0006) 0.200 | -0.0036 (-0.0053, -0.0018) <0.001 |
| P for interaction | 0.023 | 0.13 | 0.001 |
| Subgroup analysis stratified by race/ethnicity | |||
| Mexican American | -0.0026 (-0.0053, 0.0001) 0.0636 | -0.0001 (-0.0019, 0.0017) 0.9125 | -0.0045 (-0.0063, -0.0027) <0.001 |
| Other Hispanic | -0.0143 (-0.0235, -0.0052) 0.002 | -0.0107 (-0.0162, -0.0053) <0.001 | -0.0110 (-0.0170, -0.0051) <0.001 |
| Non-Hispanic White | -0.0068 (-0.0100, -0.0037) <0.001 | -0.0059 (-0.0080, -0.0039) <0.001 | -0.0089 (-0.0110, -0.0068) <0.001 |
| Non-Hispanic Black | 0.0034 (0.0002, 0.0067) 0.039 | -0.0019 (-0.0039, 0.0002) 0.082 | -0.0032 (-0.0053, -0.0011) 0.003 |
| Other races (Including multi-racial) | 0.0032 (-0.0032, 0.0095) 0.331 | 0.0035 (-0.0004, 0.0073) 0.080 | 0.0003 (-0.0038, 0.0045) 0.870 |
| P for interaction | <0.001 | <0.001 | <0.001 |
Model 1: no covariates were adjusted. Model 2: age, gender, and race/ethnicity were adjusted. Model 3: age, gender, race/ethnicity, BMI, PIR, serum calcium, and serum phosphorus. Abbreviation: BMI body mass index. BMD bone mineral density. PIR poverty income ratio.
Fig 3. The associations between γ-tocopherol and lumbar spine BMD.
Each black point represents a sample. Age, gender, race/ethnicity, PIR, BMI, serum calcium, and serum phosphorus were adjusted. Abbreviations: PIR, poverty income ratio. BMI, body mass index. BMD, bone mineral density.
The stratified analyses showed no interactive effect of γ-tocopherol on males (β = -0.0077, 95% CI: -0.0092, -0.0062, P< 0.001) and females (β = -0.0062, 95% CI: -0.0077, -0.0047, P< 0.001), (P for interaction = 0.061). The age-stratified analyses (8–13 and 14–19) also showed that the negative association was more pronounced in aged 8–13 (β = -0.0090, 95% CI: -0.0105, -0.0076, P< 0.001), but not 14–19 (β = -0.0036, 95% CI: -0.0053, -0.0018, P< 0.001) (P for interaction = 0.001) (Table 3). The race-stratified analysis showed that the negative association was more prominent in Non-Hispanic White, Mexican American, Non-Hispanic Black, and Other Hispanic, but not in Other Races in model 3 (P for interaction< 0.001) (Table 3).
Discussion
Our study first evaluates the relationship between vitamin E status and BMD in children and adolescents. Our analysis indicates that serum α- and γ- tocopherol concentration is negatively related to lumbar BMD in American children and adolescents, and this association is stronger in γ-tocopherol. For every 1umol/L increase in serum α- and γ- tocopherol, the lumbar spine BMD decreased by -0.0016 and -0.0068 g/cm2, respectively. Individuals in the highest quartile of serum α- and γ- tocopherol concentration have a -0.0223 and -0.0329 g/cm2 lower mean BMD than those in the lowest quartile, respectively. In addition, the interactive effects show that the negative association between α-tocopherol and BMD is more pronounced in females, individuals aged 8–13, Mexican American, Non-Hispanic White, and Non-Hispanic Black. Furthermore, the interactive effect of γ- tocopherol with lumbar BMD also exists in different ages and races, but not sex.
In vitro studies, a- and γ- tocopherol has been proven to impact bone metabolism through their antioxidant and anti-inflammatory activities. However, the conclusions were inconsistent [19, 26–28]. Soeta et al. [28] found that α- and γ- tocopherol could decrease the activity of alkaline phosphatase of cultured osteoblasts in the rat calvariae, especially in the early stage. Nevertheless, in another study, Ahn et al. [29] found that α- tocopherol could promote osteoblastogenesis by increasing the runt-related transcription factor 2 (RUNX-2) expression. RUNX-2 has been proven to be an upstream modulator of osteoblastic genes. The potential effect of Vitamin E on bone mechanism has been explored in animals and produced diverse findings [30–32]. Feresin et al. investigated the effects of vitamin E on rats. The study indicated that medium doses (525 mg/kg diet) and high doses (750 mg/kg) of vitamin E could reverse the bone loss of ovariectomized rats [30]. Another study by Muhammad et al. showed that vitamin E treatment for ovariectomized young rats could prevent bone mass reduction by increasing trabecular volume, number, and separation [31]. In addition, an early investigation showed that vitamin E has the potential to recover bone metabolism impaired by nicotine [32]. Nevertheless, the adverse effects of vitamin E on bone metabolism were also observed in animal experiments [19, 33]. Fujita et al. [33] found that mice deficient in α-tocopherol transfer protein have a higher BMD than wild-type mice. They indicated that α-tocopherol could enhance the activity of osteoclast fusion by inducing the expression of dendritic-cell-specific transmembrane protein [33]. Wild-type mice fed a diet supplemented with a high dose of alpha-tocopherol also experienced bone loss [33]. Several aspects could explain these inconsistent results. First, the differences in age and sex of the rat may cause variations in the results. Second, the exposure doses used in these studies are also different. Previous evidence has proven that the effect of vitamin E on bone metabolism could be dose-dependent [34]. A study by Smith et al. found that treating male rats with a high dose of α-tocopherol could prevent osteoporosis induced by hindlimb-unloading, but this was not observed in a low dose of α-tocopherol. Furthermore, the large doses of tocopherol used in the rat studies far exceeded the recommended daily vitamin E consumption in humans, which makes it hard to generalize these findings in the rat model to the population [35].
Several epidemiological studies investigating the association between vitamin E and adult BMD have also drawn inconsistent results [36, 37]. In 19 years of a follow-up study in Sweden [38], 61,422 women and 1138 men were included for analysis. The results suggested that a lower α-tocopherol intake was related to a higher hip fracture rate in women. Nevertheless, serum α-tocopherol concentration was connected to decreased fracture rate in men. A recent mendelian randomization study showed that higher serum α-tocopherol was linked with a greater BMD [37]. However, Wolf et al. [36] measured 11,068 women aged 50–79, and they observed no associations between dietary intake, serum vitamin E, and BMD after adjusting an extensive range of BMD-related covariates. Similarly, in another NHANES study, Li et al. [39] found no significant associations of serum vitamin E levels with femur BMD. An early longitudinal study conducted by MacDonald et al. [40] observed a negative association between α-tocopherol intake and BMD in the femoral neck and lumbar spine in premenopausal women aged 45–54 years. However, serum α-tocopherol level was not included in this study. Vitamin E intake calculated by dietary recall only is not entirely credible. Differences in serum vitamin E levels across populations and studies may provide an explanation for these inconsistent results [22, 41, 42]. Some previous studies in adults have indicated a positive association between vitamin E and BMD in low vitamin E populations [22, 41], whereas a negative association in those with higher vitamin E levels [42]. Thus, a vitamin E excess could be the reason for the impairment of bone health.
Our study shows a stronger effect of γ- tocopherol than α- tocopherol on BMD in children and adolescents. Gamma-tocopherol is the major form of vitamin E from dietary sources, while alpha-tocopherol is the predominant form of vitamin E supplements [11, 43]. In vivo, Gamma-tocopherol showed a more potent antioxidant and anti-inflammatory ability than alpha-tocopherol [44]. Contrary to our findings, a previous study of postmenopausal women showed that higher serum α-tocopherol levels and low serum α/γ-tocopherol value were associated with elevated BAP levels (a biomarker of bone formation) [42]. Another study of an older American population showed a negative correlation between serum levels of α-tocopherol and BMD, but the negative association was not significant in γ-tocopherol. However, the youth are in a period of rapid growth and development, and the effect of vitamin E and its isomers on bone density in children may differ from that in adults. Our study was also the first to explore the association between different vitamin E isomers and bone health in children and adolescents. Further studies with stronger evidence are needed to verify our findings and their underlying mechanisms. Another notable finding in our study is that the negative effect of vitamin E is more pronounced in individuals aged 8–13 years than in 14–19 years. Previous studies showed that children and adolescents achieved the highest BMD increase rate at around 17 years in males and 15 years in females. Thus, the negative effect of vitamin E on BMD may be attenuated after reaching these ages [45]. However, due to the lack of evidence from clinical and basic studies, more prospective studies are needed to verify our speculations.
Although there are some epidemiological studies in adults, no study has investigated the relationship between vitamin E status and BMD in children and adolescents. Vitamin E is essential in children’s rapid growth and development and is more likely deficient than in adults [46]. Therefore, vitamin E’s biological effects on BMD may differ in adults and children. Our study identifies an inverse relationship between vitamin E and BMD in children and adolescents. Vitamin E supplementation during childhood may be helpful in issues like lung function, kwashiorkor, or liver disease [47–49]. Vitamin E supplementation for children and adolescents could not improve bone health or even damage bone health.
Our study has some advantages. First, this study is conducted based on NHANES, and all analyses used MEC weights, which can be representative of the general U.S. population. Second, we selected serum α- and γ-tocopherol rather than dietary vitamin E intake as exposure variables since they could better reflect an individual’s vitamin E status [50]. Some potential limitations in this study should also be acknowledged. First, no causal association could be inferred because of the nature of the observational study. Second, we could not investigate the relationship between vitamin E and femur BMD since the lack of data in NHANES. However, studies have shown that the relationship between vitamin E and BMD may be site-specific. For instance, Mata-Granados et al. [41] evaluated the relationship between vitamin E status and osteoporosis in Spanish postmenopausal women. They found that vitamin E was positively associated with lumbar spine BMD but not femur BMD. It may be attributed to the lumbar spine having more trabecular bone than the femur. Additional studies are required to explore the association between vitamin E and BMD at various sites. Third, we did not investigate the association in children younger than eight years old since the limitation of the NHANES database. More research should be carried out to explore the association between vitamin E status and BMD in children under eight years.
Conclusions
Our study is the first study that explores the association between vitamin E and BMD in children and adolescents. The results indicate that serum α- and γ-tocopherol are negatively correlated with lumbar BMD. Age, gender, and race have a modifying effect on this relationship. Our study has important clinical implications. A higher vitamin E status for children and adolescents could not improve bone health and even damage bone health. In addition, more prospective research with more robust evidence is needed to verify our findings and their underlying mechanisms.
Acknowledgments
We acknowledge the data from the National Health and Nutrition Examination Survey (NHANES).
List of abbreviations
- BMD
bone mineral density
- PIR
poverty income ratio
- WHO
World Health Organization
- PBM
peak bone mass
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- DXA
dual-energy X-ray absorptiometry
- VIF
variance inflation facto
- BMI
body mass index
- RUNX-2
runt-related transcription factor 2
- MSC
Swedish Mammography Cohort
- ULSAM
Uppsala Longitudinal Study of Adult Men
Data Availability
All data are available from the NHANES database:https://www.cdc.gov/nchs/nhanes/.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Styrkarsdottir U, Thorleifsson G, Gudjonsson SA et al. Sequence variants in the PTCH1 gene associate with spine bone mineral density and osteoporotic fractures. Nat Commun 2016;7:10129. doi: 10.1038/ncomms10129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao PL, Cui AY, Hsu CJ et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int 2022. doi: 10.1007/s00198-022-06454-3 [DOI] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergård M et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8:136. doi: 10.1007/s11657-013-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matkovic V, Jelic T, Wardlaw GM et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest 1994;93:799–808. doi: 10.1172/JCI117034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozenberg S, Bruyère O, Bergmann P et al. How to manage osteoporosis before the age of 50. Maturitas 2020;138:14–25. doi: 10.1016/j.maturitas.2020.05.004 [DOI] [PubMed] [Google Scholar]
- 6.Boreham CA, McKay HA. Physical activity in childhood and bone health. Br J Sports Med 2011;45:877–9. doi: 10.1136/bjsports-2011-090188 [DOI] [PubMed] [Google Scholar]
- 7.Karlsson MK, Rosengren BE. Exercise and Peak Bone Mass. Curr Osteoporos Rep 2020;18:285–290. doi: 10.1007/s11914-020-00588-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui A, Xiao P, Hu B et al. Blood Lead Level Is Negatively Associated With Bone Mineral Density in U.S. Children and Adolescents Aged 8–19 Years. Front Endocrinol (Lausanne) 2022;13:928752. doi: 10.3389/fendo.2022.928752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fewtrell MS, Williams JE, Singhal A, Murgatroyd PR, Fuller N, Lucas A. Early diet and peak bone mass: 20 year follow-up of a randomized trial of early diet in infants born preterm. Bone 2009;45:142–9. doi: 10.1016/j.bone.2009.03.657 [DOI] [PubMed] [Google Scholar]
- 10.Brigelius-Flohé R. Vitamin E research: Past, now and future. Free Radic Biol Med 2021;177:381–390. doi: 10.1016/j.freeradbiomed.2021.10.029 [DOI] [PubMed] [Google Scholar]
- 11.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 2001;74:714–22. doi: 10.1093/ajcn/74.6.714 [DOI] [PubMed] [Google Scholar]
- 12.Birringer M. Analysis of vitamin E metabolites in biological specimen. Mol Nutr Food Res 2010;54:588–98. doi: 10.1002/mnfr.200900457 [DOI] [PubMed] [Google Scholar]
- 13.Xin J, Jiang X, Ben S et al. Association between circulating vitamin E and ten common cancers: evidence from large-scale Mendelian randomization analysis and a longitudinal cohort study. BMC Med 2022;20:168. doi: 10.1186/s12916-022-02366-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R, Han X, Zhang H et al. Association of vitamin E intake in diet and supplements with risk of dementia: A meta-analysis. Front Aging Neurosci 2022;14:955878. doi: 10.3389/fnagi.2022.955878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 2000;342:154–60. doi: 10.1056/NEJM200001203420302 [DOI] [PubMed] [Google Scholar]
- 16.Huang HS, Chen J, Chen CF, Ma MC. Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int 2006;70:699–710. doi: 10.1038/sj.ki.5001651 [DOI] [PubMed] [Google Scholar]
- 17.Hermizi H, Faizah O, Ima-Nirwana S, Ahmad Nazrun S, Norazlina M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague-dawley male rats after nicotine cessation. Calcif Tissue Int 2009;84:65–74. doi: 10.1007/s00223-008-9190-x [DOI] [PubMed] [Google Scholar]
- 18.Norazlina M, Chua CW, Ima-Nirwana S. Vitamin E deficiency reduced lumbar bone calcium content in female rats. Med J Malaysia 2004;59:623–30. [PubMed] [Google Scholar]
- 19.Fujita K, Iwasaki M, Ochi H et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat Med 2012;18:589–94. doi: 10.1038/nm.2659 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Hu X, Zhang J. Associations between serum vitamin E concentration and bone mineral density in the US elderly population. Osteoporos Int 2017;28:1245–1253. doi: 10.1007/s00198-016-3855-5 [DOI] [PubMed] [Google Scholar]
- 21.Shi WQ, Liu J, Cao Y, Zhu YY, Guan K, Chen YM. Association of dietary and serum vitamin E with bone mineral density in middle-aged and elderly Chinese adults: a cross-sectional study. Br J Nutr 2016;115:113–20. doi: 10.1017/S0007114515004134 [DOI] [PubMed] [Google Scholar]
- 22.Yang TC, Duthie GG, Aucott LS, Macdonald HM. Vitamin E homologues α- and γ-tocopherol are not associated with bone turnover markers or bone mineral density in peri-menopausal and post-menopausal women. Osteoporos Int 2016;27:2281–2290. doi: 10.1007/s00198-015-3470-x [DOI] [PubMed] [Google Scholar]
- 23.Forwood MR, Baxter-Jones AD, Beck TJ, Mirwald RL, Howard A, Bailey DA. Physical activity and strength of the femoral neck during the adolescent growth spurt: a longitudinal analysis. Bone 2006;38:576–83. doi: 10.1016/j.bone.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 24.Anderson JJ. Calcium, phosphorus and human bone development. J Nutr 1996;126:1153s–8s. doi: 10.1093/jn/126.suppl_4.1153S [DOI] [PubMed] [Google Scholar]
- 25.Li T, Xie Y, Wang L et al. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999–2006 and 2011–2018. Nutrients 2022;14. doi: 10.3390/nu14071523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin KY, Mo H, Soelaiman IN. A review of the possible mechanisms of action of tocotrienol—a potential antiosteoporotic agent. Curr Drug Targets 2013;14:1533–41. doi: 10.2174/13894501113149990178 [DOI] [PubMed] [Google Scholar]
- 27.Urban K, Höhling HJ, Lüttenberg B, Szuwart T, Plate U. An in vitro study of osteoblast vitality influenced by the vitamins C and E. Head Face Med 2012;8:25. doi: 10.1186/1746-160X-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeta S, Higuchi M, Yoshimura I, Itoh R, Kimura N, Aamsaki H. Effects of vitamin E on the osteoblast differentiation. J Vet Med Sci 2010;72:951–7. doi: 10.1292/jvms.09-0487 [DOI] [PubMed] [Google Scholar]
- 29.Ahn KH, Jung HK, Jung SE, Yi KW, Kim T. Microarray Analysis of Gene Expression During Differentiation of Human Mesenchymal Stem Cells Treated with Vitamin E in vitro into Osteoblasts. 2011. [Google Scholar]
- 30.Feresin RG, Johnson SA, Elam ML et al. Effects of vitamin e on bone biomechanical and histomorphometric parameters in ovariectomized rats. J Osteoporos 2013;2013:825985. doi: 10.1155/2013/825985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhammad N, Luke DA, Shuid AN, Mohamed N, Soelaiman IN. Two different isomers of vitamin e prevent bone loss in postmenopausal osteoporosis rat model. Evid Based Complement Alternat Med 2012;2012:161527. doi: 10.1155/2012/161527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norazlina M, Hermizi H, Faizah O, Nazrun AS, Norliza M, Ima-Nirwana S. Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch Med Sci 2010;6:505–12. doi: 10.5114/aoms.2010.14460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwaniec UT, Turner RT, Smith BJ et al. Evaluation of long-term vitamin E insufficiency or excess on bone mass, density, and microarchitecture in rodents. Free Radic Biol Med 2013;65:1209–1214. doi: 10.1016/j.freeradbiomed.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith BJ, Lucas EA, Turner RT et al. Vitamin E provides protection for bone in mature hindlimb unloaded male rats. Calcif Tissue Int 2005;76:272–9. doi: 10.1007/s00223-004-0269-8 [DOI] [PubMed] [Google Scholar]
- 35.Dennehy C, Tsourounis C. A review of select vitamins and minerals used by postmenopausal women. Maturitas 2010;66:370–80. doi: 10.1016/j.maturitas.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 36.Wolf RL, Cauley JA, Pettinger M et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr 2005;82:581–8. doi: 10.1093/ajcn.82.3.581 [DOI] [PubMed] [Google Scholar]
- 37.Michaëlsson K, Larsson SC. Circulating Alpha-Tocopherol Levels, Bone Mineral Density, and Fracture: Mendelian Randomization Study. Nutrients 2021;13. doi: 10.3390/nu13061940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaëlsson K, Wolk A, Byberg L, Ärnlöv J, Melhus H. Intake and serum concentrations of α-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am J Clin Nutr 2014;99:107–14. doi: 10.3945/ajcn.113.064691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Liu X. Associations of serum vitamins levels with bone mineral density in the different race-ethnicities US adults. BMC Musculoskelet Disord 2021;22:137. doi: 10.1186/s12891-021-03997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr 2004;79:155–65. doi: 10.1093/ajcn/79.1.155 [DOI] [PubMed] [Google Scholar]
- 41.Mata-Granados JM, Cuenca-Acebedo R, Luque de Castro MD, Quesada Gómez JM. Lower vitamin E serum levels are associated with osteoporosis in early postmenopausal women: a cross-sectional study. J Bone Miner Metab 2013;31:455–60. doi: 10.1007/s00774-013-0432-2 [DOI] [PubMed] [Google Scholar]
- 42.Hamidi MS, Corey PN, Cheung AM. Effects of vitamin E on bone turnover markers among US postmenopausal women. J Bone Miner Res 2012;27:1368–80. doi: 10.1002/jbmr.1566 [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin PJ, Weihrauch JL. Vitamin E content of foods. J Am Diet Assoc 1979;75:647–65 [PubMed] [Google Scholar]
- 44.Berdnikovs S, Abdala-Valencia H, McCary C et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol 2009;182:4395–405. doi: 10.4049/jimmunol.0803659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu PW, Briody JN, Ogle GD et al. Bone mineral density of total body, spine, and femoral neck in children and young adults: a cross-sectional and longitudinal study. J Bone Miner Res 1994;9:1451–8. doi: 10.1002/jbmr.5650090918 [DOI] [PubMed] [Google Scholar]
- 46.Traber MG. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr 2014;5:503–14. doi: 10.3945/an.114.006254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar R, Ferrie RP, Balmert LC et al. Associations of α- and γ-tocopherol during early life with lung function in childhood. J Allergy Clin Immunol 2020;146:1349–1357.e3. doi: 10.1016/j.jaci.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odigwe CC, Smedslund G, Ejemot-Nwadiaro RI, Anyanechi CC, Krawinkel MB. Supplementary vitamin E, selenium, cysteine and riboflavin for preventing kwashiorkor in preschool children in developing countries. Cochrane Database Syst Rev 2010;2010:Cd008147. doi: 10.1002/14651858.CD008147.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2006;24:1553–61. doi: 10.1111/j.1365-2036.2006.03161.x [DOI] [PubMed] [Google Scholar]
- 50.Greaves RF, Woollard GA, Hoad KE et al. Laboratory medicine best practice guideline: vitamins a, e and the carotenoids in blood. Clin Biochem Rev 2014;35:81–113. doi: 10.1186/bcr2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the NHANES database:https://www.cdc.gov/nchs/nhanes/.