Abstract
Ischemic heart disease (IHD) affects more than 20 million adults in the United States. Although classically attributed to atherosclerosis of the epicardial coronary arteries, nearly half of patients with stable angina and ischemic heart disease who undergo invasive coronary angiography do not have obstructive epicardial coronary artery disease. Ischemia with non-obstructive coronary arteries, or INOCA, is frequently caused by microvascular angina with underlying coronary microvascular dysfunction (CMD). Guidelines and consensus statements now recommend comprehensive assessment of coronary physiology to identify CMD in these patients, and recent advances in the invasive evaluation of the microcirculation has led to specific, quantitative, and reproducible measures of coronary microcirculatory function. Greater understanding the pathophysiology, diagnosis, and treatment of CMD holds promise to improve clinical outcomes of patients with ischemic heart disease.
Keywords: Coronary flow reserve, CFR, coronary microvascular disease, CMD, coronary microvascular dysfunction, coronary physiology, hyperemic microvascular resistance, hMR, index of microcirculatory resistance, IMR, ischemia with non-obstructive coronary arteries, INOCA, microvascular, microvascular angina, microvascular testing, resistive reserve ratio
Background
Ischemic heart disease (IHD) affects more than 20 million adults in the United States and is a leading cause of death.1 Stable ischemic heart disease is associated with angina, decreased exercise tolerance, and adverse health-related quality of life. Although angina is classically attributed to atherosclerosis of the epicardial coronary arteries, recent evidence suggests that nearly half of patients who undergo invasive coronary angiography for the evaluation of angina do not have obstructive coronary disease.2,3 This perplexing clinical scenario, first described in 1967 and later termed cardiac Syndrome X, is now referred to as ischemia with non-obstructive coronary arteries, or INOCA.4 Underlying mechanisms of INOCA include microvascular angina with coronary microvascular dysfunction (CMD), epicardial coronary spasm, and non-cardiac chest pain.5,6 Recent American and European clinical practice guidelines recognize the impact of INOCA and recommend functional assessment of the coronary microcirculation to determine mechanisms of ischemia and guide pharmacological therapy.7-9 The objective of this review is to (a) outline the anatomy of the coronary circulation and the pathophysiology of CMD, (b) describe current approaches to assess the coronary microcirculation in the cardiac catheterization laboratory, and (c) examine the clinical implications of CMD in INOCA and other cardiovascular disorders.
Anatomy and Physiology of the Coronary Microcirculation
Anatomy
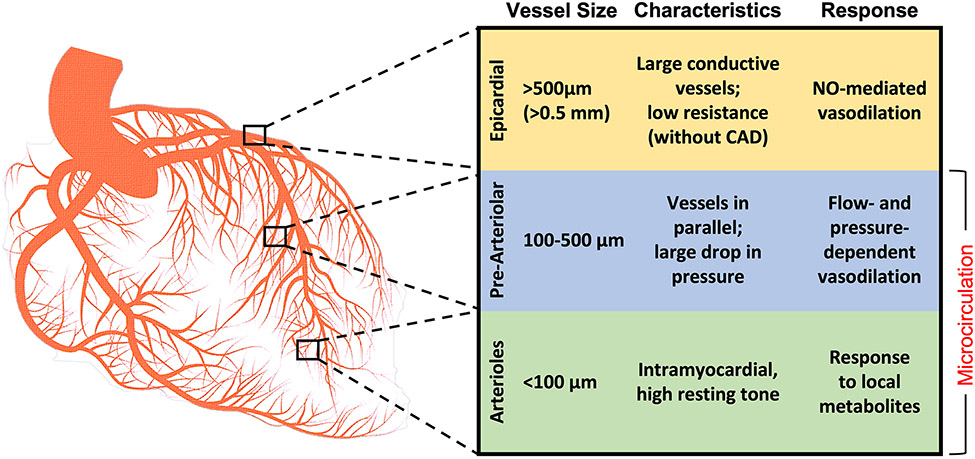
From the epicardial vessels to the myocardium, the coronary circulation can be divided into three distinct “compartments” (Figure 1). Blood enters the coronary circulation via the major epicardial coronary arteries, vessels 500 μm to 5mm in size that course predominantly along the surface of the myocardium and can be easily visualized by coronary angiography. Epicardial coronary arteries have been the focus of coronary interventions for more than half a century. Despite their important role as proximal conduit vessels, the epicardial coronaries only directly supply 5-10% of the myocardium. In normal physiologic conditions, epicardial coronary arteries offer little resistance to blood flow due to their relatively large diameters. Nitric oxide-mediated vasodilation of the epicardial coronaries accommodates increased blood flow in times of high metabolic demand. Atherosclerotic plaques in the epicardial coronaries can lead to dramatic increases in resistance to flow.10 The epicardial coronary arteries branch and taper into a vast network of arterioles to supply the myocardium.
Figure 1. Anatomy of the compartments of the coronary circulation.
CAD = Coronary artery disease; NO = Nitric oxide
Extra-myocardial pre-arteriolar vessels, which range in diameter from 100 to 500 μm, form the intermediate compartment of the coronary circulation. These pre-arteriolar vessels run in parallel, leading to physiologic drops in pressures.11 Proximal pre-arterioles in this compartment are most sensitive to changes in intravascular flow, whereas distal vessels are sensitive to changes in pressure. Vasoconstriction and vasodilation of the pre-arterioles maintains constant arteriolar pressures.12 This compartment may be responsible for CMD in patients with INOCA.
The third and final compartment of the coronary circulation consists of innumerable intramural arterioles <100μm in diameter. These vessels directly supply low-pressure myocardial capillary beds that serve as the site for nutrient and gas exchange. Arterioles in the coronary circulation have a high resting tone and dilate in response to local metabolites produced by the surrounding myocardium, including adenosine, nitric oxide, and prostaglandins.11 Thus, arterioles are responsible for the metabolic regulation of myocardial blood flow.
In normal circumstances, the three compartments of the coronary circulation work in concert to regulate myocardial blood flow in response to metabolic demands. Once maximal myocardial oxygen extraction (~75%) has been reached, additional metabolic demands must be met with increases in myocardial blood flow.13 Increases in heart rate and contractility can augment cardiac output, and vasodilatory metabolites from the myocardium decrease microcirculatory arteriolar resistance to enhance coronary blood flow (CBF).13 Thus, the microcirculation plays a pivotal role to ensure coronary perfusion matches myocardial oxygen demand.
Pathobiology of Coronary Microvascular Disease
Coronary microvascular disease has been broadly classified as microvascular dysfunction (a) in the absence of obstructive CAD or myocardial diseases, (b) in the presence of myocardial disease, (c) in the presence of obstructive CAD, or (d) from an iatrogenic cause.14 A variety of mechanisms can contribute to structural or functional abnormalities of the microcirculation.15,16 In patients without obstructive atherosclerosis or myocardial diseases, the pathogenesis of microvascular disease may include arteriolar remodeling and fibrosis, intimal proliferation, smooth muscle hypertrophy, or vessel rarefaction. Small vessel atherosclerosis, platelet activation and plugging,17 or microembolization of thrombotic material in the setting of epicardial atherosclerotic disease may also contribute.18 In the setting of myocardial disease, such as left ventricular hypertrophy, increased intramyocardial, left ventricular diastolic, and coronary venous pressures may also lead to increased resistance to microcirculatory flow.19
In addition to structural disorders of the microcirculation, functional abnormalities associated with impairment to the normal responses to neurohormonal and metabolic signaling can lead to impaired microcirculatory flow. This may include attenuated responses to or decreased synthesis of vasodilators such as nitric oxide.20,21 Coronary microvascular spasm, with episodic increases in microvascular resistance and provocation of myocardial ischemia can also occur.22
Although the exact pathophysiology of CMD is uncertain, risk factors include older age, hypertension, dyslipidemia, diabetes mellitus, cigarette smoking, and chronic inflammatory disorders, including systemic lupus erythematosus and rheumatoid arthritis.23-28 Additional investigation is needed to identify modifiable risk factors to prevent development of microcirculatory disease.
Assessing the Microcirculation in the Catheterization Laboratory
Unlike the epicardial coronary arteries, coronary microcirculation cannot be directly visualized. Thus, assessments are largely based on parameters of microcirculatory flow. Coronary flow reserve, or CFR, the original integrated measure of coronary epicardial flow and microvascular function, is defined as the ratio of hyperemic CBF to CBF at rest. Normal coronary arteries can augment blood flow >4-fold at maximal hyperemia, while a CFR <2 to <2.5 in the absence of epicardial coronary disease is abnormal and reflects microvascular dysfunction (Table 1).5,6
Table 1.
Invasively derived measures to assess coronary microvascular dysfunction
| Technique | Measurement Method |
Normal Value | Comments |
|---|---|---|---|
| Coronary Flow Reserve (CFR) | Doppler or Thermodilution | > 2.0 - 2.5 |
|
| Index of Microcirculatory Resistance (IMR) | Thermodilution (Bolus dose) | < 25 |
|
| Hyperemic microvascular resistance (hMR) | Doppler | ≤ 2 |
|
| Resistive reserve ratio (RRR) | Doppler or Thermodilution | < 1.7-3.5 |
|
| Minimal microvascular resistance (mMR) | Doppler | Not Defined |
|
| Rmicro | Thermodilution (Continuous Infusion) | < 500 Woods units |
|
| Microvascular Resistance Reserve (MRR) | Thermodilution (Continuous Infusion) | Not Defined |
|
Invasive evaluation of CFR can be performed in the catheterization laboratory using thermodilution or Doppler-based assessment of CBF. The intracoronary Doppler guidewire, first developed in the late 1980s, contains a piezoelectric ultrasound transducer mounted at the tip to measure CBF velocity.29 The current generation of wire (FloWire or ComboWire XT, Phillips Volcano) can measure the average peak velocity (APV) of CBF at a specific location using a commercially available console with dedicated software (ComboMap, Philips, Volcano). Coronary APV is typically measured at rest, and then again after induction of hyperemia with the administration of a non-endothelial dependent vasodilator such as adenosine. Doppler-derived CFR is typically simplified as the ratio of the hyperemic APV to the APV measured at rest. To estimate CBF, the vessel diameter (D) at the site of APV measurement can be determined using quantitative coronary angiography, and flow can then be calculated by the formula: CBF = 0.5 x APV x (D2 π)/4.29 In response to an infusion of acetylcholine, an endothelial-dependent vasodilator, CBF typically increases substantially. Augmentation of CBF by <50% in the presence of acetylcholine is abnormal and, in the absence of significant epicardial coronary constriction, indicates that endothelial dependent microvascular dysfunction may be present.30,31
In contrast to Doppler-based measures that measure coronary velocity at a single location, coronary thermodilution techniques assesses flow based on temperature changes across the entire vessel. This technique requires 3mL bolus injections of room temperature saline through the guide catheter, with a distal thermistor on a coronary pressure-wire recording temperatures in the coronary artery (PressureWire X, Abbott Vascular). The shaft of this wire acts as a proximal thermistor. The speed of change of the distal temperature (relative to the proximal temperature) is used to calculate the mean transit time (Tmn), which is inversely proportional to coronary flow according to the principles of indicator dilution theory.
Doppler and thermodilution approaches to measurement of coronary flow each have limitations. Doppler-derived measures of coronary flow velocity assume that flow at the transducer is parallel, laminar, and parabolic, and may not remain constant at different wire positions.32 Indeed, animal and human studies have demonstrated significant variability in Doppler wire measurements,33 and Doppler measurements tend to be technically more challenging, require more time for data acquisition, and have a steeper learning curve.34,35 Although coronary thermodilution is somewhat easier to obtain, it can be significantly impacted by changes in guide catheter position during administration of saline boluses. In an early porcine model, thermodilution-derived CFR correlated better with absolute flow measured by an external coronary flow probe than did the Doppler wire-derived CFR.36 However, in a study of 98 vessels in 40 consecutive patients, Doppler-derived CFR correlated more closely with PET-derived CFR than thermodilution-derived CFR.37 Ultimately, both techniques are considered to be valid in the assessment of the coronary microcirculation (Table 1).
Unfortunately, CFR is not specific to microcirculation and is affected by epicardial coronary stenosis and resting hemodynamics. In the absence of obstructive epicardial disease, a reduced CFR can reflect increased microcirculatory resistance to flow, an abnormal response to the standard vasodilatory stimulus, or increased resting coronary flow prior to vasodilator administration. Among patients with INOCA, high resting flow is more common in women, younger patients, and patients with fewer cardiovascular risk factors, and may represent a distinct pathology.38
Microvascular resistance indices offer an alternative method to interrogate the microcirculation.39 The index of microcirculatory resistance (IMR), first reported in 2003, is a thermodilution-based measure that reflects the minimal achievable resistance of the microcirculation with endothelial-independent vasodilators (Table 1).32 IMR is based on Ohm's law, which states that the potential difference across an ideal conductor is proportional to the current through the conductor (Voltage = Current x Resistance). Applying this to coronary physiology, the microcirculation acts as a conductor, the voltage is analogous to the difference in pressure across the microvasculature (mean distal coronary pressure [Pd] – coronary venous pressure [Pv]), and current is myocardial flow (1/Tmn). Since coronary venous pressure (Pv) is usually negligible, IMR may be calculated by the formula: IMR = Pd x Tmn. A normal value of IMR has been reported to be <25,40-42 with higher values reported in the RCA, perhaps due to longer vessel length and larger diameters accounting for a somewhat prolonged Tmn.42
Still, there are some situations in which IMR must be interpreted with caution. In the presence of a significant coronary stenosis, collateral flow to the vessel of interest may increase the coronary wedge pressure (Pw). Thus, coronary thermodilution may underestimate flow and IMR may overestimate resistance.43 To account for collateral flow, the following formula has been proposed: IMR = Pa x Tmn x ([Pd -Pw]/[Pa - Pw]).43 Since coronary wedge pressures are not routinely measured, a correction factor for IMR has been derived and validated from experimental data with coronary wedge pressures (Pw) measured during proximal vessel balloon occlusions.44,45 Corrected IMR can be calculated using Yong’s modification (corrected IMR = Pa × Tmn × [(1.35 × Pd/Pa) − 0.32]) during hyperemia.
There are a number of benefits to IMR, which remains stable in the presence of increasing epicardial stenosis,43 is relatively independent of resting hemodynamics.46 Variability in IMR is lower than that of CFR, despite changes in heart rate, blood pressure and contractility.46 Finally, IMR is highly reproducible measure over time, with low interobserver variability despite manual injection of saline boluses.47,48
Microcirculatory resistance can also be determined using a guidewire with a Doppler ultrasound transducer and simultaneous distal coronary pressure monitoring. The Doppler-derived hyperemic microvascular resistance (hMR) is analogous to IMR and is defined as the ratio of mean distal pressure to the Doppler-derived APV (Table 1).49 An hMR ≥2 is generally considered to be abnormal. In patients with INOCA, HMR >1.9 predicted recurrent chest pain in one study, although in another, a threshold of ≥2.5 provided the highest sensitivity and specificity to detect CMR-determined microvascular disease or abnormal invasive CFR.34,50 A related measure, the minimal microvascular resistance (mMR), was proposed as the ratio of hyperemic distal coronary pressure and hyperemic APV measured during wave-free period window of diastole, when microvascular resistance is at its lowest.51 Although conceptually attractive, mMR requires further study.
The resistive reserve ratio (RRR), another recently developed microcirculatory parameter, is calculated as the ratio of baseline microvascular resistance to hyperemic microvascular resistance, with higher values indicating greater vasodilatory capacity of the microcirculation (Table 1).52,53 Thresholds for abnormal RRR are not well established, but have been proposed between <1.7 – 3.5 in various populations and with both Doppler and Thermodilution derived resistance measures.54-56 In a study of 1,692 patients with INOCA, Doppler-derived RRR < 2.62 was associated with mortality and was superior to CFR to predict long-term survival.57 Low RRR was also associated with long-term outcomes in cohorts with acute MI and CAD undergoing revascularization.55,56
Continuous thermodilution is another emerging technique to assess the microcirculation.58 Temperature changes associated with intracoronary saline infusion, administered at a known rate through a dedicated monorail infusion catheter, can be measured in the distal coronary with a thermistor on a pressure-sensing coronary wire to calculate absolute coronary flow (Q). Absolute coronary flow can be derived as Q = 1.08 x Ti/T x Qi, where Ti is the temperature of saline as it exits the catheter, T is the temperature of the blood in the distal coronary during the steady-state infusion, and Qi is the saline infusion rate (in mL/min).59,60 Based on Ohms law, absolute microvascular resistance (R=Pd/Q), measured in Woods units (WU), can be obtained. Slow coronary infusions of saline (typically 8-10 mL/min) are used to assess baseline resistance at rest, while hyperemia is induced at higher saline infusion rates (15-25 mL/min).61 Continuous thermodilution Q has strong agreement with PET-derived coronary flow,62 absolute resistance >500 WU is the optimal threshold to identify patients with an IMR ≥25, 63 and both absolute flow and resistance are associated with angina.64
Based on absolute flow from continuous thermodilution measures, the Microvascular Resistance Reserve (MRR) has been proposed as an index specific for the microvasculature, independent of autoregulation and myocardial mass, and corrected for epicardial conductance. MRR can be defined as the ratio of the pure microvascular resistance at rest, as it would exist in the absence of epicardial disease affecting microcirculatory autoregulation, to the minimal microvascular resistance measured during hyperemia. Although the derivation of the formula for MRR is beyond the scope of this review, in practice, MRR can be calculated as the CFR divided by fractional flow reserve (FFR), corrected for coronary driving pressures as follows: MRR = (CFR / FFR) x (Pa at rest / Pa at hyperemia).65 This conceptually elegant and promising new measure of microcirculatory function requires additional validation.
Practical Approach to Invasive Microcirculatory Assessment
Prior to testing, patients should be advised to abstain from caffeine intake to ensure appropriate responses to hyperemic agents. When combined with acetylcholine or ergonovine reactivity testing, patients should also withhold long-acting nitrates, calcium channel blockers, and beta blockers for 48 hours prior to testing. Coronary angiography should be performed to assess for epicardial coronary stenosis and myocardial bridges. Coronary microvascular testing should be performed using a guiding catheter (preferably ≥6 French) that is stably engaged in the coronary ostium, after administration of intracoronary nitroglycerin and systemic anticoagulation.
To measure thermodilution-based CFR and IMR, a 0.014” coronary pressure–temperature sensor guidewire (Pressure Wire, Abbott Vascular) should be introduced into the guide, and residual contrast media flushed with saline. Pressure waveforms should be equalized with the pressure sensor at the tip of the guide catheter. Next, the temperature and pressure sensor should be advanced to the distal two thirds of the LAD, approximately 8 to 10 centimeters into the circumflex and placed in a large obtuse marginal branch or dominant distal vessel, or in the distal RCA prior to the bifurcation of the posterior descending artery. A 3-way stopcock and a 3-mL syringe should be connected to the manifold. Next, 3mL boluses of room-temperature saline should be briskly injected through the guide catheter to determine Tmn at rest. Measurements are performed in triplicate. If there is >30% variability between the measurements, the Tmn value that deviates most significantly from the mean value should be replaced. Once assessment of baseline flow has been completed, hyperemia is induced with intravenous adenosine (140 mcg/kg/min), or with a bolus of intracoronary papaverine (10–20 mg). Once maximal hyperemia has been achieved, typically ~2 minutes after initiation of intravenous adenosine, 3mL boluses of room temperature saline should again be briskly injected through the guide. CFR can be calculated as the ratio of Tmn at rest to Tmn at hyperemia; IMR is the product of Tmn and Pd at hyperemia. Non-hyperemic pressure ratios and fractional flow reserve (FFR) should also be assessed to determine significance of any angiographically intermediate epicardial lesions. RRR can be calculated as the ratio of baseline to hyperemic microvascular resistance (Tmn at rest x Pd at rest)/IMR.
Doppler-based measurements of CFR and hMR follow a similar sequence. After intracoronary nitroglycerin, a 0.014” coronary guidewire with a pressure sensor and Doppler crystal (ComboWire XT, Philips Volcano) should be positioned parallel to the vessel, away from the vessel wall, and manipulated to obtain a stable maximal Doppler flow signal. APV is measured at rest and again with maximal hyperemia, as previously described. The CFR is calculated as the ratio of hyperemic APV to resting APV. The hMR is calculated as the hyperemic Pd divided by the hyperemic APV. To assess endothelial dependent microvascular function, CBF at rest and during acetylcholine infusions can be estimated from Doppler-derived APV and luminal dimensions from quantitative coronary angiography.
Clinical Implications of Microcirculatory Disease
Microvascular Disease in INOCA
Nearly 50% of patients who present with chest pain have angiographically normal or non-obstructive epicardial coronary arteries (<50% stenosis) by angiography.2,3,66 A comprehensive approach to the diagnosis of INOCA, including testing for microvascular disease, can improve the care of these patients (Figure 2). In the Coronary Microvascular Angina (CorMicA) trial, 151 patients underwent blinded assessment of coronary microvascular function and provocative testing for coronary artery spasm; participants were randomly assigned to disclosure of the results or usual care.6 Overall, microvascular angina was identified in 52% of patients, coronary spasm was identified in 20%, and mixed microvascular and spasm diagnosis was present in 17%, with no discernable etiology identified in the remaining 11%.67 Although non-cardiac chest pain was presumed in 60-65% of participants prior to randomization, the results of testing improved diagnostic certainty, reduced the number of patients inappropriately diagnosed with non-cardiac chest pain, and impacted therapy in the overwhelming majority of patients in the intervention group. Although similar at baseline, patients assigned to disclosure of coronary functional testing had significantly better Angina Summary Scores and quality of life at 6 months and 1 year compared to the control group.6,68 This trial provides compelling data in support of invasive microvascular testing to guide therapy and improve symptoms in INOCA (Figure 2). Additional studies to evaluate the clinical benefit of microvascular testing without coronary spasm testing are currently underway (iCorMICA NCT04674449).
Figure 2. Diagnostic pathway for the invasive assessment of patients with INOCA.
*Assessed with Doppler APV and coronary diameter via quantitative coronary angiography to determine coronary blood flow at rest and with ACh.
INOCA = ischemia with non-obstructive coronary arteries; PET = positron emission tomography; CMR= cardiovascular magnetic resonance; CFR = coronary flow reserve; IMR = index for microvascular resistance; CMD = coronary microvascular disease; ECG = electrocardiogram; CAD = coronary artery disease; CFVR = coronary flow velocity reserve; FFR = fractional flow reserve; Ach = acetylcholine.
Although outcomes are more favorable than patients with obstructive disease, INOCA is associated with excess major adverse cardiovascular events compared with reference populations without ischemic heart disease.69 Data from Women’s Ischemia Syndrome Evaluation (WISE) registry indicate that CFR <2.3 is associated with increased risks of MACE in patients with INOCA.31,70 In an multicenter international study of INOCA patients with microvascular angina, the annual incidence of the composite of MACE was 7.7%.71 In a study-level metanalysis, CMD was associated with 5-fold greater odds for major adverse cardiovascular events compared to patients without CMD.72 Thus, novel therapies to reduce the risk of MACE in patients with microvascular disease are urgently needed.
Impact of CMD in MI and PCI
In patients with acute myocardial infarction (MI), distal embolization of thrombotic material can lead to the “no-reflow phenomenon”, characterized by myocardial tissue hypoperfusion in the presence of a patent epicardial coronary artery. No-reflow, mediated by microvascular disease, is a strong predictor for adverse outcomes post-MI.73 Quantitative assessment of the coronary microcirculation provides additional insights into MI severity and outcomes. In patients with ST segment elevation MI (STEMI), IMR post-PCI correlates with myocardial injury, echocardiographic wall motion abnormalities,74 myocardial viability by PET,75 and myocardial salvage by cardiac magnetic resonance (CMR).48 Although IMR correlates with microvascular obstruction by CMR imaging overall,76 discordances between the two measures are reported in up to a third of cases.77 Still, in a cohort of 253 patients undergoing primary PCI for STEMI with a median follow up of 2.8 years, elevated IMR ≥40 immediately after revascularization was associated with an two-fold excess hazard of long-term death or rehospitalization for heart failure, and a four-fold excess hazard of mortality.78 The Doppler-derived resistance index, hMR, has also been associated with outcomes after STEMI.35 Similarly in patients who presented with NSTEMI and underwent PCI, post-PCI elevated IMR >27 was an independent predictor of MACE over a median follow-up of 21 months.79
Even in stable patients undergoing PCI, a high baseline IMR is an independent risk factor for periprocedural MI. Among patients undergoing PCI of simple LAD lesions, pre-PCI IMR ≥27, was independently associated with a 23-fold increase in the risk of periprocedural MI.80 Furthermore, in a cohort of 572 patients undergoing successful PCI for stable ischemic heart disease, post-PCI IMR was independently associated with major adverse cardiovascular events at follow up.81
Conclusion
The coronary microcirculation represents the next frontier in the diagnosis and treatment of coronary artery disease. Recognition of the importance of coronary physiology has expanded our understanding of ischemic heart disease and European and American societal guidelines now recommend comprehensive assessment of microvascular function in patients with INOCA.7,8 Recent advances in invasive techniques and technologies to quantify microcirculatory CBF and resistance are vital to the comprehensive evaluation of coronary artery disease. Additional studies are needed to determine optimal therapies for patients with coronary microcirculatory disease in various cardiac disease states.
Key Points:
Coronary microvascular dysfunction (CMD) is an important cause of ischemia with non-obstructive coronary arteries (INOCA).
The pathophysiology of CMD is not well established.
Coronary flow reserve (CFR), the index of microcirculatory resistance (IMR), hMR, and RRR are invasively measured indices to evaluate the microcirculation.
CMD is associated with clinical outcomes in patients with INOCA and obstructive CAD.
Acknowledgements:
Dr. Smilowitz is supported, in part, by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL150315.
Footnotes
Disclosures:
Dr. Smilowitz serves on an advisory board for Abbott Vascular. The remainder of the authors report no financial relationships or conflicts of interest regarding the content herein.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. Jan 26 2022:CIR0000000000001052. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. Mar 11 2010;362(10):886–95. doi: 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. Apr 8 2008;117(14):1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562 [DOI] [PubMed] [Google Scholar]
- 4.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. Mar 14 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. International journal of cardiology. Jan 1 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068 [DOI] [PubMed] [Google Scholar]
- 6.Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. Dec 11 2018;72(23 Pt A):2841–2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 7.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. Nov 30 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 8.Kunadian V, Chieffo A, Camici PG, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. Oct 1 2020;41(37):3504–3520. doi: 10.1093/eurheartj/ehaa503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padro T, Manfrini O, Bugiardini R, et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res. Mar 1 2020;116(4):741–755. doi: 10.1093/cvr/cvaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson RF, Marcus ML, White CW. Prediction of the physiologic significance of coronary arterial lesions by quantitative lesion geometry in patients with limited coronary artery disease. Circulation. Apr 1987;75(4):723–32. doi: 10.1161/01.cir.75.4.723 [DOI] [PubMed] [Google Scholar]
- 11.Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nature reviews Cardiology. Jan 2015;12(1):48–62. doi: 10.1038/nrcardio.2014.160 [DOI] [PubMed] [Google Scholar]
- 12.Diez-Delhoyo F, Gutierrez-Ibanes E, Loughlin G, et al. Coronary physiology assessment in the catheterization laboratory. World J Cardiol. Sep 26 2015;7(9):525–38. doi: 10.4330/wjc.v7.i9.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. Jul 2008;88(3):1009–86. doi: 10.1152/physrev.00045.2006 [DOI] [PubMed] [Google Scholar]
- 14.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. Feb 22 2007;356(8):830–40. doi: 10.1056/NEJMra061889 [DOI] [PubMed] [Google Scholar]
- 15.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. May 2014;35(17):1101–11. doi: 10.1093/eurheartj/eht513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. Jun 1 2010;121(21):2317–25. doi: 10.1161/CIRCULATIONAHA.109.900191 [DOI] [PubMed] [Google Scholar]
- 17.Lanza GA, Andreotti F, Sestito A, Sciahbasi A, Crea F, Maseri A. Platelet aggregability in cardiac syndrome X. Eur Heart J. Oct 2001;22(20):1924–30. doi: 10.1053/euhj.2001.2624 [DOI] [PubMed] [Google Scholar]
- 18.Kleinbongard P, Heusch G. A fresh look at coronary microembolization. Nat Rev Cardiol. Nov 16 2021;doi: 10.1038/s41569-021-00632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. Sep 11 2003;349(11):1027–35. doi: 10.1056/NEJMoa025050 [DOI] [PubMed] [Google Scholar]
- 20.Mills I, Fallon JT, Wrenn D, et al. Adaptive responses of coronary circulation and myocardium to chronic reduction in perfusion pressure and flow. Am J Physiol. Feb 1994;266(2 Pt 2):H447–57. doi: 10.1152/ajpheart.1994.266.2.H447 [DOI] [PubMed] [Google Scholar]
- 21.Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. Jan 1993;91(1):29–37. doi: 10.1172/JCI116183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. Apr 29 2014;129(17):1723–30. doi: 10.1161/CIRCULATIONAHA.113.004096 [DOI] [PubMed] [Google Scholar]
- 23.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. Aug 2009;30(15):1837–43. doi: 10.1093/eurheartj/ehp205 [DOI] [PubMed] [Google Scholar]
- 24.Hirata K, Kadirvelu A, Kinjo M, et al. Altered coronary vasomotor function in young patients with systemic lupus erythematosus. Arthritis and rheumatism. Jun 2007;56(6):1904–9. doi: 10.1002/art.22702 [DOI] [PubMed] [Google Scholar]
- 25.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med. Sep 2011;52(9):1369–77. doi: 10.2967/jnumed.110.082883 [DOI] [PubMed] [Google Scholar]
- 26.Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. Aug 1994;90(2):808–17. doi: 10.1161/01.cir.90.2.808 [DOI] [PubMed] [Google Scholar]
- 27.Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol. Jul 1998;32(1):147–53. doi: 10.1016/s0735-1097(98)00222-8 [DOI] [PubMed] [Google Scholar]
- 28.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. Mar 24 2015;131(12):1054–60. doi: 10.1161/circulationaha.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. May 1992;85(5):1899–911. doi: 10.1161/01.cir.85.5.1899 [DOI] [PubMed] [Google Scholar]
- 30.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. Sep 2015;8(11):1445–53. doi: 10.1016/j.jcin.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 31.AlBadri A, Bairey Merz CN, Johnson BD, et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. Feb 19 2019;73(6):684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fearon WF, Balsam LB, Farouque HM, et al. Novel index for invasively assessing the coronary microcirculation. Circulation. Jul 01 2003;107(25):3129–32. doi: 10.1161/01.cir.0000080700.98607.d1 [DOI] [PubMed] [Google Scholar]
- 33.Barbato E, Aarnoudse W, Aengevaeren WR, et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur Heart J. Feb 2004;25(3):219–23. doi: 10.1016/j.ehj.2003.11.009 [DOI] [PubMed] [Google Scholar]
- 34.Williams RP, de Waard GA, De Silva K, et al. Doppler Versus Thermodilution-Derived Coronary Microvascular Resistance to Predict Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction or Stable Angina Pectoris. Am J Cardiol. Jan 1 2018;121(1):1–8. doi: 10.1016/j.amjcard.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Waard GA, Fahrni G, de Wit D, et al. Hyperaemic microvascular resistance predicts clinical outcome and microvascular injury after myocardial infarction. Heart. Jan 2018;104(2):127–134. doi: 10.1136/heartjnl-2017-311431 [DOI] [PubMed] [Google Scholar]
- 36.Fearon WF, Farouque HM, Balsam LB, et al. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. Nov 04 2003;108(18):2198–200. doi: 10.1161/01.cir.0000099521.31396.9d [DOI] [PubMed] [Google Scholar]
- 37.Everaars H, de Waard GA, Driessen RS, et al. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve: A Head-to-Head Comparison With [(15)O]H2O PET. JACC Cardiovasc Interv. Oct 22 2018;11(20):2044–2054. doi: 10.1016/j.jcin.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 38.Nardone M, McCarthy M, Ardern CI, et al. Concurrently Low Coronary Flow Reserve and Low Index of Microvascular Resistance Are Associated With Elevated Resting Coronary Flow in Patients With Chest Pain and Nonobstructive Coronary Arteries. Circ Cardiovasc Interv. Feb 9 2022:CIRCINTERVENTIONS121011323. doi: 10.1161/CIRCINTERVENTIONS.121.011323 [DOI] [PubMed] [Google Scholar]
- 39.Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. Effects of adenosine on human coronary arterial circulation. Circulation. Nov 1990;82(5):1595–606. doi: 10.1161/01.cir.82.5.1595 [DOI] [PubMed] [Google Scholar]
- 40.Luo C, Long M, Hu X, et al. Thermodilution-derived coronary microvascular resistance and flow reserve in patients with cardiac syndrome X. Circ Cardiovasc Interv. Feb 2014;7(1):43–8. doi: 10.1161/circinterventions.113.000953 [DOI] [PubMed] [Google Scholar]
- 41.Solberg OG, Ragnarsson A, Kvarsnes A, et al. Reference interval for the index of coronary microvascular resistance. EuroIntervention. Jan 22 2014;9(9):1069–75. doi: 10.4244/EIJV9I9A181 [DOI] [PubMed] [Google Scholar]
- 42.Lee JM, Layland J, Jung JH, et al. Integrated physiologic assessment of ischemic heart disease in real-world practice using index of microcirculatory resistance and fractional flow reserve: insights from the International Index of Microcirculatory Resistance Registry. Circ Cardiovasc Interv. Nov 2015;8(11):e002857. doi: 10.1161/CIRCINTERVENTIONS.115.002857 [DOI] [PubMed] [Google Scholar]
- 43.Aarnoudse W, Fearon WF, Manoharan G, et al. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. Oct 12 2004;110(15):2137–42. doi: 10.1161/01.CIR.0000143893.18451.0E [DOI] [PubMed] [Google Scholar]
- 44.Yong AS, Layland J, Fearon WF, et al. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. Jan 2013;6(1):53–8. doi: 10.1016/j.jcin.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 45.Layland J, MacIsaac AI, Burns AT, et al. When collateral supply is accounted for epicardial stenosis does not increase microvascular resistance. Circ Cardiovasc Interv. Feb 1 2012;5(1):97–102. doi: 10.1161/CIRCINTERVENTIONS.111.964718 [DOI] [PubMed] [Google Scholar]
- 46.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. May 02 2006;113(17):2054–61. doi: 10.1161/circulationaha.105.603522 [DOI] [PubMed] [Google Scholar]
- 47.Pagonas N, Gross CM, Li M, Bondke A, Klauss V, Buschmann EE. Influence of epicardial stenosis severity and central venous pressure on the index of microcirculatory resistance in a follow-up study. EuroIntervention. Jan 22 2014;9(9):1063–8. doi: 10.4244/EIJV9I9A180 [DOI] [PubMed] [Google Scholar]
- 48.Payne AR, Berry C, Doolin O, et al. Microvascular Resistance Predicts Myocardial Salvage and Infarct Characteristics in ST-Elevation Myocardial Infarction. Journal of the American Heart Association. Aug 2012;1(4):e002246. doi: 10.1161/jaha.112.002246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chamuleau SA, Siebes M, Meuwissen M, Koch KT, Spaan JA, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. American journal of physiology Heart and circulatory physiology. Nov 2003;285(5):H2194–200. doi: 10.1152/ajpheart.01021.2002 [DOI] [PubMed] [Google Scholar]
- 50.Sheikh AR, Zeitz CJ, Rajendran S, Di Fiore DP, Tavella R, Beltrame JF. Clinical and coronary haemodynamic determinants of recurrent chest pain in patients without obstructive coronary artery disease - A pilot study. International journal of cardiology. Sep 15 2018;267:16–21. doi: 10.1016/j.ijcard.2018.04.077 [DOI] [PubMed] [Google Scholar]
- 51.de Waard GA, Nijjer SS, van Lavieren MA, et al. Invasive minimal Microvascular Resistance Is a New Index to Assess Microcirculatory Function Independent of Obstructive Coronary Artery Disease. Journal of the American Heart Association. Dec 22 2016;5(12)doi: 10.1161/JAHA.116.004482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarsini R, De Maria GL, Borlotti A, et al. Incremental Value of Coronary Microcirculation Resistive Reserve Ratio in Predicting the Extent of Myocardial Infarction in Patients with STEMI. Insights from the Oxford Acute Myocardial Infarction (OxAMI) Study. Cardiovascular revascularization medicine : including molecular interventions. Dec 2019;20(12):1148–1155. doi: 10.1016/j.carrev.2019.01.022 [DOI] [PubMed] [Google Scholar]
- 53.Layland J, Carrick D, McEntegart M, et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. Jun 2013;6(3):231–6. doi: 10.1161/CIRCINTERVENTIONS.112.000180 [DOI] [PubMed] [Google Scholar]
- 54.Corcoran D, Young R, Adlam D, et al. Coronary microvascular dysfunction in patients with stable coronary artery disease: The CE-MARC 2 coronary physiology sub-study. International journal of cardiology. Sep 1 2018;266:7–14. doi: 10.1016/j.ijcard.2018.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maznyczka AM, Oldroyd KG, Greenwood JP, et al. Comparative Significance of Invasive Measures of Microvascular Injury in Acute Myocardial Infarction. Circ Cardiovasc Interv. May 2020;13(5):e008505. doi: 10.1161/CIRCINTERVENTIONS.119.008505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, Lee JM, Park J, et al. Prognostic Implications of Resistive Reserve Ratio in Patients With Coronary Artery Disease. Journal of the American Heart Association. Apr 21 2020;9(8):e015846. doi: 10.1161/JAHA.119.015846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toya T, Ahmad A, Corban MT, et al. Risk Stratification of Patients With NonObstructive Coronary Artery Disease Using Resistive Reserve Ratio. Journal of the American Heart Association. Jun 2021;10(11):e020464. doi: 10.1161/JAHA.120.020464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aarnoudse W, Van't Veer M, Pijls NH, et al. Direct volumetric blood flow measurement in coronary arteries by thermodilution. J Am Coll Cardiol. Dec 11 2007;50(24):2294–304. doi: 10.1016/j.jacc.2007.08.047 [DOI] [PubMed] [Google Scholar]
- 59.Jansen TPJ, Konst RE, Elias-Smale SE, et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries: JACC Review Topic of the Week. J Am Coll Cardiol. Oct 5 2021;78(14):1471–1479. doi: 10.1016/j.jacc.2021.08.028 [DOI] [PubMed] [Google Scholar]
- 60.Xaplanteris P, Fournier S, Keulards DCJ, et al. Catheter-Based Measurements of Absolute Coronary Blood Flow and Microvascular Resistance: Feasibility, Safety, and Reproducibility in Humans. Circ Cardiovasc Interv. Mar 2018;11(3):e006194. doi: 10.1161/CIRCINTERVENTIONS.117.006194 [DOI] [PubMed] [Google Scholar]
- 61.De Bruyne B, Adjedj J, Xaplanteris P, et al. Saline-Induced Coronary Hyperemia: Mechanisms and Effects on Left Ventricular Function. Circ Cardiovasc Interv. Apr 2017;10(4)doi: 10.1161/CIRCINTERVENTIONS.116.004719 [DOI] [PubMed] [Google Scholar]
- 62.Everaars H, de Waard GA, Schumacher SP, et al. Continuous thermodilution to assess absolute flow and microvascular resistance: validation in humans using [15O]H2O positron emission tomography. Eur Heart J. Jul 21 2019;40(28):2350–2359. doi: 10.1093/eurheartj/ehz245 [DOI] [PubMed] [Google Scholar]
- 63.Rivero F, Gutierrez-Barrios A, Gomez-Lara J, et al. Coronary microvascular dysfunction assessed by continuous intracoronary thermodilution: A comparative study with index of microvascular resistance. International journal of cardiology. Jun 15 2021;333:1–7. doi: 10.1016/j.ijcard.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 64.Konst RE, Elias-Smale SE, Pellegrini D, et al. Absolute Coronary Blood Flow Measured by Continuous Thermodilution in Patients With Ischemia and Nonobstructive Disease. J Am Coll Cardiol. Feb 16 2021;77(6):728–741. doi: 10.1016/j.jacc.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 65.De Bruyne B, Pijls NHJ, Gallinoro E, et al. Microvascular Resistance Reserve for Assessment of Coronary Microvascular Function: JACC Technology Corner. J Am Coll Cardiol. Oct 12 2021;78(15):1541–1549. doi: 10.1016/j.jacc.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 66.Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. Jun 2014;167(6):846–52 e2. doi: 10.1016/j.ahj.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 67.Ford TJ, Yii E, Sidik N, et al. Ischemia and No Obstructive Coronary Artery Disease: Prevalence and Correlates of Coronary Vasomotion Disorders. Circ Cardiovasc Interv. Dec 2019;12(12):e008126. doi: 10.1161/CIRCINTERVENTIONS.119.008126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ford TJ, Stanley B, Sidik N, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. Jan 13 2020;13(1):33–45. doi: 10.1016/j.jcin.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. Mar 2012;33(6):734–44. doi: 10.1093/eurheartj/ehr331 [DOI] [PubMed] [Google Scholar]
- 70.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. Jun 22 2010;55(25):2825–32. doi: 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimokawa H, Suda A, Takahashi J, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur Heart J. Nov 21 2021;42(44):4592–4600. doi: 10.1093/eurheartj/ehab282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of Isolated Coronary Microvascular Dysfunction With Mortality and Major Adverse Cardiac Events: A Systematic Review and Meta-Analysis of Aggregate Data. J Am Heart Assoc. May 5 2020;9(9):e014954. doi: 10.1161/JAHA.119.014954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. May 25 2010;55(21):2383–9. doi: 10.1016/j.jacc.2009.12.054 [DOI] [PubMed] [Google Scholar]
- 74.Fearon WF, Shah M, Ng M, et al. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. Feb 05 2008;51(5):560–5. doi: 10.1016/j.jacc.2007.08.062 [DOI] [PubMed] [Google Scholar]
- 75.Lim HS, Yoon MH, Tahk SJ, et al. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. Dec 2009;30(23):2854–60. doi: 10.1093/eurheartj/ehp313 [DOI] [PubMed] [Google Scholar]
- 76.Carrick D, Haig C, Ahmed N, et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation. Dec 6 2016;134(23):1833–1847. doi: 10.1161/CIRCULATIONAHA.116.022603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Maria GL, Alkhalil M, Wolfrum M, et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients With STEMI Undergoing Primary PCI. JACC Cardiovascular imaging. May 2019;12(5):837–848. doi: 10.1016/j.jcmg.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 78.Fearon WF, Low AF, Yong AS, et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. Jun 18 2013;127(24):2436–41. doi: 10.1161/circulationaha.112.000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murai T, Yonetsu T, Kanaji Y, et al. Prognostic value of the index of microcirculatory resistance after percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome. Catheter Cardiovasc Interv. Nov 15 2018;92(6):1063–1074. doi: 10.1002/ccd.27529 [DOI] [PubMed] [Google Scholar]
- 80.Ng MK, Yong AS, Ho M, et al. The index of microcirculatory resistance predicts myocardial infarction related to percutaneous coronary intervention. Circ Cardiovasc Interv. Aug 1 2012;5(4):515–22. doi: 10.1161/CIRCINTERVENTIONS.112.969048 [DOI] [PubMed] [Google Scholar]
- 81.Nishi T, Murai T, Ciccarelli G, et al. Prognostic Value of Coronary Microvascular Function Measured Immediately After Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study. Circ Cardiovasc Interv. Sep 2019;12(9):e007889. doi: 10.1161/CIRCINTERVENTIONS.119.007889 [DOI] [PubMed] [Google Scholar]