Video
Flexible fiber cholangioscope for detection of near-infrared fluorescence.
Abbreviations: NIR, near-infrared; T/B, target-to-background
Introduction
Cholangiocarcinoma is a cancer of the bile ducts and is characterized by a high mortality rate and a steadily rising incidence.1 This disease is usually diagnosed at an advanced stage when the prognosis is poor. Biliary intraepithelial neoplasia represents a precursor condition that can result in excellent patient outcomes with surgical resection if detected early. This condition is often suspected in patients with indeterminate biliary strictures.2 Because the bile ducts are small in caliber, conventional biopsy and brushings often do not produce an adequate number of cells for either cytology or histology to make a definitive diagnosis.3 Patients often either undergo major surgery only to find a benign stricture or they delay therapy for a late-stage cancer. Therefore, a new imaging technology that can access the bile ducts to identify early biliary neoplasia is desired. We aim to demonstrate proof-of-concept for a flexible fiber cholangioscope that is sensitive to near-infrared (NIR) fluorescence to detect targeted contrast agents in the bile ducts.
Description of Technology
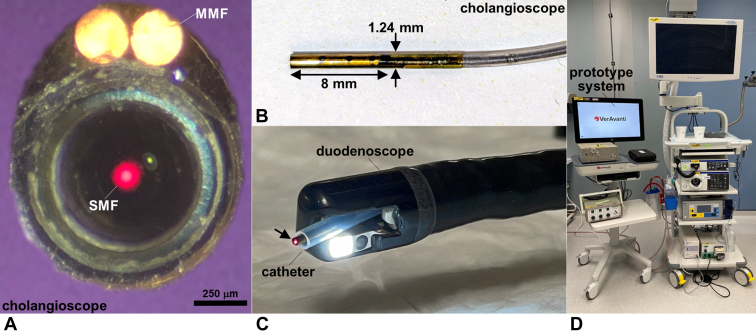
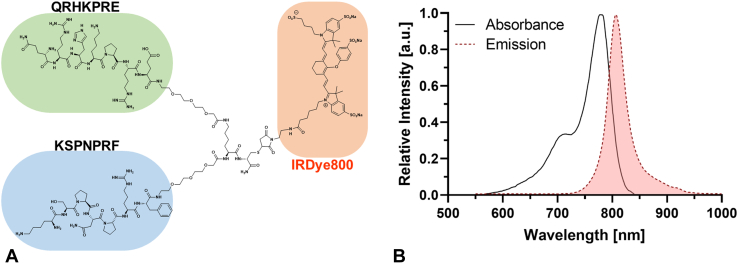
We have previously demonstrated clinical use of a flexible fiber endoscope (VerAvanti, Redmond, Wash, USA) with dimensions of 2.4 mm in diameter and 9 mm in rigid length to collect NIR fluorescence images using targeted contrast agents.4,5 This instrument was used in the human esophagus to identify Barrett's neoplasia. The instrument dimensions were scaled down in size to 1.2 mm in diameter and 8 mm in rigid length to image the bile ducts (Fig. 1A). Laser excitation at λex = 635 and 779 nm was delivered via a single-mode fiber that was scanned in a spiral pattern by a piezo tube actuator resulting in an average maximum deflection angle of ∼70° (Fig. 1B). Two multimode fibers with core diameter of 250 μm and a numerical aperture of 0.63 were attached to the periphery of the instrument to collect fluorescence and reflectance concurrently. The cholangioscope was ∼2.3 m in total length and passed forward easily through the working channel and elevator of a standard duodenoscope (Fig. 1C). The imaging system was contained on a portable cart for use as an accessory during ERCP (Fig. 1D). Monomer peptides QRHKPRE and KSPNPRF,6,7 specific for EGFR and ErbB2, respectively, were arranged in a heterodimer configuration8,9 (Fig. 2A and B). These peptides demonstrated increased intensity to stain human specimens of biliary intraepithelial neoplasia and early cholangiocarcinoma ex vivo by comparison with normal biliary epithelium using immunohistochemistry and immunofluorescence.10 IRDye800 was used to label the targeted contrast agent. This fluorophore emits in the spectrum with low sensitivity to hemoglobin absorption and tissue scattering and has reduced tissue autofluorescence.
Figure 1.
Near-infrared cholangioscope. A, En face view shows the single-mode fiber that delivers laser excitation and 2 multimode fibers (MMFs) that collect light. B, The rigid tip has dimensions of 8 mm in length and 1.24 mm in diameter. C, The cholangioscope (arrow) was passed forward through a push catheter (10F) before insertion into the working channel of a standard duodenoscope. D, The prototype imaging system was contained on a portable cart placed next to the video processor for the duodenoscope to perform ERCP.
Figure 2.
Targeted contrast agent. A, Biochemical structure is shown for a peptide heterodimer specific for EGFR (QRHKPRE) and ErbB2 (KSPNPRF) labeled with IRDye800. B, Absorbance and emission spectra shown maxima at 783 and 806 nm, respectively.
Clinical Demonstration
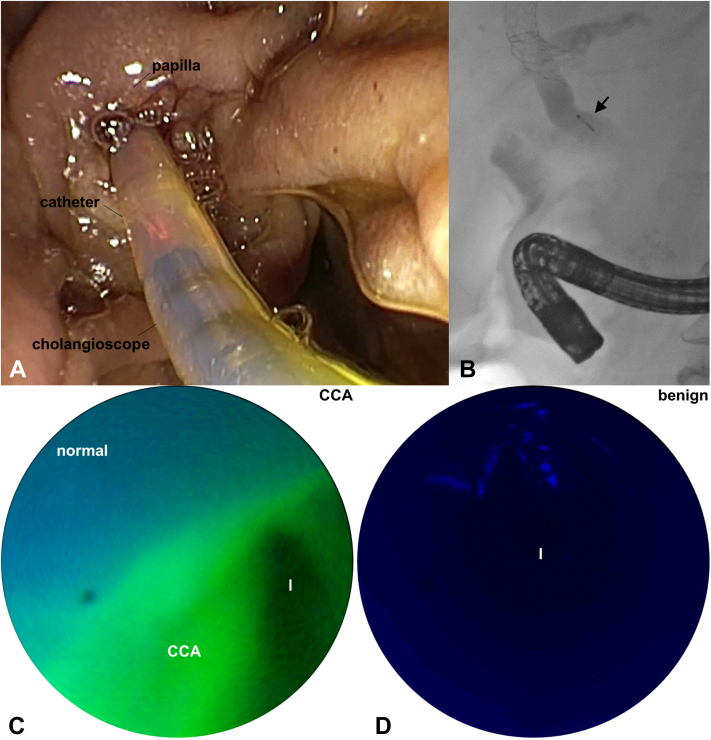
Patients with indeterminate biliary strictures scheduled previously for an elective outpatient ERCP were enrolled, and informed consent was obtained. The clinical study was regulated under Investigational New Drug #139,834, approved by the Michigan Medicine Institutional Review Board, and registered online at ClinicalTrials.gov (NCT04304781). After the targeted contrast agent was administered, the cholangioscope was advanced into the bile ducts under fluoroscopy guidance to image the stricture (Video 1, available online at www.giejournal.org; Fig. 3A and B). The fluorescence images were quantified by calculating the target-to-background (T/B) ratio from individual frames using a Chan-Vese–based algorithm.11 A stricture from a cholangiocarcinoma validated by pathology resulted in a T/B ratio of 1.62 (Fig. 3C). By comparison, a benign biliary stricture produced a T/B ratio of 1.00 (Fig. 3D). The coregistered reflectance image was used to interpret the fluorescence image. The cholangioscope was sterilized after each use.
Figure 3.
Clinical imaging. A, White-light image shows insertion of cholangioscope via push catheter into the duodenal papilla. B, Fluoroscopy shows the presence of the cholangioscope (arrow) in the common bile duct. Fluorescence images are shown in the bile duct lumen (l) of a (C) cholangiocarcinoma (CCA), and (D) benign biliary stricture.
Conclusion
We have demonstrated clinical use of a flexible fiber cholangioscope that collects NIR fluorescence images in vivo from human bile ducts. This instrument can be used with a targeted contrast agent to evaluate indeterminant biliary strictures. Larger clinical studies are needed to validate the performance of this methodology.
Acknowledgments
We thank C. Olivo for fabricating the fluorescence cholangioscopes; E. Brady and C. Nolan for clinical support; J. Chen, Y. Jiang, and S. Feng for technical support ; and L. Young for voice recordings.
Disclosure
Dr Seibel is an inventor on patents filed by the University of Washington on the fluorescence cholangioscope and is a consultant at VerAvanti, Inc. Dr Wang is an inventor on patents filed by the University of Michigan on the peptide heterodimer. All other authors disclosed no financial relationships. This study was supported in part by the National Institutes of Health (grants U01 CA189291 and U54 CA163059 to T.D.W. and E.J.S.; grant R01 CA200007 to E.J.S. and T.D.W.).
Supplementary data
Flexible fiber cholangioscope for detection of near-infrared fluorescence.
References
- 1.Gad M.M., Saad A.M., Faisaluddin M., et al. Epidemiology of cholangiocarcinoma; United States incidence and mortality trends. Clin Res Hepatol Gastroenterol. 2020;44:885–893. doi: 10.1016/j.clinre.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Novikov A., Kowalski T.E., Loren D.E. Practical management of indeterminate biliary strictures. Gastrointest Endosc Clin N Am. 2019;29:205–214. doi: 10.1016/j.giec.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Ejaz A., Cloyd J.M., Pawlik T.M. Advances in the diagnosis and treatment of patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020;27:552–560. doi: 10.1245/s10434-019-07873-z. [DOI] [PubMed] [Google Scholar]
- 4.Chen J., Jiang Y., Chang T.-S., et al. Multiplexed endoscopic imaging of Barrett's neoplasia using targeted fluorescent heptapeptides in a phase 1 proof-of-concept study. Gut. 2021;70:1010–1013. doi: 10.1136/gutjnl-2020-322945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Jiang Y, Chang T-S, et al. Detection of Barrett’s neoplasia with a near-infrared fluorescent heterodimeric peptide. Endoscopy. Epub March 17, 2022. https://doi.org/10.1055/a-1801-2406. [DOI] [PMC free article] [PubMed]
- 6.Zhou J., Joshi B.P., Duan X., et al. EGFR overexpressed in colonic neoplasia can be detected on wide-field endoscopic imaging. Clin Transl Gastroenterol. 2015;6 doi: 10.1038/ctg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi B.P., Zhou J., Pant A., et al. Design and synthesis of near-infrared peptide for in vivo molecular imaging of HER2. Bioconjug Chem. 2016;27:481–494. doi: 10.1021/acs.bioconjchem.5b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Zhou J., Gao Z., et al. Multiplexed targeting of Barrett's neoplasia with a heterobivalent ligand: imaging study on mouse xenograft in vivo and human specimens ex vivo. J Med Chem. 2018;61:5323–5331. doi: 10.1021/acs.jmedchem.8b00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Gao Z., Li G., et al. Dual-modal in vivo fluorescence and photoacoustic imaging using heterodimeric peptide. Chem Communicat. 2018;54:13196–13199. doi: 10.1039/c8cc06774k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturm M.B., Joshi B.P., Owens S.R., et al. Multiplexed imaging strategy to distinguish indeterminant biliary strictures: an ex vivo study. World J Gastroenterol Hepatol Endosc. 2020;3:1–7. doi: 10.47690/wjghe.2020.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y., Gong Y., Rubenstein J.H., et al. Toward real-time quantification of fluorescence molecular probes using target/background ratio for guiding biopsy and endoscopic therapy of esophageal neoplasia. J Med Imaging (Bellingham) 2017;4 doi: 10.1117/1.JMI.4.2.024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flexible fiber cholangioscope for detection of near-infrared fluorescence.
Flexible fiber cholangioscope for detection of near-infrared fluorescence.