Abstract
OBJECTIVE
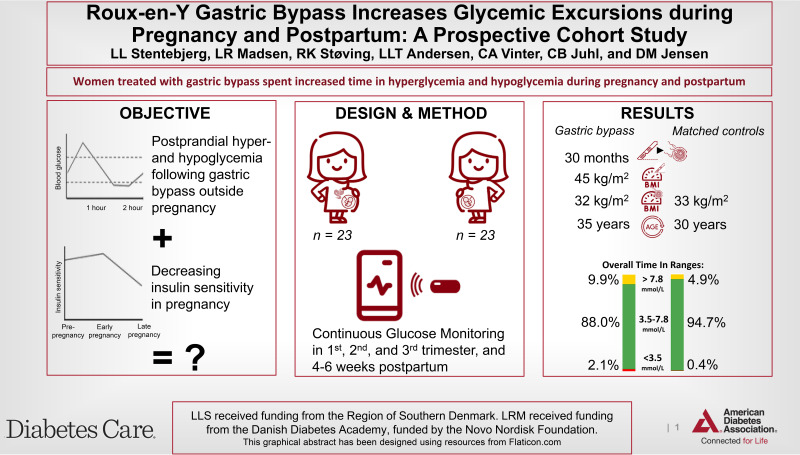
Roux-en-Y gastric bypass (RYGB) and pregnancy markedly alter glucose metabolism, but evidence on glucose metabolism in pregnancy after RYGB is limited. Thus, the aims of the Bariatric Surgery and Consequences for Mother and Baby in Pregnancy study were to investigate interstitial glucose (IG) profiles during pregnancy, risk factors associated with hypoglycemia, and the association between fetal growth and hypoglycemia in pregnant women previously treated with RYGB, compared with control participants.
RESEARCH DESIGN AND METHODS
Twenty-three pregnant women with RYGB and 23 BMI- and parity-matched pregnant women (control group) were prospectively studied with continuous glucose monitoring in their first, second, and third trimesters, and 4 weeks postpartum. Time in range (TIR) was defined as time with an IG level of 3.5–7.8 mmol/L.
RESULTS
Women with RYGB were 4 years (interquartile range [IQR] 0–7) older than control participants. Pregnancies occurred 30 months (IQR 15–98) after RYGB, which induced a reduction in BMI from 45 kg/m2 (IQR 42–54) presurgery to 32 kg/m2 (IQR 27–39) prepregnancy. Women with RYGB spent decreased TIR (87.3–89.5% vs. 93.3–96.1%; P < 0.01) owing to an approximately twofold increased time above range and increased time below range (TBR) throughout pregnancy and postpartum compared with control participants. Women with increased TBR had a longer surgery-to-conception interval, lower nadir weight, and greater weight loss after RYGB. Finally, women giving birth to small-for-gestational age neonates experienced slightly increased TBR.
CONCLUSIONS
Women with RYGB were more exposed to hypoglycemia and hyperglycemia during pregnancy compared with control participants. Further research should investigate whether hypoglycemia during pregnancy in women with RYGB is associated with decreased fetal growth.
Graphical Abstract
Introduction
Although Roux-en-Y gastric bypass (RYGB) increases fertility and decreases the risks associated with obesity in pregnancy (1,2), RYGB is also associated with alterations in glucose metabolism (3). Nutrients are delivered to the jejunum less digested, and a rapid secretion of glucagon-like peptide 1 causes a subsequent augmented insulin release that results in a disproportion in glucose and insulin levels with risk of postprandial hypoglycemia, also known as post bariatric hypoglycemia (PBH) (3,4).
During a normal pregnancy, placental hormones and adipokines cause metabolic changes that affect insulin sensitivity (5). In early pregnancy, insulin secretion increases, and insulin sensitivity decreases, remains unchanged, or increases (5,6). As pregnancy advances, insulin secretion is further increased, and insulin sensitivity decreases (5–7). There is limited evidence for the effects of these modifications on the altered glucose profiles caused by RYGB and the risk of PBH.
Our recent meta-analysis indicated that 58% of pregnant women previously treated with RYGB are exposed to PBH, defined as glucose levels <3.3 mmol/L (8). To our knowledge, only six research centers have investigated glucose profiles by testing once in mid pregnancy with an oral glucose tolerance test (OGTT) or continuous glucose monitoring (CGM) in pregnant women treated with RYGB (8). Most studies have investigated the validity and complications of the OGTT regarding diagnosis of gestational diabetes (GD), reporting PBH as a secondary outcome (9–11).
Predictive factors for PBH have been described in nonpregnant, postbariatric populations (12,13). RYGB, female sex, young age at surgery, absence of presurgery diabetes, lower presurgery BMI, lower nadir BMI, and percentage of total weight loss (%TWL) are associated with increased risk of PBH (12,13). Similar data do not appear to be available in pregnant women even though identifying risk factors for PBH may be key to prevention.
Increased risk of fetal growth restriction is evident in pregnancies after RYGB (14). Insufficient gestational weight gain (GWG) has been associated with small-for-gestational age (SGA) neonates, but it does not account for all cases (15,16). Maternal micronutrient deficiencies due to malabsorption have also been suggested, but an association with SGA has not been confirmed (15,17). Hypoglycemia has been suggested as a contributing factor to decreased fetal growth, but the evidence is conflicting. Gohier et al. (18) found an association between SGA neonates and decreased time below range (TBR) during CGM, and several other studies in which OGTT was used found a positive association between maternal glucose levels and birth weight (11,19,20).
Owing to the limited evidence on the effect of RYGB on glucose metabolism during pregnancy, we performed a prospective study comparing women who had undergone RYGB prior to pregnancy with pregnant matched control participants to determine the following unknowns: 1) whether CGM metrics (time in, below, or above range) differ between women treated with RYGB and properly matched pregnant women during pregnancy; 2) whether CGM metrics vary during the course of pregnancy and the postpartum period; 3) whether prepregnancy, presurgery, or postsurgery characteristics are associated with PBH; and 4) whether exposure to PBH is associated with decreased fetal growth.
Research Design and Methods
Study Population
This study was conducted as part of the Bariatric Surgery and Consequences for Mother and Baby in Pregnancy (BAMBI) study, which is a prospective, observational study registered with ClinicalTrials.gov (identifier NCT03713060), consisting of pregnant women treated with RYGB prior to pregnancy as well as pregnant women matched for age, parity, and prepregnancy BMI, who were the control group. Recruitment was performed at Odense University Hospital (Odense, Denmark) from April 2019 to November 2021.
Women aged 18 to 45 years with a singleton pregnancy were included in the study before 14 weeks of gestation. Pregnancies resulting in miscarriage, termination, stillbirth, or delivery of a phenotypically abnormal infant were excluded. Tobacco consumption, alcohol consumption, severe psychiatric or medical comorbidities, pregestational diabetes, and overt diabetes at the beginning of the study were also causes of exclusion. However, women with GD in a previous pregnancy were not excluded. All matched control participants underwent a 2-hour, 75-g OGTT at 24 weeks of gestation.
Of the 23 women with RYGB, 14, 7, and 2 underwent surgery in the public health care system in the Region of Southern Denmark and at private hospitals in Denmark and in Belgium, respectively. We extracted the women’s medical history, including characteristics of RYGB surgery when possible, and validated the data by interview.
Continuous Glucose Monitoring
We monitored interstitial glucose (IG) levels for 10 days in gestational weeks 12–14, 24, and 34, as well as 4–6 weeks postpartum. The Dexcom G6 sensor (Dexcom, Inc., San Diego, CA) was inserted in the subcutaneous tissue on the abdomen, and it provided 288 readings per day (1 every 5 minutes). We excluded data during the initial 24 h of monitoring due to the suboptimal accuracy of the device. The mean, SD and coefficient of variation values, as well as time in range (TIR), time above range (TAR), TBR, and time with very low IG levels were determined. Calculations were performed for 24 h, daytime (06:00 to 23:59), and nighttime (00:00 to 05:59). According to the international consensus on glucose targets in pregnancy, an IG level in target range and a very low IG level were defined as 3.5–7.8 mmol/L and <3.0 mmol/L, respectively (21), based on data from type 1 diabetes, because established target values do not exist for pregnant women or women treated with RYGB.
Fetal Growth
Gestational age was based on ultrasonography performed before gestational week 14. SGA and large-for-gestational age (LGA) neonates were defined by birth weight below the 10th or above the 90th percentile, respectively, according to sex- and gestational age–adjusted Scandinavian growth references (22).
Statistical Analyses
Data were collected and managed using the Research Electronic Data Capture (REDCap) software hosted at Open Patient Data Explorative Network (OPEN), Odense University Hospital (Odense, Denmark). For continuous variables, medians and interquartile ranges (IQR) were calculated and compared between groups by the Wilcoxon rank-sum test. For TIR, TAR, and TBR, all CGM measurements were pooled for the two groups, and means and SDs were calculated. Categorical variables are presented as counts and percentages, and they were compared by the Fisher exact test. The Cuzick test was applied to test trends across ordered groups during the course of pregnancy. Trimester-specific 24-h IG profiles were calculated by pooling 30-min IG means of each participant followed by calculating group means of 30-min intervals. The mean IG level of the matched control participants was subtracted from the mean IG level of the women with RYGB to determine the mean difference between groups. The SEs of the difference in means were used to calculate 95% CIs.
To assess the predicting effects of prepregnancy, presurgery, and postsurgery clinical parameters on PBH in pregnancy after RYGB, regression analyses were performed. The prepregnancy clinical parameters included age at conception, prepregnancy BMI, parity, gravidity, and assisted reproductive conception. Surgical clinical parameters included age at surgery, surgery-to-conception interval, presurgery BMI, post-surgery nadir BMI, post-surgery nadir weight, excess weight loss, and %TWL. Additionally, timing of measurement during pregnancy and postpartum, as well as whether RYGB had been performed, were assessed as predictive factors. Owing to the repeated measurements over time for each participant, mixed-effects logistic regression was performed to determine whether time in hypoglycemia (<3.0 mmol/L and <3.5 mmol/L) was increased. All analyses were performed using Stata Statistical Software, Release 16 (StataCorp LLC, College Station, TX).
Ethical Approval
The BAMBI study was approved by the local ethics committee of the Region of Southern Denmark (27 July 2017; approval no. S-20160134). All participants provided written informed consent.
Results
In total, 50 pregnant women were consecutively enrolled in the BAMBI cohort. During the study period, 4 women (1 with RYGB and 3 matched control participants) dropped out of the study because of miscarriage (n = 2), fear of contracting COVID-19 (n = 1), and nausea or vomiting (n = 1). As a result, 46 women were included in the BAMBI cohort, with 23 women in each group (matched 1:1). The characteristics of the study population are described in Table 1. The groups were properly matched for BMI, parity, fertility treatment, smoking, alcohol consumption, former GD, and diabetes. Because matching ages was difficult, the women treated with RYGB were 4 years (IQR 0–7) older than the matched control participants.
Table 1.
Maternal characteristics and birth outcome
| Women with RYGB | Matched control group | P | |
|---|---|---|---|
| Participants, n (%) | 23 (50) | 23 (50) | |
| Maternal age, years | 35 (31–38) | 30 (26–32) | <0.01 |
| Prepregnancy BMI, kg/m2 | 32 (27–39) | 33 (28–40) | 0.88 |
| Prepregnancy BMI groups, n (%) | |||
| 18.5–24.9 | 2 (9) | 2 (9) | 1.00 |
| 25–29.9 | 4 (17) | 4 (17) | |
| 30–34.9 | 8 (35) | 8 (35) | |
| 35–39.9 | 4 (17) | 4 (17) | |
| ≥40 | 5 (22) | 5 (22) | |
| Nulliparity, n (%) | 14 (61) | 14 (61) | 1.00 |
| Mode of conception, assisted reproductive techniques, n (%) | 5 (22) | 2 (9) | 0.41 |
| Smokers, n (%) | 0 (0) | 0 (0) | 1.00 |
| Alcohol consumption, n (%) | 0 (0) | 0 (0) | 1.00 |
| Previous gestational diabetes, n (%) | 1 (4) | 1 (4) | 1.00 |
| Previous diabetes, n (%) | 0 (0) | 0 (0) | 1.00 |
| Age at surgery, years | 29 (27–35) | ||
| Surgery-to-conception interval, months | 30 (15–89) | ||
| Presurgery weight, kg | 130 (117–157) | ||
| Nadir weight postsurgery, kg | 81 (68–99) | ||
| Nadir BMI postsurgery, kg/m2 | 28 (24–33) | ||
| Presurgery BMI, kg/m2 | 45 (42–54) | ||
| Presurgery BMI groups, n (%) | |||
| 18.5–34.9 | 1 (4) | ||
| 35–39.9 | 3 (13) | ||
| 40–44.9 | 7 (30) | ||
| 45–49.9 | 5 (22) | ||
| ≥50 | 7 (30) | ||
| %TWL | 35 (31–45) | ||
| %EWL | 82 (70–104) | ||
| Gestational length, weeks+days | 39+3 (38+2–40+1) | 39+6 (39+0–40+2) | 0.24 |
| Birth weight, g | 3,365 (3,035–3,695) | 3,630 (3,355–3,920) | 0.08 |
| SGA, n (%) | 6 (26) | 1 (4) | 0.10 |
| LGA, n (%) | 3 (13) | 2 (9) | 1.00 |
| Admission to neonatal intensive care unit | 4 (17) | 4 (17) | 1.00 |
Data are presented as median (IQR) unless otherwise indicated. %EWL, percentage of excess weight loss.
The median surgery-to-conception interval was 30 months (IQR 15–89). Before entering pregnancy, the women treated with RYGB had lost 82% of their excess body weight, corresponding to a reduction in median BMI from 45 kg/m2 (IQR 42–54) presurgery to 32 kg/m2 (IQR 27–39) prepregnancy. For the majority of the RYGB group (78%), the studied pregnancy was the first after their surgery. Fewer women treated with RYGB (n = 4; 17%), compared with the matched control participants (n = 8; 35%; NS), achieved an appropriate GWG according to the Institute of Medicine’s recommendations (23). The women treated with RYGB, who did not achieve appropriate GWG were evenly distributed with insufficient and excessive GWG, whereas the majority of the matched control participants had excessive GWG (Supplementary Table 1).
Among the women treated with RYGB, 2 were exposed to recurrent events of severe PBH, and offspring of these women had neonatal hypoglycemia upon delivery. Only 1 woman had an unexplained syncope, which was clinically suspected to be caused by PBH but not verified. Several women reported symptoms suspicious of hypoglycemia prior to pregnancy, but were never diagnosed, and one woman struggled with symptoms for years but lacked blood glucose measurements. None of the women in the matched control group had symptomatic hypoglycemia before or during the study period. There were no significant differences in obstetric outcomes between the groups (Supplementary Table 1). During pregnancy, 2 women with RYGB and 1 matched control participant were diagnosed with GDM according to national guidelines by meal-related, self-monitoring of blood glucose and OGTT, respectively.
CGM During Pregnancy and Postpartum
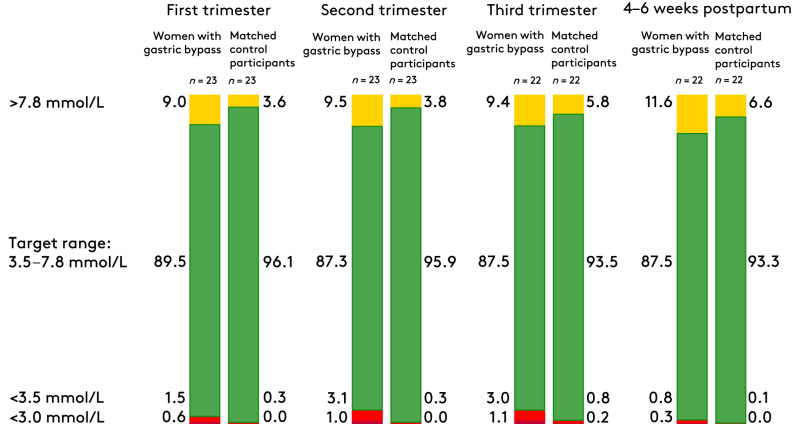
No significant differences were found when comparing the medians of mean IG levels, which ranged from 5.4 to 6.0 mmol/L for both groups during pregnancy and postpartum. Compared with matched control participants, TIR was significantly lower among women treated with RYGB, due to an increase in TAR and TBR (Table 2 and Fig. 1). TAR was increased by approximately twofold throughout pregnancy and the postpartum period among women treated with RYGB (Table 2 and Fig. 1). During the entirety of pregnancy and the postpartum period, 11 (48%) of the women treated with RYGB had a TBR greater than the international consensus for type 1 diabetes in pregnancy of 4%, compared with 2 (9%) of the matched control participants (P < 0.01) (20). Similarly, 11 (48%) of the women treated with RYGB spent increased time with IG levels <3.0 mmol/L vs. 1 (4%) in the matched control group (P < 0.01). TBR increased from the first trimester to the second and third trimesters, but TBR decreased in the postpartum period to less than TBR in early pregnancy (Table 2 and Fig. 1).
Table 2.
CGM metrics
| First trimester | Second trimester | Third trimester | Postpartum | |||||
|---|---|---|---|---|---|---|---|---|
| Women with RYGB | Matched control group | Women with RYGB | Matched control group | Women with RYGB | Matched control group | Women with RYGB | Matched control group | |
| Participants, n | 23 | 23 | 23 | 23 | 22 | 23 | 22 | 22 |
| Timing of CGM, weeks+days | 14+4 (13+6–15+3) | 14+3 (13+6–15+4) | 24+5 (24+1–25+5) | 24+4 (24+0–26+0) | 34+1 (32+3–35+4) | 34+3 (34+0–35+4) | 5+0 (4+2–5+4) | 5+0 (4+4–5+4) |
| Days CGM worn on each occasion | 8.4 (7.5–8.8) | 8.6 (7.8–8.7) | 8.0 (6.6–8.7) | 8.7 (8.4–8.8) | 8.4 (6.6–8.7) | 8.7 (7.0–8.7) | 7.6 (6.9–8.7) | 8.7 (8.3–8.7) |
| Active CGM time, % | 97 (93–100) | 100 (97–100) | 98 (92–100) | 100 (97–100) | 97 (94–100) | 100 (99–100) | 90 (86–99) | 100 (97–100) |
| Mean glucose level, mmol/L | 5.8 (5.3–6.1) | 5.9 (5.5–6.2) | 5.4 (5.2–5.9) | 5.7 (5.3–6.0) | 5.6 (5.3–5.9) | 5.8 (5.4–6.5) | 6.0 (5.4–6.5) | 6.0 (5.7–6.7) |
| Glycemic variability (coefficient of variation) | 25 (20–28) | 14 (13–16) | 27 (22–31) | 15 (14–17) | 26 (23–30) | 16 (15–19) | 23 (19–26) | 13 (11–15) |
| Proportion of time, mean (SD) | ||||||||
| Above target (>7.8 mmol/L) | 9.0 (5.1) | 3.6 (3.9) | 9.5 (5.4) | 3.8 (4.8) | 9.4 (5.9) | 5.8 (6.0) | 11.6 (6.7) | 6.6 (8.8) |
| In target (3.5–7.8 mmol/L) | 89.5 (5.0) | 96.1 (3.7) | 87.3 (6.4) | 95.9 (4.7) | 87.5 (6.5) | 93.5 (5.7) | 87.5 (6.4) | 93.3 (8.7) |
| Below target (<3.5 mmol/L) | 1.5 (2.5) | 0.3 (0.7) | 3.1 (4.5) | 0.3 (0.3) | 3.0 (4.9) | 0.8 (1.6) | 0.8 (1.1) | 0.1 (0.2) |
| Very low (<3.0 mmol/L) | 0.6 (1.1) | 0.0 (0.0) | 1.0 (1.4) | 0.0 (0.1) | 1.1 (2.3) | 0.2 (0.5) | 0.3 (0.4) | 0.0 (0.0) |
| Participants with increased time, n (%) | ||||||||
| Below target (4%) | 4 (17) | 0 (0) | 6 (26) | 0 (0) | 4 (18) | 2 (9) | 1 (5) | 0 (0) |
| Very low (1%) | 5 (22) | 0 (0) | 6 (26) | 0 (0) | 4 (18) | 1 (4) | 1 (5) | 0 (0) |
Data are presented as median (IQR) unless otherwise indicated. Significant differences are in bold.
Figure 1.
Distribution of CGM data according to trimester or postpartum. Data are reported in percentages. Adapted from the international consensus on glucose targets in pregnancy with type 1 diabetes, because no target values exist on glucose range in pregnancies after RYGB (21).
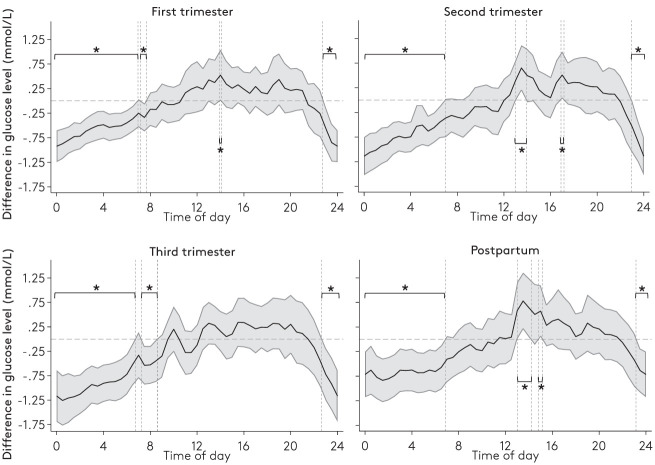
Compared with the matched control group, the glycemic variability (coefficient of variation) was significantly higher among women treated with RYGB, as a result of increased diurnal glycemic variability (Supplementary Table 2). Women treated with RYGB had a significantly lower nocturnal IG level (Fig. 2), and their mean nocturnal IG level significantly decreased during the course of pregnancy (Fig. 2 and Supplementary Table 2). In the postpartum period, the median of mean nocturnal IG levels increased to a level higher than that of the first trimester (Fig. 2 and Supplementary Table 2). Among the matched control participants, the median of mean nocturnal IG levels significantly increased from the first trimester to the postpartum period.
Figure 2.
Difference in mean temporal glucose levels across the 24-h day between women with RYBG and matched control participants. Solid lines with 95% CIs (gray areas) represent the mean IG level of the women with RYBG minus the mean IG level of the matched control participants. The CI surrounding the mean is based on the SE of the difference in means between groups. Dashed zero line indicates no difference in means. *Significant differences with 95% CIs.
Table 2 shows that there were no differences in the timing of CGM between the groups, and the application of CGM adhered to the BAMBI study protocol. The percentage of active CGM time was significantly lower among women treated with RYGB throughout pregnancy and the postpartum period compared with the matched control group, but the active CGM time exceeded the minimum recommendation of 70% by the international consensus on all occasions (21).
Risk Factors for Hypoglycemia
Compared with matched control participants, women treated with RYGB before pregnancy had an increased risk of increased time below the international consensus target (both <3.5 and <3.0 mmol/L; odds ratio [OR] 10.2; 95% CI 2.0–51.8). Increased prepregnancy body weight was associated with less time in hypoglycemia (OR 0.9; 95% CI 0.8–0.9).
Univariate analysis of women treated with RYGB indicated that increased time in hypoglycemia was associated with a longer surgery-to-conception interval (OR 89 [IQR 30–123] vs. 21 [IQR 9–35] months; P = 0.01), a lower nadir weight postsurgery (OR 77 [IQR 62–92] vs. 85 [IQR 80–106] kg; P = 0.04), and greater weight loss (%TWL 45% [IQR 36%–52%] vs. 33% [IQR 29%–35%]; P = 0.01) compared with women without increased time in hypoglycemia (Supplementary Table 3). In the multivariate analysis, none of the variables remained as independent predictive factors for hypoglycemia.
Hypoglycemia and Fetal Growth
All the neonatal anthropometrics were lower (i.e., birth weight, abdominal circumference, and length) in the offspring of women treated with RYGB, but these differences were not significant (Supplementary Table 1). The median differences of the median birth weights were 275 g (IQR −640 to 75). Compared with 1 neonate (4%) of matched control participants, 6 neonates (26%) of women treated with RYGB were SGA (NS), whereas 2 neonates (9%) of matched control participants were LGA compared with 3 neonates (13%) (NS) of women treated with RYGB.
Women treated with RYGB who gave birth to SGA neonates spent slightly increased time in both low and very low ranges compared with those who delivered appropriate-for-gestational age and LGA neonates. Furthermore, these women had a decreased TAR (Supplementary Figure 1). In contrast, women treated with RYGB who gave birth to LGA neonates spent less time in the low and very low range as well as more TAR.
Supplementary Figure 2A shows that the women who gave birth to SGA neonates, among those treated with RYGB, had lower mean IG levels during both the second and third trimesters, compared with those who did not give birth to SGA neonates. In contrast, compared with women treated with RYGB who did not give birth to LGA neonates, women who gave birth to LGA neonates had higher IG levels on most of the days when levels were measured during the second and third trimesters (Supplementary Figure 2B). In the postpartum period, the women who gave birth to SGA neonates had lower mean IG levels almost 24 h/day, with levels in 24% of the 24 h being significantly lower. Contrarily, the women who gave birth to LGA neonates had higher mean IG levels, with 5% of levels being significantly higher.
Conclusions
To our knowledge, this is the first study on CGM dynamics throughout pregnancy and the postpartum period in women treated with RYGB. Our findings demonstrated that women with RYGB had decreased TIR throughout pregnancy and postpartum compared with matched control participants, predominantly due to a twofold increase in TAR. Hypoglycemic metrics were slightly increased in mid and late pregnancy. Furthermore, the findings demonstrated a decreasing nocturnal IG level during the course of pregnancy in women with RYGB, with an increase in the postpartum period. Women with longer surgery-to-conception intervals, lower nadir weight, and greater weight loss after RYGB had increased time in hypoglycemia. Finally, women who gave birth to SGA neonates had slightly increased TBR than those who did not.
Remarkably, the percentage of time women with RYGB spent above range corresponds to previously reported data for women with GD(24), but only 2 women with RYGB were diagnosed with GDM by capillary blood glucose profiles. Screening of GD in these women is lacking evidence, resulting in potential underdiagnosis (25). These results call for further investigation.
Owing to the enhanced insulin sensitivity in early to mid-pregnancy (5,26,27), one may expect the risk of PBH to be increased at this time point. Surprisingly, an increase in both TBR and time spent in the very low range was evident in the second and third trimesters compared with the first trimester among women treated with RYGB. Furthermore, nocturnal IG levels significantly decreased as pregnancy advanced. Because insulin resistance is expected to decrease in the postpartum period (24), one may expect an increase in the hypoglycemic metrics. Instead, the median nocturnal IG levels and TAR values increased to levels higher than that of early pregnancy, and the hypoglycemic metrics decreased. In contrast, the matched control participants experienced a modest decrease in TIR during pregnancy, mainly due to an increase in TAR, corresponding to a decrease in insulin sensitivity. Thus, even though affected by pregnancy, the metabolic changes of RYGB significantly affect glucose profiles in pregnancy.
Use of CGM is expanding (21), but easily accessible metrics and their reference values are fundamental prerequisites for CGM use in clinical practice. In 2019, an international consensus issued reference values for TIR in pregnancy (21) but only included reference values for type 1 diabetes in pregnancy, which may vary from the optimal targets for the pregnant women without diabetes in the BAMBI cohort. The evidence for TIR for healthy pregnant women, women with type 2 diabetes in pregnancy, and those with GD is limited (21). Supplementary Table 4 displays the CGM metrics for the matched control participants without GD for future reference of CGM metrics in healthy, obese, pregnant women.
In the third trimester, which had the worst clinical target adherence, the matched control participants had a TIR, TAR, and TBR of 93.5%, 5.8%, and 0.8%, respectively. Given that the women were not encouraged to change their lifestyle as part of the BAMBI protocol, the 3.5–7.8 mmol/L target seemed easily achievable during pregnancy for the matched control participants despite the majority of the women being obese.
In accordance with a recent study in a nonpregnant, post bariatric population (12), we found that an increased weight loss and lower nadir weight post surgery were associated with increased time in hypoglycemia. Furthermore, younger age and female sex have been suggested as independent predictive factors of PBH (12,13). We found no difference in age at surgery for the women with and without increased time in hypoglycemia. Because of the nature of the BAMBI study pertaining to pregnancy, the BAMBI cohort represents the younger part of the population treated by RYGB. According to the Sixth International Federation for the Surgery of Obesity and Metabolic Disorder 2021 registry report covering 50 countries worldwide, the median age for both women and all patients undergoing bariatric surgery is 42 years (IQR 33–51) (28), which may prevent differences within our selected population. Without detection of independent predictive factors of hypoglycemia in pregnancy after RYGB, the presurgery counseling of women who want to undergo bariatric surgery before getting pregnant is difficult. Nevertheless, longer surgery-to-conception interval, a lower nadir weight, and greater weight loss may serve as warning signs and should increase awareness for clinicians.
According to the Pedersen hypothesis, maternal hyperglycemia results in increased fetal excretion of insulin, which, in turn, acts as a growth factor and causes fetal overgrowth (29). Consequently, women with diabetes are at increased risk of giving birth to LGA neonates (30). Similar to Gohier et al. (18), we found that LGA neonates were associated with an increased TAR among pregnant women treated with RYGB and the matched control participants in the present study. However, it remains unclear whether hypoglycemia causes decreased fetal growth, but an increased risk of fetal growth restriction among women who have undergone RYGB has been established (14). Gohier et al. (18) reported that SGA is associated with reduced TBR, suggesting that hypoglycemia is not a cause of decreased fetal growth. In contrast to the results of Gohier et al., we found a slightly increased TBR and very low range among the women treated with RYGB who gave birth to SGA neonates (Supplementary Fig. 1). This inconsistency may be caused by hyperglycemia often preceding hypoglycemia in these women. The women who did not give birth to SGA neonates in the study by Gohier et al. (18) spent more TAR compared with the women who gave birth to SGA neonates. Even though the women with RYGB spent approximately twofold increased time in hyperglycemia compared with control participants in the present study, they still delivered fewer LGA neonates than SGA neonates and had an increased risk of delivering SGA neonates compared with matched control participants. Thus, even a small increase in time spent in hypoglycemia may outweigh hyperglycemia in relation to fetal growth.
In addition to the increased risk of SGA, a meta-analysis has shown that birth weight is 226 g lower among women treated with RYGB compared with control participants (14). Our data yielded a median of differences in median birth weights of 275 g (IQR −640 to 75). Nocturnal hyperglycemia has been reported among pregnant women with GD who gave birth to LGA neonates (26). In the present study, the women treated with RYGB had significantly lower nocturnal (23:00 to 08:00) glucose levels, corresponding to at least one-third of the day throughout pregnancy (Fig. 2). This phenomenon may explain why the neonates were smaller than average without necessarily being SGA.
Interestingly, the present results show that women treated with RYGB who had SGA neonates had lower mean IG levels, whereas women treated with RYGB who had LGA neonates had higher mean IG levels in both the second and third trimesters (Supplementary Fig. 2A and B). These tendencies persisted in the postpartum period. Because the intrauterine environment no longer affects the neonate at this point, the women may be predisposed for a low or high IG level before pregnancy, and perhaps these predisposing factors cause both hypoglycemia and decreased fetal growth. Nonetheless, it is likely that both spending TBR and TAR has consequences for fetal growth. Even though this study was not powered for fetal outcomes, the present results raise concern and warrant larger prospective studies. Furthermore, these findings encourage a reduction in time spent in hypoglycemia, which, in a clinical setting, would entail raising awareness of symptoms among these women and offering them relevant dietary advice.
A strength of this study is the large amount of data acquired by application of CGM in each trimester and postpartum. Because knowledge of CGM metrics in normal pregnancy is lacking, the inclusion of a matched control group was important. Because the accuracy of glucose monitors in the hypoglycemic range have previously been questioned, we used the Dexcom G6 sensor, which has been tested for accuracy in pregnant women with diabetes and found to have acceptable accuracy in the hypoglycemic range (31).
The most important limitation was that the CGM sensor can indicate false hypoglycemic IG levels when exposed to pressure (31,32), which may occur in late pregnancy if placed inappropriately on the abdomen. This may potentially account for a few of the women in the control group during the third trimester, but this bias was not suspected in the RYGB group, in which most women were marked by their substantial weight loss with loose skin on the abdomen. Nonetheless, given that it is impossible to distinguish between this phenomenon and asymptomatic PBH in the women with RYGB, periods suspicious of erroneously low IG values were not excluded for either group. Because the Dexcom G6 sensor was not approved for application on the upper extremities at the initiation of the study, all women wore the Dexcom G6 sensor on the abdomen for all measurements. In addition, because the Dexcom G6 sensor’s urgent low IG alarm was impossible to disable, the women were alerted if their IG level dropped below 3.1 mmol/L, leading to an underestimation if the women responded by ingesting carbohydrates. For future studies, blinded devices should be preferred. To diminish the risk of compliance issues with the extensive BAMBI protocol, we did not collect data on dietary intake. Moreover, the surgical techniques may have undergone minor adaptations over time, indicating that alterations in the operation procedure may confound the finding that TBR increased with longer surgery-to-conception interval.
A nonpregnant control group consisting of either the recruited women before pregnancy or after lactation period, or female nonpregnant control participants matched for BMI and age, would have allowed us to clarify whether the hypoglycemic metrics are lower before pregnancy. However, the hypoglycemic metrics may have been higher and then decreased in early pregnancy before increasing in late pregnancy.
The BAMBI study showed increased glucose excursions in pregnancy after RYGB, with increased time spent in both hyper- and hypoglycemia compared with control participants. Evidence-based guidelines for screening of GD are warranted, given that the women with RYGB had twofold increased TAR. Moreover, the study adds to the growing evidence of PBH as an appreciable complication in pregnancy after RYGB. Research in larger cohorts should investigate whether hypoglycemia during pregnancy in women with RYGB is associated with decreased fetal growth. The use of sleeve gastrectomy, which results in fewer gastrointestinal alterations and potentially lower risk of hypoglycemia, has recently increased, surpassing that of RYGB, thereby indicating the need for studies on the effects of sleeve gastrectomy during pregnancy to determine the optimal procedure for women of childbearing age.
Article Information
Acknowledgments. The authors acknowledge the great efforts made by the women of the BAMBI cohort, making this study possible. The authors thank nurse Vibe Vestergaard as well as medical laboratory technicians Lone Hansen and Charlotte Bøtchiær Fage Olsen at Steno Diabetes Center Odense, Odense University Hospital (Odense, Denmark), for assistance during the BAMBI study period.
Funding. L.L.S. received funding from the Region of Southern Denmark. L.R.M. received funding from the Danish Diabetes Academy, founded by the Novo Nordisk Foundation (grant NNF17SA).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
The funders were not involved in the study design, data collection, data analysis, data interpretation, and writing of the report, and they did not impose any restrictions regarding the publication of the report.
Author Contributions. L.L.S., R.K.S., L.L.T.A., C.B.J., and D.M.J. designed the study protocol. L.L.S. and L.R.M. wrote the manuscript, which all authors critically reviewed. L.L.S., L.L.T.A., C.A.V., C.B.J., and D.M.J. screened and enrolled participants. L.L.S. obtained participants’ consent and collected outcome data, prepared the data for analysis, and analyzed the data in collaboration with a biostatistician. L.L.S. and D.M.J. are the guarantors of this work, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were reported in an oral presentation at the Diabetes Pregnancy Study Group, Madrid, Spain, 8–11 September 2022, and in a short oral communication presented at the European Association for the Study of Diabetes 58th Annual Meeting, Stockholm, Sweden, 20–23 September 2022. An oral presentation was presented at the 2022 meeting of the International Association of Diabetes and Pregnancy Study Groups, Sydney, New South Wales, Australia, 17–20 November 2022.
Footnotes
Clinical trial reg. no. NCT03713060, clinicaltrials.gov
See accompanying article, p. 500.
This article contains supplementary material online at https://doi.org/10.2337/figshare.21543588.
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air.
References
- 1. Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol 2018;218:573–580 [DOI] [PubMed] [Google Scholar]
- 2. Johansson K, Cnattingius S, Naslund I, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med 2015;372:814–824 [DOI] [PubMed] [Google Scholar]
- 3. Smith EP, Polanco G, Yaqub A, Salehi M. Altered glucose metabolism after bariatric surgery: what’s GLP-1 got to do with it? Metabolism 2018;83:159–166 [DOI] [PubMed] [Google Scholar]
- 4. Raverdy V, Baud G, Pigeyre M, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: a five year longitudinal study. Ann Surg 2016;264:878–885 [DOI] [PubMed] [Google Scholar]
- 5. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007;30(Suppl. 2):S112–S119 [DOI] [PubMed] [Google Scholar]
- 6. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 1999;180:903–916 [DOI] [PubMed] [Google Scholar]
- 7. Sonagra AD, Biradar SM, K D, Murthy D S J. Normal pregnancy- a state of insulin resistance. J Clin Diagn Res 2014;8:CC01–CC03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stentebjerg LL, Madsen LR, Støving RK, et al. Hypoglycemia in pregnancies following gastric bypass-a systematic review and meta-analysis. Obes Surg 2022;32:2047–2055 [DOI] [PubMed] [Google Scholar]
- 9. Freitas C, Araújo C, Caldas R, Lopes DS, Nora M, Monteiro MP. Effect of new criteria on the diagnosis of gestational diabetes in women submitted to gastric bypass. Surg Obes Relat Dis 2014;10:1041–1046 [DOI] [PubMed] [Google Scholar]
- 10. Andrade HF, Pedrosa W, Diniz MFHS, Azaredo Passos VM. Adverse effects during the oral glucose tolerance test in post-bariatric surgery patients. Arch Endocrinol Metab 2016;60:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feichtinger M, Stopp T, Hofmann S, et al. Altered glucose profiles and risk for hypoglycaemia during oral glucose tolerance testing in pregnancies after gastric bypass surgery. Diabetologia 2017;60:153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bienvenot R, Sirveaux MA, Nguyen-Thi PL, Brunaud L, Quilliot D. Symptomatic hypoglycemia after gastric bypass: incidence and predictive factors in a cohort of 1,138 consecutive patients. Obesity (Silver Spring) 2021;29:681–688 [DOI] [PubMed] [Google Scholar]
- 13. Lee CJ, Brown TT, Schweitzer M, Magnuson T, Clark JM. The incidence and risk factors associated with developing symptoms of hypoglycemia after bariatric surgery. Surg Obes Relat Dis 2018;14:797–802 [DOI] [PubMed] [Google Scholar]
- 14. Akhter Z, Rankin J, Ceulemans D, et al. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PLoS Med 2019;16:e1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akhter Z, Heslehurst N, Ceulemans D, Rankin J, Ackroyd R, Devlieger R. Pregnancy after bariatric surgery: a nested case-control study of risk factors for small for gestational age babies in AURORA. Nutrients 2021;13:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grandfils S, Demondion D, Kyheng M, et al. Impact of gestational weight gain on perinatal outcomes after a bariatric surgery. J Gynecol Obstet Hum Reprod 2019;48:401–405 [DOI] [PubMed] [Google Scholar]
- 17. Gascoin G, Gerard M, Sallé A, et al. Risk of low birth weight and micronutrient deficiencies in neonates from mothers after gastric bypass: a case control study. Surg Obes Relat Dis 2017;13:1384–1391 [DOI] [PubMed] [Google Scholar]
- 18. Gohier H, Guyard-Boileau B, Tuyeras G, et al. Glucose abnormalities and inappropriate weight gain predict negative pregnancy outcomes after gastric bypass surgery. Obes Surg 2021;31:3123–3129 [DOI] [PubMed] [Google Scholar]
- 19. Maric T, Kanu C, Muller DC, Tzoulaki I, Johnson MR, Savvidou MD. Fetal growth and fetoplacental circulation in pregnancies following bariatric surgery: a prospective study. BJOG 2020;127:839–846 [DOI] [PubMed] [Google Scholar]
- 20. Rottenstreich A, Elazary R, Ezra Y, Kleinstern G, Beglaibter N, Elchalal U. Hypoglycemia during oral glucose tolerance test among post-bariatric surgery pregnant patients: incidence and perinatal significance. Surg Obes Relat Dis 2018;14:347–353 [DOI] [PubMed] [Google Scholar]
- 21. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848 [DOI] [PubMed] [Google Scholar]
- 23. Institute of Medicine (US), National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines . Weight Gain During Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, Eds. Washington, DC: National Academies Press (US); 2010. [Google Scholar]
- 24. Law GR, Alnaji A, Alrefaii L, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care 2019;42:810–815 [DOI] [PubMed] [Google Scholar]
- 25. Shawe J, Ceulemans D, Akhter Z, et al. Pregnancy after bariatric surgery: consensus recommendations for periconception, antenatal and postnatal care. Obes Rev 2019;20:1507–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Draznin B, Aroda VR, Bakris G, et al.; American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee . 15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl. 1):S232–S243 [DOI] [PubMed] [Google Scholar]
- 27. Scott EM, Murphy HR, Kristensen KH, et al. Continuous glucose monitoring metrics and birth weight: informing management of type 1 diabetes throughout pregnancy. Diabetes Care 2022;45:1724–1734 [DOI] [PubMed] [Google Scholar]
- 28. Brown AB, Kow L, Shikora S, et al. The 6th IFSO 2021 Registry Report. Accessed 10 July 2022. Available at https://www.ifso.com/pdf/ifso-6th-registry-report-2021.pdf
- 29. Pedersen J. Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Ugeskr Laeger 1952;114:685. [PubMed] [Google Scholar]
- 30. Law GR, Ellison GT, Secher AL, et al. Analysis of continuous glucose monitoring in pregnant women with diabetes: distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care 2015;38:1319–1325 [DOI] [PubMed] [Google Scholar]
- 31. Castorino K, Polsky S, O’Malley G, et al. Performance of the Dexcom G6 continuous glucose monitoring system in pregnant women with diabetes. Diabetes Technol Ther 2020;22:943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dexcom . Why does my Dexcom G6 say I’m low at night when my blood glucose meter shows otherwise? 2021. Accessed April 2022. Available from: https://www.dexcom.com/faqs/why-does-my-dexcom-g6-say-low-at-night-my-blood-glucose-does-not