Abstract
Background
Postoperative cognitive dysfunction (POCD) is a common postoperative complication in elderly patients. The strong stress response causing by surgical trauma can induce POCD. We hypothesized that stellate ganglion block (SGB) can provide the neuroprotection to POCD by regulating the neuroendocrine response.
Methods
Sprague-Dawley male rats, 18–20 months old and weighing 550–650 g were assigned into four groups: sham surgery group (Sham), sham surgery + saline group (Sham + NS), surgery group (Surgery), and surgery + SGB group (Surgery + SGB). The change of body weight, heart rate variability analysis, behavior testing, neuronal damage, inflammatory response, neuroendocrine hormone level were evaluated by their corresponding methods.
Results
The results showed that SGB can reduce the number of both types of errors in the postoperative eight-arm maze assay, attenuate neural structural damage, inhibit neuroapoptosis, suppress inflammatory responses, increase the release of neurotrophic factors, accelerate postoperative weight recovery, and promote postoperative recovery in rats. Most importantly, SGB reduced the level of neuroendocrine hormone of TH, Cyp11b1, CRH, and SGB also activated dorsal motor nucleus of vagus (detected by c-fos immunohistochemistry).
Conclusions
Our findings indicated that SGB could be a neuroprotective therapy for the cognitive dysfunction induced by exploratory laparotomy model of POCD, which might be attributable for balancing the autonomic nervous system, regulating hypothalamic-pituitary-adrenal (HPA) axis system.
Keywords: Postoperative cognitive dysfunction, Stellate ganglion block, Neuroendocrine stress response, Autonomic nervous system, HPA axis
1. Introduction
Postoperative cognitive dysfunction (POCD) is a common postoperative complication in elderly patients and includes various degrees of impairment in cognitive function, with memory impairment being the most frequent symptom [[1], [2], [3]]. Most patients with POCD will gradually recover. However, some patients experience long-term cognitive function changes, which have severe adverse effects on the patients’ postoperative recovery and impose a heavy burden on their families and society [3]. The pathogenesis of POCD is intricate and complex, and numerous risk factors, including advanced age, pre-operative cognitive function, duration of surgery, surgical trauma stress, anesthetic drug neurotoxicity, and internal environmental disturbances may contribute to the development of POCD [[4], [5], [6]]. Clinically, more than 30% of elderly patients undergoing non-cardiac surgery have postoperative POCD and more than 50% of elderly patients develop postoperative cognitive decline 3 days after abdominal surgery [7,8]. Considering the aging population, the number of elderly patients undergoing surgery is increasing yearly. Therefore, reducing the occurrence of POCD in elderly patients is of increasing importance.
The stellate ganglion is a sympathetic ganglion formed by the fusion of the inferior cervical sympathetic ganglion and the first thoracic sympathetic ganglion [9]. Stellate ganglion block (SGB) is widely used in clinical practice to treat painful and non-painful diseases since almost a century [9,10]. SGB causes vasodilation of sympathetic innervation and regulates the autonomic nervous, endocrine, and immune systems to maintain homeostatic balance [11]. Surgical trauma can induce a stress response and trigger a strong neuroendocrine response in the sympathetic-adrenal medulla and hypothalamic-pituitary-adrenal cortex (HPA) systems, which increases excitability [12,13]. This stress response is one of the causes of POCD. In a previous study, we showed that SGB modulates the HPA axis and inhibits sympathetic excitation [14]. We hypothesized that SGB could be applied to prevent POCD.
2. Materials and methods
2.1. Ethical statement
The study was approved by the Animal Care Committee of Harbin Medical University (China) and followed national guidelines (Guidelines on Administration of Laboratory Animals in China and Guidelines on the Humane Treatment of Laboratory Animals in China).
Experiments were conducted with Sprague-Dawley male rats, 18–20 months old and weighing 550–650 g. The animals were purchased from Vital River Laboratory Animal Sales and housed in the Animal Experiment Center of the Second Hospital of Harbin Medical University.
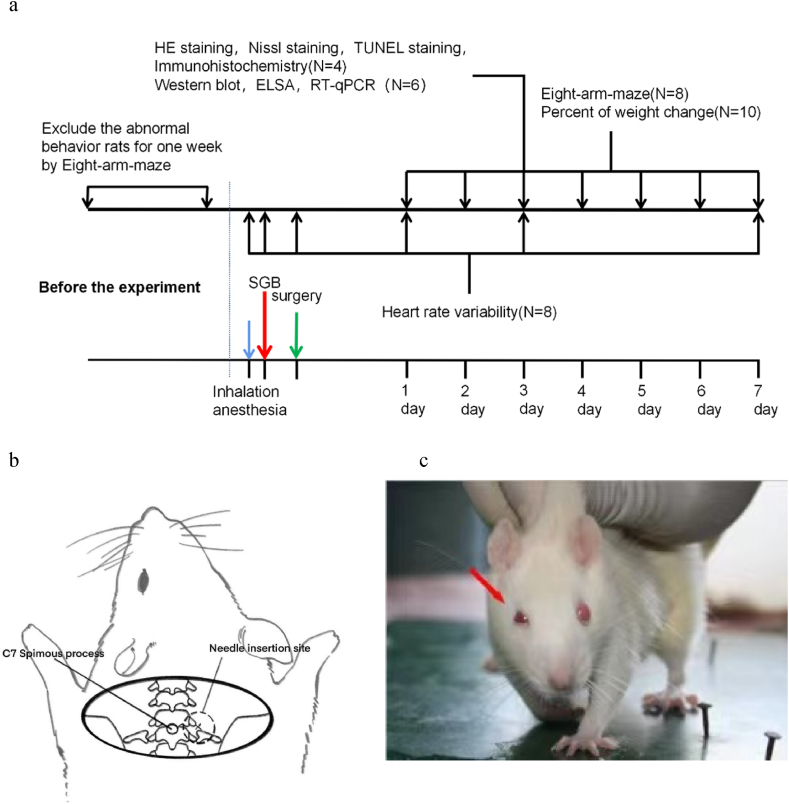
184 animals were randomly divided into four groups: sham surgery group (Sham), sham surgery + saline group (Sham + NS), surgery group (Surgery), and surgery + SGB group (Surgery + SGB). The experimental procedure is shown in Fig. 1a.
Fig. 1.
Experimental flow. (a) Experimental design and timeline; (b) Schematic of the bony markers and needle entry points for the stellate ganglion block; (c) Successful induction of the stellate ganglion block (arrow in the figure indicates ptosis).
2.2. Stellate ganglion block
Rats were anesthetized with 8% sevoflurane, placed in the prone position, marked at the spinous process of the seventh cervical vertebra (C7), and blocked in a paramedian approach to the stellate ganglion [15] (Fig. 1b). The saline group was given 0.2 mL of saline, and the SGB group was given 0.2 mL of 0.25% bupivacaine hydrochloride. Rats recovered within a few minutes, the success of the block was marked by eyelid ptosis (Fig. 1c).
2.3. Exploratory laparotomy
Rats were anesthetized with 8% sevoflurane in a transparent airtight plastic box, with tracheal intubation and an inhalation oxygen concentration of 60% air. We adjusted the sevoflurane concentration to 3–4%, and adjusted the sevoflurane concentration at any time mainly according to the intraoperative conditions of animals, during surgery. Exploratory laparotomy and sham surgery were performed as described by Martin et al. [16]. The sham group did not operate on the animals, but the anesthesia induction and postoperative medication were consistent with the surgery group. 0.2% ropivacaine was used for incision infiltration anesthesia after surgery.
2.4. Eight-arm maze
An eight-arm radial maze (Xinruan Information Technology Co. Ltd., Shanghai, China) was used to test the spatial memory of rats. Four arms were selected as target arms, food was placed, and the rats were trained. When the total number of working memory errors and reference memory errors for five consecutive training sessions did not exceed two, the rats were entered into the group to start the experiment. Working memory errors and reference memory errors were observed and recorded for 7 days after the operation [17].
2.5. Heart-rate variability testing
Standard lead II electrocardiograms were recorded before and during the procedure. Heart-rate variability (HRV) was analyzed with frequency domain analysis (AcqKnowledge 4.0 software, BIOPAC Systems Inc., Santa Barbara, CA, USA). The low frequency (LF) component reflects sympathetic and parasympathetic heart rate regulation. The high frequency (HF) component reflects parasympathetic regulation of heart rate. The LF/HF ratio was used as an indicator of cardiac autonomic balance. An elevated ratio indicated sympathetic dominance, whereas a decreased ratio suggested parasympathetic dominance. HRV was recorded at three time points: after anesthesia (T1), after SGB (T2), and after surgery (T3). In addition, HRV analysis was performed at postoperative day 1 (POD1) (T4), POD3 (T5), and POD7 (T6) before euthanasia.
2.6. Histological analysis
On POD1 and POD3, rats were anesthetized via intraperitoneal injection of 3% pentobarbital (30 mg/kg) and perfused with fixative. The heart was exposed and a perfusion needle was inserted into the left ventricle for saline perfusion. The right auricula dextra was removed to drain blood. The fixation buffer consisted of 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) buffer (pH 7.4) and was applied when the liver turned white. Brain tissue was extracted after the limb twitches stopped and each organ was sclerosed and preserved in the fixation buffer. Based on the rat brain atlas, hippocampal tissue was located and 5 μm coronal sections were prepared and embedded in paraffin-embedded for further test.
2.7. Nissl staining
For Nissl staining, 10-μm-thick rats brain slices were dewaxed, immersed in a staining solution in water for 30 min, and rinsed. Subsequently, sections were dehydrated in 95% anhydrous ethanol, permeabilized in xylene, and stained for Nissl vesicles or rough endoplasmic reticulum. Then sections were placed in neutral resin to observe the results and number of Nissl vesicles.
2.8. TUNEL staining
The in-situ cell death assay kit was used according to the manufacturer's instructions: 5-μm sections of hippocampal tissue were deparaffinized twice with xylene for 10 min each and permeabilized by ethanol gradient elution (100%, 95%, 90%, 80%, and 70%). Sections were treated with 50 μL TUNEL reaction solution for 60 min at 37 °C in a humid dark box. Subsequently, 50 μL of streptavidin-horseradish peroxidase working solution was added to the dark box for 30 min. Nuclei were fluorescently stained with DAPI and then dehydrated before the sections were decolorized and fixed. Apoptosis rates were examined and images were acquired using a light microscope (Nikon Eclipse Ti SR, Japan) at 400× magnification.
2.9. Immunohistochemistry
Immunohistochemical staining was performed on 5 μm paraffin sections. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 10 min. Sections were incubated with a primary antibody (Abcam 1:400, ab208942) at room temperature for 2 h. After washing the sections with PBS, they were incubated with biotinylated goat anti-rabbit IgG secondary antibody for 30 min. Immunoreactivity was detected by DAB (Boster) and hematoxylin re-staining. The location of the dorsal motor nucleus of the vagus nerve (DMV) at 14.06 mm was determined via stereotaxic coordinates. C-fos expression was measured in the vagal nucleus and Iba-1 expression was measured in the hippocampus CA1.
2.10. Inflammatory factor assays
2.10.1. Enzyme-linked immunosorbent assay (ELISA)
Rats were killed for blood sampling via cardiac puncture on POD3 and decapitated to obtain hippocampal tissue. Hippocampal tissue and plasma levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 were measured using ELISA (rat TNF-α, rat IL-1β, and rat IL-6 ELISA Kits, Shanghai Enzyme Link, China) according to the manufacturer's instructions.
2.11. Related protein assays
2.11.1. Western blot
Rats were decapitated to obtain hippocampal tissue on POD3. Brain tissue was immediately stored at −80 °C, followed by solubilization in lysis buffer and protease inhibitor (Sigma) at 4 °C for 1 h. After the samples were centrifuged at 12,000 g/min for 10 min, the supernatant was collected, and protein concentrations were calculated using a BCA protein assay kit (Bio-Rad). Equal amounts of proteins were loaded on SDS-PAGE gels and electrophoresis products were transferred to PVDF membranes (Invitrogen) according to the wet transfer procedure (transfer buffer: 25 mM Tris, 192 mM glycine, 20% methanol). Membranes were closed with 5% skim milk in TBST buffer and PVDF membranes were incubated with primary antibodies against caspase-3 (1:1000, Abcam), and brain-derived neurotrophic factor [BDNF (1:1000; Abcam)] at 4 °C, followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (1:5000) for 1 h at 37 °C. The final product was measured using chemiluminescent substrates. β-Actin was used as an internal reference control.
2.12. Neuroendocrine response assays
2.12.1. RT-qPCR
Tyrosine hydroxylase (TH), 11β-hydroxylase (Cyp11b1), corticotropin-releasing hormone (CRH), and c-fos were measured by quantitative real-time polymerase chain reaction (RT-qPCR) (see Supplementary Information 1 for primer sequences). Rat paraventricular nucleus and adrenal RNA was extracted with the Multisource Total RNA Miniprep Kit (AP-MN-MS-RNA-50, Axygen, USA) and then reverse transcribed into cDNA using HiScriptII reverse transcriptase (R223-01, Vazyme, China). Reverse transcription reaction products were determined via the Roche Fluorescence Quantification Kit (FastStart Universal SYBR Green Master, 04913850001, Roche, Swiss) and a Bio-Rad CFX96 assay system (Bio-Rad, CA, USA). Gene expression before the experiment was calculated using the blank group as a standard. Each experiment was conducted three times and gene expression was determined via the 2−ΔΔCT method using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference. Results were expressed as fold increase relative to normal levels.
2.13. Statistical analysis
Statistical analyses were performed using SPSS (version 20.0, Chicago, IL, USA). Data were expressed as mean ± standard error. Repeated measures analysis of variance (ANOVA) and the LSD test were used to analyze work error, reference error, and HRV. Percentages were assessed using generalized linear models for weight change. Other information was analyzed using one-way ANOVA and the LSD test. p < 0.05 was considered a statistically significant difference.
3. Results
3.1. Changes in cognitive function
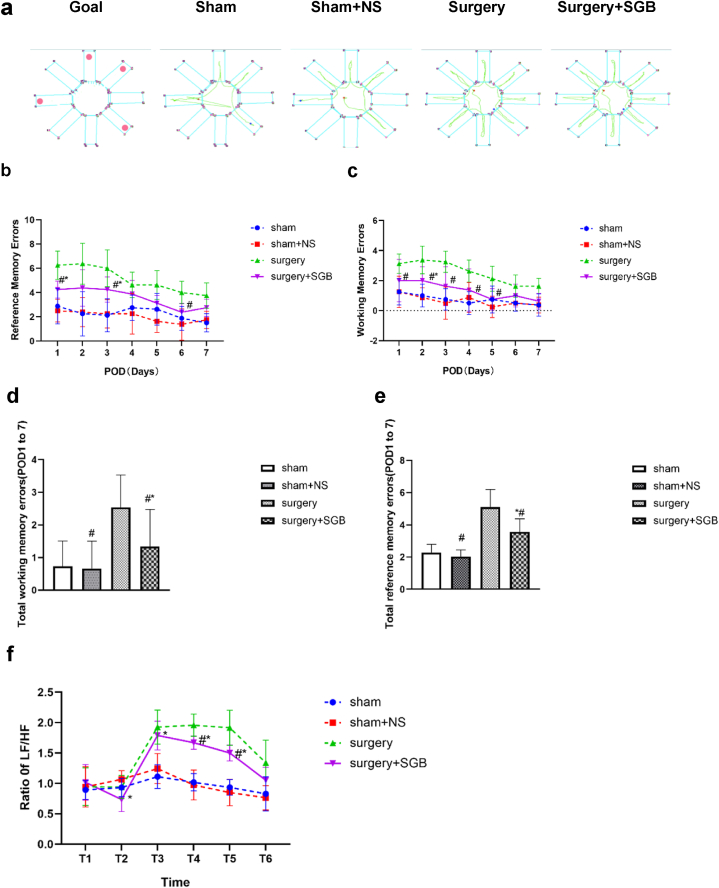
Schematic of the eight-arm maze for each group of rats were shown in Fig. 2a. There was no statistical difference between the total working and reference errors in the Sham and Sham + NS groups (P = 0.91). The total of both types of errors in the Surgery + SGB group differed significantly from that in the Sham and Surgery groups (P = 0.005) (Fig. 2b, c) and the total working and reference errors in the Surgery group differed significantly from those in the Sham group (P < 0.001) (Fig. 2d, e).
Fig. 2.
Changes in the cognitive function and comparison of heart rate variability. (a) Schematic of the eight-arm maze for each group of rats; (b) changes in working errors during 7 days after surgery for each group; (c) changes in reference errors during 7 days after surgery for each group; (d) total working errors for 7 days after surgery for each group; (e) total reference errors for 7 days after surgery for each group; (f) comparison of LF/HF values for each group (T1: after anesthesia; T2: after stellate ganglion block; T3: after surgery; T4: 1 day after surgery; T5: 3 days after surgery; T6: 7 days after surgery). Notes: *p < 0.05 compared with the Sham group; #p < 0.05 compared with the Surgery group.
LF, low frequency; HF, high frequency.
3.2. Rate of change in body weight
The rate of weight change at POD7 between the Sham and Sham + NS groups did not differ (P = 0.26). The Surgery and Surgery + SGB groups started losing weight continuously from POD2, which was statistically different from the Sham group (P = 0.003). However, the Surgery + SGB group stopped losing weight on POD4 and slowly regained weight, which was statistically different from the Surgery group (P = 0.002) (Table 1).
Table 1.
The weight change (%) in the experimental groups.
| Sham | Sham + NS | Surgery | Surgery + SGB | |
|---|---|---|---|---|
| POD 1 | 99.87 ± 0.33 | 99.64 ± 0.37 | 100.02 ± 0.45 | 100.27 ± 0.68 |
| POD 2 | 100.03 ± 0.36 | 99.41 ± 0.69 | 98.69 ± 0.61 | 99.32 ± 0.84 |
| POD 3 | 100.27 ± 0.44 | 99.54 ± 0.68 | 97.45 ± 0.75 | 98.49 ± 0.79* |
| POD 4 | 100.58 ± 0.61 | 99.88 ± 0.74 | 96.54 ± 0.85 | 97.97 ± 0.66#* |
| POD 5 | 100.76 ± 0.67 | 100.03 ± 0.71 | 95.76 ± 0.99 | 97.83 ± 1.11#* |
| POD 6 | 100.97 ± 0.71 | 100.13 ± 1.11 | 95.11 ± 122 | 97.89 ± 1.38#* |
| POD 7 | 101.23 ± 0.67 | 100.63 ± 0.55 | 94.54 ± 1.59 | 98.23 ± 1.57#* |
Data are presented as mean ± SEM; n = 10/group.
#p < 0.05 vs. Surgery group; *p < 0.05 vs. Sham group.
POD, postoperative day.
3.3. Comparison of heart rate variability
After performing SGB and before performing surgery, the LF/HF rate in the Surgery + SGB group was significantly lower (Fig. 2f; P = 0.01). After surgery, the LF/HF rates in the Surgery and Surgery + SGB groups were significantly higher than those in the Sham and Sham + NS groups, the Surgery + SGB group were significantly lower than those in the Surgery group but higher than those in the Sham and Sham + NS groups (Fig. 2f; T3: Sham vs. Surgery, P < 0.001; Sham vs. Surgery + SGB, P < 0.001, Sham + NS vs. Surgery, P < 0.001,Sham + NS vs. Surgery + SGB, P = 0.002, Surgery vs. Surgery + SGB, P = 0.71; T4: Sham vs. Surgery, P < 0.001; P < 0.001, Sham + NS vs. Surgery,P < 0.001,Sham + NS vs. Surgery + SGB, P < 0.001, Surgery vs. Surgery + SGB, P = 0.01; T5: Sham vs. Surgery, P < 0.001;P < 0.001, Sham + NS vs. Surgery, P < 0.001,Sham + NS vs. Surgery + SGB, P < 0.001, Surgery vs. Surgery + SGB, P = 0.02). On POD7, the difference between the groups was not statistically significant (Fig. 2f; T6: Sham vs. Surgery + NS, P = 0.94, Sham vs. Surgery, P = 0.11; Sham vs. Surgery + SGB, P = 0.32, Sham + NS vs. Surgery, P = 0.06,Sham + NS vs. Surgery + SGB, P = 0.25, Surgery vs. Surgery + SGB, P = 0.82).
3.4. Neuronal damage
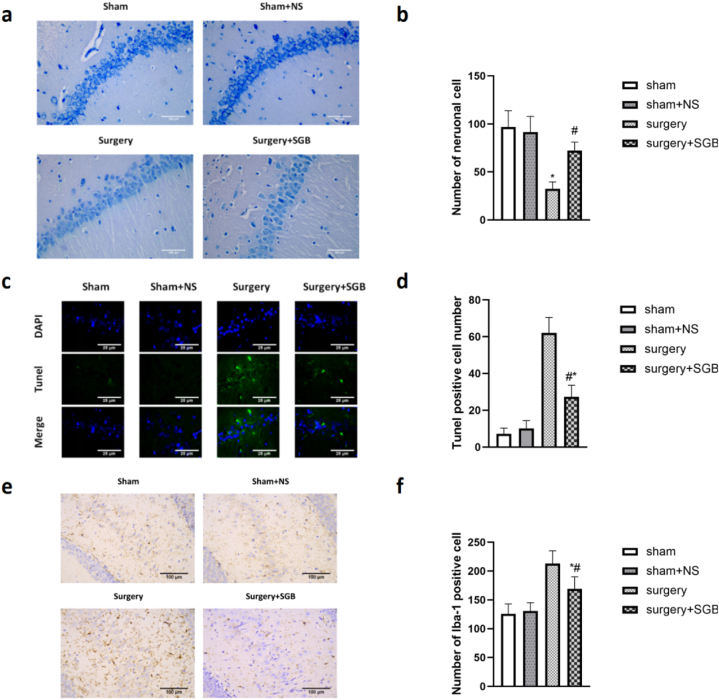
Nissl staining was performed to measure the loss of hippocampal CA1 neurons at POD3. The number of neurons between the Sham and Sham + NS groups did not differ (P = 0.93). The Surgery group lost a significantly higher number of neurons than the Sham and Sham + NS groups (Fig. 3a, b; Sham vs. Surgery, P < 0.001; Sham + NS vs. Surgery, P < 0.001). The number of lost neurons was lower in the Surgery + SGB group than in the Surgery group but significantly higher than in the Sham and Sham + NS groups (Fig. 3a, b; Surgery vs. Surgery + SGB, P < 0.001; Sham vs. Surgery + SGB, P = 0.01; Sham + NS vs. Surgery + SGB, P = 0.03).
Fig. 3.
Histological comparison of the hippocampal region in rats after surgery (a) TUNEL staining results in the hippocampal region; (b) comparison of the number of positive cells after TUNEL staining in the hippocampal region; (c) Nissl staining results in the hippocampal region; (d) comparison of the number of neurons in the hippocampal region; (e) Iba-1 immunohistochemistry in the hippocampal region (CA1); (f) comparison of the number of Iba-1-positive cells in the hippocampal region.
Notes: *p < 0.05 compared with the Sham group; #p < 0.05 compared with the Surgery group.
3.5. Apoptosis of neuronal cells
TUNEL staining was performed to detect apoptosis of neurons in hippocampal tissue at POD3 (Fig. 3). The number of positive cells between the Sham and Sham + NS groups did not differ (P = 0.98). The number of apoptotic cells in the Surgery group was significantly higher than that in the Sham and Sham + NS groups (Fig. 3e, f; Sham vs. Surgery, P < 0.001; Sham + NS vs. Surgery, P = 0.001). The number of apoptotic cells in the Surgery + SGB group was significantly lower than that in the Surgery group but higher than that in the Sham and Sham + NS groups (Fig. 3c, d; Surgery vs. Surgery + SGB, P = 0.04; Sham vs. Surgery + SGB, P = 0.05).
3.6. Microglia activity-mediated microglial activation
Immunohistochemical staining was performed to detect Iba-1 expression in hippocampal CA1 tissue at POD3 (Fig. 3). The number of Iba-1-positive cells between the Sham and Sham + NS groups did not differ (P = 0.98). The number of Iba-1-positive cells in the Surgery group was significantly higher than that in the Sham and Sham + NS groups (Fig. 3e, f; Sham vs. Surgery,P < 0.001; Sham + NS vs. Surgery, P = 0.001). Furthermore, the number of Iba-1-positive cells in the Surgery + SGB group was significantly lower than that in the Surgery group but higher than that in the Sham and Sham + NS groups (Fig. 3e, f; Surgery vs. Surgery + SGB, P = 0.04; Sham vs. Surgery + SGB, P = 0.05).
3.7. Expression of inflammatory factors in the plasma and brain tissue
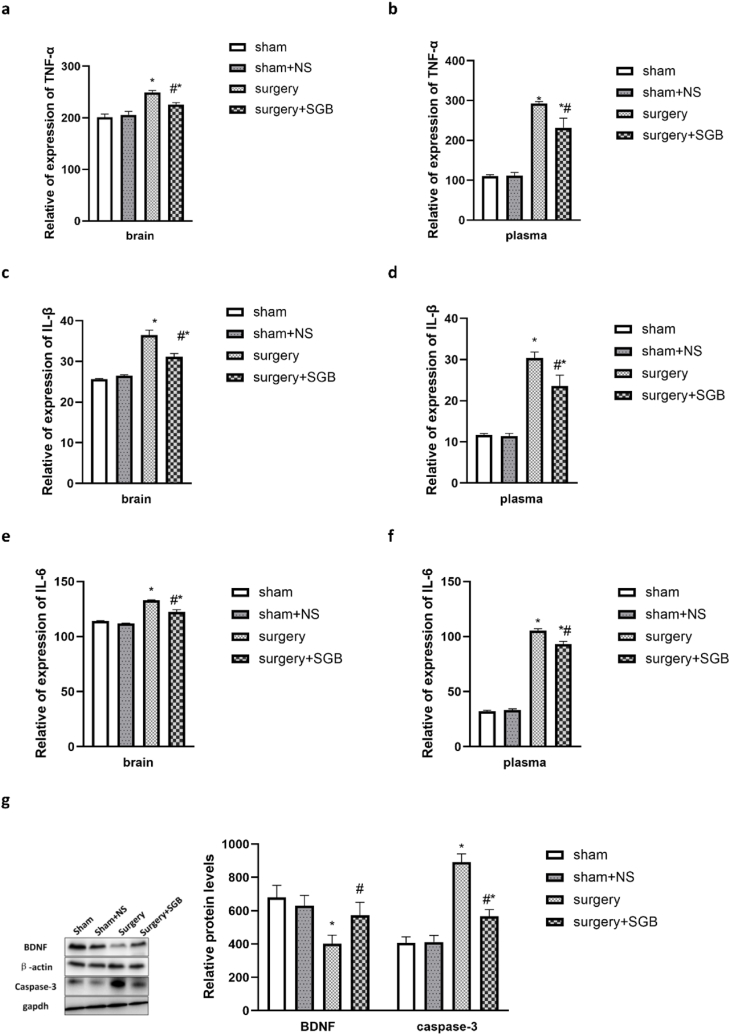
ELISA results showed consistent trends in the levels of inflammatory factors in plasma and brain tissue. TNF-α, IL-1β, and IL-6 levels between the Sham and Sham + NS groups at POD3 did not differ (Fig. 4; Sham vs. Sham + NS, TNF-α: plasma P = 0.99, brain P = 0.75; IL-1β:plasma P = 0.99, brain P = 0.82; IL-6:plasma P = 0.89, brain P = 0.75). The levels of inflammatory factors in the Surgery group were significantly higher than those in the Sham and Sham + NS groups (Sham vs. Surgery, TNF-α: plasma P < 0.001, brain P < 0.001, Fig. 4a, b; IL-1β:plasma P < 0.001, brain P < 0.001, Fig. 4c, d; IL-6:plasma P < 0.001, brain P < 0.001, Fig. 4e, f; Sham + NS vs. Surgery, TNF-α: plasma P < 0.001, brain P < 0.001; IL-1β:plasma P < 0.001, brain P < 0.001; IL-6:plasma P < 0.001, brain P < 0.001). The levels of inflammatory factors in the Surgery + SGB group were significantly lower than those in the Surgery group but significantly higher than those in the Sham and Sham + NS groups (Surgery vs. Surgery + SGB, TNF-α: brain P = 0.003, plasm P = 0.002, Fig. 4a, b; IL-1β: brain P = 0.001, plasma P = 0.003, Fig. 4c, d; IL-6: brain P < 0.001, plasma P < 0.001 Fig. 4e, f; Sham vs. Surgery + SGB,TNF-α: plasma P < 0.001, brain P = 0.005; IL-1β:plasma P < 0.001, brain P < 0.001; IL-6:plasma P < 0.001, brain P < 0.001; Sham + NS vs. Surgery + SGB,TNF-α: plasma P < 0.001, brain P = 0.01; IL-1β:plasma P < 0.001, brain P < 0.001; IL-6:plasma P < 0.001, brain P < 0.001).
Fig. 4.
Comparison of inflammatory factors and related proteins (a) TNF-α expression levels in the hippocampal region; (b) TNF-α levels in the plasma (c) IL-1β expression levels in the hippocampal region; (d) IL-1β expression levels in the plasma; (e) IL-6 expression levels in the hippocampal region; (f) IL-6 expression levels in the plasma; (g) Western blot analysis results and comparison of BDNF and caspase-3 expression levels in the hippocampal region.
Notes: *p < 0.05 compared with the Sham group; #p < 0.05 compared with the Surgery group.
3.8. Expression of BDNF and caspase-3
Western blot analysis results showed that the BDNF and Caspase-3 levels did not differ between the Sham and Sham + NS groups at POD3 (Fig. 4g; Sham vs. Sham + NS, P = 0.87, Caspase-3 P = 0.99). The BDNF levels in the Surgery group were significantly lower than those in the Sham and Sham + NS groups (Fig. 4g; Sham vs. Surgery, BDNF P < 0.001, Caspase-3 P < 0.001, Sham + NS vs. Surgery, BDNF P < 0.001, Caspase-3 P < 0.001). The BDNF levels in the Surgery + SGB group were significantly higher than those in the Surgery group but significantly lower than those in the Sham and Sham + NS groups (P = 0.01). The Caspase-3 levels in the Surgery + SGB group were significantly lower than those in the Surgery group but significantly higher than those in the Sham and Sham + NS groups (Fig. 4g; Surgery vs. Surgery + SGB, P < 0.001; Sham vs. Surgery + SGB, P = 0.02; Sham + NS vs. Surgery + SGB, P = 0.02).
3.9. Endocrine stress response
3.9.1. HPA axis change
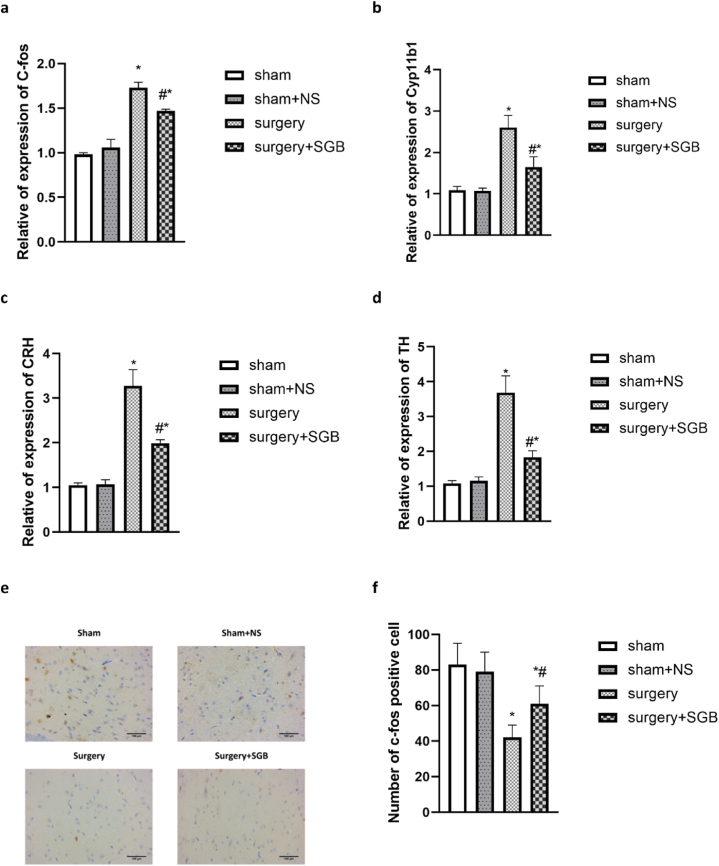
The paraventricular nucleus is an important neurosecretory nucleus in the hypothalamus and the activation of the paraventricular nucleus indicated the excitement of the HPA axis. C-fos protein can reflect the activation of neurons. RT-qPCR results showed that the level of c-fos in the Surgery group were significantly higher than those in the Sham and Sham + NS groups, and the level of c-fos were lower in the Surgery + SGB group, but it were still higher than Sham and Sham + NS groups (Fig. 5a; Surgery vs. Surgery + SGB, P = 0.002; Sham vs. Surgery + SGB, P < 0.001; Sham + NS vs. Surgery + SGB, P < 0.001).
Fig. 5.
Comparison of stress endocrine response (a) comparison of c-fos levels in the paraventricular nucleus; (b) comparison of adrenal Cyp11b1 levels; (c) comparison of CRH levels in the hypothalamus; (d) comparison of adrenal TH levels; (e) immunohistochemical images of c-fos labeled cells in the vagal nucleus; (f) comparison of c-fos positive cell numbers in the vagal nucleus.
Notes: *p < 0.05 compared with the Sham group; #p < 0.05 compared with the Surgery group.
The level of CRH and Cyp11b1 in the surgery group increased significantly in the POD1, and decreased in the Surgery + SGB group (Fig. 5b, c; CRH: Surgery vs. Surgery + SGB, P = 0.002; Sham vs. Surgery + SGB, P = 0.002; Sham + NS vs. Surgery + SGB, P = 0.002; Cyp11b1: Surgery vs. Surgery + SGB, P = 0.002; Sham vs. Surgery + SGB, P = 0.04; Sham + NS vs. Surgery + SGB, P = 0.03). It suggested that surgery stress stimulated HPA axis and released CRH and Cyp11b1, SGB regulated HPA axis excitability and inhibited the level of CRH and Cyp11b1.
3.9.2. Autonomic nervous system change
The autonomic nervous system includes the sympathetic and parasympathetic nerves. Since the sympathetic nucleus are difficult to locate and detect, we detected the vagus nucleus to reflect the imbalance of the autonomic nervous system under surgery stress. The dorsal motor nucleus of the vagus (DMV) has been acknowledge as the largest source of parasympathetic pre-ganglionic neurons. We observed a trend decreased C-fos expression in the DMV induced by surgery stress, and increased substantially with SGB therapy (Fig. 5e, f; Surgery vs. Surgery + SGB, P = 0.03; Sham vs. Surgery + SGB, P = 0.003; Sham + NS vs. Surgery + SGB, P = 0.005).
The level of TH was associated with catecholamine in body. As shown in Fig. 5, the mRNA level of TH dramatically increased in surgery group, and rats in Surgery + SGB group exhibited dramatically decreased (Fig. 5d; Sham vs. Surgery, P < 0.001; Sham vs. Surgery + SGB, P = 0.003; Sham + NS vs. Surgery, P < 0.001; Surgery vs. Surgery + SGB, P = 0.002).
4. Discussion
The results of this study confirmed that SGB protects cognitive function. SGB as a safe and effective neuroprotective approach can reduce the errors of eight-arm maze assay, attenuate neural structural damage, inhibit neuroapoptosis, suppress inflammatory responses, increase the release of neurotrophic factors, accelerate postoperative weight recovery, and promote postoperative rehabilitation in rats. We found that SGB reduced the degree of cognitive function impairment induced by surgical trauma by inhibiting the excitability of the sympathetic and HPA axes.
The definition and diagnostic criteria of POCD in animal experiments are more ambiguous than those in humans. Therefore, we used cognitive impairment as a readout to describe the effect of surgery on cognitive function in aged rats [18]. Since advanced age is one of the risk factors for POCD, we chose aged rats as experimental subjects. Furthermore, it can increase the sensitivity of the test animals to the detection index and may better fit the population characteristics of POCD occurring in clinical practice. To exclude that short-time inhalation of sevoflurane during SGB affects the experimental results, we designed an experimental group that underwent sham surgery after saline injection around the stellate ganglion. The results showed that short-time inhalation of sevoflurane did not significantly affect the experimental outcome.
We showed that SGB prevented postoperative cognitive impairment in aged rats. The behavioral tests in the eight-arm maze included working errors and reference errors, which reflect short- and long-range memory, respectively. The results showed that the cognitive function of the rats in the Surgery group was significantly diminished from POD1, while the degree of impairment in the aged rats that had undergone SGB was significantly lower than that in the Surgery group. We also found that the rats in the Surgery and SGB groups showed different degrees of recovery of cognitive function over time, with the SGB group recovering better and faster.
The postoperative weight change of rats can reflect postoperative recovery. We found that the weight of rats in the Surgery and SGB groups increased at POD1, which may be related to postoperative intestinal edema. Afterward, the bodyweight of rats in both groups decreased continuously. The weight of rats in the SGB group stopped decreasing on POD5 and gradually increased, suggesting that the rats in the SGB group may have a better prognosis than those in the Surgery group.
HRV is a non-invasive test used clinically to evaluate the regulatory function of the cardiac sinus node autonomic nervous system and to describe the oscillatory changes between the R-R intervals (R-Ri) of the continuous ECG. Studies of HRV in aged rats showed that the LF/HF ratio increases postoperatively, suggesting that surgery leads to persistent postoperative sympathetic excitation in aged rats [19]. Similarly, the LF/HF ratio decreased in aged rats in the SGB group. This result suggests that sympathetic excitation may be one of the causes of postoperative cognitive function alterations in aged rats and that SGB may protect cognitive function in aged rats by suppressing sympathetic excitation and regulating autonomic balance.
The hippocampal region of the brain has a key role in memory [20]. We performed hippocampal histology in aged rats at POD3. We found that surgery could decrease Nissl vesicle expression and damage neural structures. TUNEL staining showed that surgical stimulation could cause apoptosis of neuronal cells. Western blot detection of the caspase-3 in the hippocampal region was consistent with the histological results. These results suggest that surgical trauma can trigger neurological damage in the brain and that SGB inhibits the degree of apoptosis induced by surgical trauma while increasing the release of BDNF, further confirming the protective effect of SGB on cognitive function in aged rats.
Iba-1 reflects the activation of microglia and the impairment of the inflammatory response. Surgical trauma activates the immune system, leading to a perioperative inflammatory response and increased expression of pro-inflammatory factors IL-6, TNF-α, and IL-1β in plasma and brain tissue. Pro-inflammatory factors in the brain can increase blood-brain barrier permeability, induce an inflammatory response in the brain or directly damage neurons, cause an autoimmune response in the brain, and aggravate neuronal damage. Recent research shows SGB can prevent pathological damage of the hippocampus and maintain spatial learning and memory function in a sleep deprived rat model. This occurs due to reduced expression of IL-6, IL-1β and Caspase-3 in the hippocampus [21]. Our results showed that SGB attenuated the systemic and intracerebral inflammatory responses induced by surgical trauma and protected the cognitive function of aged rats.
The protective effect of SGB on cognitive function in aged rats was hypothesized to be linked to the activation and excitation of the neuroendocrine system due to surgical stress. To confirm our hypothesis, we conducted separate assays for two neuroendocrine response systems (sympathetic-adrenal medulla and HPA axis). Because the sympathetic nucleus is challenging to detect, we chose the dorsal vagal nucleus DMV to analyze c-fos expression. The dorsal motor nucleus of the vagus (DMV) is known to control vagal activity, while c-fos is an immediate early gene widely used for the assessment of neuronal excitability. It responds transiently to acute stimuli and peaks approximately several hours after stress [22,23]. Thus, c-fos levels at the DMV can reflect the excitability of the vagus nerve [24] and the sympathetic nerves. We also examined the expression levels of adrenal TH, the enzyme responsible for catalyzing the conversion of the amino acid l-tyrosine to dihydroxyphenylalanine (Dopa). Dopa is a precursor of dopamine, and accordingly, of norepinephrine and epinephrine. Therefore, the level of TH can reflect the sympathetic excitation of the body. Our results show that surgical trauma causes sympathetic excitation, while SGB inhibits the degree of sympathetic excitation caused by surgical trauma.
The precise regulation of organ and tissue functions by autonomic nerve requires fine-tuning of the activities of sympathetic and parasympathetic nerves. Therefore, the sympathetic and parasympathetic nervous systems do not work independently, and their activities are the result of multiple interactions at different levels of the nerve axis and at the level of peripheral effector cells. Therefore, the sympathetic and parasympathetic nerves interact with each other. The interaction between the sympathetic and parasympathetic nervous systems at the level of the central nervous system can be realized through several ways and mechanisms. These interactions may arise from the processing of information transmitted by neuronal and humoral pathways to the nucleus tractus solitarius (NTS) or the paraventricular hypothalamic nucleus (PVN) [25]. Therefore, there is mutual regulation between sympathetic and parasympathetic. Although this study did not directly find evidence that the sympathetic nucleus was activated, we can still reflect the activation of the sympathetic nerve through the vagus nerve and verify the activation of the sympathetic nerve through the expression of TH in the adrenal medulla.
The HPA axis is an important component of the neuroendocrine system, which is involved in controlling stress responses and regulating physiological processes such as digestion, immunity, mood, emotion, and energy storage and expenditure. It is also associated with the pathophysiology of multiple mood and cognitive disorders [26]. Studies have shown that the HPA axis is overactive in patients with depression. Furthermore, a correlation between HPA axis activation and cognitive performance and a potential role of HPA axis genetic variants in cognition has been described [27]. We selected the paraventricular nucleus, an important neurosecretory nucleus of the hypothalamus, for our study. Similarly, we examined the c-fos expression in the paraventricular nucleus region, which can reflect the excitability of paraventricular nucleus neurons. The expression levels of Cyp11b1, an important protein in aldosterone synthesis, and CRH, a pro-adrenal hormone-releasing hormone, which both reflect the excitability of the HPA axis, were also examined in the paraventricular nucleus [28,29]. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice [30]. BDNF is a stress and activity-dependent factor involved in many activities modulated by the HPA axis. Significantly, ectopic expression of BDNF in vivo increased CRH, whereas reduced expression of BDNF, or its receptor TrkB, decreased CRH expression and normal HPA functions [31]. Surgical trauma increased HPA axis excitability and SGB inhibited this, indicating the possible mechanism of the protective effect of SGB on postoperative cognitive function and suggesting that the HPA axis may play an important role in POCD.
The main purpose of this study was to investigate the effectiveness of SGB for the prevention of POCD and to provide a theoretical basis for the clinical application of SGB for the prevention of POCD in the elderly. At the same time, we attempted to explore the protective mechanism of cognitive function in SGB and found that dysregulation of the autonomic nervous system and HPA axis may play an important role in POCD.
Our study has a few limitations. First, the surgical trauma caused by exploratory laparotomy is limited, and is, therefore, not a good model for major procedures with a higher clinical probability of POCD. Furthermore, the pathogenesis of POCD is complex and the protective effect of SGB on cognitive function may be multifaceted. The present study analyzed and explored POCD mainly based on endocrine stress alterations. Thus, further research is needed to study other protective mechanisms.
5. Conclusion
SGB inhibited the neuroendocrine stress response induced by surgical trauma and alleviated postoperative cognitive decline in aged rats.
Author contribution statement
Xijin Deng, M.D.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tian Sun, M.D.: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Dengming Zhao, M.D.; Sa na Si ri gu leng, M.D.: Performed the experiments; Analyzed and interpreted the data.
Wenzhi Li, M.D., Ph.D.: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14337.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Needham M.J., Webb C.E., Bryden D.C. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br. J. Anaesth. 2017;119(suppl_1):i115–i125. doi: 10.1093/bja/aex354. [DOI] [PubMed] [Google Scholar]
- 2.Kotekar N., Shenkar A., Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin. Interv. Aging. 2018;13:2267–2273. doi: 10.2147/CIA.S133896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daiello L.A., Racine A.M., Yun Gou R., et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477–491. doi: 10.1097/ALN.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evered L., Silbert B., Knopman D.S., et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 2018;121(5):1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J., Luo A., Gao J., et al. The role of SIRT1 in neuroinflammation and cognitive dysfunction in aged rats after anesthesia and surgery. Am. J. Transl. Res. 2019;11(3):1555–1568. [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Hu Y., Yan E., et al. Perioperative neurocognitive dysfunction: thinking from the gut? Aging. 2020;12(15):15797–15817. doi: 10.18632/aging.103738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleh A.J., Tang G.X., Hadi S.M., et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2015;21:798–805. doi: 10.12659/MSM.893359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X.Q., Mei B., Zuo Y.M., et al. A multicentre randomised controlled trial of the effect of intra-operative dexmedetomidine on cognitive decline after surgery. Anaesthesia. 2019;74(6):741–750. doi: 10.1111/anae.14606. [DOI] [PubMed] [Google Scholar]
- 9.Raut M.S., Maheshwari A. Stellate ganglion block: important weapon in the anesthesiologists' armamentarium. J. Cardiothorac. Vasc. Anesth. 2018;32(2):e36–e37. doi: 10.1053/j.jvca.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Narouze S. Ultrasound-guided stellate ganglion block: safety and efficacy. Curr. Pain Headache Rep. 2014;18(6):424. doi: 10.1007/s11916-014-0424-5. [DOI] [PubMed] [Google Scholar]
- 11.Lipov E., Gluncic V., Lukić I.K., et al. How does stellate ganglion block alleviate immunologically-linked disorders? Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110000. [DOI] [PubMed] [Google Scholar]
- 12.Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray A., Gulati K., Rai N. Stress, anxiety, and immunomodulation: a pharmacological analysis. Vitam. Horm. 2017;103:1–25. doi: 10.1016/bs.vh.2016.09007. [DOI] [PubMed] [Google Scholar]
- 14.Wei W.W., Wei D.S., Wen Z.L., et al. Stellate ganglion block attenuates chronic stress induced depression in rats. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdi S., Yang Z. A novel technique for experimental stellate ganglion block in rats. Anesth. Analg. 2005;101(2):561–565. doi: 10.1213/01.ANE.0000159169.12425.50. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Martin T.J., Buechler N.L., Kahn W., et al. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101(1):191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Tao T., Zhao M., Yang W., et al. Neuroprotective effects of therapeutic hypercapnia on spatial memory and sensorimotor impairment via anti-apoptotic mechanisms after focal cerebral ischemia/reperfusion. Neurosci. Lett. 2014;573:1–6. doi: 10.1016/j.neulet.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 18.Glumac S., Kardum G., Karanovic N. Postoperative cognitive decline after cardiac surgery: a narrative review of current knowledge in 2019. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019;25:3262–3270. doi: 10.12659/MSM.914435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi L., Du K., Liu D., et al. Electroacupuncture brain protection during ischemic stroke: a role for the parasympathetic nervous system. J. Cerebr. Blood Flow Metabol. 2018;38(3):479–491. doi: 10.1177/0271678X17697988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekeres M.J., Winocur G., Moscovitch M. The hippocampus and related neocortical structures in memory transformation. Neurosci. Lett. 2018;680:39–53. doi: 10.1016/j.neulet.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Dai D., Zheng B., Yu Z., Lin S., et al. Right stellate ganglion block improves learning and memory dysfunction and hippocampal injury in rats with sleep deprivation. BMC Anesthesiol. 2021;21(1) doi: 10.1186/s12871-021-01486-4. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera D.G., Robertson H.A. Activation of c-fos in the brain. Prog. Neurobiol. 1996;50(2–3):83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 23.Kovács K.J. Measurement of immediate-early gene activation-c-fos and beyond. J. Neuroendocrinol. 2008;20(6):665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 24.Machhada A., Ang R., Ackland G.L., et al. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm. 2015;12(11):2285–2293. doi: 10.1016/j.hrthm.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ondicova K., Mravec B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: a minireview. Endocr. Regul. 2010 Apr;44(2):69–75. doi: 10.4149/endo_2010_02_69. [DOI] [PubMed] [Google Scholar]
- 26.Reilly E.B., Gunnar M.R. Neglect, HPA axis reactivity, and development. Int. J. Dev. Neurosci. 2019;78:100–108. doi: 10.1016/j.ijdevneu.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Menke A. Is the HPA Axis as target for depression outdated, or is there a new hope? Front. Psychiatr. 2019;10:101. doi: 10.3389/fpsyt.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freel E.M., Ingram M., Wallace A.M., et al. Effect of variation in CYP11B1 and CYP11B2 on corticosteroid phenotype and hypothalamic-pituitary-adrenal axis activity in hypertensive and normotensive subjects. Clin. Endocrinol. 2008;68(5):700–706. doi: 10.1111/j.1365-2265.2007.03116.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 30.Wosiski-Kuhn M., Erion J.R., Gomez-Sanchez E.P., Gomez-Sanchez C.E., Stranahan A.M. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology. 2014;42:165–177. doi: 10.1016/j.psyneuen.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeanneteau F.D., Lambert W.M., Ismaili N., et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 2012;109(4):1305–1310. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.