Highlights
-
•
Six GI factors estimated most likely to influence prostate motion were identified: depression, anxiety, diabetes, obesity, low physical activity, and pelvic floor disorder.
-
•
Tools to reliably, quickly and easily quantified these GI factors are available.
-
•
A comprehensive GI factor assessment package suitable to implement into the radiotherapy clinic is presented.
-
•
Unveiling patient specific GI factors upfront could guide improved personalisation of radiotherapy pre-habilitation, preparation and treatment delivery.
Keywords: Prostate cancer, Radiotherapy, Prostate motion, Gastrointestinal factors, Personalised care, Prediction model
Abstract
Purpose
A scoping literature review was conducted to identify gastrointestinal (GI) factors most likely to influence prostate motion during radiotherapy. We proffer that patient specific measurement of these GI factors could predict motion uncertainty during radiotherapy, facilitating personalised care by optimising treatment technique e.g., daily adaption or via bespoke patient pre-habilitation and preparation.
Methods
The scoping review was undertaken as per JBI guidelines. Searches were conducted across four databases: Ovid Medline®, EMBASE, CINAHL and EBSCO discovery. Articles written in English from 2010-present were included. Those pertaining to paediatrics, biological women exclusively, infectious and post-treatment GI morbidity and diet were excluded.
Common GI factors impacting men were identified and related symptoms, incidence and measurement tools examined. Prevalence among persons with prostate cancer was explored and suitable assessment tools discussed.
Results
A preliminary search identified four prominent GI-factors: mental health, co-morbidity and medication, physical activity, and pelvic floor disorder. The scoping search found 3644 articles; 1646 were removed as duplicates. A further 1249 were excluded after title and abstract screening, 162 remained subsequent to full text review: 42 mental health, 53 co-morbidity and medication, 39 physical activity and 28 pelvic floor disorder.
Six GI factors prevalent in the prostate cancer population and estimated most likely to influence prostate motion were identified: depression, anxiety, diabetes, obesity, low physical activity, and pelvic floor disorder. Reliable, quick, and easy to use tools are available to quantify these factors.
Conclusion
A comprehensive GI factor assessment package suitable to implement into the radiotherapy clinic has been created. Unveiling these GI factors upfront will guide improved personalisation of radiotherapy.
Background
Prostate cancer (PCa) accounted for 22.2% of all male cancers in Europe in 2020 [1]. More than 30% of PCa patients receive radical radiotherapy (RT) [2] with five-year progression free survival rates of 80.5–90.6% achieved [3], [4], [5], [6].
Prostate motion consisting of translational and rotational motion, shape deformation and seminal vesicle displacement independent of the gland, is well documented during RT [7], [8], [9], [10], [11], [12], [13]. Significant association between rectal volume and prostate motion is reported with the probability and magnitude of prostate motion increasing in patients with rectal distention [9], [11], [14], [15], [16], [17]. Changes in faecal load or gas have a pronounced effect on inter-fraction prostate motion and/or deformation [15], [16], whereas mobile gas pockets can in addition increase intra-fraction prostate motion [9], [11].
During conventional and moderately hypofractionated RT regimes, rectal volume is reported to decrease and stabilise as treatment weeks progress [18], [19]. Swift RT delivery reduces the risk of significant prostate motion [9], [13] especially when employing daily online IGRT. For ultra-hypofractionated prescriptions (two to seven fractions), the impact of geometrical uncertainty is greater because of less time for rectal volumes to stabilise, fewer fractions for deviations to be averaged over and typically longer beam-on time [20]. With the drive towards fewer fractions for prostate RT, further endeavours to manage motion uncertainty are warranted.
Bowel preparation protocols to improve rectal volume consistency during RT are recommended [20]. A single preparation protocol for all prostate patients is typically used, despite anecdotal evidence that efficacy varies across the population, possibly due to intrinsic patient and environmental factors. Unfortunately, tools to predict individuals’ organ variability and preparation needs do not exist, hence the reliance on standardised regimes.
To date efforts to predict individual’s prostate motion have focussed on qualitative and semi-qualitative review of rectal volume and gas on RT imaging. Rectal-filling status on imaging has been reported as a significant predictor of patient specific prostate motion [11]. However, images only capture anatomical variance at a specific moment or over a brief time. Rectal filling is the product of a chain of digestive events occurring throughout the gastrointestinal (GI) tract, many of which occur without conscious control, regulated instead by nervous, hormonal and local elements co-ordinating internal reflexes [21]. Numerous factors affect digestive function [22], in turn these factors may also influence rectal filling and impact prostate motion. If true, patient specific measurement of GI influencing factors could be used to predict prostate motion, facilitating personalisation of care, either through aligning patient’s predicted motion characteristics with the optimal treatment platform for them or by introducing bespoke prehabilitation and preparation advice.
The paucity of literature relating physiological effectors to prostate motion during RT raised the question ‘could GI factors most likely to influence prostate motion during radiotherapy be identified’. We aim to answer this question in order to create a comprehensive GI factor assessment package suitable to implement at the time of RT consent, and in doing so achieve the first step in developing a personalised prediction model.
Methods
The scoping literature review was conducted as per Joanna Briggs Institute (JBI) methodology [23], the review question was developed using the population, concepts, and context (PCC) framework [23] (Table 1). PRISMA (transparent reporting of systematic reviews and meta-analyses) flowcharts [24] guided the process of literature identification, screening, eligibility, and inclusion.
Table 1.
Scoping review literature search strategy; PCC, search terms, inclusion/exclusion criteria.
| Population | Concept | Context |
|---|---|---|
| Biological adult males. (Not limited to persons with prostate cancer due to lack of evidence identified in preliminary search) |
Factors which affect lower GI filling and volume fluctuation and therefore may influence prostate motion during radiotherapy treatment | Evidence from population surveys and from clinical or medical settings; hospital, community, social care |
| GI-factor | Specific search terms | |
| Mental health disorder | Anxiety; Anxiety disorders; Depression; Mental disorders; Anxiety score; Depression score | |
| Co-morbid conditions and medication | Comorbidity, Drug use, Drug use disorder, Musculoskeletal disorders, Neurological disorders, Dementia, Cardiovascular disease, Hypertension, Myocardial infarction, Diabetes, Chronic respiratory disease, CPOD, Cerebrovascular event, Stroke, Obese, Congestive heart failure, Peripheral vascular disease | |
| Physical activity | Physical activity; Exercise; Physical exertion; Sports | |
| Pelvic floor disorder | Pelvic floor; Pelvic floor disorder | |
| Lower GI effect search terms | ||
| Motion adj3 (bowel or rect*), Motility adj3 bowel, Dysmotility adj3 bowel, bowel pattern, colon transit time, Defecation, Gastrointestinal transit time, Gut motor activity, Intestinal peristalsis, Stool frequency, Intestinal transit, Chronic constipation, Chronic diarrhoea, Faecal loading, Rectal distension. | ||
| Literature inclusion criteria | Literature exclusion criteria | |
|
|
|
For the purpose of this review GI factors were defined as: a measurable and defined cause of altered lower GI function.
We aimed to:
-
1.
Identify and categorise common GI factors impacting men
-
2.
Examine GI symptoms caused by GI factors and their prevalence
-
3.
Explore different methods and tools used to measure GI factors and GI-symptoms
The clinical application was subsequently explored by determining the incidence of common GI factors among persons with PCa and identifying the most appropriate tools to measure these in the RT clinic.
Search strategy
Two preliminary OVID MEDLINE® searches were conducted in May 2021 to establish common terms relating to lower GI motion and morbidity and to identify predominant GI factors. Text in the title and abstract were analysed by one reviewer (SA) to provided additional search key words and phrases. To allow for inclusion of commonly used phrases, exact phrase searching was introduced, for example “gut motor activity”. Identified GI factors were scrutinised using the Medical Subject Headings (MeSH) database [25] to allow full exploration of associated academic language (Table 1). The Public Health England report ‘trends in morbidity and risk factors’ was used to establish relevant co-morbidities to a UK population [26].
Scoping review searches using the GI factors identified were run on four electronic databases to ensure comprehensive data capture: Ovid Medline®, EMBASE, CINAHL complete and EBSCO discovery. Searches were conducted during February 2022. Literature inclusion and exclusion criteria are presented in Table 1. The search was limited to literature from 2010 onwards, to optimise the clinical currency of GI factors and assessment tools sampled. Diet was excluded as its effect on rectal filling and prostate motion has been previously investigated, with no unanimous conclusion met [16], [18], [27], [28], [29]. Investigating diet requires trained professionals to calculate portion sizes and match to food composition tables [30] and food diaries posing significant time burden on the patient [31]. Considering the lack of evidence linking diet to prostate motion and the staff/patient time investment necessary to investigate diet accurately in the prostate RT clinic, it was not considered further.
Evidence selection and data extraction
Articles were exported to Mendeley version 1.19.18. Title and abstract screening was completed by one reviewer (SA). Full text review of eligible studies and data extraction, using an electronic data extraction form [32], was undertaken by one reviewer (SA). Final articles to be included were discussed and approved with two authors (AT, HMcN) and PRISMA flowcharts created for transparency of data review.
Articles found for each GI factor were sub-grouped and analysed by key theme.
Results and discussion
Literature search results
The preliminary search for GI factors (May 2021) yielded 489 articles, 87 met the inclusion/exclusion criteria. Four prominent GI factors were identified: mental health, co-morbidity and medication, physical activity and pelvic floor disorder.
The scoping review search (Feb. 2022) identified 3644 articles; 1646 were removed as duplicates. A further 1249 were excluded after title and abstract screening, 162 remained after full text review: 42 mental health, 53 co-morbidity and medication, 39 physical activity and 28 pelvic floor disorder. Fig. 1 presents the volume of literature identified, screened, and included for each GI factor.
Fig. 1.
PRISMA flow diagram for each GI factor.
Articles were sub-grouped by key theme and four sub-factors selected for each theme based on the most articles indicting a GI symptom association. GI symptoms were mapped to each sub-factor (Table 2). The review focussed on these sub-factors based on the belief that GI factors most common in a general male population are likely most relevant to a PCa population.
Table 2.
Predominant GI-factors, four most common sub-factors and their associated GI symptoms as identified in the literature.
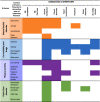 |
Research studies and population surveys for the 16 GI sub-factors are tabulated and presented in Supplementary material 1. Review articles have not been included but were used to ensure all relevant articles were incorporated.
Clinical application
Assessment of 16 sub-factors would pose significant time burden on clinical teams and patients alike in a busy RT clinic. Agreement to reduce the number of GI sub-factors for clinical investigation, concentrating on those most dominant in the literature was reached. The volume of literature supporting each sub-factor ranged from 2 to 27 research studies or population surveys. The highest volume GI sub-factors, retained for clinical investigation, were depression, anxiety, diabetes, obesity and low physical activity. The sub-categories of pelvic floor disorder were condensed to one category.
Common GI factor results, prevalence in persons with prostate cancer and assessment tools
The six GI factors identified for clinical investigation are summarised in this section, focusing on associated GI symptoms, assessment tools and prevalence rates.
Each factor was subsequently evaluated in the context of PCa, exploring incidence and defining suitable assessment tools for use in the RT clinic.
Mental health disorder
Depression literature results
Twenty-seven articles report an association between depression and disordered bowel habits: seventeen with constipation [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [51], [58], IBS eleven [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], diarrhoea three [51], [58], [59] and faecal incontinence one [44]. Four articles report more than one disorder [38], [44], [51], [58].
The ROME criteria was the primary GI disorder assessment tool used [33], [34], [35], [36], [37], [38], [40], [42], [43], [45], [48], [50], [51], [52], [53], [54], [55], [56], [57], [59] with the Bristol Stool Scale second [38], [43], [44], [46], [58], [59]. Depression was commonly assessed using the Hospital Anxiety and Depression Score (HADS) [34], [38], [41], [42], [43], [48], [51], [53], [55], [56] followed by the Patient Health Questionnaire 9 (PHQ-9) [35], [36], [44], [49], [58].
In persons with constipation, depression rate ranged from 9 to 69.8% [33], [38], [39], [40], [44], [45], [46], [51]. Depression rate in those with IBS was reported as 13.6–74.5% [38], [48], [50], [51], [52] and for diarrhoea, 11.6–15.53% [51], [58]. Depression was considerably lower in healthy control groups, 5.88–10% [39], [40], [45], [58]. Ten studies used the HADS depression scale, a score of 8–10 indicates borderline depression while a score ≥ 11 indicates clinical depression. Average scores ranged from 4 to 14.6 in constipated [38], [42], [43], 4–8.3 in IBS [49], [53], [56] and 2.5–7.5 in healthy [42], [53], [56] cohorts. In all studies with a healthy control comparison, the bowel disorder group suffered higher depression rates.
Depression and prostate cancer
Depression rate in persons with PCa is between 17% [96] and 20% [97], the estimated global rate of depression is 5% [98] and for men in the United Kingdom 9% [99]. Depression is two to three times more prevalent in cancer patients than in age matched healthy control groups [100].
Depression levels reported by PCa patients are similar across all treatment strategies; active surveillance, surgery and RT [101], although androgen deprivation therapy (ADT) may confer an additional risk [102]. The cause of depression is multi-fold; distress related to diagnosis, physical symptoms, treatment side effects and family and social concerns [96]. Depressive disorders are also associated with poorer functional and mortality outcomes following PCa diagnosis [103].
Depression assessment tools
The gold standard for assessing Major Depressive Disorder (MDD) is a structured clinical interview with a mental health professional using the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) criteria [104]. In the RT clinic however, there is neither time nor expertise to perform this assessment. The use of self-reported questionnaires to assess depression was common in the literature, HADS depression score was favoured followed by PHQ-9. When compared against the DSM-5 diagnostic criteria HADS did not assess all symptoms [97] and can therefore only provide an overview of depression, it cannot clinically identify it. PHQ-9 covered all MDD criteria so is the preferred tool.
Anxiety literature results
Sixteen articles reported an association between anxiety and disordered bowel habits. Eleven with constipation [52], [53], [54], [55], [56], [58], [59], [61], [64], [65], [69], IBS seven [56], [67], [68], [69], [71], [74], [75] and diarrhoea one [69]. Two articles report on more than one disorder [56], [69].
The ROME criteria was the primary GI disorder assessment tool used [34], [35], [36], [37], [38], [40], [43], [50], [51], [53], [56], [57] with the Bristol Stool Scale second [38], [46]. Anxiety was assessed using the Hospital Anxiety and Depression Score (HADS) [34], [38], [41], [43], [51], [53], [56] most often, followed by the Zung self-rating anxiety scale [37], [46], [47] and the General Anxiety Disorder Scale (GAD-7) [35], [36].
For persons with constipation, anxiety rate ranged from 14.8 to 42.6% [34], [38], [40], [41], [46] for those with IBS the rate was 27.4–62.1% [38], [46], [51] and for diarrhoea 18.9% [51]. One article with a healthy control group reported the rate of anxiety as 28.5% in patients without bowel dysfunction [40].
A score of 8–10 on the HADS anxiety scale indicates borderline anxiety, ≥ 11 indicates significant anxiety. Average scores ranged from 7 to 8.5 in constipated [43] 6–10.1 in IBS [53], [56] and 3.0–7.9 in healthy [53], [56] cohorts. For all studies comparing to a healthy control, the bowel disorder group suffered higher anxiety rates.
Anxiety and prostate cancer
Anxiety is one of the most common symptoms experienced by cancer patients [99], with PCa patients found to have a high prevalence of anxious symptomology [105]. In PCa patients the prevalence of anxiety pre-treatment, on treatment and post treatment is reported as 27.0%, 15.1% and 18.5% respectively [99]. In contrast the prevalence of clinical anxiety in British men over 65 years is estimated to be 8% [106]. Anxiety has been associated with poorer genitourinary and sexual functional outcomes following PCa treatment [103].
Anxiety assessment tools
HADS, Zung anxiety self-rated and GAD-7 were the most frequently used assessment scales. In lung cancer patients, Zung and HADS identified comparable levels of anxiety and were concluded to have similar detection value, HADS was deemed more acceptable for a clinic setting as it was quicker to complete, reducing patient and clinic time burden [107]. HADS and GAD-7 offer comparable and adequate diagnostic accuracy for generalised anxiety disorder in cancer patients [108], completion typically takes 2–3 min.
The DSM-5 criteria for clinical anxiety sets the benchmark for self-assessment tools. When mapped against this, HADS covered 50%, Zung anxiety self-rated scale covered 75% and GAD-7 covered 63% of relevant content [109]. Taking completion time and content into consideration, GAD-7 would be most appropriate in the RT clinic as it has good concordance with DSM-5 with minimal time expense.
Co-morbidity and medication
Diabetes
Of the 16 papers discussing bowel disorders and diabetes, including patients with Type 1 and Type 2 diabetes, nine were research articles or population surveys. Diabetes and constipation were examined in five [60], [61], [62], [63], [65], [68], diarrhoea in five [61], [62], [64], [65], [68], mixed (alternating) diarrhoea/constipation in two [61], [65], bowel motility disorders in six [63], [64], [66], [67], [68], [69] and faecal incontinence in one [62]. Many studies investigated more than one disorder [61], [62], [63], [64], [65], [68]. Little consistency in GI disorder assessment tools was present, six studies quantitively assessed GI transit time using wireless capsule endoscopy [63], [64], [66], [67] or scintigraphy [68], [69].
In persons with diabetes, constipation rate ranged from 9.9% − 67% [61], [62], [63], [65], [68], diarrhoea from 3.7 − 30% [61], [62], [64], [65], [68], mixed diarrhoea/constipation from 12.5 to 25.8% [61], [65] and faecal incontinence rate was 44% [62]. Two studies included a healthy control group and reported constipation prevalence of 54% [63] and 11.2% [65] and a diarrhoea rate of 6% [65], lower than in their comparative diabetic cohorts.
GI transit time is longer in persons with diabetes [63], [65], [66], [67], [68], [69] although comparison between the studies is difficult as different sections of GI travel are reported. An increase in rectal sensation threshold was reported by one study [62].
Diabetes and prostate cancer
More than 4.9 million people in the UK have diabetes [110]. Risks factors for type 2 diabetes are age over 40 years, south Asian, African-Caribbean, or black African decent, high blood pressure and being overweight [111]. Risk factors for PCa include age over 50 years, family history and black ethnicity [112]. With overlapping risk factors and the increasing incidence of PCa [113] a considerable number of patients may endure both diagnoses.
The rate of co-morbid diabetes in a PCa cohort is reported as 8% [114], 10% [115], 12% [116] and 19% [117], although these figures do not precisely compare to our current practice as they are dated [114], [115], [117], and internationally accrued [114], [116], [117]. Treatment of PCa with ADT is associated with an increased risk of diabetes [118] and having both cancer and diabetes is reported to lower health related quality of life and increase symptom severity [119].
Diabetes assessment tools
All review articles, bar one, describe a patient or medical record reported diabetes diagnosis. Only one study acquired blood glucose tests [69], therefore the true prevalence of diabetes may have been underestimated [110]. Acquiring a HbA1c blood test result, which gives an average blood glucose level, may prove more reliable but its utility in the RT clinic is limited due to additional cost and lack of follow-up services.
Obesity
Obesity was established by BMI for all articles [69], [70], [71], [72], [73], [74], BMI ≥ 30 was regarded as obese [70], [72], [74] severely/morbidly obese results were based on either BMI greater than 35 [69], [72], [73] or BMI greater than 40 [71].
Fourteen articles associate GI disorder with obesity, six were research articles or population surveys. Three examine constipation [70], [71], [72] two faecal incontinence [71], [72] and diarrhoea [72], flatulence [70], bowel motility disorder [69] and small intestine bacterial overgrowth [74] are considered by one article each. Three articles examine more than one bowel disorder [70], [71], [72]. Several tools were used to assess bowel disorders.
In obese and severely obese persons, constipation rates ranged from 5 to 34.4% [71], [72]. Diarrhoea rates were reported as 8.5% in obese persons rising to 11.5% in the severely obese [72], after adjusting for confounding factors this study reported that obese individuals have 60% increased odds and severely obese individuals have almost double the odds of having chronic diarrhoea compared to those with normal BMI [72]. Faecal incontinence rate varied greatly from 6.8 to 65.4% [71], [72], compared to 0% in the non-obese control group [73] rectal squeeze pressure was significantly lower in obese persons [73].
GI transit time was slower in morbidly obese versus not-obese patients [69]. Breath tests were taken for methane [70] and bacterial overgrowth [74], with higher results reported for both in obese participants; symptoms include flatulence and bloating.
Obesity and prostate cancer
Obesity is estimated to affect one in every four UK adults [120]. With obesity rates of 25% in the general population, a considerable number of obese PCa patients is expected. Especially as the prevalence of obesity is higher among people with cancer compared to the general population [121].
A recent meta-analysis concluded an 8–11% increased risk of advanced PCa and PCa specific mortality in obese men [122]. Digital rectal prostate exams are more likely to be inconclusive [123] and prostate biopsies less accurate in obese persons [124]. These confounding factors during diagnosis contribute to the risk of advanced cancer staging and PCa specific mortality [125].
The development of obesity and cardiometabolic risk factors, including dyslipidaemia, insulin resistance and elements of metabolic syndrome have also been associated with ADT treatment of PCa [126], [127], [128]. Obese persons have an increased risk of biochemical failure, development of distant metastases, PCa specific mortality and overall mortality following radical RT treatment [117]. Early studies attribute this to difficult or poor patient set-up [129], [130] however this association was not quantified on daily IGRT [117].
Obesity assessment tools
All review articles measured obesity using BMI (kg/m2). The World Health Organisation (WHO) define obesity as a BMI greater than or equal to 30 kg/m2 [131]. The National Institute for Health and Care Excellence report obesity class II as a BMI of 35–39.9 kg/m2 and obesity class III as a BMI of 40 kg/m2 or more [132]. Obesity class III is also described as ‘severely obese’ [120].
BMI is an easy and quick method of measuring overall adiposity in the RT clinic however it does not provide proportions of fat and lean mass, which may be of greater importance for the development and progression of prostate cancer [125]. Adding a waist measurement can supplement BMI and assess fat distribution. Men with a waist size of ≥ 94 cm are more likely to develop obesity related health problems [120].
Physical activity
Low physical activity/Sedentary behaviour
The classification of ‘activity’ across studies varied. Several articles mention ‘sedentary behaviour’ but actually refer to an amount of physical activity below a threshold. Sedentary behaviour is classified as “waking behaviour characterised by an energy expenditure ≤ 1.5 METs, while in a sitting, reclining, or lying posture” [76]. One metabolic equivalent (MET) is defined as the amount of oxygen consumed while sitting at rest and is equal to 3.5 ml O2 per kg body weight × min [77]. No studies refer directly to this state, prompting the decision to combine ‘low activity’ and ‘sedentary behaviour’ categories.
Eighteen articles discuss GI disorders and low physical activity or sedentary behaviour, twelve of which are research studies or population surveys. The majority make the association with constipation [40], [75], [78], [79], [80], [81], [82], [83], [84] the remaining relate to IBS [48], diarrhoea [85] or GI motility disorder [86].
The Bristol Stool Scale [75], [78], [82], [83], [84] and ROME criteria [40], [48], [80], [81] were utilised to characterise GI disorder. Little homogeneity quantifying physical activity presented; three articles used the Global Physical Activity Questionnaire developed by WHO [78], [83], [84], two applied the International physical activity questionnaire [40], [81] and the others used study specific questions.
In persons with low levels of physical activity, constipation ranged from 10.1 to 26.3% [40], [81] with lower rates of constipation in those more physically active, 6.2–11.6% [40], [81]. In studies examining constipated cohorts, low physical activity rate ranged from 28.4 to 82% [79], [83], [84] this is only slightly higher than in comparable non-constipated groups; 28.4% versus 22% [78], 82% versus 74% [83] and 49% versus 33.6% [84]. The low physical activity threshold promoting constipation cannot be established as reporting methods are so varied. In persons with low activity, the introduction of a regular physical activity programme was shown to significantly reduce total colon transit time [86].
For persons with IBS low physical activity was significantly higher than in those without [48]. A large population study revealed the rate of diarrhoea in participants not partaking in regular physical activity as 20.9%, significantly higher than those who do regularly exercise, 15.3% [85].
Physical activity and prostate cancer
Twenty-six percent of adult men in England (2019–2020) report being physically inactive, rising to 31.1% and 50.1% in the 65–74 and 75 plus age groups respectively [133]. Sedentary behaviour in the UK is high, it is estimated that the average man spends the equivalent of 78 days each year sitting down [134].
Regular physical activity is associated with a 10% risk reduction of PCa [135]. An associate between physical activity in earlier adulthood and lower PCa risk is reported [135], [136]. For persons with PCa regular physical activity can reduce the risk of local and systemic disease progression, cancer-specific and overall mortality [137]. Where ADT is prescribed, deficits in muscle strength and physical function because of ADT, can be reversed following a period of resistance training or combined resistance and aerobic exercise [138], [139], [140].
Physical activity assessment
Self-reported physical assessment questionnaires are the cheapest, simplest and most common assessment method [141]. The GPAQ and the IPAQ, frequently used, were designed to survey physical activity in large surveillance studies [142] not clinic cohorts, and with an approximate 30-minute completion time, are too onerous for patients in clinic. In contrast some articles assessed physical activity using one or two simple questions, risking limited information capture. No review articles used the Godin-Shepard Leisure-Time Physical Activity Questionnaire (GSLTPHQ), often used in oncology research [143]. It is a short self-reported questionnaire seeking information on mild, moderate and strenuous physical activity engagement offering a practical solution in the RT clinic.
A limitation of questionnaires is they are vulnerable to unreliable self-reporting and memory recall bias [144]. Accelerometers or pedometers would offer objective physical activity tracking but have associated product and monitoring costs [142]. Smartphone applications benefit from real time data capture, patient accessibility and reduced staffing burden [145], [146] however initial development is costly and low digital literacy can be a barrier for some patients [147].
Pelvic floor disorder
Fifteen articles examined dyssynergic defecation, five were research articles or population surveys linked with constipation [88], [89], [90], [91], [92], two also investigated faecal incontinence [88], [90]. Eleven articles examined rectal hyposensitivity, four of which were research articles or population surveys. Association between rectal hyposensitivity and constipation [62], [90], [93], faecal incontinence [62], [73], [90] and variable bowel disorders [62] were examined. Anorectal laxity was the focus of six articles, four were research studies or population surveys including persons with constipation [88], [90], [92] faecal incontinence [88], [90], or examining association between faecal incontinence and obesity [73]. The effect of age on pelvic floor function was discussed by seven articles two of which were research studies or population surveys.
Anorectal manometry was the primary assessment tool used to quantify pelvic floor disorders [62], [73], [88], [90], [91], [92], [93], [95]. Digital rectal examination was compared to anorectal manometry to detect dyssynergia with sensitivity and specificity results of 75% and 87% respectively [92]. Self-reported symptoms relating to straining, duration of strain, urge to defecate and incomplete evacuation were also useful to inform a diagnosis of pelvic floor disorder [91].
Dyssynergic defecation
Dyssynergic defecation is the inability to coordinate abdominal, anal and pelvic floor muscles during defecation because of inadequate rectal and or abdominal propulsive force, impaired anal relaxation or increased anal sphincter or puborectalis contraction [87]. Dyssynergia rate in constipated cohorts ranges from 53.4 to 87% [89], [91], [92]; in patients with faecal incontinence a rate of 89% was reported [88]. Abdominal pressure deficit and decreased rectal sensitivity are manometric findings associated with dyssynergic defecation, 23.2% and 13.7% of a constipated cohort and 0% and 30.1% of a faecal incontinence cohort met these thresholds respectively [90]. No studies compared to a healthy control.
Rectal hyposensitivity
Rectal sensitivity is the volume of rectal filling needed to evoke a full sensation; higher manometry volumes are indicative of rectal hyposensitivity. In persons with constipation and or faecal incontinence, rectal hyposensitivity prevalence was reported as 17% [93] and 68% [90], variation is in part due to different assessment criteria used. Rectal hyposensitivity was more common and pronounced in constipated participants compared to those with faecal incontinence [90], [93]. Rectal sensation manometry volumes were higher in participants with constipation [90], diabetes [62] and obesity [73]. One study included a healthy control group, their rectal sensation volume was the lowest reported across all studies [62].
Muscle laxity
Anorectal muscle laxity causes reduced anal resting and squeeze pressure. Normal resting pressure values of 40 mmHg–70 mmHg and squeeze pressure values of 100 mmHg–180 mmHg are referenced [88]. Aligned with these three findings outside normal ranges presented: high resting and squeeze pressure in a constipated cohort (85.2 mmHg and 190 mmHg respectively) [88] and low squeeze pressure in persons with faecal incontinence (85.4 mmHg) [90]. In a conflicting study 38 and 43% of a constipated cohort had reduced resting and squeeze pressure respectively [92]. A confounding factor is that comparator normal values vary [90], [92]. Obese individuals with faecal incontinence had similar resting pressures to those without, 62.6 mmHg vs 65.6 mmHg but squeeze pressure was lower 145.9 mmHg vs 173.8 mmHg [73].
Age
One study reports no significant impact of age on the type and rate of pathological findings diagnosed in men with evacuation disorders [94]. Caution is required as only 8% of the population sampled were male (n = 24) [94]. The second reports a significant decrease in mean resting pressure and squeeze pressure and a decrease in rectal sensitivity (although not significant) with advancing age [95].
Pelvic floor disorder and prostate cancer
Aging is a risk factors for pelvic floor disorder in men [148]. Significant pelvic floor muscle thinning is described in healthy men aged 65 years plus, compared to younger men [149], [150] with pelvic floor disorders reported to affect up to 25% of elderly men [151]. Eighty-eight percent of persons diagnosed with PCa in England in 2019–2020, were aged 60 years plus with 56% of these being 70 years or more [2]. It is therefore reasonable to assume that several patients undergoing PCa RT will have diminished pelvic floor strength.
Pelvic floor muscles are exposed to and affected by radiation, with changes in muscle activity and contractility that impact urinary and rectal function [152], [153]. Introducing pelvic floor exercises pre and during RT has been recommended as an approach to prevent urinary incontinence, reduce diarrhoea, and improve quality of life following PCa RT [154].
Pelvic floor assessment
Anorectal manometry is the gold standard assessment method and was utilised by most review articles. Being resource intensive, requiring a specialist workforce, equipment, space and time, renders its use as a scheduled pre-radiotherapy assessment tool unjustifiable.
Digital rectal examination (DRE) can reliably detect dyssynergia and normal anal sphincter tone [92]. DRE technique and grading is well defined in the literature however no standardised, quantifiable method for recording anal sphincter tone on DRE exists [155], [156], [157]. DRE is not typically done in the RT clinic and its routine use would be unnecessarily invasive. However, for patients having prostate fiducial marker insertion, rectal examination is routine, providing an opportune timepoint to assess pelvic floor tone.
An alternative non-invasive approach is self-reporting of symptoms [91]. The Vaizey scale (also known as the St Mark’s incontinence score) [158] is a widely used patient reported questionnaire containing important incontinence-specific items like frequency, type of faecal incontinence, alteration in lifestyle, and pad and/or medication use [159]. It is a recommended instrument for measurement of patient reported pelvic floor disorder [160] and takes under 3 min to complete.
Interlinking GI factors
The effect of each GI factor may be further compounded by interplay between factors. Depression rates are up to three-times higher in patients with diabetes [161]. Low physical activity and depression are associated although the direction of causality is uncertain [162]. Low activity promotes weight gain, being overweight is linked with higher rates of depression and anxiety while medication taken to treat these causes weight gain [163]. Increased abdominal pressure due to obesity increase the risk of pelvic floor disorders.
Obesity is strongly associated with type two diabetes [164]. Physical activity is effective in reducing the risk of type two diabetes [111] however obesity and diabetes typically reduce the level of physical activity a person does [165], [166]. Diabetes induced neuropathy affects the GI tract causing reduced rectal sensitivity [167] and pelvic floor dysfunction [62].
Relevance to radiotherapy outcomes
Further work is necessary to quantify the impact of GI factors on prostate motion however, the comprehensive GI factor assessment package alone holds significant merit in the RT clinic. Co-existing depression, anxiety, diabetes, obesity and physical inactivity are all negatively associated with functional and mortality outcomes in PCa as discussed. Routine upfront assessment of these factors would enable risk stratification and provide the opportunity to tailor pre, during and post RT care to individual needs, to improve patients’ quality of life and health outcomes.
GI disorder assessment
Despite the focus of this review being GI factors not GI morbidity, it would be amiss not to highlight the importance of thoroughly assessing patient’s baseline GI function before RT. The ROME criteria, most frequently used in the literature, is considered the global gold-standard tool for diagnosing functional gastrointestinal disorders [168]. However, its use in a busy clinic has been describes as ‘cumbersome’ [169]. The Bristol Stool scale offers a more convenient way for patients to describe their bowel habits [170] and coupled with medical history this can guide further GI investigations as needed.
Limitations
The scoping review was limited by the absence of a second dedicated reviewer. This is inconsistent with the JBI manual for evidence synthesis [171]. Funding was not available for a second reviewer, so an agreement was established for SA to discuss ambiguous literature with at least one other author.
Lower GI tract physiology and GI factor interaction was not considered in this review. The ‘mechanism of action’ was record in the data extraction table for each GI factor, however these findings sit outside the scope of this report so have not been included. Radiotherapy technique and the impact of bowel preparation and rectal displacement devices e.g., ProSpare™, Rectafix™ and spacers was also not discussed. Identifying GI factors upfront could facilitate more tailored use of such tools.
Excluding literature pertaining to biological female participants limits the generalisability of this work to cancers affecting them.
Conclusion
Six GI factors prevalent in the PCa population and estimated most likely to influence prostate motion during RT were identified: Depression, anxiety, diabetes, obesity, low physical activity, and pelvic floor disorders. Reliable, quick, and easy-to-use tools were suggested to quantify these factors in the RT clinic.
A comprehensive GI factor assessment package suitable to implement into the RT clinic has consequently been created (Table 3). We will introduce this package into clinical practice to establish the prevalence of GI factors in our PCa population and evaluate their effect on inter- and intrafraction prostate motion. If correlation is established between GI factors and prostate motion, these could be integrated into a prostate motion prediction model aligning patient’s predicted motion characteristics with the optimal treatment platform.
Table 3.
Estimated prevalence of GI factor in a prostate cancer population from literature, preferred assessment tool, time, and ease of assessment completion.
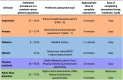 |
Introduction of this assessment package provides an opportunity to integrate health improvement measures into the PCa management pathway, with the potential to ameliorate the long-term health consequences of PCa and PCa treatment [172]. Patient specific mental health, co-morbidity, physical activity and pelvic floor function will be unveiled upfront to guide improved personalisation of RT pre-habilitation and preparation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Alison Tree reports institutional grant funding from Elekta, Varian and Accuray and Travel assistance/honoraria from Elekta, Accuray and Janssen.
Acknowledgments
Acknowledgements
We acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and The Institute of Cancer Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
The Institute of Cancer Research is supported by Cancer Research UK Programme Grant C33589/A28284. Sophie Alexander is funded by Cancer Research UK Programme Grant C33589/A28284 – Adaptive Data-driven Radiation Oncology.
Alison Tree acknowledges the support of Cancer Research UK grant numbers C7224/ A28724 and C33589/A28284.
Helen McNair is funded by a National Institute for Health Research and Health Education England (HEE/NIHR), Senior Clinical Lecturer award (ICA-SCL-2018-04-ST2-002).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100604.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.European Commission. European cancer information. https://ecis.jrc.ec.europa.eu; 2021. Accessed 27/11/2021.
- 2.National Prostate Cancer Audit (NPCA). NPCA Annual report 2020. https://www.npca.org.uk/content/uploads/. Accessed 07/09/2021.
- 3.Dearnaley D., Syndikus I., Mossop H., Khoo V., Birtle A., Bloomfield D., et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Incrocci L., Wortel R.C., Alemayehu W.G., Aluwini S., Schimmel E., Krol S., et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061–1069. doi: 10.1016/S1470-2045(16)30070-5. [DOI] [PubMed] [Google Scholar]
- 5.Catton C.N., Lukka H., Gu C.S., Martin J.M., Supiot S., Chung P.W., et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 6.Widmark A., Gunnlaugsson A., Beckman L., Thellenberg-Karlsson C., Hoyer M., Lagerlund M., et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura K., Shirato H., Seppenwoolde Y., Onimaru R., Oda M., Fujita K., et al. Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int J Radiation Oncol Biol Phys. 2002;53(5):1117–1123. doi: 10.1016/S0360-3016(02)02882-1. [DOI] [PubMed] [Google Scholar]
- 8.Millender L.E., Aubin M., Pouliot J., Shinohara K., Roach M., III Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiation Oncol Biol Phys. 2004;59(1):6–10. doi: 10.1016/j.ijrobp.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Ghilezan M.J., Jaffray D.A., Siewerdsen J.H., Van Herk M., Shetty A., Sharpe M.B., et al. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI) Int J Radiation Oncol Biol Phys. 2005;62(2):406–417. doi: 10.1016/j.ijrobp.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Frank S.J., Dong L., Kudchadker R.J., De Crevoisier R., Lee A.K., Cheung R., et al. Quantification of prostate and seminal vesicle interfraction variation during IMRT. Int J Radiation Oncol Biol Phys. 2008;71(3):813–820. doi: 10.1016/j.ijrobp.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Adamson J., Wu Q. Inferences about prostate intrafraction motion from pre-and posttreatment volumetric imaging. Int J Radiation Oncol Biol Phys. 2009;75(1):260–267. doi: 10.1016/j.ijrobp.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udrescu C., Jalade P., de Bari B., Michel-Amadry G., Chapet O. Evaluation of the respiratory prostate motion with four-dimensional computed tomography scan acquisitions using three implanted markers. Radiother Oncol. 2012;103(2):266–269. doi: 10.1016/j.radonc.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Chasseray M., Dissaux G., Lucia F., Boussion N., Goasduff G., Pradier O., et al. Kilovoltage intrafraction monitoring during normofractionated prostate cancer radiotherapy. Cancer/Radiothérapie. 2020;24(2):99–105. doi: 10.1016/j.canrad.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Padhani A.R., Khoo V.S., Suckling J., Husband J.E., Leach M.O., Dearnaley D.P. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiation Oncol Biol Phys. 1999;44(3):525–533. doi: 10.1016/S0360-3016(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 15.Oates R., Brown A., Tan A., Foroudi F., Joon M.L., Schneider M., et al. Real-time image-guided adaptive-predictive prostate radiotherapy using rectal diameter as a predictor of motion. Clin Oncol. 2017;29(3):180–187. doi: 10.1016/j.clon.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer C., Zamboglou C., Volegova-Neher N., Martini C., Nicolay N.H., Schmidt-Hegemann N.S., et al. Impact of a low FODMAP diet on the amount of rectal gas and rectal volume during radiotherapy in patients with prostate cancer–a prospective pilot study. Radiat Oncol. 2020;15(1):1–9. doi: 10.1186/s13014-020-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marnouche E.A., Hadadi K., Abdelhak M., Benlemlih M., Hommadi M., Zaghba N., et al. Evaluation of margins in pelvic lymph nodes and prostate radiotherapy and the impact of bladder and rectum on prostate position. Cancer/Radiothérapie. 2021;25(2):161–168. doi: 10.1016/j.canrad.2020.06.033. [DOI] [PubMed] [Google Scholar]
- 18.McNair H.A., Wedlake L., Lips I.M., Andreyev J., Van Vulpen M., Dearnaley D. A systematic review: effectiveness of rectal emptying preparation in prostate cancer patients. Pract Radiat Oncol. 2014;4(6):437–447. doi: 10.1016/j.prro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Tøndel H., Solberg A., Lydersen S., Jensen C.A., Kaasa S., Lund J.Å. Rectal volume variations and estimated rectal dose during 8 weeks of image-guided radical 3D conformal external beam radiotherapy for prostate cancer. Clin Transl Radiation Oncol. 2019;15:113–117. doi: 10.1016/j.ctro.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Royal College of Radiologists. On target 2: updated guidance for image-guided radiotherapy. radiotherapy-board-on-target-2-updated-guidance-image-guided-radiotherapy.pdf (rcr.ac.uk). Accessed 01.03.2022. [DOI] [PubMed]
- 21.Griffiths M. Crash Course Gastrointestinal System Updated Edition-E-Book. Elsevier Health Sciences. 2015 [Google Scholar]
- 22.Thompson G.S. Understanding anatomy & physiology: A visual, auditory, interactive approach. FA Davis. 2012 [Google Scholar]
- 23.Transparent reporting of systematic reviews and meta-analyses (PRISMA). Checklist. https://prisma-statement.org//documents/PRISMA-ScR-Fillable-Checklist_11Sept2019.pdf. Accessed 01/10/2021.
- 24.Transparent reporting of systematic reviews and meta-analyses (PRISMA). Flow diagram. https://prisma-statement.org//PRISMAStatement/FlowDiagram.aspx. Accessed 01/10/2021.
- 25.National library of medicine. Medical subject headings. https://www.nlm.nih.gov/mesh/meshhome.html. Accessed 05/06/2021.
- 26.Public Health England. Research and analysis. Chapter 3: trends in morbidity and risk factors – GOV.UK (www.gov.uk). Accessed 05/06/2021.
- 27.Nichol A.M., Warde P.R., Lockwood G.A., Kirilova A.K., Bayley A., Bristow R., et al. A cinematic magnetic resonance imaging study of milk of magnesia laxative and an antiflatulent diet to reduce intrafraction prostate motion. Int J Radiation Oncol Biol Phys. 2010;77(4):1072–1078. doi: 10.1016/j.ijrobp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Lips I.M., Kotte A.N., van Gils C.H., van Leerdam M.E. van der Heide UA, van Vulpen M. Influence of antiflatulent dietary advice on intrafraction motion for prostate cancer radiotherapy. Int J Radiation Oncol Biol Phys. 2011;81(4):e401–e406. doi: 10.1016/j.ijrobp.2011.04.062. [DOI] [PubMed] [Google Scholar]
- 29.Smitsmans M.H., Pos F.J., de Bois J., Heemsbergen W.D., Sonke J.J., Lebesque J.V., et al. The influence of a dietary protocol on cone beam CT–guided radiotherapy for prostate cancer patients. Int J Radiation Oncol Biol Phys. 2008;71(4):1279–1286. doi: 10.1016/j.ijrobp.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Foster E., Lee C., Imamura F., Hollidge S.E., Westgate K.L., Venables M.C., et al. Validity and reliability of an online self-report 24-h dietary recall method (Intake24): a doubly labelled water study and repeated-measures analysis. J Nutr Sci. 2019;8 doi: 10.1017/jns.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goris A.H., Westerterp-Plantenga M.S., Westerterp K.R. Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr. 2000 Jan 1;71(1):130–134. doi: 10.1093/ajcn/71.1.130. [DOI] [PubMed] [Google Scholar]
- 32.JBI. Scoping review network. https://jbi.global/scoping-review-network/resources. Accessed 05/06/2021.
- 33.Duan S., Liu L., Li G., Wang J., Hu Y., Zhang W., et al. Altered functional connectivity within and between salience and sensorimotor networks in patients with functional constipation. Front Neurosci. 2021;147 doi: 10.3389/fnins.2021.628880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosseinzadeh S.T., Poorsaadati S., Radkani B., Forootan M. Psychological disorders in patients with chronic constipation. Gastroenterol Hepatol Bed Bench. 2011;4(3):159–163. [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y., Tang Y.-R., Xie C., Yu T., Xiong W.-J., Lin L., et al. Influence of sleep disorders on somatic symptoms, mental health, and quality of life in patients with chronic constipation. Medicine (Baltimore) 2017;96:1–7. doi: 10.1097/MD.0000000000006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y., Tang Y., Lin L. Clinical Characteristics of Different Primary Constipation Subtypes in a Chinese Population. J Clin Gastroenterol. 2020;54:626–632. doi: 10.1097/MCG.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin Q., Duan S., Li G., Sun L., Hu Y., Hu C., et al. Sex-related differences in resting-state brain activity and connectivity in the orbital frontal cortex and insula in patients with functional constipation. Neurogastroenterol Motil. 2019;31(5):e13566. doi: 10.1111/nmo.13566. [DOI] [PubMed] [Google Scholar]
- 38.Kawamura Y., Yamamoto S., Funaki Y., Ohashi W., Yamamoto K., Ozeki T., et al. Internet survey on the actual situation of constipation in the Japanese population under 70 years old: focus on functional constipation and constipation-predominant irritable bowel syndrome. J Gastroenterol. 2020;55(1):27–38. doi: 10.1007/s00535-019-01611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mody R., Guerin A., Fok B., Lasch K.L., Zhou Z., Wu E.Q., et al. Prevalence and risk of developing comorbid conditions in patients with chronic constipation. Curr Med Res Opin. 2014;30(12):2505–2513. doi: 10.1185/03007995.2014.964854. [DOI] [PubMed] [Google Scholar]
- 40.Moezi P., Salehi A., Molavi H., Poustchi H., Gandomkar A., Imanieh M.H., et al. Prevalence of chronic constipation and its associated factors in pars cohort study: A study of 9000 adults in Southern Iran. Middle East J Digestive Dis. 2018;10(2):75. doi: 10.15171/mejdd.2018.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray H.B., Flanagan R., Banashefski B., Silvernale C.J., Kuo B., Staller K. Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Clin Gastroenterol Hepatol. 2020;18(11):2471–2478. doi: 10.1016/j.cgh.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Panarese A., Pesce F., Porcelli P., Riezzo G., Iacovazzi P.A., Leone C.M., et al. Chronic functional constipation is strongly linked to vitamin D deficiency. World J Gastroenterol. 2019;25(14):1729. doi: 10.3748/wjg.v25.i14.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh P., Surana R., Soni S., Agnihotri A., Ahuja V., Makharia G.K., et al. Cross cultural comparison of constipation profiles at tertiary care centers between India and USA. Neurogastroenterol Motil. 2018;30(8):e13324. doi: 10.1111/nmo.13324. [DOI] [PubMed] [Google Scholar]
- 44.Andy U.U., Vaughan C.P., Burgio K.L., Alli F.M., Goode P.S., Markland A.D. Shared risk factors for constipation, fecal incontinence, and combined symptoms in older US adults. J Am Geriatr Soc. 2016;64(11):e183–e188. doi: 10.1111/jgs.14521. [DOI] [PubMed] [Google Scholar]
- 45.Werth B.L., Fisher M.J., Williams K.A., Pont L.G. Chronic constipation in the community: a national survey of Australian adults. J Wound Ostomy Cont Nurs. 2020;47(3):259–264. doi: 10.1097/WON.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 46.Yu T., Shen X., Li M., Wang M., Lin L. Efficacy and predictors for biofeedback therapeutic outcome in patients with dyssynergic defecation. Gastroenterol Res Pract. 2017 doi: 10.1155/2017/1019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L., Lin Z., Lin L., Wang M., Zhang H. Functional constipation: implications for nursing interventions. J Clin Nurs. 2010;19(13–14):1838–1843. doi: 10.1111/j.1365-2702.2010.03246.x. [DOI] [PubMed] [Google Scholar]
- 48.Aljammaz K.I., Alrashed A.A., Alzwaid A.A. Irritable bowel syndrome: Epidemiology and risk factors in the adult Saudi population of the central region. Niger J Clin Pract. 2020;23(10):1414–1418. doi: 10.4103/njcp.njcp_382_19. [DOI] [PubMed] [Google Scholar]
- 49.Chan Y., So S.H., Mak A.D., Siah K.T., Chan W., Wu J.C. The temporal relationship of daily life stress, emotions, and bowel symptoms in irritable bowel syndrome—diarrhea subtype: a smartphone-based experience sampling study. Neurogastroenterol Motil. 2019;31(3):e13514. doi: 10.1111/nmo.13514. [DOI] [PubMed] [Google Scholar]
- 50.Fan W.J., Xu D., Chang M., Zhu L.M., Fei G.J., Li X.Q., et al. Predictors of healthcare-seeking behavior among Chinese patients with irritable bowel syndrome. World J Gastroenterol. 2017;23(42):7635. doi: 10.3748/wjg.v23.i42.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford A.C., Bercik P., Morgan D.G., Bolino C., Pintos-Sanchez M.I., Moayyedi P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014;39(3):312–321. doi: 10.1111/apt.12573. [DOI] [PubMed] [Google Scholar]
- 52.Lu J., Shi L., Huang D., Fan W., Li X., Zhu L., et al. Depression and structural factors are associated with symptoms in patients of irritable bowel syndrome with diarrhea. J Neurogastroenterol Motility. 2020;26(4):505. doi: 10.5056/jnm19166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okami Y., Kato T., Nin G., Harada K., Aoi W., Wada S., et al. Lifestyle and psychological factors related to irritable bowel syndrome in nursing and medical school students. J Gastroenterol. 2011;46(12):1403–1410. doi: 10.1007/s00535-011-0454-2. [DOI] [PubMed] [Google Scholar]
- 54.Satake R., Sugawara N., Sato K., Takahashi I., Nakaji S., Yasui-Furukori N., et al. Prevalence and predictive factors of irritable bowel syndrome in a community-dwelling population in Japan. Intern Med. 2015;54(24):3105–3112. doi: 10.2169/internalmedicine.54.5378. [DOI] [PubMed] [Google Scholar]
- 55.Windgassen S., Moss-Morris R., Everitt H., Sibelli A., Goldsmith K., Chalder T. Cognitive and behavioral differences between subtypes in refractory irritable bowel syndrome. Behav Ther. 2019;50(3):594–607. doi: 10.1016/j.beth.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Qin G, Liu DR, Wang Y, Yao SK. Increased expression of brain-derived neurotrophic factor is correlated with visceral hypersensitivity in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol 2019;25(2):269. https://doi.org/10.3748%2Fwjg.v25.i2.269. [DOI] [PMC free article] [PubMed]
- 57.Zhu L., Huang D., Shi L., Liang L., Xu T., Chang M., et al. Intestinal symptoms and psychological factors jointly affect quality of life of patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2015;13(1):1–8. doi: 10.1186/s12955-015-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ballou S., Katon J., Singh P., Rangan V., Lee H.N., McMahon C., et al. Chronic diarrhea and constipation are more common in depressed individuals. Clin Gastroenterol Hepatol. 2019;17(13):2696–2703. doi: 10.1016/j.cgh.2019.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jemilohun A.C., Elikwu C.J., Adeleye O.O., Ajiro T.O., Fasesan O.A., Akande K.O. Functional Diarrhoea in a Nigerian Community: Prevalence, Risk Factors and Associated Quality of Life. J Clin Diagn Res. 2020;14(10) [Google Scholar]
- 60.Lackner J., Jaccard J., Baum C., Smith A., Krasner S., Katz L., et al. Patient-reported outcomes for irritable bowel syndrome are associated with patients' severity ratings of gastrointestinal symptoms and psychological factors. Clin Gastroenterol Hepatol. 2011;9(11):957–964. doi: 10.1016/j.cgh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fagherazzi G., Gusto G., Balkau B., Boutron-Ruault M.C., Clavel-Chapelon F., Bonnet F. Functional gastrointestinal disorders and incidence of type 2 diabetes: Evidence from the E3N–EPIC cohort study. Diabetes Metab. 2016;42(3):178–183. doi: 10.1016/j.diabet.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Reszczyńska M., Kempiński R. The prevalence of enteropathy symptoms from the lower gastrointestinal tract and the evaluation of anorectal function in diabetes mellitus patients. J Clin Med. 2021;10(3):415. doi: 10.3390/jcm10030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sangnes D.A., Lundervold K., Bekkelund M., von Volkmann H.L., Berentsen B., Gilja O.H., et al. Gastrointestinal transit and contractility in diabetic constipation: A wireless motility capsule study on diabetes patients and healthy controls. UEG J. 2021;9(10):1168–1177. doi: 10.1002/ueg2.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sangnes D.A., Dimcevski G., Frey J., Søfteland E. Diabetic diarrhoea: a study on gastrointestinal motility, pH levels and autonomic function. J Intern Med. 2021;290(6):1206–1218. doi: 10.1111/joim.13340. [DOI] [PubMed] [Google Scholar]
- 65.Sommers T., Mitsuhashi S., Singh P., Hirsch W., Katon J., Ballou S., et al. Prevalence of chronic constipation and chronic diarrhea in diabetic individuals in the United States. Official J Am College Gastroenterol. 2019;114(1):135–142. doi: 10.1038/s41395-018-0418-8. [DOI] [PubMed] [Google Scholar]
- 66.Brock C., Liao D., Wegeberg A.M., Mohr D.A. The antroduodenal transition time is prolonged in adults with type 1 diabetes. Neurogastroenterol Motil. 2021;33(11):e14144. doi: 10.1111/nmo.14144. [DOI] [PubMed] [Google Scholar]
- 67.Egea Valenzuela J, Sánchez Martínez A, García Marín AV, Alberca de Las Parras F. Influence of demographic and clinical features of the patient on transit times and impact the on the diagnostic yield of capsule endoscopy. Rev Esp Enferm Dig. 2019; 111:530-6. https://doi.org/10.17235/reed.2019.5971/2018. [DOI] [PubMed]
- 68.Faria M., Pavin E.J., Parisi M.C., Lorena S.L., Brunetto S.Q., Ramos C.D., et al. Delayed small intestinal transit in patients with long-standing type 1 diabetes mellitus: investigation of the relationships with clinical features, gastric emptying, psychological distress, and nutritional parameters. Diabetes Technol Ther. 2013;15(1):32–38. doi: 10.1089/dia.2012.0158. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen N.Q., Debreceni T.L., Burgess J.E., Bellon M., Wishart J., Standfield S., et al. Impact of gastric emptying and small intestinal transit on blood glucose, intestinal hormones, glucose absorption in the morbidly obese. Int J Obes (Lond) 2018;42(9):1556–1564. doi: 10.1038/s41366-018-0012-6. [DOI] [PubMed] [Google Scholar]
- 70.Basseri R.J., Basseri B., Pimentel M., Chong K., Youdim A., Low K., et al. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol. 2012;8(1):22. [PMC free article] [PubMed] [Google Scholar]
- 71.Sileri P., Franceschilli L., Cadeddu F., De Luca E., D’Ugo S., Tognoni V., et al. Prevalence of defaecatory disorders in morbidly obese patients before and after bariatric surgery. J Gastrointest Surg. 2012;16(1):62–67. doi: 10.1007/s11605-011-1705-5. [DOI] [PubMed] [Google Scholar]
- 72.Ballou S., Singh P., Rangan V., Iturrino J., Nee J., Lembo A. Obesity is associated with significantly increased risk for diarrhoea after controlling for demographic, dietary and medical factors: a cross-sectional analysis of the 2009–2010 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2019;50(9):1019–1024. doi: 10.1111/apt.15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neto I.J., Pinto R.A., Jorge J.M., Santo M.A., Bustamante-Lopez L.A., Cecconello I., et al. Are obese patients at an increased risk of pelvic floor dysfunction compared to non-obese patients? Obes Surg. 2017;27(7):1822–1827. doi: 10.1007/s11695-017-2559-z. [DOI] [PubMed] [Google Scholar]
- 74.Roland B.C., Lee D., Miller L.S., Vegesna A., Yolken R., Severance E., et al. Obesity increases the risk of small intestinal bacterial overgrowth (SIBO) Neurogastroenterol Motil. 2018;30(3):e13199. doi: 10.1111/nmo.13199. [DOI] [PubMed] [Google Scholar]
- 75.Cheng J., Li L., Xu F., Xu Y., Lin L., Chen J.D. Poststroke constipation is associated with impaired rectal sensation. Offic J Am College Gastroenterol. 2020;115(1):105–114. doi: 10.14309/ajg.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 76.Tremblay M.S., Aubert S., Barnes J.D., Saunders T.J., Carson V., Latimer-Cheung A.E., et al. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):1–7. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jetté M., Sidney K., Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 78.Li Y., Tong W.D., Qian Y. Effect of Physical Activity on the Association Between Dietary Fiber and Constipation: Evidence from the National Health and Nutrition Examination Survey 2005–2010. J Neurogastroenterol Motility. 2021;27(1):97. doi: 10.5056/jnm20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsushita E., Okada K., Ito Y., Satake S., Shiraishi N., Hirose T., et al. Characteristics of physical prefrailty among Japanese healthy older adults. Geriatr Gerontol Int. 2017;17(10):1568–1574. doi: 10.1111/ggi.12935. [DOI] [PubMed] [Google Scholar]
- 80.Otani K., Watanabe T., Takahashi K., Nadatani Y., Fukunaga S., Hosomi S., et al. Prevalence and risk factors of functional constipation in the Rome IV criteria during a medical check-up in Japan. J Gastroenterol Hepatol. 2021;36(8):2157–2164. doi: 10.1111/jgh.15436. [DOI] [PubMed] [Google Scholar]
- 81.Sajitha N., Kumari B. Association of gender, physical activity, and fluid intake with bowel habits and symptoms of constipation. Natl J Physiol Pharmacy Pharmacol. 2021;11(6):657–661. doi: 10.5455/njppp.2021.11.04122202103052021. [DOI] [Google Scholar]
- 82.Šket R., Treichel N., Debevec T., Eiken O., Mekjavic I., Schloter M., et al. Hypoxia and inactivity related physiological changes (constipation, inflammation) are not reflected at the level of gut metabolites and butyrate producing microbial community: the PlanHab study. Front Physiol. 2017;8:250. doi: 10.3389/fphys.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taba Vakili S., Nezami B.G., Shetty A., Chetty V.K., Srinivasan S. Association of high dietary saturated fat intake and uncontrolled diabetes with constipation: evidence from the National Health and Nutrition Examination Survey. Neurogastroenterol Motil. 2015;27(10):1389–1397. doi: 10.1111/nmo.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson P.B. Associations between physical activity and constipation in adult Americans: Results from the National Health and Nutrition Examination Survey. Neurogastroenterol Motil. 2020;32(5):e13789. doi: 10.1111/nmo.13789. [DOI] [PubMed] [Google Scholar]
- 85.Ma C., Wu S., Yang P., Li H., Tang S., Wang Q. Behavioural factors associated with diarrhea among adults over 18 years of age in Beijing. China BMC Public Health. 2014;14(1):1–7. doi: 10.1186/1471-2458-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song B.K., Kim Y.S., Kim H.S., Oh J.W., Lee O., Kim J.S. Combined exercise improves gastrointestinal motility in psychiatric in patients. World J Clin Cases. 2018;6(8):207. doi: 10.12998/wjcc.v6.i8.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao S.S., Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J Neurogastroenterol Motility. 2016;22(3):423. doi: 10.5056/jnm16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrianjafy C., Luciano L., Bazin C., Baumstarck K., Bouvier M., Vitton V. Three-dimensional high-resolution anorectal manometry in functional anorectal disorders: results from a large observational cohort study. Int J Colorectal Dis. 2019;34(4):719–729. doi: 10.1007/s00384-019-03235-z. [DOI] [PubMed] [Google Scholar]
- 89.Cavallaro P.M., Staller K., Savitt L.R., Milch H., Kennedy K., Weinstein M.M., et al. The contributions of internal intussusception, irritable bowel syndrome, and pelvic floor dyssynergia to obstructed defecation syndrome. Dis Colon Rectum. 2019;62(1):56–62. doi: 10.1097/dcr.0000000000001250. [DOI] [PubMed] [Google Scholar]
- 90.Camargo H.P., Machado V.F., Parra R.S., Féres O., Rocha J.J., Feitosa M.R. Main manometric findings and potential for anorectal physical therapy in the treatment of patients with evacuation disorders. Arq Gastroenterol. 2020;57:306–310. doi: 10.1590/S0004-2803.202000000-56. [DOI] [PubMed] [Google Scholar]
- 91.Parker C.H., Tomlinson G., Correia A., Liu L.W. The use of standardized questions in identifying patients with dyssynergic defecation. J Can Assoc Gastroenterol. 2018;1(2):60–66. doi: 10.1093/jcag/gwy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tantiphlachiva K., Rao P., Attaluri A., Rao S.S. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 93.Burgell R.E., Bhan C., Lunniss P.J., Scott S.M. Fecal incontinence in men: coexistent constipation and impact of rectal hyposensitivity. Dis Colon Rectum. 2012;55(1):18–25. doi: 10.1097/dcr.0b013e318237f37d. [DOI] [PubMed] [Google Scholar]
- 94.Andrade L.C., Correia H., Semedo L.C., Ilharco J., Caseiro-Alves F. Conventional videodefecography: Pathologic findings according to gender and age. Eur J Radiol Open. 2014;1:1–5. doi: 10.1016/j.ejro.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gundling F., Seidl H., Scalercio N., Schmidt T., Schepp W., Pehl C. Influence of gender and age on anorectal function: normal values from anorectal manometry in a large caucasian population. Digestion. 2010;81(4):207–213. doi: 10.1159/000258662. [DOI] [PubMed] [Google Scholar]
- 96.Fervaha G., Izard J.P., Tripp D.A., Rajan S., Leong D.P., Siemens D.R. Depression and prostate cancer: A focused review for the clinician. In Urologic Oncology: Seminars and Original Investigations. 2019;37(4):282–288. doi: 10.1016/j.urolonc.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 97.Sharpley C.F., Christie D.R., Bitsika V. Depression and prostate cancer: implications for urologists and oncologists. Nat Rev Urol. 2020;17(10):571–585. doi: 10.1038/s41585-020-0354-4. [DOI] [PubMed] [Google Scholar]
- 98.Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watts S., Leydon G., Birch B., Prescott P., Lai L., Eardley S., et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901. doi: 10.1136/bmjopen-2013-003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caruso R., Nanni M.G., Riba M., Sabato S., Mitchell A.J., Croce E., et al. Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol. 2017;56(2):146–155. doi: 10.1080/0284186X.2016.1266090. [DOI] [PubMed] [Google Scholar]
- 101.Donovan J.L., Hamdy F.C., Lane J., Mason M., Metcalfe C., Walsh E., et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dinh K.T., Reznor G., Muralidhar V., Mahal B.A., Nezolosky M.D., Choueiri T.K., et al. Association of androgen deprivation therapy with depression in localized prostate cancer. J Clin Oncol. 2016;34(16):1905. doi: 10.1200/JCO.2015.64.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dinesh AA, Pinto SH, Brunckhorst O, Dasgupta P, Ahmed K. Anxiety, depression and urological cancer outcomes: A systematic review. InUrologic Oncology: Seminars and Original Investigations 2021 Dec 1 (Vol. 39, No. 12, pp. 816-828). Elsevier. https://doi.org/10.1016/j.urolonc.2021.08.003. [DOI] [PubMed]
- 104.National Institute for Health and Care Excellence (NICE). Depression diagnosis. https://cks.nice.org.uk/topics/depression/diagnosis/diagnosis/. Accessed 04/03/2022.
- 105.Naser A.Y., Hameed A.N., Mustafa N., Alwafi H., Dahmash E.Z., Alyami H.S., et al. Depression and anxiety in patients with cancer: a cross-sectional study. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.585534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Department of Health. No health without mental health: a cross-government mental health outcomes strategy for people of all ages. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/138253/dh_124058.pdf. Accessed 04/03/2022.
- 107.Guo C., Huang X. Hospital anxiety and depression scale exhibits good consistency but shorter assessment time than Zung self-rating anxiety/depression scale for evaluating anxiety/depression in non-small cell lung cancer. Medicine. 2021;100(8) doi: 10.1097/MD.0000000000024428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esser P., Hartung T.J., Friedrich M., Johansen C., Wittchen H.U., Faller H., et al. The Generalized Anxiety Disorder Screener (GAD-7) and the anxiety module of the Hospital and Depression Scale (HADS-A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology. 2018;27(6):1509–1516. doi: 10.1002/pon.4681. [DOI] [PubMed] [Google Scholar]
- 109.Shunmugasundaram C., Rutherford C., Butow P.N., Sundaresan P., Dhillon H.M. What are the optimal measures to identify anxiety and depression in people diagnosed with head and neck cancer (HNC): a systematic review. J Patient-Reported Outcomes. 2020;4(1):1–4. doi: 10.1186/s41687-020-00189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diabetes UK. Position statements and reports. https://www.diabetes.org.uk/professionals/position-statements-reports/statistics. Accessed 06/03/2022.
- 111.Diabetes UK. Diabetes risk factors. https://www.diabetes.org.uk/preventing-type-2-diabetes/diabetes-risk-factors. Accessed 06/03/2022.
- 112.Prostate cancer UK (PCUK). Are you at risk? https://prostatecanceruk.org/prostate-information/are-you-at-risk. Accessed 06/03/2022.
- 113.Cancer research UK (CRUK). Prostate cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer. Accessed 06/03/2022.
- 114.Janssen-Heijnen M.L., Houterman S., Lemmens V.E., Louwman M.W., Maas H.A., Coebergh J.W. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55(3):231–240. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Lemanska A., Byford R.C., Cruickshank C., Dearnaley D.P., Ferreira F., Griffin C., et al. Linkage of the CHHiP randomised controlled trial with primary care data: a study investigating ways of supplementing cancer trials and improving evidence-based practice. BMC Med Res Method. 2020;20(1):1–2. doi: 10.1186/s12874-020-01078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jefferson M., Drake R.R., Lilly M., Savage S.J., Price S.T., Halbert C.H. Co-morbidities in a retrospective cohort of prostate cancer patients. Ethn Dis. 2020;30(Suppl 1):185. doi: 10.18865/ed.30.S1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang L.S., Murphy C.T., Ruth K., Zaorsky N.G., Smaldone M.C., Sobczak M.L., et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2015;121(17):3010–3017. doi: 10.1002/cncr.29472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grossmann M., Wittert G. Androgens, diabetes and prostate cancer. Endocr Relat Cancer. 2012 Oct 1;19(5):F47–F62. doi: 10.1530/ERC-12-0067. [DOI] [PubMed] [Google Scholar]
- 119.Vissers P.A., Falzon L., van de Poll-Franse L.V., Pouwer F., Thong M.S. The impact of having both cancer and diabetes on patient-reported outcomes: a systematic review and directions for future research. J Cancer Surviv. 2016 Apr;10(2):406–415. doi: 10.1007/s11764-015-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.National Health Service. Overview, obesity. https://www.nhs.uk/conditions/obesity/. Accessed 06/03/2022.
- 121.National Academies of Sciences, Engineering, and Medicine. Incorporating weight management and physical activity throughout the cancer care continuum: Proceedings of a workshop. National Academies Press; 2018. https://doi.org/10.17226/24925. [PubMed]
- 122.World cancer research fund. Body fatness and weight gain. https://www.wcrf.org/wp-content/uploads/2021/01/Body-fatness-and-weight-gain_0.pdf. Accessed 06/03/2022.
- 123.Freedland S.J., Sun L., Kane C.J., Presti J.C., Jr, Terris M.K., Amling C.L., et al. Obesity and oncological outcome after radical prostatectomy: impact of prostate-specific antigen-based prostate cancer screening: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102(8):969–974. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 124.Buschemeyer W.C., III, Freedland S.J. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007 Aug;52(2):331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 125.Wilson R.L., Taaffe D.R., Newton R.U., Hart N.H., Lyons-Wall P., Galvão D.A. Obesity and prostate cancer: A narrative review. Crit Rev Oncol Hematol. 2022;169 doi: 10.1016/j.critrevonc.2021.103543. [DOI] [PubMed] [Google Scholar]
- 126.Collier A., Ghosh S., McGlynn B., Hollins G. Prostate Cancer, Androgen Deprivation Therapy, Obesity, the Metabolic Syndrome, Type 2 Diabetes, and Cardiovascular Disease. Am J Clin Oncol. 2012;35(5):504–509. doi: 10.1097/coc.0b013e318201a406. [DOI] [PubMed] [Google Scholar]
- 127.Morote J., Gómez-Caamaño A., Alvarez-Ossorio J.L., Pesqueira D., Tabernero A., Gómez Veiga F., et al. The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J Urol. 2015;193(6):1963–1969. doi: 10.1016/j.juro.2014.12.086. [DOI] [PubMed] [Google Scholar]
- 128.Turner L., Poole K., Faithfull S., Griffin B.A. Current and future strategies for the nutritional management of cardiometabolic complications of androgen deprivation therapy for prostate cancer. Nutr Res Rev. 2017;30(2):220–232. doi: 10.1017/S0954422417000087. [DOI] [PubMed] [Google Scholar]
- 129.Strom S.S., Kamat A.M., Gruschkus S.K., Gu Y., Wen S., Cheung M.R., et al. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006 Aug 1;107(3):631–639. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 130.Stroup S.P., Cullen J., Auge B.K., L'Esperance J.O., Kang S.K. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer. 2007 Sep 1;110(5):1003–1009. doi: 10.1002/cncr.22873. [DOI] [PubMed] [Google Scholar]
- 131.World health organisation (WHO). Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 06/03/2022.
- 132.National Institute for Health and Care Excellence (NICE). Obesity: identification, assessment and management. https://www.nice.org.uk/guidance/cg189/chapter/1-Recommendations#identification-and-classification-of-overweight-and-obesity. Accessed 06/03/2022. [PubMed]
- 133.British Heart Foundation (BHF). Physical inactivity report 2017. https://www.bhf.org.uk/informationsupport/publications/statistics/physical-inactivity-report-2017. Accessed 06/03/2022.
- 134.Kenfield S.A., Stampfer M.J., Chan J.M., Giovannucci E. Smoking and prostate cancer survival and recurrence. J Am Med Assoc. 2011;305(24):2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y., Hu F., Li D., Wang F., Zhu L., Chen W., et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011 Nov;60(5):1029–1044. doi: 10.1016/j.eururo.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 136.Sorial E., Si S., Fritschi L., Darcey E., Leavy J.E., Girschik J., et al. Lifetime recreational physical activity and the risk of prostate cancer. Cancer Causes Control. 2019;30(6):617–625. doi: 10.1007/s10552-019-01138-6. [DOI] [PubMed] [Google Scholar]
- 137.Friedenreich C.M., Stone C.R., Cheung W.Y., Hayes S.C. Physical Activity and Mortality in Cancer Survivors: A Systematic Review and Meta-Analysis, JNCI Cancer. Spectrum. February 2020;4(1):pkz080. doi: 10.1093/jncics/pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Segal R.J., Reid R.D., Courneya K.S., Malone S.C., Parliament M.B., Scott C.G., et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003 May 1;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 139.Galvao D.A., Nosaka K., Taaffe D.R., Spry N., Kristjanson L.J., McGuigan M.R., et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006 Dec 1;38(12):2045–2052. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 140.Galvao D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010 Jan 10;28(2):340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 141.Brown J.C., Winters-Stone K., Lee A., Schmitz K.H. Cancer, physical activity, and exercise. Compr Physiol. 2012;2(4):2775. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cleland C., Ferguson S., Ellis G., Hunter R.F. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behaviour of older adults in the United Kingdom. BMC Med Res Method. 2018;18(1):1–2. doi: 10.1186/s12874-018-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Amireault S., Godin G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills. 2015;120(2):604–622. doi: 10.2466/03.27.PMS.120v19x7. [DOI] [PubMed] [Google Scholar]
- 144.Seymour D.G., Ball A.E., Russell E.M., Primrose W.R., Garratt A.M., Crawford J.R. Problems in using health survey questionnaires in older patients with physical disabilities. The reliability and validity of the SF-36 and the effect of cognitive impairment. J Eval Clin Pract. 2001;7(4):411–418. doi: 10.1046/j.1365-2753.2001.00296.x. [DOI] [PubMed] [Google Scholar]
- 145.Higgins J.P. Smartphone applications for patients' health and fitness. Am J Med. 2016;129(1):11–19. doi: 10.1016/j.amjmed.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 146.Sandborg J., Henriksson P., Larsen E., Lindqvist A.K., Rutberg S., Söderström E., et al. Participants’ Engagement and Satisfaction With a Smartphone App Intended to Support Healthy Weight Gain, Diet, and Physical Activity During Pregnancy: Qualitative Study Within the HealthyMoms Trial. JMIR Mhealth Uhealth. 2021;9(3):e26159. doi: 10.2196/26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neves A.L., Jácome C., Taveira-Gomes T., Pereira A.M., Almeida R., Amaral R., et al. Determinants of the use of health and fitness mobile apps by patients with asthma: secondary analysis of observational studies. J Med Internet Res. 2021 Sep 22;23(9):e25472. doi: 10.2196/25472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lakhoo J., Khatri G., Elsayed R.F., Chernyak V., Olpin J., Steiner A., et al. MRI of the male pelvic floor. Radiographics. 2019;39(7):2003–2022. doi: 10.1148/rg.2019190064. [DOI] [PubMed] [Google Scholar]
- 149.Komemushi Y., Komemushi A., Morimoto K., Yoneda Y., Yoshimura R., Tanaka T., et al. Quantitative evaluation of age-related changes to pelvic floor muscles in magnetic resonance images from 369 patients. Geriatr Gerontol Int. 2019;19(8):834–837. doi: 10.1111/ggi.13726. [DOI] [PubMed] [Google Scholar]
- 150.Tai J.W., Sorkhi S.R., Trivedi I., Sakamoto K., Albo M., Bhargava V., et al. Evaluation of age-and radical-prostatectomy related changes in male pelvic floor anatomy based on magnetic resonance imaging and 3-dimensional reconstruction. The World Journal of Men's Health. 2021;39(3):566. doi: 10.5534/wjmh.200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Avers D., Wong R. Guccione's Geriatric Physical Therapy E-Book. Elsevier Health Sciences. 2019 [Google Scholar]
- 152.Smeenk R.J., Hoffmann A.L., Hopman W.P., van Lin E.N., Kaanders J.H. Dose-effect relationships for individual pelvic floor muscles and anorectal complaints after prostate radiotherapy. Int J Radiation Oncol Biol Phys. 2012 Jun 1;83(2):636–644. doi: 10.1016/j.ijrobp.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 153.Bernard S., Ouellet M.P., Moffet H., Roy J.S., Dumoulin C. Effects of radiation therapy on the structure and function of the pelvic floor muscles of patients with cancer in the pelvic area: a systematic review. J Cancer Surviv. 2016 Apr;10:351–362. doi: 10.1007/s11764-015-0481-8. [DOI] [PubMed] [Google Scholar]
- 154.Urvaylıoğlu A.E., Kutlutürkan S., Kılıç D. Effect of Kegel exercises on the prevention of urinary and fecal incontinence in patients with prostate cancer undergoing radiotherapy. Eur J Oncol Nurs. 2021 Apr;1(51) doi: 10.1016/j.ejon.2021.101913. [DOI] [PubMed] [Google Scholar]
- 155.Dieperink K.B., Johansen C., Hansen S., Wagner L., Andersen K.K., Minet L.R., et al. The effects of multidisciplinary rehabilitation: RePCa—a randomised study among primary prostate cancer patients. Br J Cancer. 2013;109(12):3005–3013. doi: 10.1038/bjc.2013.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ribeiro A.M., Nammur L.G., Mateus-Vasconcelos E.C., Ferreira C.H., Muglia V.F., Oliveira H.F. Pelvic floor muscles after prostate radiation therapy: morpho-functional assessment by magnetic resonance imaging, surface electromyography and digital anal palpation. Int Braz J Urol. 2020;47:120–130. doi: 10.1590/S1677-5538.IBJU.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Orkin B.A., Sinykin S.B., Lloyd P.C. The digital rectal examination scoring system (DRESS) Dis Colon Rectum. 2010;53(12):1656–1660. doi: 10.1007/DCR.0b013e3181f23c85. [DOI] [PubMed] [Google Scholar]
- 158.Vaizey C.J., Carapeti E., Cahill J.A., Kamm M.A. Prospective comparison of faecal incontinence grading systems. Gut. 1999 Jan 1;44(1):77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Terra M.P., Deutekom M., Dobben A.C., Baeten C.G., Janssen L.W., Boeckxstaens G.E., et al. Can the outcome of pelvic-floor rehabilitation in patients with fecal incontinence be predicted? Int J Colorectal Dis. 2008;23(5):503–511. doi: 10.1007/s00384-008-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bordeianou L.G., Anger J., Boutros M., Birnbaum E., Carmichael J.C., Connell K., et al. Measuring pelvic floor disorder symptoms using patient-reported instruments: proceedings of the consensus meeting of the pelvic floor consortium of the American Society of Colon and Rectal Surgeons, the International Continence Society, the American Urogynecologic Society, and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction. Tech Coloproctol. 2020;24(1):5–22. doi: 10.1007/s10151-019-02125-4. [DOI] [PubMed] [Google Scholar]
- 161.Bădescu S.V., Tătaru C., Kobylinska L., Georgescu E.L., Zahiu D.M., Zăgrean A.M., et al. The association between diabetes mellitus and depression. J Med Life. 2016;9(2):120. [PMC free article] [PubMed] [Google Scholar]
- 162.Roshanaei-Moghaddam B., Katon W.J., Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31(4):306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Pereira-Miranda E., Costa P.R., Queiroz V.A., Pereira-Santos M., Santana M.L. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36(3):223–233. doi: 10.1080/07315724.2016.1261053. [DOI] [PubMed] [Google Scholar]
- 164.Bray G.A. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 165.Fontaine K.R., Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2(3):173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 166.Morrato E.H., Hill J.O., Wyatt H.R., Ghushchyan V., Sullivan P.W. Physical activity in US adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30(2):203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 167.Søfteland E., Brock C., Frøkjær J.B., Simrén M., Drewes A.M., Dimcevski G. Rectal sensitivity in diabetes patients with symptoms of gastroparesis. Journal of Diabetes Research. 2014;2014 doi: 10.1155/2014/784841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Holtmann G.J., Talley N.J. Inconsistent symptom clusters for functional gastrointestinal disorders in Asia: is Rome burning? Gut. 2018 Nov 1;67(11):1911–1915. doi: 10.1136/gutjnl-2017-314775. [DOI] [PubMed] [Google Scholar]
- 169.Shivaji U.N., Ford A.C. Beliefs about management of irritable bowel syndrome in primary care: cross-sectional survey in one locality. Prim Health Care Res Dev. 2015 May;16(3):263–269. doi: 10.1017/S1463423614000383. [DOI] [PubMed] [Google Scholar]
- 170.Lacy B.E., Patel N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017 Oct 26;6(11):99. doi: 10.3390/jcm6110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-20-01.
- 172.Faithfull S., Lemanska A., Poole K., Aning J., Manders R., Marshall J., et al. Obesity and low levels of physical activity impact on cardiopulmonary fitness in older men after treatment for prostate cancer. Eur J Cancer Care. 2021 Nov;30(6):e13476. doi: 10.1111/ecc.13476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



