Abstract
Purpose
Long-term immunity after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in immunosuppressed patients is not well characterized. We aimed to explore the long-term natural immunity against SARS-CoV-2 in liver transplant (LT) recipients compared to the non-transplanted population (control group).
Methods
Fifteen LT recipients and 15 controls matched according to variables associated with disease severity were included at 12 months following the coronavirus disease 2019 (COVID-19) onset. Peripheral blood mononuclear cells were stimulated with peptide pools covering spike (S), nucleocapside (N), and membrane (M) proteins. Reactive CD4+ and CD8+ T cells were identified using flow cytometry, and cytokine production was evaluated in the culture supernatants using cytometric bead array. Serum anti-N and anti-S IgG antibodies were detected with chemiluminescence.
Results
The percentage of patients with a positive response in both CD4+ and CD8+ T cells against each viral protein and IL2, IL10, TNF-α, and IFN-γ levels was similar between LT recipients and controls. IFN-γ levels were positively correlated with the percentage of reactive CD4+ (p = 0.022) and CD8+ (p = 0.043) T cells to a mixture of M + N + S peptide pools. The prevalence and levels of anti-N and anti-S IgG antibodies were slightly lower in the LT recipients, but the difference was not statistically significant.
Conclusion
LT recipients exhibited a similar T cell response compared to non-transplanted individuals one year after COVID-19 diagnosis.
Keywords: Flow cytometry, Humoral immunity, Liver transplantation, Reactive T cells, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly become a public health issue profoundly affecting healthcare systems worldwide, including liver transplantation (LT) programs. Knowledge of the natural history of the disease is progressively increasing; however, the durability of adaptive immunity to SARS-CoV-2 after natural infection in immunocompromised patients remains unclear.
The induction of effective and durable immune memory, in both humoral and cellular arms, is essential to prevent severe disease and protect against reinfection. A broad immune response to SARS-CoV-2 has been well documented, with multiple epitopes of membrane (M) glycoprotein, nucleocapsid (N) phosphoprotein, and spike (S) protein being the most prominent targets of specific T and B cells.1 , 2 Previous studies have shown long-term persistence of S-specific IgG+ memory B cells and virus-specific CD4+ and CD8+ T cells3 in immunocompetent patients. In addition, early and medium-term T cell-mediated immune response4 has been described in LT recipients. Compared to nontransplanted patients, LT recipients show a lower prevalence of anti-SARS-CoV-2 IgG antibodies 12 months after COVID-195 but the long-term cellular immunity to SARS-CoV-2 in these patients has not yet been assessed.
The present study aimed to assess the long-term specific SARS-CoV-2 T cell-mediated immune response in LT recipients compared with non-transplant patients.
Methods
Study population
Fifteen LT recipients and 15 non-transplanted controls who had confirmed COVID-19 in March–April 2020 were included at 12 months following COVID-19 diagnosis to determine SARS-CoV-2-reactive T cells and humoral responses. None of the patients received therapy with immunoglobulins or convalescent plasma transfusions, active chemotherapy, or SARS-CoV-2 vaccination. LT recipients with clinical operational tolerance and controls receiving any immunosuppressive treatment were excluded.
All of the patients had been enrolled in previous studies5 including a large cohort of LT recipients and controls matched using a propensity score according to demographic features, comorbidities (diabetes, arterial hypertension, and cardiovascular disease), hospital admission, requirement of mechanical ventilation, and admission to the intensive care unit. COVID-19 was confirmed by a real-time reverse transcription-polymerase chain reaction assay6 on nasopharyngeal swab samples. Severe COVID-19 was defined as admission to the intensive care unit or requirement of mechanical ventilation. Demographic and clinical data were obtained from reliable electronic medical records.
The study was performed according to the principles of the Declaration of Helsinki and European Union Regulation 2016/679 and was approved by the local research ethics committee. All patients provided written informed consent before their inclusion in the study.
Sample collection
Peripheral venous blood was collected from each patient at Hospital General Universitario Gregorio Marañón. Serum samples were recovered from anticoagulant-free tubes after being allowed to clot at room temperature and then centrifuged for 10 min at 800×g. The recovered serum was cryopreserved in small aliquots at −80 °C until use. Freshly EDTA-anticoagulated blood was shipped at room temperature to the Hospital Universitario Puerta de Hierro Majadahonda, and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation within 4 h of collection. Samples shipped for cellular assays were anonymized and blinded to patient status until statistical analysis was performed.
In vitro stimulation of SARS-Cov-2 reactive T cells
Fresh PBMCs were stimulated with overlapping peptide pools (PepTivator peptide pools; Miltenyi Biotec, Cologne, Germany) covering the complete sequence of proteins M and N or the immunodominant sequence domains of protein S. PBMCs were seeded at a density of 5 × 106 cells/cm2 in U-bottom 96-well plates in TexMACS™ (Miltenyi Biotec) culture medium containing 1ug/ml of proteins M, N, S, or a mixture of all three proteins (M + N + S). Each experiment included negative and positive controls for stimulation, consisting of PBMCs in complete medium alone or with a polyclonal stimulator of human effector/memory T cells (Cytostim; Miltenyi Biotec), respectively. Cells were harvested after 16 h at 37 °C and 5% CO2, and culture supernatants were cryopreserved at −20 °C until use.
Analysis of SARS-CoV-2- reactive T cells
After culturing under the conditions described above, PBMCs were washed in PBS/2 mM EDTA/0.5% BSA and incubated with mouse anti-human antibodies anti-CD3-FITC (clone BW264/56), anti-CD137-PE (clone 4B4-1), anti-CD69-APC (clone FN50, all from Miltenyi Biotec), and anti-CD4-PerCp-Cy5.5 or anti-CD8-PerCp-Cy5.5 (BD Biosciences; Franklin Lakes, New Jersey, USA). Mouse anti-human isotype control-irrelevant antibodies were used as negative controls for staining. At least 30,000 CD3+CD4+ or CD3+CD8+ cells were acquired and analyzed using a FACSCalibur flow cytometer (BD Biosciences) with CELLQuest-Pro software.
SARS-CoV-2 reactive T cells were considered as CD3+CD4+ or CD3+CD8+ T lymphocytes expressing CD137 and CD69 simultaneously. The percentage of reactive T cells was calculated by subtracting non-specific reactive T cells when PBMCs were cultured in media alone. The cut-off values for a positive response were set at the mean ± 2 standard deviation (SD) of reactive T cells when PBMCs were cultured without stimuli.
Analysis of cytokine secretion after stimulation with SARS-CoV-2 peptides
Interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ were quantified in the culture supernatants by flow cytometry using the Cytometric Bead Array Human Th1/Th2 Cytokine Kit II (BD Biosciences) according to the manufacturer's instructions. Samples and standards were acquired using a FACSCalibur flow cytometer and analyzed using FCAP Array software (BD Biosciences). The final concentration of each cytokine was calculated by subtracting the concentration observed in the supernatant of cells cultured in the media alone.
Assessment of SARS-CoV-2-specific antibodies
SARS-CoV-2 IgG antibodies targeting protein N were detected in serum samples with chemiluminescence (SARS-CoV-2 IgG Reagent Kit, Abbott, Chicago, Illinois, USA)5 and antibodies targeting protein S with a quantitative chemiluminescent assay (SARS-CoV-2 IgG II Quant Reagent Kit, Abbott)5 using the ARCHITECT i2000 INSTRUMENT (Abbott).
Statistical analysis
Continuous variables are reported as median and interquartile range (IQR), and categorical variables as absolute numbers and percentages.
Demographic and clinical characteristics of LT recipients and controls or of patients classified according to the reactivity of T cells against SARS-CoV-2 protein N were compared using the Fisher correction for the chi-square test or the Mann–Whitney U test, whenever appropriate.
The Mann–Whitney U test was used to compare the percentage of reactive T cells and cytokine or IgG levels between the LT recipients and controls. Qualitative analyses of reactive T-cells against SARS-CoV-2 proteins and IgG prevalence were performed using Fisher's correction for the chi-square test.
Correlations between the percentage of reactive T cells and cytokine or serum IgG levels were calculated using Spearman's test.
Statistical analyses were made using SPSS version 25 (IBM SPSS Statistics), and graphs were generated using GraphPad Prism version 6.0 (GraphPad Software). All tested hypotheses were two-tailed and considered significant at P < 0.05.
Results
Study population and baseline characteristics
The assessment of T cell-mediated and humoral immune responses was carried out at a median time of 11.9 (IQR 11.7–12.16) months after COVID-19 diagnosis without evidence of SARS-CoV-2 reinfection. Most patients were male (73.3%), with a median age of 63 years (Table 1 ). Arterial hypertension was the most frequent comorbidity (66.7%), and none of the patients had chronic obstructive pulmonary disease. All patients presented with symptomatic COVID-19. Eleven (73.3%) LT recipients and 14 (93.3%) controls required hospital admission, but only 4 patients (13.3%) presented with severe COVID-19. None of the LT recipients received interferon beta, and they were less frequently treated with lopinavir (p = 0.005) than were controls (Table 1). At the time of COVID-19 diagnosis, all LT recipients received chronic immunosuppression, mainly tacrolimus (n = 11; 73.3%), followed by mycophenolate mofetil (n = 7; 46.7%) and everolimus (n = 4; 26.7%). Twelve months post-infection, the majority of patients received tacrolimus (n = 11; 73.3%) and everolimus (n = 6; 40.0%) (Table 1).
Table 1.
Demographical and clinical characteristics of the study group.
| LT recipients (n = 15) | Immunocompetent controls (n = 15) | p | |
|---|---|---|---|
| Age (years) | 63 (43–66) | 62 (47–72) | 0.461 |
| Sex (male) | 11 (73.3) | 11 (73.3) | >0.99 |
| Previous medical history | |||
| Diabetes mellitus | 5 (33.3) | 7 (46.7) | 0.710 |
| Hypertension | 9 (60.0) | 11 (73.3) | 0.700 |
| ACE inhibitors or ARB | 6 (40.0) | 8 (53.3) | 0.715 |
| Clinical characteristics | |||
| Non-severe COVID-19a | 14 (93.3) | 13 (86.7) | >0.99 |
| Hospital admission | 11 (73.3) | 14 (93.3) | 0.330 |
| Interval since transplantation (years) | 5.57 (2.14–12) | NA | NA |
| COVID-19 specific therapy | |||
| Lopinavir | 6 (40.0) | 14 (93.3) | 0.005 |
| Interferon beta | 0 | 10 (66.7) | 0.001 |
| Hydroxychloroquine | 13 (86.7) | 14 (93.3) | >0.99 |
| Azithromycin | 9 (60.0) | 4 (26.7) | 0.114 |
| Tocilizumab | 1 (6.7) | 1 (6.7) | >0.99 |
| Corticosteroids (boluses) | 1 (6.7) | 1 (6.7) | >0.99 |
| Immunosuppression at baseline | |||
| Tacrolimus | 11 (73.3) | NA | NA |
| Cyclosporine | 1 (6.7) | NA | NA |
| Mycophenolate | 7 (46.7) | NA | NA |
| Corticosteroids (maintenance) | 1 (6.7) | NA | NA |
| Everolimus | 4 (26.7) | NA | NA |
| Immunosuppression at month 12 | |||
| Tacrolimus | 11 (73.3) | NA | NA |
| Tacrolimus trough levels (ng/mL), n = 11 | 3.7 (1.9–4.7) | NA | NA |
| Cyclosporine | 0 | NA | NA |
| Mycophenolate | 3 (20.0) | NA | NA |
| Corticosteroids (maintenance) | 0 | NA | NA |
| Everolimus | 6 (40.0) | NA | NA |
| Everolimus trough levels (ng/mL), n = 6 | 3.25 (2.9–4.9) | NA | NA |
Data are expressed as the median (interquartile range) or n (%).
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; NA, not applicable.
Severe COVID-19 was defined as the requirement for respiratory support or admission to the intensive care unit.
SARS-Cov-2 reactive T cells
The percentage of SARS-CoV-2 reactive T cells against viral proteins was calculated in gated CD4+ and CD8+ T cells (Fig. 1 ). The percentage of SARS-CoV-2 reactive T cells in PBMCs cultured in media alone was 0.02 ± 0.014% and 0.019 ± 0.014% in CD4+ and CD8+ T cells, respectively, and the cut-off value for a positive response was set at 0.048% for CD4+ T cells and 0.047% for CD8+ T cells.
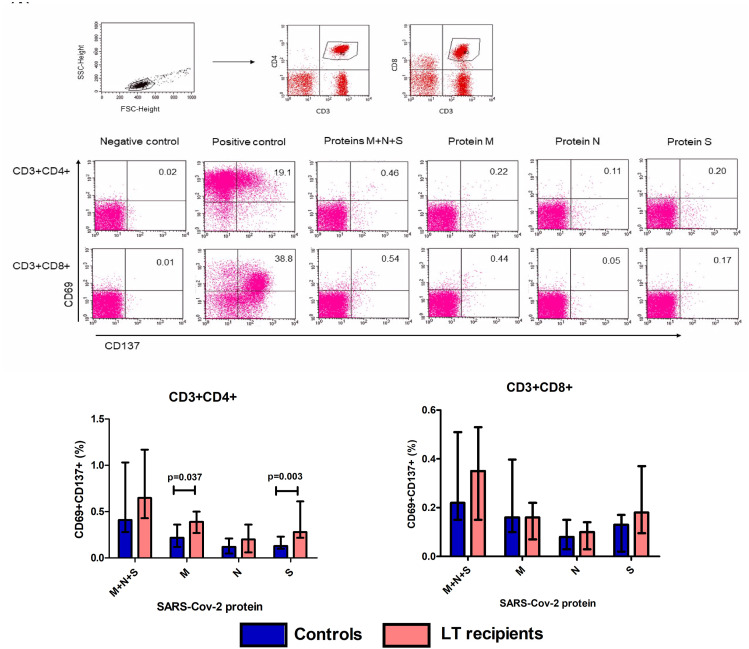
Figure 1.
Reactive CD4+ and CD8+ T cells against SARS-CoV-2 proteins. PBMC from 15 LT recipients and 15 controls were stimulated in vitro with proteins M, N, S, and a mixture of M + N + S for 16 h (A) Lymphocytes were gated according to FSC-height and SSC-height characteristics. Then, CD3+CD4+ or CD3+CD8+ T cells were selected, and SARS-CoV-2 reactive T cells were considered as those CD69+CD137+ cells in gated CD3+CD4+ or CD3+CD8+ cells (B) Example plots showing staining patterns of SARS-CoV-2 reactive T cells in gated CD3+CD4+ and CD3+CD8+ T cells in each culture condition (C) Percentage of reactive CD4+ and CD8+ T cells against each SARS-CoV-2 protein in 15 LT recipients and 15 controls. Bars show median percentage and error bars show interquartile range.
No differences were found in the percentage of reactive CD4+ (31.1 ± 13.9% and 24.3 ± 7%; p = 0.172) or CD8+ (35.1 ± 12.8% and 37.7 ± 13.3%; p = 0.847) T cells between LT recipients and controls, respectively, when PBMC were cultured with the positive control of antigenic stimulation.
All LT recipients and controls presented SARS-CoV-2 reactive CD4+ and CD8+ T cells when stimulated with a mixture of M + N + S proteins, and no differences between the two groups were found in the percentage of reactive T cells (Fig. 1). When analyzing the reactive T cells against each viral protein separately, the percentage of reactive CD8+ T cells was similar for the two study groups. However, LT recipients showed a higher percentage of CD4+ T cells reactive against S (p = 0.003) and M (p = 0.037) proteins than did the control group (Fig. 1).
Considering reactive T cells in a qualitative trait, no differences were found between the percentage of LT recipients and controls with a positive response to each viral protein (Table 2 ). All patients showed a positive CD4+ and/or CD8+ T-cell response to at least one of the viral proteins analyzed. In addition, 15 patients (eight LT recipients and seven controls) showed a positive response of both CD4+ and CD8+ T cells to all three proteins. A higher proportion of positive responses was found in CD4+ T cells than in CD8+ T cells, and protein N induced the lowest proportion of positive responses in both CD4+ and CD8+ T cells (Table 2).
Table 2.
Detectable T cells reactive to SARS-CoV-2 proteins M, N and S.
| All patients (n = 30) | LT recipients (n = 15) | Immunocompetent controls (n = 15) | p | |
|---|---|---|---|---|
| Reactive CD3+CD4+ T cells | ||||
| Against protein M | 29 (96.7) | 15 (100) | 14 (93.3) | >0.99 |
| Against protein N | 25 (83.3) | 12 (80) | 13 (86.7) | >0.99 |
| Against protein S | 30 (100) | 15 (100) | 15 (100) | >0.99 |
| Reactive CD3+CD8+ T cells | ||||
| Against protein M | 28 (93.3) | 13 (86.7) | 15 (100) | 0.483 |
| Against protein N | 17 (56.7) | 9 (60) | 8 (53.3) | >0.99 |
| Against protein S | 23 (76.7) | 12 (80) | 11 (73.3) | >0.99 |
Data are expressed as n (%).
Two LT recipients and one control failed to show a positive response to protein N in both CD4+ and CD8+ T-cells. To explore potential risk factors for a lower or null response to protein N, we compared demographic and clinical data of patients with both reactive CD4+ and CD8+ T cells [double positive (DP)] response (eight LT recipients and seven controls) and patients who did not show a DP response to protein N. All variables considered were similar for patients with and those without a DP response (Table 3 ), although LT recipients receiving tacrolimus tended to present a non-DP response to protein N (p = 0.077).
Table 3.
Demographical and clinical characteristics of the study group according to T cell response to SAR-Cov-2 protein N.
| DP response (n = 15) | No DP response (n = 15) | p | |||
|---|---|---|---|---|---|
| Age (years) | 63 | (44–69) | 63 | (43–66) | 0.539 |
| Sex (male) | 13 | (86.7) | 9 | (60.0) | 0.215 |
| Previous medical history | |||||
| Diabetes mellitus | 7 | (46.7) | 5 | (33.3) | 0.710 |
| Hypertension | 9 | (60.0) | 11 | (73.3) | 0.700 |
| ACE inhibitors or ARB | 5 | (33.3) | 8 | (60.0) | 0.272 |
| Clinical characteristics | |||||
| Non-severe COVID-19a | 14 | (93.3) | 12 | (80.0) | 0.598 |
| Hospital admission | 13 | (86.7) | 12 | (80.0) | p > 0.99 |
| COVID-19 specific therapy | |||||
| Lopinavir | 8 | (53.3) | 12 | (80.0) | 0.245 |
| Interferon beta | 4 | (26.7) | 6 | (40) | 0.700 |
| Hydroxychloroquine | 13 | (86.7) | 14 | (93.3) | p > 0.99 |
| Azithromycin | 5 | (33.3) | 8 | (53.3) | 0.462 |
| Tocilizumab | 1 | (6.7) | 1 | (6.7) | p > 0.99 |
| Corticosteroids (boluses) | 0 | (0.0) | 2 | (13.3) | 0.483 |
| Immunosuppression at baseline | (n = 8) | (n = 7) | |||
| Tacrolimus | 4 | (50) | 7 | (100) | 0.077 |
| Cyclosporine | 1 | (12.5) | 0 | (0.0) | p > 0.99 |
| Mycophenolate | 4 | (50.0) | 3 | (42.9) | p > 0.99 |
| Corticosteroids (maintenance) | 1 | (12.5) | 0 | (0.0) | p > 0.99 |
| Everolimus | 3 | (37.5) | 1 | (14.3) | 0.569 |
| Immunosuppression at month 12 | (n = 8) | (n = 7) | |||
| Tacrolimus | 4 | (40.0) | 7 | (100) | 0.077 |
| Tacrolimus trough levels (ng/mL), n = 11 | 3.6 | (2.1–6.6) | 4.2 | (1.9–4.7) | p > 0.99 |
| Mycophenolate | 2 | (25.0) | 1 | (14.3) | p > 0.99 |
| Everolimus | 3 | (37.5) | 3 | (42.9) | p > 0.99 |
| Everolimus trough levels (ng/mL), n = 6 | 3.3 | (2.1–6.6) | 3.2 | (2.7–3.2) | p > 0.99 |
| Interval since transplantation (years) | 6.9 | (3.2–18.9) | 2.4 | (1.7–8.4) | p = 0.19 |
Data are expressed as the median (interquartile range) or n (%).
DP: patients with both CD4+ and CD8+ positive response to protein N.
Severe COVID-19 was defined as the requirement for respiratory support or admission to the intensive care unit.
Cytokine production after stimulation with SARS-Cov-2 peptides
Interleukine-6 was widely detected above the maximum quantifiable level with the kit used, while IL4 concentration was below detectable levels in all culture conditions. Therefore, IL6 and IL4 production after stimulation with SARS-Cov-2 peptides was excluded from further analysis.
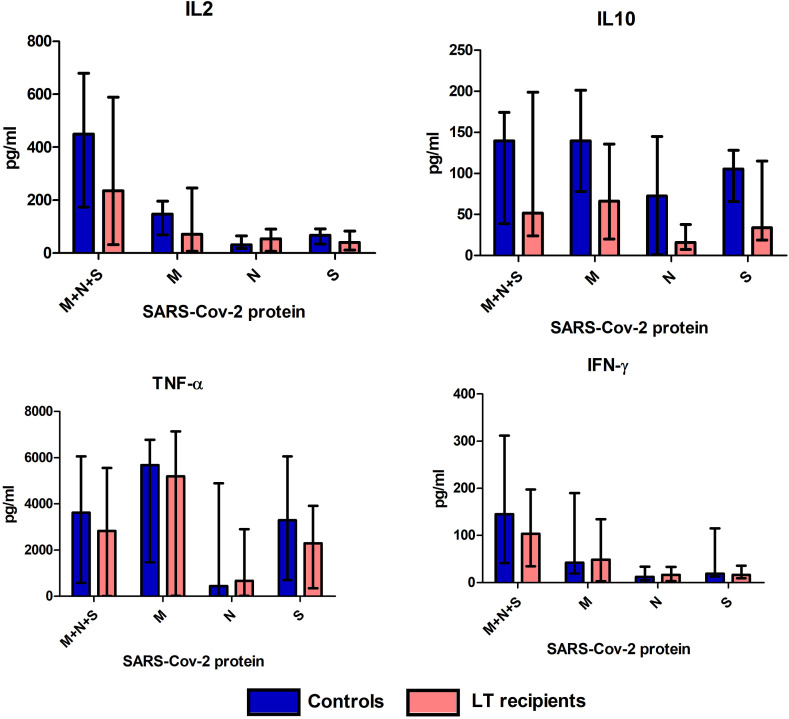
Under almost all culture conditions, IL2, IL10, TNF-α, and IFN-γ levels were slightly lower in LT recipients than in controls, although the differences were not statistically significant (Fig. 2 ).
Figure 2.
Cytokine production after stimulation with SARS-CoV-2 proteins. Concentration (pg/ml) of IL2, IL10, TNF-α, and IFN-γ in culture supernatants after stimulation with proteins M, N, S, and M + N + S in 15 controls and 15 LT recipients. Bars show median percentage and error bars show interquartile range.
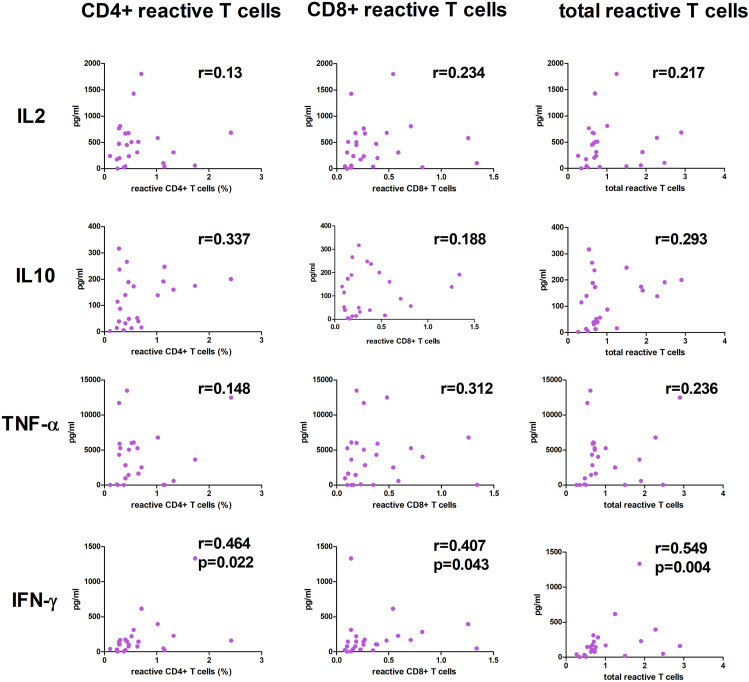
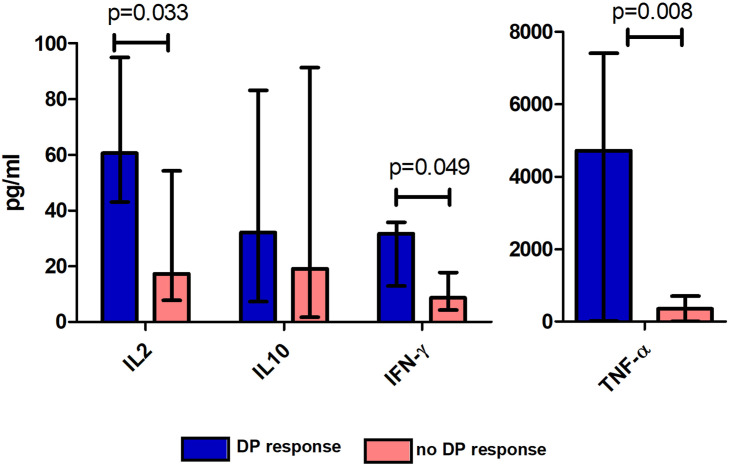
We then explored the correlation between cytokine levels and the percentage of CD4+, CD8+, or the sum of CD4+ and CD8+ (total) reactive T cells in all the included patients (n = 30). After stimulation with M + N + S proteins, a positive correlation was found between IFN-γ and the percentage of CD4+, CD8+, and total reactive T cells (Fig. 3 ). No correlation was found between cytokine levels and the percentage of reactive T cells to proteins M or S. Patients with a DP response to protein N presented higher production of IL2 (p = 0.033), TNF-α (p = 0.008), and IFN-γ (p = 0.049) than did patients without a DP response (Fig. 4 ).
Figure 3.
Correlation among cytokine levels and reactive T cells against SARS-CoV-2 proteins. Correlations between levels (pg/ml) of IL2, IL10, TNF-α, and IFN-γ in culture supernatants and reactive CD4+, CD8+ and total T cells after PBMC stimulation with proteins M + N + S. Median percentage of reactive T cells and IgG levels of all 30 patients included are shown.
Figure 4.
Cytokine production in patients with and without a DP response to SARS-Cov-2 protein N. Concentration (pg/ml) of IL2, IL10, IFN-γ (left), and TNF-α (right) in culture supernatants after stimulation with protein N in patients with and without a positive response to both CD4+ and CD8+ T cells (DP). Bars show median percentage and error bars show interquartile range for all 30 patients included.
Prevalence and quantitative assessment of IgG antibodies against SARS-CoV-2
All patients, except for two LT recipients, showed anti-S IgG antibodies at 12 months following COVID-19 diagnosis. In contrast, only five (33.3%) LT recipients and eight (53.3%) controls presented anti-N IgG antibodies. No statistical significance was reached when comparing the prevalence or levels of anti-S and anti-N IgG antibodies of the LT recipients and controls (Table 4 ).
Table 4.
Incidence and levels of anti-spike and anti-nucleocapsid IgG antibodies according to study group.
| LT recipients (n = 15) | Immunocompetent patients (n = 15) | p | |
|---|---|---|---|
| Anti-spike IgG detected; n (%) | 13 (86.7) | 15 (100.0) | 0.483 |
| Anti-spike IgG levels (UA/mL); median (IQR) | 502.3 (169.5–984.2) | 819.1 (480.9–2159.7) | 0.370 |
| Anti-nucleocapsid IgG detected; n (%) | 5 (33.3) | 8 (53.3) | 0.462 |
| Anti-nucleocapsid IgG levels; median (IQR) | 0.8 (0.11–3.57) | 1.75 (0.53–3.14) | 0.232 |
Data are expressed as median (IQR) or n (%).
Correlation between humoral and cellular response to SARS-CoV-2
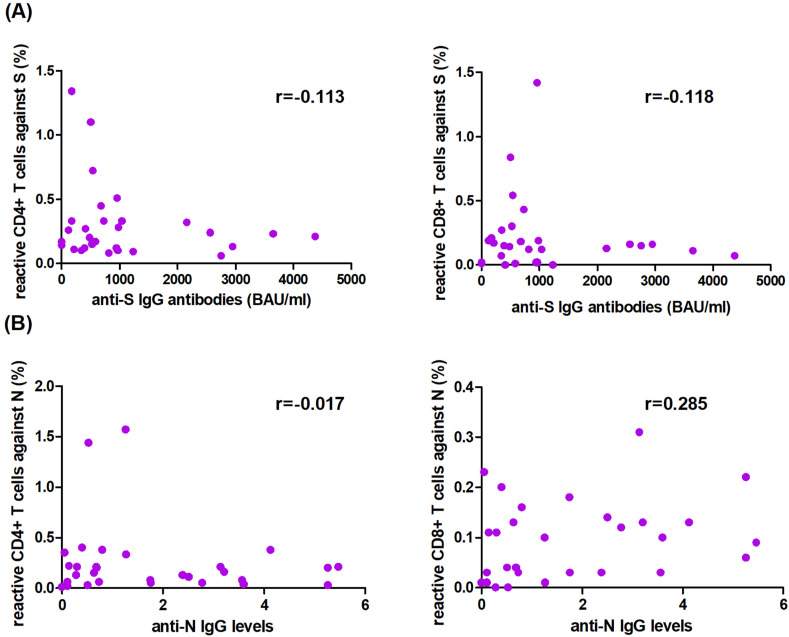
No correlation was observed between the percentage of reactive T cells against protein N or S and levels of anti-N or anti-S IgG antibodies, respectively (Fig. 5 ), in all included patients (n = 30). Remarkably, all patients lacking anti-S IgG or anti-N IgG antibodies showed CD4+ T cells reactive to protein S or N, respectively, except for the two LT recipients and one control who failed to present T cells reactive against protein N.
Figure 5.
Correlation among reactive T cells and anti-IgG levels against SARS-CoV-2 at 12 months after COVID-19 diagnosis. Correlation between (A) reactive T cells against protein S and anti-S IgG levels, and (B) reactive T cells against protein N and anti-N IgG levels.
SARS-CoV-2 reinfections beyond one year
Six patients (3 LT recipients and 3 controls) documented SARS-CoV-2 reinfection, all with asymptomatic or mild disease, at 11.2 ± 1.4 months following the study. All patients had received mRNA COVID-19 vaccines after the study, and reinfections were diagnosed at 5.7 ± 2.3 months after two (n = 1) or 3 (n = 1) doses in LT recipients and at 5.03 ± 3.02 months after one, two, or three doses in controls.
No differences in cellular or humoral responses were found in patients and controls with or without SARS-CoV-2 reinfections.
Discussion
The limited data available indicate that the SARS-CoV-2 reactive cellular response in transplant recipients and in the general population is quite similar from soon after symptom onset7 , 8 up to six-eight months later.4 , 9 Our results show that the magnitude and functionality of cellular T-cell responses against SARS-CoV-2 were similar in LT recipients and non-transplant patients 12 months following COVID-19 infection. Remarkably, all LT recipients and controls presented T cells reactive against at least one viral protein analyzed (M, N, and S), and 50% of patients showed both CD4+ and CD8+ T cells reactive against all three viral proteins.
As reported in several studies, most individuals that have recovered from COVID-19 present T-cell responses to proteins M, N, and S soon after diagnosis,7 with a stable SARS-CoV-2 T-cell repertoire of up to 3–8 months after symptoms4 , 10, 11, 12, 13 regardless of disease severity. Very few studies have analyzed the T-cell response to SARS-CoV-2 one year after infection. Zhang3 et al. found a T-cell response maintained over time in most patients at 6 and 12 months after disease onset. In a recent study performed in convalescent patients over a period of 1–11 months after COVID,14 specific response (T cell and IgG levels) was present in 90% of patients, but in contrast to our results, exposure to immunosuppressive drugs was an independent risk factor for the absence of a specific T-cell response. However, it is important to note that only 2 out of the 6 patients who were receiving immunosuppressive therapy were solid organ transplantation (SOT) recipients14; therefore, these results in the SOT population should be interpreted with caution.
The percentage of reactive T cells for each viral protein tested was highly variable, and SARS-Cov-2 reactive CD4+ T cells exceeded CD8+ T cells against each protein, as widely described,1 , 4 , 7 , 11 , 12 in both LT recipients and controls. In addition, proteins M and S induced a higher percentage of reactive T cells than did protein N in both CD4+ and CD8+ T cells.9 , 15 While most patients presented a positive response of both CD4+ and CD8+ T cells against proteins M and S, only 50% of patients presented a DP response to protein N. However, no differences were found between patients with and those without a DP response against protein N with respect to demographic and clinical characteristics, COVID-19 severity, or time elapsed since transplantation or immunosuppressive regimen in LT recipients.
In line with previous reports,1 , 3 , 7 , 12 , 15 we found polarization of reactive T cells towards a classic Th1 type, as considerable IL2, TNF-α, and IFN-γ were produced, while very little or no IL4 production was observed. Although we quantified cytokine levels in culture supernatants rather than directly analyzing their production by reactive T cells (such as intracellular staining or ELISpot), we suggest that these cytokines may be specifically secreted by T cells reactive to SARS-CoV-2 because of the positive correlation between the percentage of reactive T cells and IFN-γ levels. Moreover, the culture supernatants of patients with a DP response of both CD4+ and CD8+ T cells against protein N presented higher levels of IL2, TNF-α, and IFN-γ than did those of patients without a DP response.
No significant differences were found between LT recipients and nontransplant patients in terms of specific humoral immunity. However, we cannot conclude that humoral immunity is similar in the two groups since lower prevalence and levels of anti-S and anti-N were found in LT recipients, although statistical significance was not reached, perhaps due to the small sample size.
The proportion of patients with anti-S IgG antibodies one year after COVID-19 was very high in both study groups. In contrast, a lower prevalence of anti-N IgG antibodies was found, which is in agreement with previous studies.16 , 17 We did not find a correlation between the percentage of T cells reactive against N or S and anti-N or anti-S IgG antibody levels, as has also been reported in both SOT recipients and in the general population at 6 months following infection.4 , 12 , 13 , 18 Remarkably, the vast majority of patients lacking anti-N IgG antibodies presented CD4+ T cells reactive against N.
These results show that most LT recipients and non-transplant patients who recovered from COVID-19 have SARS-CoV-2 specific CD4+ T cells, which, along with the reported memory B-cell repertoire,11 , 12 , 19 likely contribute to the development of protective immunity for at least 12 months after SARS-CoV-2 infection. Therefore, serodiagnostic tests alone cannot be considered strong indicators of protective immunity in COVID-19 convalescent patients, and T-cell assays might be considered to investigate the history of prior infection in epidemiological studies.
Natural SARS-CoV-2 infection provides substantial and persistent immunologic protection to similar strains for a period of several months,20 and it is also cross-reactive with the highly mutated variant Omicron21 in most individuals. In this regard, none of unvaccinated patients included in this study suffered documented reinfection within one year. Indeed, reinfections were diagnosed about two years after prior infection and were probably due to Omicron since it was the predominant strain in Spain in this period. All reinfections presented with mild diseases, but since all patients were vaccinated after the study, we cannot discern whether protection from severe disease was attributable to natural immunity, vaccination, or both.
This study has several limitations that should be considered when interpreting the results. First, the relatively small number of patients may have limited the ability to detect clinical associations with cellular response. Second, a high proportion of the patients required hospitalization but presented a non-severe disease. Therefore, it is possible that the spectrum of mild and asymptomatic COVID-19 as well as the most severe disease are not adequately captured, and our results could not be extrapolated to all forms of the disease. In addition, corticosteroid boluses were rarely administered to patients included in our study, although their use is now widespread in the acute phase of COVID-19. Although it is not yet clear whether corticosteroid boluses in the acute phase impair T-cell immunity in the long term, this possibility should be considered when interpreting our results. Furthermore, given the long interval since transplantation of the patients included in our study, our results should be interpreted with caution in regards to the first year post-transplantation; a higher degree of immunosuppression during the early post-transplant period theoretically could have led to differences in immunity against SARS-CoV-2 between transplant recipients and non-transplanted patients. Finally, since all patients received SARS-CoV-2 vaccination after the study, it is not possible to determine how long the protection of natural immunity lasts. However, long-term data regarding the T-cell immunity of unvaccinated patients are scarce; therefore, our results may be of interest for strategies against future SARS-CoV-2 variants that could be less covered by currently available vaccines.
In conclusion, despite exposure to immunosuppression, LT recipients exhibited similar functional T-cell responses against SARS-CoV-2 to the reponses of matched non-transplanted individuals one year after a COVID-19 diagnosis.
Funding
This study has been supported by the grant “Beca de Investigación de la Fundación Sociedad Española de Trasplante Hepático (FSETH) 2020”. Maria J. Citores has received support from the “Catedra de Patrocinio UAM-Fundacion Lair” and Aránzazu Caballero-Marcos from the Instituto de Salud Carlos III (Rio Hortega-CM19/00247).
Data statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
None.
References
- 1.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in Humans with COVID-19 disease and unexposed individuals. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.015. 1489.e15-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;2:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J., Lin H., Ye B., Zhao M., Zhan J., Dong S., et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clin Infect Dis. 2021:ciab884. doi: 10.1093/cid/ciab884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Ruiz M., Olea B., Almendro-Vázquez P., Giménez E., Marcacuzco A., San Juan R., et al. T cell-mediated response to SARS-CoV-2 in liver transplant recipients with prior COVID-19. Am J Transplant. 2021;21:2785–2794. doi: 10.1111/ajt.16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caballero-Marcos A., Salcedo M., Alonso-Fernández R., Rodríguez-Perálvarez M., Olmedo M., Graus Morales J., et al. Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. Am J Transplant. 2021;21:2876–2884. doi: 10.1111/ajt.16599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thieme C.J., Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A., Seibert F.S., et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression. Transplantation. 2021;105:2156–2164. doi: 10.1097/TP.0000000000003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candon S., Guerrot D., Drouot L., Lemoine M., Lebourg L., Hanoy M., et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21:854–863. doi: 10.1111/ajt.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favà A., Donadeu L., Jouve T., Gonzalez-Costello J., Lladó L., Santana C., et al. A comprehensive assessment of long-term SARS-CoV-2-specific adaptive immune memory in convalescent COVID-19 solid organ transplant recipients. Kidney Int. 2022;101:1027–1038. doi: 10.1016/j.kint.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonifacius A., Tischer-Zimmermann S., Dragon A.C., Gussarow D., Vogel A., Krettek U., et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54:340–354.e6. doi: 10.1016/j.immuni.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesch M., Andréll J., et al. Persistence of SARS- CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (NY) 2021;2:281–295.e4. doi: 10.1016/j.medj.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchner T., Heinrich S., Bonifacius A., Engel B., Ruhl L., Pink I., et al. Reduced humoral but stable cellular SARS-CoV-2-specific immunity in liver transplant recipients in the first year after COVID-19. PLoS One. 2022;7 doi: 10.1371/journal.pone.0276929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konik M., Lindemann M., Zettler M., Meller L., Dolff S., Rebmann V., et al. Long-term SARS-CoV-2 specific immunity is affected by the severity of initial COVID-19 and patient age. J Clin Med. 2021;10:4606. doi: 10.3390/jcm10194606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thieme C.J., Anft M., Paniskaki K., Blazquez-Navarro A., Doevelaar A., Seibert F.S., et al. Robust T cell response toward spike, membrane, and nucleocapsid SARS-CoV-2 proteins is not associated with recovery in critical COVID-19 patients. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenwick C., Croxatto A., Coste A.T., Pojer F., André C., Pellaton C., et al. Changes in SARS-CoV-2 antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol. 2021;95 doi: 10.1128/JVI.01828-20. e01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chia W.N., Tan C.W., Foo R., Kang A.E.Z., Peng Y., Sivalingam V., et al. Serological differentiation between COVID-19 and SARS infections. Emerg Microb Infect. 2020;9:1497–1505. doi: 10.1080/22221751.2020.1780951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffner J., Backhaus I., Rimmele J., Schulz S., Möhlenkamp T., Klemens J.M., et al. Long-term course of humoral and cellular immune responses in outpatients after SARS-CoV-2 infection. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.732787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore T., Hossain R., Doores K.J., Shankar-Hari M., Fear D.J. SARS-CoV-2-specific memory B cell responses are maintained after recovery from natural infection and postvaccination. Viral Immunol. 2022;35:425–436. doi: 10.1089/vim.2022.0013. [DOI] [PubMed] [Google Scholar]
- 20.Spicer K.B., Glick C., Cavanaugh A.M., Thoroughman D. Protective immunity after natural infection with severe acute respiratory syndrome coronavirus-2 (SARS- CoV-2) - Kentucky, USA, 2020. Int J Infect Dis. 2022;114:21–28. doi: 10.1016/j.ijid.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]