Abstract
Background
Osteoporosis is often considered to be a disease of women. Over the last few years, owing to the increasing clinical and economic burden, the awareness and imperative for identifying and managing osteoporosis in men have increased substantially. With the approval of agents to treat men with osteoporosis, more economic evaluations have been conducted to assess the potential economic benefits of these interventions. Despite this concern, there is no specific overview of cost-effectiveness analyses for the treatment of osteoporosis in men.
Objectives
This study aims (1) to systematically review economic evaluations of interventions for osteoporosis in men; (2) to critically appraise the quality of included studies and the source of model input data; and (3) to investigate the comparability of results for studies including both men and women.
Methods
A literature search mainly using MEDLINE (via Ovid) and Embase databases was undertaken to identify original articles published between 1 January, 2000 and 30 June, 2022. Studies that assessed the cost effectiveness of interventions for osteoporosis in men were included. The Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases and the International Osteoporosis Foundation osteoporosis-specific guideline was used to assess the quality of design, conduct, and reporting of included studies.
Results
Of 2973 articles identified, 25 studies fulfilled the inclusion criteria, classified into economic evaluations of active drugs (n = 8) or nutritional supplements (n = 4), intervention thresholds (n = 5), screening strategies (n = 6), and post-fracture care programs (n = 2). Most studies were conducted in European countries (n = 15), followed by North America (n = 9). Bisphosphonates (namely alendronate) and nutritional supplements were shown to be generally cost effective compared with no treatment in men over 60 years of age with osteoporosis or prior fractures. Two other studies suggested that denosumab was cost effective in men aged 75 years and older with osteoporosis compared with bisphosphates and teriparatide. Intervention thresholds at which bisphosphonates were found to be cost effective varied among studies with a 10-year probability of a major osteoporotic fracture that ranged from 8.9 to 34.2% for different age categories. A few studies suggested cost effectiveness of screening strategies and post-fracture care programs in men. Similar findings regarding the cost effectiveness of drugs and intervention thresholds in women and men were captured, with slightly greater incremental cost-effectiveness ratios in men. The quality of the studies included had an average score of 18.8 out of 25 (range 13–23.5). Hip fracture incidence and mortality risk were mainly derived from studies in men, while fracture cost, treatment efficacy, and disutility were commonly derived from studies in women or studies combining both sexes.
Conclusions
Anti-osteoporosis drugs and nutritional supplements are generally cost effective in men with osteoporosis. Screening strategies and post-fracture care programs also showed economic benefits for men. Cost-effectiveness and intervention thresholds were generally similar in studies conducted in both men and women, with slightly greater incremental cost-effectiveness ratios in men.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01239-2.
Key Points for Decision Makers
| Medicines for osteoporosis and nutritional supplements are cost effective in men aged 60 years and older with prior fractures or with a diagnosis of osteoporosis. Based on expert societies’ practice guidelines, reimbursement for these active drugs should be considered as part of the standard of care. |
| Similar findings regarding the cost effectiveness of drugs and intervention thresholds in women and men with osteoporosis were captured, with a moderate increase in incremental cost-effectiveness ratios in men. Fracture risk reduction is the primary consideration in the treatment for osteoporosis irrespective of sex. |
Introduction
Osteoporosis is commonly recognized as a disease in women following menopause, which is often overlooked in men mainly because there is no aging process in men analogous to menopause with a resultant rapid loss of bone mass. In men, secondary osteoporosis is more frequent; common causes include glucocorticoid excess, hypogonadism, and alcohol abuse [1]. In particular, androgen deficiency (hypogonadism) that can result from androgen deprivation therapy for prostate cancer is accompanied by a decline in bone mineral density (BMD) within the first 6–9 months of initiation and an increase in fracture risk of nearly 20% after 5 years of therapy [2].
Osteoporotic fractures are not limited to postmenopausal women; one in five men (compared to one in two women) over 50 years of age will sustain an osteoporotic fracture in their remaining lifetime [3, 4]. A US study reported that men account for 29% of osteoporotic fractures and 25% of the cost of fractures (with the total annual expense for all osteoporosis-related fractures in the USA at approximately $57 billion in 2018, which is comparable to the annual cost of €56 billion estimated in 2019 for Europe) [5–7]. In addition, the consequences of fractures, in particular hip fractures, were shown to be greater in men than in women [8], as suggested by the increased relative risk of a subsequent fracture and mortality following the initial fractures. This rising clinical and economic burden of osteoporosis in men has led to increased attention recently.
With the availability of pharmacological therapies as well as the implementation of post-fracture care programs for the prevention of secondary fractures, cost-effectiveness assessments of these interventions have been conducted to inform decision making or to determine cost-effective osteoporosis intervention thresholds (i.e., 10-year fracture probabilities at which treatment can be cost effective). Most of the studies were conducted in women and are summarized in previous systematic reviews [9, 10]. To our knowledge, there is currently no overview of published cost-effectiveness analyses for the treatment of osteoporosis in men. Such information may inform payers about the economic value of treating osteoporosis in men, identify relevant gaps and opportunities, and provide pertinent information for further economic studies. Therefore, the objective of this study is to systematically review cost-effectiveness analyses in men with osteoporosis, to critically appraise these studies, to investigate the source of model input data, and to assess the comparability of cost-effectiveness results among studies including both men and women.
Methods
The 2020 Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [9] was used for the entire procedure (identify, select, appraise, and synthesize studies) of this systematic review. A protocol was registered in PROSPERO with the registration number CRD42022331820 [11]. Covidence software as a systematic review management tool was used to manage search results, including the removal of duplicates, abstract and title screening, and full-text screening.
Literature Search
The literature search was restricted to articles published between January 2000 and June 2022 (given the first osteoporosis-related study including men was published in 2004). MEDLINE (via Ovid) and Embase databases were searched initially in January 2022 and were updated in June 2022 using adapted search strategies (based on the previous search strategies of Li et al. [9]). As suggested by a guideline for systematic reviews of economic evaluations [12], two other economic evaluation databases were also searched: the National Health Service Economic Evaluation Database and the Cost-Effectiveness Analysis Registry, using two keywords (“osteoporosis” and “men”). However, it should be noted that updates to National Health Service Economic Evaluation database were discontinued in 2015. In addition, reference lists and citations of included articles and previously published systematic reviews (of economic evaluations of interventions for osteoporosis) were reviewed as additional studies of interest. Details of search strategies are in the Electronic Supplementary Material (ESM).
Study Selection
Peer-reviewed studies from any country or type of healthcare system were considered eligible if they contained a full economic evaluation comparing at least an intervention and a comparator in both costs and outcomes, either placebo or an alternative intervention as comparator(s). Eligible studies are cost-effectiveness analyses, cost-utility analyses, cost-benefit analyses, and cost-minimization analyses for any type of interventions or management (drugs, screening, intervention thresholds, adherence intervention, nutrition, fracture liaison services [FLS]). Particularly, studies were included if they reported outcomes for men (studies in men only, or studies including both men and women but separately reporting cost-effectiveness results for men). Non-original articles (e.g., case reports, reviews, letters to the editors, conference abstracts, opinion pieces, protocols) and studies published in non-English language were excluded [13].
Using these criteria, two reviewers (NL, CB) independently identified studies through title and abstract screening. Then, these reviewers conducted a full-text screening to determine eligibility, discrepancies were resolved by a consensus meeting with a third reviewer (MH).
Data Extraction and Synthesis
Included studies were classified into four categories: active drugs or nutritional supplements, intervention threshold, screening strategies, and post-fracture care programs. A standardized data extraction form was developed and pre-tested on a sample of five of the eligible studies to extract data from these studies by one independent reviewer (NL) and a second reviewer (CB) checked these results to assure the quality of the form. Study characteristics extracted included publication information (author, year of publication, journal), study design (country setting, target population, economic perspective, model type, time horizon, intervention and comparators, intervention duration, outcome measure, cost type, year of valuation, discount rates), study outcomes (base-case and sensitivity analyses), and funding source. It should be noted that for studies combining both women and men, only male data were extracted. The outcomes varied according to study categories. For studies investigating the cost effectiveness of active drugs or nutritional supplements, screening strategies, and post-fracture care programs, incremental cost-effectiveness ratios (ICERs) were extracted as originally reported along with the conclusions on the cost effectiveness of the intervention determined by the authors. Furthermore, information on the duration of drug/nutrition treatment, screening time and drug/nutrition treatment, and the duration of post-fracture care programs as the intervention duration was also collected. Intervention thresholds (i.e., the threshold of fracture probability at which an intervention becomes cost effective) [14] were extracted as the main study outcome of the intervention threshold studies, and the duration of drug/nutritional treatment was reported as the intervention duration.
Studies that reported outcomes separately for both men and women were further categorized into two groups according to the type of outcome (ICER or intervention threshold). In studies using ICER as the outcome, the difference in ICERs was displayed using +/− with the data from the women as the reference (+ means men had higher ICERs than women, − means men had lower ICERs than women), and the conclusion on cost effectiveness was also shown for both sexes using Yes/No. For studies using an intervention threshold as the outcome, thresholds were separately reported for men and women, and absolute change was calculated with the data from the women as the reference.
Quality Assessment
The conduct and reporting quality of included studies were appraised by two independent researchers (NL, CB) using the osteoporosis-specific guideline formulated by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases and the International Osteoporosis Foundation (ESCEO-IOF) [15]. Discrepancies were discussed and resolved with a third researcher (MH). The ESCEO-IOF guideline includes 29 items; four items (a. the FRAX® or GARVAN® tools can be used to model fracture; b. national ICUROS data if available; c. sequential therapy may be considered as intervention/comparators; d. in the absence of hip/wrist-specific efficacy data, use of non-vertebral or clinical fracture efficacy data) were not included in the scoring system as these recommendations are not compulsory or not applicable to all eligible studies. Each of the remaining 25 items was scored with a Yes, No, Partial, Not reported, or Not applicable to indicate if the requirement was fulfilled. A quality score was obtained for each study by assigning a score of 1 for any Yes, a score of 0.5 for Partially, and a score of 0 for No, not reported, and not applicable, for a total possible score of 25 points.
Additionally, another form was used to extract the source of the most important model parameters (i.e., fracture incidence, fracture cost, baseline utility and fracture disutility, baseline mortality, and excess mortality, treatment efficacy, side effects, and medication adherence/persistence) and to determine whether these data were derived from studies in men exclusively, from women, or from studies including both sexes.
Results
Study Selection
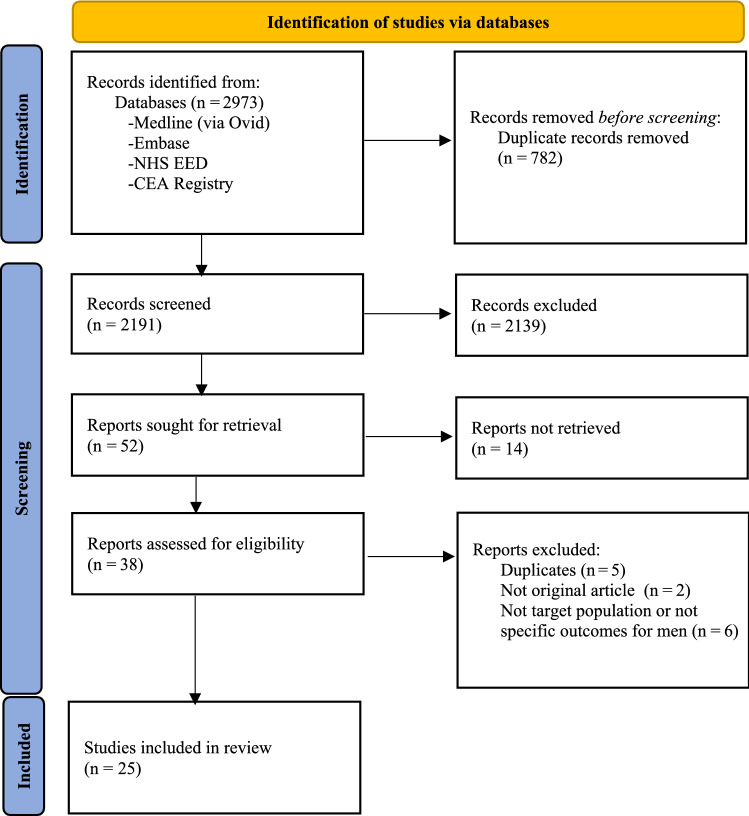
Figure 1 shows the PRISMA flow chart for the identification of studies. The database search identified 2973 records, of which 782 were removed as duplicates. Fifty-two full economic evaluations were identified after title and abstract screening. Of those, 14 articles were conference abstracts and therefore rejected; 38 studies were thus assessed for eligibility by full-text screening. Thirteen studies were subsequently excluded for reasons such as duplicates (n = 5), not the original article (n = 2), and not the target population or not specific outcomes for men (n = 6), leaving a total of 25 articles included in the analysis. No new studies were identified through screening of reference lists and citations of included articles.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) flowchart of study selection. CEA Registry Cost-Effectiveness Analysis Registry, NHS EED National Health Service Economic Evaluation Database
Overview of Included Studies
Table 1 presents the characteristics of included studies. Most assessed active drugs or nutritional (primarily vitamin D alone or with calcium) supplements (n = 12) followed by screening strategies (n = 6), intervention thresholds (n = 5), and post-fracture care programs (n = 2). Sixteen out of 25 studies were conducted before 2015, only two studies [16, 17] were published in the past 5 years. Most studies were conducted in European countries (n = 15), especially in Sweden (n = 4), Belgium (n = 3), and the UK (n = 3), followed by the USA (n = 9), with one study performed in Asia.
Table 1.
Characteristics of studies assessing the cost-effectiveness of interventions for men with osteoporosis
| Reference | Year of publication | Country | Population | Perspective | Model type | Outcome measure | Time horizon | Cost type | Discount rate (costs, QALY) | Funding source | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Active drugs or nutritional supplements | |||||||||||
| Hiligsmann et al. [27] | 2017 | France |
General population (women and men) aged 60 years and older |
Healthcare | Markov microsimulation | QALY | Lifetime | Direct costs | 3%, 3% | CNIEL | |
| Ethgen et al. [21] | 2015 | Belgium | General population, patients (women and men) with osteoporosis and a PVF or hip fracture aged 50 years and older | Healthcare | Markov microsimulation | LY | Lifetime | Direct costs | 3%, 1.5% | None | |
| Silverman et al. [25] | 2015 | USA | Men aged 75 years and older with osteoporosis | Healthcare | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% | Amgen Inc. | |
| Hiligsmann et al. [28] | 2014 | Belgium | Patients (women and men) aged 60 years and older with osteoporosis | Healthcare | Markov microsimulation | QALY | Lifetime | Direct costs | 3%, 1.5% | SMB Belgium | |
| Parthan et al. [26] | 2014 | Sweden | Men aged 75 years and older with osteoporosis | Healthcare | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% | NR | |
| Hiligsmann et al. [40] | 2013 | Belgium | Men aged 65–90 years with BMD T-score ≤−2.5 or PVF | Healthcare | Markov microsimulation | QALY | Lifetime | Direct costs | 3%, 1.5% | Servier | |
| Kouta et al. [23] | 2010 | USA | Men aged 70 years with locally advanced or high-risk localized prostate cancer starting a 2-year course of ADT after radiation therapy | Societal | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% | None | |
| Kreck et al. [18] | 2008 | Germany | Patients (women and men) with osteopenia or osteoporosis due to inflammatory bowel disease | Societal | Markov cohort | QALY | 10 years | Direct and indirect costs | 5%, 5% | German Federal Ministry of Education and Research | |
| Schousboe et al. [22] | 2007 | USA | White men aged 65 years and older with osteoporosis | Societal | Markov microsimulation | QALY | Lifetime | Direct and indirect costs | 3%, 3% | National Institutes of Health funding | |
| Van Staa et al. [20] | 2007 | UK | Patients (women and men) aged 40 years and older who were prescribed an oral GC and who were registered in the GPRD | Healthcare | Markov microsimulation | QALY | 6 years | Direct costs | 6%, 1.5% | None | |
| Borgstrom et al. [24] | 2004 | Sweden |
Men aged 55–80 years with low bone mass and radiographically identified CV |
Societal and healthcare | Markov cohort | QALY | Lifetime | Direct and indirect costs | 3%, 3% | Merck and Co., Inc. | |
| Fleurence et al. [39] | 2004 | UK | Patients (women and men) aged 70 years and older who were at high risk and general risk of fracture | Healthcare | Markov cohort | QALY | Lifetime | Direct costs | 6%, 6% |
Eli Lilly and UK Medical Research Council |
|
| Intervention threshold | |||||||||||
| Chan et al. [29] | 2017 | Taiwan, China | Patients (women and men) aged 50 years and older at different probabilities of MOF and hip fracture | Healthcare | Markov cohort | QALY | NR | Direct costs | 1.36%, 1.36% | Grant DOH102-TD-M-113-102006 | |
| Makras et al. [30] | 2015 | Greece | Patients (women and men) aged 50 years and older at different probabilities of MOF and hip fracture | Healthcare | Markov cohort | QALY | Lifetime | Direct costs | NR | NR | |
| Lippuner et al. [32] | 2012 | Switzerland | Patients (women and men) aged 50 years and older at different probabilities for a MOF | Healthcare | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% | MSD Switzerland AG | |
| Tosteson et al. [31] | 2008 | USA | Patients (women and men) aged 50 years and older at different probabilities for a hip fracture | Healthcare | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% |
National Osteoporosis Foundation and National Institutes of Health |
|
| Kanis et al. [33] | 2005 | Sweden | Patients (women and men) aged 50 years and older at different probabilities for a hip fracture | Healthcare and societal | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% |
IOF, Pfizer, Alliance for Better Bone Health, IGEA, Lilly Research Centre, Hologic, Novartis, Wyet, and Roche |
|
| Screening strategies | |||||||||||
|
Ito et al. [17] |
2020 | USA | Community-dwelling men aged 65 years who had fallen at least once in the past year | Societal | Markov cohort | QALY | Lifetime | Direct cost | 3%, 3% | NR | |
| Pisu et al. [16] | 2019 | USA | Patients (women and men) aged 65 years and older with an abdominal CT scan but without a recent DXA, and without osteoporosis treatment, or had no disqualifying conditions for an osteoporosis diagnostic screen | Healthcare | Markov cohort | QALY | 5 years | Direct costs | 3%, NR | National Institutes of Health | |
| Nayak et al. [34] | 2016 | USA | Community-dwelling men aged 50 years and older | Societal | Markov microsimulation | QALY | Lifetime | Direct costs | 3%, 3% | National Institutes of Health | |
| Schousboe et al. [35] | 2013 | USA | Caucasian patients (women and men) aged 55 years and older without a prior clinical fracture but with femoral neck osteoporosis and a 10-year hip fracture risk of ≥3% | Healthcare | Markov microsimulation | QALY | Lifetime | Direct costs | 3%, 3% | National Institutes of Health | |
| Ito et al. [37] | 2009 | USA | Community-dwelling white men aged 70 years without a history of clinical osteoporotic fractures | Societal | Markov cohort | QALY | Lifetime | Direct costs | 3%, 3% | None | |
| Schwenkglenks et al. [36] | 2007 | Switzerland | Patients (women and men) aged 50 years with osteoporosis | Healthcare | Markov microsimulation | QALY | Lifetime | Direct costs | 3%, 3% | Merck Sharp and Dohme-Chibret AG | |
| Post-fracture care programs | |||||||||||
| DPhil et al. [38] | 2017 | UK | Patients (women and men) aged 60 years and older with a hip fracture | Healthcare and societal | Markov microsimulation | QALY | Lifetime | Direct costs | 3.5%, 3.5% | NIHR HS and DR | |
| Johansson et al. [19] | 2008 | Sweden | Community-dwelling persons (women and men) aged 65 years and older | Societal | Markov cohort | QALY | 6 years | Direct and indirect costs | 3%, 3% | Stockholm County Council | |
ADT androgen deprivation therapy, BMD bone mineral density, CT computerized tomography, CV clinical vertebral fracture, DXA dual-energy X-ray absorptiometry, GC glucocorticoid, GPRD general practice research database, LY life-year, MOF major osteoporosis fracture, NR not reported, PVF prevalent vertebral fracture, QALY quality-adjusted life-year
Regarding the study population, nine studies included only men and 16 included both men and women. A wide variability in patient characteristics was observed, including patients with osteoporosis, low bone mass, or at high risk of fracture; patients or the general population with or without prior/recent fracture, men with prostate cancer beginning androgen deprivation therapy, and patients prescribed oral glucocorticoids. A healthcare perspective (typically including direct medical and non-medical costs) was used in 15 studies incorporating only direct costs, seven with a societal perspective (also including productivity losses arising from patients’ inability to work) and the remaining three studies with both societal and healthcare perspectives. All included studies used a Markov model consisting of a Markov microsimulation model (n = 10) and a Markov cohort model (n = 15). Most studies (n = 21) considered a lifetime horizon; only four studies [16, 18–20] applied a fixed time horizon such as 5, 6, or 10 years. One study [21] used the life-year as the outcome while the remaining studies used quality-adjusted life-years (QALYs). Most studies (n = 15) applied 3% as the discount rate for both costs and QALYs. Nine studies were funded by industry and another nine by national public funds while seven studies did not mention the source of funding (n = 3) or had no funding (n = 4).
Cost Effectiveness of Interventions
Table 2 reports information on the intervention and comparator, intervention duration, year of costing valuation, sensitivity analysis, and the main results of included articles. In eight studies that included active drugs (n = 8), bisphosphonates were included as the intervention in five studies [18, 20, 22–24] along with BMD testing or calcium/cholecalciferol and were compared to no treatment or nutrition supplements (sodium fluoride and/or calcium/cholecalciferol) alone. Two of these studies [20, 24] indicated that the bisphosphonate strategy alone was considered cost effective in patients aged 55 years and older with a fracture history, low bone mass, rheumatoid arthritis, or use of high-dose glucocorticoid doses (15 mg/day). Another two studies [22, 23] reported bone densitometry followed by bisphosphonates was cost effective for men aged 70 years or older with osteoporosis caused by androgen deprivation therapy, or for men aged over 65 years with a self-reported prior clinical fracture and for men aged 80–85 years with no prior fracture. Denosumab was included in two studies [25, 26] in comparison with bisphosphonates (generic alendronate, zoledronate, risedronate, and ibandronate) and teriparatide, with findings suggesting denosumab was cost effective in men aged 75 years and older with osteoporosis. Three studies [21, 27, 28] included vitamin D-fortified dairy products or calcium/vitamin D supplementation and indicated nutritional supplements were cost effective in men aged over 80 years, and in men over 60 years of age with osteoporosis when compared with usual care or no treatment.
Table 2.
Main results of studies assessing the cost-effectiveness of interventions for men with osteoporosis
| Reference | Intervention | Comparators | Intervention duration | Year of valuation | Results | |
|---|---|---|---|---|---|---|
| Base-case analysis | Sensitivity analysis | |||||
| Active drugs or nutritional supplements | ||||||
| Hiligsmann et al. [27] | Vit D-fortified dairy products | Usual care | 1 year (two dairy products per day) | 2015 EUR | In men aged over 60 years, the intake of two Vit D-fortified dairy products per day had an ICER of €106,113/QALY compared to usual care. The ICER was below a threshold of €30,000 /QALY in men aged over 80 years | One-way SA |
| Ethgen et al. [21] | CA/Vit D dietary supplementation | The absence of appropriate intake |
1 year: a daily dairy supplementation containing 1000 mg of CA and 800 IU of Vit D |
2014 EUR | The daily intake of Vit-D-rich dairy products was cost effective in general men aged over 70 years and in male patients at increased risk of osteoporotic fractures | None |
| Silverman et al. [25] | Denosumab | Bisphosphonates (generic alendronate, branded zoledronate, branded risedronate, branded ibandronate), and teriparatide |
5 years: denosumab, bisphosphonates 2 years: teriparatide |
2013 USD | Denosumab had an ICER of $16,888/QALY compared to generic alendronate and dominated all other treatments | One-way and probabilistic SA |
| Hiligsmann et al. [28] | CA/Vit D supplementation | No treatment (no CA/Vit D supplementation) | 3 years | 2012 EUR | Compared to no treatment, CA/Vit D supplementation had an ICER of €23,477/QALY and €10,250/QALY in men aged 60 years and 70 years, respectively, which was cost saving in men aged 80 years, suggesting CA/Vit D supplementation was cost effective for men with osteoporosis aged over 60 years | One-way and probabilistic SA |
| Parthan et al. [26] | Denosumab | Bisphosphonates (generic alendronate, branded zoledronate, generic risedronate, branded ibandronate), strontium ranelate, and teriparatide |
5 years: bisphosphonates, strontium ranelate, denosumab 2 years: teriparatide |
2012 EUR | Compared to other treatments, denosumab had the lowest costs and highest QALYs, indicating denosumab dominated all other treatments | One-way and probabilistic SA |
| Hiligsmann et al. [40] | Strontium ranelate | No treatment (no active drug treatment) | 3 years | 2010 EUR | Compared to no treatment, strontium ranelate had an ICER of €49,798/QALY and €25,584/QALY using ITT and PPS efficacy data, respectively in entire patients. The ICER falls below thresholds in partial patients with a BMD T-score ≤ − 2.5 or with PVF. Strontium ranelate could be considered cost effective compared with no treatment for male osteoporosis | One-way and probabilistic SA |
| Kouta et al. [23] | Selective branded alendronate therapy + BMD test | (Universal branded alendronate therapy without BMD test) or (no BMD test and no therapy) | 5 years: branded alendronate | 2008 USD | The ICERs for the strategy of a BMD test and selective alendronate therapy for patients with osteoporosis and universal alendronate therapy without a BMD test were US$66,800/QALY and $178,700/QALY, respectively, which was cost effective for those with osteoporosis | One-way SA |
| Kreck et al. [18] | Branded ibandronate+ calcium/colecalciferol | Fluoride strategy (sodium fluoride + calcium/colecalciferol), and calcium strategy (calcium/colecalciferol alone) | 42 months | 2004 EUR | The calcium strategy dominated the fluoride strategy. Compared to the calcium strategy, the ibandronate strategy had an ICER of €1,042,295/QALY in male patients aged over 65 years with a BMD T-score at baseline of −3.0. The ibandronate strategy is not cost effective | One-way, two-way, and probabilistic SA |
| Schousboe et al. [22] | Branded bisphosphonate + bone densitometry | No intervention (no drug treatment, no screening) | 5 years: oral bisphosphonate | 2004 USD | Compared with no intervention, the densitometry and follow-up treatment strategy had an ICER less than USD 50,000/QALY for men aged 65 years or older with a prior clinical fracture and for men aged 80 years or older without a prior fracture. The strategy may be cost effective in these patients | One-way and probabilistic SA |
| Van Staa et al. [20] | Branded bisphosphonate + GC | GC | 5 years: GC, bisphosphonate |
2003/2004 IB |
With the use of 5mg GCs daily, compared to no bisphosphonate, the bisphosphonate strategy had an ICER of £40,000, £43,000, £35,000 in men aged < 60 years, 60–79 years, and 80+ years, respectively. With the use of GC 15 mg daily, which was £22,000, £34,000, and £33,000, respectively. Bisphosphonates can be considered cost effective in patients with higher fracture risks and younger patients with a fracture history, low body mass index, rheumatoid arthritis, or using high GC doses | One-way SA |
| Borgstrom et al. [24] | Branded alendronate | No treatment (no active drug treatment) | 5 years | 2001 EUR | Compared to no treatment, alendronate had an ICER of €14,843/QALY and €5314/QALY from the societal and healthcare perspective, respectively. Alendronate was projected to be cost effective | One-way and probabilistic SA |
| Fleurence et al. [39] |
Scenario 1: VitD/CA Scenario 2: VitD/CA + hip protectors |
Scenario 1: hip protectors and no treatment (no VitD/CA ) Scenario 2: no treatment (no VitD/CA, no hip protector ) |
All preventive treatments would be used during the remaining lifetime of the patients. Patients would need two hip protectors every 2 years | 2000 USD | Vit D and CA alone was dominated by hip protectors in all patients. In the general-risk male population, compared to no treatment, hip protectors had an ICER of USD 47,426/QALY. In the male high-risk population, hip protectors had an ICER of USD 17,017/QALY, which was cost effective | Probabilistic SA |
| Intervention threshold | ||||||
| Chan et al. [29] | Branded alendronate | No treatment (no active drug treatment) | 5 years | 2010 USD | The hip fracture and MOF intervention threshold were 6% and 12.5% for men, respectively. For both MOF and hip fracture, interventions were cost effective only for men aged 55–85 years in the NHIA model, and for men aged over 75 years in the fracture model | One-way SA |
| Makras et al. [30] | (18% generic +82% branded) bisphosphonate-like intervention (combination of drugs) | Standard treatment (CA/Vit D) | 5 years | 2013 EUR | Drug intervention was found to be cost effective with a 10-year probability for a MOF at or above 20%, 9.5%, 9.5%, and 11% for men aged 50–54, 55–64, 65–74, and over 75 years, respectively. In addition, the drug intervention aiming at reducing the fracture risk was found to be cost effective with a 10-year probability for a hip fracture at/or above 1.4%, 1.2%, 2.3%, and 5.7% for men aged 50–54, 55–64, 65–74, and over 75 years, respectively | One-way SA |
| Lippuner et al. [32] | Branded alendronate | No treatment (no active drug treatment) | 5 years | 2008 CHF | Drug intervention was cost effective with a 10-year probability for a MOF at or above 15.1% (range 9.9–19.9%). Using the translational approach, the treatment was cost-effective or cost saving after the age of 55 years in men who had a previous fragility fracture | One-way SA |
| Tosteson et al. [31] | Generic bisphosphonate-like therapy | No treatment (no active drug treatment) | 5 years | 2005 USD | The treatment was cost effective when the 10-year hip fracture probability reached approximately 3% (range 2.4–4.9%) in men. The results were similar across race/ethnicity groups | One-way SA |
| Kanis et al. [33] | A hypothetical intervention | No treatment (no active drug treatment) | 5 years | 2001 USD | The treatment in men was cost effective with a 10-year hip fracture probability that ranged from 2.0% at 50 years to 6.46% at 80 years | One-way SA |
| Screening strategies | ||||||
|
Ito et al. [17] |
Screening strategy followed by bisphosphonate | Usual care (no screening) | Drug therapy: 5 years of alendronate therapy for those diagnosed with osteoporosis | 2019 USD | Compared to usual care, the screening strategy was cost effective having an ICER of USD 33,169/QALY. The screening strategy would become more effective and less costly for men 77 years and older | One-way and probabilistic SA |
| Pisu et al. [16] | Biomechanical computed tomography | Usual care; no screening |
BCT screening: in year 1 Drug therapy: 2 years of generic alendronate therapy for 50% of patients |
2016 USD | Compared to both usual care and no screening strategy, the BCT program was a dominant strategy, it provided greater clinical benefit at a lower cost. BCT was cost saving compared to usual care and no screening ($7000) | One-way SA |
| Nayak et al. [34] | Different bone density screening strategies | No screening |
Screening: repeat screening intervals of 5 years or 10 years Drug therapy: 5 years of bisphosphonate therapy for those screened positive with a particular strategy, or those who sustained a clinical fracture |
2014 USD | No screening was a less effective and more expensive option than all other strategies, indicating no screening was “dominated” by screening with DXA or the Osteoporosis Self-Assessment Tool at all evaluated screening initiation ages and repeat screening intervals | One-way and probabilistic SA |
| Schousboe et al. [35] | Bone density screening + (generic + branded) bisphosphonate therapy | No intervention (no screening, no drug treatment) | Drug therapy: 5 years | 2010 USD | The bone densitometry followed by drug therapy was cost effective for men aged 55, 75, and 80 years without a prior fracture when the body weight thresholds were below 67, 101, and 108 kg, respectively | One-way and probabilistic SA |
|
Ito et al. [37] |
Selective bone densitometry using the Osteoporosis Self-Assessment Tool |
No bone densitometry or universal bone densitometry |
Drug therapy: 5 years of generic alendronate therapy for those diagnosed with osteoporosis | 2006 USD | Selective bone densitometry would cost US$100,700/LY compared to the no bone densitometry strategy. Universal bone densitometry would cost US$483,500/LY compared to selective bone densitometry. When quality adjustments were introduced into the analysis, selective and universal bone densitometry became approximately 15% more cost effective | One-way SA |
| Schwenkglenks et al. [36] |
Screen-and-treat strategy (DXA followed by branded alendronate) |
No intervention (no screening, no drug treatment) |
5 years: branded alendronate The main screening ages were 65, 75, and 85 years |
2000 CHF | In men who entered the model at 50 years, the screen-and-treat strategy is not cost effective compared with no intervention for all age groups | One-way and probabilistic SA |
| Post-fracture care programs | ||||||
| DPhil et al. [38] | OG and nurse-led fracture liaison service | Usual care | NR |
2012/2013 IB |
The OG-led service was the most effective and cost-effective model of care at a threshold of £30,000/QALY. An OG-led or a nurse-led fracture liason service was cost effective when compared with usual care. If only healthcare costs are considered, an OG-led service was cost-effective at £14,525 for men aged 83 years | One-way and probabilistic SA |
| Johansson et al. [19] | A safety promotion program | Do nothing (no care was provided) | The program is 6 years | 2004 SEK | The do-nothing alternative was dominated by the post-intervention | One-way SA |
ADT androgen deprivation therapy, BCT biomechanical computed tomography, BMD bone mineral density, CA calcium, CHF Swiss Franc, DXA dual-energy X-ray absorptiometry, EUR Euro, GC glucocorticoid, GDP gross domestic product, HRQoL health-related quality of life, IB Pound, ICER incremental cost-effectiveness ratio, ITT intention-to-treat, LY life-year, MOF major osteoporosis fracture, NHIA National Health Insurance Administration, NR not reported, OG orthogeriatric, OST osteoporosis self-assessment tool, PPS per protocol studies, PVF prevalent vertebral fracture, QALY quality-adjusted life-year, SA sensitivity analysis, SEK Swedish Krona, USD US dollar, Vit D vitamin D, WTP willingness to pay
In five studies [29–33] that investigated cost-effective intervention thresholds, three used FRAX® as the measure of fracture risk. Bisphosphonates were used as the intervention in four studies (branded alendronate was used in two studies; however, the name of the bisphosphonate used in the other two studies was not mentioned) [29–32] compared with no treatment or calcium and vitamin D alone; the drug intervention was found to be cost effective with a 10-year probability of a major osteoporotic fracture (MOF) or hip fracture that ranged from 8.9 to 34.2% and from 0.8 to 7.5% for different age categories, respectively. The intervention thresholds at which an intervention is cost effective generally increases with the age of the population.
In six studies [16, 17, 34–37] that compared the screening strategy (followed by drug treatment) with no screening strategy or non-intervention strategy (both screening type and medications were not included), four studies [16, 17, 34, 35] reported that screening via bone density was cost effective in men aged 65 years and older. The screen-and-treat strategy was not cost effective compared with no intervention for all age groups as reported by Schwenkglenks and Lippuner [36]. One study [35] reported that bone densitometry followed by drug therapy was cost effective for men aged 55, 75, and 80 years without a prior fracture when the body weight thresholds were below 67, 101, and 108 kg, respectively.
Two studies [19, 38] indicated that post-fracture care programs were cost effective compared with the usual care or do-nothing alternative in men aged over 60 years with a recent fracture. For studies including active drugs, treatment costs encompassing drug costs, physician visit costs (and frequency) as well as BMD testing costs (and frequency) were removed from the analysis but are included in the ESM. These costs differ greatly among studies (with teriparatide having the highest annual cost and generic alendronate having the lowest annual cost in general). Most studies assumed a physician visit once per year and BMD testing once every 2 years.
Comparison in Cost Effectiveness Between Men and Women
Tables 3 and 4 present synthesized studies (n = 14) that reported results of different (age, fracture risk, or intervention) characteristics for men and women. These studies were further categorized by use of ICERs (Table 3, n = 9) or intervention thresholds (Table 4, n = 5) as the outcome. Specifically, nine studies [16, 18, 20, 21, 27, 28, 36, 38, 39] used ICERs as the main outcome, leading to a total of 33 comparisons from these studies. Among these, 73% (24) of comparisons reported higher ICERs in men than in women; the relative difference in ICERs was larger with increasing age and a higher fracture risk at baseline in general. Despite differences in ICERs between men and women, five studies [16, 18, 21, 28, 38] and 24 of 33 comparisons (73%) reported similar conclusions about the cost effectiveness of the intervention. The remaining 27% revealed the intervention was cost effective only for women (men yielded higher ICERs). In five studies with intervention thresholds [29–33] (containing a total of 43 comparisons), 21 out of 43 comparisons (49%) reported lower intervention thresholds for men compared with women (particularly those that assessed the 10-year probability of hip fracture in men over the age of 70 years), the other half of the comparisons indicated higher intervention thresholds in women, suggesting no major differences were identified between men and women.
Table 3.
Results of comparison between men and women for studies using ICER as the outcome (base case)
| Reference | ICER | Cost-effective (yes/no) | ||||
|---|---|---|---|---|---|---|
| Scenarios | Women | Men | Difference (women as reference) | Women | Men | |
| Hiligsmann et al. [27] | All ages | €38,526 | €106,113 | + | No | No |
| 60–69 years | €155,006 | €218,176 | + | No | No | |
| 70–79 years | €24,997 | €92,676 | + | Yes | No | |
| 80+ years | €1907 | €27,683 | + | Yes | Yes | |
| Ethgen et al. [21] | Osteoporosis | |||||
| 50 years | €300,277 | €203,563 | − | No | No | |
| 60 years | €174,359 | €121,582 | − | No | No | |
| 70 years | €74,707 | €61,349 | − | Yes | Yes | |
| 80 years | €19,910 | €24,231 | + | Yes | Yes | |
| Prevalent fracture | ||||||
| 50 years | €168,701 | €118,823 | − | No | No | |
| 60 years | €96,744 | €70,057 | − | Yes | Yes | |
| 70 years | €35,687 | €31,423 | − | Yes | Yes | |
| 80 years | €3369 | €8916 | + | Yes | Yes | |
| Hiligsmann et al. [28] | 60 years | €40,578 | €23,477 | + | Yes | Yes |
| 70 years | €7912 | €10,250 | + | Yes | Yes | |
| 80 years | −€12,815 (cost saving) | −€6723 (cost saving) | + | Yes | Yes | |
| Kreck et al. [18] | BMD T-score − 3.0 | |||||
| 65 years | €407,375 | €1,042,295 | + | No | No | |
| Van Staa et al. [20] | GC 5 mg | |||||
| < 60 years | £41,000 | £40,000 | − | No | No | |
| 60–79 years | £17,000 | £43,000 | + | Yes | No | |
| 80 + years | £5000 | £35,000 | + | Yes | No | |
| GC 15 mg | ||||||
| < 60 years | £17,000 | £22,000 | + | Yes | Yes | |
| 60–79 years | £13,000 | £34,000 | + | Yes | No | |
| 80 + years | £15,000 | £33,000 | + | Yes | No | |
| Fleurence et al. [39] | General population | |||||
| Hip pad | $11,722 | $47,426 | + | Yes | No | |
| Hip pad + VitD/CA | $25,123 | $80,998 | + | No | No | |
| High-risk population | ||||||
| Hip pad | −$450 (cost saving) | $17,017 | + | Yes | Yes | |
| Hip pad + VitD/CA | $6572 | $33,565 | + | Yes | No | |
| Pisu et al. [16] | Vs no screening | −$49,261 (cost saving) | −$4487 (cost saving) | + | Yes | Yes |
| Vs usual care | −$38,305 (cost saving) | −$4729 (cost saving) | + | Yes | Yes | |
| Schwenkglenks et al. [36] | 65 years | CHF70,995 | CHF197,460 | + | No | No |
| 75 years | CHF35,412 | CHF123,094 | + | Yes | No | |
| 85 years | CHF28,170 | CHF118,945 | + | Yes | No | |
| DPhil et al. [38] | FLS vs usual care | £20,421 | £19,955 | − | Yes | Yes |
| OG vs FLS | £22,709 | £23,407 | + | Yes | Yes | |
BMD bone mineral density, CA calcium, CHF Swiss Franc, FLS fracture liaison service, GC glucocorticoid, ICER incremental cost-effectiveness ratio, OG orthogeriatric, Vit D vitamin D, + indicates men had higher ICERs than women, − indicates men had lower ICERs than women
Table 4.
Results of comparison between men and women for studies using intervention threshold as the outcome (base case)
| References | Threshold in women | Women | Men | Absolute change (women as the reference) |
|---|---|---|---|---|
| Chan et al. [29] | 10-year probability of hip fracture | |||
| All ages | 7.0% | 6.0% | − 1.0% | |
| 10-year probability of MOF | ||||
| All ages | 15.0% | 12.5% | − 2.5% | |
| Makras et al. [30] | 10-year probability of MOF | |||
| 50 years | 20.4% | 34.2% | + 13.8% | |
| 55 years | 7.8% | 9.6% | + 1.8% | |
| 60 years | 9.1% | 9.3% | + 0.2% | |
| 65 years | 8.7% | 10.0% | + 1.3% | |
| 70 years | 9.2% | 8.9% | − 0.3% | |
| 75 years | 13.0% | 10.5% | − 2.4% | |
| 80 years | 16.0% | 11.2% | − 4.8% | |
| 85 years | 16.0% | 11.2% | − 4.8% | |
| 10-year probability of hip fracture | ||||
| 50 years | 1.7% | 1.8% | + 0.1% | |
| 55 years | 0.9% | 0.8% | − 0.1% | |
| 60 years | 1.5% | 1.5% | 0% | |
| 65 years | 1.8% | 2.2% | + 0.4% | |
| 70 years | 2.6% | 2.4% | − 0.2% | |
| 75 years | 4.7% | 4.5% | − 0.2% | |
| 80 years | 7.1% | 5.9% | − 1.2% | |
| 85 years | 7.8% | 6.6% | − 1.2% | |
| Lippuner et al. [32] | 10-year probability of MOF | |||
| All ages | 13.8% | 15.1% | + 1.3% | |
| 55 years | 14.1% | 9.9% | − 4.2% | |
| 60 years | 14.4% | 12.0% | − 4.4% | |
| 65 years | 12.8% | 13.9% | + 1.1% | |
| 70 years | 14.4% | 17.5% | + 3.1% | |
| 75 years | 14.8% | 19.9% | + 5.1% | |
| 80 years | 15.0% | 19.0% | + 4.0% | |
| 85 years | 10.8% | 13.5% | + 2.7% | |
| Tosteson et al. [31] | 10-year probability of hip fracture (white) | |||
| 50 years | 2.5% | 2.4% | − 0.1% | |
| 55 years | 2.8% | 4.2% | + 1.4% | |
| 60 years | 3.0% | 4.1% | + 1.1% | |
| 65 years | 2.8% | 3.5% | + 0.7% | |
| 70 years | 4.0% | 4.8% | + 0.8% | |
| 75 years | 4.4% | 3.9% | − 0.5% | |
| 80 years | 4.0% | 4.0% | 0% | |
| 85 years | 3.3% | 3.1% | − 0.2% | |
| Kanis et al. [33] | 10-year probability of hip fracture | |||
| 50 years | 0.9% | 1.6% | + 0.6% | |
| 55 years | 1.5% | 1.9% | + 0.4% | |
| 60 years | 2.3% | 2.6% | + 0.2% | |
| 65 years | 3.5% | 3.4% | − 0.1% | |
| 70 years | 4.8% | 4.6% | − 0.2% | |
| 75 years | 6.1% | 5.6% | − 0.5% | |
| 80 years | 7.9% | 7.1% | − 0.8% | |
| 85 years | 6.9% | 7.5% | + 0.6% | |
| 90 years | 7.7% | 7.4% | − 0.2% |
MOF major osteoporosis fracture
Quality Assessment
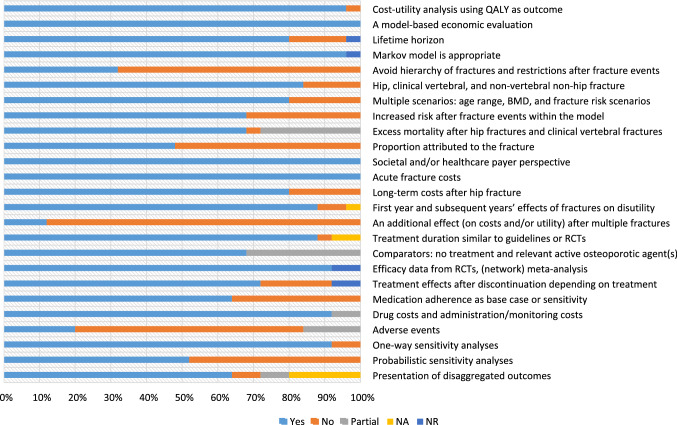
Results of the quality appraisal of the design and conduct of the economic evaluation in men with osteoporosis are presented in the ESM. The quality of included studies was relatively good with an average score of 18.8 out of 25 (range 13–23.5). The average score for studies that included active drugs or nutritional supplements, intervention thresholds, screening strategies, and post-fracture care programs as the intervention was 19.2, 17.1, 21.0, and 14.0, respectively; 44% of included studies scored more than 20 points.
Figure 2 displays the proportion of studies that included the individual items recommended in the ESCEO-IOF guidelines and whether an item was fully reported, partially reported, or not reported in the cited studies. The most frequently unreported items were ‘an additional effect on costs and/or utility after multiple fractures,’ ‘the effect of adverse events on costs and/or utility,’ ‘avoid hierarchy of fractures and restrictions after fracture events,’ and ‘proportion of excess mortality attributed to the fracture’. In addition, two items (‘comparators: no treatment and relevant active osteoporotic agents’ and ‘excess mortality after hip and clinical vertebral fractures’) were frequently partially reported.
Fig. 2.
Proportion of studies meeting individual items recommended in the Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases and the International Osteoporosis Foundation guideline (total studies: 25). BMD bone mineral density, NA not applicable, NO not reported, NR not reported, Partial partially reported, QALY quality-adjusted life-year, RCTs randomized controlled trials, Yes fully reported
Source of Model Input Data
Table 5 displays the source of model parameters for each study. For fracture incidence, specific male data were used for hip fracture in all included studies. One study [39] indicated their vertebral fracture incidence data were adjusted from female data, and the incidence of other fractures was obtained from a study combining both sexes. However, given the absence of country-specific fracture incidence data, four studies [18, 29, 32, 40] derived relevant data from other countries. The source of fracture cost data varies significantly among studies. Most studies (n = 13) obtained hip fracture cost data from studies including both men and women; only eight studies fully obtained and used male hip fracture cost data. For non-hip fracture costs, only three studies [25, 28, 32] reported the use of male-specific data. With regard to utility data, nine studies [16, 17, 19, 22, 25, 26, 31, 34, 37] used male-specific baseline utilities; however, over 90% of the studies derived disutility data from studies encompassing both sexes. In addition, nearly all studies obtained male-specific baseline mortality data, and male excess mortality data were used in half of the studies (n = 13). With regard to treatment efficacy data, only three studies [22, 34, 37] extracted male efficacy data from meta-analyses based on randomized controlled trials of alendronate in men, most studies (n = 14) used female efficacy data or obtained relevant data from studies combining both sexes. Most studies did not include treatment adverse events (n = 16) and medication adherence (n = 9) in their models. In five studies [23, 32, 34, 37, 40] that modeled the adverse events, only one study [34] included the rare but serious side effects of osteonecrosis of the jaw, and subtrochanteric femoral fracture. For studies that indicated the source of these two parameters, female data or assumptions were frequently used.
Table 5.
Source of model input data (men, women, or combined studies including both sexes)
| References | Fracture incidence | Fracture cost | Baseline utilities | Fracture disutility | Baseline mortality | Fracture effects on mortality | Treatment efficacy | Side effects | Medication adherence/persistence | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip | Non-hip | Hip | Non-hip | Hip | Non-hip | |||||||
| Active drugs or nutritional supplements | ||||||||||||
| Hiligsmann et al. [27] | M | M | C | C | C | C | C | M | M | C | NA | A (only SA) |
| Ethgen et al. [21] | M | M |
Hos: M Extra: Wb |
A | NR | NR | NR | M | C | C | NA | None |
| Silverman et al. [25] | M | M | M | M | M | C |
CV: C Others: W |
M | M | W | None | W |
| Hiligsmann et al. [28] | M | M | M | M | C | C | C | M | M | C | NA | None |
|
Parthan et al. [26] |
M | M | M | C | M | C |
CV: C Others: W |
M | M | W | None | W |
| Hiligsmann et al. [40] | M | Ma |
Hos: M Extra: Wb |
A | C | C | C | M | M | W | A and EO | W |
| Kouta et al. [23] | M | NA | C | NA | A | M | NA | M | M | A | C | A |
| Kreck et al. [18] | M | Ma | C | C | C | C | C | M |
HF: C CV: M |
Wb | None | None |
| Schousboe et al. [22] | M | M | C | C | M | C | C | M | NR | M | None | W |
| Van Staa et al. [20] | M | M | W | W | C | W | W | M | M | A | None | None |
| Borgstrom et al. [24] | M | M | M | C | NR | C and A | C and A | M | M | W | None | None |
| Fleurence et al. [39] | M |
CV: Wb Colles’ fx: M Others: C |
W | W | C | C | C | M | A | C and A | NA | C |
| Intervention threshold | ||||||||||||
| Chan et al. [29] | Ma | Ma | C | C | C | C | C | C | Ma | C | None | None |
| Makras et al. [30] | M | M | Ca | Ca | NR | NR | NR | M |
HF and CV: M Others: A |
A | None | A |
| Lippuner et al. [32] | M |
CV and WF: M Others: Ma |
M | M | Ca | Ca | Ca | M |
HF and CV: M WF: A Others: W |
C | A | C |
| Tosteson et al. [31] | M | M | C |
CV and WF: C Others: M |
M | C |
CV and WF: C Others: M |
M |
HF: C Others: none |
A | None | A |
| Kanis et al. [33] | M | M | M | M (A) | NR | C | C | M | C | A | None | None |
| Screening strategies | ||||||||||||
| Ito et al. [17] | M | M | C | C | M | C | C | M | M | W | None | C |
| Pisu et al. [16] | M | NA | M | NA | M | C | NA | M | C | W | None | W |
| Nayak et al. [34] | M | M | C | C (A for HF) | M | C | C | M | M | M | C | A |
| Schousboe et al. [35] | M | M | C | C | C (A) | C | C | M | C | C | None | W |
| Ito et al. [37] | M | M | C | C | M | C | C | M | C | M | W | C |
| Schwenkglenks et al. [36] | M | M | C | C | C | M | M | M | M | W | None | W |
| Post-fracture care programs | ||||||||||||
| DPhil et al. [38] | M | M | M | NA | C | C | NA | M | NR | NR | None | None |
| Johansson et al. [19] | M | NA | C | NA | M | C | NA | M | M | A | None | None |
A assumption, C combination, CV clinical vertebral fracture, EO expert opinion, Fx fracture, HF hip fracture, HF humerus fracture, Hos hospitalization, M men, NA not applicable, None parameters were not incorporated in the model, NR not reported, W women, WF wrist fracture
aRelevant data were extracted from other countries
bWomen data adjusted
Discussion
This systematic review identified 25 cost‑effectiveness analyses of interventions for osteoporosis in men published between 2000 and June 2022. Most of the studies assessed active drugs (n = 8) or nutritional supplements (n = 4) followed by screening strategies (n = 6), intervention thresholds (n = 5), and post-fracture care programs (n = 2). A comparison to two previous reviews [9, 10] of cost‑effectiveness analyses of drugs for postmenopausal osteoporosis by Hiligsmann et al. [10] (n = 39, between 1 January, 2008 and 31 December, 2013) and Li et al. [9] (n = 27, between 1 July, 2013 and 31 December, 2019), shows that the number of economic evaluations of interventions in men (n = 25) with osteoporosis is limited. Nearly all studies included in this review were conducted in Europe and North America, and only two studies [16, 17] were published in the past 5 years. In contrast, cost-effectiveness studies in women were performed in a large number of countries (a total of 23 countries in Europe, three in North America, three in the Asia-Pacific region, one in the Middle East, and one in Australia), and the number of publications identified were published in recent years [9]. Compared with postmenopausal women, economic evaluations in men are largely insufficient and relatively outdated, even though some of the medicines for osteoporosis are approved for use in men. This could be owing to the lack of attention given to the treatment of osteoporosis in men, and some active drugs that have yet to be approved or reimbursed for men in some countries.
With regard to active drugs (referring to anti-osteoporosis medication rather than nutritional supplements), four out of five studies [18, 20, 22–24] revealed that bisphosphonates (with/without BMD testing) were generally cost effective compared with no treatment or nutritional supplements in men aged 55 years and older with a fracture history, low bone mass, or rheumatoid arthritis. However, there was no study comparing the cost effectiveness between bisphosphonate types. More future studies are needed. Although glucocorticoid excess and hypogonadism (e.g., androgen deprivation therapy for prostate cancer) are two main factors to increase the risk of secondary osteoporosis [1] and fracture in men, only two cost-effectiveness analyses [20, 23] were published and reported that alendronate therapy in conjunction with BMD testing was cost effective in patients starting adjuvant androgen deprivation therapy for locally advanced or high-risk localized prostate cancer, and bisphosphates were cost effective in patients using high doses of glucocorticoids. However, it should be noted that heterogeneity in interventions being compared in the target population of the included studies in terms of BMD status, fracture risk, and prior fracture (yes/no) was identified, which made it difficult to conduct comparisons between the studies and synthesize data.
Only two studies (in the USA and Sweden) were identified to assess the cost effectiveness of denosumab in men with osteoporosis [25, 26], compared with 17 studies in women as reported by a recent systematic review [41]. Although both studies indicated denosumab was cost effective compared to bisphosphonates (alendronate, zoledronate, risedronate, and ibandronate), the results should be further confirmed. Moreover, considering denosumab is also approved for the treatment of bone loss in men with prostate cancer undergoing hormone ablation therapy, and for men with glucocorticoid-induced osteoporosis, future studies are highly needed to reveal the potential economic benefits of denosumab for men with glucocorticoid use or hypogonadism.
Most studies assessed the cost effectiveness in primary prevention (i.e., patients with osteoporosis) and only one study [40] compared the cost effectiveness of strontium ranelate to no treatment in various populations (BMD T-score ≤−2.5 and/or prevalent vertebral fracture), suggesting improved cost effectiveness (lower ICERs) in patients with previous fractures. No economic evaluations have been performed in men with osteoporosis treated with teriparatide despite its approval in 2002 by the US Food and Drug Administration and in 2003 by the European Medicines Agency to increase bone mass in men. Only two studies [25, 26] used it as a comparator and reported teriparatide was not cost effective compared to denosumab. The recent availability of biosimilar teriparatide could potentially affect this finding.
Recently, one study [42] reported that abaloparatide, a human parathyroid hormone-related peptide(1–34) analog, in men with osteoporosis leads to rapid and significant improvements in BMD with a safety profile similar to women, suggesting abaloparatide can be considered as an effective anabolic treatment option for men with osteoporosis. However, relevant economic data of abaloparatide in men are still lacking, and future economic studies are needed.
There is emerging economic evidence about the value of sequential therapy (anabolic agents followed by anti-resorptive agents) in postmenopausal women [43–45], no relevant studies were identified in men. Economic research on sequential therapy in men may be of interest for future research. Our review found that most cost-effectiveness analyses in men were based on bridging studies, the small-scaled studies with a shorter duration that use BMD as a surrogate endpoint to support an indication in men. When an agent in a bridging study for men increases BMD to a magnitude comparable to that observed in the larger, longer, and more extensive studies (e.g., including assessment of the effect on fracture risk) required for approval in postmenopausal women, the validation of this treatment in men is considered sufficient. This strategy is the accepted approach by regulators and payers, and acceptable from a health economics perspective [46].
Several economic evaluations included in our study were performed to assess the cost effectiveness of screening strategies for osteoporosis. Though four out of six studies (in our review) indicated BMD screening was cost effective in men, there is ongoing debate regarding the benefits of a widespread systematic screening approach for osteoporosis in men [47]. In the USA, dual-energy X-ray absorptiometry-based osteoporosis screening is recommended by some societies and guidelines (but not widely covered by insurance) for men aged over 70 years or over 50 years who have sustained a fracture [48]. Within the included studies, we found that most studies assumed/reported BMD testing once every 2 years for patients with osteoporosis. The frequency of BMD testing could, however, depend on BMD T-scores, and less frequent testing has been recommended for patients with osteopenia [49].
For patients requiring a fracture risk assessment, the threshold at which treatment should be initiated will vary according to factors such as healthcare provision, willingness to pay, and cost of medications [47]. The most recent guidelines [50, 51] suggest treating patients whose FRAX 10-year major osteoporotic fracture risk scores are ≥ 20%. However, a recently published study [52] indicated that assessment by the FRAX algorithm appears to underestimate the risk in older people, thus the therapeutic choice for these patients needs to be adjusted. Diagnostic-therapeutic decision making in real-world practice must consider a wider assessment focused on the specific needs of the individual patient [52]. Another concern is that intervention thresholds varied significantly across studies and settings because consensus on whether the threshold level should be fixed or age and sex dependent is lacking. All five studies included in our systematic review reported age- and sex-dependent thresholds, and the intervention thresholds increased with age, which was in line with National Osteoporosis Guideline Group in the UK [53].
With the wide implementation of post-fracture care programs, such as FLS, there has been an increase in the number of cost-effectiveness analyses conducted [54] and most of these studies only focused on women. Studies in our review indicated that post-fracture care programs were cost effective in men, the economic benefits of FLS in men might be further supported in future studies.
The cost-effectiveness estimations (ICERs or intervention thresholds) for men and women were quite similar. Specifically, over 70% of comparisons reported similar conclusions about the cost effectiveness of the intervention in men and women, despite men yielding higher ICERs that led to non-cost-effective estimations in the remaining few comparisons resulting mainly from differences in fracture incidence. This could be because fracture incidence at baseline was comparably lower for men than for women. It might be interesting to confirm in future studies. In addition, no major differences were identified between men and women concerning cost-effective intervention thresholds, suggesting that intervention thresholds are probably similar in men and women from an economic point of view. Given the similar ICERs and intervention thresholds between men and women, fracture risk reduction is the primary consideration in the treatment of osteoporosis irrespective of sex, which is also indicated by romosozumab for the treatment of severe osteoporosis in Australia by the Pharmaceutical Benefits Advisory Committee [55].
An osteoporosis-specific guideline [15] was used in our study for quality appraisal of the studies included. This guideline can serve as a guide for the design, conduct, and reporting of economic evaluations in osteoporosis to improve their transparency, comparability, and methodologic standards, and to further facilitate inter-study comparisons. Although the quality of the studies included in our review was relatively high, some items were frequently missing or only partially reported , which is in alignment with a previous systematic review [9], and these items deserve attention in future studies.
Regarding the source of model input data, male-specific data were commonly used for fracture incidence, baseline mortality, and baseline utility data. However, some data, for example, fracture cost and disutility, were commonly retrieved from studies including both men and women, and treatment efficacy was mostly obtained from women based on a meta-analysis or randomized controlled trial. This is not an incorrect use of data per se as the effect of fracture on utility has been shown to be similar between men and women as reported by a recent study [56] revealing that men and women had a similar trajectory of health-related quality-of-life recovery following fragility fracture at any skeletal site. Similarly, one systematic review and meta-analysis [57] reported the efficacy of treatment options to reduce osteoporotic fracture risk in men was comparable to women, therefore it might not weaken the analysis to use female data in the absence of male-specific treatment efficacy data. It is however important that male-specific data be used for several parameters owing to the differences between men and women, in particular for fracture incidence, increased risk after subsequent fractures, mortality excess, and fracture costs.
There are several implications of our review. First, this study summarizes the current economic evidence of cost-effectiveness analyses of interventions in men with osteoporosis and reveals the knowledge gap (insufficient economic data and publications) when compared with studies in women. Second, our study indicates the overall comparability of conclusions on the cost effectiveness of interventions in men and women, with greater ICERs in men. Third, we highlight that some male-specific data are needed in the design of an economic evaluation in men. Adhering to the ESCEO-IOF guideline [15] as well as CHEERS 2022 [58] is also important for future economic evaluations in osteoporosis to improve the quality of studies. These guidelines provide recommendations for the conduct and reporting of economic evaluations (in osteoporosis) and are important to improve the quality and standardization of these studies.
Our study has two main limitations. First, the osteoporosis-specific guideline is more appropriate to appraise cost-effectiveness analyses of active drugs for osteoporosis, thus some items for studies that investigated other interventions such as screening strategies and intervention thresholds might not be applicable and underscored. Second, the source of model input data in some studies cannot be identified, therefore it is difficult to make a fully precise summary on the proportion of study using male-specific data for these model parameters.
Conclusions
Our systematic review included 25 studies on the cost effectiveness of interventions for osteoporosis in men, covering active drugs or nutritional supplements, intervention thresholds, screening strategies, and post-fracture care programs between 1 January, 2000 and 30 June, 2022. Overall, anti-osteoporosis drugs and nutritional supplements are generally cost effective in men with osteoporosis. Screening strategies and post-fracture care programs also showed economic benefits for men. Cost-effectiveness and intervention thresholds were generally rather similar in studies conducted in both men and women, with slightly greater ICERs in men. More high-quality and national studies in men with osteoporosis are needed to close the current research gap and further inform decision making.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This study is sponsored by Radius Health, Inc.
Conflict of interest
Nannan Li is funded by the China Scholarship Council (grant number 201909110080). Mickaël Hiligsmann has received research grants through his institution from Amgen, Radius Health, Inc. (Radius), and ViiV, consulting fees from UCB, and lecture fees from Mylan Pharmaceuticals. Stuart Silverman has received grants from Amgen and Radius, and has received consulting fees from Amgen and Radius. Steven W. Ing has received research grants paid to his institution from Alexion, Amgen, Calcilytix, Radius, Takeda, and Ultragenyx. Andrea Singer has received research grants paid to her institution from Radius and UCB, consulting fees from Agnovos, Amgen, Radius, and UCB, and speaking fees from Amgen and Radius. Charlotte Beaudart, Jean-Yves Reginster, Nancy E. Lane, and Jane A, Cauley have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data analyzed as part of this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Authors’ contributions
NL: study rationale and design, literature selection, data extraction, quality assessment of included studies, data synthesis, writing study protocol and manuscript. CB: study rationale and design, literature search and selection, checking data extraction forms, quality assessment of included studies, writing study protocol, reviewing and revising the manuscript. JAC: reviewed data generated, reviewing and revising the manuscript. SWI reviewed data generated, reviewing and revising the manuscript. NEL reviewed data generated, reviewing and revising the manuscript. JYR reviewed data generated, reviewing and revising the manuscript. SS: reviewed data generated, reviewing and revising the manuscript. AJS reviewed data generated, reviewing and revising the manuscript. MH: study rationale and design, providing suggestions for discrepancies between two reviewers, interpretation and reflection, reviewing and revising the manuscript, the guarantor of the study.
Footnotes
The original online version of this article was revised to correct a sentence beginning "However, it should be noted...", under heading Methods, section 2.1.
Change history
3/4/2023
A Correction to this paper has been published: 10.1007/s40273-023-01255-w
References
- 1.Vilaca T, Eastell R, Schini M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022;10(4):273–283. doi: 10.1016/S2213-8587(22)00012-2. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC. Risk of fracture after androgen deprivation for prostate cancer. J Urol. 2005;174(3):929–930. doi: 10.1097/S0022-5347(01)68451-9. [DOI] [PubMed] [Google Scholar]
- 3.Adler RA. Osteoporosis in men: a review. Bone Res. 2014;2(April):1–8. doi: 10.1038/boneres.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Osteoporosis Foundation. Fragility fractures: epidemiology. https://www.osteoporosis.foundation/health-professionals/fragility-fractures/epidemiology. Accessed 17 Jan 2023.
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, Norton N, Harvey NC, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16(1):82. doi: 10.1007/s11657-020-00871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewiecki EM, Ortendahl JD, Vanderpuye-Orgle J, et al. Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus. 2019;3(9):1–7. doi: 10.1002/jbm4.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman JM. Management of osteoporosis in older men. Aging Clin Exp Res. 2021;33(6):1439–1452. doi: 10.1007/s40520-021-01845-8. [DOI] [PubMed] [Google Scholar]
- 9.Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39(2):181–209. doi: 10.1007/s40273-020-00965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiligsmann M, Evers SM, Ben Sedrine W, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205–224. doi: 10.1007/s40273-014-0231-1. [DOI] [PubMed] [Google Scholar]
- 11.NIHR. PROSPERO: international prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/. Accessed 17 Jan 2023.
- 12.Thielen FW, Van Mastrigt GAPG, Burgers LT, et al. How to prepare a systematic review of economic evaluations for clinical practice guidelines: database selection and search strategy development (part 2/3) Expert Rev Pharmacoecon Outcomes Res. 2016;16(6):705–721. doi: 10.1080/14737167.2016.1246962. [DOI] [PubMed] [Google Scholar]
- 13.Morrison A, Polisena J, Husereau D, et al. The effect of english-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 14.Kanis JA, McCloskey EV, Harvey NC, et al. Intervention thresholds and diagnostic thresholds in the management of osteoporosis. Aging Clin Exp Res. 2022;34(12):3155–3157. doi: 10.1007/s40520-022-02216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiligsmann M, Reginster J, Tosteson ANA, et al. Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the Osteoporosis Foundation. Osteoporosis Int. 2019;30(1):45–57. doi: 10.1007/s00198-018-4744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisu M, Kopperdahl DL, Lewis CE, Saag KG, Keaveny TM. Cost-effectiveness of osteoporosis screening using biomechanical computed tomography for patients with a previous abdominal CT. J Bone Miner Res. 2019;34(7):1229–1239. doi: 10.1002/jbmr.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito K. Cost-effectiveness of screening for osteoporosis in older men with a history of falls. JAMA Netw Open. 2020;3(12):e2027584. doi: 10.1001/jamanetworkopen.2020.27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreck S, Klaus J, Leidl R, et al. Cost effectiveness of ibandronate for the prevention of fractures in inflammatory bowel disease-related osteoporosis: cost-utility analysis using a Markov model. Pharmacoeconomics. 2008;26(4):311–328. doi: 10.2165/00019053-200826040-00004. [DOI] [PubMed] [Google Scholar]
- 19.Johansson P, Sadigh S, Tillgren P, Rehnberg C. Non-pharmaceutical prevention of hip fractures: a cost-effectiveness analysis of a community-based elderly safety promotion program in Sweden. Cost Eff Resour Alloc. 2008;6:1–12. doi: 10.1186/1478-7547-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Staa TP, Geusens P, Zhang B, Leufkens HGM, Boonen A, Cooper C. Individual fracture risk and the cost-effectiveness of bisphosphonates in patients using oral glucocorticoids. Rheumatology. 2007;46(3):460–466. doi: 10.1093/rheumatology/kel249. [DOI] [PubMed] [Google Scholar]
- 21.Ethgen O, Hiligsmann M, Burlet N, Reginster JY. Public health impact and cost-effectiveness of dairy products supplemented with vitamin D in prevention of osteoporotic fractures. Arch Public Health. 2015;73(1):1–7. doi: 10.1186/s13690-015-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. J Am Med Assoc. 2007;298(6):629–637. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Elkin EB, Girotra M, Morris MJ. Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med. 2010;152(10):621–629. doi: 10.7326/0003-4819-152-10-201005180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgström F, Johnell O, Jönsson B, Zethraeus N, Sen SS. Cost effectiveness of alendronate for the treatment of male osteoporosis in Sweden. Bone. 2004;34(6):1064–1071. doi: 10.1016/j.bone.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Silverman S, Agodoa I, Kruse M, Parthan A, Orwoll E. Denosumab for elderly men with osteoporosis: a cost-effectiveness analysis from the US payer perspective. J Osteoporos. 2015 doi: 10.1155/2015/627631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parthan A, Kruse M, Agodoa I, Silverman S, Orwoll E. Denosumab: a cost-effective alternative for older men with osteoporosis from a Swedish payer perspective. Bone. 2014;59:105–113. doi: 10.1016/j.bone.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Hiligsmann M, Burlet N, Fardellone P, Al-Daghri N, Reginster JY. Public health impact and economic evaluation of vitamin D-fortified dairy products for fracture prevention in France. Osteoporos Int. 2017;28(3):833–840. doi: 10.1007/s00198-016-3786-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiligsmann M, Sedrine W Ben, Bruyère O, Evers SM, Rabenda V, Reginster JY. Cost-effectiveness of vitamin D and calcium supplementation in the treatment of elderly women and men with osteoporosis. Eur J Public Health. 2015;25(1):20–5. 10.1093/eurpub/cku119. [DOI] [PubMed]
- 29.Chan DC, McCloskey EV, Chang CB, et al. Establishing and evaluating FRAX® probability thresholds in Taiwan. J Formos Med Assoc. 2017;116(3):161–168. doi: 10.1016/j.jfma.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Makras P, Athanasakis K, Boubouchairopoulou N, et al. Cost-effective osteoporosis treatment thresholds in Greece. Osteoporos Int. 2015;26(7):1949–1957. doi: 10.1007/s00198-015-3055-8. [DOI] [PubMed] [Google Scholar]
- 31.Tosteson ANA, Melton LJ, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19(4):437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippuner K, Johansson H, Borgström F, Kanis JA, Rizzoli R. Cost-effective intervention thresholds against osteoporotic fractures based on FRAX® in Switzerland. Osteoporos Int. 2012;23(11):2579–2589. doi: 10.1007/s00198-011-1869-6. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, Johnell O, Oden A, et al. Intervention thresholds for osteoporosis in men and women: a study based on data from Sweden. Osteoporos Int. 2005;16(1):6–14. doi: 10.1007/s00198-004-1623-4. [DOI] [PubMed] [Google Scholar]
- 34.Nayak S, Greenspan SL. Cost-effectiveness of osteoporosis screening strategies for men. J Bone Miner Res. 2016;31(6):1189–1199. doi: 10.1002/jbmr.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schousboe JT, Gourlay M, Fink HA, et al. Cost-effectiveness of bone densitometry among Caucasian women and men without a prior fracture according to age and body weight. Osteoporos Int. 2013;24(1):163–177. doi: 10.1007/s00198-012-1936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwenkglenks M, Lippuner K. Simulation-based cost-utility analysis of population screening-based alendronate use in Switzerland. Osteoporos Int. 2007;18(11):1481–1491. doi: 10.1007/s00198-007-0390-4. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Hollenberg JP, Charlson ME. Using the osteoporosis self-assessment tool for referring older men for bone densitometry: a decision analysis. J Am Geriatr Soc. 2009;57(2):218–224. doi: 10.1111/j.1532-5415.2008.02110.x. [DOI] [PubMed] [Google Scholar]
- 38.Leal J, Gray AM, Hawley S, et al. Cost-effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population-based study. J Bone Miner Res. 2017;32(2):203–211. doi: 10.1002/jbmr.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleurence RL. Cost-effectiveness of fracture prevention treatments in the elderly. Int J Technol Assess Health Care. 2004;20(2):184–191. doi: 10.1017/S0266462304000960. [DOI] [PubMed] [Google Scholar]
- 40.Hiligsmann M, Ben Sedrine W, Bruyère O, Reginster JY. Cost-effectiveness of strontium ranelate in the treatment of male osteoporosis. Osteoporos Int. 2013;24(8):2291–2300. doi: 10.1007/s00198-013-2272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan Y, Zeng F, Tan H, et al. Cost-effectiveness analyses of denosumab for osteoporosis: a systematic review. 2022. Osteoporos Int [Epub Ahead of Print]. 10.1007/s00198-021-06268-9. [DOI] [PubMed]
- 42.Czerwinski E, Cardona J, Plebanski R, et al. The Efficacy and Safety of Abaloparatide-SC in Men With Osteoporosis: A Randomized Clinical Trial. J Bone Miner Res. 2022;37(12):2435-2442; 10.1002/jbmr.4719 [DOI] [PMC free article] [PubMed]
- 43.Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum. 2019;49(2):184–96. 10.1016/j.semarthrit.2019.01.006. [DOI] [PubMed]
- 44.Le QA, Hay JW, Becker R, Wang Y. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother. 2019;53(2):134–143. doi: 10.1177/1060028018798034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of sequential teriparatide/alendronate versus alendronate-alone strategies in high-risk osteoporotic women in the US: analyzing the impact of generic/biosimilar teriparatide. JBMR Plus. 2019;3(11):1–11. doi: 10.1002/jbm4.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman J, Reginster JY, Boonen S, et al. Treatment of osteoporosis in men. 2013;53(1):134–44. 10.1016/j.bone.2012.11.018. [DOI] [PMC free article] [PubMed]
- 47.Dennison E. Osteoporosis Treatment: a clinical overview. Springer International Publishing, 2021.
- 48.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.UptoDate. Patient education: bone density testing (beyond the basics). https://www.uptodate.com/contents/bone-density-testing-beyond-the-basics. Accessed 17 Jan 2023.
- 50.Kanis JA, McCloskey EV, Harvey NC, Johansson H, Leslie WD. Intervention thresholds and the diagnosis of osteoporosis. J Bone Miner Res. 2015;30(10):1747–1753. doi: 10.1002/jbmr.2531. [DOI] [PubMed] [Google Scholar]
- 51.Kanis JA, Harvey N, Cooper C, et al. A systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016.;11(1):25. 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed]
- 52.Zoccarato F, Ceolin C, Trevisan C, et al. Comparison between real-world practice and application of the FRAX algorithm in the treatment of osteoporosis. Aging Clin Exp Res. 2022. 34(11):2807 -2814. 10.1007/s40520-022-02212-x. [DOI] [PMC free article] [PubMed]
- 53.Compston J, Cooper A, Cooper C, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017.12(1):43. 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed]
- 54.Wu CH, Kao IJ, Hung WC, et al. Economic impact and cost-effectiveness of fracture liaison services: a systematic review of the literature. Osteoporos Int. 2018;29(6):1227–1242. doi: 10.1007/s00198-018-4411-2. [DOI] [PubMed] [Google Scholar]
- 55.Australian Public Assessment Report for Romosozumab. https://www.tga.gov.au/sites/default/files/auspar-romosozumab-200120.pdf. Accessed 17 Jan 2023.
- 56.Talevski J, Sanders KM, Watts JJ, et al. Sex differences in recovery of quality of life 12 months post-fracture in community-dwelling older adults: analyses of the Australian arm of the International Costs and Utilities Related to Osteoporotic Fractures Study (AusICUROS) Osteoporos Int. 2022;33(1):67–75. doi: 10.1007/s00198-021-06058-3. [DOI] [PubMed] [Google Scholar]
- 57.Nayak S, Greenspan SL. Osteoporosis treatment efficacy for men: a systematic review and meta-analysis. J Am Geriatr Soc. 2017;65(3):490–495. doi: 10.1111/jgs.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiligsmann M, Li N, Cooper C, et al. Improving the reporting of economic evaluation in osteoporosis: the value of CHEERS 2022 statement. Osteoporos Int. 2022;0123456789:1–2. doi: 10.1007/s00198-022-06400-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.