Abstract
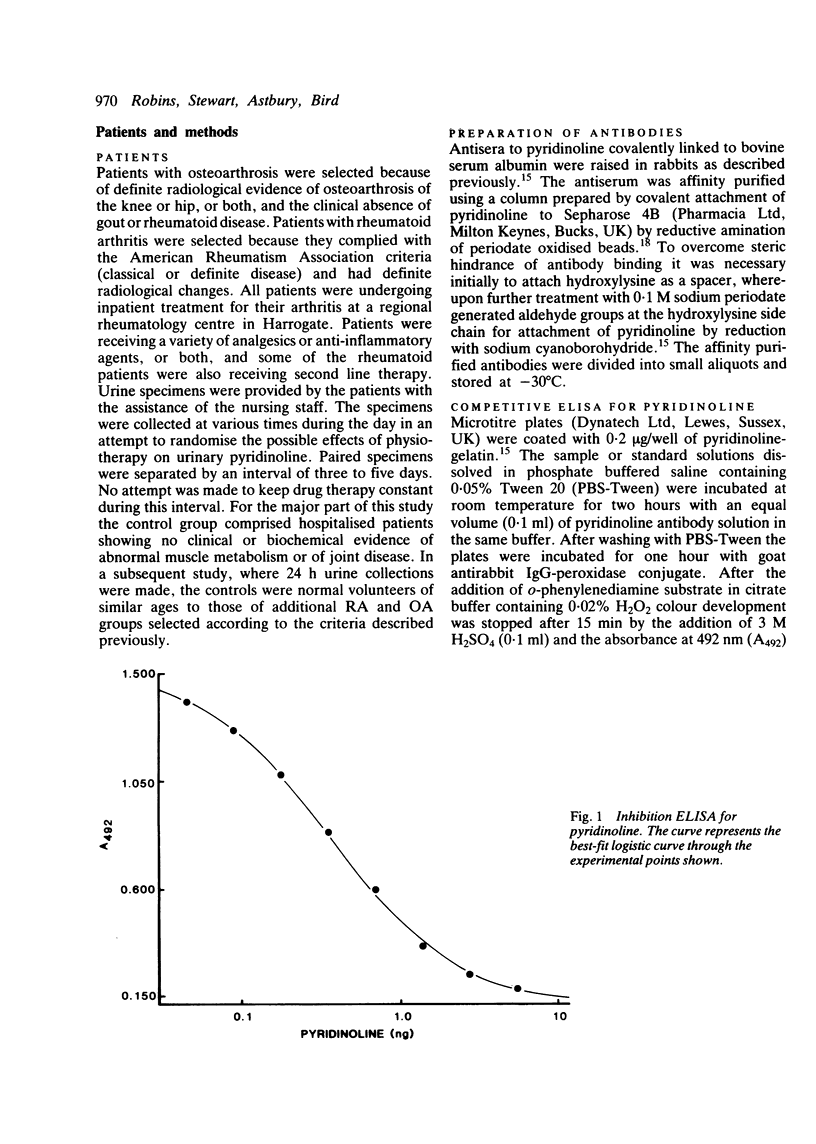
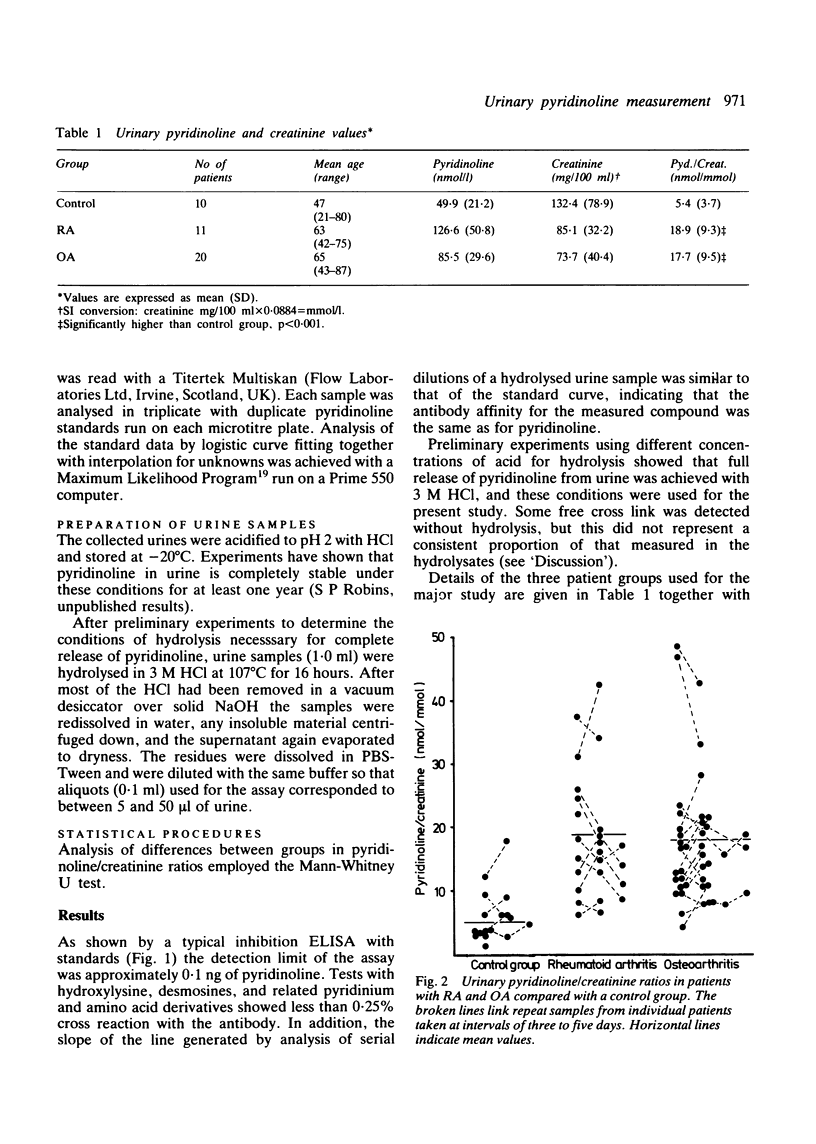
An enzyme linked immunoassay (ELISA) for the collagen cross link, pyridinoline, has been developed using affinity purified antibodies, with a sensitivity down to about 0.1 ng of cross link. Measurements of urinary pyridinoline were made in patients with rheumatoid arthritis (RA), osteoarthritis (OA), and a control group showing no signs of joint disease. Expressed relative to creatinine values, pyridinoline was significantly increased in both RA and OA groups compared with controls: these differences were much larger than could be attributed to any age related effects or to changes in urinary creatinine concentrations. These findings were confirmed by analysis of a series of 24 h urine collections which showed that the total pyridinoline excretions were significantly higher in both RA and OA groups than in the controls. As pyridinoline is much more prevalent in cartilage than in bone collagen, measurement of this compound in urine may provide an index for monitoring the increased joint destruction that occurs in arthritic disease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowski R. S., Engels C. J. Measurement of intracellular collagen degradation. Anal Biochem. 1981 Sep 15;116(2):414–424. doi: 10.1016/0003-2697(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Eyre D. R. Collagen: molecular diversity in the body's protein scaffold. Science. 1980 Mar 21;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Koob T. J., Van Ness K. P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984 Mar;137(2):380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Aging and cross-linking in human aorta. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1264–1269. doi: 10.1016/0006-291x(82)91913-1. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Moriguchi T., Ishida T., Hayashi H. The structure of pyridinoline, a collagen crosslink. Biochem Biophys Res Commun. 1978 Sep 14;84(1):52–57. doi: 10.1016/0006-291x(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Suzuki M., Uchiyama A., Miyamoto S., Inoue T. Analysis of pyridinoline, a cross-linking compound of collagen fibers, in human urine. J Biochem. 1983 Oct;94(4):1133–1136. doi: 10.1093/oxfordjournals.jbchem.a134457. [DOI] [PubMed] [Google Scholar]
- Gielen F., Dequeker J., Drochmans A., Wildiers J., Merlevede M. Relevance of hydroxyproline excretion to bone metastasis in breast cancer. Br J Cancer. 1976 Sep;34(3):279–285. doi: 10.1038/bjc.1976.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Boucek R. J. Collagen cross-linking compounds in human urine. Biochem J. 1981 Sep 1;197(3):759–762. doi: 10.1042/bj1970759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörlein D., Fietzek P. P., Kühn K. Pro-Gln: the procollagen peptidase cleavage site in the alpha1(I) chain of dermatosparactic calf skin procollagen. FEBS Lett. 1978 May 15;89(2):279–282. doi: 10.1016/0014-5793(78)80236-1. [DOI] [PubMed] [Google Scholar]
- Kelleher P. C. Urinary excretion of hydroxyproline, hydroxylysine and hydroxylysine glycosides by patients with Paget's disease of bone and carcinoma with metastases in bone. Clin Chim Acta. 1979 Mar 15;92(3):373–379. doi: 10.1016/0009-8981(79)90216-x. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I. Urinary excretion of hydroxyproline in health and disease. Int Rev Connect Tissue Res. 1970;5:93–163. doi: 10.1016/b978-0-12-363705-5.50008-7. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Fujimoto D. Crosslink of collagen in hypertrophic scar. J Invest Dermatol. 1979 Mar;72(3):143–145. doi: 10.1111/1523-1747.ep12530609. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Fox R., Krane S. M. Human collagens: differences in glycosylated hydroxylysines in skin and bone. Biochim Biophys Acta. 1971 Jan 19;229(1):119–122. doi: 10.1016/0005-2795(71)90325-4. [DOI] [PubMed] [Google Scholar]
- Robins S. P. An enzyme-linked immunoassay for the collagen cross-link pyridinoline. Biochem J. 1982 Dec 1;207(3):617–620. doi: 10.1042/bj2070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P. Cross-linking of collagen. Isolation, structural characterization and glycosylation of pyridinoline. Biochem J. 1983 Oct 1;215(1):167–173. doi: 10.1042/bj2150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P. Cross-linking of collagen. Isolation, structural characterization and glycosylation of pyridinoline. Biochem J. 1983 Oct 1;215(1):167–173. doi: 10.1042/bj2150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Cunningham L. W. Variations in human urinary O-hydroxylysyl glycoside levels and their relationship to collagen metabolism. J Clin Invest. 1970 Aug;49(8):1497–1509. doi: 10.1172/JCI106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. B., Nakane P. K. The covalent coupling of proteins to periodate-oxidized sephadex: a new approach to immunoadsorbent preparation. J Immunol Methods. 1976;12(1-2):171–181. doi: 10.1016/0022-1759(76)90107-1. [DOI] [PubMed] [Google Scholar]


