Abstract
Background
This study aimed to explore whether there is an association between androgen receptor (AR) expression and ultrasound, clinicopathological features and prognosis of breast cancer.
Methods
A total of 141 breast cancer patients were included in this retrospective study. AR expression was analyzed by immunohistochemistry. The images of B-mode, color Doppler and strain elastography from 104 patients were collected continuously, and the corresponding ultrasound characteristics were obtained. The differences in ultrasound and clinicopathological features in different AR status were analyzed. Progression-free survival (PFS) of patients was obtained through up to 90 months of follow-up; then, the effect of AR on PFS was analyzed. Subsequently, a nomogram was constructed to predict the AR status. The predictive accuracy was calculated using C-index.
Results
The positive expression of AR (AR +) was associated with lower histological grade (p = 0.034) and lower Ki-67 level (p = 0.029). Triple-negative breast cancer (TNBC) had the lowest probability of AR + (p < 0.001). The AR + group mostly showed unsmooth margin (p < 0.001), posterior acoustic shadowing (p = 0.002) and higher elasticity score (p = 0.022) on ultrasound. The echo pattern of most tumors with AR + was heterogeneous (p = 0.024) in Luminal A subtype. AR + could be a sign of a better prognosis in overall breast cancer (p < 0.001), as well as in human epidermal growth factor receptor 2 (HER2) overexpression and Luminal B subtypes (p = 0.001 and 0.025). The nomogram showed relatively reliable performance with a C-index of 0.799.
Conclusion
Our research demonstrated that AR expression was closely related to ultrasound, clinicopathological features and prognosis of breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13244-023-01387-9.
Keywords: Breast cancer, Androgen receptor, Ultrasound, Nomogram, Prognosis
Key points
In terms of clinicopathology, the probability of AR expression in different subtypes of breast cancer was various, and the positive expression of AR was associated with lower histological grade and lower Ki-67 level.
In terms of ultrasound, the positive expression of AR was associated with unsmooth margin, posterior acoustic shadowing and stiffer on strain elastography, and AR + and AR– tumors performed differently when analyzed separately according to different molecular subtypes.
The positive expression of AR could be a sign of a better prognosis in breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13244-023-01387-9.
Introduction
Breast cancer, with a global incidence of approximately 2.26 million new cases each year, has overtaken lung cancer to become the most commonly diagnosed cancer globally, which seriously threatens women's health and life [1]. Meanwhile, a proportion of patients still cannot benefit from current clinical treatments due to drug resistance, lack of effective treatment targets and other factors [2, 3]. This makes the diagnosis and treatment of breast cancer increasingly challenging. Consequently, it is urgent to explore new molecular markers and potential therapeutic targets for breast cancer to improve the prognosis. Breast cancer is a highly hormone dependent tumor [4]. As a member of the steroid receptor in the nuclear receptor superfamily, the androgen receptor (AR) plays a vital role in breast cancer, together with the estrogen receptor (ER) and progesterone receptor (PR) [5]. Moreover, it is widely expressed in osteosarcoma, tissues of the prostate, liver, cardiovascular, breast and other human tissues, among which the expression of AR in breast cancer is the third highest [6]. Recently, the development of selective androgen receptor modulators, the great effect of AR–related therapy in prostate cancer and the in-depth study of luminal androgen receptor (LAR) subtype of breast cancer have highlighted AR. Several studies have confirmed that AR can be used as a potential therapeutic target and an emerging prognostic marker to guide the clinical treatment of breast cancer [7].
The expression of molecular markers affects the biological and histological behavior of breast cancer and then affects the imaging appearances; they are inextricably related [8, 9]. Ultrasound and mammography are two distinct imaging methods. They are complementary to each other and both play the important role in the diagnosis and treatment of breast diseases [10]. Ultrasound has a variety of modalities, B-mode can show the shape of the mass, internal echo and other two-dimensional features, color Doppler can reflect the blood perfusion of the tumors, and elastography can assess the hardness of the tumors, so that, ultrasound can identify benign and malignant tumors, predict axillary lymph node metastasis and guide percutaneous biopsy and interventional therapy, among others [11, 12]. Current research focuses on the relationship between ultrasound characteristics and molecular biological expression [13]. Liu et al. [14] found that the positive expression of human epidermal growth factor receptor 2 (HER2) was related to the blood supply, lymph node metastasis and microcalcification. ER + was correlated with tumor morphology, margin and perimeter. Similarly, Zhao et al. reported that the positive expression of Ki-67 was associated with tumor diameter, blood flow grade and lymph node metastasis [15]. However, few studies have explored the relationship between ultrasound appearances and the expression of AR.
Bae et al. [16] suggested that AR + was related to calcifications with or without a mass on mammography, non-mass enhancement on MRI, irregular shape or spiculated margins on ultrasound. Candelaria et al. [17] found that the majority of the mammography of TNBC with AR + showed heterogeneously dense breast composition and high mass density, and the ultrasound showed irregular mass shape. Muller et al. [18] reported that most LAR tumors showed spiculated margins on mammography and smooth borders on ultrasound. Such studies related to AR have primarily focused on TNBC, instead of other molecular subtypes. Therefore, in this retrospective research, we aimed to determine whether AR status was related to the ultrasound, clinicopathological features or prognosis of breast cancer and to demonstrate whether such a correlation existed in different molecular subtypes.
Materials and methods
Patients
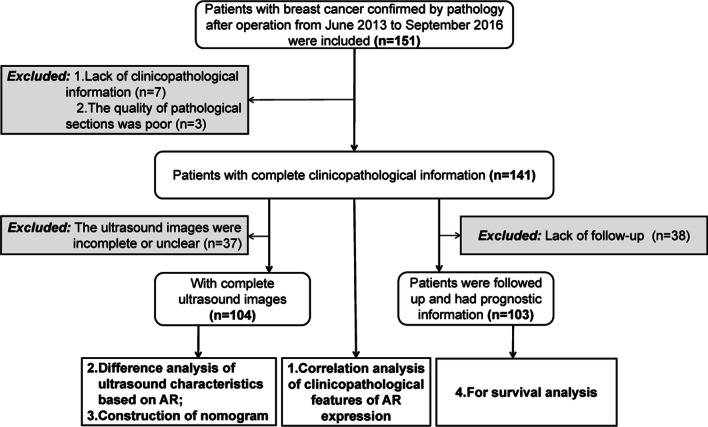
This is a retrospective study of case information collated from June 2013 to September 2016. A total of 151 breast cancer patients underwent ultrasound examination before the operation and obtained pathological sections postoperatively. However, 7 of these patients lacked clinicopathological information and the other 3 patients had poor quality of pathological sections, which led to the exclusion of these 10 patients. Finally, 141 patients were included in the study. This study was approved by the institutional ethics committee of Harbin medical university (approval number, KY-2016-127). We reviewed the clinical data and the ultrasound images of these patients during the study period. The process of selecting patients for data analysis is presented in Fig. 1.
Fig. 1.
Flow chart for patient selection. AR, androgen receptor
Baseline data collection
We reviewed the following data from the 141 patients’ medical records: age, tumor size, menopausal status (non-menopause and menopause), lymph node metastasis (absent and present), distant metastasis (absent and present), histological type (invasive ductal carcinoma and other types), histological grade (1, 2 and 3) [19] and clinical TNM stage (cT1, cT2, cT3 and cT4) [20]. According to the immunohistochemistry (IHC) results for ER, PR, Ki-67 and HER2 expression, breast cancer was divided into the following four molecular subtypes: Luminal A (ER + and/or PR + , HER2-, Ki-67 < 14%); Luminal B (ER + and/or PR + , HER2-, Ki-67 ≥ 14%; ER + and/or PR + , HER2 overexpressed); HER2 overexpression (ER-, PR-, HER2 overexpressed); TNBC (ER-, PR-, HER2-) [21].
Ultrasound examination
The ultrasound images of breast masses in 104 patients were scanned by a radiologist with 5 years of experience in breast ultrasound using a HITACHI Vision 500 system (Hitachi Medical System, Tokyo, Japan) equipped with a linear probe of 5–13 MHz. In real-time scanning, the static images of the maximum diameter of the mass during transverse and longitudinal cutting, and the dynamic images of the standard sections of B-mode, color Doppler and ultrasound strain elastography were preserved. These ultrasound images were reviewed independently by two breast radiologists with the wealth of experience of 8 and 10 years, respectively, while ignoring the IHC results. Where differences occurred, a consensus was reached. Feature extraction of B-mode ultrasound images was based on the Breast Imaging Reporting and Data System (BI-RADS) lexicon [22]. The ultrasound characteristics were included as follows: shape, orientation, hyperechoic halo, margin, posterior acoustic pattern, calcification, echo pattern, adler grade and elasticity score. 5-point elasticity scoring, as one of the evaluation systems, is highly specific in evaluating the stiffness of breast lesions [23]. The evaluation of ultrasound elastography was based on the World Federation of Ultrasound in Medicine and Biology (WFUMB) [24]. Blood flow was assessed according to Adler grade (0, 1, 2 and 3) [25].
Immunohistochemistry
The expression of AR was analyzed by IHC based on tissue microarray (TMA), which was evaluated by two pathologists with > 10 years of breast pathology experience. The TMA section was stained with AR antibody (clone AR 441, DAKO). Normal breast tissue on TMA was used for internal control. We choose known positive breast cancer tissues as positive controls. Negative controls were prepared by omitting the primary antibody. False negatives and false positives were avoided by this approach, similar to ER and PR. AR positivity is defined as ≥ 10% of tumor cells with positive nuclear staining [26]. According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline, the cutoff value of ER and PR positivity was defined as 1% [27]. The IHC score of HER2 status included 0, 1 + , 2 + and 3 + and HER2 overexpression was defined by 3 + or 2 + with a > twofold change in fluorescence in situ hybridization (FISH), while 0 and 1 + were defined as HER2 negativity [28]. Positive nuclear staining of Ki-67 ≥ 14% was defined as high expression and < 14% was defined as low expression [29].
Patient follow-up
Of the 141 patients, 103 were followed up in multiple methods. We gave priority to collecting as much detailed as possible through outpatient review information, inpatient treatment records, followed by telephone follow-ups. These patients were followed up twice a year with a 6-month cycle. Patients without recurrence were followed for at least 60 months, and those with recurrence were followed until the time of recurrence. Progression-free survival (PFS) was used as the study endpoint and was defined as the time from the operation date to tumor recurrence and death or the last follow-up date.
Statistical analysis
IBM SPSS Statistics, Version 26.0, and R Version 4.1.1 (http://www.R-project.org) were used for statistical analysis. The relationship between clinicopathological features and AR status was analyzed by univariate and multivariate logistic regression analysis; the odds ratio (OR) and 95% confidence interval (95% CI) were calculated, and the differences in ultrasound characteristics between AR + and AR– groups were evaluated using the Chi-square test or Fisher's exact test. A nomogram based on the logistic regression analysis model was constructed to predict AR status. The efficiency of the logistic regression model was calculated by receiver operating characteristic curve (ROC) and area under curve (AUC). Additionally, the performance of the nomogram was measured by C-index and calibration curve. Moreover, the Kaplan–Meier method was used to evaluate PFS. Multivariate Cox proportional hazards model was used to analyze prognostic factors. For all the analysis p values < 0.05 were considered statistically significant.
Results
The correlation between the expression of AR and clinicopathological features
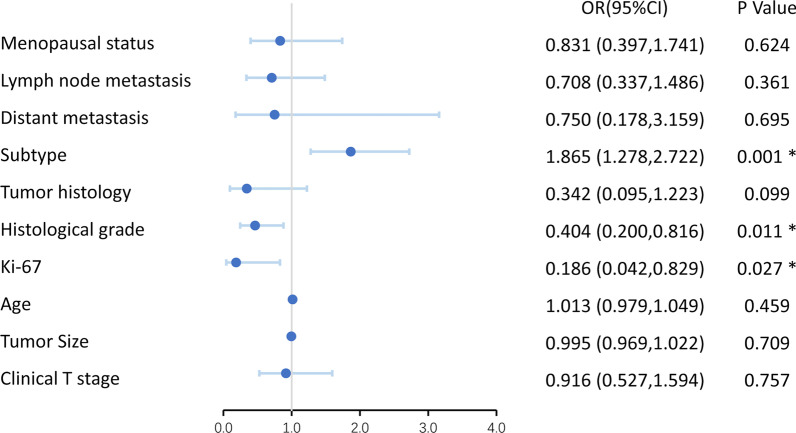
As presented in Table 1, of the 141 breast cancer patients in our study, 102 (72.34%) were AR + and 39 (27.66%) were AR–. The mean age of AR + was 52.25 ± 11.29 years (range, 34–83), and that of AR– was 50.72 ± 10.12 years (range, 33–73) at the time of diagnosis. The average size of tumor in AR + was 22.98 ± 14.46 mm (range, 7–70) and in AR– was 23.95 ± 12.09 mm (range, 7–51). A total of 55 tumors could be palpated obviously, all larger than 2 cm. We found that molecular subtype (OR: 1.865 (1.278, 2.722), p = 0.001), histological grade (OR: 0.404 (0.200, 0.816), p = 0.011) and Ki-67 level (OR: 0.186 (0.042, 0.829), p = 0.027) were significantly different between the two groups by univariate logistic regression analysis (Fig. 2). In our study, TNBC had the lowest AR positive expression probability (18.18%, 4/22, p < 0.001); further, AR + was related to lower histological grade (OR: 0.459 (0.223, 0.943), p = 0.034) and lower Ki-67 level (OR: 0.177 (0.037, 0.839), p = 0.029) by multivariate logistic regression analysis.
Table 1.
Summary data of clinicopathological features of 141 patients
| Characteristics | AR + population (n = 102) | AR– population (n = 39) | p value | ||
|---|---|---|---|---|---|
| n | Percent (%) | n | Percent (%) | ||
| Age (y) | 0.459 | ||||
| Mean ± SD | 52.25 ± 11.29 | 50.72 ± 10.12 | |||
| Subtype | 0.001* | ||||
| TNBC | 4 | 3.92 | 18 | 46.15 | |
| Luminal A | 36 | 35.29 | 6 | 15.38 | |
| Luminal B | 31 | 30.39 | 4 | 10.26 | |
| HER2 overexpression | 31 | 30.39 | 11 | 28.21 | |
| Ki-67 (%) | 0.027* | ||||
| < 14 | 23 | 22.55 | 2 | 5.13 | |
| ≥ 14 | 79 | 77.45 | 37 | 94.87 | |
| Tumor size (mm) | 0.709 | ||||
| Mean ± SD | 22.98 ± 14.46 | 23.95 ± 12.09 | |||
| Lymph node metastasis | 0.361 | ||||
| Absent | 61 | 59.80 | 19 | 48.72 | |
| Present | 41 | 40.20 | 20 | 51.28 | |
| Menopausal status | 0.624 | ||||
| Non-menopause | 45 | 44.12 | 19 | 48.72 | |
| Menopause | 57 | 55.88 | 20 | 51.28 | |
| Distant metastasis | 0.695 | ||||
| Absent | 96 | 94.12 | 36 | 92.31 | |
| Present | 6 | 5.88 | 3 | 7.69 | |
| Tumor histology | 0.099 | ||||
| Invasive ductal carcinoma | 82 | 80.39 | 36 | 92.31 | |
| Other types | 20 | 19.61 | 3 | 7.69 | |
| Clinical T stage | 0.757 | ||||
| cT1 | 62 | 60.78 | 20 | 51.28 | |
| cT2 | 29 | 28.43 | 17 | 43.59 | |
| cT3 and cT4 | 11 | 10.78 | 2 | 5.13 | |
| Histological grade | 0.011* | ||||
| 1 | 12 | 11.76 | 1 | 2.56 | |
| 2 | 50 | 49.02 | 15 | 38.46 | |
| 3 | 40 | 39.22 | 23 | 58.97 | |
y, years old; TNBC, triple-negative breast cancer; HER2, human epidermal growth factor receptor 2; AR, androgen receptor; * indicates p < 0.05
Fig. 2.
Forest map of univariate logistic regression analysis based on clinicopathological features related to AR. OR, odds ratio; CI, confidence interval; AR, androgen receptor; * indicates p < 0.05
The comparison of the ultrasound characteristics of the groups with different AR status
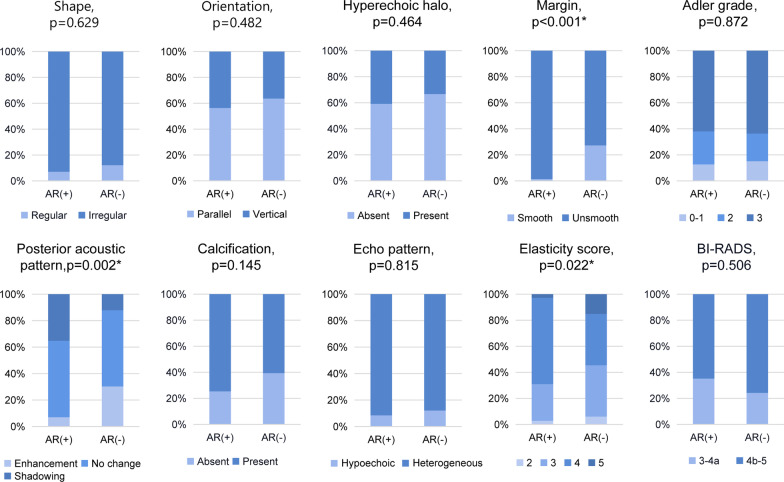
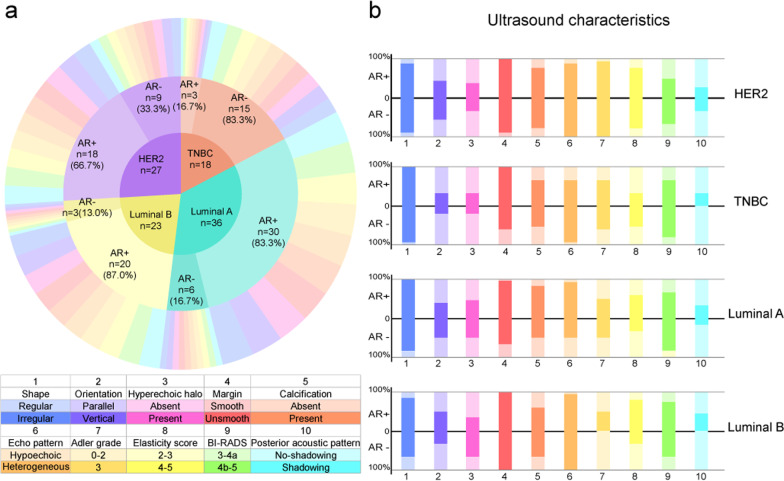
The ultrasound characteristics of AR + and AR– breast cancer were significantly different. On ultrasound, 98.59% (70/71) of AR + breast cancer showed unsmooth margin on ultrasound; however, that of AR– was 72.73% (24/33). Only 1 of 71 cases of AR + breast cancer showed smooth margin (p < 0.001). Compared with AR– breast cancer, AR + was more likely to be posterior acoustic shadowing (35.21% vs 12.12%, p = 0.002). The elasticity score of AR + breast cancer was mostly concentrated in 4 (66.20% vs 39.39%, p = 0.022) (Fig. 3, Table 2 and Additional file 1: Fig. S1). The analysis of the relationship between AR and ultrasound characteristics in different molecular subtypes showed that: in the Luminal A subtype, with the different expression of AR, the echo pattern of the tumor was different (p = 0.024). The internal echo pattern in AR + group was more heterogeneous. However, in our study, perhaps because the sample size was not large enough, no significant differences were found in ultrasound characteristics when AR expression differed in the other three subtypes (Fig. 4 and Table 3).
Fig. 3.
Comparison of ultrasound characteristics between AR + and AR– groups in the entire study cohort. BI-RADS, Breast Imaging Reporting and Data System; AR, androgen receptor; * indicates p < 0.05
Table 2.
Ultrasound characteristics of AR status in 104 patients
| Characteristics | AR + (n = 71) | AR– (n = 33) | χ2value | p value |
|---|---|---|---|---|
| Shape | 0.233 | 0.629 | ||
| Regular | 5 (7.04%) | 4 (12.12%) | ||
| Irregular | 66 (92.96%) | 29 (87.88%) | ||
| Orientation | 0.495 | 0.482 | ||
| Parallel | 40 (56.34%) | 21 (63.64%) | ||
| Vertical | 31 (43.66%) | 12 (36.36%) | ||
| Hyperechoic halo | 0.537 | 0.464 | ||
| Absent | 42 (59.15%) | 22 (66.67%) | ||
| Present | 29 (40.85%) | 11 (33.33%) | ||
| Margin | 14.493 | < 0.001* | ||
| Smooth | 1 (1.41%) | 9 (27.27%) | ||
| Unsmooth | 70 (98.59%) | 24 (72.73%) | ||
| Posterior acoustic pattern | 12.759 | 0.002* | ||
| Enhancement | 5 (7.04%) | 10 (30.30%) | ||
| No change | 41 (57.75%) | 19 (57.58%) | ||
| Shadowing | 25 (35.21%) | 4 (12.12%) | ||
| Calcification | 2.123 | 0.145 | ||
| Absent | 18 (25.35%) | 13 (39.39%) | ||
| Present | 53 (74.65%) | 20 (60.61%) | ||
| Echo pattern | 0.055 | 0.815 | ||
| Hypoechoic | 6 (8.45%) | 4 (12.12%) | ||
| Heterogeneous | 65 (91.55%) | 29 (87.88%) | ||
| Adler grade | 0.273 | 0.872 | ||
| 0–1 | 9 (12.68%) | 5 (15.15%) | ||
| 2 | 18 (25.35%) | 7 (21.21%) | ||
| 3 | 44 (61.97%) | 21 (63.64%) | ||
| Elasticity score | 9.429 | 0.022* | ||
| 2 | 2 (2.82%) | 2 (6.06%) | ||
| 3 | 20 (28.17%) | 13 (39.39%) | ||
| 4 | 47 (66.20%) | 13 (39.39%) | ||
| 5 | 2 (2.82%) | 5 (15.15%) | ||
| BI-RADS | 0.443 | 0.506 | ||
| 3-4a | 25 (35.21%) | 8 (24.24%) | ||
| 4b-5 | 46 (64.79%) | 25 (75.76%) |
BI-RADS, Breast imaging-reporting and data system; AR, androgen receptor; * indicates p < 0.05
Fig. 4.
Comparison of ultrasound characteristics between AR– and AR + groups in different molecular subtypes. a Expression of AR in different molecular subtypes. b The distribution differences of ultrasound characteristics between AR– and AR + groups in HER2 overexpression, TNBC, Luminal A and Luminal B subtypes, respectively. TNBC, triple negative breast cancer; HER2, human epidermal growth factor receptor 2; AR, androgen receptor; BI-RADS, Breast Imaging Reporting and Data System
Table 3.
Ultrasound characteristics of AR status in different molecular subtypes
| Characteristics | TNBC (n = 18) | Luminal A (n = 36) | Luminal B (n = 23) | HER2 overexpression (n = 27) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AR + (n = 3) | AR– (n = 15) | p value | AR + (n = 30) | AR– (n = 6) | p value | AR + (n = 20) | AR– (n = 3) | p value | AR + (n = 18) | AR– (n = 9) | p value | |
| Shape | 1.000 | 0.167 | 0.453 | 1.000 | ||||||||
| Regular | 0 (0.0%) | 1 (6.7%) | 0 (0.0%) | 1 (16.7%) | 3 (15.0%) | 1 (33.3%) | 2 (11.1%) | 1 (11.1%) | ||||
| Irregular | 3 (100.0%) | 14 (93.3%) | 30 (100.0%) | 5 (83.3%) | 17 (85.0%) | 2 (66.7%) | 16 (88.9%) | 8 (88.9%) | ||||
| Orientation | 1.000 | 0.677 | 1.000 | 0.695 | ||||||||
| Parallel | 2 (66.7%) | 12 (80.0%) | 18 (60.0%) | 3 (50.0%) | 10 (50.0%) | 2 (66.7%) | 10 (55.6%) | 4 (44.4%) | ||||
| Vertical | 1 (33.3%) | 3 (20.0%) | 12 (40.0%) | 3 (50.0%) | 10 (50.0%) | 1 (33.3%) | 8 (44.4%) | 5 (55.6%) | ||||
| Hyperechoic halo | 1.000 | 1.000 | 0.538 | 1.000 | ||||||||
| Absent | 2 (66.7%) | 12 (80.0%) | 16 (53.3%) | 3 (50.0%) | 13 (65.0%) | 1 (33.3%) | 11 (61.1%) | 6 (66.7%) | ||||
| Present | 1 (33.3%) | 3 (20.0%) | 14 (46.7%) | 3 (50.0%) | 7 (35.0%) | 2 (66.7%) | 7 (38.9%) | 3 (33.3%) | ||||
| Margin | 0.515 | 0.066 | / | 0.333 | ||||||||
| Smooth | 0 (0.0%) | 6 (40.0%) | 1 (3.3%) | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | ||||
| Unsmooth | 3 (100.0%) | 9 (60.0%) | 29 (96.7%) | 4 (66.7%) | 20 (100.0%) | 3 (100.0%) | 18 (100.0%) | 8 (88.9%) | ||||
| Posterior acoustic pattern | 0.167 | 0.643 | 0.253 | 1.000 | ||||||||
| No-shadowing | 2 (66.7%) | 15 (100.0%) | 20 (66.7%) | 5 (83.3%) | 11 (55.0%) | 3 (100.0%) | 13 (72.2%) | 6 (66.7%) | ||||
| Shadowing | 1 (33.3%) | 0 (0.0%) | 10 (33.3%) | 1 (16.7%) | 9 (45.0%) | 0 (0.0%) | 5 (27.8%) | 3 (33.3%) | ||||
| Calcification | 1.000 | 0.109 | 1.000 | 1.000 | ||||||||
| Absent | 1 (33.3%) | 7 (46.7%) | 5 (16.7%) | 3 (50.0%) | 8 (40.0%) | 1 (33.3%) | 4 (22.2%) | 2 (22.2%) | ||||
| Present | 2 (66.7%) | 8 (53.3%) | 25 (83.3%) | 3 (50.0%) | 12 (60.0%) | 2 (66.7%) | 14 (77.8%) | 7 (77.8%) | ||||
| Echo pattern | 0.314 | 0.024* | 1.000 | 0.538 | ||||||||
| Hypoechoic | 1 (33.3%) | 1 (6.7%) | 2 (6.7%) | 3 (50.0%) | 1 (5.0%) | 0 (0.0%) | 2 (11.1%) | 0 (0.0%) | ||||
| Heterogeneous | 2 (66.7%) | 14 (93.3%) | 28 (93.3%) | 3 (50.0%) | 19 (95.0%) | 3 (100.0%) | 16 (88.9%) | 9 (100.0%) | ||||
| Adler grade | 1.000 | 1.000 | 0.229 | 1.000 | ||||||||
| 0–2 | 1 (33.3%) | 6 (40.0%) | 15 (50.0%) | 3 (50.0%) | 10 (50.0%) | 3 (100.0%) | 1 (5.6%) | 0 (0.0%) | ||||
| 3 | 2 (66.7%) | 9 (60.0%) | 15 (50.0%) | 3 (50.0%) | 10 (50.0%) | 0 (0.0%) | 17 (94.4%) | 9 (100.0%) | ||||
| Elasticity score | 1.000 | 0.374 | 0.155 | 1.000 | ||||||||
| 2–3 | 2 (66.7%) | 7 (46.7%) | 12 (40.0%) | 4 (66.7%) | 4 (20.0%) | 2 (66.7%) | 4 (22.2%) | 2 (22.2%) | ||||
| 4–5 | 1 (33.3%) | 8 (53.3%) | 18 (60.0%) | 2 (33.3%) | 16 (80.0%) | 1 (33.3%) | 14 (77.8%) | 7 (77.8%) | ||||
| BI-RADS | 1.000 | 0.643 | 1.000 | 0.683 | ||||||||
| 3-4a | 1 (33.3%) | 3 (20.0%) | 10 (33.3%) | 1 (16.7%) | 5 (25.0%) | 1 (33.3%) | 9 (50.0%) | 3 (33.3%) | ||||
| 4b-5 | 2 (66.7%) | 12 (80.0%) | 20 (66.7%) | 5 (83.3%) | 15 (75.0%) | 2 (66.7%) | 9 (50.0%) | 6 (66.7%) | ||||
TNBC, triple-negative breast cancer; HER2, human epidermal growth factor receptor 2; AR, androgen receptor; BI-RADS, breast imaging reporting and data system; * indicates p < 0.05
The nomogram for predicting AR status
Construction of logistic regression model
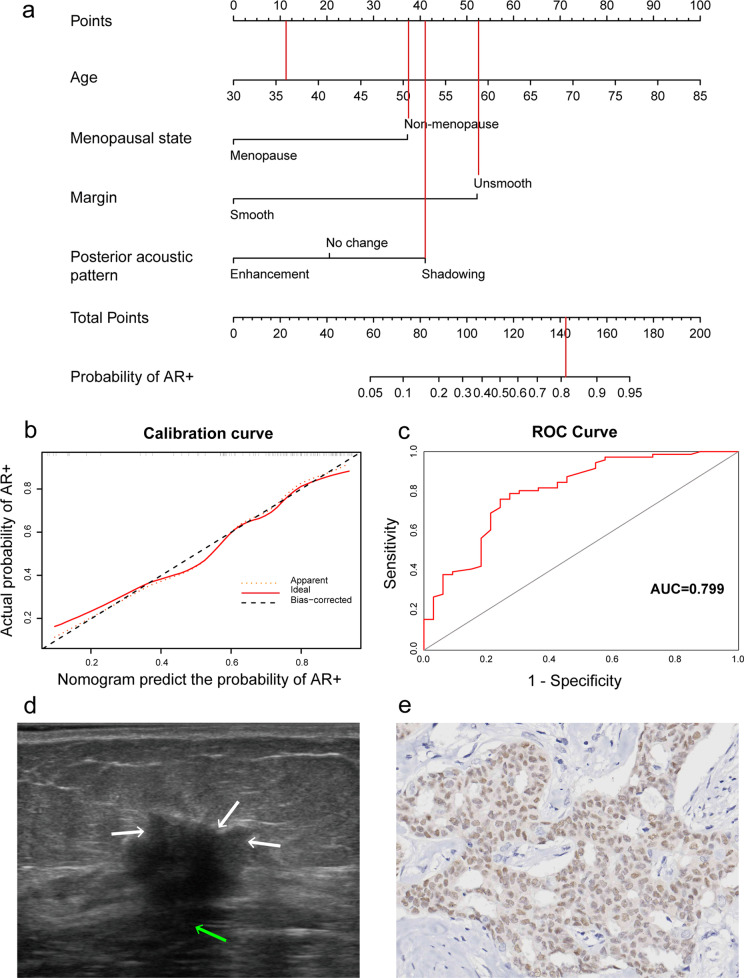
We included the clinical features available before surgery (age, tumor size and menopausal status) and statistically significant ultrasound features (margin, posterior acoustic pattern and elasticity score) in the multivariate logistic regression analysis (Table 4). The results showed that age (OR: 1.105 (1.023, 1.194), p = 0.011), posterior acoustic pattern (OR: 2.930 (1.193, 7.192), p = 0.019) and margin (OR: 14.984 (1.625, 138.161), p = 0.017) were positively correlated with the expression of AR, while menopausal status (OR: 0.135 (0.029, 0.634), p = 0.011) was negatively correlated. This meant that the probability of AR positive expression was higher in patients who were older, with posterior acoustic shadowing, with unsmooth margins, and non-menopause. Based on the statistically significant variables of multivariate regression, the final logistic model was established to predict the AR status. The model showed excellent diagnostic efficiency with the Hosmer-Lemeshow goodness of fit test for AR (χ2 = 3.545, p = 0.896), and AUC of 0.799 (Fig. 5c).
Table 4.
Multivariate logistic regression analysis of selected variables (clinical features and ultrasound characteristics)
| Variables | β | S.E | Wald | p value | Odds radio | 95% LCL | 95% UCL |
|---|---|---|---|---|---|---|---|
| Menopausal status | − 1.999 | 0.787 | 6.451 | 0.011* | 0.135 | 0.029 | 0.634 |
| Tumor size | − 0.019 | 0.025 | 0.557 | 0.455 | 0.981 | 0.934 | 1.031 |
| Age | 0.100 | 0.039 | 6.426 | 0.011* | 1.105 | 1.023 | 1.194 |
| Elasticity score | − 0.013 | 0.38 | 0.001 | 0.974 | 0.987 | 0.469 | 2.081 |
| Posterior acoustic pattern | 1.075 | 0.458 | 5.503 | 0.019* | 2.930 | 1.193 | 7.192 |
| Margin | 2.707 | 1.133 | 5.704 | 0.017* | 14.984 | 1.625 | 138.161 |
LCL, lower control limit; UCL, upper control limit; * indicates p < 0.05
Fig. 5.
An application example of nomogram to predict the AR status. a A nomogram was constructed from four informative features. b Calibration curve for evaluating nomogram effectiveness fitted well. c Receiver operating characteristic curve of the model for predicting AR status. a and d In a 36-year-old (11 points) non-menopausal (37.5 points) woman with breast cancer, the B-mode ultrasound showed the tumor was 15 mm in diameter, the margin of the tumor was unsmooth (52.5 points), and the posterior acoustic pattern was shadowing (41 points) and BI-RADS 4c. The total points were about 142 and the probability of AR + predicted by our nomogram was about 82% in this case. e The immunohistochemistry result of the patient: nuclear staining was more than 10%, the expression of AR was positive, original magnification × 400. ROC, receiver operating characteristic; AUC, area under curve; AR, androgen receptor
Development and validation of nomogram
Subsequently, the nomogram was constructed according to the logistic regression model. In the nomogram, the menopausal status, age, margin and posterior acoustic pattern were distributed according to their risk coefficient. Each patient can calculate the total points by accumulating the corresponding points of each feature according to their conditions to evaluate the AR status (Fig. 5a). There was no obvious deviation in the calibration curve, with the C-index of 0.799, which showed the nomogram performed well (Fig. 5b). An example of the nomogram application is shown in Fig. 5. The patient's total points were approximately 142, and the probability of AR + was 82%. The result of IHC confirmed that the expression of AR was positive.
The correlation between the expression of AR and PFS in breast cancer
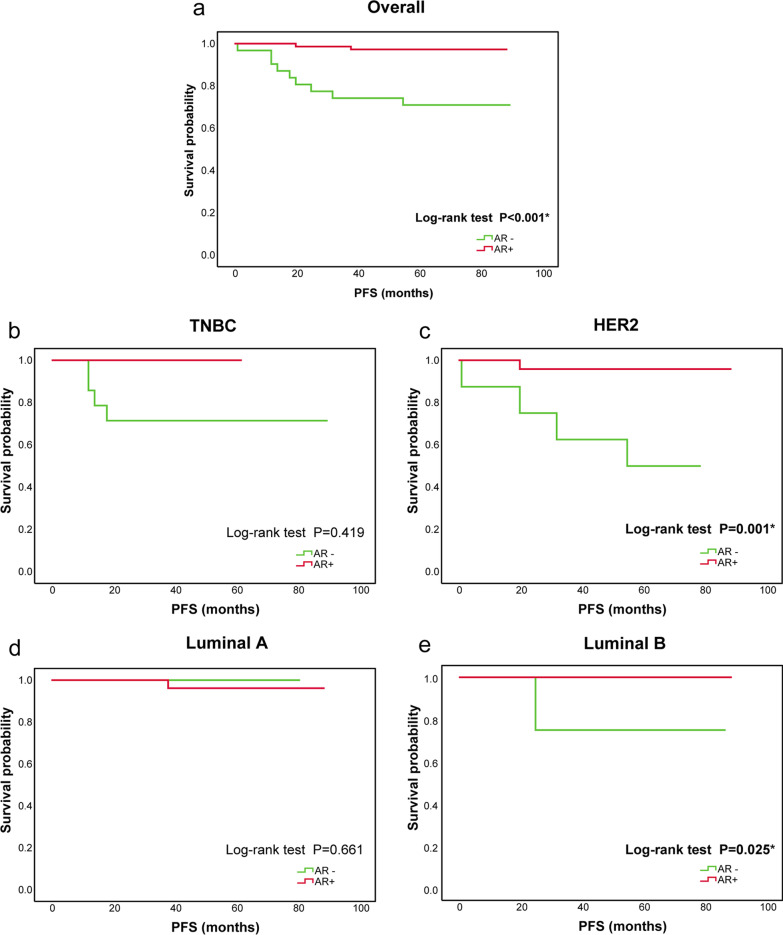
In this study, a total of 103 patients (AR + = 72, AR– = 31) were followed up from 1 to 90 months (median, 63 months). Of the 103 patients, 11 cases experienced disease progression. Only 2 patients (2.78%, 2/72) in the AR + group experienced disease recurrence, compared with 9 (29.03%, 9/31) in the AR– group. The 5-year survival rate of AR + and AR– groups was 97.22% and 70.97% for PFS, respectively. After the Log-rank test, there was a statistically significant difference between the two groups (χ2 = 16.895, p < 0.001). According to AR status, the Kaplan–Meier plot of PFS for the entire study cohort is presented in Fig. 6a. In general, it can be observed that the prognosis of patients with AR + was significantly better than that of AR– (Additional file 1: Fig. S1). In addition, the results showed that AR status (HR: 0.135 (0.028, 0.664), p = 0.014) and tumor size (HR: 1.103 (1.048, 1.162), p < 0.001) were independent prognostic factors for breast cancer by multivariate Cox regression analysis. In the survival analysis of different subtypes, it was found that the clinical outcomes of Luminal B (p = 0.025) and HER2 overexpression subtypes (p = 0.001) were consistent with that of the entire study cohort; the patients with AR + had favorably clinical outcomes (Fig. 6b– e).
Fig. 6.
Prognostic role of AR in different breast cancer patients. a Overall patients (n = 103), 2 cases (2.78%,2/72) in the AR + group vs 9 cases (29.03%,9/31) in the AR- group experienced disease progression. b TNBC patients(n = 16), no patient (0/2) in the AR + group versus 4 cases (28.57%,4/14) in the AR– group experienced disease progression. c HER2 overexpression subtype patients(n = 32), 1 case (4.17%,1/24) in the AR + group versus 4 cases (50%,4/8) in the AR- group experienced disease progression. d Luminal A subtype patients(n = 31), 1 case (3.85%,1/26) in the AR + group versus no patient (0/5) in the AR– group experienced disease progression. e Luminal B subtype patients(n = 24), no patient (0/20) in the AR + group versus 1 case (25%,1/4) in the AR– group experienced disease progression. TNBC, triple negative breast cancer; HER2, human epidermal growth factor receptor 2; AR, androgen receptor; PFS, progression-free survival; * indicates p < 0.05
Discussion
It is generally acknowledged that AR plays an important role in the physiology and pathology of men; nevertheless, it also counts a great deal in female breast cancer. Many studies have proven that gene expression can affect the imaging performance of tumors.[13, 30] Previous studies on AR have been done on the imaging of TNBC [16–18]. However, all molecular subtypes were included in our study, and there were significant differences in clinicopathological features and ultrasound characteristics between AR– and AR + groups in the entire study cohort. Then, there were also variations between the AR– and AR + breast cancer in different subtypes, grouped by molecular subtype. Finally, we also found that AR + breast cancer patients tend to have better prognosis.
Consequently, AR + breast cancer usually had lower histological grade and lower Ki-67 level. The expression of AR was also related to the molecular subtypes of breast cancer. Ki-67 exists in proliferating cells and can evaluate breast cancer prognosis as a marker of proliferation [31]. The higher the Ki-67 index, the faster the tumor proliferation and the worse prognosis of patients [32]. The histological grade is also an important factor in evaluating the prognosis. Tumors with high histological grade usually proliferate rapidly and differentiate poorly, indicating adverse clinical outcomes [33]. Consequently, it can reveal that the tumor with AR + can obtain better clinical results by inhibiting cell proliferation, which is consistent with the results of our survival analysis. We also found that the probability of AR + in TNBC was the lowest, just accounting for 18.18%, compared with HER2 overexpression (73.81%), Luminal A (85.71%) and Luminal B (88.57%). It may be related to the high degree of malignancy, strong invasiveness, rapid development, easy recurrence and low survival rate of TNBC.
As we can see in the research, AR + tumors usually show unsmooth margin, posterior acoustic shadowing and higher elasticity score (that is, stiffer) on ultrasound. Ultrasound elastography can semi-quantitatively measure the hardness of tumor in a routine examination [34]. The hardness of tumors is a characteristic of the extracellular matrix regulated by collagen cross-linking [35]. It has been found that there is insufficient vessel supply in stiffer tumors, which leads to the proportion of cancer cells decreases and is replaced by fibrosis. Therefore, the tumor showed a higher elasticity score on ultrasound [36]. Our results showed that AR + breast tumors were stiffer, which indicates that a higher degree of fibrosis and a relatively lower proportion of cancer cells in the AR + group lead to the slow progression of cancer. The posterior acoustic pattern is determined by the proportion of fibrous tissue and breast glands in the tumor. Posterior acoustic shadowing is caused by the decrease in sound beam penetration due to the proliferation of connective tissue, which is usually regarded as a sign of lower-grade malignant tumors [37]. The ultrasound findings of the margins of masses with good prognosis are controversial. Some researchers suggested that unsmooth margin is highly related to a malignant tumor with a poor prognosis [38]. However, we found that most AR + tumors showed unsmooth margins and better prognosis, which was supported by many studies [39]. Some studies have argued that the proliferation in varying degrees of collagen fibers around breast tumors leads to unsmooth margin on ultrasound images, which may inhibit the rapid infiltration of tumor cells [35]. Hence, the lower-grade malignant tumors tend to show unsmooth margin, the higher-grade tumors may show smooth margin [39, 40]. Therefore, it is clear that AR can be seen as a marker of a favorable outcome in breast cancer. Perhaps because the sample size was not large enough, we did not find more significant differences in ultrasound performance between the AR– and AR + groups in each molecular subtype; notwithstanding, only one distinctive characteristic was found in the Luminal A subtype. The echo pattern of tumor is related to fibrosis, cellular components and necrosis [41]. Some studies have found that the internal echo of locally advanced tumors showed hypoechoic [42]. Our results showed that AR– group was more likely to show homogeneous hypoechoic in Luminal A breast cancer, which indicates that the malignant degree of AR– group is higher than AR + group. Taken together, further investigation of this finding is warranted.
We initially constructed a simple nomogram to predict the AR status based on our findings that AR is indeed related to ultrasound and clinicopathological features. Nomogram is a model that can reflect the evaluation value intuitively, and contains various characteristics. It can not only be used to the prediction, but also to verify the correlation [43]. Our nomogram was established according to menopausal status, margin, posterior acoustic pattern and age, easily obtained in preoperative diagnosis and treatment. The nomogram demonstrated satisfactory performance with a C-index of 0.799, which proved the close correlations between the selected features and AR status. This is the main purpose to construct the nomogram in our study. Meanwhile, the initially successful construction of the nomogram also plays a solid foundation for the further study. With the in-depth study of AR in breast cancer, AR–related therapy is gradually applied in clinical practice [44]. In future studies with larger samples, we will focus on non-invasive and rapid prediction of AR status by clinical and imaging characteristics before surgery. This will provide valuable information for the formulation of clinical treatment plans.
Survival analysis showed that AR status and tumor size were independent prognostic factors of PFS. Besides, in the entire study cohort, the clinical outcomes of AR + patients were significantly better than that of AR–. Moreover, we analyzed the effect of the AR status on prognosis in different subtypes and found that the results in HER2 overexpression and Luminal B subtypes were consistent with the overall results, which were in accordance with the results of Jiang et al.[45]. Given experimental data have shown cross-talk between AR and HER2 pathways, which can influence the prognosis of HER2 + breast cancer [44]. However, our study showed that there was no significant difference in prognosis between the AR– and AR + groups in the TNBC and Luminal A subtypes. In our study, the cohort of TNBC was the smallest, with only 16 cases, and the AR positive expression rate of TNBC was also the lowest among the four molecular subtypes. Meanwhile, Gao et al. [46] suggested that Luminal A subtype had the best prognosis among the four molecular subtypes. Our research also found that only one of 31 Luminal A patients had recurrent disease, which supports the above view. Therefore, this study did not find the effect of AR status on the prognosis of TNBC and Luminal A subtypes. However, experiments have found that AR can interfere with ER binding to estrogen-related elements and inhibit the proliferative effect of ER, thereby promoting cancer cell apoptosis [47]. Another study has also shown that the patients with AR + TNBC had better survival outcomes [48]. Taken together, these studies indicate that AR is important in each molecular subtype of breast cancer, and therefore, further exploration is urgently needed.
Altogether, we studied the correlation between AR status and ultrasound, clinicopathological features and clinical outcomes in breast cancer, although our research still has some limitations. First, the sample size is not large enough, which might lead to bias in the results. Our study can be regarded as the initial exploration, and the sample size should continue to be expanded to analyze different results caused by the expression of AR in different subtypes. In order to explore whether AR is related to more detailed categorization of BI-RADS, it is also necessary to expand the sample size. Second, we did not include more features, such as the number of lymph node metastasis, peritumoral vascular invasion and blood flow resistance index, among others. And we only included strain elastography, without evaluating shear wave elastography. Additionally, mammogram, MRI and other imaging features were not included in this study. Then, we did not evaluate the diagnostic differences between radiologists. Finally, the simple nomogram was mainly used to verify the correlation, and the subsequent research will improve it for better prediction.
In conclusion, AR is closely related to the clinicopathological features and prognosis of breast cancer. Moreover, the ultrasound findings of breast cancer with different expression of AR are also different. As a new molecular marker of breast cancer and an important prognostic factor, AR will play an increasingly important role in diagnosing and treating breast cancer. The above results will help to better understand various biological functions of AR and provide more information for the treatment of breast cancer.
Supplementary Information
Additional file 1. Fig. S1. Examples of ultrasound and IHC images of two patients with breast cancer.
Abbreviations
- AR
Androgen receptor
- ASCO/CAP
American Society of Clinical Oncology/College of American Pathologists
- AUC
Area under curve
- BI-RADS
Breast Imaging Reporting and Data System
- CI
Confidence interval
- C-index
Concordance index
- ER
Estrogen receptor
- FISH
Fluorescence in situ hybridization
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- IHC
Immunohistochemistry
- LAR
Luminal androgen receptor
- OR
Odds ratio
- PFS
Progression-free survival
- PR
Progesterone receptor
- ROC
Receiver operating characteristic curve
- TMA
Tissue microarray
- TNBC
Triple-negative breast cancer
- WFUMB
World Federation of Ultrasound in Medicine and Biology
Author contributions
XDZ, HC and LZ designed the project. Ultrasound images and clinicopathological data collection were done by NNH, PTW, XXZ and WF. Follow-up data collection was done by HH, SL, HQK and FHP. Data analysis was done by PH and DTZ. XDZ and HC drafted the initial manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 82272001, 82102072, 81701705 and 81974265), College Students' Innovative Entrepreneurial Training Plan Program (grant number 202210226004), Outstanding Youth Program of Heilongjiang Natural Science Foundation (YQ2022148).
Availability of data and materials
The imaging data and code that support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Harbin medical university (approval number, KY-2016-127). Informed consent was not required because this study was a retrospective report of cases, which is a retrospective analysis of clinical data. The need of informed consent was waived by the ethical committee of the Harbin medical university.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xudong Zhang and Hao Cui contributed equally to this work and equal first authors.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietze EC, Sistrunk C, Miranda-Carboni G, O'Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Q, Niu Y, Liu N, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011;22:1288–1294. doi: 10.1093/annonc/mdq586. [DOI] [PubMed] [Google Scholar]
- 5.Vesin P, Cattan D. Epithelial cancer and monoclonal immunoglobulins. Sem Hop. 1975;51:817–821. [PubMed] [Google Scholar]
- 6.Huang R, Han J, Liang X, et al. Androgen receptor expression and Bicalutamide antagonize androgen receptor inhibit beta-catenin transcription complex in estrogen receptor-negative breast cancer. Cell Physiol Biochem. 2017;43:2212–2225. doi: 10.1159/000484300. [DOI] [PubMed] [Google Scholar]
- 7.Gucalp A, Traina TA. The androgen receptor: is it a promising target? Ann Surg Oncol. 2017;24:2876–2880. doi: 10.1245/s10434-017-5961-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Li J. Research on the correlation between ultrasonographic features of breast cancer and expressions of ER, CD34 and p53. J BUON. 2018;23:372–377. [PubMed] [Google Scholar]
- 9.Elias SG, Adams A, Wisner DJ, et al. Imaging features of HER2 overexpression in breast cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1464–1483. doi: 10.1158/1055-9965.EPI-13-1170. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Tong Z, Chen K, et al. Interpretation of breast cancer screening guideline for Chinese women. Cancer Biol Med. 2019;16:825–835. doi: 10.20892/j.issn.2095-3941.2019.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Zheng Y, Wu XF, et al. Value of contrast-enhanced ultrasound area ratio in identifying benign and malignant small breast masses. Gland Surg. 2020;9:1486–1494. doi: 10.21037/gs-20-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samiei S, de Mooij CM, Lobbes MBI, Keymeulen K, van Nijnatten TJA, Smidt ML. Diagnostic performance of noninvasive imaging for assessment of axillary response after neoadjuvant systemic therapy in clinically node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2021;273:694–700. doi: 10.1097/SLA.0000000000004356. [DOI] [PubMed] [Google Scholar]
- 13.Cui H, Zhang D, Peng F, et al. Identifying ultrasound features of positive expression of Ki67 and P53 in breast cancer using radiomics. Asia Pac J Clin Oncol. 2021;17:e176–e184. doi: 10.1111/ajco.13397. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Xiong W, Xu JM, Liu YX, Zhang J. Correlations between the expression of C-erB-2, CD34 and ER in breast cancer patients and the signs of conventional ultrasonography and ultrasound elastography. Eur Rev Med Pharmacol Sci. 2018;22:5539–5545. doi: 10.26355/eurrev_201809_15815. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Yang X, Fu L, Yu K. Associations of estrogen receptor, progesterone receptor, human epidemic growth factor receptor-2 and Ki-67 with ultrasound signs and prognosis of breast cancer patients. Cancer Manag Res. 2021;13:4579–4586. doi: 10.2147/CMAR.S276422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae MS, Park SY, Song SE, et al. Heterogeneity of triple-negative breast cancer: mammographic, US, and MR imaging features according to androgen receptor expression. Eur Radiol. 2015;25:419–427. doi: 10.1007/s00330-014-3419-z. [DOI] [PubMed] [Google Scholar]
- 17.Candelaria RP, Adrada BE, Wei W, et al. Imaging features of triple-negative breast cancers according to androgen receptor status. Eur J Radiol. 2019;114:167–174. doi: 10.1016/j.ejrad.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller M, Guth U, Varga Z, et al. Clinical imaging of the heterogeneous group of triple-negative breast cancer. Anticancer Res. 2020;40:2125–2131. doi: 10.21873/anticanres.14171. [DOI] [PubMed] [Google Scholar]
- 19.Tan PH, Ellis I, Allison K, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77:181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 20.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedgwick E. The breast ultrasound lexicon: breast imaging reporting and data system (BI-RADS) Semin Roentgenol. 2011;46:245–251. doi: 10.1053/j.ro.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Yerli H, Yilmaz T, Kaskati T, Gulay H. Qualitative and semiquantitative evaluations of solid breast lesions by sonoelastography. J Ultrasound Med. 2011;30:179–186. doi: 10.7863/jum.2011.30.2.179. [DOI] [PubMed] [Google Scholar]
- 24.Barr RG, Nakashima K, Amy D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 2: breast. Ultrasound Med Biol. 2015;41:1148–1160. doi: 10.1016/j.ultrasmedbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Adler DD, Carson PL, Rubin JM, Quinn-Reid D. Doppler ultrasound color flow imaging in the study of breast cancer: preliminary findings. Ultrasound Med Biol. 1990;16:553–559. doi: 10.1016/0301-5629(90)90020-d. [DOI] [PubMed] [Google Scholar]
- 26.Venema CM, Bense RD, Steenbruggen TG, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther. 2019;200:135–147. doi: 10.1016/j.pharmthera.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 29.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA. Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol. 2010;20:1111–1117. doi: 10.1007/s00330-009-1656-3. [DOI] [PubMed] [Google Scholar]
- 31.de Azambuja E, Cardoso F, de Castro G, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng C, Zhao H, Tian W, Hu C, Zhao H. Predicting the expression level of Ki-67 in breast cancer using multi-modal ultrasound parameters. BMC Med Imaging. 2021;21:150. doi: 10.1186/s12880-021-00684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamb PM, Perry NM, Vinnicombe SJ, Wells CA. Correlation between ultrasound characteristics, mammographic findings and histological grade in patients with invasive ductal carcinoma of the breast. Clin Radiol. 2000;55:40–44. doi: 10.1053/crad.1999.0333. [DOI] [PubMed] [Google Scholar]
- 34.Zhi H, Ou B, Xiao XY, et al. Ultrasound elastography of breast lesions in chinese women: a multicenter study in China. Clin Breast Cancer. 2013;13:392–400. doi: 10.1016/j.clbc.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Chang JM, Park IA, Lee SH, et al. Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol. 2013;23:2450–2458. doi: 10.1007/s00330-013-2866-2. [DOI] [PubMed] [Google Scholar]
- 36.Chamming's F, Latorre-Ossa H, Le Frere-Belda MA, et al. Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol. 2013;23:2079–2086. doi: 10.1007/s00330-013-2828-8. [DOI] [PubMed] [Google Scholar]
- 37.Au-Yong IT, Evans AJ, Taneja S, et al. Sonographic correlations with the new molecular classification of invasive breast cancer. Eur Radiol. 2009;19:2342–2348. doi: 10.1007/s00330-009-1418-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Sui X, Zhou S, Hu L, Huang X. Correlation of conventional ultrasound characteristics of breast tumors with axillary lymph node metastasis and Ki-67 expression in patients with breast cancer. J Ultrasound Med. 2019;38:1833–1840. doi: 10.1002/jum.14879. [DOI] [PubMed] [Google Scholar]
- 39.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol. 2010;194:1160–1166. doi: 10.2214/AJR.09.2355. [DOI] [PubMed] [Google Scholar]
- 40.Aho M, Irshad A, Ackerman SJ, et al. Correlation of sonographic features of invasive ductal mammary carcinoma with age, tumor grade, and hormone-receptor status. J Clin Ultrasound. 2013;41:10–17. doi: 10.1002/jcu.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamaki K, Sasano H, Ishida T, et al. The correlation between ultrasonographic findings and pathologic features in breast disorders. Jpn J Clin Oncol. 2010;40:905–912. doi: 10.1093/jjco/hyq070. [DOI] [PubMed] [Google Scholar]
- 42.Watermann DO, Tempfer CB, Hefler LA, Parat C, Stickeler E. Ultrasound criteria for ductal invasive breast cancer are modified by age, tumor size, and axillary lymph node status. Breast Cancer Res Treat. 2005;89:127–133. doi: 10.1007/s10549-004-1478-6. [DOI] [PubMed] [Google Scholar]
- 43.Luo C, Zhong X, Wang Z, et al. Prognostic nomogram for patients with non-metastatic HER2 positive breast cancer in a prospective cohort. Int J Biol Markers. 2019;34:41–46. doi: 10.1177/1724600818824786. [DOI] [PubMed] [Google Scholar]
- 44.Kono M, Fujii T, Lim B, Karuturi MS, Tripathy D, Ueno NT. Androgen receptor function and androgen receptor-targeted therapies in breast cancer: a review. JAMA Oncol. 2017;3:1266–1273. doi: 10.1001/jamaoncol.2016.4975. [DOI] [PubMed] [Google Scholar]
- 45.Jiang HS, Kuang XY, Sun WL, et al. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget. 2016;7:41285–41293. doi: 10.18632/oncotarget.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao JJ, Swain SM. Luminal A breast cancer and molecular assays: A Review. Oncologist. 2018;23:556–565. doi: 10.1634/theoncologist.2017-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hickey TE, Selth LA, Chia KM, et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat Med. 2021;27:310–320. doi: 10.1038/s41591-020-01168-7. [DOI] [PubMed] [Google Scholar]
- 48.Rampurwala M, Wisinski KB, O'Regan R. Role of the androgen receptor in triple-negative breast cancer. Clin Adv Hematol Oncol. 2016;14:186–193. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig. S1. Examples of ultrasound and IHC images of two patients with breast cancer.
Data Availability Statement
The imaging data and code that support the findings of this study are available from the corresponding author upon request.