Abstract
The objective of the present study was to develop tofu with soybean-water soluble extract coagulated with cardoon flower (F1) and magnesium chloride (MgCl2, F2). The produced tofu was characterized in terms of physical, chemical, microbiological, and sensory properties during 14 days of storage. The yield of F1 was higher (p < 0.05) (195 g/100 soybean seeds) than F2 (162 g/100 soybean seeds). F1 presented higher moisture, protein, acidity, syneresis, and lipids when compared with F2, and a reduction of these contents during the storage. F1 presented lower hardness, stickiness, springiness, and cohesiveness compared with F2. The acceptability of F1 showed a score of 6.00 and F2 of 4.68, and the purchase intention was 3.22 for F1 and 2.23 for F2. This study recommended the use of cardoon flower at 35% level as it has great potential as a coagulant for the elaboration of tofu with higher yield, and acceptability and reasonable purchasing intention.
Keywords: Soybean, Cardoon flower extract, Texture, Physical properties, Sensory evaluation
Introduction
Tofu is one of the most important traditional soybean foods in Eastern countries (Yang et al. 2021a). It presents nutritional qualities, such as free cholesterol, ash, polyunsaturated fatty acids (Losado et al. 2018; Tres et al. 2019; Yang et al. 2021b; Scherer et al. 2021b), and high quality protein compared to meat, fish, and cheese, which can be consumed by vegan individuals as a substitute for animal food products (Khoder et al. 2020). Consumption of tofu in the world is increasing due to its nutrients, such as protein, ash, vitamins, omega-3 fatty acids, and isoflavones (Guo et al. 2017).
Tofu manufacturing involves the processing of water-soluble soybean extract by maceration and grinding the grains, followed by heating, coagulation, and molding. The extract heating will favor thermal inactivation of lipoxygenase enzymes that promote astringent flavor. Coagulation of the soluble extract is the key process to obtaining high quality tofu. Texture and yield are affected by the type and concentration of coagulant (Stanojevic et al. 2020).
The kind of neutral flavor of tofu makes texture an important attribute for quality and consumer acceptance. Therefore, several factors could affect this characteristic, such as the soybean cultivar, processing, and type of coagulant, among others (Rigo et al. 2015; Dahmer et al. 2018; Cantelli et al. 2022). Salt coagulants are commonly used in tofu manufacturing, and magnesium chloride (MgCl2) is the most traditional and widely used industrially (Arii et al. 2021). This salt promotes rapid solidification, resulting in an undesirable erratic and granular texture, coarse and hard aspects. An alternative is the use of vegetable coagulants to meet consumer requirements, with soft texture, flavor, mild and umami characteristics (Schmidt et al. 2017; Shen and Kuo 2017; Jun et al. 2019).
The use of vegetable proteases as milk coagulants (Faion et al. 2020) makes the products suitable for vegetarian consumers (Schmidt et al. 2017). A natural source of those enzymes is found in cardoon flowers (Cynara cardunculus), which affects the flavor and texture of the products (Barracosa et al. 2021). Cardoon flowers or cardoon extract is used for promote specific proteolysis in milk coagulation due mainly to the activity of aspartic proteases (cardosins A and B). However, coagulation of tofu using cardoon flower extract is not reported in the literature. So, the innovation of this work is the use of novel coagulant from cardoon flower extract in the obtention of tofu. Therefore, the aim of this work was to use cardoon flower extract to coagulate water soluble extract of soybean cultivar Vmax in comparison to the traditional coagulant (MgCl2). The tofu samples were characterized in terms of physical, chemical, microbiological, and sensory properties during 14 days of storage.
Materials and methods
Materials
Commercial soybean (Glycine max (L.) Merrill) cultivar Vmax (conventional) was provided by the Brazilian Agricultural Research Corporation (Embrapa) – National Wheat Research Center, Brazil. All the chemicals and reagents for the analyses were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Cardoon flower samples were purchased from Viana do Castelo of Portugal (Portugal) in dry form. The MgCl2 was purchased from Vetec® (Brazil).
Water soluble soybean extract
Water soluble soybean extract was obtained using hulled beans according to Scherer et al. (2021). One hundred and fifty grams of washed soybeans were soaked in 500 mL of water for 16 h (22 °C). The soybean grains were added in deionized water at 90 °C in a weight ratio of 1:8 before blending. Then the mixture was blended (Blender M. Vitrory, model HP 12, Brazil) for 3 min at medium–high speed and filtered through a vacuum (model TE-058, Tecnal, Brazil) with cloth 100-mesh screen.
Cardoon flower coagulant extract
The cardoon flower coagulant extract was obtained using 3.5 g of flower in 25 g of salt (NaCl), macerated with grit and pistil, and this mixture was added in 1 L of water-soluble soybean extract.
Tofu preparation
The tofu was elaborated according to the methodology adapted from Benassi et al. (2011). The coagulants studied were the extract from cardoon flower and MgCl2 (standard formulation). The tofu samples were named as follows: F1—coagulated with cardoon flower extract, while F2—coagulated with MgCl2.The conditions of coagulation of F1 and F2 are obtained by preliminary tests (data not shown). Water soluble soybean extract (2 L) was heated for 10 min at 90 °C before being cooled to the appropriate coagulation temperature (cardoon flower, 30 °C and MgCl2, 70 °C). For MgCl2 coagulation, 5 g of the salt was diluted in 40 mL of distilled water and added to 2 L of water-soluble soybean extract at 70 °C for 20 min. And for the coagulation of cardoon flowers, 2 mL of extract was used for every 2 L of water-soluble soybean extract, at 30 °C and 60 min. After the addition of the coagulant, the water-soluble soybean extract was homogenized and left to stand for coagulation. The tofu samples obtained were placed in plastic containers with lids, and stored at 4 °C for 14 days.
Chemical analysis
The composition of tofu was analyzed by using the standard method of Institute Adolfo Lutz (IAL 2008). The moisture content was determined by drying at 105 °C to a constant weight. The protein was obtained by the Kjeldahl method with a conversion factor of 6.25. Lipids were evaluated by the Soxhlet method. After calcination at 550 °C for 6 h, ash was obtained by a gravimetric method. Kunitz Trypsin Inhibitor (KTI) was determined according to Hamerstrand et al. (1981). Urease activity index was obtained according to AOAC (2005). Phytic acid was determined by spectrophotometry (Latta and Eskin 1980). The yield was obtained in accordance with Benassi et al. (2011).
The antioxidant activities of tofu, water soluble soybean extract, and cardoon flower extract were analyzed by the methodology of DPPH (Miranda and Fraga 2006). The DPPH radical (%) was obtained for the antioxidant activity (AA%). After evaluating the ideal range concentration, it was calculated the inhibitory concentration (IC50) of the extract needed to inhibit 50% of the DPPH radicals obtained from the linear regression curve (r = 0.99).
Physical properties
The syneresis was determined by the drainage method of Hassan et al. (1996).
The pH was analyzed by potentiometric (Digimed, Model DM-20) (IAL 2008). Acidity was determined by titration with sodium hydroxide (0.1 N) and was calculated as g lactic acid/100 g.
The instrumental color was measured in a colorimeter (Minolta Chroma Meter, model CR-400, Japan), using the following parameters: L* (lightness), a* + (tendency to be more red) and b* + (tendency to be more yellow), hue angle (H* amount of pigment) and chroma (C* color perception) indices.
Textural properties such as hardness, springiness, cohesiveness, and gumminess of tofu samples were obtained by using a texture analyzer (CT3, Brookfield) fitted with a 5 kg load cell. A cylindrical stainless-steel probe with a 35 mm diameter, a pretest speed 1 mm/s, test speed of 2 mm/s, post-test speed of 2 mm/s, load cell 25,4 mm of diameter was used.
Microstructure observation
For microstructural analysis with a scanning electron microscope (SEM), the samples were sliced (3 × 2 × 2 mm) and submerged in 2.5% glutaraldehyde. After rinsing, the samples were frozen (CL 600–80) at − 80 °C for 24 h, and dehydrated (Liotop L101AISI304) for 24 h. The dried samples of tofu were sputter-coated (Sputter Coater SCD 050—Balzers) with gold prior to analysis with SEM (Zeiss, model EVO LS25) at an acceleration voltage of 10.0 kV.
Microbiological analysis
Microbiological analyses of Salmonella sp., Staphylococcus coagulase positive, thermo-tolerant Coliforms and B. cereus were performed according to recommendations and requirements of the RDC Number 60 (Brazil 2019).
Sensory evaluation
The sensory evaluation (global acceptance and purchase intention) was performed in individual booths at 22 °C under white light. Samples of tofu were taken from the refrigerator (4 °C) and maintained at room temperature for 30 min, before serving. Samples were cut into blocks (4 × 4 × 2 cm) and offered in petri dishes to untrained panelists/consumers (n = 60). Samples were labeled with 3-digit codes in random order. For the consumer acceptance test, a structured hedonic scale with 9 points (9 = like a lot, and 1 = dislike a lot) was applied (Queiroz and Treptow 2006). The experiment was approved under CPEA number 12952719.3.0000.5351 by the Research Ethics Committee (URI-Erechim) and registered at Brazil Plataform.
Statistical analysis
Experiments were carried out in triplicate, and data was analyzed using Statistica 5.0 software. The statistical significance of differences between means was determined by the T-Student and Tukey tests with a 95% confidence level. Pearson’s correlation coefficient analysis and principal component analysis (PCA) were determined using XLSTAT (2019 free) software.
Results and discussion
Chemical and physical characterization of tofu
The moisture, pH, ash, lipids, proteins, color parameters, KTI, ureactic activity, phytic acid, acidity, and syneresis of tofu samples F1 (cardoon flower) and F2 (MgCl2) are presented in Table 1. The moisture of tofu samples was between 79.92% and 85.79% with significant differences (p < 0.05) during the storage. According to Ciabotti et al. (2009) tofu with a moisture content between 80 and 97% is considered soft, which was observed in the present study, where F1 can be considered softer than F2. Lee et al. (2019) found results similar to those of this work, with values of 79.34, 81.88 and 82.25 g/100 g of tofu. Tofu F1 showed high water retention capacity (high moisture content), which could be due to the low crosslinking between protein molecules (Zheng et al. 2020). The low crosslinking provides a soft mass, allowing air gaps within the network (Jayasena et al. 2014). The low reticulation provides a soft mass, letting loose the comprehensive organization and leaving many air gaps within the network (Jayasena et al. 2014). However, considering the storage days, it is observed that the moisture content decreased for all tofu samples evaluated.
Table 1.
Chemical properties of the tofu prepared with different coagulants during the storage (1, 7, and 14 days)
| Analysis | Days of Storage | Coagulant | |
|---|---|---|---|
| Cardoon flower (F1) | MgCl2 (F2) | ||
| Moisture (g/100 g) | 1 | 85.79aA ± 0.37 | 80.97bA ± 0.15 |
| 7 | 85.46aA ± 0.16 | 80.13bB ± 0.14 | |
| 14 | 83.15aB ± 0.05 | 79.92bC ± 0.04 | |
| pH | 1 | 5.40bA ± 0.02 | 5.49aA ± 0.01 |
| 7 | 5.34bB ± 0.04 | 5.42aB ± 0.02 | |
| 14 | 5.29bB ± 0.04 | 5.36aC ± 0.01 | |
| Ash (g/100 g) | 1 | 0.76bA ± 0.02 | 0.86aA ± 0.01 |
| 7 | 0.72bB ± 0.01 | 0.83aB ± 0.01 | |
| 14 | 0.68bC ± 0.01 | 0.79aC ± 0.01 | |
| Lipid (g/100 g) | 1 | 4.25aA ± 0.02 | 4.15bA ± 0.03 |
| 7 | 4.20aA ± 0.01 | 4.10bB ± 0.01 | |
| 14 | 4.16aB ± 0.02 | 4.07bC ± 0.01 | |
| Protein (g/100 g) | 1 | 12.18aA ± 0.01 | 11.26bA ± 0.05 |
| 7 | 11.49aB ± 0.01 | 11.20bAB ± 0.06 | |
| 14 | 11.25aC ± 0.02 | 11.15bB ± 0.01 | |
| KTI (mg.IT/g) | 1 | 1.16aA ± 0.03 | 1.15aA ± 0.01 |
| 7 | 1.13aA ± 0.01 | 1.15aA ± 0.01 | |
| 14 | 1.12aA ± 0.01 | 1.14aA ± 0.02 | |
| Ureatic activity (pH) | 1 | 0.06aA ± 0.02 | 0.05aA ± 0.03 |
| 7 | 0.06aA ± 0.02 | 0.05aA ± 0.02 | |
| 14 | 0.06aA ± 0.02 | 0.06aA ± 0.02 | |
| Phytic acid (g/100 g) | 1 | 1.39aA ± 0.04 | 1.37aA ± 0.02 |
| 7 | 1.38aA ± 0.03 | 1.36aA ± 0.02 | |
| 14 | 1.38aA ± 0.02 | 1.35aA ± 0.01 | |
| Acidity (% lactic acid) | 1 | 0.43aB ± 0.03 | 0.30bC ± 0.02 |
| 7 | 0.94aA ± 0.07 | 0.57bB ± 0.03 | |
| 14 | 1.00bA ± 0.03 | 1.25aA ± 0.05 | |
| Syneresis (%) | 1 | 7.17aA ± 1.25 | 6.67aA ± 0.76 |
| 7 | 4.53aB ± 0.07 | 1.40bB ± 0.10 | |
| 14 | 1.84aC ± 0.06 | 0.88bC ± 0.01 | |
*Means (± Standard Deviation) followed by equal letters lowercase/uppercase indicate that there is no significant difference at the 5% level (Student’s t-test and Tukey test) in the row/column, respectively
The yield of tofu F1 was higher (195 g/100 soybean seed) than F2 (162 g/100 soybean seed). The tofu with a high yield also has a high moisture content, suggesting that yield and moisture content are highly correlated.
The pH and acidity values (Table 1) differed significantly (p < 0.05) between the tofu samples (F1 and F2). It was observed that there was a reduction in pH and an increase in acidity for all tofu samples during the storage. As coagulant extract (cardoon flower) has a pH of 5.13, it may be one of the factors that favors soymilk coagulation, resulting in a more acid tofu when compared to that obtained with traditional coagulant F2. According to Hendrawati et al. (2021), the coagulant can affect the pH of tofu, which also affects the solubility of proteins, and quality of the texture. Using conventional soybean cultivars, Schmidt et al. (2017) found pH values similar to those of this work using different coagulants: kiwi, ginger, and lemon. Fasoyiro (2014) developed tofu using soybean variety TGX-1448-IE coagulated with roselle dried flower calyx and obtained a pH of 5.8, similar to that obtained in this work.
During storage, there was a decrease in the protein, lipid, and ash values (Table 1), which could be attributed to syneresis (liquid loss) and protein exudation. This reduction over storage time is due to the release of whey, which causes migration of the components. This behavior was also observed by Schimidt et al. (2017) during the tofu storage, where they related the release of ash from the whey during storage to salt migration from tofu to whey. The syneresis during storage is generally accepted and can be related to increased cross-linking among protein molecules (Khoder et al. 2020).
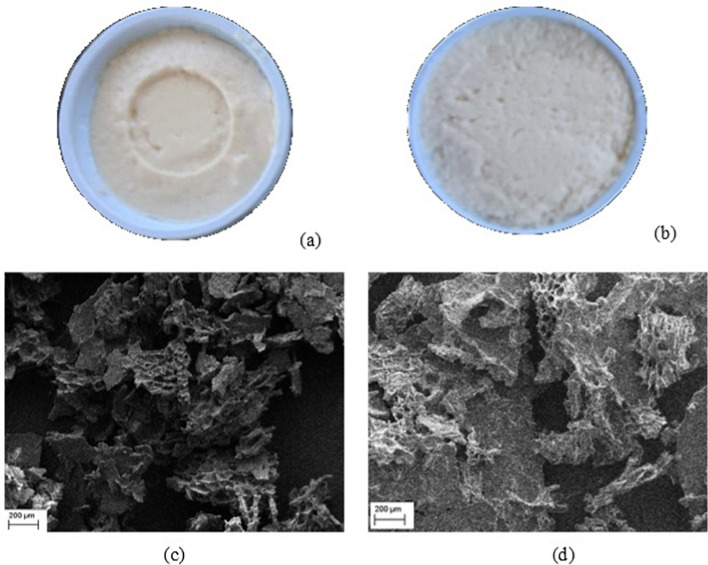
It can be highlighted that the protein content of F1 was significantly higher than F2 (p < 0.05). This high content can affect the gel structure, resulting in a visual aspect that is more homogeneous (Fig. 1a). According to Zhang et al. (2018) tofu coagulated with MgCl2 reduces the interaction of protein molecules, resulting in a firm product, due to the release of water from the gel structure. This behavior was also observed in the presented study in Fig. 1b. So, the quality and yield of tofu can be affected by the type of coagulant used. The changes in these characteristics were observed in the microstructure of tofu samples (Fig. 1c and d), where the structure of protein aggregates was held together with different homogeneity across the tofu samples. In F2, a compact structure with thicker aggregates and fewer pores (Fig. 1c) was formed when compared to tofu coagulated with cardoon flower extract (Fig. 1d).
Fig. 1.
Visual appearance of tofu prepared with different coagulants a cardoon flower extract—F1, and b MgCl2—F2. Scanning electron microscopy (SEM) of c F1 and d F2 tofu. Scale bar = 200 µm
Ureatic activity, phytic acid, and Kunitz Trypsin Inhibitor (KTI) values were similar in both tofu samples during the studied storage (Table 1). Ureatic activity index values were in the range considered for inactivation of the enzyme (0.05 to 0.30 pH unit) (Olguin et al. 2003). The low phytic acid values found are interesting, as they can provide certain functional actions (Kumar et al. 2005). All tofu samples showed minimum residual levels of KTI. Since KTI is an anti-nutritional factor, it must be reduced or inactivated in soy-based foods (Gu et al. 2017). Therefore, it is observed that the thermal processing to obtain the water-soluble extract to produce the tofu could inactivate 93% of the trypsin inhibitor present in soybean (18.46 mg.IT/g). Furthermore, it is observed that KTI values for tofu were lower (Table 1) than those for soymilk (2.13 ± 0.22 mg.IT/g) (Scherer et al. 2021a), indicating that the process of transforming the extracts into tofu could contribute to obtaining a healthier product.
In general, in tofu, a white or light yellow color is a desired quality parameter. Because of that, soybean grains with yellow or clear hilum are preferred. Grains of the conventional cultivar Vmax have a clear hilum, which contributes to the light coloration of the developed tofu (Fig. 1a and b). On the 1st day of storage, the F1 showed a little bit darker (L*) than F2 and has a tendency to yellowish color, with significant difference (p > 0.05). With storage, a slight reduction in the intensity of luminosity L* and chromaticity was observed for all tofu samples (p < 0.05). Tofu prepared with cardoon flower showed higher a* values (p < 0.05) than that with MgCl2 (Table 2), but the b* values did not differ (p > 0.05) across samples. The Hue* did not differ for both coagulants at the 1st and 7th days of storage (p < 0.05). C* was different for both coagulants (p < 0.05).
Table 2.
Physical property (color) of the tofu prepared with different coagulants during the storage of 1, 7, and 14 days
| Color | Days of Storage | Coagulant | |
|---|---|---|---|
| Cardoon flower(F1) | MgCl2 (F2) | ||
| L* | 1 | 81.58aA ± 0.06 | 80.58bA ± 0.06 |
| a* | 0.35aA ± 0.03 | 0.13bA ± 0.02 | |
| b* | 13.78bA ± 0.01 | 14.79aA ± 0.01 | |
| ºHue | 1.55aA ± 0.01 | 1.56aA ± 0.01 | |
| C* | 13.78bA ± 0.01 | 14.79aA ± 0.01 | |
| L* | 7 | 79.83aB ± 0.06 | 79.86aB ± 0.26 |
| a* | 0.39aA ± 0.02 | 0.10bAB ± 0.05 | |
| b* | 13.48aB ± 0.26 | 13.67aB ± 0.30 | |
| ºHue | 1.54aA ± 0.01 | 1.56aA ± 0.01 | |
| C* | 13.49aAB ± 0.26 | 13.67aA ± 0.30 | |
| L* | 14 | 79.37aC ± 0.33 | 79.62aB ± 0.32 |
| a* | 0.40aA ± 0.04 | 0.06bB ± 0.02 | |
| b* | 13.45aB ± 0.16 | 12.93aC ± 0.40 | |
| ºHue | 1.54bA ± 0.01 | 1.57aA ± 0.01 | |
| C* | 13.46aB ± 0.16 | 12.93bB ± 0.04 | |
*Means (± Standard Deviation) followed by equal letters lowercase/uppercase indicate that there is no significant difference at the 5% level (Student’s t-test and Tukey test) in the row/column, respectively
Coagulation performed with cardoon flower extract in the presence of protease enzymes, hydrolyzes peptide bonds in soymilk, acting near the ends or in internal areas of the polypeptide chains (Rawlings et al. 2010). So, cardoon extract is a good coagulant due to the ease of processing and improved yield of tofu.
One of the most important factors in determining consumer acceptance, especially for semi-solid foods (tofu), is texture. Texture attributes such as hardness, viscosity, elasticity, and cohesiveness are shown in Table 3. The hardest tofu was F2 (6.64 N, Table 3), which is related to the coagulant used (MgCl2). According to Wang et al. (2018), MgCl2 produces tofu gel with a hard and granular texture due to its rapid clotting ability. On the other hand, the tofu F1 exhibited lower hardness. So, according to Joo and Cavender (2020) it needs less force to break through the structure initially, showing greater moisture. It was possible to verify that all tofu samples showed a tendency to increase hardness during storage (Table 3), which may be related to the decrease in moisture (Table 1), thus making the protein gel matrix more dense with the past storage days, the same behavior observed by Ullah et al. (2019).
Table 3.
Texture profile (hardness, stickiness, springiness, and cohesiveness) of tofu produced from grains of soybean cultivar Vmax, coagulated with two different coagulants (F1 and F2) at 1st, 7th, and 14th days of storage
| Texture | Days of storage | Tofu | |
|---|---|---|---|
| Cardoon flower (F1) | MgCl2 (F2) | ||
| Hardness (N) | 1 | 0.89bB ± 0.09 | 6.64aC ± 0.17 |
| 7 | 1.15bA ± 0.01 | 7.68aB ± 0.08 | |
| 14 | 1.20bA ± 0.12 | 7.91aA ± 0.15 | |
| Stickiness (N) | 1 | 0.39bA ± 0.05 | 3.31aA ± 0.07 |
| 7 | 0.42bA ± 0.02 | 3.44aA ± 0.05 | |
| 14 | 0.46bA ± 0.06 | 3.47aA ± 0.09 | |
| Springiness (mm) | 1 | 7.55bA ± 0.07 | 7.97aA ± 0.05 |
| 7 | 7.19bB ± 0.05 | 7.65aB ± 0.05 | |
| 14 | 6.31bC ± 0.04 | 7.36aC ± 0.07 | |
| Cohesiveness | 1 | 0.52bA ± 0.04 | 0.63aA ± 0.01 |
| 7 | 0.50bA ± 0.01 | 0.59aB ± 0.01 | |
| 14 | 0.44bB ± 0.03 | 0.57aB ± 0.02 | |
*Means (± Standard Deviation) followed by equal letters lowercase/uppercase indicate that there is no significant difference at the 5% level (Student’s t-test and Tukey test) in the row/column, respectively
Microbiological, sensory evaluation and antioxidant activity of tofu
Before performing the sensory evaluation, tofu products were subjected to microbiological analysis, and was it observed that the thermo-tolerant coliforms (< 1.5 × 101 MPN/g), Staphylococcus coagulase positive (< 1 CFU/g), Salmonella sp. (25 g/absence) and B. cereus (< 1 CFU/g) were within the values established in the RDC n° 60 (Brazil 2019). These results demonstrate that the product was safe for the panelists involved in sensory analysis.
The sensory evaluation (acceptance) showed a significant difference (p > 0.05) between tofu F1 (6.00) and F2 (4.68), which correspond to the scale “liked moderately and liked a lot”, respectively, that also presented the purchase intention of 3.22 to 2.23. It is verified that the F1 was preferred by the panelists, and this better acceptability may be associated with the soft characteristics of this tofu.
In relation to IC50 the tofu F1, cardoon flower extract, and water-soluble soybean extract present values of 0.15 ± 0.2 mg/mL, 0.31 ± 0.01 mg/mL, and, 0.59 ± 0.02 mg/mL, respectively. This antioxidant activity value of tofu may be due to the association of phenolic compounds from cardoon flower with soymilk proteins (Gominho et al. 2018).
Principal components (PCA) and correlation analysis
The results of Pearson correlation and principal component analysis (PCA) of the physical–chemical, sensory and texture variables of tofu formulations (F1-cardoon flower extract) and F2-MgCl2), at the 1st, 7th and 14th days of storage are presented in Table 4 and Fig. 2.
Table 4.
Pearson’s correlation matrix for moisture, pH, ash, lipid, protein, color L*, a*, b*, C*, °Hue, Kunitz Trypsin Inhibitor (KTI), ureatic activity, phytic acid, acidity, yield, syneresis, acceptance, texture parameters (hardness, stickiness, springiness, and cohesiveness) variables of tofu coagulated with cardoon flower (F1) and MgCl2 (F2), during the 1st, 7th and 14th days of storage
| Variable | Moisture | pH | Ash | Lipid | Protein | L* | a* | b* | Hue | C* | KTI | Ureatic act | Phytic acid | Acidity | Yied | Syneresis | Acceptance | Hardness | Stickiness | Springiness | Cohesiveness | Chewiness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | 1 | |||||||||||||||||||||
| pH | − 0.339 | 1 | ||||||||||||||||||||
| Ash | − 0.666 | 0.826 | 1 | |||||||||||||||||||
| Lipid | 0.703 | − 0.123 | − 0.304 | 1 | ||||||||||||||||||
| Protein | 0.788 | 0.050 | − 0.290 | 0.649 | 1 | |||||||||||||||||
| L* | 0.422 | 0.539 | 0.198 | 0.463 | 0.822 | 1 | ||||||||||||||||
| a* | 0.898 | − 0.558 | − 0.738 | 0.660 | 0.534 | 0.115 | 1 | |||||||||||||||
| b* | − 0.013 | 0.709 | 0.553 | 0.169 | 0.119 | 0.489 | − 048 | 1 | ||||||||||||||
| Hue | − 0.893 | 0.584 | 0.756 | − .648 | − .519 | − .090 | − 0.999 | 0.083 | 1 | |||||||||||||
| C* | − 0.009 | 0.707 | 0.550 | 0.172 | 0.121 | 0.489 | − 0.043 | 1.000 | 0.078 | 1 | ||||||||||||
| KTI | − 0.017 | 0.527 | 0.405 | 0.397 | 0.379 | 0.596 | − 0.168 | 0.376 | 0.191 | 0.375 | 1 | |||||||||||
| Ureatic act | 0.175 | − 0.240 | − 0.161 | − 0.054 | 0.116 | − 0.010 | 0.225 | − 0.085 | − 0.224 | − 0.084 | 0.126 | 1 | ||||||||||
| Phytic acid | 0.543 | − 0.045 | − 0.250 | 0.557 | 0.447 | 0.258 | 0.572 | 0.205 | − 0.554 | 0.207 | 0.450 | 0.193 | 1 | |||||||||
| Acidity | − 0.099 | − 0.765 | − 0.509 | − 0.216 | − 0.407 | − 0.726 | 0.069 | − 0.826 | − 0.099 | − 0.826 | − 0.452 | 0.210 | − 0.145 | 1 | ||||||||
| Yied | 0.928 | − 0.590 | − 0.801 | 0.589 | 0.610 | 0.150 | 0.972 | − 0.187 | − 0.974 | − 0.182 | − 0.209 | 0.217 | 0.540 | 0.126 | 1 | |||||||
| Syneresis | 0.651 | 0.302 | − 0.070 | 0.557 | 0.946 | 0.927 | 0.375 | 0.358 | − 0.352 | 0.360 | 0.485 | 0.062 | 0.445 | − 0.600 | 0.432 | 1 | ||||||
| Acceptance | 0.927 | − 0.596 | − 0.802 | 0.593 | 0.609 | 0.155 | 0.969 | − 0.195 | − 0.973 | − 0.191 | − 0.240 | 0.188 | 0.495 | 0.122 | 0.997 | 0.428 | 1 | |||||
| Hardness | − 0.944 | 0.508 | 0.748 | − 0.600 | − 0.639 | − 0.227 | − 0.969 | 0.080 | 0.969 | 0.076 | 0.223 | − 0.172 | − 0.505 | − 0.019 | − 0.988 | − 0.491 | − 0.991 | 1 | ||||
| Stickiness | − 0.936 | 0.568 | 0.789 | − 0.583 | − 0.622 | − 0.178 | − 0.965 | 0.167 | 0.968 | 0.163 | 0.249 | − 0.182 | − 0.485 | − 0.091 | − 0.995 | − 0.450 | − 0.999 | 0.996 | 1 | |||
| Springiness | − 0.291 | 0.880 | 0.715 | − 0.046 | 0.137 | 0.560 | − 0.602 | 0.544 | 0.624 | 0.541 | 0.612 | − 0.131 | − 0.106 | − 0.639 | − 0.611 | 0.328 | − 0.620 | 0.556 | 0.597 | 1 | ||
| Cohesiveness | − 0.600 | 0.757 | 0.808 | − 0.099 | − 0.215 | 0.260 | − 0.726 | 0.482 | 0.743 | 0.479 | 0.594 | − 0.125 | − 0.242 | − 0.432 | − 0.803 | − 0.021 | − 0.810 | 0.771 | 0.804 | 0.843 | 1 | |
| Chewiness | − 0.940 | 0.541 | 0.767 | − 0.608 | − 0.626 | − 0.193 | − 0.972 | 0.126 | 0.974 | 0.122 | 0.231 | − 0.162 | − 0.499 | − 0.050 | − 0.993 | − 0.459 | − 0.997 | 0.997 | 0.998 | 0.579 | 0.786 | 1 |
Values in bold are different from 0 with a significance level alpha = 0.05 (r > 0.55)
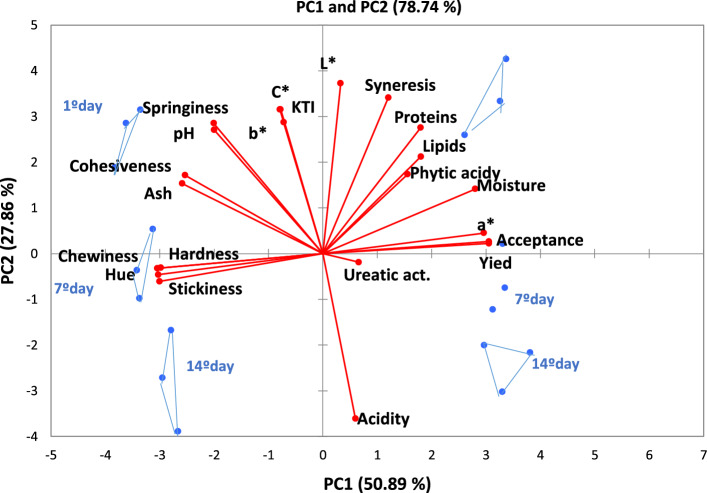
Fig. 2.
Principal component analysis of the tofu coagulated with cardoon flower (F1) and MgCl2 (F2) during the storage (1, 7 and 14 days)
According to PCA, in which variables are represented as vectors, the longer the vector, the better explanation of the variability between the variables (Fig. 2). The samples on each storage day are represented by triangles, being each vertex replication of n = 3.
The first (PC1) and second (PC2) dimensions explained 78.74% of the total variance. PC1 accounted for 50.89%, while PC2 accounted for 27.86%. A good discrimination between the two tofu formulations (F1 and F2) was observed. This discrimination was justified by the type of coagulant used in the formulations (F1 = cardoon flower and F2 = standard MgCl2).
Formulation F2 had higher values for texture parameters (springiness, hardness, stickiness, and cohesiveness), KTI, b* and C* color, hue, pH, and ash. Formulation F1, especially for the 1st day of storage, showed higher values for protein, phytic acid, syneresis, total lipid, moisture, and L* color. At the 7th day of storage, yield, a* color, ureatic activity and acceptance had the higher values, while acidity was higher at the 14th day of storage.
The values obtained through Pearson's correlation (Table 4) confirm the relationship between the parameters observed in the principal component analysis (Fig. 2). Moisture content was positively correlated (p > 0.05) with protein, lipids, a* color, yield, syneresis, and acceptance. However, moisture was negatively correlated with all texture parameters, except for springiness (r = − 0.291).
Protein was positively correlated (p > 0.05) with L* color, yield, syneresis, and acceptance; and negatively correlated with hardness, and stickiness. Acceptance was positively correlated (p < 0.05) with moisture, lipids, protein, a* color, and yield, and negatively correlated (p < 0.05) with the texture parameters (hardness, stickiness, springiness, and cohesiveness), showing that when texture characteristics are higher, the acceptance of the product tends to decrease.
Conclusion
The use of cardoon flower extract as a tofu coagulant by using grains of the conventional soybean cultivar Vmax, provides a soft tofu with a higher yield, good acceptability, and purchase intent. These results confirm that the use of this new coagulant is satisfactory compared with the use of a traditional coagulant (MgCl2). Results of the sensory analysis also show the potential for use of cardoon flowers in human nutrition or food processing.
Acknowledgements
The authors would like to thank the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel Brazil (CAPES)—Finance Code 001, the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (Fapergs), and the URI for the financial and structure support.
Abbreviations
- F1
Tofu coagulated with cardoon flower extract
- F2
Tofu coagulated with MgCl2
- MgCl2
Magnesium chloride
- NaCl
Sodium chloride
- KTI
Kunitz Trypsin Inhibitor
- DPPH
2,2-Diphenyl-1-picryl-hydrazyl-hydrate
- SEM
Scanning electron microscope
- RDC
Resolution of directory college
- CPEA
Certificate of presentation of ethical appreciation
- PCA
Principal component analysis
- CFU
Colony-forming unit
- MPN
Most Probable Number
- IC50
Half-maximal inhibitory concentration
- PC1
Principal component 1
- PC2
Principal component 2
- r
Pearson correlation coefficient
- AA
Antioxidant activity
Author contributions
All authors contributed equally to this work.
Funding:
Not applicable.
Availability of data and material
All data are available in the work.
Code availability
XLSTAT (2019 free).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Consent to participate
The contribution of all the authors have been mentioned with credits.
Consent for publication
The presented manuscript has not been submitted and published anywhere.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC (2005) Official methods of analysis of the association of official analytical chemists (method 900.02, 994.12, 996.06, 996.01). Gaithersburg, Maryland
- Arii Y, Sano Y, Nishizawa K. Direct comparison of the tofu-like precipitate formation by adding different coagulants: magnesium chloride and glucono-δ-lactone. Heliyon. 2021;7:e07239. doi: 10.1016/j.heliyon.2021.e07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barracosa P, Simões I, Martins AP, et al. Biochemical diversity of cardoon flowers (Cynara cardunculus L.): Predicting PDO Mediterranean cheese textures. Food Biosci. 2021;39:100805. doi: 10.1016/j.fbio.2020.100805. [DOI] [Google Scholar]
- Benassi VDT, Benassi MDT, Prudencio SH. Brazilian soybean cultivars: characteristics for tofu production and acceptance by the consumer market. Semin Ciências Agrárias. 2011;32:1901–1914. doi: 10.5433/1679-0359.2011v32Suplp1901. [DOI] [Google Scholar]
- Brazil (2019) Normative Instruction n° 60, 23 December 2019. Establishes lists of microbiological standards for foods
- Cantelli KC, Carrão-panizzi MC, Moreira F, et al. Evaluation of packaging systems with O2-absorbers on quality of minimally processed soybean sprouts. Food Sci Technol Int. 2022;1:108201322210848. doi: 10.1177/10820132221084863. [DOI] [PubMed] [Google Scholar]
- Dahmer AM, Rigo AA, Steffens J, et al. Thermal treatment for soybean flour processing with high-quality color and reduced Kunitz trypsin inhibitor. J Food Process Eng. 2018;41:12925. doi: 10.1111/jfpe.12925. [DOI] [Google Scholar]
- da Scherer GCR, Ambrósio N, Fernandes IA, et al. Maximization of maceration step of obtaining water-soluble soy extract process. Braz J Dev. 2021;7:28197–28215. doi: 10.34117/bjdv7n3-507. [DOI] [Google Scholar]
- da Scherer GCR, Colet R, Cavalheiro D, et al. Properties of tofu coagulated with cardoon flower (Cynara Cardunculus L.) Res Soc Dev. 2021;10:e207101623576. doi: 10.33448/rsd-v10i16.23576. [DOI] [Google Scholar]
- Faion AM, Menegotto ALL, Fernandes IA, et al. Production of Serra da Estrela cheese from ultrafiltered sheep’s milk. Emir J Food Agric. 2020 doi: 10.9755/ejfa.2020.v32.i5.2109. [DOI] [Google Scholar]
- Gominho J, Curt MD, Lourenço A, et al. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenerg. 2018;109:257–275. doi: 10.1016/j.biombioe.2018.01.001. [DOI] [Google Scholar]
- Gu E-J, Kim DW, Jang G-J, et al. Mass-based metabolomic analysis of soybean sprouts during germination. Food Chem. 2017;217:311–319. doi: 10.1016/j.foodchem.2016.08.113. [DOI] [PubMed] [Google Scholar]
- Guo S, Zhu X, Loh XJ. Controlling cell adhesion using layer-by-layer approaches for biomedical applications. Mater Sci Eng C. 2017;70:1163–1175. doi: 10.1016/j.msec.2016.03.074. [DOI] [PubMed] [Google Scholar]
- Hamerstrand GEE, Black LTT, Glover JDD. Trypsin inhibitors in soy products: modification of the standard analytical procedure. Cereal Chem. 1981;58:42. [Google Scholar]
- Hassan AN, Frank JF, Schmidt KA, Shalabi SI. Textural properties of yogurt made with encapsulated nonropy lactic cultures. J Dairy Sci. 1996;79:2098–2103. doi: 10.3168/jds.S0022-0302(96)76583-9. [DOI] [Google Scholar]
- Hendrawati TY, Audini K, Ismiyati et al (2021) Effects and characterization of different soybean varieties in yield and organoleptic properties of tofu. Results Eng 11:100238. 10.1016/j.rineng.2021.100238
- IAL (2008) Instituto Adolfo Lutz. Métodos físico-químicos para análise de alimentos
- Jayasena V, Tah WY, Nasar-Abbas SM. Effect of coagulant type and concentration on the yield and quality of soy-lupin tofu. Qual Assur Saf Crop Foods. 2014;6:159–166. doi: 10.3920/QAS2012.0176. [DOI] [Google Scholar]
- Joo KH, Cavender GA. Investigation of tofu products coagulated with trimagnesium citrate as a novel alternative to nigari and gypsum: Comparison of physical properties and consumer preference. LWT. 2020;118:108819. doi: 10.1016/j.lwt.2019.108819. [DOI] [Google Scholar]
- Jun J, Jung M, Jeong I, et al. Effects of crab shell extract as a coagulant on the textural and sensorial properties of tofu (soybean curd) Food Sci Nutr. 2019;7:547–553. doi: 10.1002/fsn3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoder RM, Yin T, Liu R, et al. Effects of nano fish bone on gelling properties of tofu gel coagulated by citric acid. Food Chem. 2020;332:127401. doi: 10.1016/j.foodchem.2020.127401. [DOI] [PubMed] [Google Scholar]
- Kumar V, Rani A, Rajpal S, et al. Phytic acid in Indian soybean: genotypic variability and influence of growing location. J Sci Food Agric. 2005;85:1523–1526. doi: 10.1002/jsfa.2151. [DOI] [Google Scholar]
- Latta M, Eskin M. A simple and rapid colorimetric method for phytate determination. J Agric Food Chem. 1980;28:1313–1315. doi: 10.1021/jf60232a049. [DOI] [Google Scholar]
- Losado VAMVAM, Cantelli KCKC, Steffens J, et al. Improvement in soybean sprouts production with ultrasound power. Bol Do Cent Pesqui Process Aliment. 2018;35:1–9. doi: 10.5380/bceppa.v35i2.60274. [DOI] [Google Scholar]
- Miranda A, Fraga C (2006) Free radical scavenger activity: determination of the antioxidant profile of bioactive substances. In: Practical studies for medicinal chemistry
- Olguin MC, Hisano N, D’Ottavio AE, et al. Nutritional and antinutritional aspects of an Argentinian soy flour assessed on weanling rats. J Food Compos Anal. 2003;16:441–449. doi: 10.1016/S0889-1575(03)00005-X. [DOI] [Google Scholar]
- Queiroz MI, Treptow RO (2006) Análise sensorial para avaliação da qualidade dos alimentos. Editora FURG - Fundação Universidade Federal do Rio Grande
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo AA, Dahmer AM, Steffens C et al (2015) Characterization of soybean cultivars genetically improved for human consumption. Int J Food Eng v:1–7. 10.18178/ijfe.1.1.1-7
- Schmidt J, Cantelli KC, Steffens C, et al. Effects of vegetable coagulants in the production and storage of tofu. Glob Sci Technol. 2017;10:188–198. [Google Scholar]
- Shen Y-R, Kuo M-I. Effects of different carrageenan types on the rheological and water-holding properties of tofu. LWT. 2017;78:122–128. doi: 10.1016/j.lwt.2016.12.038. [DOI] [Google Scholar]
- Stanojevic SP, Barać MB, Pešić MB, Vucelic-Radovic BV. Protein composition and textural properties of inulin-enriched tofu produced by hydrothermal process. LWT. 2020;126:109309. doi: 10.1016/j.lwt.2020.109309. [DOI] [Google Scholar]
- Tres G, Steffens J, Steffens C. Tofu obtained with lemon coagulant and soybean concentrate. Rev Tecnol. 2019;28:85–100. doi: 10.4025/revtecnol.v28i1.48693. [DOI] [Google Scholar]
- Ullah I, Hu Y, You J, et al. Influence of okara dietary fiber with varying particle sizes on gelling properties, water state and microstructure of tofu gel. Food Hydrocoll. 2019;89:512–522. doi: 10.1016/j.foodhyd.2018.11.006. [DOI] [Google Scholar]
- Wang X, Zeng M, Qin F, et al. Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018;242:459–465. doi: 10.1016/j.foodchem.2017.09.044. [DOI] [PubMed] [Google Scholar]
- Yang JS, Li L. The gel properties and gastric digestion kinetics of a novel lactic acid bacteria fermented tofu: Focusing on the effects of transglutaminase. LWT. 2021;143:110998. doi: 10.1016/j.lwt.2021.110998. [DOI] [Google Scholar]
- Yang JS, Li L, et al. The gel properties and gastric digestion kinetics of a novel lactic acid bacteria fermented tofu: Focusing on the effects of transglutaminase. LWT. 2021;143:110998. doi: 10.1016/j.lwt.2021.110998. [DOI] [Google Scholar]
- Zhang Q, Wang C, Li B, et al. Research progress in tofu processing: from raw materials to processing conditions. Crit Rev Food Sci Nutr. 2018;58:1448–1467. doi: 10.1080/10408398.2016.1263823. [DOI] [PubMed] [Google Scholar]
- Zheng L, Regenstein JM, Teng F, Li Y. Tofu products: a review of their raw materials, processing conditions, and packaging. Compr Rev Food Sci Food Saf. 2020;19:3683–3714. doi: 10.1111/1541-4337.12640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the work.
XLSTAT (2019 free).