Abstract
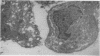
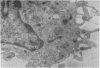
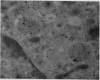
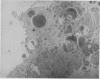
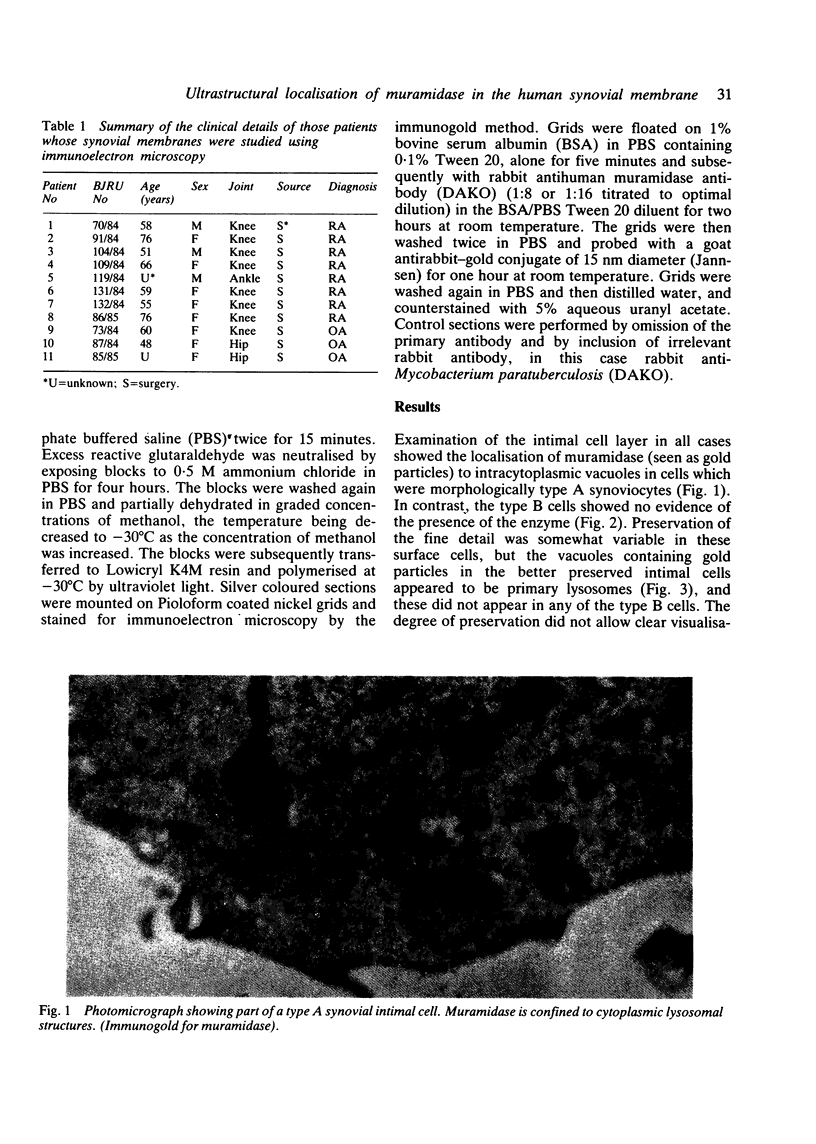
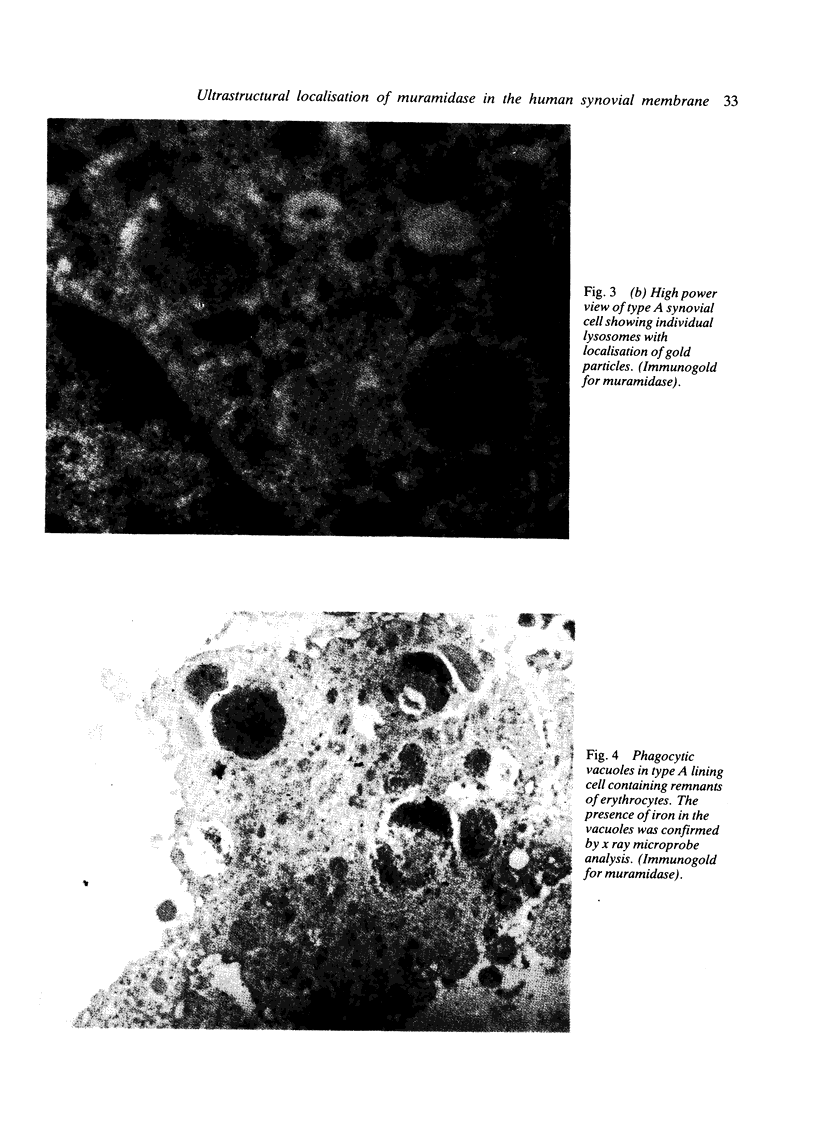
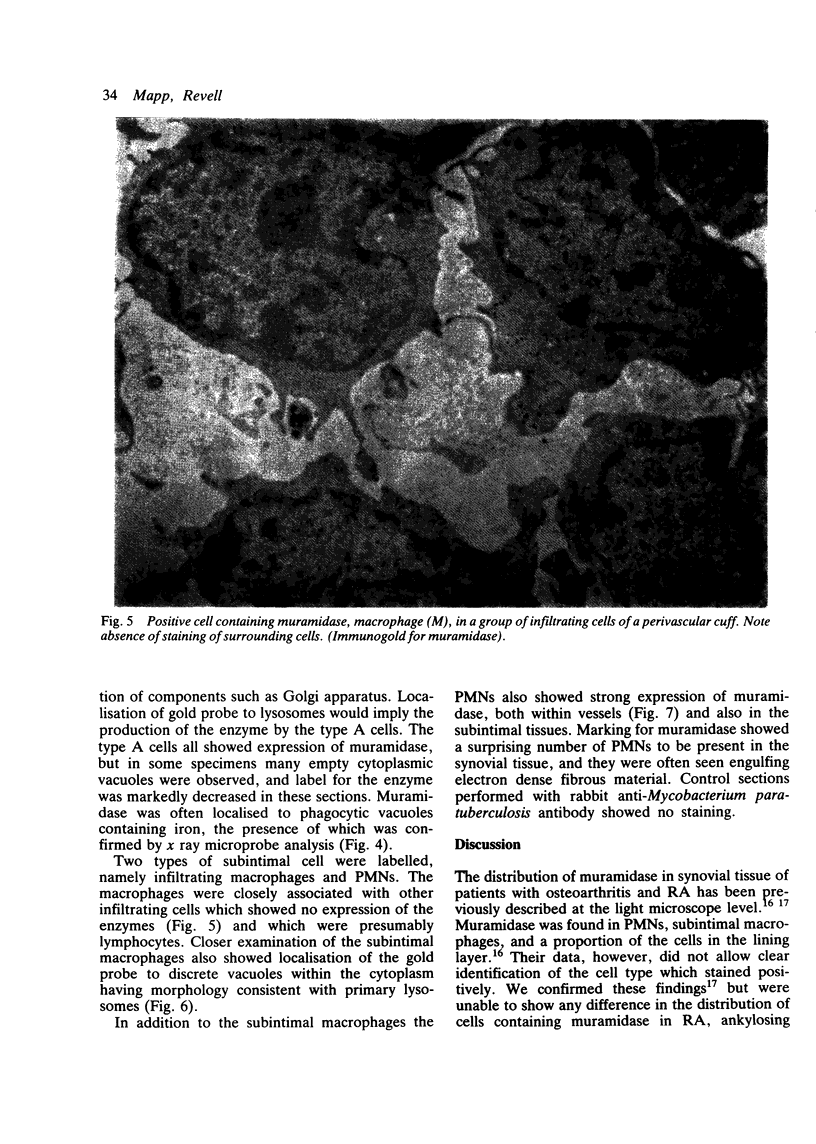
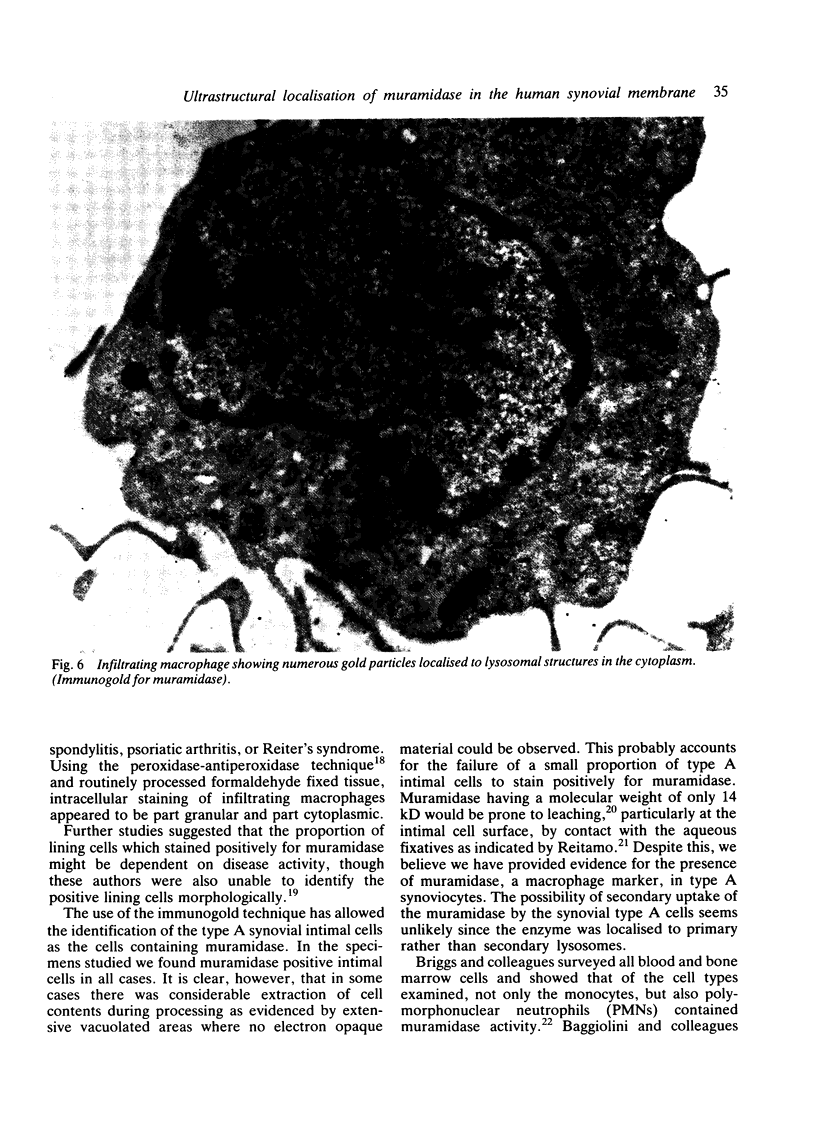
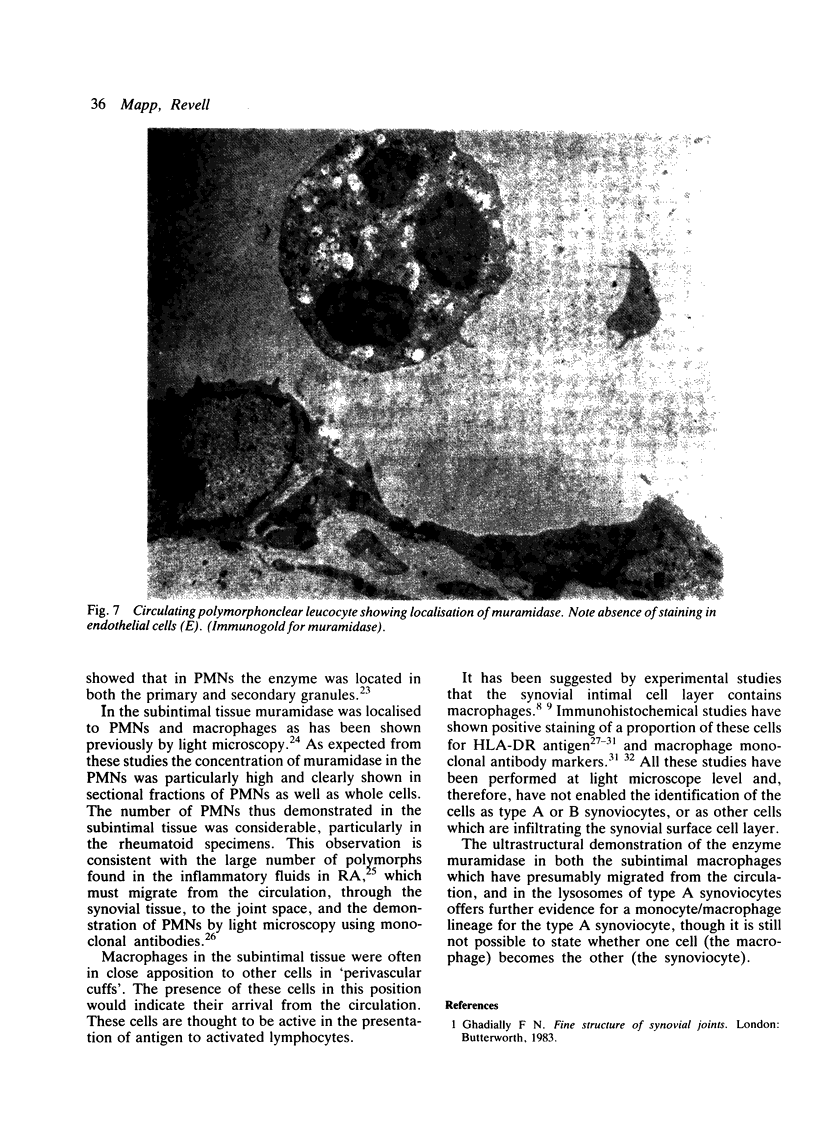
The synovial intimal cell layer comprises two morphological types of cell, A and B, with an intermediate type also postulated. Type A cells show features in common with other cells of the mononuclear phagocyte system, while type B cells appear similar to fibroblasts and are assumed to have synthetic activity. Muramidase is a marker of mononuclear phagocytic cells, and we have investigated the synovial membrane for the presence of this enzyme in cells by an immunogold labelling technique. Muramidase was localised within intracytoplasmic vacuoles in subintimal macrophages and type A synoviocytes. This finding provides further evidence that type A cells are closely related to macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLAND P., NOVIKOFF A. B., HAMERMAN D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962 Aug;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. S., Perillie P. E., Finch S. C. Lysozyme in bone marrow and peripheral blood cells. J Histochem Cytochem. 1966 Feb;14(2):167–170. doi: 10.1177/14.2.167. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Dreher R. Origin of synovial type A cells during inflammation. An experimental approach. Immunobiology. 1982 Apr;161(3-4):232–245. doi: 10.1016/S0171-2985(82)80079-X. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Willoughby D. A. Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann Rheum Dis. 1982 Apr;41(2):177–182. doi: 10.1136/ard.41.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Taylor T. D. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem. 1974 Jun;22(6):401–413. doi: 10.1177/22.6.401. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Glauert A. M., Barratt M. E., Green R. The pig synovium. I. The intact synovium in vivo and in organ culture. J Anat. 1976 Dec;122(Pt 3):663–680. [PMC free article] [PubMed] [Google Scholar]
- Fritz P., Müller J., Braun U., Laschner W., Saal J. G., Rautenstrauch H., Reiser H. Distribution of lysozyme in synovial tissue of patients with osteoarthritis and rheumatoid arthritis demonstrated by different enzyme histochemical methods. Rheumatol Int. 1982;2(1):41–47. doi: 10.1007/BF00541270. [DOI] [PubMed] [Google Scholar]
- Førre O., Thoen J., Lea T., Dobloug J. H., Mellbye O. J., Natvig J. B., Pahle J., Solheim B. G. In situ characterization of mononuclear cells in rheumatoid tissues, using monoclonal antibodies. No reduction of T8-positive cells or augmentation in T4-positive cells. Scand J Immunol. 1982 Oct;16(4):315–319. doi: 10.1111/j.1365-3083.1982.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Geiler G., Riedel U. Detection by the PAP technique of lysozyme-containing synoviocytes and their quantity in rheumatoid arthritis and osteoarthrosis. Histochem J. 1985 May;17(5):562–563. doi: 10.1007/BF01003191. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N. Overview article: the articular territory of the reticuloendothelial system. Ultrastruct Pathol. 1980 Apr-Jun;1(2):249–264. doi: 10.3109/01913128009141422. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella T. D., Baum J., Ziff M. Studies of isolated synovial living cells of rheumatoid and nonrheumatoid synovial membranes. Arthritis Rheum. 1970 Nov-Dec;13(6):734–753. doi: 10.1002/art.1780130603. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Klein W., Rosenbauer K. A., Rupprecht L., Krämer J., Huth F. Beitrag zur Kenntnis der juvenilen monartikulären rheumatoiden Arthritis. Virchows Arch A Pathol Pathol Anat. 1972;357(4):359–368. [PubMed] [Google Scholar]
- Klockars M., Pettersson T. Lysozyme concentrations in synovial fluid, pleural fluid and thoracic duct lymph in rheumatoid arthritis. Scand J Rheumatol. 1985;14(1):69–75. doi: 10.3109/03009748509102021. [DOI] [PubMed] [Google Scholar]
- Klockars M., Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem. 1975 Dec;23(12):932–940. doi: 10.1177/23.12.1104708. [DOI] [PubMed] [Google Scholar]
- Malmquist J., Thorell J. I., Wolheim F. A. Lactoferrin and lysozyme in arthritic exudates. Acta Med Scand. 1977;202(4):313–318. doi: 10.1111/j.0954-6820.1977.tb16834.x. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975 Feb;28(2):124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- PETIT J. F., JOLLES P. PURIFICATION AND ANALYSIS OF HUMAN SALIVA LYSOZYME. Nature. 1963 Oct 12;200:168–169. doi: 10.1038/200168a0. [DOI] [PubMed] [Google Scholar]
- Palmer D. G., Selvendran Y., Allen C., Revell P. A., Hogg N. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985 Mar;59(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Reitamo S. Lysozyme antigenicity and tissue fixation. Histochemistry. 1978 Apr 4;55(3):197–207. doi: 10.1007/BF00495759. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Wynne-Roberts C. R., Anderson C. Light- and electron-microscopic studies of normal juvenile human synovium. Semin Arthritis Rheum. 1978 May;7(4):279–286. doi: 10.1016/0049-0172(78)90026-4. [DOI] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]