Abstract
With the fast development of synthetic chemistry, the introduction of functional group into organic molecules has attracted increasing attention. In these reactions, the difunctionalization of unsaturated bonds, traditionally with one nucleophile and one electrophile, is a powerful strategy for the chemical synthesis. In this work, we develop a different path of electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles. Under metal-free and external oxidant-free conditions, a series of structurally diverse heteroatom-containing compounds hardly synthesized by traditional methods (such as high-value alkoxy-substituted phenylthioacetates, α-thio, α-amino acid derivatives as well as α-amino, β-amino acid derivatives) are obtained in synthetically useful yields. In addition, the procedure exhibits mild reaction conditions, excellent functional-group tolerance and good efficiency on large-scale synthesis. Importantly, the protocol is also amenable to the key intermediate of bioactive molecules in a simple and practical process.
Subject terms: Synthetic chemistry methodology, Reaction mechanisms, Catalyst synthesis, Electrocatalysis, Green chemistry
The difunctionalization of diazo compounds, traditionally with one nucleophile and one electrophile, is a powerful strategy. Here, the authors develop a path of electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles.
Introduction
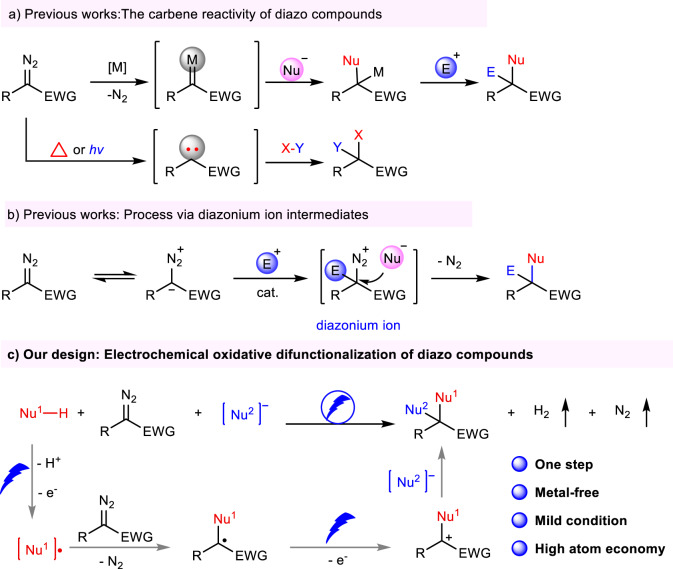
Diazo compounds are a class of charge-neutral organic compounds containing diazo groups bound to carbon atoms that can resonate in different structures1–3. In this regard, chemists have been devoting continuous efforts to pursuing the versatile transformations of diazo compounds for the synthesis of a variety of high-value molecules, such as pharmaceuticals and natural products4–8. Among them, the difunctionalization of diazo compounds has emerged as an important tool for the formation of challenging carbon-carbon and carbon-heteroatom bonds with the introduction of multiple functional groups9–17. Over the last decades, transition-metal (such as Rh, Cu, Fe, Pd, etc) catalyzed difunctionalization of diazo compounds with nucleophiles and electrophiles has undergone a flourishing development because of the unique reaction properties of metal carbene (Fig. 1a)18–27. In addition, thermolysis or photolysis induced carbene transfer reactions provide alternative routes for the difunctionalization of diazo compounds28–32. On the other hand, due to the electron-rich nature of the resonance structures of the diazonium compounds, the diazonium compounds can react with electrophiles through a carbene-free mechanism. And then the diazonium ion intermediate is captured by nucleophile in an intermolecular or intramolecular manner to construct target molecules (Fig. 1b)33–37. Despite these excellent developments, a more attractive, a green and atom-economical approach to realize the difunctionalization of diazo compounds with readily available starting materials is still highly anticipated and remains a relevant challenge. As shown in Scheme 1c, if two different, but readily available nucleophiles are employed in the difunctionalization of diazo compounds under mild conditions, it will present an interesting and different avenue to discovery and build organic molecules38–40.
Fig. 1. Reaction models of diazo compounds.
a The carbene reactivity of diazo compounds. b Process via diazonium ion intermediates. c Our design: Electrochemical oxidative difunctionalization of diazo compounds.
In recent years, the electrochemistry-induced reactions have drawn much attention due to the high reaction efficiency, innate scalability and sustainability of the electrolytic process41–47. The past decade has witnessed the rapid development of difunctionalization of unsaturated bonds in organic synthesis48–54. Despite significant success in the area, most of these transformations are limited to the functionalization of two carbon atoms of alkenes and alkynes because of the intrinsic reaction nature55–61. In order to expand the application of other unsaturated bonds, we developed the electrochemical oxidative carbon-atom difunctionalization of isocyanides for the construction of multisubstituted imino sulfide ethers, isothioureas as well as α-amino amides62–64. Based on our previous efforts in electro-chemistry, we envision that tandem single-electron oxidation may realize the difunctionalization of diazo compounds with two different nucleophiles via electrochemical approach: a radical generated from single-electron oxidation of a nucleophile on anode could be captured by diazo compound to form a carbon radical intermediate. The fast second single-electron oxidation of the intermediate suppressed the decomposition of the radical, and further was attacked by another nucleophile to generate the desired difunctionalization molecules (Fig. 1c).
Herein, we develop a different path of electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles. A series of structurally diverse heteroatom-containing compounds hardly synthesized by traditional methods (such as high-value alkoxy-substituted phenylthioacetates, α-thio, α-amino acid derivatives as well as α-amino, β-amino acid derivatives) are obtained in synthetically useful yields.
Results and discussion
Condition optimization for electrochemical oxidative difunctionalization of diazo compounds
We started to investigate the electrochemical oxidative difunctionalization of ethyl diazoacetate (2) with 4-fluorothiophenol (1) as the radical source and methanol (3) as the nucleophile. Although there are several challenges in this transformation: (1) competitive difunctionalization with same nucleophiles; (2) competitive monofunctionalization instead of difunctionalization owing to properties of intermediate, to our delight, utilizing carbon rod as anode, and platinum plate as cathode under 10 mA constant current in undivided cell, the desired ethyl 2-((4-fluorophenyl)thio)−2-methoxyacetate (4) was obtained selectively with 70% isolated yield (Table 1, entry 1). No better reaction efficiency was observed in this electrolysis by using other solvent system, such as CH3CN/DME (4:2) and CH3CN/DCE (4:2) (Table 1, entries 2 and 3). The use of carbon rod as cathode or graphite felt as anode resulted in a decrease in yield (Table 1, entries 4 and 5). The addition of NaOAc or HOAc to the reaction showed a negative effect on product formation (Table 1, entries 6 and 7). The increase or decrease of the reaction current slightly reduced the product yield (Table 1, entries 8 and 9). While a lower ethyl diazoacetate of 1.5 mmol or a lower amount of methanol showed a decrease in the amount of the target compound (Table 1, entries 10 and 11). Further research showed that the reaction was feasible at ambient temperature (Table 1, entry 12). The reaction proceeds smoothly in an air atmosphere, which highlights the utility of this protocol (Table 1, entry 13). The electricity was critical for the transformation (Table 1, entry 14).
Table 1.
Optimization of the reaction conditions
| Entry | Variation from standard conditions | Yield/%a |
|---|---|---|
| 1 | none | 75 (70)b |
| 2 | CH3CN/DME (4/2, 6 mL) | 70 |
| 3 | CH3CN/DCE (4/2, 6 mL) | 62 |
| 4 | C ( + ) | C (−) instead of C ( + ) | Pt (−) | 55 |
| 5 | CF ( + ) | Pt (−) instead of C ( + ) | Pt (−) | 51 |
| 6 | NaOAc (0.5 mmol) | 61 |
| 7 | HOAc (0.5 mmol) | 52 |
| 8 | 20 mA, 2.5 h | 63 |
| 9 | 5 mA, 10 h | 54 |
| 10 | 1.5 mmol 2 | 65 |
| 11 | 0.1 mL 3 | 61 |
| 12 | rt | 57 |
| 13 | under air | 60 |
| 14 | no electric current | 0 |
Standard conditions: 1 (0.5 mmol), 2 (2.0 mmol), 3 (0.5 mL), Et4NBF4 (0.5 mmol), CH3CN (6.0 mL) in an undivided cell with carbon rod as anode, platinum as cathode (1.5 × 1.5 cm2), constant current = 10 mA, 50 °C, N2, 5 h.
a19F NMR yield, 3-fluorotoluene as an internal standard.
bYield of isolated product.
Scope of substrates
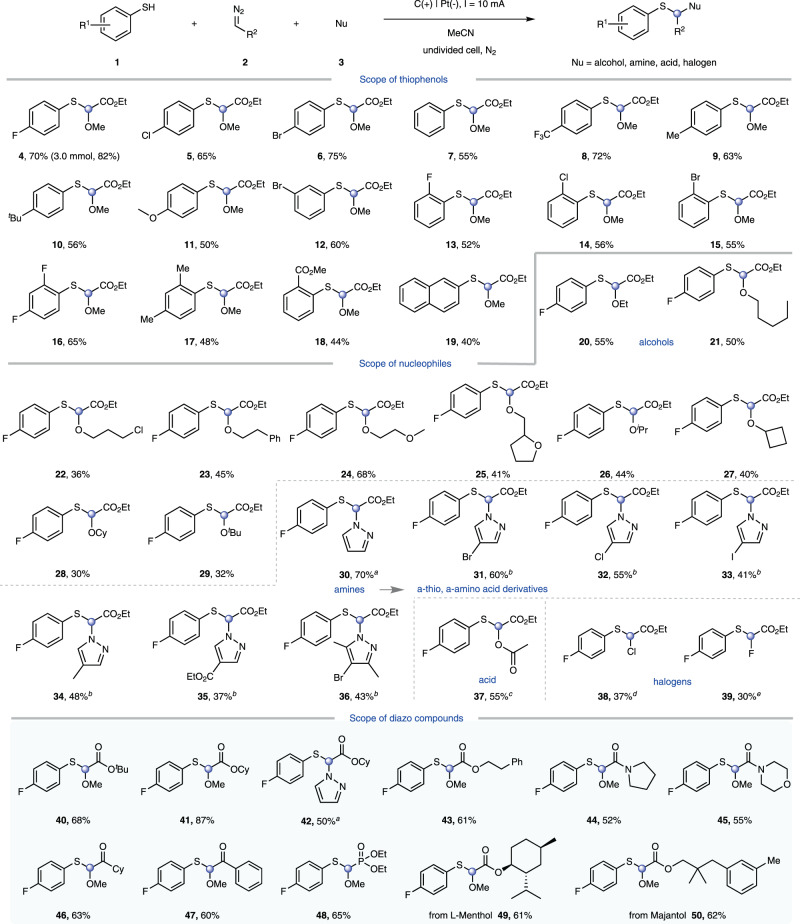
With the confirmation of optimized conditions, the scope of thiophenol was explored under standard conditions (Fig. 2). Firstly, the electronic effect of aryl-substitution was investigated with various para-substituted thiophenols. It was found that halogenated thiophenols (such as aryl fluoride 4, aryl chloride 5 and aryl bromide 6) were tolerated, which could induce subsequent catalyst conversion of the products under the corresponding conditions. Gratifyingly, product 4 was obtained with a higher yield (82%) in the scale-up of this electrosynthesis. The unsubstituted thiophenol also readily adopted in this protocol (7). Moreover, thiophenols with electron-withdrawing groups (CF3, CO2Me) or electron-donating groups (Me, tBu, and OMe) were able to afford the corresponding products in satisfactory yields (8–11, 18). This reaction could proceed effectively with thiophenols containing meta-group and sterically impeding ortho- halogen/methyl/ester functionality, which delivered a variety of target molecules in moderate yields (12–18). Naphthalene-2-thiol was also compatible with this transformation (19). With respect to nucleophile coupling partner, we selected 4-fluorobenzenethiol 1 to interrogate the performance of a range of alcohols, azoles and acids under the electrochemical conditions. The catalytic efficiency of the reaction showed little effect on the chain length of primary alcohols (20–21). 3-chloropropan-1-ol was tolerated to deliver the desired product albeit in a modest yield (22). The reaction was performed with other fatty alcohols, such as phenethyl alcohol (23), 2-methoxyethanol (24) and tetrahydrofurfuryl alcohol (25). Secondary alcohols and tertiary alcohol could be used successfully in the electrolysis, representing a practical route to construct C-S and C-O bonds build on the same carbon atom (26–29). Importantly, a series of α-thio, α-amino acid derivatives were obtained successfully with amine substrates obtaining various groups, such as halogen, methyl and ester, showcasing the versatility of this reaction platform (30–36). Besides alcohols and azoles, acetic acid was also a suitable nucleophile for this protocol (37). Finally, fluorinated or chlorinated products could be accessed by utilizing lithium chloride (LiCl) or triethylamine trihydrofluoride (Et3N·3HF) as halogen sources (38–39).
Fig. 2. Scope of thiophenols, nucleophiles and diazo compounds.
Standard conditions: thiolating agents (0.5 mmol), diazo compounds (2.0 mmol), alcohols (0.5 mL), Et4NBF4 (0.5 mmol), MeCN (6 mL), carbon rod as anode, platinum as cathode, undivided cell, constant current = 10 mA, 50 oC, N2, 5 h, yield is that of the isolated product. aUsed pyrazole (2.0 mmol) instead of alcohol. bUsed pyrazole (2.0 mmol) instead of alcohol, constant current = 10 mA, rt, 7 h.cUsed HOAc (0.5 mL) instead of alcohol. dUsed LiCl (2.0 mmol), H2O (50 μL) instead of alcohol, constant current = 10 mA, 50 oC, 7 h. eUsed Et3N·3HF (0.5 mL) instead of alcohol, constant current = 10 mA, 50 oC, 7 h.
We next investigated the generality of diazo compounds under the established conditions. tert-Butyl 2-diazoacetate, cyclohexyl 2-diazoacetate and phenethyl 2-diazoacetate could be smoothly converted into the corresponding difunctionalization products in moderate to good yields (40–43). Besides diazoacetates, other diazo compounds were used in the transformation. To our delight, diazoamide, diazoalkylketone, diazoarylketone and diazophosphonate were also coupling partners, which could react smoothly with thiophenol and methanol, providing a series of functionalized products (44–48). Pleasingly, segments of natural products (such as menthol and majantol) were amenable to this protocol, which further highlighted the potential value of this methodology (49–50).
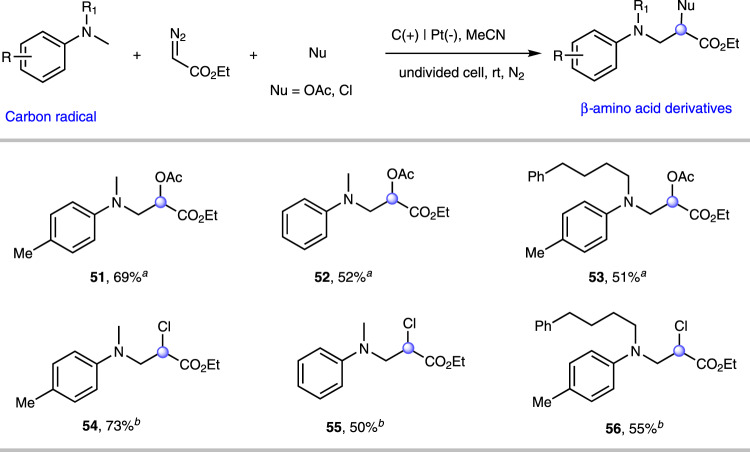
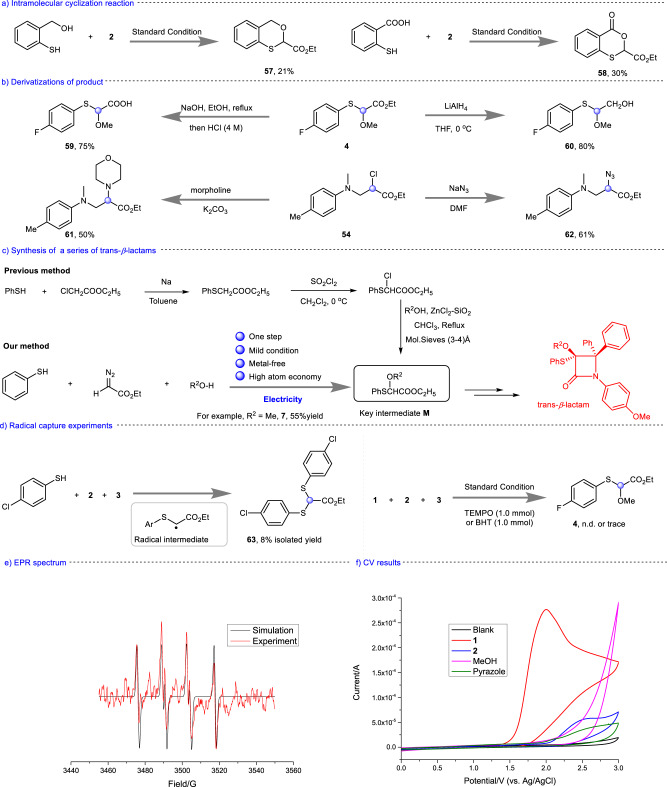
To further expand the scope of the difunctionalization of diazo, we envisaged the use of carbon radicals instead of above sulfur radicals (Fig. 3). Unexpectedly, we found that N, N-dimethylaniline was compatible with the difunctionalization of diazo. After a series of optimization of conditions, the target product ethyl−2-acetoxy-3-(methyl(p-tolyl)amino)propanoate (51) was obtained in 69% isolated yield by using carbon rod as an anode, platinum plate as a cathode at room temperature and a constant current of 10 mA in MeCN system. The similar β-amino acid derivatives (52–53) were isolated via electrochemical oxidative C(sp3)-H activation. Using LiCl as a nucleophile, this transformation could proceed smoothly to construct C-C and C-Cl bonds build on the same carbon atom simultaneously (54–56). hydride in tetrahydrofuran. The carboxy and oxhydryl provide a functional group handle for further derivatization. In addition, the active chlorine moiety was then subjected to transformations of other high-value functional groups. For example, α-chloro, β-amino acid ester was treated with morpholine to obtain α-amino, β-amino acid derivative in the presence of K2CO3. When 54 was then treated with NaN3, the expected α-azide, β-amino acid derivative was obtained in 61% yield. Importantly, β-lactam compounds are widely used in antibiotics, inhibitors, and drug structures. And alkoxy-substituted phenylthioacetates are important precursors (M) for their synthesis. It had been reported in relevant literature that the preparation of alkoxy substituted phenylthioacetate (M) required the use of hazardous and sensitive SO2Cl2 and metal sodium as well as multi-step synthesis. Herein, we have developed a green, fast, scalable, and metal-free method to synthesize the key intermediate M with high atom efficiency and the only release of hydrogen and nitrogen (Fig. 4c).
Fig. 3. Scope of substrates of carbon radicals.
Conditions: aaniline (0.5 mmol), ethyl diazoacetate (0.3 mmol), HOAc (0.5 mL), nBu4NBF4 (1.0 mmol), MeCN (6 mL), carbon rod as anode, platinum as cathode, undivided cell, constant current = 10 mA, rt, N2, 2 h; isolated yield. baniline (0.7 mmol), ethyl diazoacetate (0.3 mmol), LiCl (0.3 mmol), H2O (50 μL), HOAc (0.5 mL), nBu4NBF4 (1.0 mmol), MeCN (6 mL), carbon rod as anode, platinum as cathode, undivided cell, constant current = 10 mA, rt, N2, 2.25 h.
Fig. 4. The application of the method and the mechanistic experiments.
a Intramolecular cyclization reaction. b Derivatizations of product. c Synthesis of a series of trans-β-lactams. d Radical capture experiments. e EPR spectrum. f CV results.
Synthetic applications
We have performed the intramolecular cyclization reaction. Using 2-mercaptobenzyl alcohol as a substrate, the target product (57) was isolated with 21% yield. Using 2-mercaptobenzoic acid as a substrate, the target product (58) was isolated with 30% yield (Fig. 4a). The versatility of alkoxy-substituted phenylthioacetate was further explored (Fig. 4b). For instance, the carboxylation process of 4 led to the formation of 2-((4-fluorophenyl)thio)−2-methoxyacetic acid (59) in 75% yield by using NaOH as base. 4 could also be reduced to 2-((4-fluorophenyl)thio)-2-methoxyethan-1-ol (60) with lithium aluminum
Mechanistic studies
To more deeply study the mechanism, we carried out a series of control experiments. Under the standard conditions, ethyl 2,2-bis((4-chlorophenyl)thio)acetate 63 was isolated in 8% yield, which indicated the radical intermediate was involved in the transformation (Fig. 4d). The target product 4 was undetected when 2.0 equivalent of TEMPO was added to the reaction system. Adding 2.0 equivalent BHT into the reaction resulted in trace amounts of product 4. These experiments indicated the electrolysis may undergo a radical process (Scheme 4d). In addition, we also carried out electron paramagnetic resonance (EPR) experiments (Fig. 4e). A S-centered radical was rapidly trapped by 5,5-dimethyl-1-pyrroline N-oxide (DMPO) to form a relatively stable radical (g = 2.0070, AN = 13.40 G, AH = 14.88 G). The cyclic voltammetry (CV) experiments were carried out to further understand the details of the oxidation process (Fig. 4f). The results showed that the 4-fluorothiophenol (1) has an oxidation potential of 1.98 V vs. Ag/AgCl, while the oxidation potential of ethyl diazoacetate (2) for Ag/AgCl was over 2.60 V. These results indicated that 4-fluorothiophenol was preferentially oxidized under optimized conditions.
Based on the above mechanism studies and related literature reports, we proposed a possible mechanism for the electrochemical oxidative difunctionalization of diazo, as shown in Fig. 5. Firstly, thiophenol lost a proton to product sulfur anion and further was oxidized by a single-electron-transfer to obtain a sulfur radical at the anode. The sulfur radical could be captured by diazo compound to generate a carbon radical I with the release of nitrogen. Subsequently, radical I underwent rapid single-electron oxidation to generate cation II. Finally, the intermediate II was subjected to intermolecular nucleophilic attack by the nucleophile to form the desired product. The proton was reduced on the cathode to release hydrogen gas.
Fig. 5. Proposed reaction mechanism.
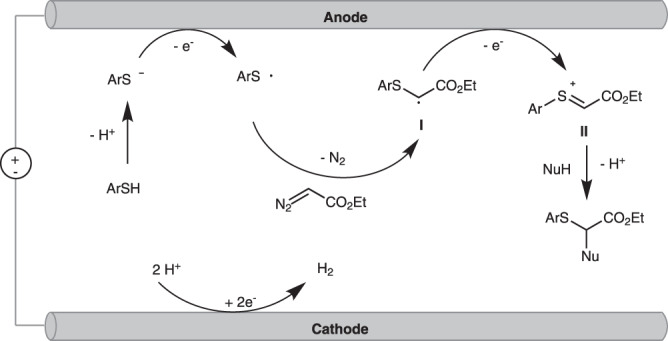
Electrochemical oxidative difunctionalization of diazo.
In conclusion, an interesting electrochemical oxidative difunctionalization of diazo compounds has been developed. Cheap and commercially available radical sources (such as S and C) and nucleophiles (such as alcohol, amine, acid and halogen anion) could be employed to perform the reaction. This simple procedure avoids the use of transition metals and external oxidants, the pre-activation of substrates (such as electrophiles) as well as harsh conditions. Excellent functional group compatibility, good large-scale synthesis efficiency and diverse synthetic application further highlight the strengths of this transformation.
Methods
In an oven-dried undivided three-necked bottle (10 mL) equipped with a stir bar, Et4NBF4 (0.5 mmol) was added. The bottle was equipped with carbon rod as the anode and platinum plate (1.5 cm × 1.5 cm × 1 mm) as the cathode. Under nitrogen atmosphere, thiophenol (0.5 mmol), diazo compounds (2.0 mmol), alcohols (0.5 mL) and MeCN (6.0 mL) were injected respectively into the tube via syringes. The reaction mixture was stirred and electrolyzed at a constant current of 10 mA for 5 h at 50 °C. The reaction mixture was washed with water and extracted with EA (10 mL × 3). The organic layers were combined, dried over Na2SO4 and concentrated in vacuum. The pure product was purified by silica gel flash column chromatography.
Supplementary information
Acknowledgements
This work was supported by the National Key R&D Programof China (No. 2022YFA1505100 and 2021YFA1500104), a project fundedby the China Postdoctoral Science Foundation (2021M702516), the National Natural Science Foundationof China (22031008), Guangdong Basic and Applied Basic Research Foundation (2023A1515012260) and the Science Foundation of Wuhan (2020010601012192), the Fundamental Research Funds for the Central Universities (2042022rc0030).
Author contributions
D.Y. and Z.G. conceived the work. D.Y., Z.G., P.W., H.Y., and A.L., designed the experiments and analyzed the data. D.Y., Y.P., and S.Z. performed the experiments. Z.G., D.Y., Z.H., H.A., D.G., H.Y., and A.L. described the paper.
Peer review
Peer review information
Nature Communications thanks Irene Bosque, Hai-Tao Tang, and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files or from the authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dongfeng Yang, Zhipeng Guan.
Contributor Information
Dong Gu, Email: DGu@whu.edu.cn.
Hong Yi, Email: hong.yi@whu.edu.cn.
Aiwen Lei, Email: aiwenlei@whu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-37032-8.
References
- 1.Ye T, McKervey MA. Organic Synthesis with.alpha.-Diazo Carbonyl Compounds. Chem. Rev. 1994;94:1091–1160. [Google Scholar]
- 2.Qi X, Lan Y. Recent Advances in Theoretical Studies on Transition-Metal-Catalyzed Carbene Transformations. Acc. Chem. Res. 2021;54:2905–2915. doi: 10.1021/acs.accounts.1c00075. [DOI] [PubMed] [Google Scholar]
- 3.Durka J, Turkowska J, Gryko D. Lightening Diazo Compounds? ACS Sustain. Chem. Eng. 2021;9:8895–8918. [Google Scholar]
- 4.Ford A, et al. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015;115:9981–10080. doi: 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Qiu D, Wang J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017;117:13810–13889. doi: 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]
- 6.Dong S, Liu X, Feng X. Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds. Acc. Chem. Res. 2022;55:415–428. doi: 10.1021/acs.accounts.1c00664. [DOI] [PubMed] [Google Scholar]
- 7.Jurberg ID, Davies HML. Blue light-promoted photolysis of aryldiazoacetates. Chem. Sci. 2018;9:5112–5118. doi: 10.1039/c8sc01165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong M, Liang X, Gao Z, Lei A, Pan Y. Synthesis of Isoxazolines and Oxazines by Electrochemical Intermolecular [2 + 1 + n] Annulation: Diazo Compounds Act as Radical Acceptors. Org. Lett. 2019;21:9300–9305. doi: 10.1021/acs.orglett.9b03306. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Q, Zhang Y, Wang J. Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions. Acc. Chem. Res. 2013;46:236–247. doi: 10.1021/ar300101k. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Stivanin ML, Jurberg ID, Koenigs RM. Visible light-promoted reactions with diazo compounds: a mild and practical strategy towards free carbene intermediates. Chem. Soc. Rev. 2020;49:6833–6847. doi: 10.1039/d0cs00224k. [DOI] [PubMed] [Google Scholar]
- 11.He Y, et al. Recent advances in transition-metal-catalyzed carbene insertion to C–H bonds. Chem. Soc. Rev. 2022;51:2759–2852. doi: 10.1039/d1cs00895a. [DOI] [PubMed] [Google Scholar]
- 12.Son S, Fu GC. Copper-Catalyzed Asymmetric [4+1] Cycloadditions of Enones with Diazo Compounds To Form Dihydrofurans. J. Am. Chem. Soc. 2007;129:1046–1047. doi: 10.1021/ja068344y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, et al. Redox-Neutral Rhodium-Catalyzed C-H Functionalization of Arylamine N-Oxides with Diazo Compounds: Primary C(sp3)-H/C(sp2)-H Activation and Oxygen-Atom Transfer. Angew. Chem. Int. Ed. 2015;54:12121–12126. doi: 10.1002/anie.201505302. [DOI] [PubMed] [Google Scholar]
- 14.Conde A, et al. Iron and Manganese Catalysts for the Selective Functionalization of Arene C(sp2)−H Bonds by Carbene Insertion. Angew. Chem. Int. Ed. 2016;55:6530–6534. doi: 10.1002/anie.201601750. [DOI] [PubMed] [Google Scholar]
- 15.Solé D, Mariani F, Bennasar ML, Fernández I. Palladium-Catalyzed Intramolecular Carbene Insertion into C(sp3)−H Bonds. Angew. Chem. Int. Ed. 2016;55:6467–6470. doi: 10.1002/anie.201602020. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, et al. Dirhodium(II)/Xantphos-Catalyzed Relay Carbene Insertion and Allylic Alkylation Process: Reaction Development and Mechanistic Insights. J. Am. Chem. Soc. 2021;143:11799–11810. doi: 10.1021/jacs.1c05701. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Zhang Y, Wang J. Catalytic Cascade Reactions Involving Metal Carbene Migratory Insertion. ACS Catal. 2013;3:2586–2598. [Google Scholar]
- 18.Huang H, Guo X, Hu W. Efficient Trapping of Oxonium Ylides with Imines: A Highly Diastereoselective Three-Component Reaction for the Synthesis of β-Amino-α-hydroxyesters with Quaternary Stereocenters. Angew. Chem. Int. Ed. 2007;46:1337–1339. doi: 10.1002/anie.200604389. [DOI] [PubMed] [Google Scholar]
- 19.Hu W, et al. Cooperative Catalysis with Chiral Brønsted Acid-Rh2(OAc)4: Highly Enantioselective Three-Component Reactions of Diazo Compounds with Alcohols and Imines. J. Am. Chem. Soc. 2008;130:7782–7783. doi: 10.1021/ja801755z. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D, Zhou J, Xia F, Kang Z, Hu W. Bond cleavage, fragment modification and reassembly in enantioselective three-component reactions. Nat. Commun. 2015;6:5801. doi: 10.1038/ncomms6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou C-Y, et al. Dirhodium Carboxylates Catalyzed Enantioselective Coupling Reactions of α-Diazophosphonates, Anilines, and Electron-Deficient Aldehydes. Angew. Chem. Int. Ed. 2012;51:11376–11380. doi: 10.1002/anie.201206551. [DOI] [PubMed] [Google Scholar]
- 22.Chen D-F, Zhao F, Hu Y, Gong L-Z. C−H Functionalization/Asymmetric Michael Addition Cascade Enabled by Relay Catalysis: Metal Carbenoid Used for C−C Bond Formation. Angew. Chem. Int. Ed. 2014;53:10763–10767. doi: 10.1002/anie.201406098. [DOI] [PubMed] [Google Scholar]
- 23.Yuan W, Szabo KJ. Rhodium-Catalyzed Oxy-Aminofluorination of Diazoketones with Tetrahydrofurans and N-Fluorobenzenesulfonimide. ACS Catal. 2016;6:6687–6691. [Google Scholar]
- 24.Yuan W, Eriksson L, Szabo KJ. Rhodium-Catalyzed Geminal Oxyfluorination and Oxytrifluoro-Methylation of Diazocarbonyl Compounds. Angew. Chem. Int. Ed. 2016;55:8410–8415. doi: 10.1002/anie.201602137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hari DP, Waser J. Copper-Catalyzed Oxy-Alkynylation of Diazo Compounds with Hypervalent Iodine Reagents. J. Am. Chem. Soc. 2016;138:2190–2193. doi: 10.1021/jacs.6b00278. [DOI] [PubMed] [Google Scholar]
- 26.Hari D, P. Waser J. Enantioselective Copper-Catalyzed Oxy-Alkynylation of Diazo Compounds. J. Am. Chem. Soc. 2017;139:8420–8423. doi: 10.1021/jacs.7b04756. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, et al. Catalytic asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of sulfonium ylides. Nat. Chem. 2017;9:970–976. doi: 10.1038/nchem.2789. [DOI] [PubMed] [Google Scholar]
- 28.Ovalles SR, Hansen JH, Davies HML. Thermally Induced Cycloadditions of Donor/Acceptor Carbenes. Org. Lett. 2011;13:4284–4287. doi: 10.1021/ol201628d. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, et al. Catalyst-Free S–S Bond Insertion Reaction of a Donor/Acceptor-Free Carbene by a Radical Process: A Combined Experimental and Computational Study. Eur. J. Org. Chem. 2016;22:3872–3877. [Google Scholar]
- 30.Zhang X, Zheng Y, Qiu L, Xu X. Thermally induced N–S bond insertion reaction of diazo compounds with N-sulfenylsuccinimides: synthesis of sulfides and mechanism studies. Org. Biomol. Chem. 2018;16:70–76. doi: 10.1039/c7ob02488f. [DOI] [PubMed] [Google Scholar]
- 31.Jana S, Pei C, Empel C, Koenigs RM. Photochemical Carbene Transfer Reactions of Aryl/Aryl Diazoalkanes—Experiment and Theory. Angew. Chem. Int. Ed. 2021;60:13271–13279. doi: 10.1002/anie.202100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hommelsheim R, Guo Y, Yang Z, Empel C, Koenigs RM. Blue-Light-Induced Carbene-Transfer Reactions of Diazoalkanes. Angew. Chem. Int. Ed. 2019;58:1203–1207. doi: 10.1002/anie.201811991. [DOI] [PubMed] [Google Scholar]
- 33.Döben N, Yan H, Kischkewitz M, Mao J, Studer A. Intermolecular Acetoxyaminoalkylation of α-Diazo Amides with (Diacetoxyiodo) benzene and Amines. Org. Lett. 2018;20:7933–7936. doi: 10.1021/acs.orglett.8b03504. [DOI] [PubMed] [Google Scholar]
- 34.Wang N-N, et al. Metal-free C(sp3)–H functionalization: oxidative carbo-oxygenation of α-diazo carbonyls via radical dediazotization. Chem. Commun. 2016;52:5144–5147. doi: 10.1039/c6cc00816j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, et al. Catalytic Asymmetric Homologation of α-Ketoesters with α-Diazoesters: Synthesis of Succinate Derivatives with Chiral Quaternary Centers. Angew. Chem. Int. Ed. 2013;52:10883–10886. doi: 10.1002/anie.201305478. [DOI] [PubMed] [Google Scholar]
- 36.Gao L, Kang BC, Ryu DH. Catalytic Asymmetric Insertion of Diazoesters into Aryl-CHO Bonds: Highly Enantioselective Construction of Chiral All-Carbon Quaternary Centers. J. Am. Chem. Soc. 2013;135:14556–14559. doi: 10.1021/ja408196g. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Zhang Y, Wang J. Geminal difunctionalization of α-diazo arylmethylphosphonates: synthesis of fluorinated phosphonates. Org. Biomol. Chem. 2016;14:10444–10453. doi: 10.1039/c6ob01858k. [DOI] [PubMed] [Google Scholar]
- 38.Lu L, Li H, Lei A. Oxidative Cross-Coupling Reactions between Two Nucleophiles. Chin. J. Chem. 2022;40:256–266. [Google Scholar]
- 39.Yi H, et al. Recent Advances in Radical C–H Activation/Radical Cross-Coupling. Chem. Rev. 2017;117:9016–9085. doi: 10.1021/acs.chemrev.6b00620. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, et al. Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem. Rev. 2015;115:12138–12204. doi: 10.1021/cr500431s. [DOI] [PubMed] [Google Scholar]
- 41.Jiao K-J, Xing Y-K, Yang Q-L, Qiu H, Mei T-S. Site-Selective C–H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis. Acc. Chem. Res. 2020;53:300–310. doi: 10.1021/acs.accounts.9b00603. [DOI] [PubMed] [Google Scholar]
- 42.Novaes LFT, et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021;50:7941–8002. doi: 10.1039/d1cs00223f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Y, Xu K, Zeng C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018;118:4485–4540. doi: 10.1021/acs.chemrev.7b00271. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Y, Lei A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Reactions. Acc. Chem. Res. 2019;52:3309–3324. doi: 10.1021/acs.accounts.9b00512. [DOI] [PubMed] [Google Scholar]
- 45.Xiong P, Xu H-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019;52:3339–3350. doi: 10.1021/acs.accounts.9b00472. [DOI] [PubMed] [Google Scholar]
- 46.Moeller KD. Using Physical Organic Chemistry To Shape the Course of Electrochemical Reactions. Chem. Rev. 2018;118:4817–4833. doi: 10.1021/acs.chemrev.7b00656. [DOI] [PubMed] [Google Scholar]
- 47.Yan M, Kawamata Y, Baran PS. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017;117:13230–13319. doi: 10.1021/acs.chemrev.7b00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siu JC, Fu N, Lin S. Catalyzing electrosynthesis: a homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 2020;53:547–560. doi: 10.1021/acs.accounts.9b00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Cui T, Zhao X, Liu P, Sun P. Electrochemical Difunctionalization of Alkenes by a Four-Component Reaction Cascade Mumm Rearrangement: Rapid Access to Functionalized Imides. Angew. Chem. Int. Ed. 2020;59:3465–3469. doi: 10.1002/anie.201913332. [DOI] [PubMed] [Google Scholar]
- 50.Qin J-H, Luo M-J, An D-L, Li J-H. Electrochemical 1,2-Diarylation of Alkenes Enabled by Direct Dual C–H Functionalizations of Electron-Rich Aromatic Hydrocarbons. Angew. Chem. Int. Ed. 2021;60:1861–1868. doi: 10.1002/anie.202011657. [DOI] [PubMed] [Google Scholar]
- 51.Ke J, Liu W, Zhu X, Tan X, He C. Electrochemical Radical Silyl-Oxygenation of Activated Alkenes. Angew. Chem. Int Ed. 2021;60:8744–8749. doi: 10.1002/anie.202016620. [DOI] [PubMed] [Google Scholar]
- 52.Huang H, Lambert TH. Electrophotocatalytic Acetoxyhydroxylation of Aryl Olefins. J. Am. Chem. Soc. 2021;143:7247–7252. doi: 10.1021/jacs.1c01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong X, Roeckl JL, Waldvogel SR, Morandi B. Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science. 2021;371:507–514. doi: 10.1126/science.abf2974. [DOI] [PubMed] [Google Scholar]
- 54.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 55.Cai C-Y, Zheng Y-T, Li J-F, Xu H-C. Cu-Electrocatalytic Diazidation of Alkenes at ppm Catalyst Loading. J. Am. Chem. Soc. 2022;144:11980–11985. doi: 10.1021/jacs.2c05126. [DOI] [PubMed] [Google Scholar]
- 56.Fu N, Sauer GS, Lin S. Electrocatalytic Radical Dichlorination of Alkenes with Nucleophilic Chlorine Sources. J. Am. Chem. Soc. 2017;139:15548–15553. doi: 10.1021/jacs.7b09388. [DOI] [PubMed] [Google Scholar]
- 57.Lu L, Siu JC, Lai Y, Lin S. An Electroreductive Approach to Radical Silylation via the Activation of Strong Si–Cl Bond. J. Am. Chem. Soc. 2020;142:21272–21278. doi: 10.1021/jacs.0c10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siu JC, Parry JB, Lin S. Aminoxyl-Catalyzed Electrochemical Diazidation of Alkenes Mediated by a Metastable Charge-Transfer Complex. J. Am. Chem. Soc. 2019;141:2825–2831. doi: 10.1021/jacs.8b13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siu JC, et al. Electrochemical Azidooxygenation of Alkenes Mediated by a TEMPO–N3 Charge-Transfer Complex. J. Am. Chem. Soc. 2018;140:12511–12520. doi: 10.1021/jacs.8b06744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye K-Y, et al. Anodically Coupled Electrolysis for the Heterodifunctionalization of Alkenes. J. Am. Chem. Soc. 2018;140:2438–2441. doi: 10.1021/jacs.7b13387. [DOI] [PubMed] [Google Scholar]
- 61.Cai C-Y, Xu H-C. Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles. Nat. Commun. 2018;9:3551. doi: 10.1038/s41467-018-06020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guan Z, et al. Electrochemical Oxidative Carbon-Atom Difunctionalization: Towards Multisubstituted Imino Sulfide Ethers. Angew. Chem. Int. Ed. 2021;60:1573–1577. doi: 10.1002/anie.202011329. [DOI] [PubMed] [Google Scholar]
- 63.Guan Z, et al. Electrochemically selective double C(sp2)–X (X = S/Se, N) bond formation of isocyanides. Chem. Sci. 2021;12:14121–14125. doi: 10.1039/d1sc04475c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guan Z, et al. Electrochemical synthesis of α-amino amides via C(sp3)–H bond activation. Green Chem. 2022;24:3964–3968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files or from the authors upon request.