Abstract
Background
The VALID Act is a legislative effort that, if enacted, would alter the regulatory requirements of laboratory developed tests (LDTs) used for clinical testing in the United States. Benzodiazepines, which are primarily excreted into urine as glucuronidated metabolites such as lorazepam, cross-react poorly with FDA-cleared immunoassays, leading to false-negatives. This shortfall can be addressed with LDTs created by adding glucuronidase to the immunoassay reagents producing “high sensitivity” assays that detect glucuronidated metabolites.
Methods
Precision and stability of two high-sensitivity (HS) benzodiazepine immunoassays from Roche and Thermo Scientific were evaluated using manufacturer-supplied quality control (QC) material and glucuronidated QC material. The immunoassays were directly compared to an LC-MS/MS LDT benzodiazepine assay to determine clinical sensitivity/specificity using urine specimens (n = 82 for Thermo Scientific; n = 265 for Roche). The clinical impact of the HS LDT immunoassay was determined by analyzing clinical testing results 60 days before and after its implementation.
Results
The precision and clinical sensitivity/specificity of the HS-Thermo Scientific and HS-Roche benzodiazepine assays were acceptable. The reagent stability of the HS-Thermo Scientific immunoassay was poor, whereas the HS-Roche immunoassay was stable. After implementation of the HS-Roche benzodiazepine immunoassay as an LDT, there was a 30-fold increase (p-value: < 0.00001) in the percentage of lorazepam confirmations.
Conclusions
We demonstrate the development and validation of an immunoassay LDT with improved sensitivity for glucuronidated benzodiazepines. This LDT can detect glucuronidated benzodiazepines in clinical urine specimens and is stable for 60 days. Importantly, we were able to validate the immunoassay as an LDT by utilizing an LC-MS/MS LDT.
Keywords: Laboratory Developed Tests, VALID Act, High Sensitivity, Benzodiazepine, LC-MS/MS, Immunoassay, Beta-glucuronidase
Introduction
Benzodiazepines are among the most frequently prescribed drugs for the treatment of anxiety, insomnia, convulsions, and, in combination with opiates, acute and chronic pain [1], [2]. To ensure compliance, pain management centers perform urine drug testing to verify that patients are only taking their prescribed medication. This class of drug is also subject to misuse and is frequently included in urine drugs of abuse testing panels. Immunoassays are commonly used to screen for benzodiazepines with hyphenated mass spectrometry techniques being used to identify specific drugs and metabolites [3]. Urine concentrations of benzodiazepines and their metabolites can vary by several orders of magnitude depending on a variety of factors including the drug, dose, and time of urine collection, making this analysis challenging [4]. While immunoassays are popular due to their high-throughput and short turn-around-times, they are subject to interferences or poor-cross reactivity resulting in both false positives and false negatives. Confirmatory testing for benzodiazepines by liquid chromatography - tandem mass spectrometry (LC-MS/MS) or gas chromatography - mass spectrometry (GC–MS) helps rule out false positive results; however, certain benzodiazepines, especially those that are excreted as glucuronide metabolites, are subject to false negative screening results [1], [3].
Benzodiazepine immunoassays have been known to have poor cross-reactivity for glucuronidated benzodiazepine metabolites [5]. Lorazepam, oxazepam, and temazepam metabolize directly to their glucuronic acid form and are a source of false negatives for currently available commercial benzodiazepine immunoassays [4], [6], [7]. Lorazepam, which is excreted almost exclusively as a glucuronide, is especially subject to false negative results. In the 1990 s, Beck et al. demonstrated the importance of adding beta-glucuronidase to automated benzodiazepine immunoassays. They added a beta-glucuronidase enzyme to their benzodiazepine immunoassay reagent pack and improved sensitivity for glucuronidated benzodiazepine metabolites [8], [9]. This has prompted other laboratorians to evaluate the modification of commercially available benzodiazepine immunoassays by adding beta-glucuronidase to hydrolyze the glucuronide moiety [10], [11], [12]. In the clinical laboratory, such modification results in the creation of a laboratory developed test (LDT) [13].
In the United States, clinical laboratories are currently regulated by the Centers for Medicare and Medicaid Services (CMS) under the Clinical Laboratory Improvement Amendments (CLIA). The Food and Drug Administration (FDA) defines an LDT as an “in vitro diagnostic test that is manufactured and used within a single laboratory” [14]. CMS accepts this definition, and nearly all LC-MS/MS and GC–MS assays are considered LDTs that must meet all requirements for high complexity testing [15].
Furthermore, CMS considers any modification to an FDA-cleared or -approved assay to be the same as creating a new test, and thus, by definition, an LDT. In late 2018, Congress released the Verifying Accurate Leading-edge In Vitro Clinical Test (IVCT) Development Act (VALID) for public comment [16]. A strict interpretation of the VALID discussion draft would require laboratories to incur significant costs and increased regulatory burden to implement a modified FDA-cleared/approved assay or mass spectrometry-based assay as an LDT [17].
In this study, two benzodiazepine immunoassays that incorporate the addition of beta-glucuronidase to form “highly sensitive (HS)” assays—Thermo Scientific High Sensitivity (HS-Thermo) cloned enzyme donor immunoassay (CEDIA) Benzodiazepines assay and a laboratory-modified version of the FDA-cleared Roche Benzodiazepines Plus assay (HS-Roche)—were evaluated using urine specimens encountered in a routine hospital setting. The HS-Thermo assay is marketed as “high sensitivity” due to its improved sensitivity for glucuronidated benzodiazepine metabolites (e.g., lorazepam-glucuronide). Since it is not FDA-cleared, it must be validated and implemented in the clinical laboratory as an LDT. In addition, we evaluated a second assay (HS-Roche), which is a laboratory modified version of the FDA-cleared Roche Benzodiazepines Plus assay, to which a recombinant room temperature beta-glucuronidase enzyme was added. This modification results in the creation of an LDT that would be subject to the increased operational burden and scrutiny proposed in the VALID Act. These two immunoassay LDTs were validated using an LC-MS/MS benzodiazepine LDT as the reference method. Precision, accuracy, clinical sensitivity, clinical specificity, and stability were assessed for each assay. A retrospective study was conducted to evaluate the clinical impact of implementing these LDTs. This study demonstrates the role that LDTs (both immunoassay and LC-MS/MS) have in healthcare and highlights the importance of preserving innovation essential for the practice of laboratory medicine.
Materials and methods
Specimens
Excess urine specimens from a total of 347 subjects were collected under the University of California, San Diego (UCSD) Institutional Review Board (IRB) protocol 181656, in accordance with the World Medical Association's Code of Ethics (Declaration of Helsinki) for experiments involving human subjects. The UCSD IRB deemed that informed consent was not necessary as this study used existing specimens. These specimens were initially screened for drugs of abuse on Roche automated (6000/8000) analyzers. Specimens returning a positive immunoassay result were reflexed to the clinical toxicology laboratory for confirmatory analysis by LC-MS/MS. Additionally, 82 urine specimens were collected to evaluate the HS-Thermo assay; these specimens were unique and separate from the 265 urine specimens collected to evaluate the HS-Roche assay.
Glucuronidated quality control material
930 ng/mL of lorazepam-glucuronide in human urine was used as a positive quality control (UTAK Laboratories; equivalent to 600 ng/mL free lorazepam).
Kura-B-ONE Beta-glucuronidase
The Kura-B-ONE beta-glucuronidase is an all-in-one hydrolysis room temperature beta-glucuronidase (activity ≥ 12,000 PS-U/mL; Kura Biotech).
Benzodiazepine plus immunoassay
The Roche Benzodiazepines Plus (Catalog #: 04490789190) is an in vitro diagnostic test based on the kinetic interaction of microparticles in solution (KIMS) for the qualitative and semi-quantitative detection of benzodiazepines in human urine on Roche/Hitachi Cobas c systems at a cutoff concentration of 200 ng/mL. For the purpose of this manuscript, we refer to this as the “Roche” assay and it does not contain beta-glucuronidase. Calibration was performed as specified in the manufacturer's package insert. Quality controls (PreciPos and PreciNeg) specified in the manufacturer's package insert were run once every 24 h or prior to patient testing on the day the assay was performed. This FDA-cleared immunoassay does not utilize a glucuronidase hydrolysis step. The positive cutoff for this assay was set at 1000 mabs, corresponding to 200 ng/mL of the nordiazepam calibrator.
High sensitivity benzodiazepine plus immunoassay (HS-Roche)
The High Sensitivity Benzodiazepines Plus assay (referred to as the “HS-Roche” assay) is an LDT version of the Roche Benzodiazepine Plus assay that includes the addition of beta-glucuronidase to hydrolyze glucuronide metabolites. The hydrolysis of the glucuronide converts benzodiazepine glucuronides into the parent drug, improving the detection of these common urine metabolites. Briefly, 1 mL of Kura B-One beta-glucuronidase (≥12,000 PS-U/mL) was added to position B (R1) of the cassette pack (MULTI pack). The volume was empirically derived by comparing the mabs response of free lorazepam to the mabs response of an equimolar concentration of glucuronidated-lorazepam until similar mabs response was observed (data not shown). No other modifications were made to the reagent pack and the assay was calibrated (200 ng/mL nordiazepam) and run as described in the manufacturer's package insert. Positive quality controls (930 ng/mL lorazepam-glucuronide) and negative quality controls (PreciNeg DATIII; 150 ng/mL nordiazepam) were run once every 24 h or prior to patient samples. The positive cutoff for this assay was set at 1000 mabs, corresponding to 200 ng/mL of nordiazepam.
Thermo Scientific CEDIA immunoassay (HS-Thermo)
The Thermo Scientific CEDIA Benzodiazepine Assay (REF: 100094-CJF; ThermoFisher Scientific) is approved for criminal justice and forensic use only and has the option to utilize recombinant beta-glucuronidase to enhance the detection of benzodiazepines that are primarily excreted as glucuronidated metabolites. We refer to this assay as the “HS-Thermo” assay. Preparation of the reagents was performed according to the manufacturer's product insert instructions, and the assay was calibrated (200 ng/mL oxazepam) and run as described in the manufacturer's package insert. Briefly, reagents were added to R1 (Enzyme Acceptor; 18 mL) and R3 (Enzyme Donor; 18 mL) of a Roche open channel MULTI pack cassette. The Enzyme Acceptor reagent included 325 µL of the beta-glucuronidase that was provided with the reagent kit and was added upon reagent reconstitution. The cassette was loaded on the Roche cobas c 502 as an open channel assay. Two levels of quality control material included with the assay kit were run daily: MGC Primary DAU Low Control (150 ng/mL oxazepam) and MGC Primary DAU High Control (250 ng/mL oxazepam). The positive cutoff for this assay was set at 1000 mabs, corresponding to 200 ng/mL of oxazepam.
Confirmatory isotope dilution LC-MRM-MS/MS benzodiazepine panel
Urine specimens were confirmed for 10 benzodiazepines using liquid chromatography and multiple reaction monitoring tandem mass spectrometry (LC-MRM-MS/MS; Supplementary Fig. 1). The panel monitored quantifier, qualifier, and deuterated internal standard transition ions for α-OH alprazolam, 7-aminoclonazepam, desalkylflurazepam, 2-OH ethyl flurazepam, lorazepam, α-OH midazolam, nordiazepam, oxazepam, temazepam, and α-OH triazolam. A positive confirmatory result was defined as ≥ 20 ng/mL. Singly-confirmed specimens are defined as specimens in which only one unique benzodiazepine was detected. Multiply-confirmed specimens are defined as specimens in which two or more benzodiazepines were detected. Quality control material %CVs are shown in Supplementary Table 1. This panel was used for routine drugs of abuse confirmatory analysis at UCSD Health and was validated according to Clinical & Laboratory Standards Institute (CLSI) 62-A guidance on LC-MS methods.
Stratification of clinical urine specimens for sensitivity and specificity evaluations
Eighty-two and 256 urine specimens were collected in two separate batches for evaluating the Thermo Scientific and Roche high-sensitivity immunoassays, respectively. The Roche Benzodiazepines Plus immunoassay was performed on the Roche cobas c502 module to screen the specimens as either positive or negative. For comparison purposes, a true positive was defined as any specimen containing total benzodiazepines ≥ 20 ng/mL as determined by LC-MRM-MS/MS, and a true negative was any specimen containing total benzodiazepines ≤ 200 ng/mL as determined by LC-MRM-MS/MS.
Sensitivity and specificity
Clinical sensitivity and specificity were determined using standard formulas [18].
Retrospective analysis of benzodiazepine testing of clinical urine specimens
Results of urine specimens tested for benzodiazepines were determined by searching the hospital information system for tests performed 60 days before and 60 days after implementation of the HS-Roche assay. None of the specimens that were part of this analysis had additional testing performed.
Statistical analyses
Statistical significance of differences between the proportion of benzodiazepine confirmations 60 days before and 60 days after implementation of the HS-Roche assay was determined using a two proportion Z-test to compare the proportion of positive benzodiazepine confirmations with a single benzodiazepine, multiple benzodiazepines, a single benzodiazepine (alprazolam, lorazepam, oxazepam, and temazepam), and lorazepam only [19].
Results
Precision profiles of two Commercial High-Sensitivity benzodiazepine assays
The precision profile of the Thermo Scientific High Sensitivity Benzodiazepine assay was evaluated over 5 days (Supplementary Table 2). Within-day, between-day, and total %CV for the positive quality control (QC) (250 ng/mL oxazepam; MGC Multi Drug Primary – DAU Control High) were 4.0%, 10.3%, and 5.6%, respectively. Within-day, between-day, and total %CV for the negative QC (150 ng/mL oxazepam; MGC Multi Drug Primary – DAU Control Low) were 6.8%, 6.3%, and 6.7%, respectively. The precision profile for the HS-Roche assay was performed over a single day for within-run precision and across 15 days for total precision (Supplementary Table 3). The observed %CVs for within-run and precision across 15 days were < 2%. The within-run precision (n = 20) for the positive QC (930 ng/mL Lorazepam Glucuronide) and negative QC (PreciNeg) was < 2%. Precision across 15 days (n = 26) was calculated for 930 ng/mL of lorazepam glucuronide and the corresponding amount of free lorazepam, assuming 100% glucuronide hydrolysis (600 ng/mL of free lorazepam) (Supplementary Table 3). Observed total %CVs were < 2% for 930 ng/mL of lorazepam glucuronide. Importantly, analysis of the glucuronidated quality control material returned a negative result when beta-glucuronidase was omitted from the reagent pack.
Clinical sensitivity and specificity
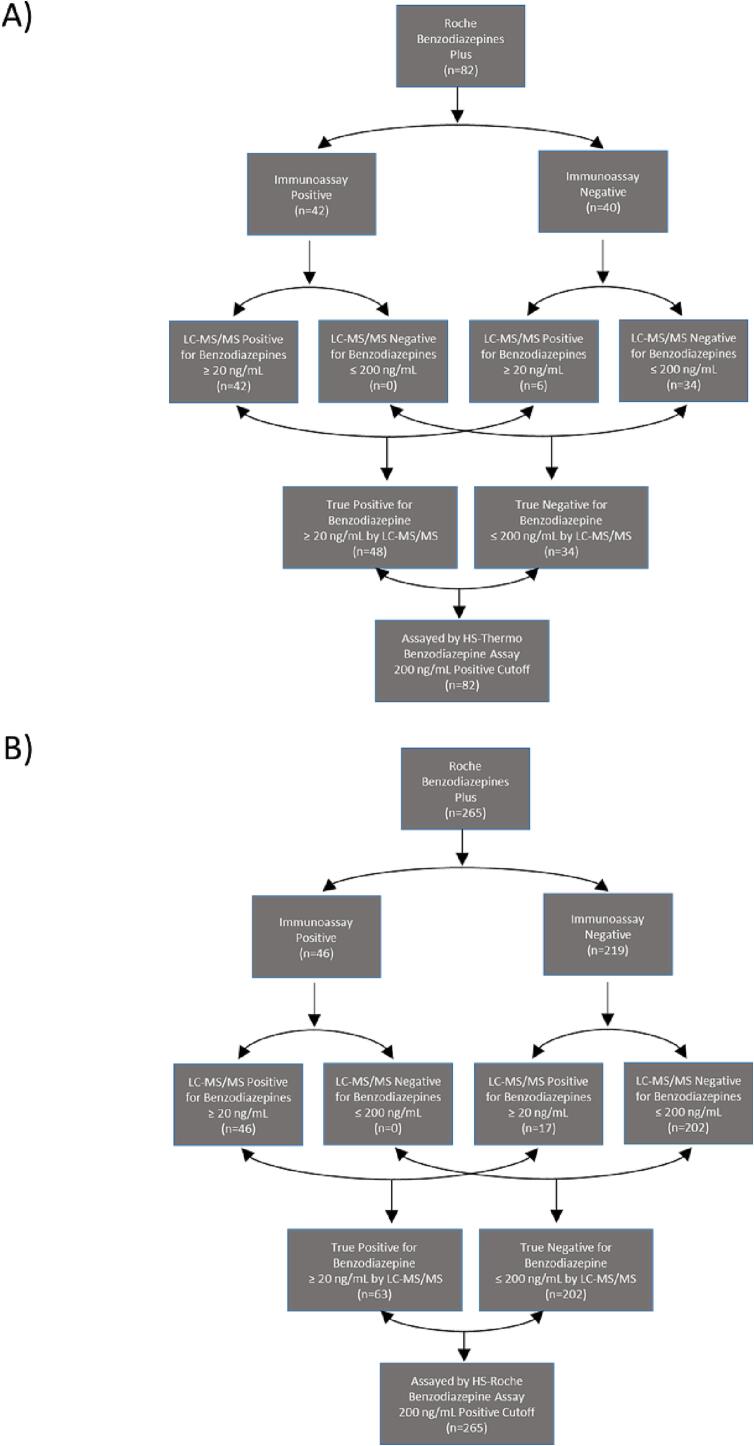
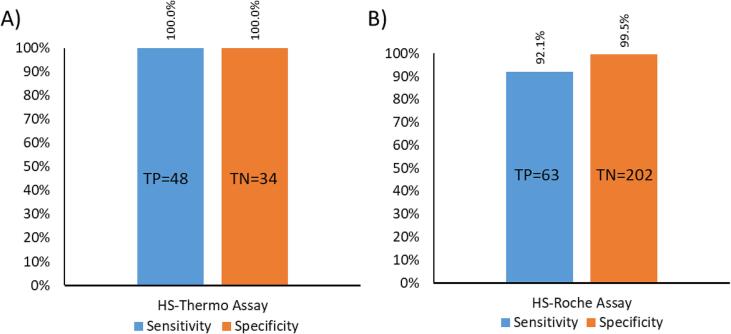
The clinical sensitivity and specificity of the HS-Thermo assay were assessed by classifying clinical urine specimens into true positive and true negative cohorts for each assay using LC-MS/MS as the reference method (Fig. 1A and 1B). All clinical specimens were first screened during live testing using the Roche Benzodiazepines Plus assay and preliminarily classified as immunoassay positive or negative; stratification of the cohort was then finalized using LC-MS/MS as the reference method. The total cohort for evaluating the HS-Thermo assay was divided into 48 true positives and 34 true negatives (total n = 82), while the cohort for the HS-Roche assay was divided into 63 true positives and 202 true negatives (total n = 265). There were no false negatives or false positives when evaluating the HS-Thermo assay, resulting in 100% clinical sensitivity and specificity (Fig. 2A). In contrast, there were five false negatives and one false positive observed when evaluating the HS-Roche assay, resulting in a clinical sensitivity and specificity of 92.1% and 99.5%, respectively (Fig. 2B).
Fig. 1.
Classification of Clinical Specimens Used to Evaluate Commercial HS Benzodiazepine Immunoassays
Clinical urine specimens were screened during live testing using the Roche Benzodiazepine Plus immunoassay and categorized based on the screening result (positive vs. negative). LC-MS/MS analysis was used to stratify the samples based on the level of total benzodiazepines to determine the sensitivity and specificity (Y-axis) of the HS-Thermo (A) and HS-Roche (B) benzodiazepine immunoassays in clinical specimens. True positives and true negatives were classified based on the total concentration of benzodiazepines within a specimen as measured by LC-MS/MS (true positive ≥ 20 ng/mL; true negative ≤ 200 ng/mL).
Fig. 2.
Clinical Sensitivity and Specificity of Commercial High Sensitivity Benzodiazepine Immunoassays.
A) Sensitivity and specificity (Y-axis) of the HS-Thermo benzodiazepine immunoassay in clinical specimens. Bar-graphs representing sensitivity (blue) and specificity (orange) are indicated for each immunoassay. True positives (TP) and true negatives (TN) are indicated within each bar. B) Sensitivity and specificity (Y-axis) of the HS-Roche benzodiazepine immunoassays in clinical specimens. Bar-graphs representing sensitivity (blue) and specificity (orange) are indicated for each immunoassay. True positives (TP) and true negatives (TN) are indicated within each bar.
Stability studies of a glucuronidated quality control
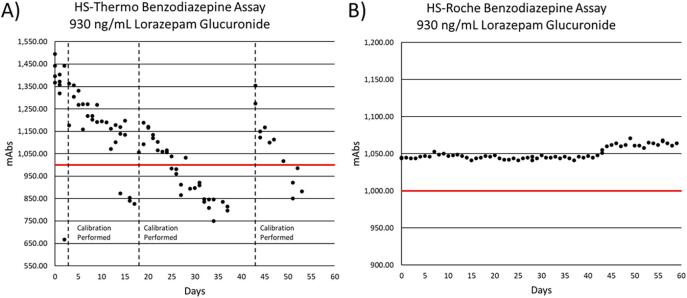
Using 930 ng/mL of lorazepam-glucuronide as a positive QC, the reagent stability of the HS-Roche and HS-Thermo assays were monitored for 59 and 53 days, respectively. The positive QC was assayed to monitor reagent stability and efficiency of glucuronide hydrolysis by observing the response (mabs) over time. Performance of the HS-Thermo assay degraded rapidly following calibration (Fig. 3A), requiring three separate calibrations on Days 3, 18, and 43 as the positive QC returned results below the positive cutoff value of 1000 mabs units (corresponding to 200 ng/mL of benzodiazepine). The assay was not calibrated from Days 26 to 37 to ensure that the downward trend of observed values continued below the 1000 mabs cutoff. The mean time to calibration was 14.3 days (Standard Deviation: 11 days). In contrast, performance of the HS-Roche assay was stable and did not require calibration over the 59 days that were monitored, with an observed %CV of 0.8% across 59 days (Fig. 3B).
Fig. 3.
Reagent Stability of Commercial High Sensitivity Benzodiazepine Immunoassays.
A) Observed mabs units (Y-axis) for 930 ng/mL lorazepam-glucuronide positive control material were plotted across 53 days (X-axis) for the HS-Thermo benzodiazepine assay to monitor reagent stability. Days where re-calibration was required were indicated by a vertical dotted line. The cutoff for a positive result (200 ng/mL of benzodiazepines) is indicated by a solid red line at 1000 mabs units. B) Observed mabs units (Y-axis) for 930 ng/mL lorazepam-glucuronide positive control material were plotted across 59 days (X-axis) for the HS-Roche benzodiazepine assay to monitor reagent stability. The cutoff for a positive result (200 ng/mL of benzodiazepines) is indicated by a solid red line at 1000 mabs units.
Retrospective analysis of live testing performance
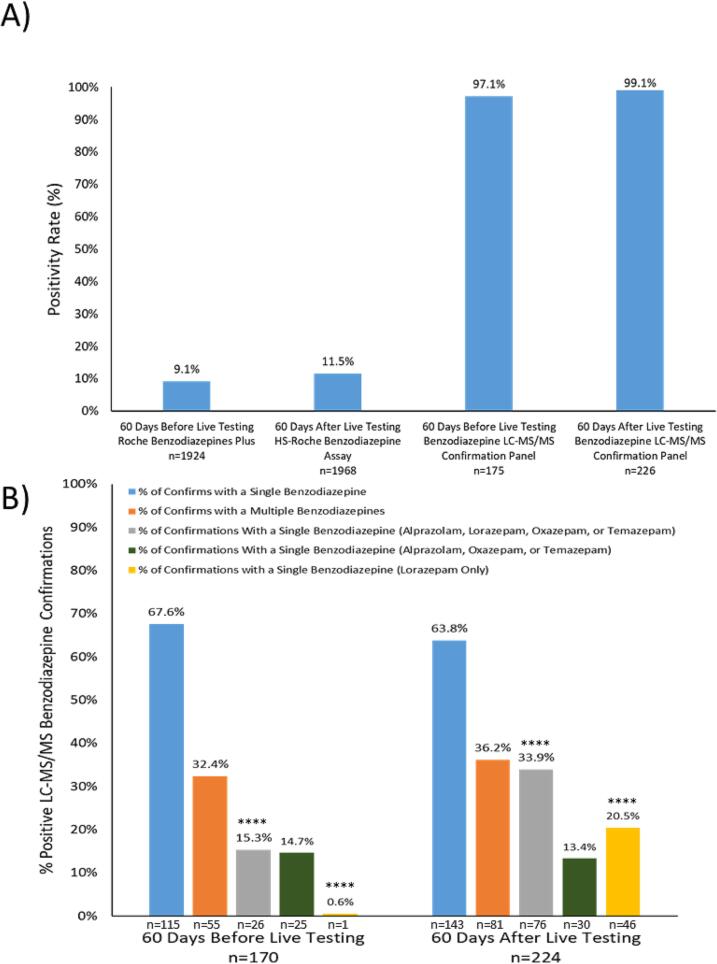
Based on the findings during assay validation, the HS-Roche Benzodiazepines Plus assay was implemented as a LDT for patient testing at UCSD Health on August 30th, 2022. To evaluate live testing performance of the immunoassay LDT, a retrospective analysis of the screen and confirm testing approach that is implemented at UCSD Health for benzodiazepines was performed 60 days before and after the LDT was launched (Fig. 4). The positivity rate of benzodiazepines in clinical urine specimens at UCSD Health 60 days before the launch of the HS Benzodiazepine LDT was 9.1% (n = 1924) with an LC-MS/MS confirmation rate of 97.1% (Fig. 4A). In contrast, the positivity rate in the following 60 days post-launch increased by 2.4%, to 11.5% of the total number of urine specimens assayed (n = 1968) with an LC-MS/MS confirmation rate of 99.1% (Fig. 4A). The percentage of immunoassay positive urine specimens that were found with single benzodiazepines (blue-bars) versus multiple benzodiazepines (orange-bars) by the LC-MS/MS confirmation panel is shown in Fig. 4B for before and after the LDT launch date. There were no obvious differences in the % of urine specimens with singly-confirmed benzodiazepines (67.6% vs 63.8%; before and after) and multiply-confirmed benzodiazepines (32.4% vs 36.2%; before and after). However, by stratifying the data further and focusing on benzodiazepines that are known to be glucuronidated, there was more than a 2-fold increase in the % of urine specimens (15.3% vs 33.9%; before and after) with a singly-confirmed benzodiazepine that was either alprazolam, lorazepam, oxazepam, or temazepam (gray-bars). In contrast, there was a 1.3% decrease when comparing the % of urine specimens with a singly-confirmed benzodiazepine that was either alprazolam, oxazepam, or temazepam (green-bars); 14.7% vs 13.4%; before and after. Finally, by looking at the % of confirmations that were identified to contain lorazepam only, a more than 30-fold increase was observed (0.6% vs 20.5%; before and after) since the launch of the LDT (p-value: < 0.00001).
Fig. 4.
Retrospective Analysis of Clinical Testing Performance of the HS-Roche benzodiazepine LDT.
A) Positivity rates (Y-axis) of the benzodiazepine screening (Immunoassay) and confirmatory (LC-MS/MS) assays offered at UCSD Health before and after the launch of the HS-Roche benzodiazepine LDT assay are shown. The number of tests (n) performed for each assay is indicated below each bar on the X-axis, and the percentage of positive results out of the total number of tests performed is indicated above each bar. B) The positivity rates (Y-axis) of the LC-MS/MS benzodiazepine confirmation panel are shown for urine specimens with singly-confirmed (blue-bar), multiply-confirmed (orange-bar), singly-confirmed alprazolam/lorazepam/oxazepam/temazepam (gray-bar), singly-confirmed alprazolam/oxazepam/temazepam (green-bar), and singly-confirmed lorazepam-only (yellow-bar). The number of positive specimens (n) for each category is indicated below each bar and the total number of specimens tested (n) subjected to confirmatory testing (60 days before and after LDT implementation) are indicated in bold. Statistical significance comparing the proportions of positive benzodiazepine confirmations with a single benzodiazepine, multiple benzodiazepines, single benzodiazepine (alprazolam, lorazepam, oxazepam, and temazepam) before and after the HS-Roche benzodiazepine LDT was implemented was determined by a two proportion Z-test (p-values < 0.00001 are indicated by ****).
Discussion
The regulation and implementation of LDTs in the clinical laboratory is essential for the practice of laboratory medicine. Clinical laboratories generally develop and implement LDTs to meet unmet analytical and/or clinical needs [17], [20]. LDTs have played a critical role at the frontlines of in vitro diagnostic innovation and are generally implemented when there are no FDA cleared options. In this study, we demonstrate the clinical utilization of two LDTs: an immunoassay and one performed by LC-MS/MS. It is important to note that the LC-MS/MS LDT was essential for validating the HS-Roche LDT. Without a well-validated LC-MS/MS assay to provide definitive identification of specific benzodiazepines it would have been impossible to validate the immunoassay LDT that incorporated the addition of beta-glucuronidase. Utilization of the LC-MS/MS LDT was essential in revealing the presence of false negatives within specimens screened by the FDA cleared Roche Benzodiazepine Plus assay for each respective cohort; 6 for the cohort used to evaluate HS-Thermo (Fig. 1A) and 17 for the cohort used to evaluate HS-Roche (Fig. 1B). Furthermore, the use of quality control material containing a glucuronidated benzodiazepine was also essential for identifying stability issues with the HS-Thermo assay. Interestingly, this stability issue was not observed when testing the non-glucuronidated control material supplied with the reagent kit.
The concentration of lorazepam-glucuronide in the positive QC was chosen based on the claimed cross-reactivity of free lorazepam described in the manufacturer’s product insert of the Roche Benzodiazepines Plus assay, which is 62% [21]. This corresponds to 600 ng/mL of free lorazepam, assuming 100% hydrolysis of the glucuronide and a theoretical benzodiazepine concentration of 372 ng/mL when taking into account the cross-reactivity of lorazepam in the assay. During the validation of the HS-Roche immunoassay, five false negatives were observed. Interestingly, four of these specimens were positive by LC-MS/MS for lorazepam with concentrations ranging from 213 to 338 ng/mL and returned mabs values ranging from 941 to 999 (positive cutoff: 1000 mabs). The last false negative contained 216 ng/mL of 7-amino-clonazepam (by LC-MS/MS after hydrolysis), which has a claimed cross-reactivity of 39% according to the manufacturer’s product insert, and returned a mabs value of 934. Taking into account these claimed cross-reactivities, it appears that the immunoassay would return a negative value as the effective concentration of benzodiazepines in each specimen was beneath the 200 ng/mL cutoff.
A profound impact was observed when retrospectively evaluating benzodiazepine LC-MS/MS confirmation data for a period of 60 days before and after the high sensitivity immunoassay LDT was implemented; there was an observed 34-fold increase (p-value < 0.00001) in the percentage of positive LC-MS/MS benzodiazepine confirmations that contained only lorazepam. A statistically significant (p-value < 0.00001) difference was observed in the percentage of positive singly-confirmed LC-MS/MS benzodiazepines which contained only alprazolam, lorazepam, oxazepam, or temazepam; all excreted in varying proportions as glucuronidated metabolites [6], [7], [22]. However, this difference was due to the large increase in the number of specimens containing only lorazepam, as no significant difference was observed (p-value: 0.71) when comparing the percentage of positive singly-confirmed LC-MS/MS benzodiazepine confirmations that contained only alprazolam, oxazepam, or temazepam (Fig. 4B). Furthermore, the percentage of immunoassay-positive specimens that confirmed negative when analyzed by LC-MS/MS decreased by 2% (2.9% vs 0.9%) following implementation of the HS-Roche assay; however, this difference was found to be insignificant (p-value greater than 0.01). It is worth noting that while a single false positive was observed during the validation of the HS-Roche immunoassay, it is possible that this specimen contained a benzodiazepine not included in our targeted LC-MS/MS confirmatory benzodiazepine panel, such as etizolam or flualprazolam [23], [24].
The LDT is a powerful tool in the clinical laboratory that requires substantial validation and ongoing quality monitoring once implemented. The VALID Act and its impact on clinical laboratories providing LDTs as part of their testing menus is still being discussed. This study reinforces the importance of LDTs in laboratory medicine and their role in meeting unmet clinical needs and providing clinically optimal testing services for patients. The appropriate level of regulatory oversight can help ensure clinical validity of LDTs and patient safety; however, overregulation comes with more than just additional financial costs but also increased operational burdens and discourages innovation in a rapidly evolving field due to technological advancements. The increased regulatory requirements outlined in the VALID Act impacts all LDTs and could potentially discourage clinical laboratories from conducting their own internal studies to develop an LDT or better characterize assay performance, as any modifications made may come with an increased risk of regulatory scrutiny and operational burden. Moving forward, it is important for laboratory professionals to continually bring forth compelling arguments which underline the importance of LDTs and the dangers of overregulation as opposed to appropriate regulation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the staff in the University of California San Diego Health clinical laboratories for their help identifying specimens for clinical sensitivity and specificity testing. We also thank Dawn Francisco, Heather Tone, Nick Asfour, Amy Rockefeller, Joseph (Thad) Anderson, and Ernestine Ferrer for valuable technical expertise and support. We would like to thank Waters Corporation and Roche Diagnostics for supporting the clinical chemistry fellowship at the University of California San Diego.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2023.02.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fitzgerald R.L., Rexin D.A., Herold D.A. Detecting benzodiazepines: immunoassays compared with negative chemical ionization gas chromatography/mass spectrometry. Clin Chem. 1994 Mar;40(3):373–380. [PubMed] [Google Scholar]
- 2.Oldenhof E., Anderson-Wurf J., Hall K., Staiger P.K. Beyond Prescriptions Monitoring Programs: The Importance of Having the Conversation about Benzodiazepine Use. J Clin Med. 2019 Dec 4;8(12):2143. doi: 10.3390/jcm8122143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qriouet Z., Qmichou Z., Bouchoutrouch N., Mahi H., Cherrah Y., Sefrioui H. Analytical Methods Used for the Detection and Quantification of Benzodiazepines. J Anal Methods Chem. 2019 Sep;5(2019):2035492. doi: 10.1155/2019/2035492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L le, Ren X xin, He Y, Cui G feng, Liu J jia, Jia J, et al. Pharmacokinetics of Diazepam and Its Metabolites in Urine of Chinese Participants. Drugs RD. 2022 Mar;22(1):43–50. [DOI] [PMC free article] [PubMed]
- 5.Meatherall R. Benzodiazepine screening using EMIT II and TDx: urine hydrolysis pretreatment required. J Anal Toxicol. 1994 Dec;18(7):385–390. doi: 10.1093/jat/18.7.385. [DOI] [PubMed] [Google Scholar]
- 6.Kyriakopoulos A.A., Greenblatt D.J., Shader R.I. Clinical pharmacokinetics of lorazepam: a review. J Clin Psychiatry. 1978 Oct;39(10 Pt 2):16–23. [PubMed] [Google Scholar]
- 7.Dinis-Oliveira R.J. Metabolic profile of oxazepam and related benzodiazepines: clinical and forensic aspects. Drug Metab Rev. 2017 Nov;49(4):451–463. doi: 10.1080/03602532.2017.1377223. [DOI] [PubMed] [Google Scholar]
- 8.Beck O., Lafolie P., Odelius G., Boreus L.O. Immunological screening of benzodiazepines in urine: improved detection of oxazepam intake. Toxicol Lett. 1990 Jun;52(1):7–14. doi: 10.1016/0378-4274(90)90160-n. [DOI] [PubMed] [Google Scholar]
- 9.Beck O., Lafolie P., Hjemdahl P., Borg S., Odelius G., Wirbing P. Detection of benzodiazepine intake in therapeutic doses by immunoanalysis of urine: two techniques evaluated and modified for improved performance. Clin Chem. 1992 Feb;38(2):271–275. [PubMed] [Google Scholar]
- 10.Mina A., McNeice L., Banukumar S., Vazquez S. Optimisation of Benzodiazepine Immunoassay Using Glucuronidase Enzymatic Hydrolysis: A Comparison of Five Different Glucuronidase Enzymes. J Biosci Med. 2022;10(01):7–15. [Google Scholar]
- 11.French D., Stone J.A., Chang J.S., Wu A.H.B. Choosing the Right Benzodiazepine Assay: Impact on Clinical Decision Making. Lab Med. 2010 Apr 1;41(4):196–200. [Google Scholar]
- 12.Dixon R.B., Floyd D., Dasgupta A. Limitations of EMIT benzodiazepine immunoassay for monitoring compliance of patients with benzodiazepine therapy even after hydrolyzing glucuronide metabolites in urine to increase cross-reactivity: comparison of immunoassay results with LC-MS/MS values. Ther Drug Monit. 2015 Feb;37(1):137–139. doi: 10.1097/FTD.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 13.68 FR 3703, Jan. 24, 2003; 68 FR 50724, Aug. 22, 2003.
- 14.Food and Drug Administration. Draft Guidance “Framework for Regulatory Oversight of Laboratory Developed Tests. October 3, 2014.
- 15.Centers for Medicare and Medicaid Services. Background document on CLIA oversight of LDTs.
- 16.Senators Bennet, Hatch & Reps. Bucshon, DeGette Release Draft Legislation to Modernize FDA Regulation of Diagnostic Tests [Internet]. Michael Bennet. [cited 2022 Nov 8]. Available from: https://www.bennet.senate.gov/public/index.cfm/2018/12/senators-bennet-hatch-reps-bucshon-degette-release-draft-legislation-to-modernize-fda-regulation-of-diagnostic-tests.
- 17.Genzen J.R. Regulation of Laboratory-Developed Tests: A Clinical Laboratory Perspective. Am J Clin Pathol. 2019 Jul 5;152(2):122–131. doi: 10.1093/ajcp/aqz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front Public Health. 2017 Nov;20(5):307. doi: 10.3389/fpubh.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hittner J.B., Fasina F.O. Statistical methods for comparing test positivity rates between countries: Which method should be used and why? Methods San Diego Calif. 2021 Nov;195:72–76. doi: 10.1016/j.ymeth.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snozek C.L.H. FDA-Cleared versus Laboratory-Developed Tests: Why Start from Scratch When Kits Are Available? J Appl Lab Med. 2017 Jul 1;2(1):130–131. doi: 10.1373/jalm.2016.021832. [DOI] [PubMed] [Google Scholar]
- 21.Roche Benzodiazepines Plus Method sheet: 0004490789190c501V9.0; 2014-03, V 9.0 English.
- 22.Fraser A.D., Bryan W., Isner A.F. Urinary screening for alprazolam and its major metabolites by the Abbott ADx and TDx analyzers with confirmation by GC/MS. J Anal Toxicol. 1991 Feb;15(1):25–29. doi: 10.1093/jat/15.1.25. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell C.W., Sadler C.A., Tolia V.M., Ly B.T., Saitman A.M., Fitzgerald R.L. Overdose of Etizolam: The Abuse and Rise of a Benzodiazepine Analog. Ann Emerg Med. 2015 Apr;65(4):465–466. doi: 10.1016/j.annemergmed.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Blumenberg A, Hughes A, Reckers A, Ellison R, Gerona R. Flualprazolam: Report of an Outbreak of a New Psychoactive Substance in Adolescents. Pediatrics [Internet]. 2020 Jul 1 [cited 2020 Aug 15];146(1). Available from: https://pediatrics.aappublications.org/content/146/1/e20192953. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.