Abstract
Currently, there is greater production and consumption of craft beer due to its appreciated sensory characteristics. Unlike conventional beer, craft beers provide better health benefits due to their varied and high content of phenolic compounds (PCs) and also due to their alcohol content, but the latter is controversial. The purpose of this paper was to report on the alcoholic fraction and PCs present in craft beers and their influence on health. Despite the craft beer boom, there are few studies on the topic; there is a lot of field to explore. The countries with the most research are the United States > Italy > Brazil > United Kingdom > Spain. The type and amount of PCs in craft beers depends on the ingredients and strains used, as well as the brewing process. It was determined that it is healthier to be a moderate consumer of alcohol than to be a teetotaler or heavy drinker. Thus, studies in vitro, with animal models and clinical trials on cardiovascular and neurodegenerative diseases, cancer, diabetes and obesity, osteoporosis and even the immune system suggest the consumption of craft beer. However, more studies with more robust designs are required to obtain more generalizable and conclusive results. Finally, some challenges in the production of craft beer were detailed and some alternative solutions were mentioned.
Keywords: Craft beer, Hops (Humulus lupulus), Phenolic compounds, Prenylflavonoid, Xanthohumol, Prenylnaringenin
Graphical abstract
Highlights
-
•
Craft beers have different styles that depend on unconventional ingredients.
-
•
Craft beer could dethrone conventional beer for its potential health benefits.
-
•
Probiotic, polyphenol-rich, low-alcohol craft beers are promising alternatives.
-
•
Laws that support craft brewers are needed to overcome barriers that limit production.
-
•
Craft beers are not pasteurized, but can be safe using non-thermal processes.
1. Introduction
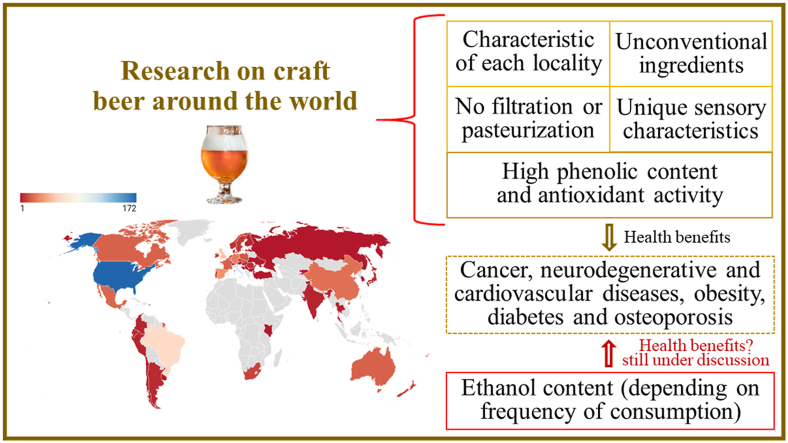
Evidence indicates that beer was produced thousands of years before Christ (Breda et al., 2022). Thanks to discoveries and changes in beer production around the world, it is now the most consumed alcoholic beverage (Salantă et al., 2020a). The brewing process is standard (Aredes et al., 2021) with limited beer styles that do not meet the needs and preferences of consumers. As a result, beer production changed significantly in the early 1990s since the emergence of microbreweries in the US (Legun et al., 2022). This led to the production of craft beers with more flavor such as India Pale Ale (IPA) beers and more bitterness such as Hazy IPA style beers (Legun et al., 2022). New Zealand consumers (22–60 years old, 39% female) preferred novelty, intriguing and complex beers over ordinary, simple beers (Cardello et al., 2016). Italian consumers (aged 18–72 years, 52% female) indicated that they prefer craft beers because of their remarkable authenticity (41.8% frequency), because they are more natural (22.9%) and tastier (22.3%) than commercial beers (Lerro et al., 2020). Italian consumers (aged 25–40 years, 50% female) indicated that they prefer craft beer for its differentiated sensory characteristics (Rivaroli et al., 2022). Authenticity, creativity and innovation characterize craft beers (Breda et al., 2022). Fig. 1 shows a brief consensus on the characteristics of the craft brewery and craft beer.
Fig. 1.
Characteristics of craft breweries and craft beers. Prepared based on Březinová (2021); Jardim et al. (2018); Liguori et al. (2020); Morgan et al. (2022); The Beer Times (2022).
In the beginning, beer was produced by a few companies (Březinová, 2021) and this homogeneity forced more authentic beers to be produced. Historically, Jaeger et al. (2020) noted that the craft beer industry originated in the 1960s in the US when Fritz Maytag, a small brewery acquired the Anchor Steam Beer Company. One of the innovations implemented was the formulation of beers of different styles from non-conventional raw materials such as rice and corn, as well as other fruits and spices (Jaeger et al., 2020). According to Milburn and Guertin-Martín (2020), the craft beer industry was spurred by H.R Bill 1337 signed by President Jimmy Carter in 1978 to allow home brewing in the United States. They defended this hypothesis because approximately 90% of craft beer producers have started their business at home.
Industrial beer has an established place in the market, but the organoleptic quality and versatility of craft beer have allowed it to gain a place in consumer preferences (Poveda, 2019). In 2016 there were more than 19 thousand breweries in the world and approximately 94% were considered craft breweries (Baiano, 2021). What else would characterize craft beers? According to Salantă et al. (2020b), the consumption of foods/drinks that have health benefits is booming. A scientific mapping detected that past research focused on the brewing process, but current publications would focus on human health (Pallottino et al., 2020). Moderate consumption of beer provides health benefits due to its nutritional value and mainly bioactive content, represented by phenolic compounds (PCs) (Burini et al., 2021). The ethanol content of beers has also been associated to the prevention of diseases (Osorio-Paz et al., 2020).
It is not intended to replace or compete with wine, which is also considered to provide health benefits, or other foods rich in PCs. On the contrary, taking advantage of the fact that beer consumption is approximately 4, 5 and 7 times higher than the consumption of cider, spirits and wine, respectively (STATISTA, 2022), offering craft beers with better characteristics is shown as a healthier alternative. In this scenario, the purpose of this study was to conduct a review on the PCs present in craft beers. The main findings on the influence of PCs on health were emphasized, also discussing the role of the alcoholic fraction. It should be clarified that part of the information shown is based on data on industrial beers, since information on craft beers is very scarce.
2. Worldwide interest in craft beer
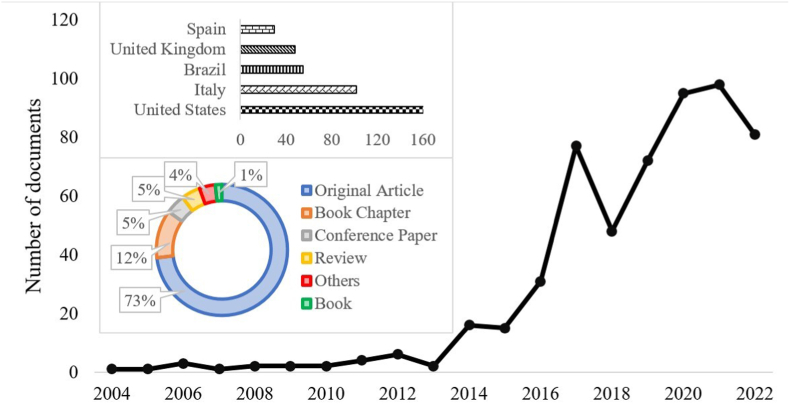
Fig. 2 shows that the field of craft beers is still under-explored (584 papers), but research is increasing exponentially (R2: 0.8998) with the maximum peak in 2020 (110 papers). A bibliometric study detected that researchers focused more on craft beer science since 2015, but participation is transient (in many cases with only one publication per author), indicating that the field of study is not highly developed (Durán-Sánchez et al., 2022). In the last 5 years (period 2018–2022), the number of published papers is more than 70% of the total number of articles. More than 70% of papers are original articles and only ≈5% are review articles; therefore, this study is necessary. Therefore, this study is necessary to summarize recent research on the field of study and present the current scenario with critical perspectives. The five countries with the highest scientific production on the topic are: United States > Italy > Brazil > United Kingdom > Spain. Similar results were shown in Baiano (2021): United States > Italy > Britain > Brazil > Australia > France > Spain > Canada. The advantage of the United States (30% of total documents) is because it is the largest beer producer after China. With 8386 craft breweries, the United States produced 13.6% of the total volume of beer (Brewers Association, 2020). Baiano (2021) pointed out that countries with large beer productions such as Mexico, China, Belgium, Ireland and the Czech Republic have few publications about craft beer. Interestingly, in countries with strong brewing industries, the craft beer market is developing slowly (Baiano, 2021). The production of conventional beers (and the number of breweries) and craft beers is not proportional and the number of microbreweries is not as important as their size because it directly influences production. Czech Republic with more than 410 craft breweries in 2019 and Ireland with 75 craft breweries had a market share of 2% in 2019 (Březinová, 2021) and 2.8% in 2017 (McMahon, 2019), respectively. On the other hand, in countries such as Belgium, large beer companies tend to absorb craft breweries and the products become just another variety in the catalog (Poelmans and Swinnen, 2018). This affects interest in craft beers and their research, as consumers believe they are industrially produced. In China, craft beer production is limited because it is difficult to acquire quality local ingredients, so imports significantly increase production costs (Li et al., 2018). In addition, since there are no laws designed specifically for craft breweries, microbrewers must follow the stringent regulations for industrial producers (Li et al., 2018). Similarly, Mexican government measures such as the application of a 26.5% special tax on the production and sale of beer regardless of the size of the brewery blocked the growing production of craft beer (Baiano, 2021).
Fig. 2.
Number of publications on craft beer in Scopus. Search string: Title-Abstract-Keywords (“craft beer” OR “artisanal beer”). Only English-language documents published until December 2022.
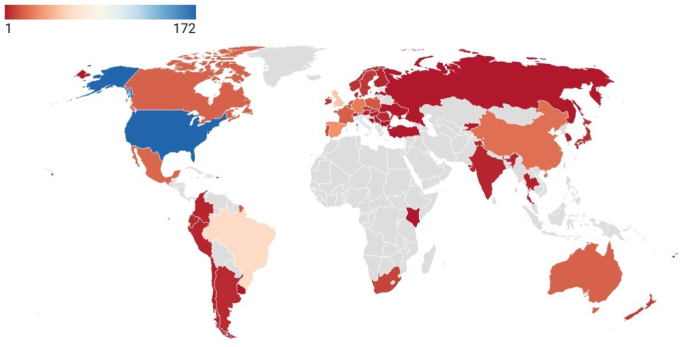
Fig. 3 shows a map with all the countries that have contributed to the topic. In Africa, only South Africa (10 papers) and barely Kenya (1) show scientific production. Sub-Saharan Africa is primarily the least developed region in beer research; however, in recent years the African market has attracted the attention of the brewing industry, which is looking to expand (Rogerson, 2019). This was because Africa began to produce craft beers with indigenous ingredients, very different from the craft beers produced in the rest of the world (Rogerson, 2019). The emergence began in the craft beer sector in South Africa. This is associated with the results obtained, as scientific production in South Africa started in 2017, but growth is slow. Similarly, many Asian countries lack scientific production, but are now part of the craft beer market (Baiano, 2021). Therefore, we hope that in the coming years more countries will join the research on this topic.
Fig. 3.
Scientific production worldwide. The bar indicates publications by country according to color. The map was made with Datawrapper (Datawrapper GmbH, Berlin, Germany) according to the results obtained from the Scopus search. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Compounds of interest in beer
Beer is rich in carbohydrates (≤6.1 g/100 mL), proteins (0.3–0.5 g/100 mL) vitamins (up to 30 mg vitamin C/100 mL) and minerals (up to 110 mg potassium/100 mL) (Mellor et al., 2020). Based on the evaluation of beers from Asia, South America and Europe, the consumption of 500 mL can provide up to 15.5% of the daily calcium requirement according to U.S. standards, and phosphorus, potassium and chlorine can provide up to 3% of the daily value (Styburski et al., 2018). Chromium (8.87 mg/L), arsenic (8.81 mg/L), selenium (4.14 mg/L), barium (90.72 mg/L), and lead (9.98 mg/L) were identified in Brazilian beers (Moreira et al., 2006). It was also reported that beers have vitamins A, D, E, K and C, thiamine, riboflavin, niacin, vitamin B6, folate, vitamin B12, biotin, magnesium, ion, sodium, zinc, selenium (Sohrabvandi et al., 2012), chloride, silica, sodium, magnesium, copper and manganese (Habschied et al., 2020).
Beer is also rich in phytochemicals and is noted for its high content of PCs (12–52 mg/100 mL) (Mellor et al., 2020). The PCs identified in different craft beers are shown in Table 1. It was determined that the total phenolic content (TPC) of Brazilian craft beers can cover from 0.2 to 11% of the daily intake value (460.15 mg/day) (Silva et al., 2021). In turn, the amount and type of phenols present in craft beer significantly influences its sensory characteristics (Ambra et al., 2021).
Table 1.
Identification of PCs in craft beers from different countries.
| Reference | Type of beer | Technique | Compounds identified |
|---|---|---|---|
| Anderson et al. (2021) | From the United States | LC-QTOF-MS | Catechin, benzoic acid, chlorogenic acid, caffeic acid, cinnamic acid, esculin, myricetin, quercetin, vanillin, naringin, and naringin |
| Breda et al. (2022) | From Portugal | HPLC | Gallic acid, ferulic acid, caffeic acid, p-coumaric acid, trans-cinnamic acid, luteolin, gallocatechin, epicatechin, quercetin, catechin and kaempferol-3-O-glucoside |
| Cortese et al. (2020) | Ego, Alter, Triplo Malto, Ubi, Fiat Lux” and Maior | HPLC-ESI-MS/MS | Gallic acid, 3-caffeoylquinic acid, 4-caffeoylquinic acid, epicatechin, caffeic acid, trans-ferulic acid, vanillic acid, syringic acid, trans-p-coumaric acid, sinapic acid, 4-hydroxybenzoic acid, quercetin, isoxanthohumol, 8-prenylnaringenin, catechin, xanthohumol, cohumulone, humulone, colupulone, and lupulone |
| dos Santos et al. (2020) | Made with brown, red and black rice. | HPLC–ESI-MS | Peonidin, cyanidin, cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside, cyanidin-3,5-O-diglucoside and peonidin-3-O-glucoside |
| Marques et al. (2017) | Brown Pote, Classic American Pilsner, Irish Red Ale and American Pale Ale | HPLC-DAD | Gallic acid, p-coumaric acid, ferulic acid and caffeic acid |
| Nardini and Garaguso (2020) | Made with fruit and fruit by-products | HPLC-DAD | Chlorogenic acid, sinapic acid, p-coumaric acid, caffeic acid, vanillic acid, syringic acid, and ferulic acid |
| Petrucci et al. (2020) | American Pale Ale, India Pale Ale and Amber Ale | HPLC-PDA-ESI-MS/MS | Caffeic acid, ferulic acid, sinapic acid, p-coumaric acid, 5-caffeoylquinic acid, gallic acid, 3,4-dihydroxybenzoic acid, syringic acid, vanillic acid, p-hydroxybenzoic acid, m-hydroxybenzoic acid, quercetin, rutin, and kaempferol |
| Silva et al. (2021) | From Brazil | HPLC-DAD | Caffeic acid, epicatechin, trans-ferulic acid, p-coumaric acid, formononetin, kaempferol, quercetin, catechin and hydrated rutin |
| Zapata et al. (2019) | Made with quince of different varieties | HPLC-DAD-ESI-MS | 3-O-caffeoylquinic acid, 3-O-coumaroylquinic acid, 5-O-caffeoylquinic acid, 5-O-coumaroylquinic acid, and 3,5-di-O-caffeoylquinic acid |
LC: liquid chromatography, HPLC: high performance liquid chromatography, QTOF: quadrupole time-of-flight, MS: mass spectroscopy, DAD: diode array detector, ESI: electrospray ionization, PDA: photodiode array detector.
Ethanol is also a crucial compound in beer and has been positively associated with flavor and aroma (Mellor et al., 2020), the content of which can vary between 4 and 6% in craft beers (Brewers Association, 2011), depending on the ingredients used as well as the type of strain (Table 2). Low-alcohol or non-alcoholic beers can also be produced. When a wheat beer was dealcoholized, the alcohol content decreased from 5.31 to 0.05% vol (Müller et al., 2021). The alcohol content in a quinoa beer fermented with Pichia myanmarensis ranged from 0.27 to 0.48% vol (Prasad et al., 2022).
Table 2.
Alcohol content, TPC and AA in craft beers.
| Reference | Style | Yeasts | Differential adjuncts | Alcohol (% v/v) | TPC (mg GAE/mL) | AA |
|---|---|---|---|---|---|---|
| Horn et al. (2021) | Imperial Stout | N.I. | Dark Munich malt and oats malt | 10.0 | 3.70 | 73.82%* |
| American Ipa | N.I. | Pale malt | 4.10 | 3.40 | 77.13%* | |
| Belgian Blond Ale | N.I. | Pilsen Belgian malt. aromatic malt and sugar | 7.00 | 2.61 | 64.82%* | |
| Catharina Sour | N.I. | Pale malt and peach extract | 6.60 | 1.88 | 51.63%* | |
| Witbier | N.I. | Pilsen malt, wheat not malted, coriander and orange | 5.00 | 1.58 | 38.50%* | |
| Capece et al. (2018) | N.I. | S. cerevisiae boulardii (SB) | N.I. | 3.83 | 0.31 | 2.30%* |
| N.I. | S. cerevisiae B2–S + SB | N.I. | 4.53 | 0.32 | 2.63 mg ET/L* | |
| N.I. | S. cerevisiae B2-M + SB | N.I. | 4.24 | 0.35 | 3.18 mg ET/L* | |
| N.I. | S. cerevisiae C3–S + SB | N.I. | 3.80 | 0.33 | 2.54 mg ET/L* | |
| N.I. | S. cerevisiae C3-M + SB | N.I. | 3.04 | 0.31 | 3.50 mg ET/L* | |
| N.I. | S. cerevisiae M4-S + SB | N.I. | 4.36 | 0.36 | 4.12 mg ET/L* | |
| N.I. | S. cerevisiae M4-M + SB | N.I. | 5.72 | 0.40 | 4.23 mg ET/L* | |
| N.I. | S. cerevisiae MT-15-S + SB | N.I. | 4.12 | 0.30 | 0.75 mg ET/L* | |
| N.I. | S. cerevisiae MT-15-M + SB | N.I. | 3.35 | 0.35 | 3.54 mg ET/L* | |
| N.I. | S. cerevisiae P4–S + SB | N.I. | 2.83 | 0.32 | 2.06 mg ET/L* | |
| N.I. | S. cerevisiae P4-M + SB | N.I. | 4.42 | 0.41 | 7.11 mg ET/L* | |
| Piva et al. (2021) | Pilsner | S. cerevisiae. | Ocimum selloi leaves (extract; AF, 0,05%) | 5.73 g/100 mL | 0.37 | 69.00%* |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (extract; AF, 0,1%) | 5.96 g/100 mL | 0.37 | 69.00%* | |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (natura; AF, 0,1%) | 6.62 g/100 mL | 0.37 | 54.90%* | |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (natura; AF, 0,5%)) | 6.48 g/100 mL | 0.36 | 50.30%* | |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (extract; DF, 0,05%) | 5.68 g/100 mL | 0.37 | 69.00%* | |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (extract; DF, 0,1%) | 5.71 g/100 mL | 0.37 | 83.50%* | |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (natura; DF, 0,1%) | 6.14 g/100 mL | 0.36 | 66.80%* | |
| Pilsner | S. cerevisiae | Ocimum selloi leaves (natura; DF, 0,5%) | 6.71 g/100 mL | 0.36 | 62.00%* | |
| Petrucci et al. (2021) | Pale Ale | S. cerevisiae Safale S-04 | Pale Ale malt (100%) | – | 0.36 | 0.75 mmol ET/L* |
| Pale Ale | S. cerevisiae Safale S-04 | Pale Ale (95%) and Caraamber (5%) malts | – | 0.45 | 0.78 mmol ET/L* | |
| Pale Ale | S. cerevisiae Safale S-04 | Pale Ale (85%) and Caraamber (15%) malts | – | 0.46 | 0.85 mmol ET/L* | |
| Liguori et al. (2020) | India Pale Ale | N.I. | Pils (60%), Pale Ale (39%) and melanoidinic (1%) malts | 5.40 | 0.36 | 1.25 mmol ET/L* |
| Dark Ale | N.I. | Munich (70%), Belgian Dark (20%) and Abbey (10%) malts | 3.80 | 0.91 | 1.52 mmol ET/L* | |
| Blond Ale | N.I. | Pale Ale malt (100%) | 6.60 | 0.35 | 0.79 mmol ET/L* | |
| Nardini and Garaguso (2020) | Ale | N.I. | Cherries var. Griotta (30%) | 6.00 | 0.77 | 3.53 mM ET/L** |
| Ale | N.I. | Cherries var. Corniolo, Ravenna and Graffione (20%) | 5.80 | 0.75 | 3.41 mM ET/L** | |
| Ale | N.I. | Raspberries (30%) | 5.00 | 0.47 | 3.41 mM ET/L** | |
| Lambic | N.I. | Raspberries (10%) | 5.80 | 0.54 | 2.35 mM ET/L** | |
| Ale | N.I. | Peaches (20%) | 8.00 | 0.51 | 1.98 mM ET/L** | |
| Ale | N.I. | Apricots (20%) | 7.00 | 0.45 | 1.86 mM ET/L** | |
| Ale | N.I. | Grapes (20%) | 8.00 | 0.63 | 2.81 mM ET/L** | |
| Ale | N.I. | Plums (20%) | 7.00 | 0.60 | 1.93 mM ET/L** | |
| Ale | N.I. | Oranges peels (0,5%) | 6.00 | 0.64 | 2.67 mM ET/L** | |
| Ale | N.I. | Apples (2%) | 5.20 | 0.40 | 1.62 mM ET/L** | |
| Humia et al. (2020) | Pale Ale | S. cerevisiae Safale US-05 | Beauregard sweet potato (30–70%) | 3.85-5.05 | 0.18-0.23 | 16.05–18.79 μL (IC50)* |
| Adamenko et al. (2020) | Pale Ale | Saccharomycodes ludwigii WSL 17 | Red-colored Cornelian cherry juice (10%) | 9.30 | ≈0.35 | ≈1.40 mmol ET/L* |
| Gasiński et al. (2020b) | Saison | S. cerevisiae diastaticus | Hawthorn juice (10%) | 3.54 g/100 mL | 0.41 | 2.18 mmol ET/L* |
| Saison | S. cerevisiae diastaticus | Hawthorn fruit (10%) | 3.72 g/100 mL | 0.28 | 0.44 mmol ET/L* | |
| Gasiński et al. (2020a) | N.I. | S. cerevisiae US-05 | Mango juice (20%) | 4.13 | 0.27 | 2.05 mmol ET/L* |
| N.I. | S. cerevisiae US-05 | Mango pulp (20%) | 4.27 | 0.22 | 1.53 mmol ET/L* | |
| N.I. | S. cerevisiae US-05 | Raw mango (20%) | 4.63 | 0.23 | 1.72 mmol ET/L* | |
| N.I. | S. cerevisiae US-05 | Heated mango (20%) | 4.49 | 0.23 | 1.72 mmol ET/L* | |
| Đorđević et al. (2016) | Pilsner | N.I. | Melissae folium extract (0,45 ml/L) | – | 0.36 | 3.05 mM ET* |
| Pilsner | N.I. | Thymi herba extract (0,50 ml/L) | – | 0.38 | 3.72 mM ET* | |
| Pilsner | N.I. | Juniperi fructus extract (0,65 ml/L) | – | 0.37 | 3.14 mM ET* | |
| Pilsner | N.I. | Urticae radix extract (0,55 ml/L) | – | 0.32 | 2.85 mM ET* | |
| Pilsner | N.I. | Lupuli strobuli extract (0,70 ml/L) | – | 0.32 | 2.83 mM ET* | |
| Bustos et al. (2019) | Amauta Porter | S. cerevisiae | Parastrephia lucida leaves (0,1%) | 5.30 | 0.48 | 1.38 mmol ET/mL** |
| Amauta Porter | S. cerevisiae | Parastrephia lucida leaves (0,5%) | 5.20 | 0.50 | 1.53 mmol ET/mL** | |
| Amauta Porter | S. cerevisiae | Parastrephia lucida leaves (1%) | 5.20 | 0.56 | 1.76 mmol ET/mL** | |
| Amauta Porter | S. cerevisiae | Parastrephia lucida leaves (5%) | 5.30 | 0.80 | 3.34 mmol ET/mL** | |
| Guglielmotti et al. (2020) | Pale Ale | S. cerevisiae Safale S-04 | Olea europaea L. leaves (9.9 g/L) | – | ≈0.52 mg/mL | ≈0.31 ET*** |
| Pale Ale | S. cerevisiae Safale S-04 | Infusion of Olea europaea L. (9.9 g/L leaves) | – | ≈0.69 mg/mL | ≈0.40 ET*** | |
| Pale Ale | S. cerevisiae Safale S-04 | Atomized extract of Olea europaea L. (9.9 g/L leaves) | – | ≈0.70 mg/mL | ≈0.45 ET*** | |
| Marques et al. (2017) | American Pale Ale | S. cerevisiae Safale US-05 | Château Pale Ale, Château Pilsen, Château Munich, Château Cara Ruby and Château Biscuit malts, Columbus and Chinook hops | 5.88 | 0.52 mg/mL | 46.7%* |
| Brown Poter | S. cerevisiae Safale US-05 | Château Pale Ale, Château Pilsen, Château Munich, Château Melano, Château Cara Gold and Château Chocolate malts, Northern and Fuggle hops | 5.13 | 0.53 mg/mL | 48.5%* | |
| Classic American Pilsner | S. pastorianus | Château Pale Ale, Château Pilsen, Château Munich and Château Melano malts, Warrior, Sladek and Premiant hops | 5.74 | 0.45 mg/mL | 29.4%* | |
| Irish Red Ale | S. cerevisiae Safale US-05 | Malta Château Pale Ale, malta Château Pilsen, malta Château Melano, Northern Brewer and Fuggle hops | 5.00 | 0.48 mg/mL | 35.7%* | |
| dos Santos et al. (2020) | N.I. | S. cerevisiae diastaticus | Brown rice | 3.95 | ≈0.50 μg/mL | – |
| N.I. | S. cerevisiae diastaticus | Red rice | 4.50 | ≈0.70 μg/mL | – | |
| N.I. | S. cerevisiae diastaticus | Black rice | 3.90 | ≈1.00 μg/mL | – | |
| Zapata et al. (2019) | American amber ale | Saccharomyces | Quince var. PUM | 5.52 | 0.17 mg pirogalol/g | 0.72 mmol ET/100 g** |
| American amber ale | Saccharomyces | Quince var. ZM9 | 5.51 | 0.18 mg pirogalol/g | 0.73 mmol ET/100 g** | |
| American amber ale | Saccharomyces | Quince var. Vranja | 5.62 | 0.16 mg pirogalol/g | 0.72 mmol ET/100 g** | |
| Leskošek-Čukalović et al. (2010) | N.I. | N.I. | Lemon balm (0,2-0,3%) | – | – | 5.4 μL/mL (IC50)* |
| N.I. | N.I. | Thyme (0,2-0,3%) | – | – | 3.3 μL/mL (IC50)* |
N.I.: No information; AF: Addition before fermentation; DF: Addition after fermentation; IC50: half maximum inhibitory concentration. *DPPH assay, ** ABTS assay, *** Not specified.
Considering that conventional beer has nutrients and bioactive compounds of interest, depending on innovation in ingredients and processes, craft beers with better characteristics can be developed, which will be shown in the following sections.
4. Determinants of craft beer composition: emphasis on PCs
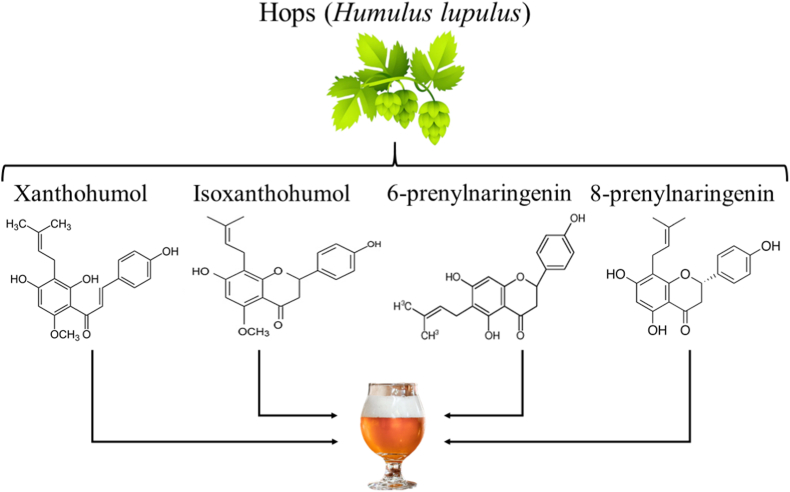
The presence and amount of nutrients and bioactive compounds (mainly PCs) in beer depends on ingredients such as malted grains and cereals (barley, wheat, oats and rice), hops, adjuncts such as fruits and spices, and microbes (Saccharomyces yeasts or co-fermenting bacteria of the genus Lactobacillus) (Simone et al., 2021). The main PCs in beer are obtained from malt and hops (Salantă et al., 2020a). The most representative PCs are xanthohumol (XH), isoxanthohumol (IXH) and other prenylflavonoids such as 6-prenylnaringenin (6-PN) and 8-prenylnaringenin (8-PN), which are obtained from hops (Fig. 4) (Osorio-Paz et al., 2020).
Fig. 4.
Main PCs in beer obtained from hops.
The objective of diversification in craft brewing is to obtain products with unique aromas, colors and flavors, but also with health benefits like other PC-rich foods. For example, antioxidant activity (AA) varied significantly in craft beers fermented with different S. cerevisiae strains (Postigo et al., 2021). Viana et al. (2021) evaluated the influence of four S. cerevisiae strains on PCs and AA of Pale Ale-style craft beers; a better polyphenolic profile and higher AA were obtained with strain US-05. Postigo et al. (2021) brewed craft beer with more than 100 strains of the genus Saccharomyces and the results were compared with beers brewed with the commercial yeast S. cerevisiae Safale S-04. Strain G 520 produced the highest amount of melatonin (a hormone with antioxidant properties) and provided higher AA (Postigo et al., 2021). The incorporation of the probiotic yeast S. cerevisiae boulardii in craft beers brewed with traditional S. cerevisiae increased TPC and AA (Capece et al., 2018).
Regarding the use of alternative ingredients, Nardini and Garaguso (2020) brewed craft beers with 8 different fruits/fruit by-products; TPC, total flavonoid content and AA were higher by 32.3, 51.33 and 42.73%, respectively, compared to commercial beers. Filho et al. (2021) evaluated the influence of spice addition on TPC and AA of green (15 days post-bottling) and aged (196 days post-bottling) red ale craft beers. The highest TPC (295 and 221 mg gallic acid equivalents (GAE)/L), the best AA by DPPH (0.65 and 0.56 mmol Trolox equivalent (ET)/L) and FRAP (408 and 350 mmol ET/L) assay in green and aged craft beers were obtained by adding turmeric extract (25 and 50%) + black pepper extract (37.5 and 20%) + aromatic hops (37.5 and 30%) (Filho et al., 2021). The addition of leaves (0.1 and 0.5%) or extract (0.05 and 0.1%) of Ocimum selloi before or after fermentation in the brewing of a Pilsner craft beer increased TPC from 291.2 to 360.6–371.9 μg GAE/mL and AA from 45.1 to 50.3–82.5% (Piva et al., 2021).
5. PCs and AA of craft beers
AA is especially attributed to the PCs content, which are found in proportions of approximately 70% in barley and 30% in hops (Capece et al., 2018). As mentioned, in craft beers, non-conventional ingredients are also a rich source of PCs (and AA), but hops are not usually substituted for their bitterness and characteristic PCs: XH, IXH, 6-PN and 8-PN. Table 2 shows the TPC and AA of different craft beers. Horn et al. (2021) determined the content of flavonoids and tannins in Brazilian craft beers, ranging from 0.84 to 3.06 mg quercetin equivalents (QE)/mL and 0.63–1.40 mg GAE/mL, respectively. The AA value (DPPH assay) ranged from 38.50 to 73.82% and correlated strongly with TPC (Horn et al., 2021). The TPC of Italian craft beers ranged from 65.6 to 105.3 μg/kg (Cortese et al., 2020). The TPC, total flavonoid content, AA by FRAP, ABTS and ORAC assay of craft beers from Chile, United Kingdom, Hawaii, and Denmark ranged from 503.97 to 1366.85 mg GAE/L, 439.23–716.15 mg EQ/L, 1.28–4.12 mmol ET/L, 1.32–2.74 mmol ET/L, and 4.71–28.75 mmol ET/L, respectively (Bustos et al., 2019). TPC, ortho-diphenols and flavonoids in craft beers from Portugal ranged from 303 to 1614 g AG/mL, 106–2216 g AG/mL, and 54–750 g catechin (CAT)/mL, respectively. AA by DPPH and ABTS assay was between 0.850 and 12.109 mmol Trolox/L, and 1.142 and 10.913 mmol Trolox/L, respectively (Breda et al., 2022).
The total flavonoid content of craft beers brewed with sweet potato variety Beauregard (30–70%) was 16.02–21.31 mg EQ/L (Humia et al., 2019). AA by ABTS and FRAP assay of a non-alcoholic craft beer with 10% red-colored Cornelian cherry juice was approximately 1.3 and 1.8 μmol ET/L, respectively (Adamenko et al., 2020). AA by ABTS and FRAP assay of craft beers with juice and fruit (10% each) of hawthorn was approximately 2.04 and 1.35, and 1.36 and 0.87 mmol ET/L, respectively (Gasiński et al., 2020b). AA by ABTS and FRAP assay of craft beers with mango juice and pulp (20% each) was approximately 1.74 and 1.69, and 1.25 and 1.32 mmol ET/L, respectively (Gasiński et al., 2020a). Total flavonoid content and AA by FRAP and ORAC assay in craft beers with addition of Parastrephia lucida leaves (0.1–5%) ranged from 0.35 to 0.60 mg/mL, from 2.17 to 5.46 mmol ET/L, and from 10.14 to 30.58 mmol ET/L, respectively (Bustos et al., 2019). The total flavonoid content of craft beers brewed with rice malt or rice malt with defatted rice bran was 2.73 and 5.34 mg/L, respectively (Prestes et al., 2019).
6. Health benefits of craft beer consumption: Role of phenolic compounds and ethanol
PCs are widely studied for their ability to scavenge reactive oxygen species and free radicals that cause oxidative damage and cellular stress (Vazquez-Cervantes et al., 2021). Caon et al. (2021) analyzed the influence of moderate consumption of commercial beer and craft beer for 30 days on redox status and liver integrity of Wistar rats exposed to carbon tetrachloride. Craft beers had higher TPC (catechin, rutin, epicatechin, caffeic acid and isoxanthohumol) and AA than traditional beers. Neither beer had harmful effects on the organism of the rats, but neither did they have a hepatoprotective effect. However, imperial red craft beer increased the enzymatic and non-enzymatic redox state; the activity of catalase (CAT), superoxide dismutase 2 (SOD2) and SOD3 was enhanced (Caon et al., 2021).
It is known that excessive ethanol consumption generates harmful effects on human health due to its toxicity; for example, the incidence of cirrhosis was positively correlated with alcohol intake in women in the United Kingdom (Simpson et al., 2019). Healthy men and women (mean age of 29.4 years) drank (330 mL/day for 3 weeks) industrial (11.9 g ethanol) and craft beer (23.8 g ethanol) with similar folate, vitamin B6 and B12 content, but different ethanol concentration (Rossi et al., 2021). Consumption of industrial beer reduced homocysteine levels in the blood, while consumption of craft beer increased the concentration due to the higher ethanol content (Rossi et al., 2021). Moderate ethanol consumption has been linked with multiple health benefits (Osorio-Paz et al., 2020); therefore, the consumption of craft beer (with low alcohol concentration) may be more beneficial than it appears. People who consume alcohol moderately and gradually have a lower risk of different diseases and a lower mortality rate compared to those who do not consume alcohol (or other alcoholic beverage) or those who consume alcohol in excess.
Despite the findings presented, the discussion continues as to whether or not it is beneficial to consume non-alcoholic beers. It should be considered that there are vulnerable people who cannot consume alcohol regardless of the dose, such as children, adolescents, pregnant women, people with certain heart, liver or pancreatic diseases. In this section, health benefits provided by the consumption of alcoholic and non-alcoholic beers (mainly craft beer) are mentioned in order to have a broad overview on the individual and/or synergistic effect of PCs (highlighting those obtained from hops) and ethanol. There are several studies on the brewing of craft beers, but they focus on sensory and physicochemical characteristics, nutrient content and bioactive compounds. Since studies on the influence of the consumption of craft beers on health are scarce, studies on beer and related beverages will be shown to serve for discussion and to suggest the potential of craft beers.
Because the design of the studies is often questioned, we show all the details of the animal and human studies in Table S1 and provide our opinion in the corresponding section. First, most studies are in vitro (mainly in the Cancer section) because they are relatively inexpensive and simple, but do not simulate the conditions and metabolic changes in the organism. There are also a considerable number of studies with rat and mouse models because they grow rapidly, are tractable and share high genetic similarities with humans, so the results can be easily extrapolated. To be applicable to humans, the experiments must be prolonged, the sample must be large and with varied characteristics, namely age, diet, physiological state and microflora, which were not met in many of the studies evaluated. For example, Seitz et al. (2021) (Cancer section) selected a sample of only 17 mice (10 in the control group and 7 in the intervention group) aged 10 weeks, did not mention lifestyle or diet before or during the intervention, and the experiment lasted only 11 days. Another problem is that the studies do not specify important data such as sample number like the study by Park et al. (2022) (Diabetes and obesity section) whose design was complete (according to the characteristics we evaluated), but the experiment lasted 4 days. Thus, the studies do not evaluate long-term outcomes. All studies with rat and mouse models were carried out under controlled environmental conditions, but it would be ideal to evaluate different conditions to obtain more generalizable results.
On the other hand, clinical trials or in vivo studies in humans are rare because they are more complex and are generally performed after several in vitro and in vivo studies. As in in vivo studies, the participants, the intervention and the conditions before and during the intervention must meet several characteristics (geographic location, race/ethnicity, gender, age, number and frequency of doses). Only studies on cardiovascular diseases and with incomplete designs were found, such as Bassus et al. (2004) who examined 12 subjects (only men) and the intervention only lasted 3 h; they did not mention the type of study, standardization and monitoring of lifestyle or diet during the intervention. Alvarez et al. (2009a) only included female participants, did not mention age range or standardization of lifestyle or diet before the intervention, and only included non-alcoholic beers as samples. The range of duration between studies was from 3 h to 45 days and although the lifestyle or diet during the intervention was standardized, the nature of the study limits the control of environmental conditions, which would lead to inconclusive results. We note another feature that would affect the robustness of the study. Padro et al. (2018) monitored compliance with the adaptation period, intervention, and lifestyle or diet during the intervention by telephone contact and an interview at the end of the intervention, and although they were meticulous with the details, the validity of the results depends on the responsibility of the participants. In summary, since the study designs were not described in detail and had shortcomings, although the findings would support the beneficial effects of craft beer they should be approached with caution.
6.1. Cardiovascular diseases
The cardioprotective effect of PCs and ethanol has been reported. According to studies in rats, different PCs have decreased the concentration of plasma cholesterol in the blood, bad cholesterol (LDL) and triglyceride levels, in addition to preventing thrombosis and atherosclerosis, decreasing platelet aggregation, and reducing the risks of myocardial infarction (Scalbert et al., 2005). PCs also increase plasma nitric oxide levels leading to a lower risk of cardiovascular disease (Fernández-Solà, 2015). With respect to ethanol, it prevents atherosclerosis and reduces the risk of heart disease by inhibiting platelet aggregation and improving fibrinogenesis in the blood. It also improves lipid metabolism, lowering cholesterol and triglyceride levels (Sohrabvandi et al., 2012).
The consumption of etanol (4%), alcoholic beer (4%) and non-alcoholic beer (1 L/h for 3 h) by men (aged 19–36 years) reduced thrombin generation, monocyte platelet aggregate and the expression of the platelet activation marker CD62 and activated fibrinogen receptor (Bassus et al., 2004). Alcoholic beer had the same effects in addition to showing procoagulant action (Bassus et al., 2004). For 4 weeks, men (mean age of 61 years) consumed 660 mL of alcoholic beer per day (30 g etanol, 1209 mg PCs) or 990 mL of non-alcoholic beer per day (<1 g ethanol, 1243 mg PCs) (Chiva-Blanch et al., 2015). There were no changes in the glucose profile, adiposity or weight of the individuals. By PCs content, non-alcoholic beer consumption reduced proinflammatory cytokines and leukocyte adhesion molecules. Due to ethanol, alcoholic beer improved the lipid profile (Chiva-Blanch et al., 2015). Non-alcoholic beer (250 mL/twice a day for 45 days) reduced carbonyl group and thiobarbituric acid reactive substances in plasma of women (mean age of 64.5 years) (Alvarez et al., 2009b). The concentration of antioxidants such as α-tocopherol and erythrocyte glutathione was increased and the lipid profile was improved (Alvarez et al., 2009b). Daily consumption (for 1 month) of 660 mL of alcoholic beer (30 g ethanol, 1208 mg PCs) or 660 mL of non-alcoholic beer (ethanol free and 828 mg PCs) in men and half in women (both aged 40–60 years) did not generate changes in weight or other aspects of health (Padro et al., 2018). Consumption of both beers reduced the oxidative susceptibility of LDL cholesterol and enhanced the antioxidant properties of good cholesterol (HDL). Consumption of alcoholic beer promoted cholesterol efflux (Padro et al., 2018).
Men (mean age of 61 years) with high cardiovascular risk consumed 660 mL of alcoholic or non-alcoholic beer (with an equivalent TPC) or gin (with an equivalent ethanol content) per day for 4 weeks, finding that the consumption of both beers considerably increased the number of endothelial progenitor cells, but gin consumption did not cause this effect (Chiva-Blanch et al., 2014). Because of this, the positive effect was attributed to ethanol of the beers (Chiva-Blanch et al., 2014). This also occurred when healthy men (mean age of 25 years) exposed to oxygen-induced arterial stiffness and oxidative stress consumed beer, wine or vodka (Krnic et al., 2011). All beverages provided protection against arterial stiffness, but only wine provided defense against oxidative stress due to its high TPC (2.5 g GAE/L) compared to beer (0.4 g GAE/L) (Krnic et al., 2011). Neto et al. (2017) determined that beers with higher TPC provided more pronounced vasodilator effects.
Regarding studies in animal models, the intake of craft beer fortified (for 4 weeks) with XH was evaluated in male Wistar rats with induced pulmonary arterial hypertension (Silva et al., 2019). Pulmonary vascular remodeling (mainly in the right ventricle) was observed; ethanol consumption (5.2%) was also evaluated, but did not show significant beneficial effects (Silva et al., 2019). Non-alcoholic beer consumption (42 mL/kg body weight per day) for 20 weeks in knockout mice provided protection against atherosclerosis (Martinez et al., 2011).
6.2. Cancer
There is sufficient evidence to show that beer or craft beer consumption helps to counteract carcinogenesis. According to animal and human studies, beer intake prevents tumor growth and metastasis by inhibiting angiogenesis, a nutrient and oxygen supply process necessary for the growth of malignant tumors (Sohrabvandi et al., 2012). This potential is due to the chemopreventive, anti-inflammatory and antioxidant characteristics of prenylflavonoids and bitter acids (α or humulones and β or lupulones) from hops (Osorio-Paz et al., 2020). This is based on the induction of detoxifying enzymes and inhibition of the metabolism of procarcinogens; PCs can inhibit inflammation- and cancer-related transcription factors: NF-κB, TGF-β, TNF-α and vascular endothelial growth factor (Serwe et al., 2012; Negrão et al., 2013). It was also reported that they can induce tumor apoptosis, preventing tumor formation or growth (Scalbert et al., 2005). In addition, in the early stages PCs can inhibit inflammatory signals of angiogenesis (Sohrabvandi et al., 2012). Low alcohol content in craft beers or moderate consumption may also promote decreased cancer risk. In a study involving analysis of data from 19,149 cases and 362,340 controls worldwide, lung cancer risk was not associated with light or moderate consumption of alcoholic beverages, but was associated with heavy consumption (Brenner et al., 2019). However, in a multiethnic cohort study with data from 190,698 participants, beer and wine consumption was associated with colorectal cancer risk (Park et al., 2019).
Administration of different PCs in animal models before or after the application of a carcinogenic agent reduced the number/size of tumors located in the stomach, mouth, colon, kidney, skin, liver, breast, etc. (Osorio-Paz et al., 2020). Specifically, moderate consumption of alcoholic or non-alcoholic beer, or supplementation of compounds such as XH, IXH, 6- and 8-PN have been successful as a potential therapeutic treatment or to prevent the risk of leukemia, cholangiocarcinoma, breast, laryngeal, colon, kidney, prostate and pancreatic cancer (Infante-Rivard et al., 2002; Busch et al., 2015; Li et al., 2016; Saito et al., 2018) in vitro, in rats/mice or in humans. Table 3 shows further studies in vitro and with results based on the IC50 value.
Table 3.
Anticancer effect of beer intake or its compounds on different cell lines.
| Reference | Raw materials/compounds | Human cell line | Treatment time (h) | Concentration (IC50, μM) |
|---|---|---|---|---|
| Ambrož et al. (2019) | XH | Colorectal (SW480) | 72 | 3.6 |
| Colorectal (SW620) | 72 | 7.3 | ||
| IXH | Colorectal (SW480) | 72 | 40.4 | |
| Colorectal (SW620) | 72 | 43.7 | ||
| 6-PN | Colorectal (SW480) | 72 | 14.8 | |
| Colorectal (SW620) | 72 | 13.7 | ||
| 8-PN | Colorectal (SW480) | 72 | 56.3 | |
| Colorectal (SW620) | 72 | 24.9 | ||
| Hudcová et al. (2014) | XH | Colorectal (HT-29) | 96 | 1.2 |
| Colorectal (SW620) | 96 | 2.5 | ||
| IXH | Colorectal (HT-29) | 96 | 16.9 | |
| Colorectal (SW620) | 96 | 37.3 | ||
| Bartmańska et al. (2018) | XH | Ovarian (A2780) | 72 | ≈2 |
| Breast (MDA-MB-231) | 72 | ≈8 | ||
| Breast (T-47D) | 72 | ≈8 | ||
| Prostate (PC-3) | 72 | ≈8 | ||
| Prostate (DU 145) | 72 | ≈6 | ||
| α, β-dihydroxanthohumol | Ovarian (A2780) | 72 | ≈1.9 | |
| Breast (MDA-MB-231) | 72 | ≈10 | ||
| Breast (T-47D) | 72 | ≈7 | ||
| Prostate (PC-3) | 72 | ≈16 | ||
| Prostate (DU 145) | 72 | ≈13 | ||
| IXH | Ovarian (A2780) | 72 | ≈8 | |
| Breast (MDA-MB-231) | 72 | ≈40 | ||
| Breast (T-47D) | 72 | ≈26 | ||
| Prostate (PC-3) | 72 | ≈50 | ||
| Prostate (DU 145) | 72 | ≈50 | ||
| 6-PN | Ovarian (A2780) | 72 | ≈35 | |
| Breast (MDA-MB-231) | 72 | ≈50 | ||
| Breast (T-47D) | 72 | ≈25 | ||
| Prostate (PC-3) | 72 | ≈75 | ||
| Prostate (DU 145) | 72 | ≈75 | ||
| 8-PN | Ovarian (A2780) | 72 | ≈25 | |
| Breast (MDA-MB-231) | 72 | ≈50 | ||
| Breast (T-47D) | 72 | ≈15 | ||
| Prostate (PC-3) | 72 | ≈50 | ||
| Prostate (DU 145) | 72 | ≈62 | ||
| Roehrer et al. (2019) | XH | Breast (MCF-7) | 144 | 7.1 |
| Analogue XH C | Breast (MCF-7) | 144 | 1.70 | |
| Hop extract enriched with XH (65–85%) | Breast (MCF-7) | 144 | 2.2 | |
| Engelsgjerd et al. (2019) | XH | Neuroblastoma (NGP) | 96 | ≈12.00 |
| Neuroblastoma (SH-SY-5Y) | 96 | ≈12.00 | ||
| Neuroblastoma (SK–N-AS) | 96 | ≈12.00 | ||
| Cho et al. (2018) | XH | Fibrosarcoma (HT1080) | 48 | 10.60 |
| Delmulle et al. (2006) | XH | Prostate (PC-3) | 48 | 13.20 |
| Prostate (DU145) | 48 | 12.30 | ||
| Desmethylxanthohumol | Prostate (PC-3) | 48 | 49.90 | |
| Prostate (DU145) | 48 | 53.80 | ||
| 6-PN | Prostate (PC-3) | 48 | 18.40 | |
| Prostate (DU145) | 48 | 29.10 | ||
| 8-PN | Prostate (PC-3) | 48 | 33.50 | |
| Prostate (DU145) | 48 | 43.10 | ||
| Stompor et al. (2017) | 8-PN | Glioblastoma (U-118 MG) | 24 | 138 |
| Merinas-Amo et al. (2021) | Lyophilised blond ale beer | Leukemia (HL-60) | 72 | 62.5 mg/mL |
| Lyophilised stout ale beer | Leukemia (HL-60) | 72 | 15.63 mg/mL | |
| Tyrosol | Leukemia (HL-60) | 72 | 127 mg/mL | |
| Alonso-Esteban et al. (2019) | Hop seed extract | Hepatocellular (HepG2) | – | 278 mg/La |
| Lung (NCI–H460) | – | 184 mg/La | ||
| Cervical (HeLa) | – | 244 mg/La | ||
| Breast (MCF-7) | – | 251 mg/La | ||
| Liver (PLP2) | – | >400 mg/La |
GI50: concentration for 50% of the maximum inhibition of cell proliferation.
Administration of xanthohumol (10 mg/kg/day for 11 days) inhibited the growth of melanoma metastasis in the liver in mice (10 weeks old) (Seitz et al., 2021). Administration of a formulation based on malts and hops (3–300 mg/kg/day for 14 weeks, 5 times per week) showed a protective effect on the first and second phase of colon cancer in mice (Tedesco et al., 2021).
6.3. Neurodegenerative diseases
Light or moderate alcohol consumption influenced the reduction of dementia in people older than 54 years (Sohrabvandi et al., 2012). One study evaluated the effect of consumption of beer, wine or spirits (with equivalent volume of alcohol) on the incidence of dementia in women aged 38–68 years (Mehlig et al., 2008). Wine was associated with greater longevity and lower incidence of dementia, beer showed the same potential, but to a lesser extent, and spirits did not show these properties (Mehlig et al., 2008). In a similar study, only the intake (weekly and monthly) of wine was linked with a lower risk of dementia in people over 64 years because it has a higher amount of PCs than beer, considering a traditional beer, since a craft beer may have higher phenolic potential (Truelsen et al., 2002). Regular consumption of alcoholic beverages was found to be healthier than being a abstainer or heavy drinker.
The consumption of non-alcohol beer (5 mL/day for 3 months, 0.9% v/v) was also evaluated in rats intoxicated with aluminum nitrate (Merino et al., 2018). Beer inhibited in vitro the activity of acetylcholinesterase and activated in vivo the transcription factor Nrf2, which is associated with the expression of detoxifying agents and antioxidants, thus providing protection against neurodegenerative effects due to intoxication and stabilizing/improving the rats' behavior (Merino et al., 2018). Extracts of dark beer > non-alcoholic beer > lager beer showed a high potential against induced oxidative stress and cell death in human and rat cancer cell lines, in addition to properly modulating adenosine receptors (Alonso-Andrés et al., 2019). Old mice treated with 1 or 5 mg XH/kg daily for 30 days had a significant reduction of proinflammatory cytokines and proapoptotic markers, protecting against age-related brain damage (Rancán et al., 2017). Craft beer consumption (6–7 mL/day for 4 months) improved cognitive functions of mice (8 weeks old) by reducing the amount of amyloid peptides and inflammatory cytokines (Cecarini et al., 2022).
6.4. Diabetes and obesity
In vitro and in vivo experiments have shown properties of PCs to prevent or combat various metabolic disorders. Diabetes and obesity were emphasized in this section because of the relationship between them. As mentioned in the Cardiovascular Diseases section, the literature states that craft beer consumption (due to its alcoholic fraction and mainly due to the polyphenolic fraction) could help to reduce triglyceride and bad cholesterol (LDL) levels, in addition to increasing good cholesterol (HDL) levels. PCs can stimulate glucose uptake, induce insulin release by pancreatic β-cells and even inhibit gluconeogenesis (Scalbert et al., 2005). XH, IXH, 6-PN and 8-PN helped to improve adipocyte metabolism and glucose tolerance in mice with obesity and diabetes (Everard et al., 2012).
Lima-Fontes et al. (2017) analyzed the effect of supplementation with ethanol, stout beer or stout beer with 10 mg XH/L for 5 weeks in Wistar rats with induced type I diabetes. In contrast to treatment with stout beer or ethanol, XH-enriched stout beer prevented alterations in the catabolic state of the liver (Lima-Fontes et al., 2017). When XH (2.5 mg/kg bw/day for 8 weeks) was fed to mice, glucose intolerance and body weight gain were inhibited (Mahli et al., 2019). The expression of inflammatory markers and alpha collagen was reduced, in addition to decreased immune cell infiltration and the activation of hepatic stellate cells (Mahli et al., 2019). Costa et al. (2017) evaluated the effect of supplementation with XH (10 mg/L) or 8-PN (10 mg/L) for 20 weeks in mice fed a high-fat diet plus + 0.1% ethanol. Both treatments prevented body weight gain, lowered blood glucose, cholesterol, triglyceride and LDL levels, increased HDL levels and improved insulin sensitivity. Specifically, acetyl-CoA carboxylase activity and lipogenic enzyme expression were decreased by inducing skeletal muscle and hepatic AMPK activation. Other signaling pathways related to fatty acid uptake were also modulated (Costa et al., 2017). In another study, XH intake (0.3 or 0.6 mg/kg bw/day for 12 weeks) in mice reduced their body weight gain, glucose, cholesterol, triglyceride and LDL levels. The effects were XH dose-dependent, unlike the increase in HLD, as the lower dose had a greater effect than the higher dose (Miranda et al., 2016). Administration of 8-PN (50 mg/kg/day for 4 days) in mice (7 weeks old) showed anti-diabetic effect by regulating glucose homeostasis (Park et al., 2022).
6.5. Osteoporosis
Beer can reduce the risk of osteoporosis due to its content of PCs, bitter acids and also because it is a good source of silicon (Sohrabvandi et al., 2012). In in vitro and in vivo studies, treatment with various PCs restored or decreased bone mineral density loss, increased the number of osteoblasts and reduced dentin resorption (Scalbert et al., 2005). It was also reported that moderate ethanol consumption helped prevent the development of osteoporosis (Redondo et al., 2018). Moderate consumption of beer and wine increased bone mineral density in the lumbar spine and hip in men (mean age of 61.5 years), premenopausal women (mean age of 48.3 years) and postmenopausal women (mean age of 62.5 years) compared to abstainers (Tucker et al., 2009). In constrast, the consumption of liquor (high levels of alcohol) produced negative effects on the bone mineral density of the participants (Tucker et al., 2009). Hops extract (1 or 2 g/kg/day/2 months, [6 days per week]) provided protection against osteoporosis in mice (9 weeks old) by inhibiting oxidative stress and β-amyloid deposition (Xia et al., 2023).
Studies in human cells analyzed the effect of XH as a therapeutic treatment for osteoclast-associated diseases (Jeong et al., 2011; Suh et al., 2013; Li et al., 2016). XH prevented the formation of early stage osteoclasts and also stimulated their differentiation by regulating the RUNX2 gene. Osteoblast activity was also inactivated, preventing bone resorption and destruction due to the ability of XH to inhibit the NF-κB and Ca2+/NFATc1 signaling pathway, preventing the expression of osteoclastogenesis-related transcription factors and the expression of osteoclast genes linked with bone resorption. Finally, XH induced the expression of osteogenic marker genes (Jeong et al., 2011; Suh et al., 2013; Li et al., 2016). These properties are of interest to prevent diseases such as arthritis, periodontitis and osteoporosis.
6.6. Immune system
For many years, oxidation and inflammation have been associated with multiple diseases. Inflammation is the immune system's response to face attacks from internal or external agents. Inflammatory responses generate oxidative stress which, in turn, aggravates the severity of the inflammatory state. Based on Vazquez-Cervantes et al. (2021), the correct coordination of adaptive and innate immune responses achieves control of the initiated inflammation, which leads to homeostasis of the organism. If inflammation occurs in excess, various age-related disorders (oxidative stress and aging) may develop. Compounds with high antioxidant, anti-inflammatory and/or immunomodulatory activity are currently of interest. These characteristics are fulfilled by the compounds present in beer and mainly in craft beer.
Moderate alcohol consumption in healthy adults (330 mL/day for women and twice as much for men for one month) increased immunoglobulin G, M and A (IgG, IgM and IgA) and anti-inflammatory cytokines IL-2, -4 and -10 (Romeo et al., 2007). It also increased the production of IFN-γ and CD3+ lymphocytes, but only in women (Romeo et al., 2007). In another study, alcoholic and non-alcoholic beer decreased the production of proinflammatory cytokines (Chiva-Blanch et al., 2015).
In in vivo and in vitro assays, treatments with hop extracts rich in PCs and bitter acids modulated the levels of proinflammatory and anti-inflammatory cytokines. Mitigation of signaling pathways such as TLR2 and TLR4, repolarization of proinflammatory macrophage M1 to anti-inflammatory macrophage M2 and decreased levels of inflammatory markers such NO, COX-2 enzyme and PGE2 were reported (Hougee et al., 2006; Lupinacci et al., 2009; Schink et al., 2018). Specifically, XH also showed these properties by preventing or ameliorating ulcerative colitis and osteoarthritis in human cells and in mice (Cho et al., 2018; Chen et al., 2021).
On the other hand, according to Yoo et al. (2020); Zheng et al. (2020), the microbiome plays a role in the development of components (proteins, carbohydrates, vitamins) of the immune system, while the immune system sets the stage for symbiosis between host and microorganisms. Homeostasis and diseases depend on the microbiota-immune system interaction and, in turn, on the environmental conditions of the host, diet, lifestyle, etc. If the balance is disturbed, irregular immune responses could cause inflammation and oxidative damage, or they can also induce the proliferation of pathogens and lead to infections (Yoo et al., 2020; Zheng et al., 2020).
In the craft beer scenario, probiotics provide health benefits to consumers due to their role in the gut microbiota. S. boulardii is the most widely used probiotic, it is safe, efficient, with higher resistance, better immunomodulatory and antimicrobial properties (Pais et al., 2020). S. boulardii is shown to be the ideal fermenter or co-fermenter for probiotic craft beers (with and without alcohol) with high numbers of viable cells, higher AA and PCs content, and improved sensory characteristics (Capece et al., 2018; Mulero-Cerezo et al., 2019; Palomino-Vasco et al., 2019; Senkarcinova et al., 2019; Silva et al., 2019, 2021; Pereira de Paula et al., 2021). S. boulardii regulates the gut microbiota by producing antimicrobial metabolites and some with immunomodulatory activity (Canani et al., 2011; Mellor et al., 2020). Regarding other probiotics, craft beer was successfully brewed with S. cerevisiae S-04 and Lactobacillus paracasei L26 (Canani et al., 2011). The polyphenolic content also positively influences the gut microbiota. The intake of PCs induces the production of short-chain fatty acids, which improves intestinal permeability, reduces intestinal inflammation and endotoxemia (Redondo et al., 2018).
Consumption of gin (without PCs) in excess increased bacteria of the genus Clostridium in the gut microbiota (Quesada-Molina et al., 2019). Consumption of alcoholic beer or non-alcoholic beer (355 mL per day for 30 days) favored the proliferation of Bacteroidetes, indicating a well-balanced microbiota. Alcohol in beer reduced the enrichment effect of microbiota diversity (Hernández-Quiroz et al., 2020). González-Zancada et al. (2020) determined that moderate beer consumption reduced the population of Clostridiaceae and increased the population of Blautia, Pseudobutyrivibrio, Butyrivibrio and Johnsonella. Abstainers or occasional beer consumers showed no changes in the diversity of their microbiota (González-Zancada et al., 2020). In this case, the PCs acted as prebiotics that enrich the beneficial microorganisms (Quesada-Molina et al., 2019) causing the inhibition of the proliferation of pathogens ¿. According to the evaluation of phenolic acids in beer, they are absorbed in the gastrointestinal tract and remain in the blood after metabolism (Nardini et al., 2006). Likewise, XH administration regulated the gut microbiota in a mouse model (Liu et al., 2022) and in humans (Langley et al., 2021). The consumption of different beers positively modulated the composition of gut microbiota in humans and the effect was positively associated with the content of PCs (Martínez-Montoro et al., 2022).
7. Current challenges and future outlook
PCs impart unique sensory characteristics to beers in general, but some can cause undesirable flavor and aroma. Guaiacol imparts a clove flavor, which is undesirable in some beers (Postigo et al., 2021). Similarly, isovaleric acid, 4-ethylphenol, 4-ethylcatechol and 4-ethylguaiacol are responsable for aromas or flavors of band-aids, antiseptic, smoked bacon, cloves, spice, barnyard, horse stable, rancidity, cheese and sweaty animals in fermented beverages such as beer (Alston et al., 2021). In this context, even if a craft beer is rich in PCs and with potential health benefits, the ingredients and operating parameters must be carefully controlled so that it is also sensorially pleasing.
Craft beer may also contain compounds that are harmful to health because it is not pasteurized, despite the use of different ingredients in its preparation. Biogenic amines stand out because they can cause hypotension, hypertension, migraine, gastrointestinal distress and pseudoanaphylaxis (Koller and Perkins, 2022). Tyramine > putrescine > cadaverine > histamine > phenylethylamine was detected in Central European craft beers and in higher concentrations at the end of the best before date (Lorencová et al., 2020). In craft beer samples from Spain, agmatine > ethanolamine > putrescine > tyramine was detected (Palomino-Vasco et al., 2019) and in another study putrescine > tyramine > histamine > cadaverine > tryptamine > 2-phenylethylamine was detected (Poveda, 2019). Other toxic compounds detected in craft beers included acrolein, ethyl carbamate, formaldehyde, acetaldehyde, furfuryl alcohol and furfural (Hernandes et al., 2020).
In the same line, due to the lack of heat treatment and filtration processes, craft beers are susceptible to microbial contamination and deterioration of their sensory and physicochemical characteristics. In addition, the use of unconventional ingredients rich in organic matter increases craft beer spoilage. According to Liping (2018), a large number of spoiled craft beers were found in China in the summer of 2018. Garofalo et al. (2015) detected Lactobacillus brevis, enterobacteria and other bacteria of the genus Staphylococcus and Acetobacter in spoiled craft beers. 68% of craft beer samples from Spain evaluated had the presence of exogenous microorganisms (yeasts > lactic acid bacteria (LAB) > acetic bacteria) and the concentration was proportional to the number of ingredients used in the brewing process (López et al., 2020). The most common microorganisms of concern are LAB due to their relationship with the production of biogenic amines. Lactobacillus, Leuconostoc and Pediococcus were identified in craft beers in Spain; and when isolated and inoculated into commercial beers, the concentration (Lactobacillus [putrescine and tyramine] > Pediococcus [putrescine] > Leuconostoc [putrescine]) of biogenic amines was considerable (Rodríguez-Saavedra et al., 2020). Higher concentrations of ochratoxin A (mycotoxin) were also detected in craft beers than in industrial beers (Rodríguez-Saavedra et al., 2020). Aflatoxin B1, aflatoxin B2, aflatoxin M1, HT-2 toxins, T-2 toxins, zearalenone, β-zearalenone, zearalenone-14-sulfate, ochratoxin A, deoxynivalenol, deoxynivalenol 3-glucoside, fumonisin B1 and fumonisin B3 were detected in craft beers from 42 countries (Peters et al., 2017).
Craft brewers should implement strategies to avoid economic losses due to the deterioration of the quality of craft beers. Producing craft beers with undesirable characteristics also significantly affects the reputation of the product and the producer, which could lead to ruin considering the high level of competition in the market. Craft breweries lack microbiological laboratories for the rapid detection and identification of spoilage microorganisms (Rodríguez-Saavedra et al., 2020); therefore, analyses can be performed in external laboratories, but craft brewers do not have the necessary financial resources. An efficient and feasible alternative is to establish a plan of gradual cleaning and disinfection (based on regulations) in all areas of the brewery to limit contamination (Garofalo et al., 2015). Manzano et al. (2011) significantly reduced the presence of LAB and yeasts in craft beers in Italy when they implemented strict hygiene practices in the brewery.
Non-thermal preservation techniques that do not significantly affect the characteristics of the beer could also be used. These include the use of high hydrostatic pressures, cold plasma, pulsed electric fields, irradiation, microwaves and ultrasound. Treatment with high hydrostatic pressure significantly reduced the concentration of S. cerevisiae ascospores in beers, without affecting the overall flavor (Milani and Silva, 2016). High hydrostatic pressures inhibited the development of lactic acid bacteria and aerobic bacteria in cloudy beers, in addition to improving turbidity, without affecting the physicochemical and sensory characteristics, but did increase the color value (Yin et al., 2016). Pulsed electric fields were efficient in microbial decontamination in red wines, without affecting their sensory and enological characteristics (Delso et al., 2023). Ohmic heating produced sterile fruit craft beers with different but acceptable chemical and sensory characteristics (Fanari et al., 2020). Peña-Gómez et al. (2020) used filtration through silica microparticles as a cold pasteurization technique and obtained microbiologically stable craft beers with sensory characteristics without significant differences compared to untreated samples. These technologies could also induce the increase of PCs, which was tested in several foods (Jacobo-Velázquez et al., 2021). This should also be investigated in depth in order to offer higher quality craft beers. Depending on the processing conditions, high-pressure processing increased the PCs content in filtered and unfiltered beers (Štulíková et al., 2021). In one study, pulsed electric fields and ultrasound did not significantly affect the content of PCs in wines during two months of storage, whereas high hydrostatic pressures induced an increase and higher stability depending on the type of wine (van Wyk et al., 2021). As can be seen, the use of non-thermal pasteurization processes in beer is scarce, mainly in the field of craft beer. This may be due to the fact that craft beers are produced on a small scale and by small producers who cannot acquire these technologies. First, government support is required through laws that exclusively support microbrewers. Another alternative is for them to form associations with multiple advantages, namely, a) attracting the attention of the government; b) access loans to enhance technical/technological capacity; c) acquire raw materials in greater volume and at lower cost; d) improve competitiveness and obtain better market opportunities.
8. Conclusions
Craft beer is constantly innovating to meet the needs of the most demanding consumers. Its differentiated sensory characteristics are consolidating it as a product that could dethrone commercial beer, and more quickly in sectors with greater purchasing power. There is a wide variety of craft beers made with different raw materials, strains and even processes that define their content of macronutrients, micronutrients and phytochemicals. Due to the high and varied content of PCs, craft beers have potential to provide health benefits, just like other superfoods. Low-alcohol craft beers or moderate consumption are also shown to be better alternatives to being teetotal, although the benefits of alcohol are still under discussion. However, more studies with animal models and mainly clinical trials with complete and robust designs are required to obtain more generalizable and conclusive results. There are some challenges such as the presence of toxic compounds and undesirable microorganisms in craft beer because it does not undergo thermal processing to maintain its unique characteristics. It is suggested to implement strict hygiene measures to reduce the contamination and to evaluate the effect of non-thermal pasteurization treatments because, according to the literature, it could also enhance bioactive compounds in craft beer. Finally, the creation of exclusive laws for craft brewers and their association can help overcome the economic, technical and technological barriers that limit craft beer production almost everywhere in the world.
CRediT authorship contribution statement
Vicente Amirpasha Tirado-Kulieva: Conceptualization, Methodology, Formal analysis, Writing – original draft. Ernesto Hernández-Martínez: Methodology, Formal analysis, Writing – review & editing, Supervision. Hans Himbler Minchán-Velayarce: Formal analysis, Writing – review & editing, Supervision. Sandra Eloisa Pasapera-Campos: Conceptualization, Writing – original draft. Olivia Magaly Luque-Vilca: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to Freepik (https://www.freepik.com/) for the images (free license) used in the figures. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Handling Editor: Professor A.G. Marangoni
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100477.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
No data was used for the research described in the article.
References
- Adamenko K., Kawa-Rygielska J., Kucharska A.Z. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020;312 doi: 10.1016/j.foodchem.2019.125968. [DOI] [PubMed] [Google Scholar]
- Alonso-Andrés P., Martín M., Albasanz J.É.L. Modulation of adenosine receptors and antioxidative effect of beer extracts in in vitro models. Nutrients. 2019;11:1258. doi: 10.3390/nu11061258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Esteban J.I., Pinela J., Barros L., Ćirić A., Soković M., Calhelha R.C., et al. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crop. Prod. 2019;134:154–159. doi: 10.1016/j.indcrop.2019.04.001. [DOI] [Google Scholar]
- Alston J.M., Arvik T., Hart J., Lapsley J.T. Brettanomics I: the cost of brettanomyces in California wine production. J. Wine Econ. 2021;16:4–31. doi: 10.1017/jwe.2020.20. [DOI] [Google Scholar]
- Alvarez J.R.M., Bellés V.V., López-Jaén A.B., Marín A.V., Codoñer-Franch P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition. 2009;25:182–187. doi: 10.1016/j.nut.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Alvarez J.R.M., Bellés V.V., López-Jaén A.B., Marín A.V., Codoñer-Franch P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition. 2009;25:182–187. doi: 10.1016/j.nut.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Ambra R., Pastore G., Lucchetti S. The role of bioactive phenolic compounds on the impact of beer on health. Molecules. 2021;26:486. doi: 10.3390/molecules26020486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrož M., Lněničková K., Matoušková P., Skálová L., Boušová I. Antiproliferative effects of hop-derived prenylflavonoids and their influence on the efficacy of oxaliplatine, 5-fluorouracil and irinotecan in human colorectalC cells. Nutrients. 2019;11:879. doi: 10.3390/nu11040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H.E., Liden T., Berger B.K., Schug K.A. Target profiling of beer styles by their iso-α-acid and phenolic content using liquid chromatography–quadrupole time-of-flight–mass spectrometry. J. Separ. Sci. 2021;44:2764–2772. doi: 10.1002/jssc.202100173. [DOI] [PubMed] [Google Scholar]
- Aredes R.S., Peixoto F.C., Sphaier L.A., Marques F.F. de C. Evaluation of craft beers through the direct determination of amino acids by capillary electrophoresis and principal component analysis. Food Chem. 2021;344 doi: 10.1016/j.foodchem.2020.128572. [DOI] [PubMed] [Google Scholar]
- Baiano A. Craft beer: an overview. Compr. Rev. Food Sci. Food Saf. 2021;20:1829–1856. doi: 10.1111/1541-4337.12693. [DOI] [PubMed] [Google Scholar]
- Bartmańska A., Tronina T., Popłoński J., Milczarek M., Filip-Psurska B., Wietrzyk J. Highly cancer selective antiproliferative activity of natural prenylated flavonoids. Molecules. 2018;23:2922. doi: 10.3390/molecules23112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassus S., Mahnel R., Scholz T., Wegert W., Westrup D., Kirchmaier C.M. Effect of dealcoholized beer (bitburger drive®) consumption on hemostasis in humans. Alcohol Clin. Exp. Res. 2004;28:786–791. doi: 10.1097/01.ALC.0000125353.93310.49. [DOI] [PubMed] [Google Scholar]
- Breda C., Barros A.I., Gouvinhas I. Characterization of bioactive compounds and antioxidant capacity of Portuguese craft beers. Int. J. Gastron. Food Sci. 2022;27 doi: 10.1016/j.ijgfs.2022.100473. [DOI] [Google Scholar]
- Brenner D.R., Fehringer G., Zhang Z.F., Lee Y.C.A., Meyers T., Matsuo K., et al. Alcohol consumption and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium and the SYNERGY study. Cancer Epidemiol. 2019;58:25–32. doi: 10.1016/j.canep.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewers Association American craft beer guide. 2011. https://cdn.brewersassociation.org/wp-content/uploads/2017/04/American_Craft_Beer_Guide_English.pdf Available at:
- Brewers Association Promoting and protecting American craft brewers. 2020. https://www.brewersassociation.org/ Available at:
- Březinová M. Beer industry in the Czech republic: reasons for founding a craft brewery. Sustainability. 2021;13:9680. doi: 10.3390/su13179680. [DOI] [Google Scholar]
- Burini J.A., Eizaguirre J.I., Loviso C., Libkind D. Non-conventional yeasts as tools for innovation and differentiation in brewing. Rev. Argent. Microbiol. 2021;53:359–377. doi: 10.1016/j.ram.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Busch C., Noor S., Leischner C., Burkard M., Lauer U.M., Venturelli S. Anti-proliferative activity of hop-derived prenylflavonoids against human cancer cell lines. Wien Med. Wochenschr. 2015;165:258–261. doi: 10.1007/s10354-015-0355-8. [DOI] [PubMed] [Google Scholar]
- Bustos L., Soto E., Parra F., Echiburu-Chau C., Parra C. Brewing of a porter craft beer enriched with the plant Parastrephia lucida: a promising source of antioxidant compounds. J. Am. Soc. Brew. Chem. 2019;77:261–266. doi: 10.1080/03610470.2019.1644478. [DOI] [Google Scholar]
- Canani R.B., Cucchiara S., Cuomo R., Pace F., Papale F. Saccharomyces boulardii: a summary of the evidence for gastroenterology clinical practice in adults and children. Eur. Rev. Med. Pharmacol. Sci. 2011;15:809–822. [PubMed] [Google Scholar]
- Caon G., Morrone M., Feistauer L., Sganzerla D., Moreira J.C.F. Moderate beer consumption promotes silymarin-like redox status without affecting the liver integrity in vivo. Food Biosci. 2021;43 doi: 10.1016/j.fbio.2021.101307. [DOI] [Google Scholar]
- Capece A., Romaniello R., Pietrafesa A., Siesto G., Pietrafesa R., Zambuto M., et al. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018;284:22–30. doi: 10.1016/j.ijfoodmicro.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Cardello A.V., Pineau B., Paisley A.G., Roigard C.M., Chheang S.L., Guo L.F., et al. Cognitive and emotional differentiators for beer: an exploratory study focusing on “uniqueness. Food Qual. Prefer. 2016;54:23–38. doi: 10.1016/j.foodqual.2016.07.001. [DOI] [Google Scholar]
- Cecarini V., Gogoi O., Bonfili L., Veneruso I., Pacinelli G., De Carlo S., et al. Modulation of gut microbiota and neuroprotective effect of a yeast‐enriched beer. Nutrients. 2022;14:2380. doi: 10.3390/nu14122380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Li Z., Hong H., Wang N., Chen J., Lu S., et al. Xanthohumol suppresses inflammation in chondrocytes and ameliorates osteoarthritis in mice. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111238. [DOI] [PubMed] [Google Scholar]
- Chiva-Blanch G., Condines X., Magraner E., Roth I., Valderas-Martínez P., Arranz S., et al. The non-alcoholic fraction of beer increases stromal cell derived factor 1 and the number of circulating endothelial progenitor cells in high cardiovascular risk subjects: a randomized clinical trial. Atherosclerosis. 2014;233:518–524. doi: 10.1016/j.atherosclerosis.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Chiva-Blanch G., Magraner E., Condines X., Valderas-Martínez P., Roth I., Arranz S., et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: a randomized feeding trial. Nutr. Metabol. Cardiovasc. Dis. 2015;25:36–45. doi: 10.1016/j.numecd.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Cho J.M., Yun S.M., Choi Y.H., Heo J., Kim N.J., Kim S.H., et al. Xanthohumol prevents dextran sulfate sodium-induced colitis via inhibition of IKKβ/NF-κB signaling in mice. Oncotarget. 2018;9:866–880. doi: 10.18632/oncotarget.23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese M., Gigliobianco M.R., Peregrina D.V., Sagratini G., Censi R., Di Martino P. Quantification of phenolic compounds in different types of crafts beers, worts, starting and spent ingredients by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2020;1612 doi: 10.1016/j.chroma.2019.460622. [DOI] [PubMed] [Google Scholar]
- Costa R., Rodrigues I., Guardão L., Rocha-Rodrigues S., Silva C., Magalhães J., et al. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017;45:39–47. doi: 10.1016/j.jnutbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Delmulle L., Bellahcène A., Dhooge W., Comhaire F., Roelens F., Huvaere K., et al. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine. 2006;13:732–734. doi: 10.1016/j.phymed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Delso C., Berzosa A., Sanz J., Ignacio Á., Raso J. Microbial decontamination of red wine by pulsed electric fields (PEF) after alcoholic and malolactic fermentation: effect on Saccharomyces cerevisiae, oenococcus oeni, and oenological parameters during storage. Foods. 2023;12:278. doi: 10.3390/foods12020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Đorđević S., Popović D., Despotović S., Veljović M., Atanacković M., Cvejić J., et al. Extracts of medicinal plants as functional beers additives. Chem. Ind. Chem. Eng. Q. 2016;22:301–308. doi: 10.2298/CICEQ150501044D. [DOI] [Google Scholar]
- dos Santos J.P., Acunha T. dos S., Prestes D.N., Rombaldi C.V., El Halal S.L.M., Vanier N.L. From brown, red, and black rice to beer: changes in phenolics, γ-aminobutyric acid, and physicochemical attributes. Cereal Chem. 2020;97:1148–1157. doi: 10.1002/cche.10335. [DOI] [Google Scholar]
- Durán-Sánchez A., de la Cruz del Río-Rama M., Álvarez-García J., Oliveira C. Vol. 12. SAGE Open; 2022. (Analysis of Worldwide Research on Craft Beer). [DOI] [Google Scholar]
- Engelsgjerd S., Kunnimalaiyaan S., Kandil E., Clark Gamblin T., Kunnimalaiyaan M. Xanthohumol increases death receptor 5 expression and enhances apoptosis with the TNF-related apoptosis-inducing ligand in neuroblastoma cell lines. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Geurts L., van Roye M., Delzenne N.M., Cani P.D. Tetrahydro iso-alpha acids from hops improve glucose homeostasis and reduce body weight gain and metabolic endotoxemia in high-fat diet-fed mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanari M., Forteschi M., Sanna M., Piu P.P., Porcu M.C., D’hallewin G., et al. Pilot plant production of craft fruit beer using Ohmic-treated fruit puree. J. Food Process. Preserv. 2020;44 doi: 10.1111/jfpp.14339. [DOI] [Google Scholar]
- Fernández-Solà J. Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat. Rev. Cardiol. 2015;12:576–587. doi: 10.1038/nrcardio.2015.91. [DOI] [PubMed] [Google Scholar]
- Filho R.C.N., Galvan D., Effting L., Terhaag M.M., Yamashita F., Benassi M. de T., et al. Effects of adding spices with antioxidants compounds in red ale style craft beer: a simplex-centroid mixture design approach. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130478. [DOI] [PubMed] [Google Scholar]
- Garofalo C., Osimani A., Milanović V., Taccari M., Aquilanti L., Clementi F. The occurrence of beer spoilage lactic acid bacteria in craft beer production. J. Food Sci. 2015;80:M2845–M2852. doi: 10.1111/1750-3841.13112. [DOI] [PubMed] [Google Scholar]
- Gasiński A., Kawa-Rygielska J., Szumny A., Czubaszek A., Gasior J., Pietrzak W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (mangifera indica) Molecules. 2020;25:3033. doi: 10.3390/molecules25133033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiński A., Kawa-Rygielska J., Szumny A., Gąsior J., Głowacki A. Assessment of volatiles and polyphenol content, physicochemical parameters and antioxidant activity in beers with dotted hawthorn (Crataegus punctata) Foods. 2020;9:775. doi: 10.3390/foods9060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Zancada N., Redondo-Useros N., Díaz L.E., Gómez-Martínez S., Marcos A., Nova E. Association of moderate beer consumption with the gut microbiota and SCFA of healthy adults. Molecules. 2020;25:4772. doi: 10.3390/molecules25204772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmotti M., Passaghe P., Buiatti S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020;85:2278–2285. doi: 10.1111/1750-3841.15318. [DOI] [PubMed] [Google Scholar]
- Habschied K., Živković A., Krstanović V., Mastanjević K. Functional beer—a review on possibilities. Beverages. 2020;6:51. doi: 10.3390/beverages6030051. [DOI] [Google Scholar]
- Hernandes K.C., Souza-Silva É.A., Assumpção C.F., Zini C.A., Welke J.E. Carbonyl compounds and furan derivatives with toxic potential evaluated in the brewing stages of craft beer. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2020;37:61–68. doi: 10.1080/19440049.2019.1675911. [DOI] [PubMed] [Google Scholar]
- Hernández-Quiroz F., Nirmalkar K., Villalobos-Flores L.E., Murugesan S., Cruz-Narváez Y., Rico-Arzate E., et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol. 2020;85:77–94. doi: 10.1016/j.alcohol.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Horn P.A., Pedron N.B., Junges L.H., Rebelo A.M., da Silva Filho H.H., Zeni A.L.B. Antioxidant profile at the different stages of craft beers production: the role of phenolic compounds. Eur. Food Res. Technol. 2021;247:439–452. doi: 10.1007/s00217-020-03637-2. [DOI] [Google Scholar]
- Hougee S., Faber J., Sanders A., Van Den Berg W.B., Garssen J., Smit H.F., et al. Selective inhibition of COX-2 by a standardized CO2 extract of Humulus lupulus in vitro and its activity in a mouse model of zymosan-induced arthritis. Planta Med. 2006;72:228–233. doi: 10.1055/s-2005-916212. [DOI] [PubMed] [Google Scholar]
- Hudcová T., Bryndová J., Fialová K., Fiala J., Karabín M., Jelínek L., et al. Antiproliferative effects of prenylflavonoids from hops on human colon cancer cell lines. J. Inst. Brew. 2014;120:225–230. doi: 10.1002/jib.139. [DOI] [Google Scholar]
- Humia B.V., Santos K.S., Barbosa A.M., Sawata M., Mendonça M. da C., Padilha F.F. Beer molecules and its sensory and biological properties: a review. Molecules. 2019;24:1568. doi: 10.3390/molecules24081568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humia B.V., Santos K.S., Schneider J.K., Leal I.L., de Abreu Barreto G., Batista T., et al. Physicochemical and sensory profile of Beauregard sweet potato beer. Food Chem. 2020;312 doi: 10.1016/j.foodchem.2019.126087. [DOI] [PubMed] [Google Scholar]
- Infante-Rivard C., Krajinovic M., Labuda D., Sinnett D. Childhood acute lymphoblastic leukemia associated with parental alcohol consumption and polymorphisms of carcinogen-metabolizing genes. Epidemiology. 2002;13:277–281. doi: 10.1097/00001648-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Jacobo-Velázquez D.A., Santana-Gálvez J., Cisneros-Zevallos L. Designing next-generation functional food and beverages: combining nonthermal processing technologies and postharvest abiotic stresses. Food Eng. Rev. 2021;13:592–600. doi: 10.1007/s12393-020-09244-x. [DOI] [Google Scholar]
- Jaeger S.R., Worch T., Phelps T., Jin D., Cardello A.V. Preference segments among declared craft beer drinkers: perceptual, attitudinal and behavioral responses underlying craft-style vs. traditional-style flavor preferences. Food Qual. Prefer. 2020;82 doi: 10.1016/j.foodqual.2020.103884. [DOI] [Google Scholar]
- Jardim C.C., de Souza D., Machado I.C.K., Pinto L.M.N., Ramos R.C.S., Garavaglia J. Sensory profile, consumer preference and chemical composition of craft beers from Brazil. Beverages. 2018;4:106. doi: 10.3390/beverages4040106. [DOI] [Google Scholar]
- Jeong H.M., Han E.H., Jin Y.H., Choi Y.H., Lee K.Y., Jeong H.G. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem. Biophys. Res. Commun. 2011;409:82–89. doi: 10.1016/j.bbrc.2011.04.113. [DOI] [PubMed] [Google Scholar]
- Koller H., Perkins L.B. Brewing and the chemical composition of amine-containing compounds in beer: a review. Foods. 2022;11:257. doi: 10.3390/foods11030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnic M., Modun D., Budimir D., Gunjaca G., Jajic I., Vukovic J., et al. Comparison of acute effects of red wine, beer and vodka against hyperoxia-induced oxidative stress and increase in arterial stiffness in healthy humans. Atherosclerosis. 2011;218:530–535. doi: 10.1016/j.atherosclerosis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Langley B.O., Ryan J.J., Hanes D., Phipps J., Stack E., Metz T.O., et al. Xanthohumol microbiome and signature in healthy adults (the XMaS trial): safety and tolerability results of a phase I triple-masked, placebo-controlled clinical trial. Mol. Nutr. Food Res. 2021;65:1–14. doi: 10.1002/mnfr.202001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legun K., Comi M., Vicol M. New aesthetic regimes: the shifting global political ecology of aroma hops. Geoforum. 2022;128:148–157. doi: 10.1016/j.geoforum.2021.12.004. [DOI] [Google Scholar]
- Lerro M., Marotta G., Nazzaro C. Measuring consumers' preferences for craft beer attributes through Best-Worst Scaling. Agric. Food Econ. 2020;8:1–13. doi: 10.1186/s40100-019-0138-4. [DOI] [Google Scholar]
- Leskošek-Čukalović I., Despotović S., Nedović V., Lakić N., Nikšić M. New type of beer - beer with improved functionality and defined pharmacodynamic properties. Food Technol. Biotechnol. 2010;48:384–391. [Google Scholar]
- Li F., Shi Y., Boswell M., Rozelle S. In: Economic Perspectives on Craft Beer: A Revolution in the Global Beer Industry. Garavaglia C., Swinnen J., editors. Palgrave Macmillan; Milan: 2018. Potential of Good's buffers to inhibit denaturation of myofibrillar protein upon freezing; pp. 457–484. [DOI] [Google Scholar]
- Li Y., Wang K., Yin S., Zheng H., Min D. Xanthohumol inhibits proliferation of laryngeal squamous cell carcinoma. Oncol. Lett. 2016;12:5289–5294. doi: 10.3892/ol.2016.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori L., De Francesco G., Perretti G., Albanese D. 2020 IEEE International Workshop on Metrology for Agriculture and Forestry, MetroAgriFor 2020 - Proceedings. 2020. Characterization of the main physico-chemical parameters in three styles of craft beer; pp. 314–318. [DOI] [Google Scholar]
- Lima-Fontes M., Costa R., Rodrigues I., Soares R. Xanthohumol restores hepatic glucolipid metabolism balance in type 1 diabetic wistar rats. J. Agric. Food Chem. 2017;65:7433–7439. doi: 10.1021/acs.jafc.7b02595. [DOI] [PubMed] [Google Scholar]
- Liping Z. Isolation and molecular identification of Lactobacillus brevis from spoilage craft beer in China. Lect. Notes Electr. Eng. 2018;444:597–603. doi: 10.1007/978-981-10-4801-2_61. [DOI] [Google Scholar]
- Liu W., He K., Wu D., Zhou L., Li G., Lin Z., et al. Natural dietary compound xanthohumol regulates the gut microbiota and its metabolic profile in a mouse model of alzheimer's disease. Molecules. 2022;27:1281. doi: 10.3390/molecules27041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M.G., Roche E., Rodríguez E. Contaminant microbiota in craft beers. J. Microbiol. Biotechnol. Food Sci. 2020;9:1181–1186. doi: 10.15414/JMBFS.2020.9.6.1181-1186. [DOI] [Google Scholar]
- Lorencová E., Salek R.N., Černíková M., Buňková L., Hýlková A., Buňka F. Biogenic amines occurrence in beers produced in Czech microbreweries. Food Control. 2020;117 doi: 10.1016/j.foodcont.2020.107335. [DOI] [Google Scholar]
- Lupinacci E., Meijerink J., Vincken J.P., Gabriele B., Gruppen H., Witkamp R.F. Xanthohumol from hop (Humulus lupulus L.) Is an efficient inhibitor of monocyte chemoattractant protein-1 and tumor necrosis factor-α release in LPS-Stimulated RAW 264.7 mouse macrophages and U937 human monocytes. J. Agric. Food Chem. 2009;57:7274–7281. doi: 10.1021/jf901244k. [DOI] [PubMed] [Google Scholar]
- Mahli A., Seitz T., Freese K., Frank J., Weiskirchen R., Abdel-Tawab M., et al. Therapeutic application of micellar solubilized xanthohumol in a western-type diet-induced mouse model of obesity, diabetes and non-alcoholic fatty liver disease. Cells. 2019;8:359. doi: 10.3390/cells8040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano M., Iacumin L., Vendrame M., Cecchini F., Comi G., Buiatti S. Craft beer microflora identification before and after a cleaning process. J. Inst. Brew. 2011;117:343–351. doi: 10.1002/j.2050-0416.2011.tb00478.x. [DOI] [Google Scholar]
- Marques D.R., Cassis M.A., Quelhas J.O.F., Bertozzi J., Visentainer J.V., Oliveira C.C., et al. Characterization of craft beers and their bioactive compounds. Chem. Eng. Trans. 2017;57:1747–1752. doi: 10.3303/CET1757292. [DOI] [Google Scholar]
- Martínez‐Montoro J.I., Quesada‐Molina M., Gutiérrez‐Repiso C., Ruiz‐Limón P., Subiri‐Verdugo A., Tinahones F.J., et al. Effect of moderate consumption of different phenolic‐content beers on the human gut microbiota composition: a randomized crossover trial. Antioxidants. 2022;11:696. doi: 10.3390/antiox11040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez N., Urpi-Sarda M., Martinez-Gonzalez M.A., Andres-Lacueva C., Mitjavila M.T. Dealcoholised beers reduce atherosclerosis and expression of adhesion molecules in apoE-deficient mice. Br. J. Nutr. 2011;105:721–730. doi: 10.1017/S0007114510004289. [DOI] [PubMed] [Google Scholar]
- McMahon C. Britain's craft beer “gold rush” has slowed to a drip. Here's how the Irish sector is shaping up. 2019. https://fora.ie/irish-craft-beer-closures-4604271-Apr2019/ Available at:
- Mehlig K., Skoog I., Guo X., Schütze M., Gustafson D., Waern M., et al. Alcoholic beverages and incidence of dementia: 34-Year follow-up of the prospective population study of women in Göteborg. Am. J. Epidemiol. 2008;167:684–691. doi: 10.1093/aje/kwm366. [DOI] [PubMed] [Google Scholar]
- Mellor D.D., Hanna-Khalil B., Carson R. A review of the potential health benefits of low alcohol and alcohol-free beer: effects of ingredients and craft brewing processes on potentially bioactive metabolites. Beverages. 2020;6:25. doi: 10.3390/beverages6020025. [DOI] [Google Scholar]
- Merinas-Amo T., Merinas-Amo R., Font R., Del Río Celestino M., Alonso-Moraga Á. Toxicological and epigenetic studies of two types of ale beer, tyrosol and iso-alpha humulone. Processes. 2021;9:485. doi: 10.3390/pr9030485. [DOI] [Google Scholar]
- Merino P., Santos-López J.A., Mateos C.J., Meseguer I., Garcimartín A., Bastida S., et al. Can nonalcoholic beer, silicon and hops reduce the brain damage and behavioral changes induced by aluminum nitrate in young male Wistar rats? Food Chem. Toxicol. 2018;118:784–794. doi: 10.1016/j.fct.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Milani E.A., Silva F.V.M. Nonthermal pasteurization of beer by high pressure processing: modelling the inactivation of saccharomyces cerevisiae ascospores in different alcohol beers. High Pres. Res. 2016;36:595–609. doi: 10.1080/08957959.2016.1190354. [DOI] [Google Scholar]
- Milburn T., Guertin-Martín F.A. Tapping into environmental harm in brewing: an exploration of pollution and waste in beer production. Crit. Criminol. 2020;28:407–423. doi: 10.1007/s10612-019-09465-5. [DOI] [Google Scholar]
- Miranda C.L., Elias V.D., Hay J.J., Choi J., Reed R.L., Stevens J.F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch. Biochem. Biophys. 2016;599:22–30. doi: 10.1016/j.abb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S., Vives A.E.S., Zucchi O.L.A.D., De Jesus E.F.O., Nascimento Filho V.F., Brienza S.M.B. Analysis of beers from Brazil with synchrotron radiation total reflection X-ray fluorescence. J. Radioanal. Nucl. Chem. 2006;270:167–171. doi: 10.1007/s10967-006-0325-0. [DOI] [Google Scholar]
- Morgan D.R., Lane E.T., Styles D. Crafty marketing: an evaluation of distinctive criteria for “craft” beer. Food Rev. Int. 2022;38:913–929. doi: 10.1080/87559129.2020.1753207. [DOI] [Google Scholar]
- Mulero-Cerezo J., Briz-Redón Á., Serrano-Aroca Á. Saccharomyces cerevisiae var. boulardii: valuable probiotic starter for craft beer production. Appl. Sci. 2019;9 doi: 10.3390/app9163250. [DOI] [Google Scholar]
- Müller M., Gastl M., Becker T. Key constituents, flavour profiles and specific sensory evaluation of wheat style non-alcoholic beers depending on their production method. J. Inst. Brew. 2021;127:262–272. doi: 10.1002/jib.663. [DOI] [Google Scholar]
- Nardini M., Garaguso I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020;305 doi: 10.1016/j.foodchem.2019.125437. [DOI] [PubMed] [Google Scholar]
- Nardini M., Natella F., Scaccini C., Ghiselli A. Phenolic acids from beer are absorbed and extensively metabolized in humans. J. Nutr. Biochem. 2006;17:14–22. doi: 10.1016/j.jnutbio.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Negrão R., Duarte D., Costa R., Soares R. Isoxanthohumol modulates angiogenesis and inflammation via vascular endothelial growth factor receptor, tumor necrosis factor alpha and nuclear factor kappa B pathways. Biofactors. 2013;39:608–622. doi: 10.1002/biof.1122. [DOI] [PubMed] [Google Scholar]
- Neto J.R.O., de Oliveira T.S., Ghedini P.C., Vaz B.G., Gil E. de S. Antioxidant and vasodilatory activity of commercial beers. J. Funct.Foods. 2017;34:130–138. doi: 10.1016/j.jff.2017.04.019. [DOI] [Google Scholar]
- Osorio-Paz I., Brunauer R., Alavez S. Beer and its non-alcoholic compounds in health and disease. Crit. Rev. Food Sci. Nutr. 2020;60:3492–3505. doi: 10.1080/10408398.2019.1696278. [DOI] [PubMed] [Google Scholar]
- Padro T., Muñoz-García N., Vilahur G., Chagas P., Deyà A., Antonijoan R.M., et al. Moderate beer intake and cardiovascular health in overweight individuals. Nutrients. 2018;10:1237. doi: 10.3390/nu10091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais P., Almeida V., Yılmaz M., Teixeira M.C. Saccharomyces boulardii: what makes it tick as successful probiotic? J. Fungi. 2020;6:78. doi: 10.3390/jof6020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallottino F., Cimini A., Costa C., Antonucci F., Menesatti P., Moresi M. Bibliometric analysis and mapping of publications on brewing science from 1940 to 2018. J. Inst. Brew. 2020;126:394–405. doi: 10.1002/jib.625. [DOI] [Google Scholar]
- Palomino-Vasco M., Acedo-Valenzuela M.I., Rodríguez-Cáceres M.I., Mora-Diez N. Automated chromatographic method with fluorescent detection to determine biogenic amines and amino acids. Application to craft beer brewing process. J. Chromatogr. A. 2019;1601:155–163. doi: 10.1016/j.chroma.2019.04.063. [DOI] [PubMed] [Google Scholar]
- Park S., Sim K.-S., Hwangbo Y., Park S.-J., Kim Y.-J., Kim J.-H. Naringenin and phytoestrogen 8-prenylnaringenin protect against islet dysfunction and inhibit apoptotic signaling in insulin-deficient diabetic mice. Molecules. 2022;27:4227. doi: 10.3390/molecules27134227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Wilkens L.R., Setiawan V.W., Monroe K.R., Haiman C.A., Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am. J. Epidemiol. 2019;188:67–76. doi: 10.1093/aje/kwy208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Gómez N., Ruiz-Rico M., Pérez-Esteve É., Fernández-Segovia I., Barat J.M. Microbial stabilization of craft beer by filtration through silica supports functionalized with essential oil components. LWT--Food Sci. Technol. 2020;117 doi: 10.1016/j.lwt.2019.108626. [DOI] [Google Scholar]
- Pereira de Paula B., de Souza Lago H., Firmino L., Fernandes Lemos Júnior W.J., Ferreira Dutra Corrêa M., Fioravante Guerra A., et al. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT--Food Sci. Technol. 2021;135 doi: 10.1016/j.lwt.2020.110233. [DOI] [Google Scholar]
- Peters J., Van Dam R., Van Doorn R., Katerere D., Berthiller F., Haasnoot W., et al. Mycotoxin profiling of 1000 beer samples with a special focus on craft beer. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci R., Di Matteo P., De Francesco G., Mattiello L., Perretti G., Russo P. Novel fast identification and determination of free polyphenols in untreated craft beers by HPLC-PDA-ESI-MS/MS in SIR mode. J. Agric. Food Chem. 2020;68:7984–7994. doi: 10.1021/acs.jafc.0c02802. [DOI] [PubMed] [Google Scholar]
- Petrucci R., Di Matteo P., Sobolev A.P., Liguori L., Albanese D., Proietti N., et al. Impact of dealcoholization by osmotic distillation on metabolic profile, phenolic content, and antioxidant capacity of low alcoholic craft beers with different malt compositions. J. Agric. Food Chem. 2021;69:4816–4826. doi: 10.1021/acs.jafc.1c00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva R.C., Verdan M.H., Mascarenhas Santos M. do S., Batistote M., Cardoso C.A.L. Manufacturing and characterization of craft beers with leaves from Ocimum selloi Benth. J. Food Sci. Technol. 2021;58:4403–4410. doi: 10.1007/s13197-020-04925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans E., Swinnen J. In: Economic Perspectives on Craft Beer: A Revolution in the Global Beer Industry. Garavaglia C., Swinnen J., editors. Palgrave Macmillan; Milan: 2018. Belgium: craft beer nation? pp. 137–160. [DOI] [Google Scholar]
- Postigo V., García M., Cabellos J.M., Arroyo T. Wine saccharomyces yeasts for beer fermentation. Fermentation. 2021;7:290. doi: 10.3390/fermentation7040290. [DOI] [Google Scholar]
- Poveda J.M. Biogenic amines and free amino acids in craft beers from the Spanish market: a statistical approach. Food Control. 2019;96:227–233. doi: 10.1016/j.foodcont.2018.09.012. [DOI] [Google Scholar]
- Prasad C.G.D., Vidyalakshmi R., Baskaran N., Anand M.T. Influence of Pichia myanmarensis in fermentation to produce quinoa based non-alcoholic beer with enhanced antioxidant activity. J. Cereal. Sci. 2022;103 doi: 10.1016/j.jcs.2021.103390. [DOI] [Google Scholar]
- Prestes D.N., Spessato A., Talhamento A., Gularte M.A., Schirmer M.A., Vanier N.L., et al. The addition of defatted rice bran to malted rice improves the quality of rice beer. LWT--Food Sci. Technol. 2019;112 doi: 10.1016/j.lwt.2019.108262. [DOI] [Google Scholar]
- Quesada-Molina M., Muñoz-Garach A., Tinahones F.J., Moreno-Indias I. A new perspective on the health benefits of moderate beer consumption: involvement of the gut microbiota. Metabolites. 2019;9:272. doi: 10.3390/metabo9110272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancán L., Paredes S.D., García I., Muñoz P., García C., López de Hontanar G., et al. Protective effect of xanthohumol against age-related brain damage. J. Nutr. Biochem. 2017;49:133–140. doi: 10.1016/j.jnutbio.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Redondo N., Nova E., Díaz-Prieto L.E., Marcos A. Effects of moderate beer consumption on health. Nutr. Hosp. 2018;35:41–44. doi: 10.20960/nh.2286. [DOI] [PubMed] [Google Scholar]
- Rivaroli S., Calvo-Porral C., Spadoni R. Using food choice questionnaire to explain Millennials' attitudes towards craft beer. Food Qual. Prefer. 2022;96 doi: 10.1016/j.foodqual.2021.104408. [DOI] [Google Scholar]
- Rodríguez-Saavedra M., González de Llano D., Moreno-Arribas M.V. Beer spoilage lactic acid bacteria from craft brewery microbiota: microbiological quality and food safety. Food Res. Int. 2020;138 doi: 10.1016/j.foodres.2020.109762. [DOI] [PubMed] [Google Scholar]
- Roehrer S., Stork V., Ludwig C., Minceva M., Behr J. Analyzing bioactive effects of the minor hop compound xanthohumol C on human breast cancer cells using quantitative proteomics. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson C.M. African traditional beer: changing organization and spaces of South Africa's sorghum beer industry. African Geogr. Rev. 2019;38:253–267. doi: 10.1080/19376812.2019.1589735. [DOI] [Google Scholar]
- Romeo J., Wärnberg J., Nova E., Díaz L.E., González-Gross M., Marcos A. Changes in the immune system after moderate beer consumption. Ann. Nutr. Metab. 2007;51:359–366. doi: 10.1159/000107679. [DOI] [PubMed] [Google Scholar]
- Rossi F., Spigno G., Luzzani G., Bozzoni M.E., Donadini G., Rolla J., et al. Effects of the intake of craft or industrial beer on serum homocysteine. Int. J. Food Sci. Nutr. 2021;72:93–98. doi: 10.1080/09637486.2020.1760219. [DOI] [PubMed] [Google Scholar]
- Saito K., Matsuo Y., Imafuji H., Okubo T., Maeda Y., Sato T., et al. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci. 2018;109:132–140. doi: 10.1111/cas.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salantă L.C., Coldea T.E., Ignat M.V., Pop C.R., Tofană M., Mudura E., et al. Functionality of special beer processes and potential health benefits. Processes. 2020;8:1613. doi: 10.3390/pr8121613. [DOI] [Google Scholar]
- Salantă L.C., Coldea T.E., Ignat M.V., Pop C.R., Tofană M., Mudura E., et al. Non-alcoholic and craft beer production and challenges. Processes. 2020;8:1382. [Google Scholar]
- Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Schink A., Neumann J., Leifke A.L., Ziegler K., Fröhlich-Nowoisky J., Cremer C., et al. Screening of herbal extracts for TLR2-and TLR4-dependent anti-inflammatory effects. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz T., Hackl C., Freese K., Dietrich P., Mahli A., Thasler R.M., et al. Xanthohumol, a prenylated chalcone derived from hops, inhibits growth and metastasis of melanoma cells. Cancers. 2021;13:511. doi: 10.3390/cancers13030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkarcinova B., Graça Dias I.A., Nespor J., Branyik T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT--Food Sci. Technol. 2019;100:362–367. doi: 10.1016/j.lwt.2018.10.082. [DOI] [Google Scholar]
- Serwe A., Rudolph K., Anke T., Erkel G. Inhibition of TGF-β signaling, vasculogenic mimicry and proinflammatory gene expression by isoxanthohumol. Invest. N. Drugs. 2012;30:898–915. doi: 10.1007/s10637-011-9643-3. [DOI] [PubMed] [Google Scholar]
- Silva A.F., Faria-Costa G., Sousa-Nunes F., Santos M.F., Ferreira-Pinto M.J., Duarte D., et al. Anti-remodeling effects of xanthohumol-fortified beer in pulmonary arterial hypertension mediated by ERK and AKT inhibition. Nutrients. 2019;11:583. doi: 10.3390/nu11030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L.C., de Souza Lago H., Rocha M.O.T., de Oliveira V.S., Laureano-Melo R., Stutz E.T.G., et al. Craft beers fermented by potential probiotic yeast or lacticaseibacilli strains promote antidepressant-like behavior in Swiss webster mice. Probiotics Antimicrob. Proteins. 2021;13:698–708. doi: 10.1007/s12602-020-09736-6. [DOI] [PubMed] [Google Scholar]
- Simone N. De, Russo P., Tufariello M., Fragasso M., Solimando M., Capozzi V., et al. Autochthonous biological resources for the production of regional craft beers: exploring possible contributions of cereals, hops, microbes, and other ingredients. Foods. 2021;10:1831. doi: 10.3390/foods10081831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R.F., Hermon C., Liu B., Green J., Reeves G.K., Beral V., et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health. 2019;4:e41–e48. doi: 10.1016/S2468-2667(18)30230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabvandi S., Mortazavian A.M., Rezaei K. Health-related aspects of beer: a review. Int. J. Food Prop. 2012;15:350–373. doi: 10.1080/10942912.2010.487627. [DOI] [Google Scholar]
- STATISTA Consumo de bebidas alcohólicas a nivel mundial en 2021, por segmento. 2022. https://es.statista.com/estadisticas/635627/consumo-mundial-de-las-principales-bebidas-con-alcohol-del-mundo/#:~:text=La cerveza es%2C sin duda,millones de litros de cerveza Available at:
- Stompor M., Uram Ł., Podgórski R. In vitro effect of 8-prenylnaringenin and naringenin on fibroblasts and glioblastoma cells-cellular accumulation and cytotoxicity. Molecules. 2017;22:1092. doi: 10.3390/molecules22071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štulíková K., Voldřichová K., Dostálek P. Influence of pasteurization and high pressure processing on the antioxidant activity of filtered and unfiltered lager beer. J. Food Nutr. Res. 2021;60:229–235. [Google Scholar]
- Styburski D., Janda K., Baranowska-Bosiacka I., Łukomska A., Dec K., Goschorska M., et al. Beer as a potential source of macroelements in a diet: the analysis of calcium, chlorine, potassium, and phosphorus content in a popular low-alcoholic drink. Eur. Food Res. Technol. 2018;244:1853–1860. doi: 10.1007/s00217-018-3098-0. [DOI] [Google Scholar]
- Suh K.S., Rhee S.Y., Kim Y.S., Lee Y.S., Choi E.M. Xanthohumol modulates the expression of osteoclast-specific genes during osteoclastogenesis in RAW264.7 cells. Food Chem. Toxicol. 2013;62:99–106. doi: 10.1016/j.fct.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Tedesco I., Spagnuolo C., Bilotto S., Izzo A.A., Borrelli F., Rigano D., et al. Antioxidant and chemopreventive effect of aliophen® formulation based on malts and hops. Antioxidants. 2021;10:29. doi: 10.3390/antiox10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Beer Times Estudio afirma que el 94% de las cervecerías del mundo son artesanales. 2022. https://www.thebeertimes.com/nuevo-estudio-afirma-94-las-cervecerias-del-mundo-artesanales/ Available at:
- Truelsen T., Thudium D., Grønbæk M. Amount and type of alcohol and risk of dementia: the copenhagen city heart study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]
- Tucker K.L., Jugdaohsingh R., Powell J.J., Qiao N., Hannan M.T., Sripanyakorn S., et al. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am. J. Clin. Nutr. 2009;89:1188–1196. doi: 10.3945/ajcn.2008.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk S., Hong L., Silva F.V.M. Non-thermal high pressure processing, pulsed electric fields and ultrasound preservation of five different table wines. Beverages. 2021;7:69. doi: 10.3390/beverages7040069. [DOI] [Google Scholar]
- Vazquez-Cervantes G.I., Ortega D.R., Ayala T.B., de la Cruz V.P., Esquivel D.F.G., Salazar A., et al. Redox and anti-inflammatory properties from hop components in beer-related to neuroprotection. Nutrients. 2021;13:2000. doi: 10.3390/nu13062000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana A.C., Pimentel T.C., Borges do Vale R., Clementino L.S., Januario Ferreira E.T., Magnani M., et al. American pale Ale craft beer: influence of brewer's yeast strains on the chemical composition and antioxidant capacity. LWT--Food Sci. Technol. 2021;152 doi: 10.1016/j.lwt.2021.112317. [DOI] [Google Scholar]
- Xia T., Zhang J., Guo Y., Jiang Y., Qiao F., Li K., et al. Humulus lupulus L. Extract protects against senior osteoporosis through inhibiting amyloid β deposition and oxidative stress in APP/PS1 mutated transgenic mice and osteoblasts. Molecules. 2023;28:583. doi: 10.3390/molecules28020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Dong J., Yu J., Chang Z., Qian Z., Liu M., et al. A preliminary study about the influence of high hydrostatic pressure processing on the physicochemical and sensorial properties of a cloudy wheat beer. J. Inst. Brew. 2016;122:462–467. doi: 10.1002/jib.344. [DOI] [Google Scholar]
- Yoo J.Y., Groer M., Dutra S.V.O., Sarkar A., McSkimming D.I. Gut microbiota and immune system interactions. Microorganisms. 2020;8:1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata P.J., Martínez-Esplá A., Gironés-Vilaplana A., Santos-Lax D., Noguera-Artiaga L., Carbonell-Barrachina Á.A. Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. LWT--Food Sci. Technol. 2019;103:139–146. doi: 10.1016/j.lwt.2019.01.002. [DOI] [Google Scholar]
- Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.